Abstract
Aim
This study explored the clinical and economic impact of clinical pharmacological advice (CPA) (based on therapeutic drug monitoring [TDM] results, and on patients' characteristics and co‐medications) on personalized linezolid therapy in a tertiary care hospital.
Methods
A 1 year retrospective analysis of quality indicators of CPA (clinicians' adherence rate to CPA, pre‐post rate of linezolid trough concentrations within the desired range and cost balance analysis) was conducted.
Results
Five hundred and forty‐four CPAs were provided to clinicians during 2014 for personalizing linezolid therapy in 168 patients. Clinicians' adherence to CPAs was very high (94.7%). The pre‐post rate of linezolid C min distribution showed a favourable impact of CPA on patient care (pre‐post ratio of C min within the desired range + 23.4%, pre, 51.2% vs. post, 74.6%). Overall, linezolid dosage was mainly reduced (56.9% of cases), whereas dose augmentation was needed only in a minority of cases (7.7%). Cost balance analysis showed that overall 1258 standard doses of linezolid (unitary dose 600 mg) were spared for treating 168 patients with a personalized dosage for a median duration of 11 days (range 3–128 days) with a cost saving of 60038.05 €.
Conclusion
Active computerized advice elaborated by the clinical pharmacologist on the basis of TDM results and of patient's pathophysiological data and co‐medications may be cost‐effective for personalizing linezolid treatment.
Keywords: clinical pharmacological advice, cost balance analysis, linezolid, therapeutic drug monitoring
What is Already Known About this Subject
Targeting plasma trough concentration (C min) of linezolid between 2 and 7 mg l−1 may improve safety outcomes during linezolid therapy while retaining efficacy.
Therapeutic drug monitoring (TDM) may poorly influence clinicians' prescribing attitudes whenever it is not supported by appropriate suggestions concerning dosage adjustments.
Cost benefit analysis may influence stakeholder decisions about implementation of TDM programmes.
What this Study Adds
Clinical pharmacological advice (CPA) may enable very high adherence rates by clinicians to dosing recommendations based on TDM of linezolid.
TDM‐based CPA may consistently increase the rate of desired and safe C min (+ 23.4%) during linezolid therapy.
TDM‐based CPA may allow consistent cost saving especially for long term therapy with linezolid.
Introduction
Clinical pharmacology is devoted to promote the rational use of drugs and in the era of personalized medicine, drug dosage individualization has fully become part of its mission 1. Therapeutic drug monitoring (TDM) is considered a valuable tool for achieving this goal in several clinical settings, especially when dealing with low therapeutic index drugs 2.
In order to maximize the usefulness of TDM results for clinicians, the TDM report should be integrated with timely clinical pharmacological advice (CPA) 3. The CPA is a TDM‐based procedure managed by the clinical pharmacologists whose aim is to provide clinicians with the most appropriate dosing regimen for a given drug according to the patient's pathophysiological conditions and/or to each potential drug–drug interaction due to co‐medications 4.
In the last decade TDM has had a resurgence in the field of antimicrobial chemotherapy, mostly driven by the worldwide increase in bacterial resistance challenging the need for optimal antimicrobial exposure 2. Among others, TDM has proved of utmost importance in maximizing the pharmacodynamics of the beta‐lactams against Gram‐negatives. This is especially true for critically ill patients who may exhibit wide pharmacokinetic variability as a consequence of the severity of sepsis and of unstable clinical conditions 5, 6.
Recently, several authors have also advocated a role for TDM for linezolid. Linezolid is an oxazolidinone antibiotic active against multi‐drug resistant (MDR) Gram‐positives, whose plasma exposure after a standard fixed dosing regimen was shown to vary greatly in daily clinical practice 7, 8. Linezolid is approved for the treatment of pneumonia and of skin and soft tissue infections. However, its use has also been progressively extended in recent years to other indications 9, including bone and joint infections, MDR tuberculosis or systemic nocardiosis 10, 11, 12. This is probably related to its favourable pharmacokinetic/pharmacodynamic profile (excellent bioavailability, good tissue penetration, non‐renal non‐hepatic elimination). Wide interinvividual pharmacokinetic variability of linezolid was attributed to drug–drug interactions and/or to variability in patients' renal function 13, 14, 15, 16, 17. A therapeutic range has been proposed for linezolid trough concentrations (C min) and for area under the plasma concentration vs. time curve (AUC) with the intent of minimizing both the risk of treatment failure and that of dose‐dependent thrombocytopenia 17, 18, 19.
Generally, TDM is a well‐accepted clinical practice which may improve the quality of care at a patient's bedside while preventing health care costs associated both with treatment failure and with the occurrence of drug‐related toxicity. Nevertheless, only a rather limited number of studies have assessed its cost‐effectiveness 20 and sound evidence in favour of this has been ascertained only for aminoglycosides 21, 22 and for vancomycin 23, 24. In times of increasing financial pressure on hospital chief executive officers, assessment of the economic impact of new TDM approaches should be carefully considered.
This study explored quality indicators of clinical pharmacological advice for personalized linezolid dosing based on TDM in a tertiary care hospital setting.
Methods
This retrospective 1 year study (carried out between January 1 2014 and December 31 2014) analyzed all the CPAs elaborated for linezolid dose adjustments at the Institute of Clinical Pharmacology of the Azienda Ospedaliero‐Universitaria of Udine, Italy.
Linezolid TDM and CPA elaboration
At our Institute TDM of linezolid has been carried out since 2003 with a thrice weekly schedule (Monday, Wednesday and Friday). A streamlined process based on three subsequent phases (pre‐analytical phase, analytical phase and post‐analytical phase) has been implemented over time with the intent of enabling the elaboration of timely CPA 3. Briefly, during the pre‐analytical phase, blood samples, after collection in the early morning of the scheduled day, are rapidly brought to the Institute together with a paper form previously filled in by the attending physician with the most relevant clinical information (patient's demographics, indication for linezolid use, total daily dose and route of administration of linezolid, underlying diseases, co‐medications). In the analytical phase, samples are processed in the laboratory by means of a validated HPLC method, as previously described 25, 26. In the post‐analytical phase, TDM results are inserted by the technicians in the hospital intranet system and made available to clinical pharmacologists for CPA elaboration. The CPA suggests to clinicians how to adapt dosage of linezolid taking into account both TDM results and patient data 19. The adaptation is usually based on empirical proportional dose modification. In the case of oral administration, whenever the recommended single dose of linezolid was different from the standard 600 mg, the suspension formulation was used. The desired therapeutic range of linezolid (C min between 2 and 7 mg l−1 enabling AUC(0,24 h) between 160 and 300 mg l−1 h−1) is based on the findings of a previous study of our group 19. Maintenance of C min and AUC within these intervals was shown to improve safety outcomes (low risk of linezolid‐induced thrombocytopenia) while retaining appropriate efficacy in adult patients with infections requiring prolonged treatment with linezolid 19. CPAs are made available to the attending physicians through the hospital intranet system, usually within the mid‐afternoon of the same day of the TDM analysis.
Assessment of clinical efficacy and safety
Clinical outcome was defined as cured, improved or dead for other reasons according to linezolid treatment response. A patient was classified as cured if signs and symptoms of the infection disappeared after linezolid treatment, as improved in cases of partial clinical and/or laboratory evidence of response to linezolid with subsequent de‐escalation to other Gram‐positive medications and as dead for other reasons in cases of death unrelated to the infection for which linezolid was administered. Criteria of exclusion from this analysis were emipiric combination therapy due to suspected mixed Gram‐positive/Gram‐negative infections, withdrawal of linezolid therapy because of Gram‐negative bacterial infections or fungal infections and death because of other underlying diseases. As far as safety evaluation was concerned, thrombocytopenia was defined as a reduction greater than 30% of platelet count from baseline.
Linezolid consumption balance analysis
Linezolid consumption balance analysis was conducted for each course of therapy. First, the total amount of linezolid (expressed in mg) administered to each patient during the whole treatment course was calculated. Then, the theoretical total amount of linezolid that the same patient would have received if treated for the same duration with the fixed daily dosage of 600 mg every 12 h was calculated. Finally, the difference between the two was divided by the unitary dose, namely 600 mg, to define the number of standard doses that were spared or used in excess.
Cost balance analysis
Cost balance analysis was conducted for each linezolid therapy course. First, the real cost of the linezolid optimized therapy was calculated by adding to the cost of the drug administered day by day to each patient that of the TDM analyses and of the CPA elaboration (OPT‐Reg). Then, the theoretical cost of a standard course of therapy of the same duration based on the fixed‐dosage of 600 mg every 12 h (ST‐Reg) was calculated. Cost balance was the result of the difference between OPT‐Reg and ST‐Reg.
Quality indicators of CPA
Three quality indicators of CPAs were defined: 1) the rate of clinicians' adherence to the CPAs recommendations, 2) the pre‐post rate of linezolid C min within the desired range and 3) the cost saving analysis of the linezolid treatment courses (OPT‐Reg vs. ST‐Reg).
Statistical analysis
The Kolmogorov–Smirnov test was used to assess whether data were normally or non‐normally distributed. Accordingly, mean ± SD or median and interquartile range (IQR) were used for descriptive statistics. Continuous variables were compared by means of the Student's t‐test or the Mann–Whitney test, as appropriate. P value <0.05 was required for achievement of statistical significance.
All statistical analysis was performed with Systat version 13 (Systat Software, Inc., USA).
Results
A total of 544 CPAs were elaborated during 2014 for optimizing linezolid therapy in 168 patients. CPAs and patients characteristics are summarized in Table 1.
Table 1.
Clinical pharmacological advice and patients characteristics
| Clinical pharmacological advice (CPA) | |
|---|---|
| Total number of CPAs | 544 |
| Applicant units, n (%) | |
| Medical ward | 73 (43.5) |
| Surgical ward | 66 (39.3) |
| ICU | 29 (17.2) |
| Patients' demographics | |
| Total number of patients | 168 |
| Age (years), mean ± SD | 62.8 ± 17.1 |
| Gender (male/female) | 117/51 |
| Body weight (kg), mean ± SD | 75.1 ± 15.5 |
| Indications for linezolid use, n (%) | |
| Bone and joint infections | 44 (26.2) |
| Hospital‐acquired pneumonia | 35 (20.8) |
| Severe sepsis/septic shock | 31 (18.4) |
| CNS infections | 19 (11.3) |
| Skin and soft tissue infections | 18 (10.7) |
| Cardiosurgical infections/endocarditis | 17 (10.1) |
| Intra‐abdominal infections | 2 (1.2) |
| Nocardiosis | 1 (0.6) |
| MDR‐tuberculosis | 1 (0.6) |
| Number of CPAs per patient, median (IQR) | 2.0 (1.0–4.0) |
| Type of CPA suggestions, n (%) | |
| At first TDM | |
| Dose confirmation | 86/168 (51.2) |
| Dose reduction | 59/168 (35.2) |
| Dose increase | 23/168 (13.7) |
| At subsequent TDM | |
| Dose confirmation | 270/376 (71.8) |
| Dose reduction | 74/376 (19.7) |
| Dose increase | 32/376 (8.5) |
| Linezolid daily dosage, n (%) | |
| At first TDM (n = 168) | |
| 600 mg every 12 h | 168/168 (100) |
| At subsequent TDM (n = 376) | |
| 600 mg every 12 h | 127/376 (33.8) |
| 450 mg every 12 h | 97/376 (25.8) |
| 600 mg every 24 h | 95/376 (25.3) |
| 450 mg every 24 h | 22/376 (5.8) |
| 450 mg every 8 h | 21/376 (5.6) |
| 600 mg every 8 h | 8/376 (2.1) |
| 300 mg every 24 h | 6/376 (1.6) |
| Route of linezolid administration (oral/i.v.) (%) | 75.6/24.4 |
| Linezolid Cmin (mg l−1), median (IQR) | 5.0 (2.9–8.0) |
| Duration of optimized therapy (days), median (IQR) | 11.0 (5.0–27.0) |
CPA, clinical pharmacological advice; CNS, central nervous system; ICU, intensive care units; i.v., intravenous route of administration; oral, oral route of administration; MDR, multi drug‐resistant; SST, skin and soft tissue; TDM, therapeutic drug monitoring
Most CPA applications for linezolid dosage personalization came from the medical wards (43.5%). Bone and joint infections, hospital acquired pneumonia and severe sepsis/septic shock accounted for more than half (56.6%) of the indications for linezolid use.
At first TDM instance, while patients were receiving the standard 600 mg every 12 h dosage, linezolid C min was within the desired range in 51.2% of cases, and the CPAs confirmed the initial dosage. However, in the remaining 48.8% of cases CPAs recommended dosage adjustments due to inappropriate drug exposure (dosage decrease in 35.2% of cases and dosage increase in 13.7% of cases).
For 117 out of 168 patients (69.6%) a re‐application for CPA was submitted in subsequent occasions (n = 376). Median (IQR) number of CPAs per patient was 2.0 (1.0–4.0) and 22.6% of patients (38/168) had ≥5 CPAs during the treatment course. Median (IQR) duration of linezolid therapy was 11 days (5.0–27.0), with a wide range of variability (3–118 days). The rate of subsequent CPAs further suggesting the need for dosage adjustments decreased to 28.2% of cases (dosage decrease in 19.7%, dosage increase in 8.5% of cases).
Interestingly, the rate of clinicians' adherence to the provided CPAs was very high (356/376, 94.7%) (Figure 1) and the rate of linezolid C min within the desired range greatly improved over time (Figure 2). Among the 38 patients who had five or more CPAs applied, the incremental rate was 23.4% (from 51.2% to 74.6%).
Figure 1.
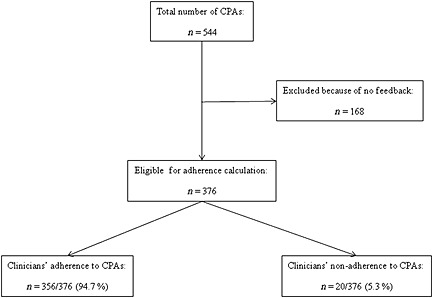
Algorithm for calculation of clinicians' adherence to clinical pharmacological advice for personalization of linezolid therapy
Figure 2.
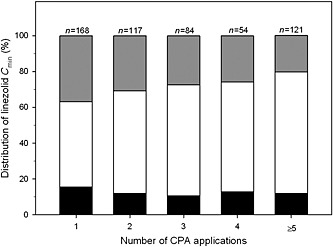
Distribution of linezolid trough concentrations (C
min) in relation to the number of applications for clinical pharmacological advice (CPA). The desisred range was a C
min between 2 and 7 mg l−1.  under the desired range,
under the desired range,  over the desired range,
over the desired range,  within the desired range
within the desired range
Linezolid consumption balance analysis showed that the personalization of therapy led to a consistent sparing in terms of standard doses (1258 doses spared in 168 patients). The relationship between the number of standard doses which were spared per each patient and the duration of therapy was assessed by non‐linear regression analysis, and was best explained by a polynomial cubic regression equation (r 2 = 0.607, P < 0.001).
In regard to clinical outcome, 58.3% of patients (98/168) were fully assessable for linezolid efficacy [23.2% (39/168) were non‐evaluable because of emipiric combination therapy against mixed Gram‐positive/Gram‐negative bacteria, 9.5% (16/168) were excluded because linezolid therapy was stopped due to Gram‐negative bacterial infections or fungal infections and 8.9% (15/168) were excluded because of death related to other underlying diseases]. Overall, 98.0% of assessable patients had a favourable clinical outcome (96/98 overall, 78/98 of whom were cured and 18/98 were improved). In the other two patients (2/98, 2.0%) linezolid therapy was stopped due to drug‐related toxicity (hyperlactacidaemia in one case and neurotoxicity in the other). Interestingly, both of these patients had supra‐therapeutic linezolid C min > 10 mg l−1 during their therapeutic course. With regards to safety, thrombocytopenia occurred in 17.3% of patients (29/168).
Cost balance analysis showed that the OPT‐Reg led to a cost saving of 60038.05 € for the healthcare system in 2014. Estimation of the theoretical cost saving for OPT‐Reg was performed in a hypothetical patient on the basis of the aforementioned reported non‐linear regression analysis concerning linezolid consumption sparing (Figure 3). The analysis showed that cost saving may become statistically significant (P < 0.05) when treatment is longer than 14 days and may remain significant irrespective of making one to three TDM re‐assessments weekly (Figure 4).
Figure 3.
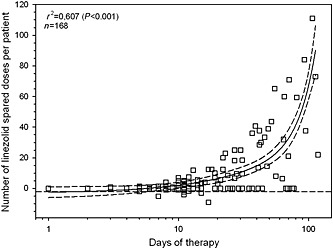
Non‐linear regression analysis between the number of standard doses of linezolid (unitary dose 600 mg) spared per each patient thanks to dosing personalization and the length of therapy. Dotted line identifies no saving
Figure 4.
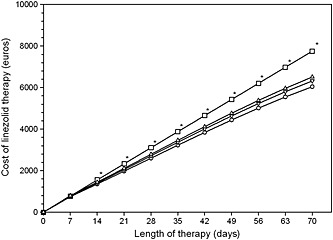
Estimation of optimized linezolid therapy on the basis of once, twice and thrice weekly CPA applications (OPT‐Reg) in comparison with that of the standard fixed dosing regimen (ST‐Reg). Asterisks denote significant difference (P < 0.001) between cost estimates of all of the OPT‐Reg vs. that of the ST‐Reg.  ST‐Reg,
ST‐Reg,  OPT‐Reg with 1 CPA weekly,
OPT‐Reg with 1 CPA weekly,  OPT‐Reg with 2 CPAs weekly,
OPT‐Reg with 2 CPAs weekly,  OPT‐Reg with 3 CPAs weekly
OPT‐Reg with 3 CPAs weekly
Discussion
This study explored the impact that TDM‐based clinical pharmacological advice may have on personalization of linezolid therapy by means of three different quality indicators.
Nowadays TDM has become an invaluable tool for individualizing dosing regimens of several antimicrobial drugs 27, 28, 29. However, those TDM services which provide test results without appropriate interpretation and dosage recommendations may generate costs with limited clinical benefits 13, 20.
Generally, whenever TDM results are not coupled with well‐articulated explanatory advice, the application rate of dosage adjustments by clinicians is reported to be quite low. In a retrospective study assessing the appropriateness of TDM for antidepressant drugs among 191 patients, application of the required dosage adjustments in the absence of suggestions was carried out only in 30% of cases 14. Similarly, in a 5 year observational study involving 475 intensive care unit adult patients who had TDM of vancomycin, overall application rate of dosage adjustments by clinicians themselves by means of pre‐defined standardized self‐explanatory local guidelines was only around 50%. This pushed clinicians to issue a call for active computerized decision support 30.
Our study supports this need, as it showed that interpretation of TDM results by the clinical pharmacologist on the basis of a patient's pathophysiological data and of co‐medications may provide clinicians with active computerized advice leading to very high adherence rates in application of linezolid dosage individualization. We believe that these findings may be explained by at least two factors. The first one is represented by the supply of an extensive programme of educational courses that we delivered during the past 3 years 3. These courses were focused on making hospital physicians and nurses aware of the relevance that personalization of antimicrobial therapy is gaining nowadays for optimal health care management of critically ill infected patients 31. This greatly improved the attention that was paid by the health care personnel in providing the clinical pharmacologists with all of the patients’ data that are necessary for getting valuable CPAs. The second one is represented by the very short turnaround time that we guarantee for our well‐articulated computerized CPAs. This usually allows clinicians prompt application of dosage adjustments at the patient's bedside, generally within a few hours from applying on the scheduled days.
The high adherence rate of clinicians to our advice was coupled with a consistent increase of the pre‐post rate of optimized linezolid C min. This confirms the high reliability of the advice in suggesting appropriate dosage adjustments. The fact that linezolid exposure was sub‐optimal in about half of the cases at first the TDM while patients were receiving the standard 600 mg every 12 h dosage further supports the role of TDM for personalizing linezolid therapy. Our findings are confirmatory of the high pharmacokinetic variability of linezolid previously observed by several authors, including our group 7, 14, 16, 17, 19, 26. This variability was attributed mainly to potential drug–drug interactions 19 and/or to variation in renal function 32 and/or to critical illness 7, and in several cases may be responsible for drug overexposure leading to severe adverse events, as thrombocytopenia and/or hyperlactacidaemia 15, 17, 19. This may be especially relevant for long term treatment, as it may occur for bone and joint infections which represented the main indication for linezolid use in our cohort.
The OPT‐Reg allowed a favourable clinical outcome in almost all of the assessable patients and thrombocytopenia occurred in less than one fifth of cases. Overall, these data seem to support both the efficacy and the safety of this strategy, even if prospective confirmatory studies are obviously warranted.
Linezolid balance consumption analysis and cost balance analysis showed that application of our CPAs for personalizing therapy in 168 patients led to an overall saving of more than 1200 standard linezolid doses with a cost saving of more or less 60 000 €. Interestingly, cost saving may become especially relevant for long term treatments lasting more than 2 weeks. This is frequently the case for bone and joint infections which are usually treated with antimicrobials for 6–8 weeks. Additionally, it may be speculated that this approach may prevent additional costs related to adverse events and/or to treatment failure.
We acknowledge that our study has some limitations. The retrospective design is probably the most relevant. Moreover, it must be recognized that the proposed therapeutic range for linezolid C min was not validated against atypical pathogens and Nocardia. However, favourable clinical outcome in a patient with cerebral nocardiosis was previously reported with this strategy by our group 33. Lastly, we did not consider in the cost balance analysis other potential sources for additional costs, such as those related to the time for CPA elaboration by the clinical pharmacologist or that related to the pharmacist's time for making the required dose adjustments for the intravenous route. However, these costs are indeed very difficult to quantify and may vary according to the skill of the operator.
In conclusion, our study showed that active computerized advice elaborated by the clinical pharmacologist on the basis of TDM results and of patients’ pathophysiological data and co‐medications may be cost‐effective for personalizing linezolid treatment. These findings should represent the basis for a prospective clinical study evaluating the impact of linezolid dose optimization on clinical outcome and drug tolerability.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, FP had consultancy with and received speaking honoraria from Pfizer in the previous 3 years, MB received speaking honoraria from Pfizer in the previous 3 years and PC and LD had no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years. All the authors had no other relationships or activities that could appear to have influenced the submitted work.
Pea, F. , Cojutti, P. , Dose, L. , and Baraldo, M. (2016) A 1 year retrospective audit of quality indicators of clinical pharmacological advice for personalized linezolid dosing: one stone for two birds?. Br J Clin Pharmacol, 81: 341–348. doi: 10.1111/bcp.12806.
References
- 1. Birkett D, Brosen K, Cascorbi I, Gustafsson LL, Maxwell S, Rago L, Rawlins M, Reidenberg M, Sjoqvist F, Smith T, Thuerman P, Walubo A, Orme M, Sjoqvist F. Clinical pharmacology in research, teaching and health care: considerations by IUPHAR, the International Union of Basic and Clinical Pharmacology. Basic Clin Pharmacol Toxicol 2010; 107: 531–59. [DOI] [PubMed] [Google Scholar]
- 2. Eliasson E, Lindh JD, Malmstrom RE, Beck O, Dahl ML. Therapeutic drug monitoring for tomorrow. Eur J Clin Pharmacol 2013; 69: 25–32. [DOI] [PubMed] [Google Scholar]
- 3. Pea F, Dose L, Cojutti P, Baraldo M, Fontana F, Favaretti C, Furlanut M. Educational and organizational interventions to improve the usefulness of clinical pharmacological advice for personalized drug dosing based on therapeutic drug monitoring. Basic Clin Pharmacol Toxicol 2014; 115: 432–7. [DOI] [PubMed] [Google Scholar]
- 4. Watson IP, Yatscoff JR. Therapeutic drug monitoring. Ther Drug Monit 1997; 19: 125. [Google Scholar]
- 5. Roberts JA, Abdul‐Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL, International Society of Anti‐Infective Pharmacology , the Pharmacology and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases . Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 2014; 14: 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roberts JA, Hope WW, Lipman J. Therapeutic drug monitoring of beta‐lactams for critically ill patients: unwarranted or essential? Int J Antimicrob Agents 2010; 35: 419–20. [DOI] [PubMed] [Google Scholar]
- 7. Zoller M, Maier B, Hornuss C, Neugebauer C, Dobbeler G, Nagel D, Holdt LM, Bruegel M, Weig T, Grabein B, Frey L, Teupser D, Vogeser M, Zander J. Variability of linezolid concentrations after standard dosing in critically ill patients: a prospective observational study. Crit Care 2014; 18: R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong H, Wang X, Dong Y, Lei J, Li H, You H, Wang M, Xing J, Sun J, Zhu H. Clinical pharmacokinetic/pharmacodynamic profile of linezolid in severely ill intensive care unit patients. Int J Antimicrob Agents 2011; 38: 296–300. [DOI] [PubMed] [Google Scholar]
- 9. Woodford N, Livermore DM. Infections caused by Gram‐positive bacteria: a review of the global challenge. J Infect 2009; 59: S4–16. [DOI] [PubMed] [Google Scholar]
- 10. Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug‐resistant and extensively drug‐resistant tuberculosis. Lancet Infect Dis 2010; 10: 621–9. [DOI] [PubMed] [Google Scholar]
- 11. Moylett EH, Pacheco SE, Brown‐Elliott BA, Perry TR, Buescher ES, Birmingham MC, Schentag JJ, Gimbel JF, Apodaca A, Schwartz MA, Rakita RM, Wallace RJ Jr. Clinical experience with linezolid for the treatment of nocardia infection. Clin Infect Dis 2003; 36: 313–8. [DOI] [PubMed] [Google Scholar]
- 12. Papadopoulos A, Plachouras D, Giannitsioti E, Poulakou G, Giamarellou H, Kanellakopoulou K. Efficacy and tolerability of linezolid in chronic osteomyelitis and prosthetic joint infections: a case–control study. J Chemother 2009; 21: 165–9. [DOI] [PubMed] [Google Scholar]
- 13. Cojutti P, Maximova N, Crichiutti G, Isola M, Pea F. Pharmacokinetic/pharmacodynamic evaluation of linezolid in hospitalized paediatric patients: a step toward dose optimization by means of therapeutic drug monitoring and Monte Carlo simulation. J Antimicrob Chemother 2015; 70: 198–206. [DOI] [PubMed] [Google Scholar]
- 14. Pea F, Cadeo B, Cojutti PG, Pecori D, Bassetti M. Linezolid underexposure in a hypothyroid patient on levothyroxine replacement therapy: a case report. Ther Drug Monit 2014; 36: 687–9. [DOI] [PubMed] [Google Scholar]
- 15. Pea F, Scudeller L, Lugano M, Baccarani U, Pavan F, Tavio M, Furlanut M, Rocca GD, Bresadola F, Viale P. Hyperlactacidemia potentially due to linezolid overexposure in a liver transplant recipient. Clin Infect Dis 2006; 42: 434–5. [DOI] [PubMed] [Google Scholar]
- 16. Richards GA, Brink AJ. Therapeutic drug monitoring: linezolid too? Crit Care 2014; 18: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsuji Y, Hiraki Y, Matsumoto K, Mizoguchi A, Kobayashi T, Sadoh S, Morita K, Kamimura H, Karube Y. Thrombocytopenia and anemia caused by a persistent high linezolid concentration in patients with renal dysfunction. J Infect Chemother 2011; 17: 70–5. [DOI] [PubMed] [Google Scholar]
- 18. Hiraki Y, Tsuji Y, Hiraike M, Misumi N, Matsumoto K, Morita K, Kamimura H, Karube Y. Correlation between serum linezolid concentration and the development of thrombocytopenia. Scand J Infect Dis 2012; 44: 60–4. [DOI] [PubMed] [Google Scholar]
- 19. Pea F, Viale P, Cojutti P, Del Pin B, Zamparini E, Furlanut M. Therapeutic drug monitoring may improve safety outcomes of long‐term treatment with linezolid in adult patients. J Antimicrob Chemother 2012; 67: 2034–42. [DOI] [PubMed] [Google Scholar]
- 20. Touw DJ, Neef C, Thomson AH, Vinks AA, Cost‐Effectiveness of Therapeutic Drug Monitoring Committee of the International Association for Therapeutic Drug Monitoring and Clinical Therapeutics . Cost‐effectiveness of therapeutic drug monitoring: a systematic review. Ther Drug Monit 2005; 27: 10–7. [DOI] [PubMed] [Google Scholar]
- 21. Streetman DS, Nafziger AN, Destache CJ, Bertino AS Jr. Individualized pharmacokinetic monitoring results in less aminoglycoside‐associated nephrotoxicity and fewer associated costs. Pharmacotherapy 2001; 21: 443–51. [DOI] [PubMed] [Google Scholar]
- 22. van Lent‐Evers NA, Mathot RA, Geus WP, van Hout BA, Vinks AA. Impact of goal‐oriented and model‐based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost‐effectiveness analysis. Ther Drug Monit 1999; 21: 63–73. [DOI] [PubMed] [Google Scholar]
- 23. Darko W, Medicis JJ, Smith A, Guharoy R, Lehmann DE. Mississippi mud no more: cost‐effectiveness of pharmacokinetic dosage adjustment of vancomycin to prevent nephrotoxicity. Pharmacotherapy 2003; 23: 643–50. [DOI] [PubMed] [Google Scholar]
- 24. Fernandez de Gatta MD, Calvo MV, Hernandez JM, Caballero D, San Miguel JF, Dominguez‐Gil A. Cost‐effectiveness analysis of serum vancomycin concentration monitoring in patients with hematologic malignancies. Clin Pharmacol Ther 1996; 60: 332–40. [DOI] [PubMed] [Google Scholar]
- 25. Pea F, Furlanut M, Cojutti P, Cristini F, Zamparini E, Franceschi L, Viale P. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob Agents Chemother 2010; 54: 4605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pea F, Viale P, Lugano M, Pavan F, Scudeller L, Della Rocca G, Furlanut M. Linezolid disposition after standard dosages in critically ill patients undergoing continuous venovenous hemofiltration: a report of 2 cases. Am J Kidney Dis 2004; 44: 1097–102. [DOI] [PubMed] [Google Scholar]
- 27. Roberts JA, Norris R, Paterson DL, Martin JH. Therapeutic drug monitoring of antimicrobials. Br J Clin Pharmacol 2012; 73: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 2009; 53: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014; 74: 839–54. [DOI] [PubMed] [Google Scholar]
- 30. Pea F, Lugano M, Baccarani U, Della Rocca G, Viale P. Biliary pharmacodynamic exposure to linezolid in two liver transplant patients. J Antimicrob Chemother 2014; 69: 567–8. [DOI] [PubMed] [Google Scholar]
- 31. Pea F, Viale P. The antimicrobial therapy puzzle: could pharmacokinetic‐pharmacodynamic relationships be helpful in addressing the issue of appropriate pneumonia treatment in critically ill patients? Clin Infect Dis 2006; 42: 1764–71. [DOI] [PubMed] [Google Scholar]
- 32. Nukui Y, Hatakeyama S, Okamoto K, Yamamoto T, Hisaka A, Suzuki H, Yata N, Yotsuyanagi H, Moriya K. High plasma linezolid concentration and impaired renal function affect development of linezolid‐induced thrombocytopenia. J Antimicrob Chemother 2013; 68: 2128–33. [DOI] [PubMed] [Google Scholar]
- 33. Pea F, Cojutti P, Pagotto A, Cristini F, Furlanut M, Viale P. Successful long‐term treatment of cerebral nocardiosis with unexpectedly low doses of linezolid in an immunocompromised patient receiving complex polytherapy. Antimicrob Agents Chemother 2012; 56: 3438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


