Abstract
The ACTOne cannabinoid receptor 1 functional system is comprised of transfected HEK cells with the parental cyclic nucleotide gated channel (CNG) co-transfected with cannabinoid receptor 1 (CB1). The ACTOne CB1 cell line was evaluated for cAMP driven fluorescence by optimizing experimental conditions for sensitivity to forskolin and CP 55,940, reading time point, reliability of cell passage number, and pertussis inactivation of Gi/o.
Keywords: ACTOne, Cannabinoids, CB1, Pharmacology, cAMP
Specifications table
| Subject area | Biology |
| More specific subject area | Pharmacology |
| Type of data | Graph |
| How data was acquired | Biotek Synergy 2 Plate reader |
| Data format | Raw fluorescent values and normalized values |
| Experimental factors | Forskolin concentration, cell passage number, reading time point, pertussis concentration |
| Experimental features | HEK cells with CNG+CB1 receptor were optimized for evaluating ligand Gi/o and Gs pharmacology at the CB1 receptor |
| Data source location | Memphis, TN, USA |
| Data accessibility | Data in the article |
Value of the data
-
•
These data establish a new functional cell line that evaluates putative CB1 ligands for pharmacologic activity.
-
•
The fluorescent detection of cAMP changes allows for a high throughput method of detection that can then be compared to the parental cell line to determine off-target influences.
-
•
This system can also be pretreated with pertussis toxin to reveal CB1 Gs coupling specific to cannabinoid ligands.
1. Data
The ACTOne CB1 cell line was optimized for experimental conditions related to 96 well plate reader detection of cAMP driven fluorescence. Cells were probed for optimal forskolin stimulation of cAMP driven fluorescence, the concentration of CB1 agonist CP 55,940 needed to suppress cAMP driven fluorescence, as well as the time point for most stable fluorescent signal. Furthermore, the robustness of the ACTOne CB1 cells was evaluated with respect to cell passage number and the ability of the cell line to reveal Gs coupled responses following pertussis toxin Gi/o inactivation.
2. Experimental design, materials and methods
2.1. Plating conditions
Cells were plated in DMEM with 10% FBS and 1% P/S at 50,000 cells/100 µL well in 96 well poly-d-lysine plates and left overnight at 37 °C, 5% CO2. Prior to testing, 100 µL of Membrane Potential dye was added to each well, then the plate was then incubated for 1 hour in the dark at room temperature. Plates were read (Ex 540 nm, Em 590 nm) at baseline, and then after addition of compounds plates were read for 60 min.
2.2. Forskolin and CP 55,490 optimization
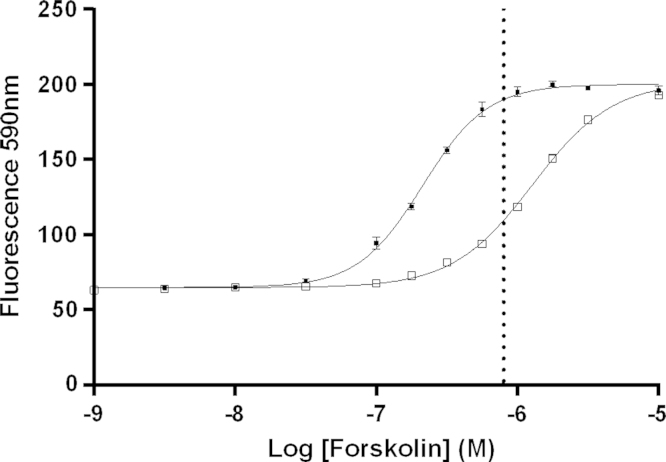
ACTOne HEK-CNG+CB1 cells were evaluated for optimal sensitivity to forskolin driven cAMP fluorescent signal by exposing the cells to increasing concentrations of forskolin from 10−4 to 10−9 M (Fig. 1). Fluorescent signal plateau began at 800 nM forskolin, and suppression of forskolin signal using CB1 agonist CP 55,940 at 1 µM resulted in a significant dextral shift in the EC50 (212.8 vs. 1284 nM, p<0.0001) (Fig. 1).
Fig. 1.
Forskolin effects in ACTOne CB1 cells (black circles) and the suppression by CP 55,940 (white squares), N=6, error is SEM. Vertical line is 800 nM forskolin.
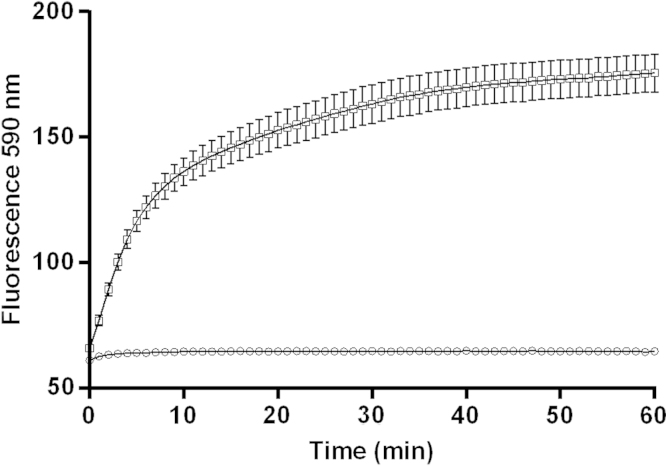
Evaluation of the optimal time point for fluorescent signal was determined to not be significantly different from 30 to 60 min, but a time point of 50 min was appropriate for technical efficiency and coincided with manufacturer׳s recommendations (Fig. 2) [1]. Baseline did not change during the incubation.
Fig. 2.
Kinetic evaluation of cAMP driven fluorescent signal at 1 min intervals in ACTOne CB1 cells with no forskolin (baseline – white circles) and 800 nM forskolin (white squares). N=20, error is SEM.
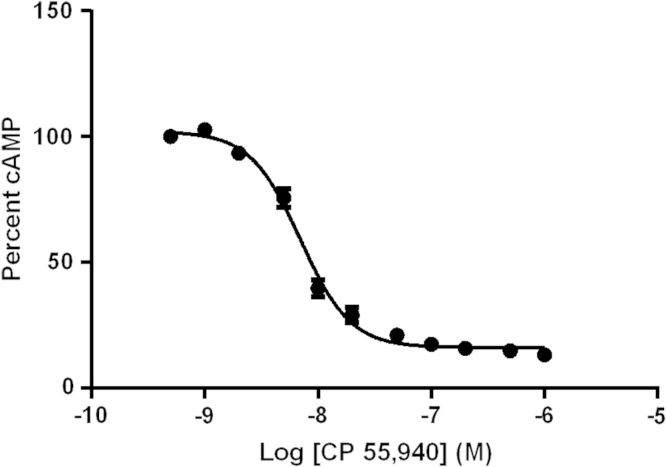
Response of ACTOne CB1 cells was normalized to forskolin as maximum signal and a blank baseline as minimum signal. The normalized response of ACTOne CB1 cells was determined to be robust and reproducible with respect to CP 55,490 suppression of cAMP from passages 3–36 (Fig. 3).
Fig. 3.
Normalized signal cAMP driven fluorescence of CP 55,490 in ACTOne CB1 cells from passage 3 to 36. N=26 from all control runs in [2] with EC50 7.00±1.2 nM, error bars are SEM.
2.3. Pertussis toxin optimization
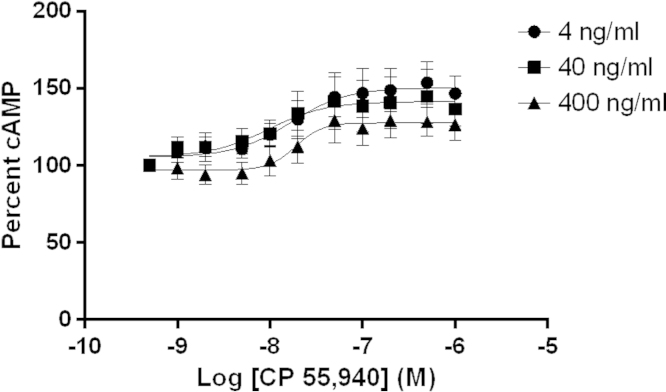
The optimal pertussis toxin (PTx) dose of 4 ng/mL was determined to reveal CB1 Gs coupling associated with CP 55,940 (Fig. 4). While increasing concentrations of PTx did not result in statistically significant changes in cAMP driven fluorescence, signal was lower at 400 ng/mL and visual inspection revealed lower cell confluence at higher PTx concentrations (Fig. 5).
Fig. 4.
Pertussis inactivation of Gi/o in ACTOne CB1 cells reveals Gs coupling. N=6, error is SEM.
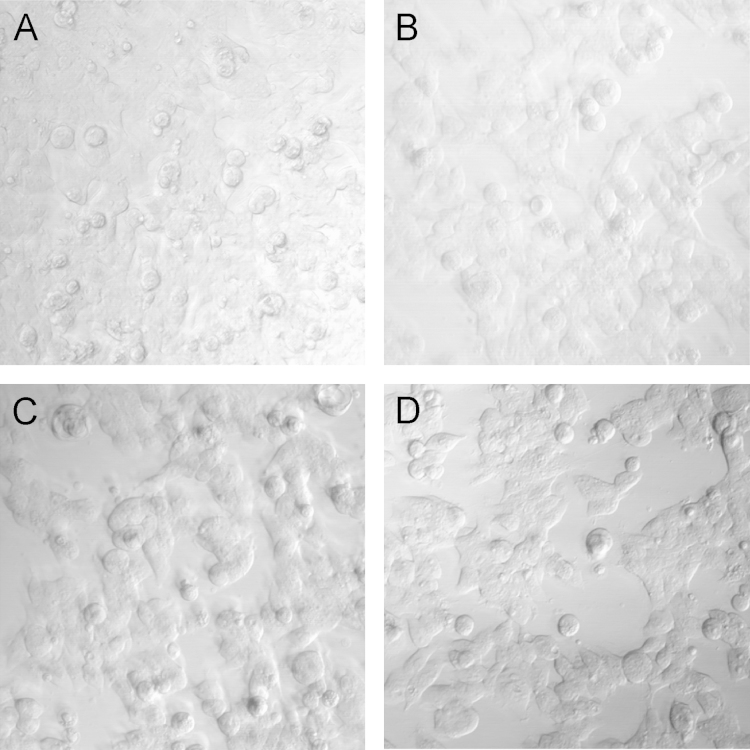
Fig. 5.
Overnight treatment of ACTOne CB1 cells with PTx: (A) 0 ng/mL, (B) 4 ng/mL, (C) 40 ng/mL, and (D) 400 ng/mL revealed lower cell confluence with higher concentrations.
2.4. Kinetic evaluation
Experiments with ACTOne CB1 cells were run for 60 min and cAMP signals were evaluated at one minute intervals. Codex Biosolutions recommended reading fluorescence at 50 min [1]. Fluorescent signal was not significantly different from 60 min (p=0.792, t-test).
2.5. Normalization
ACTOne assay data were normalized using a feature scaling equation:
where forskolin only stimulation is the experimental maximum B, a blank well with dye and cells only is the experimental minimum A, normalized value is Xʹ, Xmax is 100%, and Xmin is 0%. Data were then analyzed using non-linear regression analysis.
2.6. Pertussis Inactivation studies
Pertussis (PTx) dose response was performed in ACTOne CB1 cells with 0, 4, 40, or 400 ng/mL (Fig. 4). Cells were incubated overnight with PTx and run against CP 55,940 the next day. Visual inspected indicated that higher concentrations of PTx resulted in lower cell attachment to plates (Fig. 5). 4 ng/mL of PTx resulted in the strongest CP 55,940 Gs effect on cAMP while giving the best cellular confluence.
Acknowledgements
Dr. Jimmy Lu from Codex Biosolutions provided both the ACTOne cell lines and the support to get the assay started. Funding for Chaela Presley was supplied by the University of Tennessee Neuroscience Institute and College of Pharmacy. Funding for Ammaar Abidi was supplied by the University of Tennessee College of Dentistry.
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j. dib.2016.03.086.
Appendix A. Supplementary material
Supplementary material
References
- 1.Data Sheet – ACTOne GPCR Cannabinoid Receptor 1. 〈http://codexbiosolutions.com/pdflink/8fb161784c98c551b6c15e91ff63178cCB-80300-205-Data%20sheet-b.pdf: Codex Biosolutions〉.
- 2.Presley C.S., Abidi A.H., Moore B.M. Cannabinoid receptor 1 ligands revisited: pharmacologic assessment in the ACTOne system. Anal. Biochem. 2016;498:8–28. doi: 10.1016/j.ab.2015.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material