Abstract
Clinical pharmacology is a medical specialty whose practitioners teach, undertake research, frame policy, give information and advice about the actions and proper uses of medicines in humans and implement that knowledge in clinical practice. It involves a combination of several activities: drug discovery and development, training safe prescribers, providing objective and evidence‐based therapeutic information to ethics, regulatory and pricing bodies, supporting patient care in an increasingly subspecialized arena where co‐morbidities, polypharmacy, altered pharmacokinetics and drug interactions are common and developing and contributing to medicines policies for Governments. Clinical pharmacologists must advocate drug quality and they must also advocate for sustainability of the Discipline. However for this they need appropriate clinical service and training support. This Commentary discusses strategies to ensure the Discipline is supported by teaching, training and policy organizations, to communicate the full benefits of clinical pharmacology services, put a monetary value on clinical pharmacology services and to grow the clinical pharmacology workforce to support a growing clinical, academic and regulatory need.
Keywords: clinical pharmacology, health workforce, leadership
Introduction
The World Health Organization (WHO) recently published a major position paper on clinical pharmacology 1. It drew ideas from the International Union of Basic and Clinical Pharmacology (IUPHAR) and the Council for International Organizations of Medical Sciences and complements Aronson's ‘manifesto’ (2010) by providing a vision for the specialty 2. This includes recognition of and contribution to global health, and leadership in the area of biologics and personalized medicines, in addition to the more ‘traditional’ roles.
We concur with Aronson's definition of clinical pharmacology as a discipline that ‘teaches, does research, frames policy, gives information and advice about the actions and proper uses of medicines in humans and implements that knowledge in clinical practice’. We support the tremendous leadership roles in medicines and healthcare that have been highlighted by the WHO, by this Journal, the British Pharmacological Society 3 and the European VOICE 4. So the vision is there. The challenge is finding an implementation strategy. That is the purpose of this Commentary.
Our proposal is divided into three sections:
engaging health system/services managers and policy makers to ensure the full benefit from clinical pharmacology services is achieved,
putting a monetary value on clinical pharmacology and
ensuring adequate recruitment of doctors into the specialty.
Achievement of the first aim is a pre‐requisite for the other aims.
Communicating the full benefits of clinical pharmacology services (Figure 1)
Figure 1.
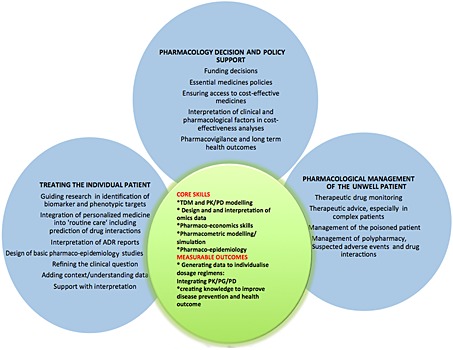
The translational, multidisciplinary and facilitative role of a clinical pharmacologist. The three pillars of comparative benefit include pharmacology decision and policy support, the pharmacological management of patients and the pharmacologist's contribution to personalized medicine. The latter includes integrating genetic knowledge into the phenotype of the clinical presentation, using therapeutic drug monitoring and other clinical information to predict choice and dose of therapy. The core skills are those multispecialty and multidisciplinary skill ‐sets in the clinical pharmacologists' armamentarium that enable individualized dosing
‘OMICS’ is a neologism for disciplines, such as pharmacogenomics, epigenomics, transcriptomics, proteomics and metabolomics, that involve studies of cellular biological pathways.
Clinical pharmacology (CP) works effectively at many interfaces. In Figure 1 we have enumerated the diverse range of skills that clinical pharmacologists possess and the types of support that they provide, to ensure that every patient gets the right drug at an individualized dose. This support covers every stage of the lifecycle of drugs, from discovery through to the formulation of medicines policies. This diverse range of skill sets includes the design of medicines based on pharmacology and disease pathophysiology, molecular biology, ‘‐ omics' and computational systems analysis. It ranges from providing context and interpretation for the large amounts of data emerging from all of these approaches to providing clinical focus and policy relevance for the increasing volumes of data being generated by pharmaco‐epidemiology networks of large population databases. ‘Personalized medicine’ to a clinical pharmacologist is the understanding and quantifying of the drug response, and the tailoring of choice and dose of medicine appropriately. The drug phenotypic information, i.e. dose and response to therapy, so often missing in discussions of traditional ‘‐omics' helps guide the interpretation of genomic, proteomic and other types of omics data. This information can reduce the risk of gene or protein ‘targeted’ therapies being developed to treat what may be an epiphenomenon, such as has been seen with expression of growth factors in some tumours 5.
It is important to consider the roles of clinical pharmacologists in relation to the challenges that are facing healthcare systems. Managers are well aware of the demands of an ageing population, leading to cohorts of individuals with multiple chronic diseases requiring long term therapy with multiple drugs, with the potential for poor adherence, drug interactions and adverse effects. They are also aware of the lack of integration of care, of duplication and wastage and the use of unnecessary tests and treatments. In our view this ‘appropriateness agenda’ is an important articulation point for clinical pharmacology and the specialty should engage more actively in the growing ‘Choosing wisely’ movement 6 (refer to Box 1). The developing Royal Australasian College of Physicians ‘EVOLVE’ framework and the Academy of Medical Royal Colleges in the UK 7, 8 are based in the vision of this movement, a movement that is ideally suited for a contribution of clinical pharmacology into broad based healthcare policy and practice.
Box 1
Choosing wisely
Choosing wisely is a programme initiated by the American Board of Internal Medicine (now being used in several other countries) that aims to promote conversations between clinicians and patients to choose care that is:
Supported by evidence
Not duplicative of other tests or procedures already received
Free from harm
Truly necessary
It aims to identify tests or procedures commonly used in specialty fields whose necessity should be questioned and discussed.
Clinical pharmacology makes a major contribution to teaching and training. In many countries there has been a large increase in the numbers of students and health professionals (other than medical graduates) who will be new prescribers. It is important they are taught the principles of quality use of medicines and the impact of altered clinical states on pharmacokinetic and dynamic processes including by people with knowledge and skills of drugs (i.e. not just specialist practitioners). Prescribers cannot be trained out of a drug formulary, devoid of knowledge of physiology and pathophysiology and the effects of these on drug response. They should receive instruction from unconflicted experts in an unbiased manner that highlights both the strengths and limitations of the evidence available. Internationally accredited teaching documents and curricula available for adaptation to all countries and systems would be a great step forward here. The UK has recently taken a lead in this area with a variety of curriculum documents and e‐learning tools, such as the ‘Prescribe’ project, a joint collaboration between the Department of Health, Medical Schools Council and the British Pharmacological Society to deliver a national eLearning solution to develop safe and effective prescribing amongst UK medical students. This is building on decades of international work developed by IUPHAR and contributed to by many authors on this manuscript.
Some have in fact argued that clinical pharmacology is a research and teaching specialty and therefore these academic roles should be funded by Universities. However the discipline makes a large contribution to planning and delivery of clinical care at institutional regional and national levels. This has recently led to its recognition as a medical specialty in the European Union, a recognition that has existed in many Commonwealth countries for decades. Such recognition is a prerequisite for acceptance by other health professionals and governments/institutions, particularly when it comes to the payment for clinical pharmacology services.
The significant contribution to clinical care was also highlighted in the recent report from the British Pharmacological Society ‘Prescription for the NHS: recognizing the value of clinical pharmacology and therapeutics’, which identified six domains (care provision, clinical toxicology, medicines policy and management, education and training, working with industry and experimental medicine) where the discipline has an important role in any healthcare system [3]. For these reasons, it is important that healthcare systems (and Universities) employ clinical pharmacologists. Lack of clinical pharmacologists will have an impact on all the domains listed above, amplifying the problem that we already face of poor knowledge of drugs and the effects of disease on exposure to and response of drugs 9, 10.
Putting a monetary value on clinical pharmacology (Box 2)
At a clinical level the primary focus of clinical pharmacology must remain on quality, safety and patient‐centred care but it is also important to emphasize the economic value of clinical pharmacology, recovered through reducing drug expenditures and the costs of unnecessary medications and avoidable adverse effects. Savings may result from discontinuation of an inappropriate medication (direct costs), reduction in bed‐stay and re‐admissions (which have a large value), prevention of an adverse drug reaction or recognition of a drug‐related diagnosis, which prevents further unnecessary tests, consultation or even surgery (which all have costs that can be measured). As examples, some of the authors currently sit on committees examining national registration and re‐imbursement issues (e.g. PHARMAC ‐ Pharmaceutical Management Agency, New Zealand and the German Gemeinsamer Bundesausschuss).
Service on hospital and regional drug committees, contribution to policy documents involving medicines, evaluation of literature to make better decisions, interactions with Industry around providing appropriate clinical support and information for particular drugs, education and training of prescribers, and advice and evaluation of regulatory and pricing documents are also important aspects of a clinical pharmacologist's workload that generate clear benefits to health systems. These activities are often provided ‘pro bono’ but should be valued and re‐imbursed, perhaps as outpatient visits in our concurrent specialties (e.g. internal medicine, cardiology) currently are.
Clinical pharmacologists not only operate as independent practitioners but can make a valuable contribution to a multidisciplinary team (e.g. in clinical pharmacokinetics and therapeutics in transplantation or infectious diseases). This has been addressed in some US and European Comprehensive Cancer Centres with the inclusion of clinical pharmacologists on ‘Tumour Boards’, and is a model for the discipline outside of oncology. We believe that doing without clinical pharmacology should be likened to practising medicine without pathology or radiology support, possible but of diminished quality. In contradistinction, there are many examples of the value of such collaborations, for example the re‐introduction of metformin, thalidomide and aspirin, genetic testing for the prevention of adverse drug reactions 11, 12, 13 and choosing an appropriate drug 12 or dose 14. These examples have created large gains in healthcare for little cost because of the leadership of clinical pharmacologists in clinical research teams. It is vital that our health payers are aware of these and future contributions.
We believe lobbying efforts directed at governments and hospitals/health services, medical schools, national registration/licensing boards and national scientific committees are vital to retain consultant/teaching posts in clinical pharmacology that, in turn, are necessary to provide high quality clinical pharmacology training. We also need to address the supply side, the training and consultant positions and the appropriately trained people to fill them.
Growing the clinical pharmacology workforce
Over the last 20 years we have witnessed the loss of training and consultant posts in clinical pharmacology around the world. Young clinicians have trained to be high level clinical pharmacologists but devoid of academic or clinical positions, have taken secure and well‐paid jobs in Industry or other physician specialties. Hospital and university employment contracts do not compete well with those in Industry. However, for many doctors the differential income is not the key issue. Rather, for young professionals facing a career option with no secure job prospects, training in academic clinical pharmacology is not a popular option because the value of this career is unrecognized. The direct result of this is that when someone does retire there is often nobody capable and well trained to fill the position. There is a need to develop an all‐embracing recruitment policy, and medical students interested in pharmacology, physiology, chemistry and/or with analytical or mathematical skills in particular should be approached early and mentored into clinical pharmacology posts. Specialist training in clinical pharmacology needs to be reviewed with links facilitated from residency and intern training wherever possible.
The second issue for trainees considering a career in clinical pharmacology is the changing requirements of the position. Specifically, a clinical pharmacologist in 2020 will be expected to have an enlarged skill base – in particular, knowledge, input to and clinical relevance of the ‘omics’ sciences, especially for those working in drug discovery 15. In the near future clinical focus on the relevance of the huge amounts of biostatistical medicines data being generated in genetics, proteomics and metabolomics will be needed. Specifically, knowledge of how these data can help develop safe and effective new drugs or re‐use of the existing therapies in more individualized ways. Another important and developing field is the interpretation of the complex analytical approaches to determining causality in the relationships between drug use and outcomes measured in large administrative databases and compilations of electronic health records 16. The role of clinical pharmacologists in regulatory agencies and their committees, such as the Therapeutic Goods Administration (Australia) and the European Medicines Agency are pivotal. Clinical pharmacologists also serve important roles on ethics committees and formulary committees.
This international group of clinical pharmacologists believe it is an opportune time to strengthen efforts to outwardly embrace the changing clinical and research paradigm in drug discovery and practice, and demonstrate the clinical service, regulatory and research support that clinical pharmacology can offer when incorporated into healthcare. Specifically, we need to use direct strategies (Box 2) to ensure that governments, universities and healthcare organizations are cognizant of the huge benefit of clinical pharmacology available at relatively low cost, so that more clinical pharmacologists can be trained and employed. For this, convincing data on both financial and clinical benefits are needed. Clinical pharmacologists are rightly relied upon to advocate for drug quality, optimal use of medicines, availability of and access to affordable medicines globally but can only perform these vital roles if there is good and well connected leadership at a policy and regulatory level and if appropriate positions in clinical pharmacology clinical services and academia in our health services and academic institutions, respectively, are supported.
Box 2
Strategies to ensure the vision becomes a reality
1. Ensure governments, health maintenance organizations (HMOs), consumer health groups and medical schools can see and measure the benefits a clinical pharmacologist adds to the healthcare and academic organization (improved patient care via correct choice and dose of drug, reduced numbers of adverse drug reactions and interactions, cost savings in pharmaceutical utilization and reducing inappropriate drug concentration testing and urine tests, enhanced design of clinical trials, correct interpretation of drug‐related laboratory tests, stopping of futile or unhelpful therapies and teaching and guidance of clinical staff in therapeutics).
2. Develop a plan for a career structure that will attract medical students and young doctors to choose the specialty of clinical pharmacology to ensure the level of clinical service is expanded given an increasingly elderly population with multiple co‐morbidities and exposed to polypharmacy.
3. Improve funding of clinical pharmacology consultations. Some countries (e.g. Germany) do not allow clinical pharmacologists to claim medical insurance rebates for medicines services‐related clinics, unlike their other clinical subspecialty colleagues. In Australia, many drug concentration and genetic tests are Government funded but the clinical interpretation of these results and the associated clinical visits, arguably the most important component of these tests, are not. In many countries, specialties that bring in income have been favoured over those that save money. Remuneration has also to reflect changing health care practice as we move from the genetics ‘revolution’ to a reassessment of how to use administrative and other data in the interests of optimizing therapy.
4. Provide strong leadership from clinical pharmacologists across several scientific and clinical specialities in primary and in secondary care and healthcare organizations as well as promoting and supporting younger clinical pharmacologists into leadership roles.
5. Ensure that development of new pharmacological agents involves the expertise of clinical pharmacologists (much as statistical methods require input of a statistician) at different stages of pre‐clinical, clinical and post marketing development and use.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
MS was supported in part by the Robert Bosch Stiftung, Stuttgart, Germany and by the Deutsche Forschungsgemeinschaft (MS, KFO Grant SCHW 858/1‐2). MP is a NIHR Senior Investigator and is supported by the MRC Centre in Drug Safety Science.
Martin, J. H. , Henry, D. , Gray, J. , Day, R. , Bochner, F. , Ferro, A. , Pirmohamed, M. , Mörike, K. , and Schwab, M. (2016) Achieving the World Health Organization's vision for clinical pharmacology. Br J Clin Pharmacol, 81: 223–227. doi: 10.1111/bcp.12803.
Note: The World Health Organization's document referred to in this article was published under the aegis of the WHO, International Union of Clinical and Basic Pharmacology and Council for International Organizations of Medical Sciences.
References
- 1.Available at http://www.who.int/medicines/areas/quality_safety/safety_efficacy/who_cioms2012/en/index.html (last accessed April 2015).
- 2. Aronson JK. A manifesto for clinical pharmacology from principles to practice. Br J Clin Pharmacol 2010; 70: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescription for the NHS: recognising the value of clinical pharmacology and therapeutics. Available at https://www.bps.ac.uk/BPSMemberPortal/media/BPSWebsite/BPS_A_prescription_for_the_NHS_FINAL_SP(1).pdf (last accessed 12 November 2015).
- 4. Orme M, Sjoqvist F. Clinical pharmacology in European health care: outcome of a questionnaire study in 31 countries. Eur J Clin Pharmacol 2013; 69: 1635–9. [DOI] [PubMed] [Google Scholar]
- 5. Martin J, Phillips E, Thomas D, Somogyi A. Adding the ‘medicines’ back into personalized medicine to improve cancer treatment outcomes. Br J Clin Pharmacol 2015; 80: 929–31. Accepted (ED‐00315‐15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Available at http://www.choosingwisely.org.au (last accessed 12 November 2015).
- 7. Malhotra A, Maughan D, Ansell J, Lehman R, Gray D, Stephenson T. Choosing wisely in the UK: the Academy of Medical Royal Colleges' initiative to reduce the harms of too much medicine. BMJ 2015; 350: 2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Available at http://members.racp.edu.au/index.cfm?objectid=3B8173E6‐F3B9‐C2FD‐24B7E7353586BDEA (last accessed 12 November 2015).
- 9. Doran E, Iedema J, Ryan L, Coombes I. Fatal rhabdomyolysis following voriconazole and simvastatin. Aust Prescr 2012; 35: 88–9. [Google Scholar]
- 10. Coombes ID, Stowasser DA, Coombes JA, Mitchell C. Why do interns make prescribing errors? A qualitative study. Med J Aust 2008; 188: 89–94. [DOI] [PubMed] [Google Scholar]
- 11. Pirmohamed M. Personalized pharmacogenomics: predicting efficacy and adverse drug reactions. Annu Rev Genomics Hum Genet 2014; 15: 349–70. [DOI] [PubMed] [Google Scholar]
- 12. Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jägel‐Guedes E, Rugina S, Kozyrev O, Flores J, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A. HLA‐B*5701 screening for hypersensitivity to abacavir. N Engl J Med 2008; 358: 568–79. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Wang J, Zhao LM, Peng W, Shen GQ, Xue L, Zheng XX, He XJ, Gong CY, Miao LY. Strong association between HLA‐B*1502 and carbamazepine‐induced stevens‐johnson syndrom. And toxic epidermal necrolysis in mainland Han Chinese patients. Eur J Clin Pharmacol 2011; 67: 885–7. [DOI] [PubMed] [Google Scholar]
- 14. Johansson I, Ingelman‐Sundberg M. Genetic polymorphism and toxicology–with emphasis on cytochrome P450. Toxicol Sci 2011; 120: 1–13. [DOI] [PubMed] [Google Scholar]
- 15. Meyer U, Zanger U, Schwab M. Omics and drug response. Annu Rev Pharmacol Toxicol 2013; 53: 475–502. [DOI] [PubMed] [Google Scholar]
- 16. Zakim D, Schwab M. Data collection as a barrier to personalized medicine. Trends Pharmacol Sci 2015; 36: 68–71. [DOI] [PubMed] [Google Scholar]


