Abstract
Transgenic mice, which express active Fyn tyrosine kinase under the control of a glial fibrillary acidic protein promoter, have been produced. This promoter induces protein expression in the initiation stage of myelination in the peripheral nervous system (PNS) “Phosphorylation of cytohesin-1 by Fyn is required for initiation of myelination and the extent of myelination during development (Yamauchi et al., 2015 [1])”. Herein we provide the data regarding myelination-related protein markers and myelin ultrastructure in transgenic mice.
Keywords: Fyn, Myelination, Peripheral nervous system, Myelin marker protein, Signaling
Specifications table
| Subject area | Biology |
|---|---|
| More specific subject area | Neurobiology, molecular and cellular neuroscience, developmental biology |
| Type of data | Figure |
| How data was acquired | Electron microscopy, immunoblotting |
| Data format | Raw data, analyzed data |
| Experimental factors | g-Ratios (numerical ratios of axon diameter to diameter of the axon׳s outermost myelinated fibers) for analyzing myelin thickness |
| Experimental features | Electron microscopic analysis, immunoblot |
| Data source location | National Research Institute for Child Health and Development, Tokyo, Japan |
| Data accessibility | Data is available with this article |
Value of the data
-
•
This data set is of value to the scientific community to need the information for molecules controlling myelination.
-
•
The data can provide the method of studying the initiation of myelination in vivo.
-
•
The data may promote further research on signaling molecules controlling myelination in vivo.
1. Data
The data shared in this article is the biochemical analysis for myelination-related proteins in active Fyn transgenic mice. The data also provides myelin ultrastructure in transgenic mice.
2. Experimental design, materials and methods
We generated transgenic mice expressing active Fyn at the relevant developmental stage. The sciatic nerves of these mice were then analyzed through electron microscopy at 3 days postnatal and through immunoblotting of proteins such as myelin markers.
2.1. Data from Fyn transgenic mice
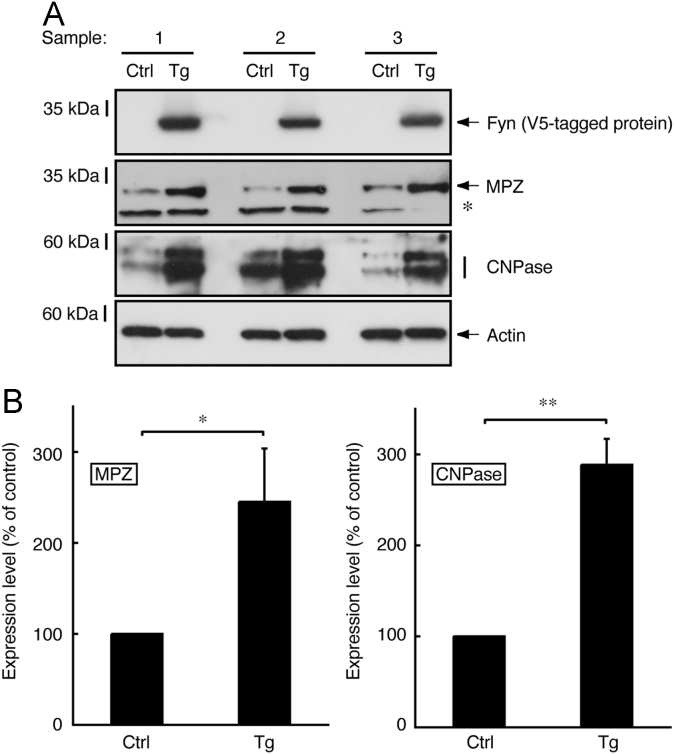
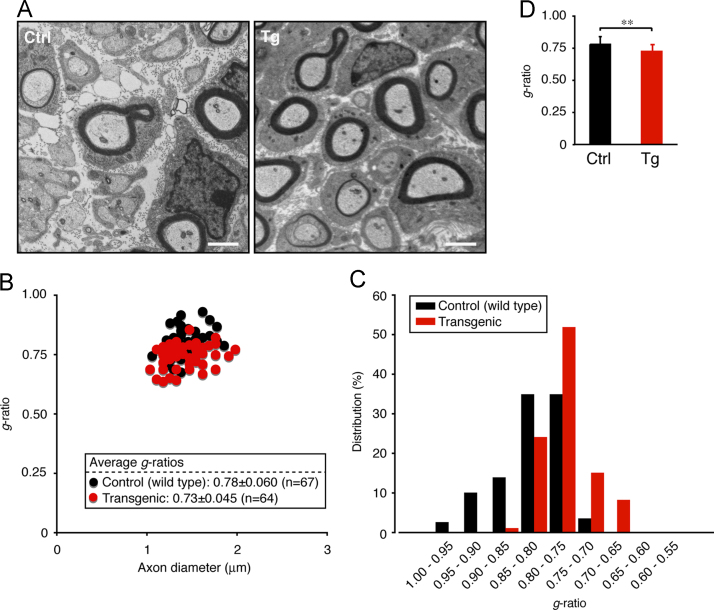
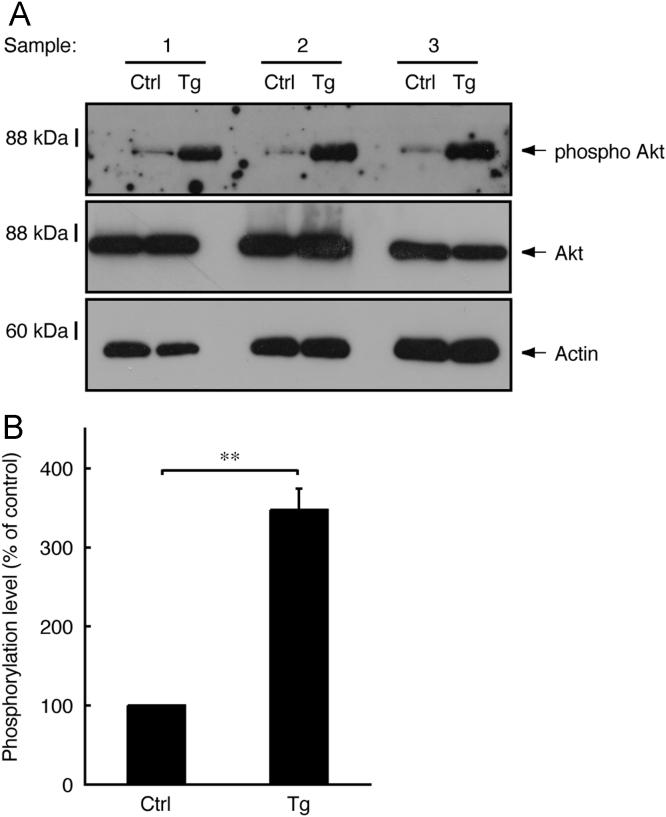
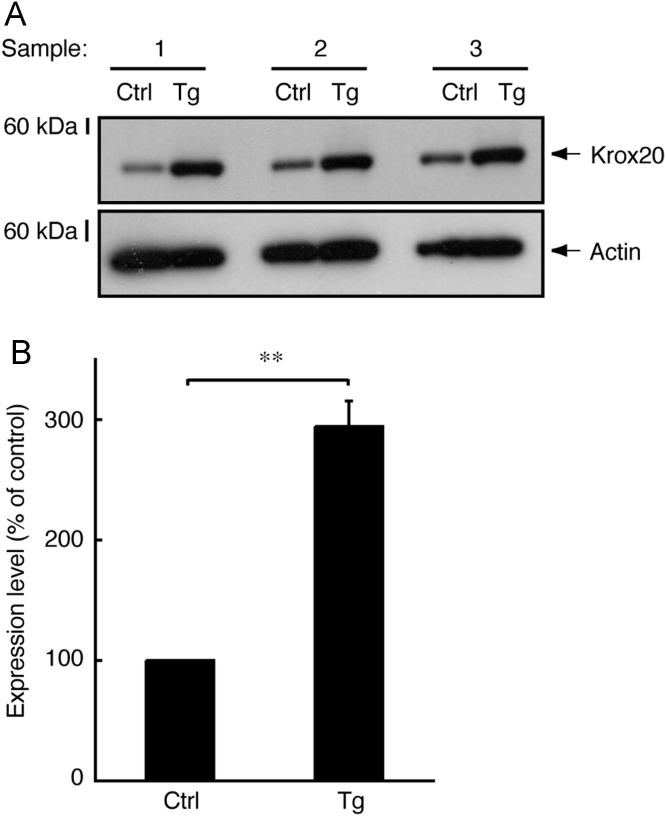
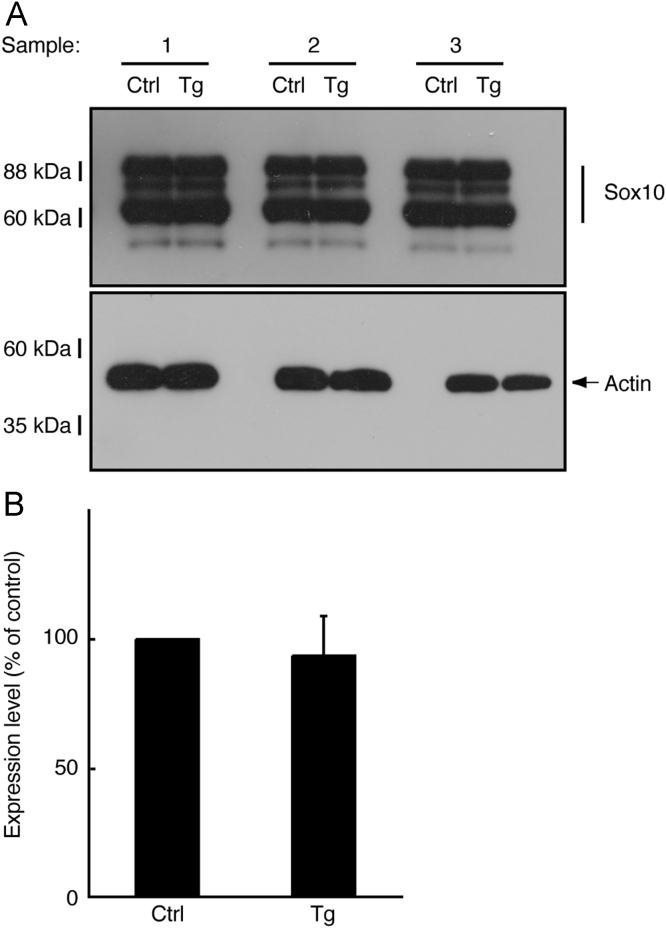
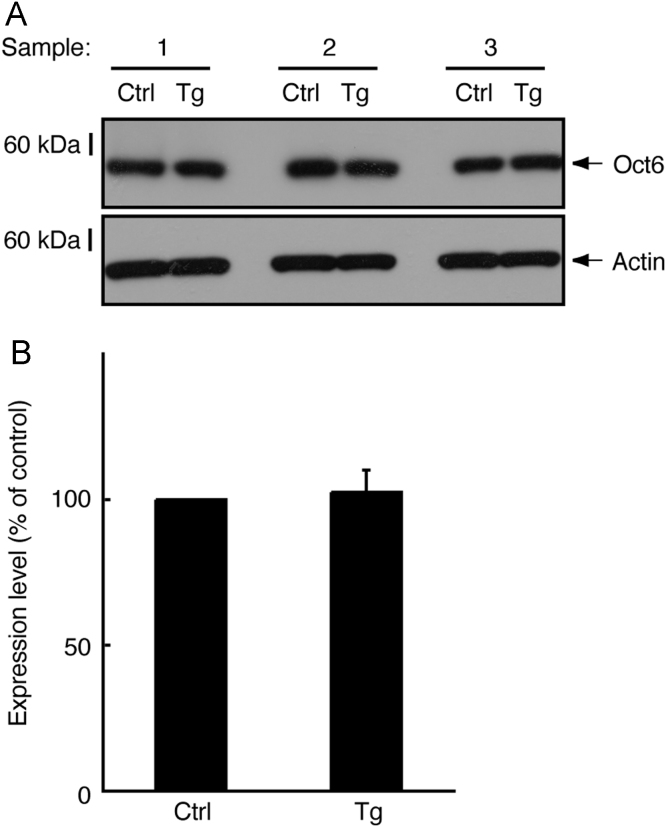
In immunoblotting, neonatal transgenic mice expressing active Fyn exhibited increased expression levels of myelin marker proteins such as myelin protein zero (MPZ, also called P0) and 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase) (Fig. 1A and B). In electron microscopic analysis, transgenic mice exhibited smaller g-ratios, indicating increased myelin thickness, in the sciatic nerves (0.73±0.045 in the transgenic mice compared to 0.78±0.060 in the control mice). Since the g-ratio is the numerical ratio of an axon׳s diameter to the diameter of the axon׳s outermost myelinated fibers [1], [2], [3], a smaller g-ratio indicates a thicker myelin sheath (Fig. 2A–D). In immunoblotting with an antibody specific for phosphorylated Akt kinase (active Akt), increased phosphorylation was observed in samples from transgenic mouse nerves (Fig. 3A and B). Akt is one of the central signal transducers controlling myelination [2], [3], [4], [5]. The myelination-associated transcription factor Krox20 [4], [5] was also increased in transgenic mouse nerves (Fig. 4, A and B). On the other hand, levels of Sox10 (Fig. 5A and B) and Oct6 (Fig. 6A and B), transcription factors expressed in Schwann cell lineage cells [4], [5], were comparable in transgenic mice and controls.
Fig. 1.
Increased expression of myelin marker proteins MPZ and CNPase in transgenic mice expressing active Fyn. (A) Tissue lysates (n=3 mouse samples) from 3-day-old sciatic nerves of active Fyn transgenic (Tg) and control (Ctrl) mice were used for immunoblotting with an anti-V5 tag (for V5-tagged active Fyn), MPZ, CNPase, or actin antibody. Control actin proteins are also shown. The positions of non-specific bands are indicated by an asterisk. (B) The scanned bands (MPZ and CNPase blots) were densitometrically analyzed for quantification. Data were evaluated using Student׳s t-test (**p<0.01; *p<0.05; n=3).
Fig. 2.
Transgenic mice exhibit increased myelin thickness. (A) Representative electron micrographs of 3-day-old transgenic (Tg) or control (Ctrl) mouse sciatic nerve cross sections are shown. The scale bars indicate 1 μm. (B) The g-ratios for 3 mice are plotted for axon diameters. The average g-ratios are also shown in the graph. (C) Distribution of the g-ratios is shown for axon diameters. (D) The g-ratios (n=67 control wild type mouse nerves and n=64 transgenic mouse nerves) were evaluated using Student׳s t-test (**p<0.01).
Fig. 3.
Elevated phosphorylation of Akt in transgenic mice. (A) Tissue lysates (n=3) from 3-day-old sciatic nerves of transgenic (Tg) and control (Ctrl) mice were used for immunoblotting with an anti-phosphorylated pan-Akt, pan-Akt, or actin antibody. (B) The scanned bands were densitometrically analyzed for quantification. Data were evaluated using Student׳s t-test (**p<0.01; n=3).
Fig. 4.
Increased expression of Krox20 in transgenic mice. (A) Tissue lysates (n=3) from 3-day-old sciatic nerves of transgenic (Tg) and control (Ctrl) mice were used for immunoblotting with an anti-Krox20 or actin antibody. (B) The scanned bands were densitometrically analyzed for quantification. Data were evaluated using Student׳s t-test (**p<0.01; n=3).
Fig. 5.
Expression of Sox10 was comparable in transgenic mice and controls. (A) Tissue lysates (n=3) from 3-day-old sciatic nerves of transgenic (Tg) and control (Ctrl) mice were used for immunoblotting with an anti-Sox10 or actin antibody. (B) The scanned bands were densitometrically analyzed for quantification.
Fig. 6.
Expression of Oct6 was comparable in transgenic mice and controls. (A) Tissue lysates (n=3) from 3-day-old sciatic nerves of transgenic (Tg) and control (Ctrl) mice were used for immunoblotting with an anti-Oct6 or actin antibody. (B) The scanned bands were densitometrically analyzed for quantification.
2.2. Generation of active Fyn transgenic mice
A DNA fragment (~4.5 kb) containing the SV40 enhancer, a mouse glial fibrillary acidic protein (GFAP) promoter specific for the neonatal stage of Schwann cells in the PNS [1], [6], [7], V5-epitope-tagged active Fyn (isolated Src homology domain 1 [1]), an artificial intron, and human chorionic gonadotropin polyA units [1], [7] was digested from the vector backbone (~3.5 kb) with NcoI, purified, and injected into fertilized BDF1 oocytes. Transgenic founder mice and established transgenic mice were routinely identified using the KAPA genomic PCR kit (KAPA Biosystems, Wilmington, MA, USA) with the specific primer pair 5′-CCGGAATTCGAATATTAGCTAGGAGTTTCAGAAAGGGGGCCTG-3′ and 5′-CCGGAATTCACTAGTGGGACTATGGTTGCTGACTAATTGAGATGC-3′. PCR was performed in 35 cycles, each consisting of denaturation at 94 °C for 0.5 min, annealing at 65 °C for 0.5 min, and extension at 0.5 °C for 1 min. The transgenic allele yielded PCR bands for 322 bases. One transgenic founder was obtained from every 240 fertilized oocyte injections. Transgenic founders were mated to wild type C57BL/6JJms mice. The transgene was stably maintained for at least 3 generations. Male mice were used for experiments when their gender was distinguishable.
2.3. Immunoblotting
Mouse sciatic nerves were lysed in lysis buffer (50 mM HEPES-NaOH, pH 7.5, 20 mM MgCl2, 150 mM NaCl, 1 mM dithiothreitol, 1 mM phenylmethane sulfonylfluoride, 1 μg/ml leupeptin, 1 mM EDTA, 1 mM Na3VO4, and 10 mM NaF) containing detergents (0.5% NP-40, 1% CHAPS, and 0.1% SDS). The presence of these detergents is important for myelin protein isolation [7], [8]. Equal amounts of the proteins (20 μg total proteins) in centrifuged cell supernatants were heat-denatured for immunoblotting using the MiniProtean TetraElectrophoresis and TransBlot TurboTransfer System (Bio-Rad, Hercules, CA, USA). The transferred membranes were blocked with the Blocking One kit (Nacalai Tesque, Kyoto, Japan) and immunoblotted using primary antibodies, followed by peroxidase-conjugated secondary antibodies (Nacalai Tesque). The bound antibodies were detected using the ImmunoStar Zeta kit (Wako, Osaka, Japan). The scanned bands were densitometrically analyzed for quantification using UN-SCAN-IT Gel software (Silk Scientific, Orem, UT, USA). The following antibodies were used: polyclonal anti-MPZ and monoclonal anti-actin from MBL (Aichi, Japan); polyclonal anti-CNPase, monoclonal anti-pan-Akt, and monoclonal phosphorylated pan-Akt (active, phosphorylated Ser-473) from Cell Signaling Technology (Danvers, MA, USA); anti-Krox20, anti-Oct6, and anti-Sox10 from Abcam (Cambridge, UK); and anti-V5 epitope from Nacalai Tesque.
2.4. Electron microscopic analysis
Mouse sciatic nerves were fixed with 2% paraformaldehyde and 2% glutaraldehyde in 0.1% cacodylate buffer [1], [7]. The tissues were postfixed with buffered 2% osmium tetroxide, dehydrated with an ethanol gradient, treated with acetone, and embedded in epoxy resin. Ultrathin sections of cross sections were stained with uranyl acetate and lead citrate, then observed and photographed with the Hitachi H-7600 or JEOL JEM-2010 electron microscope system. Myelinated nerves in the cross sections were randomly selected, and the g-ratio was calculated for each axon and as an average.
2.5. Statistical analysis
Data are presented as means±SD from independent experiments. Intergroup comparisons were performed using unpaired Student׳s t test. Differences were considered significant when p value was less than 0.05.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
This work was supported by Grants-in-Aid both for Scientific Research from the Japanese MEXT and from the Japanese MHLW. This work was also supported by grants from Innovative Areas’ Scientific Research (the Glial Assembly area and the Comprehensive Brain Science Network area), the Suzuken Science Foundation, and the Takeda Science Foundation.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2016.03.096.
Appendix A. Supplementary material
Supplementary material
References
- 1.Yamauchi J., Miyamoto Y., Torii T., Takashima S., Kondo K., Kawahara K., Nemoto N., Chan J.R., Tsujimoto G., Tanoue A. Phosphorylation of cytohesin-1 by Fyn is required for initiation of myelination and the extent of myelination during development. Sci. Signal. 2012;5:ra69. doi: 10.1126/scisignal.2002802. [DOI] [PubMed] [Google Scholar]
- 2.Taveggia C., Feltri M.L., Wrabetz L. Signals to promote myelin formation and repair. Nat. Rev. Neurol. 2010;6:276–287. doi: 10.1038/nrneurol.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira J.A., Lebrun-Julien F., Suter U. Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 2012;35:123–134. doi: 10.1016/j.tins.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Nave K.A., Werner H.B. Myelination of the nervous system: mechanisms and functions. Annu. Rev. Cell. Dev. Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- 5.Salzer J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015;7:a020529. doi: 10.1101/cshperspect.a020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monje P.V., Soto J., Bacallao K., Wood P.M. Schwann cell dedifferentiation is independent of mitogenic signaling and uncoupled to proliferation: role of cAMP and JNK in the maintenance of the differentiated state. J. Biol. Chem. 2010;285:31024–31036. doi: 10.1074/jbc.M110.116970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torii T., Miyamoto Y., Onami N., Tsumura H., Nemoto N., Kawahara K., Kato M., Kotera J., Nakamura K., Tanoue A., Yamauchi J. in vivo expression of the Arf6 guanine-nucleotide exchange factor cytohesin-1 in mice exhibits enhanced myelin thickness in nerves. J. Mol. Neurosci. 2013;51:522–531. doi: 10.1007/s12031-013-0018-4. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto Y., Torii T., Yamamori N., Ogata T., Tanoue A., Yamauchi J. Akt and PP2A reciprocally regulate the guanine nucleotide exchange factor Dock6 to control axon growth of sensory neurons. Sci. Signal. 2013;6:ra15. doi: 10.1126/scisignal.2003661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material