Abstract
Aim
Two inhaler devices (Respimat® and HandiHaler®) are available for tiotropium, a long acting anticholinergic agent. We aimed to analyze drug utilization, off‐label usage and generalizability of the TIOSPIR trial results for both devices.
Methods
Patients aged ≥18 years exhibiting at least one documented prescription of tiotropium in the database of the Association of Statutory Health Insurance Physicians, Bavaria, Germany, were included (years 2004–2008). Annual period prevalence rates (PPRs) were calculated stratified by age, gender and inhaler devices. Off‐label usage (patients lacking a chronic obstructive pulmonary disease (COPD) diagnosis) and the proportion of patients meeting the inclusion and exclusion criteria of the TIOSPIR trial were analyzed.
Results
Between 2004 and 2008, PPRs increased and varied between 49.2 and 74.5 per 10 000 persons for HandiHaler® and between 1.5 and 9.3 per 10 000 persons for Respimat®. Small differences regarding patient characteristics existed between the two inhaler devices. Only about 30% (HandiHaler® 32.1%, Respimat® 30.0%) of the database patients receiving tiotropium could be theoretically included in the TIOSPIR trial.
Conclusions
Comparing the two tiotropium devices, no clinically relevant differences regarding patient and prescribing characteristics were revealed. Results of the TIOSPIR trial were generalizable only to a minority of our study patients, underlining the need for real‐life data.
Keywords: COPD, real‐life usage, secondary data analysis, TIOSPIR trial, tiotropium
What is Already Known About This Subject
After the TIOSPIR trial, there were still some safety concerns regarding tiotropium Respimat® in, for example, cardiovascular patients.
Comparing characteristics of patients receiving tiotropium Respimat® or HandiHaler® is crucial in identifying populations at risk.
Up to now, generalizability of TIOSPIR trial results to a real‐life population is unclear, hampering risk–benefit weighting.
What this Study Adds
In a claims database covering 10.5 million patients, similar characteristics were found comparing patients receiving Respimat® or HandiHaler®.
For both devices, only about 30% of patients receiving tiotropium could be potentially included in the TIOSPIR trial.
Further studies examining safety concerns in ‘real‐life’ patients receiving tiotropium are crucial.
Introduction
Tiotropium, a long acting anticholinergic compound, is recommended as controller medication for patients suffering from chronic obstructive pulmonary disease (COPD) 1. Two different tiotropium inhaler devices are available on the market, dry powder inhalation (DPI, HandiHaler®), which was authorized in the European Union in 2002 and Soft Mist™ inhaler (SMI, Respimat®), which was approved in 2007. The recommended daily dose for Respimat® is 5 μg compared with 18 μg for HandiHaler®, as the Respimat® aerosol contains a higher fraction of fine particles leading to higher pulmonary drug deposition. Comparing the systemic exposure in patients receiving tiotropium by Respimat® or HandiHaler®, pharmacokinetic data are somewhat conflicting 2, 3, 4. In addition, safety data comparing both inhaler devices are also oppositional. In a cohort study, an increased risk of all‐cause mortality and cardiovascular/cerebrovascular death was found for patients using Respimat® compared with HandiHaler®, particularly in patients exhibiting coexisting cardiovascular diseases 5. Similarly, a meta‐analysis revealed an increased risk of overall death for patients using Respimat® compared with HandiHaler®, with more evident risk increases for cardiovascular death in patients with severe COPD and at higher daily doses of tiotropium 6.
In contrast, in a recently published randomized controlled trial (RCT, i.e. TIOSPIR), two Respimat® dosages (2.5 μg or 5 μg once daily) were not inferior to HandiHaler® (18 μg once daily) regarding the mortality risk 7. Nevertheless, an increased risk of adjudicated myocardial infarctions was detected when both Respimat® dosage groups were combined by conducting a re‐analysis of the TIOSPIR trial 8. In addition, the TIOSPIR trial's generalizability was critically discussed owing to, for example, excluding patients with renal impairment or cardiac comorbidities 9, but detailed analyses focusing on real‐life population coverage are lacking 10.
The objectives of our study were twofold, first, to assess the real‐life usage of both tiotropium devices and to identify patients potentially having an increased risk of safety issues by comparing patient characteristics of Respimat® or HandiHaler® users with regard to age, gender, indication (off‐label use by indication), cardiovascular and respiratory comorbidities, and co‐medication in a large German database and second, to assess the generalizability of the TIOSPIR trial by quantifying the proportion of database patients theoretically meeting the inclusion and exclusion criteria of the TIOSPIR trial.
Methods
Study design and data source
A drug utilization study was conducted including the years 2004–2008 in the database of the Association of Statutory Health Insurance Physicians, Bavaria, Germany (‘Kassenärztliche Vereinigung Bayerns’, KVB) 11. This population‐based database was established in 2001 and covers approximately 85% (i.e. 10.5 million people) of the Bavarian population (excluding those with private insurance). It contains all accounting information from Bavarian physicians including outpatient data on diagnoses, medical services performed and drug utilization of statutory health insurance patients. All diagnoses from general practitioners and specialists are recorded quarterly (i.e. the quarter but not the precise date is documented for each diagnosis or prescription). A patient is documented in the database only when consulting a physician. Pharmaceuticals are recorded only if they were prescribed and filled at the pharmacy. The German modification of the International Statistical Classification of Diseases and Related Health Problems (ICD‐10‐GM) was used for coding diagnoses and the Anatomical Therapeutic Chemical (ATC) classification system was used for coding drugs. All analyses were performed using anonymized data. Neither German law nor the professional code of conduct for physicians requires an ethical review for research with routinely collected anonymized data.
Study population
Patients aged ≥18 years having at least one documented prescription of tiotropium HandiHaler® (central pharmaceutical numbers (CPN) 02286532, 01686873, 02286549, 03649221) or tiotropium Respimat® (CPN 04913588, 04913594, 04913625) within the study period were included in the analysis. The study entry date was defined as the quarter of the first tiotropium prescription in the study period.
Definition of off‐label use, comorbidities and co‐medication
Patients exhibiting at least one documented COPD diagnosis in the study period (irrespective of any other additional respiratory diagnoses) were considered as ‘on‐label’ patients. Taking into account the labelling status of tiotropium during the study period, all other patients were considered as off‐label users. Patients exhibiting a diagnosis coded with one‐digit ICD‐10‐GM code ‘I’ in their medical history were considered as patients suffering from cardiovascular comorbidity. Additionally, patients with a documented diagnosis of heart failure (ICD‐10‐GM code I50), chronic ischaemic heart disease (I25) and arrhythmias including atrial fibrillation and flutter (I48), and other cardiac arrhythmias (I49) were defined as patients with a cardiovascular comorbidity of particular interest. Cardiovascular drugs (ATC one‐digit: ‘C’) were analyzed stratified by therapeutic groups (ATC three digits). Drug classes were considered for respiratory co‐medication as follows: selective β2‐adrenoreceptor agonists (R03AC), adrenergics and other drugs for obstructive airway diseases (R03AK), anticholinergics (R03BB), systemic selective β2‐adrenoreceptor agonists (R03CC), xanthines (R03DA), and xanthines and adrenergics (R03DB).
TIOSPIR trial
In the TIOSPIR trial, some seven inclusion and 29 exclusion criteria were applied to define the study population 12. In total, 21 of these criteria were reproducible in our database. Lung function parameters, for example, remained undocumented in the Bavarian database, whereas other criteria (e.g. comorbidities) were available in most cases (e‐Table 1[Link]). A sensitivity analysis was performed using additional ICD‐10‐GM codes for the exclusion criteria ‘Myocardial infarction within the last 6 months’ (I25.21), ‘Hospitalization for cardiac failure (NYHA Class III or IV) during the last year’ (I50.13) and ‘Known significant symptomatic prostatic hyperplasia or bladder‐neck obstruction. Subjects whose symptoms were controlled on treatment may have been included’ (N40).
Statistical analysis
All metric and normally distributed variables were reported as mean ± standard deviation (SD). Non‐normally distributed variables were presented as median (first quartile–third quartile). Categorical variables were presented as frequency and percentage. For the comparison of groups, the Mann–Whitney U‐test was used for metric variables and the chi‐square test or Fisher's exact test for categorical variables. P values <0.05 were considered statistically significant.
Annual period prevalence rates (PPRs) were calculated using the number of patients with at least one prescription of interest during the year of interest (numerator) divided by the total number of compulsorily insured Bavarians at midyear of the year of interest (1 July; denominator). Annual PPRs per 10 000 persons were calculated stratified by age (10 year age groups [18–19 years, 20–29 years, 30–39 years […], ≥90 years]), gender and inhaler device. As Respimat® was launched on the European market in 2007, analyses for this device are restricted to the years 2007 and 2008, whereas the HandiHaler® PPR‐related analyses were conducted for the whole study period (2004–2008). Detailed analyses regarding for example comorbidities and co‐medication were conducted for the year 2008 only.
Results
Drug utilization
In 2008, some 69 812 patients (approximately 0.7% of all insured people) were treated with tiotropium (Table 1). Almost 89% of these patients used tiotropium via HandiHaler®. Respimat® users were slightly younger (67.9 ± 12.3 years) than patients using HandiHaler® (69.5 ± 12.2 years; P < 0.0001). The proportion of females was higher in Respimat® compared with HandiHaler® users (46.9% vs. 44.6%; P < 0.0001). The highest PPRs were found in patients aged between 70 and 79 years (2004–2006) and 80 and 89 years (2007–2008) for HandiHaler® and between 80 and 89 years for Respimat® (2007–2008; e‐Table 2[Link]).
Table 1.
Baseline characteristics stratified for inhaler device (year 2008)
| HandiHaler® | Respimat® | |||
|---|---|---|---|---|
| Patient characteristics | ||||
| Total (patients) | 62 036 | 7776 | ||
| Proportion of females (n [patients], %) | 27 641 (44.6%) | 3649 (46.9%) | ||
| Age (years) (mean ± SD) | 69.5 ± 12.2 | 67.9 ± 12.3 | ||
| Cardiovascular comorbidities | ||||
| Patients with at least one cardiovascular comorbidity (n [patients], %) | 54 308 (87.5%) | 6586 (84.7%) | ||
| Patients with at least one cardiovascular comorbidity of particular interest (n [patients], %) | 32 632 (52.6%) | 3679 (47.3%) | ||
| Five most frequent cardiovascular comorbidities (n [patients], %) | Essential (primary) hypertension (41 052 [75.6%]) | Essential (primary) hypertension (4838 [73.5%]) | ||
| Chronic ischaemic heart disease (18 995 [35.0%]) | Chronic ischaemic heart disease (2108 [32.0%]) | |||
| Heart failure (17 731 [32.6%]) | Heart failure (1862 [28.3%]) | |||
| Varicose veins of lower extremities (10 209 [18.8%]) | Varicose veins of lower extremities (1213 [18.4%]) | |||
| Atrial fibrillation and flutter (9357 [17.2%]) | Other cardiac arrhythmias (1094 [16.6%]) | |||
| Cardiovascular co‐medication | ||||
| Patients with at least one cardiovascular co‐medication (n [patients], %) | 47 095 (75.9%) | 5311 (68.3%) | ||
| Cardiovascular co‐medication (n [patients], %) | Cardiac therapy (C01) | 11 376 (18.3%) | 1126 (14.5%) | |
| Antihypertensives (C02) | 2611 (4.2%) | 247 (3.2%) | ||
| Diuretics (C03) | 25 612 (41.3%) | 2485 (32.0%) | ||
| Peripheral vasodilators (C04) | 935 (1.5%) | 93 (1.2%) | ||
| Vasoprotectives (C05) | 1226 (2.0%) | 135 (1.7%) | ||
| β‐adrenoceptor blocking agents (C07) | 17 368 (28.0%) | 1858 (23.9%) | ||
| Calcium channel blockers (C08) | 14 263 (23.0%) | 1497 (19.3%) | ||
| Agents acting on the renin–angiotensin system (C09) | 31 557 (50.9%) | 3413 (43.9%) | ||
| Lipid modifying agents (C10) | 15 001 (24.2%) | 1517 (19.5%) | ||
| Respiratory co‐medication | ||||
| Patients with at least one respiratory co‐medication (n [patients], %) | 46 339 (74.7%) | 5541 (71.3%) | ||
| Respiratory co‐medication (n [patients], %) [at least 1% in one group] | Inhaled short acting β2‐adrenoceptor agonists | Salbutamol | 14 384 (23.2%) | 1889 (24.3%) |
| Fenoterol | 2736 (4.4%) | 361 (4.6%) | ||
| Inhaled short acting β2‐adrenoceptor agonist combinations | Fenoterol and cromoglicic acid | 9551 (15.4%) | 1352 (17.4%) | |
| Reproterol and cromoglicic acid | 445 (0.7%) | 78 (1.0%) | ||
| Oral short acting β2‐adrenoceptor agonist combinations | Clenbuterol and ambroxol | 1149 (1.9%) | 168 (2.2%) | |
| Inhaled short acting anticholinergics | Ipratropium | 3237 (5.2%) | 490 (6.3%) | |
| Inhaled long acting β2‐adrenoceptor agonists | Salmeterol | 832 (1.3%) | 101 (1.3%) | |
| Formoterol | 12 922 (20.8%) | 1248 (16.0%) | ||
| Inhaled long acting β2‐adrenoceptor agonist combinations | Salmeterol and fluticasone | 10 595 (17.1%) | 1251 (16.1%) | |
| Formoterol and beclometasone | 2775 (4.5%) | 612 (7.9%) | ||
| Formoterol and budenoside | 12 046 (19.4%) | 1169 (15.0%) | ||
| Xanthines | Theophylline | 12 975 (20.9%) | 1413 (18.2%) | |
| Leukotriene receptor antagonists | Montelukast | 1222 (2.0%) | 195 (2.5%) | |
| Inhaled corticosteroids | Beclomethasone | 1807 (2.9%) | 282 (3.6%) | |
| Budesonide | 7284 (11.7%) | 783 (10.1%) | ||
PPR increase was revealed for both inhaler devices within the study period. The PPRs varied between 49.2 and 74.5 per 10 000 persons for HandiHaler® (2004–2008) and between 1.5 and 9.3 per 10 000 persons for Respimat® (2007–2008; e‐Table 2[Link]). For both devices, PPRs for males were higher than those for females (Figure 1). Regarding age groups, the most prominent PPR increase for HandiHaler® was found in patients aged at least 80 years (80–89 years: +97.7%; ≥90 years: +154.1%) compared with Respimat® patients aged ≥90 years (+1217.2%; e‐Table 2[Link]).
Figure 1.
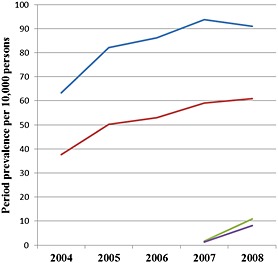
Period prevalence rates per 10 000 persons stratified by inhaler device and gender.  HandiHaler® males,
HandiHaler® males,  HandiHaler® females,
HandiHaler® females,  , Respimat® males,
, Respimat® males,  Respimat® females
Respimat® females
Comorbidities and co‐medications
In 2008, the proportion of patients with at least one cardiovascular disease (ICD‐10‐GM code ‘I’) in their medical history was slightly higher in patients using HandiHaler® (87.5%) compared with Respimat® (84.7%; P < 0.0001; Table 1). Limiting the prevalence analysis to patients with at least one cardiovascular comorbidity of particular interest (heart failure, chronic ischaemic heart disease, atrial fibrillation and flutter, and other cardiac arrhythmias), the respective values were 52.6% (HandiHaler®) and 47.3% (Respimat®, P < 0.0001). For both patient groups, essential hypertension, chronic ischaemic heart disease and heart failure were the most frequent cardiovascular diseases with higher prevalence rates in patients using HandiHaler®.
The proportion of patients receiving at least one cardiovascular co‐medication was higher in HandiHaler® patients (75.9%) than in Respimat® patients (68.3%; P < 0.0001). For both devices, the most frequent cardiovascular compound was ‘agents acting on the renin–angiotensin system’ (HandiHaler® n = 31 557 (50.9%); Respimat® n = 3413 (43.9%)), whereas the most frequently prescribed respiratory co‐medications were salbutamol (HandiHaler® n = 14 384 (23.2%); Respimat® n = 1889 (24.3%)) and theophylline (HandiHaler® n = 12 975 (20.9%); Respimat® n = 1413 (18.2%); Table 1)).
Off‐label use
The proportion of patients with off‐label HandiHaler® prescriptions was highest in 2004 (n = 5590 patients (13.9%)) and lowest in 2006 (n = 6077 (10.9%); Table 2). A small increase in the proportion of patients with off‐label prescriptions was revealed for Respimat® from 10.5% (n = 130) in 2007 to 14.0% (n = 1086) in 2008 (Table 2).
Table 2.
Number and proportion of patients with on‐label and off‐label prescriptions stratified by inhaler device
| Year | HandiHaler ® (n, %) | Respimat ® (n, %) | ||
|---|---|---|---|---|
| On‐label | Off‐label | On‐label | Off‐label | |
| 2004 | 34 631 (86.1%) | 5590 (13.9%) | – | – |
| 2005 | 46 606 (87.8%) | 6466 (12.2%) | – | – |
| 2006 | 49 903 (89.1%) | 6077 (10.9%) | – | – |
| 2007 | 55 064 (89.0%) | 6788 (11.0%) | 1109 (89.5%) | 130 (10.5%) |
| 2008 | 54 880 (88.5%) | 7156 (11.5%) | 6690 (86.0%) | 1086 (14.0%) |
Regarding age groups, we found the highest proportion of patients receiving off‐label prescriptions for HandiHaler® in the youngest age groups (18–19 years: 51.5–82.4%; 20–29 years: 46.6–53.0%), whereas the lowest proportion was found in patients aged between 60 and 69 years (8.5–11.6%, [percentage range between 2004 and 2008]). A similar age‐related pattern was found for Respimat® (data not shown).
More than one‐third of the off‐label patients had a documented diagnosis of asthma (HandiHaler® n = 2674 (37.4%); Respimat® n = 438 (40.3%)). Other relevant off‐label diagnoses were acute respiratory tract infections (e‐Table [Link]3).
TIOSPIR trial
Only approximately 30% (HandiHaler® 32.1%; Respimat® 30.0%) of our study patients theoretically met all the criteria for participating in the TIOSPIR trial. Between the two devices, no relevant differences were detected regarding exclusion criteria. Most patients were theoretically excluded because of a (concurrent) diagnosis of asthma (HandiHaler® n = 23 701 (56.2%); Respimat® n = 3140 (57.7%)); Table 3; multiple counting of exclusion criteria). For both devices, patients potentially excluded/not included in the TIOSPIR trial were slightly younger than potentially included patients. There were no gender‐related differences between potentially included or excluded patients for both inhaler devices (e‐Table 4[Link]). In a sensitivity analysis considering additional co‐morbidities as exclusion criteria (ICD‐10‐GM codes I25.21, I50.13, and N40), the proportion of patients excluded from the TIOSPIR trial increased to 73.0% for patients using HandiHaler® and to 74.3% for patients using Respimat®.
Table 3.
Exclusion criteria stratified for inhaler device (multiple counting)
| Category | Explicit criterion | Code category/unit used in KVB | Explicit code/threshold used in KVB | Not included/excluded HandiHaler ® | Not included/excluded Respimat ® | |
|---|---|---|---|---|---|---|
| Inclusion criteria | Gender | Gender | Male/female | Male/female | 0 | 0 |
| Age | Age | years | ≥40 | 769 (1.8%) | 169 (3.1%) | |
| Clinical diagnosis | COPD | ICD‐10‐GM | J44 | 7156 (17.0%) | 1086 (20.0%) | |
| Exclusion criteria | Concomitant cardiovascular disease | Myocardial infarction within the last 6 months | ICD‐10‐GM | I21, I22, I25.20 | 4535 (10.8%) | 528 (9.7%) |
| Hospitalization for heart failure within 12 months | ICD‐10‐GM | I50.14 | 564 (1.3%) | 62 (1.1%) | ||
| Unstable or life threatening arrhythmia requiring intervention or change in drug therapy within 12 months | ICD‐10‐GM | I44.2, I45.9, I46, I47.0, I47.2, I49.0 | 1306 (3.1%) | 158 (2.9%) | ||
| Other significant lung diseases | Active tuberculosis | ICD‐10‐GM | A15, A16, A17, A18, A19 | 1507 (3.6%) | 200 (3.7%) | |
| Asthma | ICD‐10‐GM | J45, J46 | 23 701 (56.2%) | 3140 (57.7%) | ||
| Cystic fibrosis | ICD‐10‐GM | E84 | 46 (0.1%) | 3 (0.1%) | ||
| Clinically evident bronchiectasis (not excluded for radiographic evidence of bronchiectasis if they were not being treated for this condition) | ICD‐10‐GM | J47 | 712 (1.7%) | 101 (1.9%) | ||
| Interstitial lung disease | ICD‐10‐GM | J60‐J70, J80, J82, J84 | 0 | 0 | ||
| Pulmonary thromboembolism | ICD‐10‐GM | I26 | 2159 (5.1%) | 252 (4.6%) | ||
| Cancer | Malignancies (other than treated basal cell carcinoma) requiring therapy within the last 5 years (resection, radiation, chemotherapy or biological treatments) | ICD‐10‐GM | C00–C97 except C44 | 11 458 (27.2%) | 1376 (25.3%) | |
| Others | Known moderate to severe renal impairment (as judged by the investigator) | ICD‐10‐GM | N18.3, N18.4, N18.5 | 2592 (6.1%) | 257 (4.7%) | |
| Drug abuse within 12 months | ICD‐10‐GM | F11.2, F12.2, F13.2, F19.0 | 261 (0.6%) | 42 (0.8%) | ||
| Alcohol abuse within 12 months | ICD‐10‐GM | F10, K70, Z72.0 | 3883 (9.2%) | 521 (9.6%) | ||
| Known narrow angle glaucoma | ICD‐10‐GM | H40.2 | 266 (0.6%) | 34 (0.6%) | ||
| Known hypersensitivity to anticholinergic drugs, lactose or other components of the HandiHaler® or Respimat® inhalation solution delivery system | ICD‐10‐GM | E73.‐ | 250 (0.6%) | 34 (0.6%) | ||
| Symptomatic bladder neck obstruction | ICD‐10‐GM | N32.0 | 523 (1.2%) | 52 (1.0%) | ||
| Child‐bearing potential not on contraceptives | ATC Age | No G03A ≤ 45 years | 856 (2.0%) | 165 (3.0%) | ||
| Unstable COPD | Plans for lung transplantation or lung volume reduction surgery | ICD‐10‐GM | Z94.2, Z94.3, T86.3, T86.81, U55.2, U55.3 | 23 (0.1%) | 5 (0.1%) | |
| Total | 42 151 (67.9%) | 5443 (70.0%) |
KVB, Kassenärztliche Vereinigung Bayerns database.
Discussion
By analyzing a large population‐based German claims database, we found an increase in prescriptions for HandiHaler® (years 2004–2008) and Respimat® devices (2007–2008), particularly in elderly patients. There were only minor differences regarding cardiovascular comorbidities and co‐medications between patients receiving HandiHaler® or Respimat®. For both devices, a similar proportion of patients received tiotropium off‐label with the highest rates in the youngest patients suffering from asthma. Of all patients documented in our database receiving tiotropium, only one‐third theoretically met the criteria for participating in the TIOSPIR trial, underlining the highly selected patient samples of RCTs.
Drug utilization
In 2008, only 0.7% of all insured people received at least one tiotropium prescription (combined analysis for both devices). The prevalence of tiotropium estimated in our study seems to be reliable for three reasons: (i) focusing on the main indication, COPD, European prevalence estimates range between 2.1% and 26.1% depending on, for example, the country and included age groups 13, (ii) the results of the BOLD study showed for a German region that almost half of patients with COPD are grouped into stage 2 or higher and (iii) the guidelines recommend tiotropium for COPD stage 2–4 1, 14. Similar to other studies focusing on COPD, we found the highest tiotropium prevalence estimates in elderly men 14, 15. Nevertheless, differences in the methods used for defining COPD cases (e.g. questionnaires, spirometry data) limit the comparability of study results 13.
Currently, only limited device‐related drug utilization data are available. In a cross‐sectional analysis assessing patient preferences with inhaler technique and incorrect use of inhaler devices in Portuguese patients attending an outpatient clinic in 2013, HandiHaler® was used by 17% of patients, whereas Respimat® was used by only 4% of patients 16. Similarly, in a multinational online survey conducted in 2010, most COPD patients were using HandiHaler® 17. To sum up the available evidence, HandiHaler® is more widely used than Respimat®.
Comorbidities and co‐medications
In our study focusing on cardiovascular comorbidities, we found that three‐quarters of all patients receiving tiotropium had a concomitant diagnosis of hypertension, whereas one‐third exhibited a diagnosis of chronic ischaemic heart disease or heart failure. Comparing patient characteristics for both devices, patients using Respimat® had slightly fewer cardiovascular comorbidities (all cardiovascular comorbidities and cardiovascular comorbidities of particular interest) than patients using HandiHaler®, a fact that might be related to a somewhat lower age. In a recently published review analyzing the burden of comorbidities in COPD patients, lower prevalence rates were reported for cardiovascular diseases compared with our study 18. These differences might be related to including patients not receiving tiotropium in most studies (stage 1 according to GOLD), resulting in younger and healthier study populations compared with our study. In addition, differences regarding age groups, definitions of comorbidities or national regulations concerning documentation modalities may also contribute to different results.
Focusing on respiratory co‐medication, the short acting β2‐adrenoceptor agonist, salbutamol, and theophylline were the most frequently prescribed compounds. Whereas salbutamol is an effective bronchodilator and recommended according to the guidelines, the high proportion of patients receiving theophylline is a matter of concern due to a narrow therapeutic range, but similar drug utilization data for theophylline were also reported in an Italian study covering the years 2006–2008 19. Reflecting theophylline‐related changes in COPD guidelines in recent years 1, 20, a 65% decrease in theophylline defined daily doses was found between 2004 and 2012 for Germany according to national drug consumption data 21, 22. From a general point of view, by co‐prescribing beta‐2‐agonists and/or theophylline to patients receiving tiotropium, one cannot exclude an, for example, increased risk for cardiovascular side effects due to pharmacodynamic drug‐drug interactions.
Off‐label use
In our study, 10–14% of all patients received tiotropium off‐label without relevant clinical differences between the two devices regarding off‐label indications (e‐Table [Link]3). In both patient groups, asthma was the most frequent off‐label indication. In recent years, evidence has accumulated for using tiotropium in asthmatic patients 23. In particular, when tiotropium was added to inhaled corticosteroids [ICS]/salmeterol, relevant clinical endpoints (e.g. asthma exacerbations) were reduced 24. Recently, tiotropium Respimat® has been approved by the European Medicines Agency (EMA) and the Federal Drug Administration (FDA) for the treatment of asthma. However, during the study period, using tiotropium in patients with asthma not having a concomitant diagnosis of COPD should be considered as off‐label treatment taking into account formerly effective guidelines 25, 26, 27.
TIOSPIR trial
Of all patients receiving tiotropium, only approximately 30% were theoretically eligible for the TIOSPIR trial considering inclusion and exclusion criteria. Thus, the results of the TIOSPIR trial stating a similar mortality risk comparing HandiHaler® and Respimat® are not generalizable to two‐thirds of patients receiving tiotropium under real‐life conditions. In a recently published study, Scichilone et al. estimated the proportion of patients not meeting the inclusion and exclusion criteria of RCTs published up to the year 2012 based on the clinical records of COPD outpatients at 83% 10. Similar to our results, concomitant lung diseases other than COPD were the most common exclusion criteria even in patients primarily diagnosed as COPD patients. In contrast to our study using claims data, Scichilone et al. were able to include additional parameters (e.g. lung function), explaining the slightly higher proportion of excluded patients compared with our results 10. Nevertheless, in the sensitivity analysis using additional ICD codes to define a few exclusion criteria, a slightly higher proportion of excluded patients was found.
From a medical and methodological point of view, both inclusion and exclusion criteria limit the generalizability of RCTs regarding efficacy and safety 28, 29. For tiotropium, which is (i) primarily renally excreted after pulmonary uptake and (ii) mainly used in elderly COPD patients frequently suffering from cardiovascular comorbidities, the exclusion of patients with cardiovascular and renal comorbidities might be justified with regard to minimizing risks for the study participants. Nevertheless, the external validity of such trials is moderate taking into account the results from Scichilone et al. and our own study 10. By excluding ‘real‐life’ patients, as mentioned above, important research questions addressing risk estimates for special populations cannot be answered sufficiently, as shown by several replies made to the TIOSPIR trial 8, 9, 30. Furthermore, absolute and relative contraindications covering, for example, comorbidities are neglected by physicians to a relevant extent under real‐life conditions 31. This fact underlines the urgent need to conduct pragmatic trials to get ‘the whole picture’ in terms of a realistic assessment of benefits and risks for patients with a potentially increased risk of adverse drug reactions 29.
Strengths and limitations
Lung function parameters were not documented in the claims database used in this study. Hence, we were unable to re‐check whether a documented diagnosis of COPD was valid or whether a diagnosis of COPD was present but not documented in the database. As described previously, an accurate diagnosis of COPD is a crucial but often unmet need in clinical practice even if lung function parameters are available 32, 33. Owing to the lack of lung function parameters, we were not able to stratify our analysis by the severity of COPD. For feasibility and validity reasons, we abstained from using surrogates (e.g. prescriptions of antibiotics) as estimates for COPD exacerbation rates. Second, we cannot exclude an underestimation of on‐label usage in patients with a documented COPD diagnosis before 2004 because of the limited observation period of 5 years. Nevertheless, by using a 5 year study period, it seems reasonable that patients suffering from COPD were coded at least once as COPD patients. Third, our off‐label analyses were focused on indication‐related off‐label usage, whereas other aspects (e.g. dose or age) remained not covered in this study. However, indication‐related off‐label usage is the most relevant off‐label use even in children with a relevant age‐related labelling 34, 35. Fourth, several inclusion and exclusion criteria were not sufficiently covered in our database and, by using surrogates, we cannot completely exclude an under‐ or overestimation of the proportion of patients not meeting the TIOSPIR criteria. However surrogates were only used for a minority of criteria and the major criteria were covered in our study (e.g. respiratory diseases other than COPD). Underlining this assumption, similar results were found in a sensitivity analysis using additional ICD codes for some exclusion criteria. Fifth, a few time‐dependent exclusion criteria (e.g. acute respiratory infection/acute exacerbation 4 weeks prior to randomization) were not applicable in our study due to the quarterly documentation in our database. Hence, we abstained from including these criteria, leading to an underestimation of potentially excluded patients. As a major strength, we used a large German database (10.5 million people) covering 85% of the Bavarian population, allowing a comprehensive assessment of real‐life prescribing for the two tiotropium devices.
In conclusion, our study analyzing claims data from 10.5 million patients between 2004 and 2008, we found an increase in prescriptions for two tiotropium devices (HandiHaler®, Respimat®). Despite some published reservations regarding the safety of the Respimat® device in particular patient groups, we revealed only slight differences between the two devices with regard to age, gender, cardiovascular and respiratory comorbidities, co‐medication and off‐label use in clinical practice. Of all patients receiving tiotropium, approximately 30% would theoretically meeting the inclusion and exclusion criteria of the TIOSPIR trial, limiting the generalizability of this trial to a relevant extent. Hence, further studies are urgently required to assess and compare the safety of both tiotropium devices in vulnerable, real‐life patient populations.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work. SS reports personal fees from Rottapharm Madaus (Cologne, Germany) and travel costs for an investigator meeting were reimbursed by Bayer HealthCare AG (Leverkusen, Germany) outside the submitted work. PT reports personal fees from Rottapharm Madaus (Cologne, Germany) outside the submitted work. JF and RR belong to EFPIA (European Federation of Pharmaceutical Industries and Association) member companies in the IMI JU, and costs related to their part in the research were carried by the respective company as in‐kind contributions under the IMI JU scheme.
This study was conducted as part of the PROTECT consortium (Pharmacoepidemiological Research on Outcomes of Therapeutics by a European ConsorTium, www.imi‐protect.eu), which is a public–private partnership coordinated by the European Medicines Agency.
Funding
The PROTECT project has received support from the Innovative Medicines Initiative Joint Undertaking (www.imi.europa.eu) under Grant Agreement no. 115 004, the resources of which are composed of financial contributions from the European Union's Seventh Framework Programme (FP7/2007–2013) and the in‐kind contribution of EFPIA companies. In addition, as a special form of the IMI JU grant, Utrecht University received a direct financial contribution from Pfizer.
Supporting information
e‐Table 1 Inclusion and exclusion criteria of the TIOSPIR trial
e‐Table 2 Period prevalence per 10 000 persons stratified by age group and inhaler device
e‐Table 3 Number and proportion of off‐label indications (year 2008, multiple counting of off‐label indications per prescription)
e‐Table 4 Characteristics of patients potentially excluded/ not included in the TIOSPIR trial
Supporting info item
Schmiedl, S. , Fischer, R. , Ibanez, L. , Fortuny, J. , Thürmann, P. , Ballarin, E. , Ferrer, P. , Sabaté, M. , Rottenkolber, D. , Gerlach, R. , Tauscher, M. , Reynolds, R. , Hasford, J. , and Rottenkolber, M. (2016) Tiotropium Respimat® vs. HandiHaler®: real‐life usage and TIOSPIR trial generalizability. Br J Clin Pharmacol, 81: 379–388. doi: 10.1111/bcp.12808.
References
- 1. Global Initiative for Chronic Obstructive Lung disease (GOLD) . Global strategy for the diagnosis, management and prevention of COPD. 2014. [cited 10/OCT/2015]; Available from: http://www.goldcopd.org
- 2. Garcia AA. On comparing different devices of inhalation products. Respir Med 2009; 103: 1774–5; author reply 76. [DOI] [PubMed] [Google Scholar]
- 3. van Noord JA, Cornelissen PJ, Aumann JL, Platz J, Mueller A, Fogarty C. The efficacy of tiotropium administered via Respimat Soft Mist inhaler or HandiHaler in COPD patients. Respir Med 2009; 103: 22–9. [DOI] [PubMed] [Google Scholar]
- 4. Hohlfeld JM, Sharma A, van Noord JA, Cornelissen PJ, Derom E, Towse L, Peterkin V, Disse B. Pharmacokinetics and pharmacodynamics of tiotropium solution and tiotropium powder in chronic obstructive pulmonary disease. J Clin Pharmacol 2014; 54: 405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verhamme KM, Afonso A, Romio S, Stricker BC, Brusselle GG, Sturkenboom MC. Use of tiotropium Respimat Soft Mist inhaler versus HandiHaler and mortality in patients with COPD. Eur Respir J 2013; 42: 606–15. [DOI] [PubMed] [Google Scholar]
- 6. Dong YH, Lin HH, Shau WY, Wu YC, Chang CH, Lai MS. Comparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta‐analysis of randomised controlled trials. Thorax 2013; 68: 48–56. [DOI] [PubMed] [Google Scholar]
- 7. Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, Dusser D, Joseph E, Kattenbeck S, Koenen‐Bergmann M, Pledger G, Calverley P. Tiotropium respimat inhaler and the risk of death in COPD. N Engl J Med 2013; 369: 1491–501. [DOI] [PubMed] [Google Scholar]
- 8. Loke YK, Singh S, Furberg CD. Tiotropium and the risk of death in COPD. N Engl J Med 2014; 370: 480–1. [DOI] [PubMed] [Google Scholar]
- 9. Verhamme KM, van Blijderveen N, Sturkenboom MC. Tiotropium and the risk of death in COPD. N Engl J Med 2014; 370: 481–2. [DOI] [PubMed] [Google Scholar]
- 10. Scichilone N, Basile M, Battaglia S, Bellia V. What proportion of chronic obstructive pulmonary disease outpatients is eligible for inclusion in randomized clinical trials? Respiration 2014; 87: 11–7. [DOI] [PubMed] [Google Scholar]
- 11. KVB . The Bavarian Association of Statutory Health Insurance Physicians (Kassenärztliche Vereinigung Bayerns). [cited 10/OCT/2015]; Available from: http://www.kvb.de/
- 12. Wise RA, Anzueto A, Calverley P, Dahl R, Dusser D, Pledger G, Koenen‐Bergmann M, Joseph E, Cotton D, Disse B. The tiotropium safety and performance in Respimat trial (TIOSPIR), a large scale, randomized, controlled, parallel‐group trial‐design and rationale. Respir Res 2013; 14: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atsou K, Chouaid C, Hejblum G. Variability of the chronic obstructive pulmonary disease key epidemiological data in Europe: systematic review. BMC Med 2011; 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska‐Mogilnicka E, Group BCR. International variation in the prevalence of COPD (the BOLD study): a population‐based prevalence study. Lancet 2007; 370: 741–50. [DOI] [PubMed] [Google Scholar]
- 15. Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging 2014; 9: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chorao P, Pereira AM, Fonseca JA. Inhaler devices in asthma and COPD–an assessment of inhaler technique and patient preferences. Respir Med 2014; 108: 968–75. [DOI] [PubMed] [Google Scholar]
- 17. Molimard M, Colthorpe P. Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners. J Aerosol Med Pulm Drug Deliv 2015; 28: 219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis 2014; 9: 871–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cazzola M, Segreti A, Bettoncelli G, Calzetta L, Cricelli C, Pasqua F, Rogliani P. Change in asthma and COPD prescribing by Italian general practitioners between 2006 and 2008. Prim Care Respir J 2011; 20: 291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Global Initiative for Chronic Obstructive Lung disease (GOLD) . Global strategy for the diagnosis, management, and prevention of COPD (updated 2004) 2004. [cited 10/OCT/2015]; Available from: http://www.goldcopd.org/uploads/users/files/GOLDWkshp2004Clean.pdf
- 21. Lemmer B. Bronchospasmolytika und Antiasthmatika In: Arzneiverordnungs‐Report 2005, eds. Schwabe U, Paffrath D, Heidelberg: Springer ‐ Verlag, 2006. [Google Scholar]
- 22. Lemmer B. Bronchospasmolytika und Antiasthmatika In: Arzneiverordnungs‐Report 2013, eds. Schwabe U, Paffrath D, Heidelberg: Springer‐Verlag Berlin, 2013. [Google Scholar]
- 23. Beeh KM, Moroni‐Zentgraf P, Ablinger O, Hollaenderova Z, Unseld A, Engel M, Korn S. Tiotropium respimat(R) in asthma: a double‐blind, randomised, dose‐ranging study in adult patients with moderate asthma. Respir Res 2014; 15: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodrigo GJ, Castro‐Rodriguez JA. What is the role of tiotropium in asthma?: a systematic review with meta‐analysis. Chest 2015; 147: 388–96. [DOI] [PubMed] [Google Scholar]
- 25. Arzneimittelkommission der deutschen Ärzteschaft . Empfehlungen zur Therapie des Asthma bronchiale im Erwachsenenalter. AVP‐Sonderheft Therapieempfehlungen 2001.
- 26. Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention 2003, 2003. [cited 10/OCT/2015]; Available from: http://www.ginasthma.org
- 27. Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention 2005, 2005. [cited 10/OCT/2015]; Available from: http://www.ginasthma.org
- 28. Halpin DM. Lessons from the major studies in COPD: problems and pitfalls in translating research evidence into practice. Prim Care Respir J 2010; 19: 170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saturni S, Bellini F, Braido F, Paggiaro P, Sanduzzi A, Scichilone N, Santus PA, Morandi L, Papi A. Randomized controlled trials and real life studies. Approaches and methodologies: a clinical point of view. Pulm Pharmacol Ther 2014; 27: 129–38. [DOI] [PubMed] [Google Scholar]
- 30. Mathioudakis AG, Chatzimavridou‐Grigoriadou V, Evangelopoulou E, Mathioudakis GA, Siafakas NM. Comparative mortality risk of tiotropium administered via handihaler or respimat in COPD patients: are they equivalent? Pulm Pharmacol Ther 2014; 28: 91–7. [DOI] [PubMed] [Google Scholar]
- 31. Meyer CN. Use of tiotropium respimat versus HandiHaler and mortality in patients with COPD. Eur Respir J 2014; 43: 1816–8. [DOI] [PubMed] [Google Scholar]
- 32. Izquierdo JL, Martin A, de Lucas P, Rodriguez‐Gonzalez‐Moro JM, Almonacid C, Paravisini A. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis 2010; 5: 241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma 2006; 43: 75–80. [DOI] [PubMed] [Google Scholar]
- 34. Baiardi P, Ceci A, Felisi M, Cantarutti L, Girotto S, Sturkenboom M, Baraldi E. In‐label and off‐label use of respiratory drugs in the Italian paediatric population. Acta Paediatr 2010; 99: 544–9. [DOI] [PubMed] [Google Scholar]
- 35. Schmiedl S, Fischer R, Ibanez L, Fortuny J, Klungel OH, Reynolds R, Gerlach R, Tauscher M, Thurmann P, Hasford J, Rottenkolber M. Utilisation and off‐label prescriptions of respiratory drugs in children. PLoS One 2014; 9: e105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
e‐Table 1 Inclusion and exclusion criteria of the TIOSPIR trial
e‐Table 2 Period prevalence per 10 000 persons stratified by age group and inhaler device
e‐Table 3 Number and proportion of off‐label indications (year 2008, multiple counting of off‐label indications per prescription)
e‐Table 4 Characteristics of patients potentially excluded/ not included in the TIOSPIR trial
Supporting info item


