Abstract
Aims
The aim of the study was to analyze the interaction between celecoxib and low dose aspirin for COX‐1 binding and its consequences on the aspirin‐mediated antiplatelet effects.
Methods
We investigated ex vivo the interaction between celecoxib and aspirin for COX‐1 binding and measured the resulting antiplatelet effects. We applied mechanism‐based pharmacokinetic−pharmacodynamic (PKPD) modelling to analyze these data and to predict in vivo platelet aggregation for different doses and administration schedules of aspirin and celecoxib.
Results
The predictions of the PK‐PD model were consistent with results from previous studies that investigated interaction between aspirin and celecoxib. The modelling results indicate that celecoxib can attenuate to a limited extent the in vivo antiplatelet effects of low dose aspirin. The extent of this interaction can be substantial (up to 15% increase in platelet aggregation by 200 mg day−1 celecoxib when combined with low dose aspirin) during the first days of aspirin administration in patients who are already treated with celecoxib, and it cannot be prevented by separate administration of the interacting drugs.
Conclusions
At the recommended therapeutic doses, celecoxib can attenuate to a limited extent the in vivo antiplatelet effects of low dose aspirin. Patients receiving a combination of low dose aspirin and the recommended doses of celecoxib were not identified to have increased risk of cardiovascular and cerebrovascular events due to competition between these drugs for COX‐1 binding. Interaction between low dose aspirin and other COX‐2 inhibitors and its clinical consequences requires further investigation.
Keywords: celecoxib, COX‐1 enzyme, drug−drug interaction, low dose aspirin anti‐aggregation effect, pharmacokinetic−pharmacodynamic modelling
What is Already Known About This Subject
Celecoxib and other COX‐2 selective inhibitors interact with COX‐1 and can interfere with the antiplatelet effects of aspirin.
What This Study Adds
We investigated the interaction between celecoxib and aspirin and its consequences for platelet aggregation using ex vivo experiments and pharmacokinetic−pharmacodynamic modelling analysis.
Celecoxib can attenuate to a limited extent the in vivo antiplatelet effects of low dose aspirin, but the consequences of this interaction for the risk of cardiovascular and cerebrovascular events in patients treated concomitantly with low dose aspirin and celecoxib remains unknown.
Introduction
The constitutive and the inducible cyclo‐oxygenase enzymes (COX‐1 and COX‐2, respectively) are prostaglandin‐endoperoxide synthases, membrane‐associated heme‐containing homodimers (composed of two 70 kDa subunits), responsible for conversion of arachidonic acid into precursors for numerous important biological mediators, including thromboxanes (Tx), prostaglandins and prostacyclin. COX‐1 and COX‐2 enzymes are important pharmacological targets that are inhibited by non‐steroidal anti‐inflammatory drugs (NSAIDs).
Most of the NSAIDs inhibit both COX‐1 and COX‐2 isoenzymes (e.g. ibuprofen, diclofenac and others), induce a variety of pharmacological effects and are predominantly used to manage diseases that are accompanied by fever and pain. However, pharmacological activities can differ widely between the individual NSAIDs. Specifically, low dose aspirin (75–100 mg day−1) induces irreversible inhibition of platelet COX‐1 leading to reduced production of thromboxane A2 (and other eicosanoids) and a decrease in platelet aggregation and blood clotting. Due to these pharmacological effects, low dose aspirin is widely used in patients with cardiovascular risk factors to prevent vascular thrombotic events like heart attacks and strokes [1, 2]. COX‐2‐selective inhibitors, such as celecoxib, were designed to target predominantly the COX‐2 isoenzyme, and to reduce inflammation and pain while minimizing COX‐1‐mediated effects, for example, gastrointestinal adverse drug reactions.
Because of the wide availability and frequency of use of NSAIDs that affect different isotypes of the COX enzyme, there is high potential for drug−drug interactions between the individual NSAID agents. Interactions between the specific NSAIDS have been analyzed in numerous studies using in vitro and ex vivo experimental systems, and in clinical experimental settings. Specifically, interaction between aspirin and ibuprofen has attracted the attention of several research groups and was thoroughly investigated. Outcomes of clinical studies [1, 3, 4] and of pharmacokinetic−pharmacodynamic modeling (PK‐PD) analysis [5‐8] indicated that ibuprofen can interfere with the antiplatelet effect of aspirin at the clinically‐relevant dosages and render aspirin less effective when used for cardioprotection and stroke prevention. Therefore, concomitant administration of ibuprofen and aspirin should be avoided [2, 9].
Possible interactions of COX‐2‐selective inhibitors such as celecoxib with other NSAIDs gained importance due to the reports of an increased incidence of cardiovascular events in patients who are treated with COX‐2 inhibitors. At the present time, there is some controversy regarding the interaction between celecoxib (a selective COX‐2 inhibitor) and low dose aspirin (that induces anti‐aggregation effects via irreversible inactivation of COX‐1) (see Table 1). No significant differences in platelet aggregation response induced ex vivo by arachidonic acid or other platelet agonists were found in clinical studies performed in healthy volunteers [4, 10] and in patients with osteoarthritis and stable ischaemic disease [3] who received different doses of aspirin and celecoxib (see Table 1).
Table 1.
Summary of studies that analyzed the interaction between low dose aspirin and celecoxib and the resulting antiplatelet effects
| Study | Subjects | Treatments and sample collection time | Analyzed parameters | Conclusions |
|---|---|---|---|---|
| Wilner et al. [10] | Healthy volunteers (aged 18–48 years, n = 8–9) | The patients received celecoxib 200 mg twice daily or matched placebo orally for 4 days. On day 5, all volunteers received 325 mg aspirin orally concomitantly with 20 mg celecoxib or with placebo orally, and samples were collected before and 2 and 8 h after this dose. | Serum TxB2 levels, platelet aggregation response by aggregometry | Celecoxib did not affect the platelet aggregation response to arachidonic acid in healthy volunteers receiving aspirin |
| Renda et al. [3] | Osteoarthritis and stable ischaemic disease patients (aged 45–73 years, n = 29) | The patient were undergoing long term treatment with 100 mg aspirin once daily orally, and received 200 mg celecoxib or placebo twice daily orally for 7 days. Samples were collected before and 4 and 24 h after the last dose. | Platelet TxB2 and LPS‐stimulated PGE2 production in whole blood, urinary 11‐dehydro‐TxB2 excretion rate, platelet aggregation by turbidimetric analysis | Celecoxib did not interfere with the inhibition of platelet COX‐1 activity and function by aspirin |
| Gladding et al. [4] | Healthy volunteers (n = 24) | Volunteers received three doses of 200 mg celecoxib or other NSAIDs or placebo orally at 12 h intervals. 2 h after the last dose, the patients received 300 mg aspirin orally, and 10 h later (24 h after the last NSAID dose) samples were collected. | PFA‐100 closure time | Celecoxib did not demonstrate any significant antiplatelet effect or reduce the antiplatelet activity of aspirin |
| Rimon et al. [12] | Healthy beagle dogs (10–12 kg, n = 6) | The dogs received 1.16 mg kg−1 aspirin once daily orally and/or 1.43 mg kg−1 celecoxib twice daily orally for 3 days, and samples were collected. | Platelet aggregation response by aggregometry | Administration of celecoxib to dogs interferes with the ability of a low dose of aspirin to inhibit arachidonic acid‐induced ex vivo platelet aggregation. |
Contrary to the results of these studies, celecoxib (at a dose corresponding to 140 mg human dose) did attenuate the anti‐aggregation effects of high dose aspirin (at a dose corresponding to 322 mg human dose) in a dog model of thrombosis [11]. Detailed recent biochemical and pharmacological investigation revealed that celecoxib and other coxibs are able to bind quite tightly to one subunit (i.e. the regulatory subunit) of the COX‐1 enzyme [12]. This binding did not affect the normal catalytic activity of the enzyme, but it did interfere with the inhibition of COX‐1 by aspirin in experiments in intact cells and proteosomal preparations, and in studies with purified COX‐1 enzyme in vitro [12]. Attenuation of the aspirin effect on ex vivo platelet aggregation was observed in samples that were collected from dogs treated with low dose aspirin (at a dose corresponding to 81 mg daily human dose) and celecoxib (at a dose corresponding to 100 mg twice daily human dose).
This controversy regarding the interaction between celecoxib and aspirin may originate from several reasons, related to the differences in the drug doses and administration times that were applied in the individual studies, and differences in the efficiency of platelet aggregation in humans vs. experimental animals. In addition, results of ex vivo platelet aggregation responses in the presence of potent aggregation inducers (e.g. arachidonic acid), that were applied in all the above‐mentioned studies [3, 4, 10, 12], cannot be easily compared since different platelet agonists and laboratory instruments were used (see Table 1 and [13]). Due to use of aggregation inducers at high concentrations, this test may overestimate the in vivo platelet aggregation and its contribution to blood clotting. Other tests can be used for analysis of in vivo platelet COX‐1 (e.g. serum TxB2 levels [14–16]) and of low dose aspirin pharmacological activity (e.g. in vivo blood clotting time), but their use in clinical settings is uncommon.
Due to the wide clinical use of aspirin and celecoxib and the clinical importance of an interaction between these drugs, we sought to investigate this interaction and its clinical consequences. The objective of this study was to analyze the interaction between celecoxib and aspirin for COX‐1 binding and its consequences for the aspirin‐mediated antiplatelet effects at therapeutic doses and different administration schedules of these drugs. To this end, we established an ex vivo assay of interaction between celecoxib and aspirin for COX‐1 binding and measured the resulting antiplatelet effects. Subsequently, we applied mechanism‐based PK‐PD modelling to analyze these data and to predict in vivo platelet aggregation for different doses and administration schedules of aspirin and celecoxib.
Methods
Volunteers
Healthy male and female volunteers were recruited as plasma donors for the purpose of this study. The inclusion criteria were age of 18–50 years, lack of chronic diseases (including blood clotting and cardiovascular diseases, metabolic disorders, infectious diseases, etc.), and no use of medications. The study was approved by the Soroka Hospital Research Ethics Committee (study approval number 0068–15‐SOR) and conducted according to the Declaration of Helsinki. All volunteers gave written informed consent prior to their recruitment.
Materials
Aspirin and celecoxib were generously provided by Teva Pharmaceutical Industries Ltd (Kfar Saba, Israel). Arachidonic acid solution (5 mg ml‐1) was purchased from Helena Laboratories (Beaumont, TX, USA).
Platelet aggregation assay
We applied analysis of platelet aggregation in the 96‐well plates format which has undergone extensive validation and comparison with the ‘standard’ tests of platelet aggregation by other researchers [17–19] and our research group. Blood samples (30–50 ml) were collected by venipuncture into tri‐sodium citrate (3.2%) tubes, and were centrifuged at 180 g for 15 min to obtain platelet rich plasma (PRP). Aspirin (0–10 μm) and celecoxib (0–2 μm, each drug individually or together) or control solution (saline) were added to the PRP aliquots, and these samples were incubated at room temperature for 1 h. Then, 200 μl from each of the samples were transferred to 96 wells plate, and 25 μl of 5 mg ml‐1 arachidonic acid solution (or 25 μl of saline for the negative control samples) were added to the wells. The plate was vortexed for 30 s, and immediately after that u.v. absorbance at 595 nm was determined using a plate reader (Model 680, Bio‐Rad Ltd.) every 15 s for 16 min, while the plates were kept at 37°C and were subjected to vigorous shaking in the plate reader between the reading cycles [19]. Changes in absorbance were converted to % aggregation by reference to the absorbance of the negative control (PRP with saline, without aspirin or celecoxib) and of the positive control samples (PRP with arachidonic acid solution, without aspirin or celecoxib). The calculation of the platelet aggregation response was based on the endpoint value, without taking into account the slope of the graph, as commonly applied in clinical practice [20], especially when arachidonic acid is used as an aggregation agonist (that induces a single wave response) [21].
The PK‐PD model and modelling analysis
The applied model was based on the available pharmacokinetic and pharmacodynamic data of COX‐1 enzyme and its interaction with aspirin and celecoxib. The turnover of COX‐1 enzyme and its interaction with the drugs (aspirin and celecoxib, see Figure 1) were assumed to follow the following equations:
| (1) |
| (2) |
| (3) |
Figure 1.
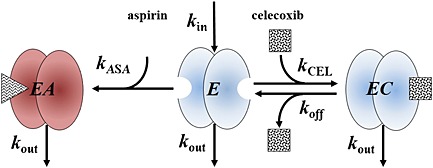
The model of aspirin and celecoxib interaction with two different subunits of COX‐1 enzyme. Free and celecoxib‐bound COX‐1, but not the aspirin‐bound COX‐1, contribute to platelet aggregation
where EA, E, and EC are the concentrations of aspirin‐bound, unbound and celecoxib‐bound COX‐1 enzyme, respectively, C ASA and C CEL are the plasma concentrations of aspirin and celecoxib, respectively, that were known in ex vivo studies, or were calculated in in vivo simulations assuming one compartmental pharmacokinetic behaviour (see Table 2; equations not shown), k ASA and k CEL are the second order rate constants of aspirin and celecoxib binding to the COX‐1 enzyme (the rate of reaction is proportional to the concentration of the individual reactants, C ASA and E, and C CEL and E, respectively), k off is the COX‐1−celecoxib complex dissociation rate constant, k in is the zero order rate constant of COX‐1 production and k out is the first order rate constant of COX‐1 elimination that equals k in divided by the plasma COX‐1 concentration in the absence of drug treatment ([COX‐1]0). Similar to previous studies [5, 8], it was assumed that the degradation kinetics of the COX‐1 enzyme was not affected by its association with the studied drugs (i.e. k out represents the degradation rate constant of the unbound, aspirin‐bound, and celecoxib‐bound COX‐1 enzyme).
Table 2.
The pharmacokinetic and pharmacodynamic parameters that were collected from the scientific literature or were estimated. The coefficients of variation for the estimated parameters are presented in brackets
| Group | Parameter | Units | Value | Source |
|---|---|---|---|---|
| ASA PK | k a ASA | h−1 | 1.1 | Hong et al. [5] |
| k 10 ASA | h−1 | 2.1 | ||
| V/F ASA | l | 10.0 | ||
| CEL PK | k a CEL | h−1 | 1.084 | Celecoxib prescribing information [33] |
| k 10 CEL | h−1 | 0.0619 | ||
| V/F CEL | l | 429 | ||
| COX‐1 turnover | [COX‐1]0 | μm | 2.676.10−4 | Bautista et al. [34] |
| k in | μm h−1 | 1.208.10−6 | ||
| ASA and CEL interaction with COX‐1 | k ASA | 1/(μm .h) | 0.124 (14%) | Estimated |
| k CEL | 1/(μm .h) | 12.32 (31%) | ||
| k off | h−1 | 19.84 (52%) |
The platelet aggregation effect was assumed to be exerted by the unbound and celecoxib‐bound COX‐1 enzyme (E and EC, respectively, and not the aspirin‐bound enzyme) and was calculated as % of the initial platelet aggregation effect (in absence of drugs) using the following equation:
| (4) |
where it is assumed that [COX‐1]0, the plasma COX‐1 concentration in absence of drug treatment, is equal to the sum of concentrations of COX‐1 enzyme in all of its forms (E, EC, and EA).
Estimation of the k ASA, k CEL and k off values was performed based on simultaneous analysis of the ex vivo platelet aggregation data points from aspirin and celecoxib samples (each drug individually or together) and the positive and negative controls using Monolix 4.2.1 software (Lixoft, Orsay, France) with maximum likelihood estimation based on a SAEM (stochastic approximation version of EM) algorithm with a Markov Chain Monte Carlo (MCMC) procedure. Success of the individual model was judged by the fits (observed vs. predicted data), visual predictive checks, the percentage of the standard errors of the parameters and the values of the Akaike Information Criterion and the Bayesian Information Criterion. The fitted parameters were assumed to be log‐normally distributed. A combined (additive and proportional components) model of variance was used to describe the residual variability of the parameters.
Model‐based predictions
All predictions were executed utilizing MATLAB® 7.11 (The MathWorks, Inc., Natick, MA, USA). The system of equations was based on equations 1–4, turnover of platelet COX‐1 enzyme and the pharmacokinetics of aspirin and celecoxib (see Table 2). These equations were solved using MATLAB®’s ode23s command, a variable order method for solving a system of stiff differential equations.
Sensitivity analysis
We used the developed PK‐PD model to predict the effect of alterations of individual parameter values (increase or decrease by 10%) on the antiplatelet effect of low dose aspirin during co‐treatment with celecoxib in ex vivo and in vivo experimental settings. In vivo sensitivity analysis was based on simulations for a patient who constantly receives 81 mg day−1 aspirin and 100 mg celecoxib twice daily (the first daily dose of celecoxib and the daily dose of aspirin are taken at the same time).
Comparison of the model‐based predictions with the results of previous studies
The experimental settings (i.e. drugs doses, administration and sampling times) of the clinical and pre‐clinical studies that investigated effect of celecoxib on aspirin antiplatelet effects (see Table 1) were used as inputs for the developed PK‐PD model. The time course of predicted antiplatelet effects was compared with the results reported in the individual studies.
Model‐based simulations of aspirin‐celecoxib co‐treatment scenarios
We predicted the time courses of plasma drug concentrations and of platelet aggregation for different administration schedules of aspirin and celecoxib using the developed PK‐PD model. The administration schedules included 75–325 mg daily aspirin doses, alone or in combination with 200 mg daily celecoxib doses, at the same time or at different times during the same day. In all these simulations, area under the effect vs. time curve (AUEC) values were calculated (i.e. area below the 100% platelet aggregation vs. time line).
Results
Ex vivo interaction between aspirin and celecoxib and its description by the PK‐PD model
The data obtained in ex vivo experiments (the observed values, Figure 2) indicate that celecoxib interfered with the antiplatelet effects of aspirin in a concentration‐dependent fashion. For instance, platelet aggregation increased from 74.8 ± 4.6 to 93.0 ± 18.4 (2.5 μm aspirin alone or in presence of 2 μm celecoxib) and from 2.0 ± 2.0 to 56.5 ± 5.9 (10 μm aspirin alone or in presence of 2 μm celecoxib). The developed PK‐PD model described appropriately the platelet aggregation data observed ex vivo for different concentrations of aspirin and celecoxib, except of a single data point (10 μm aspirin and 0 μm celecoxib, see Figure 2A) and allowed prediction of the extent of drug interactions for a wide range of concentrations of interacting drugs (see Figure 2B). The values of the estimated pharmacodynamic parameters (k ASA, k CEL, k off) are shown in Table 2. Sensitivity analysis using the applied PK‐PD model revealed that the magnitude of antiplatelet effects was more sensitive to the changes of k ASA, as compared with k CEL and k off (7.3%, −2.4% and 2.2% change in platelet aggregation effect, respectively, following a 10% increase in the corresponding parameter value, see Figure 3A).
Figure 2.
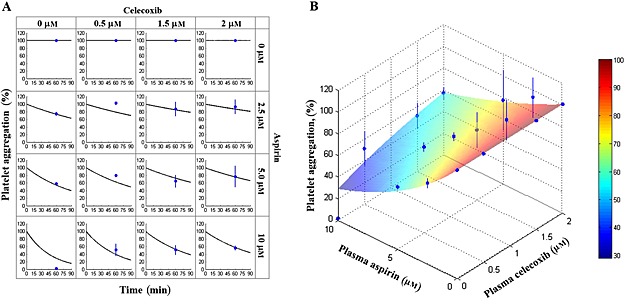
Aspirin‐celecoxib observed vs. predicted data. Samples of platelet‐rich plasma from healthy volunteers were incubated for 60 min with different concentrations of aspirin or/and celecoxib. Arachidonic acid was added and platelet aggregation was measured. The observed values are shown as blue dots and lines (average ± SD of four studies), and modelling‐based predictions for studied aspirin and celecoxib concentrations (A, black lines) and for the wide range of aspirin and celecoxib concentrations (B, color‐coded surface) are shown
Figure 3.
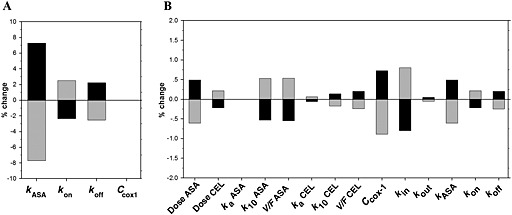
Sensitivity analysis: effect of alterations of individual parameter values (increase or decrease by 10%) on the anti‐aggregation effect of low dose aspirin during co‐administration with celecoxib. (A) Ex vivo sensitivity analysis and (B) in vivo sensitivity analysis, based on simulations for a patient who is constantly treated with 81 mg day−1 aspirin and 100 mg twice daily celecoxib (the first daily dose of celecoxib is given together with aspirin). ( ) 10% increase, (
) 10% increase, ( ) 10% decrease
) 10% decrease
Comparison of the model‐based predictions with the results of previous studies
Simulations using the developed PK‐PD model predicted rapid increase and decline in aspirin plasma concentrations, and more gradual increase and decrease in plasma concentrations of celecoxib (see Figure 4). Indeed, due to the differences in the volume of distribution, elimination half‐life values (see Table 2) and the doses, pharmacokinetic curves of aspirin were characterized by higher fluctuations (higher peak and lower trough values), as compared with celecoxib.
Figure 4.
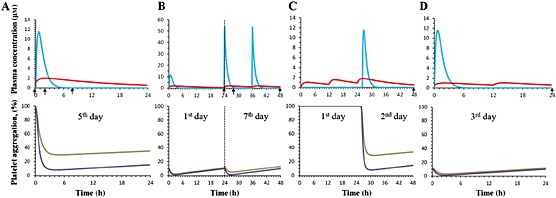
Modelling‐based predictions based on protocols of previous studies of aspirin and celecoxib interaction (see Table 1). (A) Wilner et al. [10], (B) Renda et al. [3], (C) Gladding et al. [4], (D) Rimon et al. [12]. Arrows, sample collection times. Upper graphs: ( ) ASA, (
) ASA, ( ) CEL; lower graphs: (
) CEL; lower graphs: ( ) ASA+CEL, (
) ASA+CEL, ( ) ASA.
) ASA.
The developed PK‐PD model predicted some increase in platelet aggregation in vivo due to administration of celecoxib (i.e., aspirin vs. aspirin with celecoxib administration) for experimental settings of studies by Wilner et al. (from 9.2% to 30.5% at 8 h, Figure 4A) and Gladding et al. (from 14.7% to 34.0% at 48 h, Figure 4C). On the other hand, a minor change in platelet aggregation due to administration of celecoxib was predicted for the experimental settings of studies by Renda et al. (from 10.0% to 13.1% at 48 h, Figure 4B) and Rimon et al. (from 10.0% to 11.6% at 24 h, Figure 4D).
Model‐based simulations of aspirin‐celecoxib co‐treatment scenarios
Simultaneous administration of aspirin (100 mg day−1) and celecoxib (200 mg day−1) is expected to produce aspirin plasma concentrations in the 0–11.5 μm range (without accumulation between the daily doses) and celecoxib plasma concentrations in the 0.38–1.35 μm range (with some accumulation between the daily doses; Figure 5A).
Figure 5.
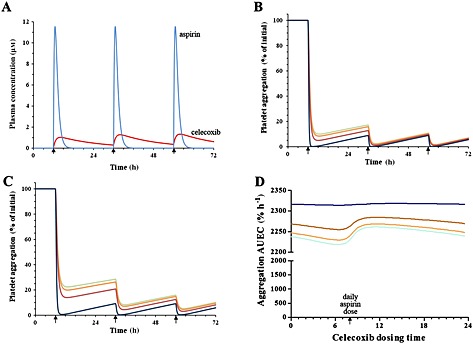
Modelling‐based predictions of different clinical scenarios. (A) predicted plasma concentrations of aspirin (81 mg day−1) and celecoxib (200 mg day−1) in a typical patient, (B) predicted time course of platelet aggregation in a patient who starts to take different daily doses of aspirin, (C) predicted time course of platelet aggregation in a patient who starts to take different daily doses of aspirin in combination with 200 mg day−1 celecoxib and (D) predicted efficiency of platelet aggregation in a patient constantly treated with different daily doses of aspirin (at 08.00 h) in combination with 200 mg day celecoxib (simultaneously with aspirin or at other dosing times, see the arrows). ( ) 75mg, (
) 75mg, ( ) 81 mg, (
) 81 mg, ( ) 100 mg, (
) 100 mg, ( ) 325 mg
) 325 mg
In absence of celecoxib treatment, 75–325 mg day−1 aspirin administration is expected to decrease the platelet aggregation in a dose‐dependent fashion on the first day of treatment, but starting from the second day and after that, these aspirin doses are expected to induce antiplatelet effects of similar magnitude (Figure 5B). Addition of 200 mg day−1 celecoxib to the treatment schedule (administered simultaneously with the daily dose of aspirin) is expected to interfere substantially with the antiplatelet effects of aspirin on the first treatment day, but the extent of this interaction is expected to decline on subsequent treatment days (Figure 5C).
At steady‐state conditions (75–325 mg day−1 aspirin and 200 mg day−1 celecoxib), the timing of aspirin vs. celecoxib daily dose had a minor effect on the predicted aspirin anti‐aggregation effects (see Figure 5D). The highest dose of aspirin (325 mg day−1) is expected to induce the maximal extent of antiplatelet effects that are not mitigated by celecoxib administration (simultaneously with aspirin or at other times during the day). For lower aspirin doses (75–100 mg day−1), timing of celecoxib administration of 200 mg day−1 celecoxib is expected to affect to a limited extent the magnitude of interaction between these drugs (e.g. 2220%.h for celecoxib administered 2 h before aspirin vs. 2262%.h for celecoxib administered 2 h after aspirin, the minimal and maximal antiplatelet effects, respectively; i.e. 1.9% difference in the AUEC values, see Figure 5D).
In vivo sensitivity analysis
The results of the sensitivity analysis indicate that at steady‐state conditions (81 mg day−1 aspirin and 100 mg celecoxib twice daily) the alterations in several parameters can affect the extent of interaction between the studied drugs (Figure 5B). The pharmacokinetic factors with the highest impact were the dose of aspirin, its volume of distribution and elimination rate constant (Dose ASA, V/F ASA, k 10 ASA). The change in the absorption rate constants of both drugs (k a ASA, k a CEL) and in the pharmacokinetic parameters of celecoxib had much lower impact on the antiplatelet effects. The pharmacodynamic factors with the highest impact were the COX‐1 turnover parameters ([COX‐1]0, k in) and the rate constant of COX‐1 association with aspirin (k ASA).
Discussion
Our study is based on the results of previous investigations that analyzed an interaction between aspirin and ibuprofen. In these studies, ibuprofen was found to interfere substantially with the antiplatelet effects of low dose aspirin [5, 8]. Subsequently, it was suggested that other NSAIDs that can bind the COX‐1 enzyme (‘COX‐1 selective’, ‘COX‐2 selective’ and non‐selective agents), can interfere with aspirin binding and acetylation of COX‐1 and increase the risk of thromboembolic effects (heart attacks and strokes) [22]. The major parameters that govern the extent of this drug−drug interaction are the pharmacokinetic properties of the individual drugs, drug doses and their timing, and drug−COX‐1 enzyme affinities.
To determine the interaction between celecoxib and aspirin due to competition for COX‐1 binding, we applied the previously published mechanism‐based PK‐PD model [5, 8], to analyze ex vivo experimental data and to predict in vivo antiplatelet effects. Our analysis revealed that celecoxib can efficiently mitigate the antiplatelet effects of aspirin ex vivo (Figure 2). However, the magnitude of this interaction in in vivo settings was limited (Figure 4 and 5). The major factors that were found to affect in vivo platelet aggregation were the rate of turnover of COX‐1 enzyme vs. the changes in the plasma concentrations of the investigated drugs (both these factors are absent in in vitro settings). The combined effects of the individual parameters on platelet aggregation in vivo are complex, and specialized tools (PK‐PD models and appropriate software) are needed to reveal the interaction between aspirin and celecoxib at the clinically relevant doses and administration schedules of these drugs.
PK‐PD modelling results indicate that that celecoxib can attenuate to a limited extent the in vivo antiplatelet effects of low dose aspirin. The extent of this interaction can be substantial during the first days of aspirin administration in patients who are already treated with celecoxib, and it cannot be prevented by separate administration of the interacting drugs. On the other hand, for a patient treated chronically with low dose aspirin, addition of celecoxib at the recommended therapeutic doses is not expected to mitigate the aspirin antiplatelet effects. At high doses or overdose, however, celecoxib will be able to compete efficiently with aspirin for COX‐1 binding in vivo, and can interfere with the antiplatelet effects of low dose aspirin. Temporary increase in aspirin dose can be used to prevent increased platelet aggregation after addition of celecoxib. However, decisions regarding these aspirin dose adjustments should take into account celecoxib dose and administration time (i.e. higher increase in aspirin dose for higher doses of celecoxib, especially if it is taken simultaneously with aspirin, Figure 5).
The mechanistic PK‐PD modelling approach that was applied in this study allows detailed and systematic analysis of the COX‐1‐mediated platelet aggregation pathway and its modulation by specific drugs. However, the PK‐PD modelling based predictions should be verified in carefully planned clinical studies to determine the antiplatelet effects in patients taking different doses of aspirin and celecoxib. The consequences of this interaction as a risk factor for vascular thrombotic events (heart attacks and strokes) in patients concomitantly treated with aspirin and celecoxib also require clarification. Due to the complexity of thrombosis mechanisms [2, 23, 24], reduced platelet aggregation has a limited effect on the risk of vascular thrombotic events. Hence, specially designed clinical studies (long duration prospective or retrospective studies with high numbers of participants) are required to establish the effect of the aspirin−celecoxib interaction on the incidence of vascular thrombotic events.
It is possible that some categories of patients are especially prone to the celecoxib‐induced inhibition of aspirin function and are at higher risk of vascular thrombotic events than others. Specifically, patients with low exposure to aspirin (due to reduced bioavailability, reduced absorption due to food−drug or drug−drug interactions, or low adherence to aspirin treatment) and high exposure to celecoxib (due to enhanced bioavailability or overdose) will maintain efficient platelet aggregation activity and may be at risk of heart attacks and strokes. Reduced sensitivity to aspirin was found in patients with type two diabetes [23, 25–27], metabolic syndrome [28] and other conditions [29], and patients with these pathologies may have increased risk of vascular thrombotic events. Twice daily dosing of low dose aspirin was demonstrated in several studies to maintain efficient antiplatelet effects in diabetes two patients and to prevent diabetes complications [30–32]. Interaction of celecoxib with aspirin at these dosing schedules and its effects on platelet aggregation and on risk of heart attacks and strokes in diabetes patients should be investigated in future clinical studies. It is possible that the interaction of aspirin with celecoxib will pose substantial risks for the above‐mentioned or other patient groups, and will require changes in doses of these drugs or switching to other pharmacological agents. In addition, interaction of aspirin with other coxibs (e.g. etoricoxib, valdecoxib, parecoxib, lumiracoxib) and NSAIDs requires detailed investigation in an in vitro, ex vivo and in vivo setting to reveal the risks of this interaction in different groups of patients with and without cardiovascular risk factors (such as diabetes, metabolic diseases, etc.). Currently, we are performing experiments to determine competition of other NSAIDs (rofecoxib, etoricoxib, ibuprofen, naproxen, NS‐398) with aspirin for COX‐1 binding. Results of these studies (obtained in ex vivo experimental settings) and their clinical implications will be reported in our future publications.
In conclusion, celecoxib, a selective inhibitor of the COX‐2 enzyme, interacts with the COX‐1 enzyme and can potentially interfere with its inhibition by aspirin. We measured ex vivo the interaction between aspirin and celecoxib and applied a mechanism‐based PK‐PD model to analyze these data and to predict the interaction between aspirin and celecoxib at therapeutic doses and different administration schedules of these drugs. The modelling results indicate that celecoxib can attenuate to a limited extent the in vivo antiplatelet effects of aspirin. The extent of this interaction can be substantial during the first days of aspirin administration in patients who are already treated with celecoxib, and it cannot be prevented by separate administration of the interacting drugs. Implications of an interaction between low dose aspirin and celecoxib for the risk of cardiovascular and cerebrovascular events in patients who receive these drugs warrant detailed analysis. Moreover, interaction between low dose aspirin and other COX‐2 inhibitors and its clinical consequences require further investigation.
Competing Interests
There was no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
We wish to thank the volunteers who participated in this study.
We thank Professor Tim Warner (The William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK) for his valuable recommendations on ex vivo measurement of antiplatelet effects of the studied drugs.
Contributors
All authors provided critical review of all drafts of the manuscript, had full editorial control of the manuscript and provided their final approval of all content. MR and DS contributed to the design and execution of the study, and analysis and interpretation of data. GR contributed to the design of the study, and interpretation of data. OP contributed to the design and execution of the study, and interpretation of data.
Ruzov, M. , Rimon, G. , Pikovsky, O. , and Stepensky, D. (2016) Celecoxib interferes to a limited extent with aspirin‐mediated inhibition of platelets aggregation. Br J Clin Pharmacol, 81: 316–326. doi: 10.1111/bcp.12801.
References
- 1. Catella‐Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, Vyas SN, FitzGerald GA. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 2001; 345: 1809–17. [DOI] [PubMed] [Google Scholar]
- 2. Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012; 141 (2 Suppl): e89S–119S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Renda G, Tacconelli S, Capone ML, Sacchetta D, Santarelli F, Sciulli MG, Zimarino M, Grana M, D'Amelio E, Zurro M, Price TS, Patrono C, De Caterina R, Patrignani P. Celecoxib, ibuprofen, and the antiplatelet effect of aspirin in patients with osteoarthritis and ischemic heart disease. Clin Pharmacol Ther 2006; 80: 264–74. [DOI] [PubMed] [Google Scholar]
- 4. Gladding PA, Webster MW, Farrell HB, Zeng IS, Park R, Ruijne N. The antiplatelet effect of six non‐steroidal anti‐inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteers. Am J Cardiol 2008; 101: 1060–3. [DOI] [PubMed] [Google Scholar]
- 5. Hong Y, Gengo FM, Rainka MM, Bates VE, Mager DE. Population pharmacodynamic modelling of aspirin‐ and ibuprofen‐induced inhibition of platelet aggregation in healthy subjects. Clin Pharmacokinet 2008; 47: 129–37. [DOI] [PubMed] [Google Scholar]
- 6. Goltsov A, Maryashkin A, Swat M, Kosinsky Y, Humphery‐Smith I, Demin O, Goryanin I, Lebedeva G. Kinetic modelling of NSAID action on COX‐1: focus on in vitro/in vivo aspects and drug combinations. Eur J Pharm Sci 2009; 36: 122–36. [DOI] [PubMed] [Google Scholar]
- 7. Goltsov A, Lebedeva G, Humphery‐Smith I, Goltsov G, Demin O, Goryanin I. In silico screening of nonsteroidal anti‐inflammatory drugs and their combined action on prostaglandin H synthase‐1. Pharmaceuticals 2010; 3: 2059–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Awa K, Satoh H, Hori S, Sawada Y. Prediction of time‐dependent interaction of aspirin with ibuprofen using a pharmacokinetic/pharmacodynamic model. J Clin Pharm Ther 2011; 37: 469–74. [DOI] [PubMed] [Google Scholar]
- 9. FDA . Information for Healthcare Professionals: Concomitant Use of Ibuprofen and Aspirin. 2006. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm125222.htm. Accessed on June 30, 2015.
- 10. Wilner KD, Rushing M, Walden C, Adler R, Eskra J, Noveck R, Vargas R. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J Clin Pharmacol 2002; 42: 1027–30. [PubMed] [Google Scholar]
- 11. Hennan JK, Huang J, Barrett TD, Driscoll EM, Willens DE, Park AM, Crofford LJ, Lucchesi BR. Effects of selective cyclooxygenase‐2 inhibition on vascular responses and thrombosis in canine coronary arteries. Circulation 2001; 104: 820–5. [DOI] [PubMed] [Google Scholar]
- 12. Rimon G, Sidhu RS, Lauver DA, Lee JY, Sharma NP, Yuan C, Frieler RA, Trievel RC, Lucchesi BR, Smith WL. Coxibs interfere with the action of aspirin by binding tightly to one monomer of cyclooxygenase‐1. Proc Natl Acad Sci USA 2010; 107: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Renda G, Zurro M, Malatesta G, Ruggieri B, De Caterina R. Inconsistency of different methods for assessing ex vivo platelet function: relevance for the detection of aspirin resistance. Haematologica 2010; 95: 2095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reilly IA, FitzGerald GA. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood 1987; 69: 180–6. [PubMed] [Google Scholar]
- 15. Patrono C, Baigent C, Hirsh J, Roth G. Antiplatelet drugs: American college of chest physicians evidence‐based clinical practice guidelines (8th edition). Chest 2008; 133 (6 Suppl): 199S–233S. [DOI] [PubMed] [Google Scholar]
- 16. Santilli F, Rocca B, De Cristofaro R, Lattanzio S, Pietrangelo L, Habib A, Pettinella C, Recchiuti A, Ferrante E, Ciabattoni G, Davi G, Patrono C. Platelet cyclooxygenase inhibition by low‐dose aspirin is not reflected consistently by platelet function assays: implications for aspirin ‘resistance’. J Am Coll Cardiol 2009; 53: 667–77. [DOI] [PubMed] [Google Scholar]
- 17. Bednar B, Condra C, Gould RJ, Connolly TM. Platelet aggregation monitored in a 96 well microplate reader is useful for evaluation of platelet agonists and antagonists. Thromb Res 1995; 77: 453–63. [DOI] [PubMed] [Google Scholar]
- 18. Moran N, Kiernan A, Dunne E, Edwards RJ, Shields DC, Kenny D. Monitoring modulators of platelet aggregation in a microtiter plate assay. Anal Biochem 2006; 357: 77–84. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong PC, Dhanji AR, Truss NJ, Zain ZN, Tucker AT, Mitchell JA, Warner TD. Utility of 96‐well plate aggregometry and measurement of thrombi adhesion to determine aspirin and clopidogrel effectiveness. Thromb Haemos 2009; 102: 772–8. [DOI] [PubMed] [Google Scholar]
- 20.Arachidonic Acid reagent leaflet, Catalogue No. 5364, Helena Laboratories, Beaumont, TX, USA.
- 21. Zhou L, Schmaier AH. Platelet aggregation testing in platelet‐rich plasma: description of procedures with the aim to develop standards in the field. Am J Clin Pathol 2005; 123: 172–83. [DOI] [PubMed] [Google Scholar]
- 22. Stepensky D, Rimon G. Competition between low‐dose aspirin and other NSAIDs for COX‐1 binding and its clinical consequences for the drugs' antiplatelet effects. Expert Opin Drug Metab Toxicol 2015; 11: 41–52. [DOI] [PubMed] [Google Scholar]
- 23. Ohmori T, Yatomi Y, Nonaka T, Kobayashi Y, Madoiwa S, Mimuro J, Ozaki Y, Sakata Y. Aspirin resistance detected with aggregometry cannot be explained by cyclooxygenase activity: involvement of other signaling pathway(s) in cardiovascular events of aspirin‐treated patients. J Thromb Haemost 2006; 4: 1271–8. [DOI] [PubMed] [Google Scholar]
- 24. Armstrong PC, Truss NJ, Ali FY, Dhanji AA, Vojnovic I, Zain ZN, Bishop‐Bailey D, Paul‐Clark MJ, Tucker AT, Mitchell JA, Warner TD. Aspirin and the in vitro linear relationship between thromboxane A2‐mediated platelet aggregation and platelet production of thromboxane A2. J Thromb Haemost 2008; 6: 1933–43. [DOI] [PubMed] [Google Scholar]
- 25. Pulcinelli FM, Biasucci LM, Riondino S, Giubilato S, Leo A, Di Renzo L, Trifiro E, Mattiello T, Pitocco D, Liuzzo G, Ghirlanda G, Crea F. COX‐1 sensitivity and thromboxane A2 production in type one and type two diabetic patients under chronic aspirin treatment. Eur Heart J 2009; 30: 1279–86. [DOI] [PubMed] [Google Scholar]
- 26. Tang WH, Stitham J, Gleim S, Di Febbo C, Porreca E, Fava C, Tacconelli S, Capone M, Evangelista V, Levantesi G, Wen L, Martin K, Minuz P, Rade J, Patrignani P, Hwa J. Glucose and collagen regulate human platelet activity through aldose reductase induction of thromboxane. J Clin Invest 2011; 121: 4462–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rocca B, Santilli F, Pitocco D, Mucci L, Petrucci G, Vitacolonna E, Lattanzio S, Mattoscio D, Zaccardi F, Liani R, Vazzana N, Del Ponte A, Ferrante E, Martini F, Cardillo C, Morosetti R, Mirabella M, Ghirlanda G, Davi G, Patrono C. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low‐dose aspirin in patients with and without diabetes. J Thromb Haemost 2012; 10: 1220–30. [DOI] [PubMed] [Google Scholar]
- 28. Smith JP, Haddad EV, Taylor MB, Oram D, Blakemore D, Chen Q, Boutaud O, Oates JA. Suboptimal inhibition of platelet cyclooxygenase‐1 by aspirin in metabolic syndrome. Hypertension 2012; 59: 719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kawai VK, Avalos I, Oeser A, Oates JA, Milne GL, Solus JF, Chung CP, Stein CM. Suboptimal inhibition of platelet cyclooxygenase one by aspirin in systemic lupus erythematosus: association with metabolic syndrome. Arthritis Care Res (Hoboken) 2014; 66: 285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spectre G, Arnetz L, Ostenson CG, Brismar K, Li N, Hjemdahl P. Twice daily dosing of aspirin improves platelet inhibition in whole blood in patients with type two diabetes mellitus and micro‐ or macrovascular complications. Thromb Haemost 2011; 106: 491–9. [DOI] [PubMed] [Google Scholar]
- 31. Capodanno D, Patel A, Dharmashankar K, Ferreiro JL, Ueno M, Kodali M, Tomasello SD, Capranzano P, Seecheran N, Darlington A, Tello‐Montoliu A, Desai B, Bass TA, Angiolillo DJ. Pharmacodynamic effects of different aspirin dosing regimens in type two diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv 2011; 4: 180–7. [DOI] [PubMed] [Google Scholar]
- 32. Bethel MA, Harrison P, Sourij H, Sun Y, Tucker L, Kennedy I, White S, Hill L, Oulhaj A, Coleman RL, Holman RR. Randomized controlled trial comparing impact on platelet reactivity of twice‐daily with once‐daily aspirin in people with type two diabetes. Diabet Med 2015. [DOI] [PubMed] [Google Scholar]
- 33.CELEBREX® (celecoxib) prescribing information, FDA, January 2011.
- 34. Bautista AP, Buckler PW, Towler HM, Dawson AA, Bennett B. Measurement of platelet life‐span in normal subjects and patients with myeloproliferative disease with indium oxine labelled platelets. Br J Haematol 1984; 58: 679–87. [DOI] [PubMed] [Google Scholar]


