Abstract
Aims
Denosumab is a fully human monoclonal immunoglobulin G2 antibody that inhibits bone resorption and increases bone mass and strength. The present clinical study assessed serum and seminal fluid pharmacokinetics following a single denosumab dose in healthy men, and evaluated whether denosumab in seminal fluid poses any risk to a fetus in the event of unprotected sexual intercourse with a pregnant partner.
Methods
An open‐label, single‐dose study in 12 healthy men was conducted over a 106‐day period. Subjects received a single subcutaneous dose of 60‐mg denosumab on day 1. Serum and seminal fluid samples were collected at specified time points to assess denosumab pharmacokinetics. Adverse events were recorded.
Results
Denosumab was measurable at low concentrations in seminal fluid (~2% of serum concentrations). The mean [standard deviation (SD)] maximum observed drug concentration (Cmax) was 6170 (2070) ng ml–1 (serum) and 100 (81.9) ng ml–1 (seminal fluid). The median time to Cmax (tmax) was 8 days (serum) and 21 days (seminal fluid). The mean (SD) area under the plasma concentration–time curve (AUC) from time zero to the time of the last quantifiable concentration (AUClast) was 333 000 (122 000) day•ng ml–1 (serum) and 5220 (4880) day•ng ml–1 (seminal fluid). The mean (SD) Cmax and AUC ratios between seminal fluid and serum were 0.0217 (0.0154) and 0.0170 (0.0148), respectively. Using conservative assumptions for ejaculate volume (6 ml), vaginal absorption (100%) and placental transfer (100%), the measured mean denosumab seminal fluid Cmax would result in fetal exposure that was more than 110 times below the preclinically derived ‘no effect level’ for denosumab.
Conclusions
These results indicate a negligible risk to a fetus exposed to denosumab via seminal fluid transfer to a pregnant partner.
Keywords: denosumab, distribution, fetus, placental transfer, seminal fluid, therapeutic protein
What is Already Known About this Subject
No published data are available on therapeutic monoclonal antibody (mAb) concentrations in human seminal fluid.
There is a lack of clinical data to indicate whether male patients who receive a monoclonal antibody therapeutic such as denosumab should be required to use a barrier method of contraception.
What this Study Adds
The data presented here indicate that the risk of denosumab in seminal fluid resulting in fetal exposure to this mAb is negligible and that any theoretical exposure that might occur is highly unlikely to be pharmacologically relevant. These observations may be generally relevant to the class of therapeutic mAbs.
Introduction
Osteoporosis is a skeletal disorder characterized by low bone mass and strength which predisposes individuals to an increased risk of fracture 1. Although osteoporosis and osteoporotic fractures are most commonly associated with postmenopausal women, osteoporosis also occurs in men. Recent data from the National Osteoporosis Foundation estimate that approximately 2 million men in the United States alone have osteoporosis, and approximately 12 million more are at risk 2, 3.
Denosumab is a fully human immunoglobulin (Ig) G2 monoclonal antibody (mAb) with a high affinity (Kd = 3 × 10−12 M) and specificity for receptor activator of nuclear factor‐kappaB ligand (RANKL) that can bind to and neutralize the activity of human RANKL similarly to the action of endogenous osteoprotegerin. In blocking RANKL, denosumab inhibits osteoclast formation, function and survival, thereby decreasing bone resorption and increasing bone mass and strength in both cortical and trabecular bone 4. The United States Food and Drug Administration (US FDA) granted approval to use denosumab to increase bone mass in men with osteoporosis at high risk of fracture. During review of the denosumab marketing application, the US FDA raised concern about the potential risk for fetal transmission of denosumab in seminal fluid during unprotected sexual intercourse with a pregnant partner. As a consequence, the approved label informed physicians of this potential risk, pending further investigation. The clinical study described here was conducted to investigate denosumab levels in the seminal fluid of denosumab‐treated men to evaluate the true potential of risk to a fetus. It should be noted that prior to December 2014, there was an overall lack of consensus on labelling requirements regarding the use of contraception in men who receive potentially genotoxic or teratogenic drug compounds. Subsequently, in December 2014, the US FDA issued the Pregnancy and Lactation Label Rule (PLLR), which now provides a dedicated subsection for discussion of male contraception requirements when there are human or animal data that suggest a potential for drug‐associated effects to the fetus.
The effects of RANKL inhibition on fetal development have been studied in: (i) an enhanced pre‐postnatal (ePPN) study in cynomolgus monkeys in which denosumab doses of 50 mg kg–1 were administered throughout pregnancy 5, 6, and (ii) genetically engineered ‘knockout’ mice or using other biological inhibitors of the RANK/RANKL pathway – namely, osteoprotegerin–Fc 7, 8, 9. Some of the biological effects of denosumab on the fetus are consistent across these animal models and with the known on‐target effects of RANKL inhibition, supporting the use of the biological ‘no observed effect level’ (NOEL) for denosumab in the prospectively calculated ‘safe’ concentration threshold in seminal fluid (see Discussion and Table 1). These effects include bone abnormalities; an absence of axillary, inguinal, mandibular and mesenteric lymph nodes; reduced haematopoiesis; tooth malalignment; and decreased neonatal growth. However, differences were seen, in that there was no effect on tooth eruption in the infant monkeys and no effect on mammary gland development in pregnant females.
Table 1.
Seminal fluid denosumab concentration back calculations
| Banholzer et al. 13 Calculation | Conservative Calculation | |
|---|---|---|
| Fetal serum concentration (Embryo–fetal C max = denosumab NOEL) | 22.9 ng ml–1 | 22.9 ng ml–1 |
| % Transfer from maternal circulation to embryo/fetus assumption | 10%* | 100%† |
| Maternal C max | = fetal serum concentration × 10 22.9 ng ml–1 × 10 = 229 ng ml–1 | = fetal serum concentration = 22.9 ng ml–1 |
| Maternal vaginally absorbed dose, assuming instantaneous dilution of dose into maternal volume of distribution (3000 ml) | 229 ng ml–1 × 3000 ml = 687 μg | 22.9 ng ml–1 × 3000 ml = 68.7 μg |
| Absorption from vagina | Assumption: 10% = maternal absorbed dose × 10 687 μg × 10 = 6870 μg | Assumption: 100% = maternal absorbed dose = 68.7 μg |
| Denosumab concentration in seminal fluid = vaginal dose/volume of seminal fluid (6 ml) | 6870 μg ÷ 6 ml = 1145 μg ml–1 | 68.7 μg ÷ 6 ml = 11.5 μg ml –1 |
First trimester estimate of fetal–maternal ratio;
Based on late gestation IgG placental transfer data. Cmax, maximum observed drug concentration; IgG, immunoglobulin G; NOEL, no observed effect level.
Further investigation of fetal exposure to an IgG2 mAb following biweekly intravaginal administration to cynomolgus monkeys throughout pregnancy yielded data demonstrating that only 0.01% of the intravaginal dose reaches the maternal circulation during the mid‐to‐late third trimester, with no detectable exposure in the conceptus 10. Similar results were seen with an IgG4 mAb 11. These in vivo animal study results indicate that male‐mediated mAb drug transfer may not pose a health risk to the pregnant female partner and her fetus, especially as mAbs are not likely to be bioavailable to the developing conceptus.
In contrast to some small‐molecule drugs, for which human seminal fluid chemical concentrations have been reported to be generally similar to or lower than blood concentrations 12, mAbs have physicochemical characteristics that limit passive transmission through biological membranes 13. Accordingly, in a study conducted by Raux et al., the seminal fluid : serum ratio for total endogenous IgG2 in 22 healthy volunteers was estimated to be 0.3% 14. As such, seminal fluid concentrations of therapeutic mAbs should also be low. However, no published data are available on therapeutic mAb concentrations in human seminal fluid.
Denosumab nonclinical safety, pharmacology and clinical study data, together with conservative assumptions derived from published literature pertaining to ejaculate volume, vaginal absorption and placental transfer of mAbs, were used to calculate prospectively a hypothetical denosumab concentration in seminal fluid below which there would be negligible potential for fetal risk (see Methods section for calculations). In the present clinical study, denosumab concentrations in the seminal fluid of denosumab‐treated men were compared with corresponding serum denosumab concentrations. These results were then evaluated against the above‐mentioned prospectively calculated denosumab seminal fluid concentration of 11.5 μg ml–1, below which there would be minimal or no biological activity or impact of denosumab and which would be considered safe for a fetus.
Currently, limited human data are available to indicate whether male patients who receive a mAb therapeutic such as denosumab should be required to use any form of contraception. The data presented here, together with other recently published data 10, 11, indicate that the risk of denosumab in seminal fluid resulting in fetal exposure to denosumab is negligible and that any theoretical exposure that might occur is highly unlikely to be pharmacologically relevant. These observations may be generally relevant to the class of therapeutic monoclonal antibodies.
Methods
Study design
This study was an open‐label, single centre, single‐dose, Phase 1b clinical trial in healthy male participants age 40–65 years. The enrollment period included a 21‐day screening period and a 106‐day treatment‐free, follow‐up period (Figure 1). Subjects received 60 mg denosumab by subcutaneous injection on day 1 and were considered to be enrolled once they had received this. All subjects were instructed to take ≥1000 mg calcium and ≥800 IU vitamin D daily. Subjects returned to the study centre on an outpatient basis for additional seminal fluid and blood sample collections for pharmacokinetic measurements (days 1, 10, 22, 36, 50, 78 and 106) and for safety assessments (chemistry: days 1, 10, 36, 78 and 106; antibody analysis: days 1 and 106). Use of concomitant medication was assessed at each study visit (days 1, 10, 22, 36, 50, 78 and 106). Adverse event and serious adverse event assessments were made throughout the study. If subjects tested positive for neutralizing antibodies to denosumab at the final scheduled study visit, they were scheduled to return for additional follow‐up testing. If an end‐of‐study test result demonstrated a significant clinical or laboratory abnormality, follow‐up of the subject occurred until resolution of the abnormality or until it was considered clinically stable by the investigator.
Figure 1.
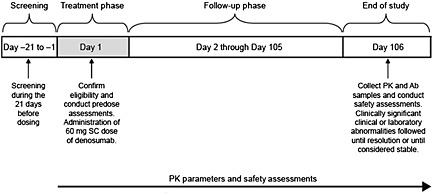
Study design and treatment schema. Ab, antibody; PK, pharmacokinetics; SC, subcutaneous
The commercial product denosumab was used in this study and was manufactured and provided by Amgen as a sterile, clear, colourless to slightly yellow, preservative‐free solution in a 1‐ml prefilled syringe for single use.
Selection of study participants
This study was conducted at one centre in the United States. Subjects were eligible for participation if they were men, aged 40–65 years, with no history or evidence of a clinically significant disorder, condition or disease that would pose a risk to subject safety or interfere with the study evaluation, procedures or completion. Key exclusion criteria included denosumab exposure in the past 12 months and participation in another drug study within 1 month or 5 half‐lives (whichever was longer) of ending treatment.
All subjects provided written informed consent to participate before any protocol‐specific screening procedures or any investigational products were administered. The study was conducted in accordance with applicable country regulations and the International Conference on Harmonisation (ICH) Good Clinical Practice (GCP) Guidelines. An institutional review board approved the study.
Study endpoints and safety
The study objective was to assess the concentration of denosumab in seminal fluid (primary endpoint) and serum (secondary endpoint). All subjects who received a dose of denosumab and had ≥1 measurable pharmacokinetic parameter were included in the pharmacokinetic analyses. All subjects who received a dose of denosumab were included in the safety analyses.
Concentration of denosumab
Concentrations of denosumab in seminal fluid and serum were measured by a validated enzyme‐linked immunosorbent assay at PPD Inc. (Richmond, VA, USA). The lower limit of quantification was 20 ng ml–1. Concentrations below 20 ng ml–1 were set to zero before the pharmacokinetic data analysis.
Before initiation of this clinical study, the concentration of denosumab in seminal fluid below which there would be no potential risk to the fetus was determined. Hypothetical seminal fluid denosumab concentrations were back‐calculated using a fetal serum concentration of 22.9 ng ml–1 as a starting point as this was the NOEL for denosumab in cynomolgus monkeys. As the binding site for denosumab is fully homologous in cynomolgus monkeys and humans, and the pharmacokinetic and pharmacodynamic properties of denosumab in these two species are very similar, the cynomolgus monkey was determined to be a suitable species for establishing the NOEL for the present clinical study.
Two sets of hypothetical calculations of the concentration of denosumab in seminal fluid below which there would be no potential for fetal risk were undertaken prospectively (Table 1). The initial assumptions were based on those described in a recent publication by Banholzer et al. for estimating drug exposure in a pregnant human female and her fetus as a result of seminal transmission and vaginal absorption following sexual intercourse with a male treated with a small molecule or therapeutic mAb 13. The assumptions underpinning this calculation have been validated by recently published data 10, 11, 15. Using the assumptions of Banholzer et al. 13, a denosumab seminal fluid concentration of 1145 μg ml–1 would be required to attain fetal exposure equal to the serum NOEL of 22.9 ng ml–1. Using a second method that applied more conservative assumptions of equal fetal and maternal exposure and 100% absorption of denosumab from the vagina, a denosumab seminal fluid concentration of 11.5 μg ml–1 would be required to attain fetal exposure equal to the serum NOEL of 22.9 ng ml–1. This estimate is 100‐fold below the Banholzer et al. estimate. Below this concentration of denosumab in seminal fluid, there would be no potential that a fetus would be exposed to biologically active concentrations of this agent.
Results
Subject disposition
The study was conducted between June and October 2013. A total of 12 subjects were screened and enrolled in the study. All subjects received investigational product, completed the study, and were included in the pharmacokinetic and safety analysis sets. No important protocol deviations were reported.
Baseline demographics
Baseline demographics and characteristics are summarized in Table 2. All subjects were men and 11 (91.7%) were white (the race for one subject was categorized as ‘Other’). The mean [standard deviation (SD)] age was 56.6 (7.0) years, the height was 177.52 (7.11) cm, the weight was 87.66 (15.80) kg and the body mass was 27.73 (4.21) kg m–2.
Table 2.
Baseline demographics and characteristics
| All subjects n = 12 | |
|---|---|
| Male – n (%) | 12 (100) |
| Race – n (%) | |
| White | 11 (91.7) |
| Other | 1 (8.3) |
| Age (years) – mean (SD) | 56.6 (7.0) |
| Height (cm) – mean (SD) | 177.5 (7.1) |
| Weight (kg) – mean (SD) | 87.7 (15.8) |
| Body mass index (kg m–2) – mean (SD) | 27.73 (4.21) |
SD, standard deviation.
Denosumab pharmacokinetics in serum and seminal fluid
The pharmacokinetic set included 84 serum and 83 seminal fluid denosumab samples from a total of 12 men. Of these, one subject had two samples excluded from the data analysis and individual concentration summary tables because the samples were collected at unscheduled visits.
Mean (± SD) serum and seminal fluid denosumab concentration–time profiles and descriptive statistics of pharmacokinetic parameter estimates after a single 60‐mg subcutaneous dose of denosumab are shown in Figure 2 and Table 3, respectively. The mean (± SD) maximum observed drug concentration (Cmax) values in the serum and seminal fluid samples were 6170 (± 2070) and 100 (± 81.9) ng ml–1, respectively. The median (range) tmax values in the serum and seminal fluid samples were.8.0 (7.9–21) and 21 (8.0–49) days, respectively. The mean (± SD) area under the plasma concentration–time curve (AUC) from time zero to the time of the last quantifiable concentration (AUClast) values were 333 000 (± 122 000) day•ng ml–1 for serum and 5220 (± 4880) day•ng ml–1 for seminal fluid. The time to last quantifiable denosumab concentration in seminal fluid was observed as early as day 36 and ranged up to day 106 (end of study). The mean (± SD) Cmax ratio (seminal fluid/serum), calculated by dividing the maximum seminal fluid concentration by the serum concentration at the corresponding time point, was 0.0217 (± 0.0154). The mean (± SD) AUC ratio for the 106‐day dosing period was 0.0170 (± 0.0148). The mean (± SD) day 106 (end of study) concentration ratio was 0.0197 (± 0.0318).
Figure 2.
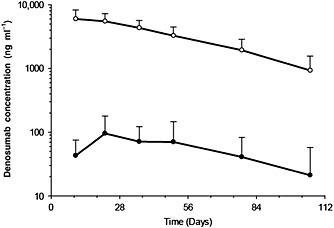
Serum and seminal fluid denosumab concentration–time profiles following a single subcutaneo us administration of denosumab 60 mg to healthy male volunteers. Data are means and standard deviations.  , Serum (N = 11–12);
, Serum (N = 11–12);  , Semen (N = 11–12).
, Semen (N = 11–12).
Table 3.
Descriptive statistics of serum and seminal fluid denosumab pharmacokinetic parameter estimates
| PK parameter | Descriptive statistics | Serum | Seminal fluid |
|---|---|---|---|
| C max (ng ml –1 ) | N | 12 | 12 |
| Mean (SD) | 6170 (2070) | 100 (81.9) | |
| Median (min, max) | 5780 (3300, 9030) | 85.2 (0.00, 301) | |
| CV% | 33.5 | 81.8 | |
| t max (days) | N | 12 | 11 |
| Median (min, max) | 8.0 (7.9, 21) | 21 (8.0, 49) | |
| AUC last (day•ng ml –1 ) | N | 12 | 12 |
| Mean (SD) | 333 000 (122 000) | 5220 (4880) | |
| Median (min, max) | 280 000 (170 000, 534 000) | 4260 (0.00, 17 100) | |
| CV% | 36.7 | 93.5 | |
| AUC ratio (seminal fluid/serum) | N | – | 12 |
| Mean (SD) | – | 0.0170 (0.0148) | |
| Median (min, max) | – | 0.0141 (0.00, 0.0463) | |
| CV% | – | 87.0 | |
| C max ratio (seminal fluid/serum) | N | – | 11 |
| Mean (SD) | – | 0.0217 (0.0154) | |
| Median (min, max) | – | 0.0161 (0.00582, 0.0493) | |
| CV% | – | 70.9 | |
| Day 106 ratio (seminal fluid/serum) | N | – | 11 |
| Mean (SD) | – | 0.0197 (0.0318) | |
| Median (min, max) | – | 0.00 (0.00, 0.0976) | |
| CV% | – | 161.2 |
AUC, area under the drug concentration–time curve; AUClast, AUC from time zero to the time of the last quantifiable concentration; AUC ratio (seminal fluid/serum), ratio of seminal fluid AUC to serum AUC for the 106‐day dosing period; Cmax, maximum observed drug concentration; Cmax ratio (seminal fluid/serum), ratio of maximum seminal fluid concentration to the serum concentration at the corresponding time point; CV%, % coefficient of variation; Day 106 ratio (seminal fluid/serum), ratio of seminal fluid concentration to the serum concentration at the last study time point (Day 106); PK, pharmacokinetic; SD, standard deviation; tmax, time to reach Cmax.
As mentioned above, the prospectively calculated estimate of 11.5 μg ml–1 denosumab in seminal fluid was determined as the threshold concentration below which there would be no risk to the fetus. The mean Cmax value in seminal fluid observed in the study was 100 ng ml–1 (0.1 μg ml–1), which is more than 110 times below the prospectively calculated conservative concentration of 11.5 μg ml–1. Additionally, based on the assumptions of Banholzer et al. 13, the mean Cmax value observed in seminal fluid was more than 11 000 times below the concentration required to result in an embryo–fetal serum Cmax equal to the serum NOEL.
No denosumab‐treated subjects tested positive for binding or neutralizing anti‐denosumab antibodies in the present study. Therefore, the effect of anti‐denosumab antibodies on denosumab pharmacokinetics was not evaluated.
Safety
Treatment‐emergent adverse events were reported for five of the 12 subjects (41.7%) (Tables 4 and 5). One subject (8.3%) had positional vertigo, which was reported as a serious adverse event [Common Terminology Criteria for Adverse Events (CTCAE) Grade 3] and was not considered by the investigator to be related to denosumab. All other adverse events were reported as mild (Grade 1) to moderate (Grade 2) in severity. None of the subjects had adverse events that led to discontinuation of treatment or from the study.
Table 4.
Summary of subject incidence of treatment‐emergent adverse events
| All subjects n = 12, n (%) | |
|---|---|
| All treatment‐emergent adverse events | 5 (41.7) |
| Serious adverse events | 1 (8.3) |
| Leading to discontinuation of investigational product | 0 (0) |
| Treatment‐related treatment‐emergent adverse events | 3 (25.0) |
| Serious adverse events | 0 (0) |
| Leading to discontinuation of investigational product | 0 (0) |
Table 5.
Treatment‐emergent adverse events by preferred term
| All Subjects n = 12, n (%) | |
|---|---|
| All treatment‐emergent adverse events | 5 (41.7) |
| Fall * | 2 (16.7) |
| Musculoskeletal pain | 2 (16.7) |
| Upper respiratory tract infection | 2 (16.7) |
| Diabetes mellitus * | 1 (8.3) |
| Fatigue | 1 (8.3) |
| Hypercholesterolaemia | 1 (8.3) |
| Hypophosphatemia | 1 (8.3) |
| Rectal haemorrhage * | 1 (8.3) |
| Vertigo positional * | 1 (8.3) |
Preferred terms were coded using MedDRA version 16.1.
Not related to investigational product.
Laboratory values were also assessed in all subjects and none were reported as CTCAE Grade 3 or above during the study. An adverse event of hypophosphatemia (Grade 2), considered to be related to denosumab, was reported in one subject on study day 9 and resolved on day 16. No intervention was required for resolution of the subject's hypophosphatemia. All other chemistry parameters, including calcium concentrations, were within laboratory reference ranges at all time points throughout the study.
The adverse events reported during the study were mild to moderate in severity and were consistent with those in previous studies and the identified risks for denosumab. In addition, no new safety risks were identified.
Discussion
The present study addressed the potential for fetal exposure to denosumab in seminal fluid from unprotected sexual intercourse with a pregnant partner. The results demonstrated that denosumab was present in seminal fluid samples at low concentrations, with mean Cmax and AUC values representing approximately 2% of the concurrent serum exposure (Table 3). The maximum denosumab seminal fluid concentration observed was 0.301 μg ml–1, which is at least 38 times below the concentration of 11.5 μg ml–1 required to attain an embryo–fetal serum Cmax equal to the serum NOEL (22.9 ng ml–1), based on conservative calculations (assuming 100% vaginal and placental transfer from a 6‐ml ejaculate), and >3800 times below the concentration of 1145 μg ml–1 based on the assumptions of Banholzer et al. 13. In addition, the maximum amount of denosumab delivered to a female partner following vaginal intercourse would result in maternal and fetal exposures approximately 11 000 times lower than the prescribed 60 mg subcutaneous dose administered. The vaginal absorption data derived from cynomolgus monkeys 10 demonstrated that maternal and fetal IgG2 plasma concentrations were only approximately 0.01% of the administered intravaginal dose. Thus, the conservative calculations assuming 100% vaginal absorption described above is also likely to be overestimating fetal exposure up to 10 000‐fold. These data suggest that male‐mediated mAb transfer via seminal fluid does not present a health risk to the female partner and is not bioavailable to the developing conceptus.
The estimates of vaginal dose, maternal absorbed dose and placental transfer of mAbs from the maternal circulation were based on data from the available literature and were selected to be conservative. The vaginal dose was estimated as the drug concentration in seminal fluid (ng ml–1) multiplied by seminal fluid volume (ml). The fluid volume capacity of the vagina is maximally 5 ml, based on studies of vaginal microbicide gels 16, 17. Banholzer et al. conducted hypothetical fetal exposure calculations for mAb biologics in seminal fluid and used a seminal fluid volume of 6 ml 13. Therefore, a seminal fluid volume of 6 ml was used in the present study. This is conservative as vaginal leakage occurs at fluidic volumes greater than 3 ml 18, 19, and 6 ml is equivalent to the 90th percentile for seminal fluid volume based on the most recent World Health Organization reference values for human semen characteristics 20.
The hypothetical maternal Cmax or maternal vaginally absorbed dose was determined as the vaginal dose multiplied by the percentage absorbed (10% or 100%) and divided by the maternal volume of distribution (3000 ml). The molecular weight, lipophilicity and ionization characteristics of mAbs such as denosumab are likely to limit the vaginal absorption of these large molecules from seminal fluid 12. Banholzer et al. assumed 10% absorption from the vagina into the maternal circulation to calculate the maternal absorbed dose 13. The conservative calculations presented herein assumed 100% absorption of the vaginal dose into the maternal circulation, which is tenfold higher than that used by Banholzer et al. 13.
Other clinical considerations include the transfer of denosumab from maternal circulation to fetus during pregnancy. The amount of placental transfer can be calculated by multiplying the maternal systemic concentration by the fetal/maternal ratio. During pregnancy, immunoglobulins are transferred from mother to fetus. Studies have shown differences in the placental transfer of Ig subtypes as pregnancy progresses 21, 22, 23, 24. As denosumab is an IgG2 mAb, placental transfer is expected to be very limited early in gestation (during organogenesis) 25 and increases as pregnancy progresses, with the largest amount transferred during the third trimester, but at a rate lower than that of other Ig subtypes in humans 21, 22, 23, 24. Banholzer et al. assumed an early gestation estimate of 10% placental transfer from the maternal to fetal circulation 13. In a study during the first trimester of human pregnancy, Jauniaux et al. demonstrated that the materno–fetal Ig transfer was closer to 1% 26. For the purposes of the conservative calculations presented herein, a fetal/maternal ratio of 100% was used, which is tenfold higher than the ratio used by Banholzer et al. 13.
Although the no‐effect dose for denosumab‐induced teratogenicity is not known, a Cmax of 22.9 ng ml–1 was identified in cynomolgus monkeys, at which no biologic effects (NOEL) of denosumab (and therefore, no inhibition of RANKL) were observed. Based on the highest observed denosumab seminal fluid concentration measured in men (0.301 μg ml–1), and assuming 100% vaginal and placental transfer from a 6‐ml ejaculate, female and fetal exposure via seminal fluid would be up to 0.6 ng ml–1. As mentioned earlier, this is at least 110 times lower than the NOEL in cynomolgus monkeys, indicating that the female partner or fetus would not be exposed to pharmacologically relevant concentrations of denosumab via seminal fluid.
The results from the present clinical study, together with other recently published data on vaginal and placental transfer of IgG2 mAbs, provide compelling evidence that the risk of exposing a fetus to denosumab through seminal fluid is negligible and that any theoretical exposure that might occur is highly unlikely to be pharmacologically relevant. These observations may be generally relevant to the class of therapeutic mAbs.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author) and declare: WS, EL, OE, GM, JB, DP, EN, SK and JGS had support from Amgen Inc. for the submitted work; WS, EL, OE, GM, JB, DP, EN, SK and JGS are/were employees of Amgen Inc. within the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
This work was supported by Amgen Inc. All authors participated in the analysis and interpretation of data, the writing of the report, and the decision to submit the manuscript for publication. Michelle N. Bradley of Amgen Inc. provided assistance with drafting and submitting the manuscript.
References
Sohn, W. , Lee, E. , Kankam, M. K. , Egbuna, O. , Moffat, G. , Bussiere, J. , Padhi, D. , Ng, E. , Kumar, S. , and Slatter, J. G. (2016) An open‐label study in healthy men to evaluate the risk of seminal fluid transmission of denosumab to pregnant partners. Br J Clin Pharmacol, 81: 362–369. doi: 10.1111/bcp.12798.
Martin K. Kankam was the principal investigator for this study.
References
- 1. National Institutes of Health (NIH). Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement 2000; 17: 1–45. [PubMed] [Google Scholar]
- 2. National Osteoporosis Foundation . Just for men [Online]. Available at http://www.nof.org/articles/justformen (last accessed July 13 2015).
- 3. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson‐Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 2014; 29: 2520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dempster DW, Lambing CL, Kostenuik PJ, Grauer A. Role of RANK ligand and denosumab, a targeted RANK ligand inhibitor, in bone health and osteoporosis: a review of preclinical and clinical data. Clin Ther 2012; 34: 521–36. [DOI] [PubMed] [Google Scholar]
- 5. Bussiere JL, Pyrah I, Boyce R, Branstetter D, Loomis M, Andrews‐Cleavenger D, Farman C, Elliott G, Chellman G. Reproductive toxicity of denosumab in cynomolgus monkeys. Reprod Toxicol 2013; 42: 27–40. [DOI] [PubMed] [Google Scholar]
- 6. Boyce RW, Varela A, Chouinard L, Bussiere JL, Chellman GJ, Ominsky MS, Pyrah IT. Infant cynomolgus monkeys exposed to denosumab in utero exhibit an osteoclast‐poor osteopetrotic‐like skeletal phenotype at birth and in the early postnatal period. Bone 2014; 64: 314–25. [DOI] [PubMed] [Google Scholar]
- 7. Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira‐dos‐Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph‐node organogenesis. Nature 1999; 397: 315–23. [DOI] [PubMed] [Google Scholar]
- 8. Capparelli C, Morony S, Warmington K, Adamu S, Lacey D, Dunstan CR, Stouch B, Martin S, Kostenuik PJ. Sustained antiresorptive effects after a single treatment with human recombinant osteoprotegerin (OPG): a pharmacodynamic and pharmacokinetic analysis in rats. J Bone Miner Res 2003; 18: 852–8. [DOI] [PubMed] [Google Scholar]
- 9. Stolina M, Dwyer D, Ominsky MS, Corbin T, Van G, Bolon B, Sarosi I, Mccabe J, Zack DJ, Kostenuik P. Continuous RANKL inhibition in osteoprotegerin transgenic mice and rats suppresses bone resorption without impairing lymphorganogenesis or functional immune responses. J Immunol 2007; 179: 7497–505. [DOI] [PubMed] [Google Scholar]
- 10. Moffat GJ, Davies R, Kwon G, Retter MW, Chellman GJ, Kanapuram S, Moore M, Loomis M, Wang W, Pyrah IT. Investigation of maternal and fetal exposure to an IgG2 monoclonal antibody following biweekly intravaginal administration to cynomolgus monkeys throughout pregnancy. Reprod Toxicol 2014; 48: 132–7. [DOI] [PubMed] [Google Scholar]
- 11. Breslin WJ, Hilbish KG, Page TJ, Coutant DE. Assessment of fetal exposure risk following seminal excretion of a therapeutic IgG4 (T‐IgG4) monoclonal antibody using a rabbit model. Reprod Toxicol 2014; 48: 124–31. [DOI] [PubMed] [Google Scholar]
- 12. Klemmt L, Scialli AR. The transport of chemicals in semen. Birth Defects Res B Dev Reprod Toxicol 2005; 74: 119–31. [DOI] [PubMed] [Google Scholar]
- 13. Banholzer ML, Buergin H, Wandel C, Schmitt G, Gocke E, Peck R, Singer T, Reynolds T, Mannino M, Deutsch J, Doessegger L. Clinical trial considerations on male contraception and collection of pregnancy information from female partners. J Transl Med 2012; 10: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raux M, Finkielsztejn L, Salmon‐Ceron D, Bouchez H, Excler JL, Dulioust E, Grouin JM, Sicard D, Blondeau C. IgG subclass distribution in serum and various mucosal fluids of HIV type 1‐infected subjects. AIDS Res Hum Retroviruses 2000; 16: 583–94. [DOI] [PubMed] [Google Scholar]
- 15. Moffat GJ, Retter MW, Kwon G, Loomis M, Hock MB, Hall C, Bussiere J, Lewis EM, Chellman GJ. Placental transfer of a fully human IgG2 monoclonal antibody in the cynomolgus monkey, rat, and rabbit: a comparative assessment from during organogenesis to late gestation. Birth Defects Res B Dev Reprod Toxicol 2014; 101: 178–88. [DOI] [PubMed] [Google Scholar]
- 16. Barnhart KT, Pretorius ES, Shaunik A, Timbers K, Nasution M, Mauck C. Vaginal distribution of two volumes of the novel microbicide gel cellulose sulfate (2.5 and 3.5 mL). Contraception 2005; 72: 65–70. [DOI] [PubMed] [Google Scholar]
- 17. Barnhart KT, Pretorius ES, Shera DM, Shabbout M, Shaunik A. The optimal analysis of MRI data to quantify the distribution of a microbicide. Contraception 2006; 73: 82–7. [DOI] [PubMed] [Google Scholar]
- 18. Barnhart KT, Pretorius ES, Timbers K, Shera D, Shabbout M, Malamud D. In vivo distribution of a vaginal gel: MRI evaluation of the effects of gel volume, time and simulated intercourse. Contraception 2004; 70: 498–505. [DOI] [PubMed] [Google Scholar]
- 19. Barnhart KT, Izquierdo A, Pretorius ES, Shera DM, Shabbout M, Shaunik A. Baseline dimensions of the human vagina. Hum Reprod 2006; 21: 1618–22. [DOI] [PubMed] [Google Scholar]
- 20. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16: 231–45. [DOI] [PubMed] [Google Scholar]
- 21. Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol 1996; 36: 248–55. [DOI] [PubMed] [Google Scholar]
- 22. Kane SV, Acquah LA. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am J Gastroenterol 2009; 104: 228–33. [DOI] [PubMed] [Google Scholar]
- 23. Pentsuk N, van der Laan JW. An interspecies comparison of placental antibody transfer: new insights into developmental toxicity testing of monoclonal antibodies. Birth Defects Res B Dev Reprod Toxicol 2009; 86: 328–44. [DOI] [PubMed] [Google Scholar]
- 24. Palmeira P, Quinello C, Silveira‐Lessa AL, Zago CA, Carneiro‐Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012; 2012: 985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Conference on Harmonisation. ICH Guideline M3 (R2): Guidance on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals. June 2009. [PubMed]
- 26. Jauniaux E, Jurkovic D, Gulbis B, Liesnard C, Lees C, Campbell S. Materno–fetal immunoglobulin transfer and passive immunity during the first trimester of human pregnancy. Hum Reprod 1995; 10: 3297–300. [DOI] [PubMed] [Google Scholar]


