Abstract
Objectives
To assess the impact of exposure to single-dose nevirapine (sdNVP) on virological response in young Ugandan/Zimbabwean children (<3 years) initiating antiretroviral therapy (ART), and investigate other predictors of response.
Design
Observational analysis within the ARROW randomised trial.
Methods
sdNVP exposure was ascertained by caregiver’s self-report when the child initiated NNRTI based ART. Viral load (VL) was assayed retrospectively over median 4.1 years follow-up. Multivariable logistic regression models were used to identify independent predictors of VL <80 copies/ml 48 and 144 weeks after ART initiation (backwards elimination, exit p=0.1).
Results
Median (IQR) age at ART initiation was 17 (10-23) months in 78 sdNVP exposed children versus 21 (14-27) months in 289 non-exposed children (36% vs 20% <12 months). At week 48, 49/73 (67%) sdNVP exposed and 154/272 (57%) non-exposed children had VL<80 copies/ml (adjusted (a)OR=2.34 [1.26-4.34] p=0.007); 79% and 77% had VL<400copies/ml. Suppression was significantly lower in males (p=0.009), those with higher pre-ART VL (p=0.001), taking syrups (p=0.05) and with lower self-reported adherence (p=0.04). At week 144, 55/73 (75%) exposed and 188/272 (69%) non-exposed had <80 copies/ml (aOR=1.75 [0.93-3.29] p=0.08). There was no difference between children with and without previous sdNVP exposure in intermediate/high-level resistance to NRTIs (p>0.3) or NNRTIs (p>0.1) (n=88) at week 144.
Conclusion
Given the limited global availability of lopinavir/ritonavir, its significant formulation challenges in young children, and the significant paediatric treatment gap, tablet fixed-dose-combination nevirapine-based ART remains a good alternative to syrup lopinavir-based ART for children, particularly those over one year and even if exposed to sdNVP.
Keywords: HIV, Africa, children, antiretroviral therapy, viral load, sdNVP
INTRODUCTION
Despite effectively reducing mother-to-child HIV transmission, single dose nevirapine (sdNVP) given to the mother and/or the infant at delivery has important limitations. First, the drug’s long-half-life, especially at birth when metabolism is limited, means sub-therapeutic levels can persist for long periods of time. Second, its low genetic barrier to high-level resistance caused by single point mutations favour the emergence of resistant variants in a substantial proportion of recipients; variants can also be transmitted to infants via breastmilk[1].
Studies have documented poorer response to nevirapine-containing combination antiretroviral therapy (ART) subsequently initiated by mothers exposed to sdNVP[2, 3]. The poorer virological response to nevirapine- vs lopinavir-containing regimens in the P1060 trials of infants exposed[4], and non-exposed[5], to sdNVP led WHO to recommend universal ART initiation with lopinavir/ritonavir-containing regimens in children <3 years[6]. However, further analysis of pooled P1060 data[7] found no impact of sdNVP on a composite endpoint of viral load (VL) failure (>400 copies/ml at week 24 or >4000 copies/ml subsequently) or death, which occurred in 13/84 (19%) sdNVP-exposed (median age 8 months; CD4% 19%) versus 30/145 (21%) non-exposed (20 months; 15%) children initiating nevirapine-based ART. Other evidence supporting poorer response to nevirapine-containing regimens in sdNVP-exposed infants is limited. One small study found virological failure by 6 months in 10/15 sdNVP-exposed infants vs 1/15 non-exposed infants (median age 1 month at initiation of nevirapine-based ART)[8]. Another found only 38% of 35 sdNVP-exposed Ugandan children (median age 6 months; CD4% 16%) versus 68% of 69 non-exposed children (22 months; 12%) had VL<400 copies/ml 48 weeks after initiating non-nucleoside-reverse-transcriptase-inhibitor (NNRTI)-based ART[9], but did not estimate associations adjusted for receipt of nevirapine vs efavirenz (respectively 97% vs 3% sdNVP-exposed, 71% vs 29% non-exposed). In contrast, another Ugandan study found 76% of 44 sdNVP exposed children (median age 20 months; CD4% 14%) versus 80% of 48 non-exposed children (median 7.8 years; 8%) had VL <400 copies/ml 48 weeks after ART initiation with nevirapine-based regimens[10].
WHO guidelines now recommend all children <5 years initiate ART regardless of immune or clinical status, and that those <3 years initiate protease-inhibitor (PI)-based regimens. However, lopinavir/ritonavir availability is limited and for young children, the only current formulation is an unpalatable liquid with cold-chain requirements, providing management challenges at lower-level health facilities. Where first-line lopinavir-containing ART is not feasible, WHO 2013 guidelines suggest non-nucleoside-reverse-transcriptase-inhibitor (NNRTI)-based regimens should be initiated as an alternative, because mortality in untreated young children is very high; the NNRTI of choice is nevirapine, because dosing of efavirenz is challenging in young children[11]. Understanding whether sdNVP is associated with substantially greater risks of virological failure in children initiating nevirapine-based ART aged >1 month of age therefore continues to have programmatic relevance, particularly in sub-Saharan Africa where most HIV-infected children live and where rollout of universal combination ART for pregnant women (Option B+) is gathering pace. Furthermore, a substantial proportion of African women still have no or incomplete antenatal care and deliver their babies at home, where the risk of receiving no interventions at all to prevent mother-to-child transmission (pMTCT) remains high. We therefore compared VL response between children initiating nevirapine-based ART aged <3 years with and without previous sdNVP exposure in the ARROW trial.
METHODS
Analyses included 367 previously untreated (except for prevention of mother-to-child-transmission) Ugandan/Zimbabwean children initiating nevirapine-based ART aged 3 months–<36 months in the ARROW trial (ISCRTN24791884). Three children <36 months (32, 35, 35 months) initiated efavirenz-based ART and were excluded. The trial recruited from March 2007-November 2008: before this, and during recruitment, sdNVP to the mother and child was the national pMTCT strategy. ART taken by the mother during pregnancy, delivery, or breastfeeding, and (separately) ART taken by the child were determined by self-report at enrolment.
Children were randomised 1:1 to clinically driven monitoring vs laboratory plus clinical monitoring for toxicity (haematology/biochemistry) and efficacy (CD4s). Children were also randomised 1:1:1 in a factorial design to open-label lamivudine+abacavir+NNRTI continuously (Arm-A, no zidovudine) versus induction-maintenance with 4-drug lamivudine+abacavir+NNRTI+zidovudine for 36 weeks, then either lamivudine+abacavir+NNRTI (Arm-B; short-term zidovudine) or lamivudine+abacavir+zidovudine (Arm-C; long-term zidovudine). Children were recruited from three centres in Uganda and one in Zimbabwe. All children were examined by a doctor at screening, randomisation, weeks 4, 8, and 12, then every 12 weeks. Every 4–6 weeks, children were reviewed by a nurse and adherence assessed using a questionnaire completed by the carer. The trial was approved by Research Ethics Committees in Uganda, Zimbabwe and the UK. Caregivers gave written consent.
VL was assayed retrospectively on stored plasma samples at 0, 4, 24, 36, 48 and 144 weeks post ART initiation, and the last study visit before trial closure on 16 March 2012 in all children. VL was additionally assayed 24-weekly after week 48 in children enrolled after June 2008 (immunology/virology substudy); and in an overlapping subset at, and 48 and 96 weeks after, a subsequent randomisation to once versus twice daily lamivudine+abacavir (which were virologically equivalent[12]). Assays were run using Abbott m2000rt (Uganda) and Roche Amplicor 1.5 (Zimbabwe). The closest measurement to 4, 24, 36 and 48 weeks on ART, and then 24-weekly (in equally spaced windows) was used in analyses, which used a lower detection limit of 80 copies/ml because many low volume samples had to be diluted 1:2. Samples with >1000 copies/ml at week 48 or 144, or any timepoint in the once/twice daily study, were genotyped (reverse transcriptase only). The closest genotype to week 144 from week 48 through to trial end was used for analysis. Major NRTI mutations were defined according to IAS 2013[13], and drug susceptibility predicted using the Stanford algorithm version 7[10].
Pre-ART characteristics of sdNVP exposed and non-exposed children were compared using chi-squared tests for categorical factors and Wilcoxon rank-sum tests for continuous values. Predictors of suppression <80 copies/ml 48 and 144 weeks after ART initiation were identified using logistic regression (backwards elimination; exit p=0.1 to develop an explanatory model), forcing into the models sdNVP (the primary exposure), age at ART initiation (a major known confounder) and ART-strategy randomisation (because,at week 144, triple NRTI maintenance (Arm-C) was virologically inferior to 2NRTI+NNRTI (Arms A and B) in the trial as a whole[14]). The 80 copies/ml threshold was chosen to provide the most sensitive investigation of the possible impact of low-level resistant variants following sdNVP exposure. Other factors considered were pre-ART WHO stage, CD4%, weight/height-for-age Z-scores (WHO reference[15]) and VL; gender, trial centre, CD4 monitoring randomisation; current or initial ART taken as all syrups; and whether the caregiver reported missed ART doses (in the last 4 weeks; percentage of scheduled visits in the last 48 weeks). Missing data were very few, so models included complete cases only. Nonlinearity in the effects of continuous predictors was explored using natural cubic splines with three knots at the 10th, 50th, and 90th centiles[16]. Interactions between variables included in final models were investigated where heterogeneity p<0.05. In additional main effect models, the primary caregiver (mother/other) and socioeconomic variables at ART initiation (physical house structure; electricity; household assets) were also included. As children in Arm-C stopped NNRTI at week 36, secondary analyses considered only Arms A and B receiving long-term NNRTI. All analyses were performed using Stata 13.1 (StataCorp). All p-values are two-sided.
RESULTS
78/367 (21%) children aged 3-<36 months initiating nevirapine-based ART had received sdNVP (Supplementary Figure 1): 51 to both the mother and child, four to the child alone, 20 to the mother alone (administration to child may not have been recorded) and 3 where the specific regimen was unknown (assumed to be sdNVP). Additional zidovudine was not recorded as received in any of these children, likely reflecting their age at enrolment given that WHO 2006 pMTCT guidelines (including 1 week zidovudine[17]) were adopted during 2008. The mother was more likely to be the primary caregiver of children who had received sdNVP (99% vs 78% non-exposed, p<0.001). Children receiving sdNVP were younger at ART initiation (median 17 vs 21 months, p=0.0008; 36% vs 20% <12 months) and therefore had slightly higher CD4 counts (914 vs 704 cells/μl p=0.003), but did not differ significantly in pre-ART CD4% (median 14%), weight-for-age Z-score (−2.2) and other pre-ART characteristics (Table 1).
Table 1. Characteristics of sdNVP exposed and non-exposed children at ART initiation and 48 and 144 weeks later.
| sdNVP (n=78) | No sdNVP (n=289) | P* | |
|---|---|---|---|
| Male | 43 (55%) | 134 (46%) | 0.17 |
| At ART initiation | |||
| Age (months) | |||
| Median (IQR) | 17 (10-23) | 21 (14-27) | 0.0008 |
| 3 – <6 months | 1 (1%) | 3 (1%) | |
| 6 – <12 months | 27 (35%) | 54 (19%) | |
| 12 – <24 months | 34 (44%) | 120 (42%) | |
| 24 – <36 months | 16 (21%) | 112 (39%) | |
| CD4 (cells/μl): median (IQR) | 914 (658-1337) | 704 (475-1101) | 0.003 |
| CD4% | 15 (11-20) | 14 (10-19) | 0.31 |
| Weight-for-age Z-score: median (IQR) | −2.1 (−3.4 to −1.1) | −2.3 (−3.5 to −1.4) | 0.53 |
| Weight (kg): median (IQR) | 7.8 (6.4-10.0) | 8.5 (7.0-10.0) | 0.08 |
| Height-for-age Z-score: median (IQR) | −2.9 (−4.0 to −2.1) | −2.9 (−3.8 to −2.0) | 0.77 |
| VL (copies/ml): median (IQR) | 757100 (192100-2076700)** | 476400 (184500-1253100)*** | 0.18 |
| WHO stage 3/4 | 57 (73%) | 204 (71%) | 0.84 |
| Randomized treatment strategy | 0.20 | ||
| Arm-A (3TC/ABC/NNRTI throughout) | 21 (27%) | 98 (34%) | |
| Arm-B (3TC/ABC/NNRTI throughout, ZDV until week 36) | 31 (40%) | 85 (29%) | |
| Arm-C (3TC/ABC/ZDV throughout, NNRTI until week 36) | 26 (33%) | 106 (37%) | |
| Initial ART as all syrups | 74 (95%) | 272 (94%) | 0.80 |
| Allocated monitoring strategy | 0.27 | ||
| Routine CD4 monitoring | 32 (41%) | 139 (48%) | |
| No CD4 monitoring | 46 (59%) | 150 (52%) | |
| Country/centre | 0.39 | ||
| Uganda/Entebbe | 5 (7%) | 37 (13%) | |
| Uganda/JCRC | 19 (24%) | 67 (23%) | |
| Uganda/PIDC | 33 (42%) | 123 (43%) | |
| Zimbabwe/Harare | 21 (27%) | 62 (21%) | |
| Primary carer | <0.001 | ||
| Mother | 77 (99%) | 224 (78%) | |
| Other | 1 (1%) | 64 (22%) | |
| Missing*** | 0 | 1 | |
| House structure | 0.57 | ||
| Poor | 15 (19%) | 43 (15%) | |
| Adequate | 17 (22%) | 58 (20%) | |
| Good | 45 (58%) | 183 (64%) | |
| Missing*** | 1 | 5 | |
| Electricity | 0.07 | ||
| No | 19 (25%) | 103 (36%) | |
| Yes | 58 (75%) | 185 (64%) | |
| Missing*** | 1 | 1 | |
| Affluence score: mean† | 2.6 | 2.5 | 0.43 |
| Week 48: N alive and in follow-up | 74 | 276 | |
| Current ART as all syrups | 54 (73%) | 158 (57%) | 0.01 |
| Missed doses in last 4 weeks | 1 (1%) | 25 (9%) | 0.04 |
| % visits to date with missed doses in last 4 weeks: mean | 7.9 | 9.9 | 0.15 |
| Week 144: N alive and in follow-up | 73 | 273 | |
| Current ART as all syrups | 5 (7%) | 10 (4%) | 0.24 |
| Missed doses in last 4 weeks | 2 (3%) | 19 (7%) | 0.18 |
| % visits in last 48 weeks with missed doses in last 4 weeks: mean | 6.6 | 8.3 | 0.46 |
Chi-squared tests for categorical factors and Wilcoxon rank-sum tests for continuous values unless otherwise indicated
n=76 (2 missing baseline VLs)
n=288 (1 missing baseline VL)
Mode assumed in multivariate analyses
Number of the following items in the house: fridge, radio, television, landline, mobile, motorbike, bicycle, car. Missing for 1 child in the sdNVP non-exposed group, mode assumed in multivariate analyses
350/367 children (95%) were alive and in follow-up 48 weeks after ART initiation, with VL measurements available in 345/350 (99%) (Supplementary Figure 1). 14 children had died and 3 had been lost. At 48 weeks, sdNVP-exposed children were more likely to receive ART as all syrups vs any tablets (73% vs 57% in non-exposed, p=0.02) and less likely to have missed doses in the last 4 weeks (1% vs 9% p=0.04) (Table 1). 144 weeks after ART initiation, 346 children (94%) were alive and in follow-up, 345 with VL available (4 lost to follow-up since 48 weeks). Only 10/367 (3%) children switched to protease-inhibitor-containing regimens during follow-up, 1 for toxicity (week 14; hepatitis on nevirapine) and 9 for first-line clinical/immunologic failure (median 153 weeks, range 88-253; 2 (3%) sdNVP-exposed, 7 (2%) non-exposed). Overall 95.5% and 94.5% of child-time through 48 weeks was spent on nevirapine-containing ART in sdNVP-exposed and non-exposed children, and 91.8% and 92.6% through 144 weeks, respectively (only including children randomised to long-term nevirapine-containing regimens (Arms A and B) from week 36 onwards). Most first-line nevirapine substitutions were to efavirenz for tuberculosis co-treatment or rash. In sdNVP-exposed and non-exposed children, 84.3% and 79.9% of child-time through 48 weeks was spent receiving ART as all syrups vs any tablets, and 37.9% and 21.1% from 48-144 weeks.
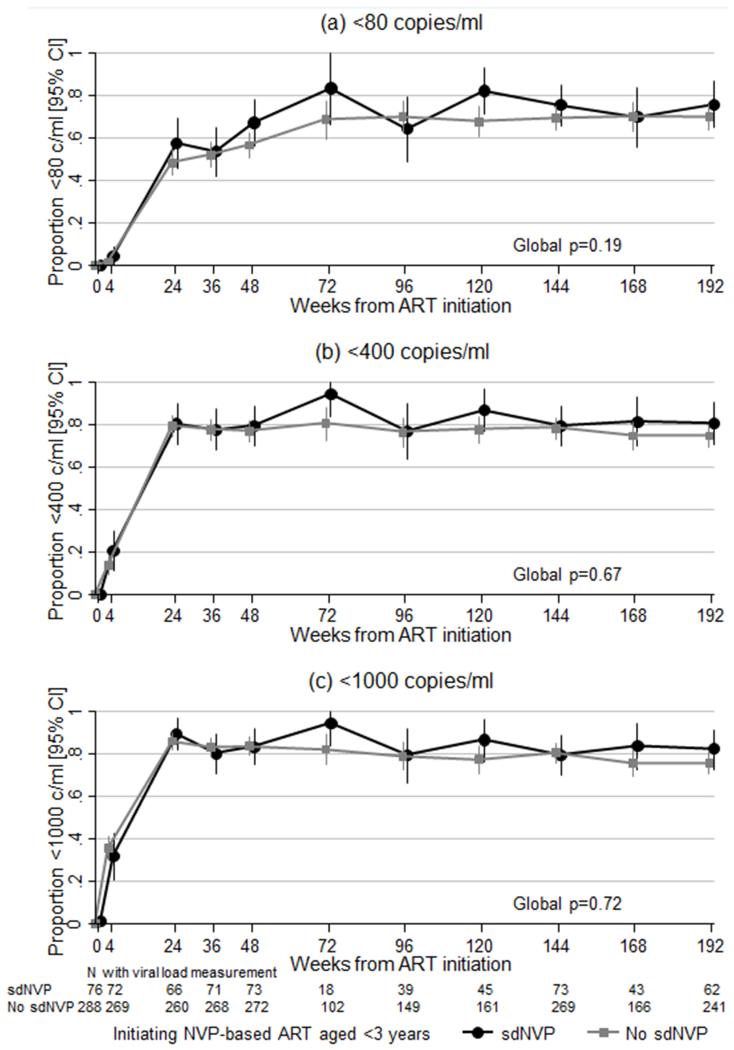
Overall, there was no evidence that suppression <80 copies/ml was any poorer in sdNVP-exposed vs non-exposed, with similar results for <400 and <1000 copies/ml (p>0.1; Figure 1). Mean VL reduction from baseline to week 4 was 2.5 and 2.4 log10 in sdNVP-exposed and non-exposed respectively (unadjusted difference +0.1 [95% CI −0.1,+0.3] p=0.41 n=339).
Figure 1.
Suppression (a) <80, (b) <400 and (c) <1000 copies/ml over time
At week 48, 49/73 (67%) sdNVP-exposed and 154/272 (57%) non-exposed children were <80 copies/ml (adjusted (a)OR=2.34 [1.26-4.34] p=0.007 n=342 complete cases) indicating, if anything, better suppression with sdNVP exposure. At week 144, 55/73 (75%) exposed and 188/272 (69%) non-exposed were <80 copies/ml (aOR=1.75 [0.93-3.29] p=0.08 n=343 complete cases).
At week 48, suppression <80 copies/ml was lower in males (p=0.009), those with higher pre-ART VL (p=0.001), currently taking all ART as syrups (p=0.05) and whose caregivers reported lower adherence (p=0.04) (Table 2). Suppression was non-significantly poorer in children who were younger at ART initiation (p=0.11). Initiating ART with all syrups versus any tablets (rather than week 48 formulation) was not associated with suppression at week 48 (p=0.80). There was no evidence of interaction between sdNVP and age (p=0.70) or any other factors in the final model (p>0.2), or between these factors and ART-strategy randomisation (p>0.3).
Table 2. Independent predictors of VL <80 copies/ml 48 and 144 weeks after ART initiation.
| Week 48 (N=342) | Week 144 (N=343) | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Main models | ||||
| sdNVP exposure (yes vs no) | 2.34 [1.26-4.34] | 0.007 | 1.75 [0.93-3.29] | 0.08 |
| Age at ART initiation (per year younger) | 0.70 [0.46-1.08] | 0.11 | 0.72 [0.50-1.05] | 0.09 |
| Allocated treatment strategy, vs Arm-A (3TC/ABC/NNRTI throughout) | 0.20 | 0.22* | ||
| Arm-B (3TC/ABC/NNRTI throughout, ZDV until week 36) | 0.77 [0.43-1.38] | 0.38 | 0.78 [0.42-1.44] | 0.43 |
| Arm-C (3TC/ABC/ZDV throughout, NNRTI until week 36) | 1.30 [0.74-2.31] | 0.36 | 0.60 [0.33-1.07] | 0.08 |
| Pre-ART VL (per log10 higher) | 0.55 [0.38-0.79] | 0.001 | 0.57 [0.39-0.83] | 0.003 |
| Male (vs female) | 0.53 [0.33-0.85] | 0.009 | 0.10 ** | |
| Current ART as all syrups (vs tablets) | 0.56 [0.31-1.01] | 0.05 | 0.13 *** | |
| Missed doses in last 4 weeks (yes vs no) | 0.35 [0.13-0.94] | 0.04 | 0.92 |
Note: multivariable models based on backwards elimination (exit p=0.1) on complete cases from all factors in Table 1, forcing sdNVP, age at ART initiation and ART-strategy randomisation into the model. Italics shows effect from adding variables into the final model. No evidence of non-linearity in age at week 48 (p=0.9) or 144 (p=0.6).
Arm-C vs A and B combined OR=0.67 [0.41-1.10] p=0.12.
adjusted (for factors above) OR=0.67 [0.41-1.08] p=0.10.
adjusted (for factors above) OR=0.40 [0.12-1.33] p=0.13; only 4% children not taking at least one drug as tablet formulation at week 144.
At week 144, suppression remained lower in children who were younger at ART initiation (p=0.09) and those with higher pre-ART VL (p=0.003). The effect of gender was in the same direction as at week 48 but non-significant (p=0.10). Suppression was also non-significantly lower in children who were on maintenance with triple NRTI (Arm-C) vs 2NRTI+NNRTI (Arms A and B) (p=0.12). Almost all children (96%) were receiving ART as tablets by week 144 reducing power to detect effects of syrups which were in the same direction as week 48 (p=0.13). There was no evidence that missing ART doses in the last 4 weeks (p=0.92) or the proportion of follow-up visits in the last 48 weeks reporting missed doses in the last 4 weeks (p=0.71) affected suppression. Considering interactions, there was some evidence that the, if anything, slightly better suppression in sdNVP-exposed children was greater at lower pre-ART VLs, with little difference in children with pre-ART VL >1,000,000 copies/ml (interaction p=0.04) (Supplementary Table 1). Although this interaction was not statistically significant at 48 weeks (p=0.26), results were qualitatively similar. There was also some evidence that the lower suppression in those who were younger at ART initiation was restricted to those on maintenance with 2NRTI+NNRTI (Arms A and B) vs triple NRTI (Arm-C) (interaction p=0.01; Supplementary Table 1). This interaction was not apparent at 48 weeks (p=0.88). There was no evidence of interaction between sdNVP and age (p=0.63) or any other factors retained in the final model (p>0.6) and were no other statistically significant interactions between ART-strategy randomisation and any factors in the final model (p>0.05).
In subsequent models, the primary caregiver (mother/other) and socioeconomic variables were also included as potential confounders between sdNVP and VL suppression. Suppression <80 copies/ml was greater in children in households that were more affluent at ART initiation (week 48: aOR=1.14 per affluence point (defined in Table 1) [95% CI 0.98-1.32] p=0.10; week 144: 1.19 [1.01-1.39] p=0.04). Suppression at week 144 was also independently greater in households with electricity (aOR=1.65 [0.99-2.74] p=0.05). There was no evidence of any independent effects of caregiver or other socioeconomic factors at either timepoint (p>0.2), and no evidence that the slightly better suppression with sdNVP was mediated by any of these factors (estimated aOR for sdNVP exposed vs non-exposed >1.6 across all models at week 48 and 144).
Results were broadly similar categorising sdNVP as received by both mother and child, child alone or mother alone (where administration to child may not have been recorded) (week 48 aOR vs no sdNVP: 2.27 both, 1.47 child alone (n=4), 2.83 mother alone (heterogeneity p=0.86); week 144: 1.85, 0.67, 1.97 respectively (heterogeneity p=0.63)). Results were also similar restricting to children on long-term NNRTI (Arms A and B).
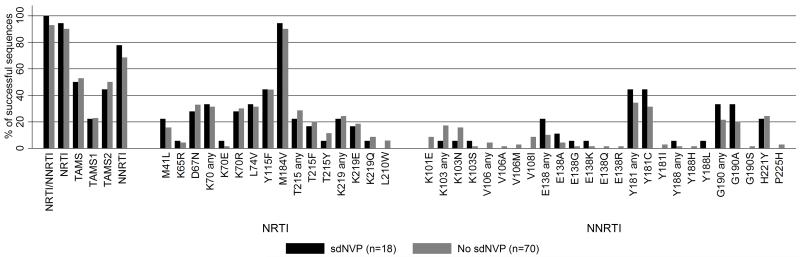
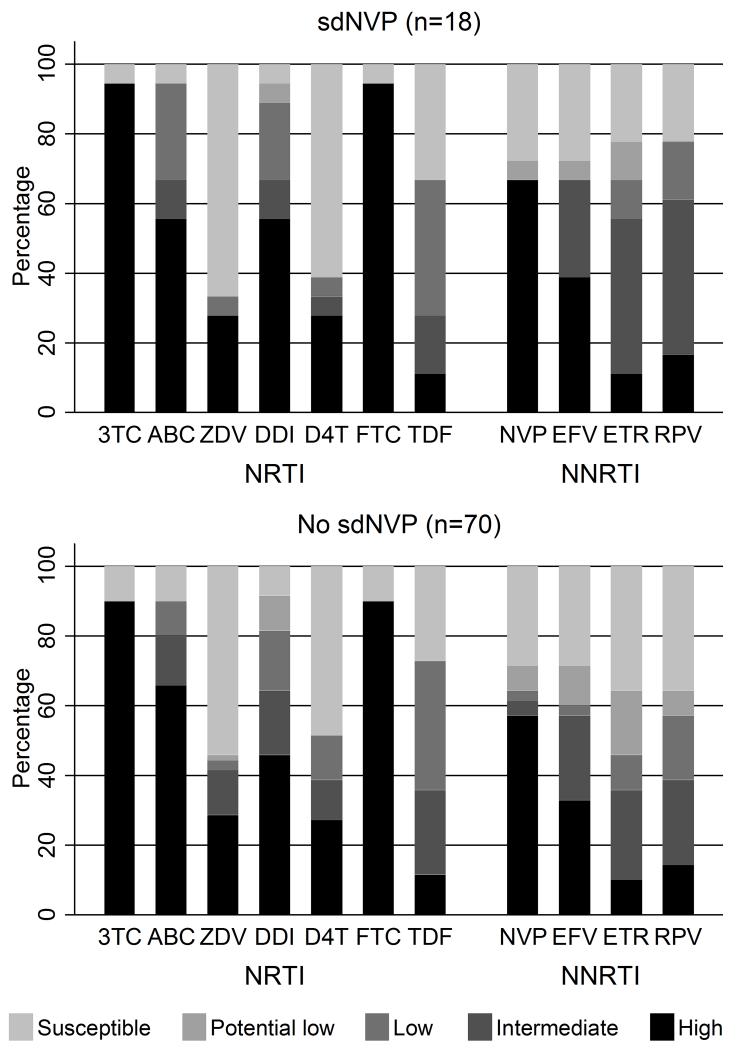
18 (23%) sdNVP exposed versus 70 (24%) non-exposed children had an available genotype, a median (IQR) [range] 144 (133-147) [48-228] weeks from ART initiation respectively (ranksum p=0.55). 14 (78%) vs 48 (69%) children respectively had one or more IAS major NNRTI mutations (median 1 vs 1 respectively per child, p=0.85) and 17 (94%) vs 63 (90%) respectively had one or more IAS NRTI mutations (median 3 vs 3 respectively per child, ranksum p=0.74; Figure 2). Median VL at the genotype was 4800 vs 16700 copies/ml respectively (p=0.17). There was no evidence of difference between children with and without previous sdNVP exposure in the percentage with intermediate/high level resistance to any NRTIs (p>0.3) or NNRTIs (p>0.1) (Figure 3). Of the 9 children switched for first-line failure, 5/5 on maintenance with 2NRTI+NNRTI (Arms A and B) vs 2/4 triple NRTI (Arm-C) had one or more IAS major NNRTI mutations at switch (median 2 vs 0.5 respectively per child, p=0.01).
Figure 2.
Prevalence of major IAS drug resistance mutations by sdNVP exposure
Footnote 1: sdNVP vs no sdNVP: K103 any: p=0.29; K103N: p=0.45; E138 any: p=0.23; Y181 any: p=0.43; Y181C: p=0.40. All others p>0.2
Figure 3.
Overall resistance to NRTI and NNRTI drugs in children with and without previous sdNVP exposure
Footnote 1: 3TC=lamivudine, ABC=abacavir, ZDV=zidovudine, DDI=didanosine, D4T=stavudine, FTC=emtricitabine, TDF=tenofovir, NVP=nevirapine, EFV=efavirenz, ETR=etravirine, RPV=rilpivirine
DISCUSSION
Although WHO guidelines recommend all HIV-infected infants and young children aged <3 years initiate ART with lopinavir-containing regimens[6], the only licenced lopinavir formulation in this age group is an oral solution, which is expensive, requires cold-chain, contains a high percentage of ethanol and is contraindicated in premature/very young infants. A sprinkle ‘pellet’ formulation is not yet licensed or commercially available, and caregivers still had major problems with its taste in children aged 1-4 years[18]. Practically therefore, particularly outside large urban centres, the decision facing many healthcare workers is whether to initiate ART with a non-lopinavir-containing regimen or not treat the infant/child at all. The latter leads to very high risks of early mortality and morbidity[11]. The former almost invariably means a nevirapine-based regimen given the challenges of efavirenz dosing in young children. Here we have shown in a relatively young cohort without severe immunodeficiency (median age 18 months, almost all ≥6 months; median CD4% 14%) that prior self-reported sdNVP receipt is not associated with poorer virological response to nevirapine-containing ART. This was similar for younger and older children in the cohort. Our findings are consistent with one of two previous Ugandan studies, where the non-sdNVP-exposed cohort were considerably older and more immunosuppressed[10], and the P1060 cohort[7]. Furthermore, we found that sdNVP exposure was not associated with increased NRTI or NNRTI resistance accompanying VL>1000 copies/ml on ART. In the other studies to observe differences in VL response, nevirapine-based treatment was initiated closer to birth (median 1 and 6 months of age)[8, 9]. WHO 2013 pMTCT guidelines now recommend universal triple ART to all pregnant and breastfeeding women, and a 6-week course of daily nevirapine to the infant[6]. This might put those infected despite pMTCT at greater risk of developing resistance than previously.
We adjusted for potential confounders including age at ART initiation, ART-strategy randomisation and also socioeconomic variables at ART initiation. It is therefore unclear why suppression remained slightly better with sdNVP exposure, possibly due to chance. As expected, high pre-ART VL strongly predicted poorer virological suppression at both 48 and 144 weeks. Interestingly and importantly, however, the impact of receiving ART with all syrups versus any tablets was equivalent to initiating ART with a 1 log10 higher VL. This impact of receiving ART with all syrups vs any tablets was also of similar magnitude to the difference in VL response between lopinavir-containing vs nevirapine-containing regimens in P1060 where all children received syrups/solutions[7]. As triple-drug nevirapine-based fixed-dose-combination (FDC) tablets are available for children from 3kg[19], this suggests that a tablet nevirapine-based regimen might have similar virological responses to a syrup lopinavir-based regimen in young infants/children. This may be particularly the case if nevirapine dose-escalation is not used in these young children who have considerably faster nevirapine clearance than older children, and where initiating nevirapine at full dose led to similar plasma levels 2 weeks after ART initiation as older children initiating with half-dose[20]. A strategy of initiating ART with full-dose nevirapine has been shown to be safe and effective in children, with 78% <250 copies/ml 96 weeks after ART initiation and no nevirapine reactions among children <2 years[21]. A cross-over pharmacokinetic substudy demonstrated significantly lower lamivudine plasma levels with syrup vs tablet administration[22] in young children; whether this could also contribute to poorer VL response with syrups is unclear. Caregivers, and children able to express a preference, strongly prefer tablet formulations for multiple reasons including the number, weight, transportation and conspicuousness of syrup[23].
Caregivers administered all drugs, so it is unclear why males had poorer VL suppression; studies have sometimes[24], but not always[25], found this in older children, but it has typically been ascribed to better adherence and health behaviour in girls which is not relevant to this young cohort. We also found some suggestion that younger age (<3 years) was associated with poorer short-term virological suppression independently of pre-ART VL, formulation, adherence and gender; longer-term this was restricted to those on maintenance with 2NRTI+NNRTI vs triple NRTI. In the ARROW trial as a whole, we previously demonstrated VL responses were as good in children under 3 years as over 3 years[14]. This illustrates the substantial variation with age that categorization can mask, given the specific and numerous challenges in medication administration as infants become toddlers, and then small children.
Although approximately a third of children were randomised to 3NRTI+NNRTI for 36 weeks then 3NRTI (Arm-C), any inferior VL response during this first 36 weeks would likely be reflected longer-term and so primary analyses included all children. However results were similar restricting to those on NNRTI-containing regimens long-term. Another study limitation is that sdNVP-exposure was based on self-report, in contrast to previous trials where medical records/health cards were reviewed[4, 5]. Baseline genotypes based on either bulk or minority sequence are not available so we are unable to investigate this further. However, given the young age of the cohort at recruitment in 2007-2008 it is plausible that self-report was reasonably accurate, as it would not have required substantial recall, although whether sdNVP was administered to the mother, child or both may be less accurate. In children receiving sdNVP, the primary caregiver was more likely to be the mother, unlikely to be explained by the slightly younger age of the sdNVP group, suggesting other caregivers might not have been aware of sdNVP use. However, self-report is undoubtedly what would be used in programmes. Although we focussed on suppression <80 copies/ml, arguing that this would provide the most sensitive test of the impact of minority NNRTI resistant variants, results were similar using higher VL thresholds of 400 and 1000 copies/ml (data not shown). The fact that it took ~72 weeks on ART for these young children to fully suppress to <80 copies/ml, despite most being <400 copies/ml by 24 weeks, with very few treatment changes, highlights the importance of evaluating virological suppression over the longer-term in children.
Given our findings, and no detrimental effect of sdNVP-exposure on subsequent response to nevirapine-based ART in most other paediatric studies, tablet-FDC nevirapine-based ART continues to be a good alternative to syrup lopinavir-based ART for children of all ages, particularly where PI regimens are not feasible and in those over one year, and even if exposed to sdNVP. Concerns about sdNVP exposure may reduce over the coming years now immediate ART initiation is recommended once HIV infection is identified in infants[6]. However, the significant treatment gap, with only 34% of children in need receiving ART[26], suggests treating young children will likely remain a significant challenge. The wide availability of triple-drug nevirapine-based FDCs is an additional advantage, given the limited global availability of lopinavir/ritonavir and its significant formulation challenges in young children. This message is particularly important for ART rollout to primary health facilities which is a priority for all African countries and requires that healthcare workers test and treat children alongside adults.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the children, caregivers and staff from all the centres participating in the ARROW trial, and the ARROW Trial Steering Committee for access to data.
Source of Funding: The main ARROW trial was funded by the UK Medical Research Council and the UK Department for International Development (DFID); ViiV Healthcare/GlaxoSmithKline donated first-line drugs for ARROW and provided funding for VL assays and genotyping. AJP is funded by the Wellcome Trust (093768/Z/10/Z).
Affiliations
MRC/UVRI Uganda Research Unit on AIDS, Entebbe, Uganda: P Munderi, P Nahirya-Ntege, R Katuramu, J Lutaakome, F Nankya, G Nabulime, I Sekamatte, J Kyarimpa, A Ruberantwari, R Sebukyu, G Tushabe, D Wangi, M Musinguzi, M Aber, L Matama, D Nakitto-Kesi Joint Clinical Research Centre, Kampala, Uganda: P Mugyenyi, V Musiime, R Keishanyu, VD Afayo, J Bwomezi, J Byaruhanga, P Erimu, C Karungi, H Kizito, WS Namala, J Namusanje, R Nandugwa, TK Najjuko, E Natukunda, M Ndigendawani, SO Nsiyona, R Kibenge, B Bainomuhwezi, D Sseremba, J Tezikyabbiri, CS Tumusiime, A Balaba, A Mugumya, F Nghania, D Mwebesa, M Mutumba, E Bagurukira, F Odongo, S Mubokyi, M Ssenyonga, M Kasango, E Lutalo, P Oronon University of Zimbabwe, Harare, Zimbabwe: KJ Nathoo, MF Bwakura-Dangarembizi, F Mapinge, E Chidziva, T Mhute, T Vhembo, R Mandidewa, M Chipiti, R Dzapasi, C Katanda D Nyoni, GC Tinago, J Bhiri, S Mudzingwa, D Muchabaiwa, M Phiri, V Masore, CC Marozva, SJ Maturure, S Tsikirayi, L Munetsi, KM Rashirai, J Steamer, R Nhema, W Bikwa, B Tambawoga, E Mufuka Baylor College of Medicine Children’s Foundation Uganda, Mulago Hospital Uganda: A Kekitiinwa, P Musoke, S Bakeera-Kitaka, R Namuddu, P Kasirye, A Babirye, J Asello, S Nakalanzi, NC Ssemambo, J Nakafeero, J Tikabibamu, G Musoba, J Ssanyu, M Kisekka MRC Clinical Trials Unit at UCL, London, UK: DM Gibb, MJ Thomason, AS Walker, AD Cook, AJ Szubert, B Naidoo-James, MJ Spyer, C Male, AJ Glabay, LK Kendall, J Crawley, AJ Prendergast
Independent ARROW Trial Monitors: I Machingura, S Ssenyonjo. Trial Steering Committee: I Weller (Chair), E Luyirika, H Lyall, E Malianga, C Mwansambo, M Nyathi, F Miiro, DM Gibb, A Kekitiinwa, P Mugyenyi, P Munderi, KJ Nathoo, AS Walker; Observers S Kinn, M McNeil, M Roberts, W Snowden. Data and Safety Monitoring Committee: A Breckenridge (Chair), A Pozniak, C Hill, J Matenga, J Tumwine. Endpoint Review Committee (independent members): G Tudor-Williams (Chair), H Barigye, HA Mujuru, G Ndeezi; Observers: S Bakeera-Kitaka, MF Bwakura-Dangarembizi, J Crawley, V Musiime, P Nahirya-Ntege, A Prendergast, M Spyer.
Economics Group: P Revill, T Mabugu, F Mirimo, S Walker, MJ Sculpher.
Footnotes
Conflicts of Interest
No conflicts of interest.
ARROW_virology_sdNVP_supplementary_digital_content_v1.0_141219.docx
Contributor Information
Philippa MUSOKE, Baylor-Uganda, Paediatric Infectious Diseases Clinic, Mulago Hospital, Kampala, Uganda; Makerere University College of Health Sciences, Kampala, Uganda.
Alexander J SZUBERT, MRC Clinical Trials Unit at University College London, London, UK.
Victor MUSIIME, Joint Clinical Research Centre, Kampala, Uganda; Makerere University College of Health Sciences, Kampala, Uganda.
Kusum NATHOO, University of Zimbabwe College of Health Sciences, Harare, Zimbabwe.
Patricia NAHIRYA-NTEGE, Medical Research Council/Uganda Virus Research Institute Uganda Research Unit on AIDS, Entebbe, Uganda.
Kuda MUTASA, Zvitambo Institute for Maternal and Child Health Research, Harare, Zimbabwe.
David Eram WILLIAMS, Joint Clinical Research Centre, Kampala, Uganda.
Andrew J. PRENDERGAST, Queen Mary University of London, London, UK
Moira SPYER, MRC Clinical Trials Unit at University College London, London, UK.
A Sarah WALKER, MRC Clinical Trials Unit at University College London, London, UK.
Diana M GIBB, MRC Clinical Trials Unit at University College London, London, UK.
REFERENCES
- 1.Arrive E, Newell ML, Ekouevi DK, Chaix ML, Thiebaut R, Masquelier B, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 2.Lockman S, McIntyre J, Zheng Y, Chipato T, Conradie F, Sawe F, et al. Antiretroviral Therapies in Women after Single-Dose Nevirapine Exposure. New England Journal of Medicine. 2010;363:1499–1509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntyre J, Hughes M, Mellors J, Zheng Y, Hakim J. Efficacy of ART with NVP+TDF/FTC vs LPV/r+TDF/FTC among antiretroviral-naive women in Africa: OCTANE Trial 2/ACTG A5208; 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA: CROI. 2010. [Google Scholar]
- 4.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral Treatment for Children with Peripartum Nevirapine Exposure. New England Journal of Medicine. 2010;363:1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus Ritonavir-Boosted Lopinavir for HIV-Infected Children. New England Journal of Medicine. 2012;366:2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organisation . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: 2013. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html. [PubMed] [Google Scholar]
- 7.Lindsey JC, Hughes MD, Violari A, Eshleman SH, Abrams EJ, Bwakura-Dangarembizi M, et al. Predictors of Virologic and Clinical Response to Nevirapine versus Lopinavir/Ritonavir-based Antiretroviral Therapy in Young Children With and Without Prior Nevirapine Exposure for the Prevention of Mother-to-child HIV Transmission. The Pediatric Infectious Disease Journal. 2014;33:846–854. doi: 10.1097/INF.0000000000000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, et al. Response to Antiretroviral Therapy after a Single, Peripartum Dose of Nevirapine. New England Journal of Medicine. 2007;356:135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 9.Musiime V, Ssali F, Kayiwa J, Namala W, Kizito H, Kityo C, et al. Response to Nonnucleoside Reverse Transcriptase Inhibitor-Based Therapy in HIV-Infected Children with Perinatal Exposure to Single-Dose Nevirapine. AIDS Research and Human Retroviruses. 2009;25:989–996. doi: 10.1089/aid.2009.0054. [DOI] [PubMed] [Google Scholar]
- 10.Musoke P, Barlow-Mosha L, Bagenda D, Mudiope P, Mubiru M, Ajuna P, et al. Response to antiretroviral therapy in HIV-infected Ugandan children exposed and not exposed to single-dose nevirapine at birth. Journal of Acquired Immune Deficiency Syndromes. 2009;52:560–568. doi: 10.1097/qai.0b013e3181b93a5a. [DOI] [PubMed] [Google Scholar]
- 11.Marston M, Becquet R, Zaba B, Moulton LH, Gray G, Coovadia H, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40:385–396. doi: 10.1093/ije/dyq255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musiime V, Kasirye P, Naidoo-James B, Nahirya P, Mhute T, Mugarura L, et al. Randomised comparison of once versus twice daily abacavir and lamivudine among 669 HIV-infected children in the ARROW trial (Poster 997); 7th Conference on Retroviruses and Opportunistic Infections; Atlanta, Georgia. 2013. [Google Scholar]
- 13.Johnson VA, Calvez V, Gunthard HF, Paredes R, Pillay D, Shafer RW, et al. Update of the drug resistance mutations in HIV-1: March 2013. Top Antivir Med. 2013;21:6–14. [PMC free article] [PubMed] [Google Scholar]
- 14.ARROW Trial team Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. The Lancet. 2013;381:1391–1403. doi: 10.1016/S0140-6736(12)62198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. World Health Organization; Geneva: 2006. [Google Scholar]
- 16.Hess K. Assessing time-by-covariate interactions in proportional hazards regression models using cubic spline functions. Statistics in Medicine. 1994;13:1045–1062. doi: 10.1002/sim.4780131007. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organisation . Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants in resource-limited settings: Towards universal access. Recommendations for a public health approach. Geneva: 2006. [Google Scholar]
- 18.Musiime V, Fillekes Q, Kekitiinwa A, Kendall L, Keishanyu R, Namuddu R, et al. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in african HIV-infected children. J Acquir Immune Defic Syndr. 2014;66:148–154. doi: 10.1097/QAI.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 19.Fillekes Q, Mulenga V, Kabamba D, Kankasa C, Thomason MJ, Cook A, et al. Pharmacokinetics of nevirapine in HIV-infected infants weighing 3 kg to less than 6 kg taking paediatric fixed dose combination tablets. AIDS. 2012;26:1795–1800. doi: 10.1097/QAD.0b013e32835705fd. [DOI] [PubMed] [Google Scholar]
- 20.Fillekes Q, Mulenga V, Kabamba D, Kankasa C, Thomason MJ, Cook A, et al. Is nevirapine dose-escalation appropriate in young, African, HIV-infected children? AIDS. 2013;27:2111–2115. doi: 10.1097/QAD.0b013e3283620811. [DOI] [PubMed] [Google Scholar]
- 21.Mulenga V, Cook A, Walker AS, Kabamba D, Chijoka C, Ferrier A, et al. Strategies for nevirapine initiation in HIV-infected children taking pediatric fixed-dose combination “baby pills” in Zambia: a randomized controlled trial. Clin Infect Dis. 2010;51:1081–1089. doi: 10.1086/656628. [DOI] [PubMed] [Google Scholar]
- 22.Kasirye P, Kendall L, Adkison K, Tumusiime C, Ssenyonga M, Bakeera-Kitaka S, et al. Pharmacokinetics of Antiretroviral Drug Varies With Formulation in the Target Population of Children With HIV-1. Clinical Pharmacology & Therapeutics. 2012;9:272–280. doi: 10.1038/clpt.2011.225. [DOI] [PubMed] [Google Scholar]
- 23.Nahirya-Ntege P, Cook A, Vhembo T, Opilo W, Namuddu R, Katuramu R, et al. Young HIV-infected children and their adult caregivers prefer tablets to syrup antiretroviral medications in Africa. PLoS One. 2012;7:e36186. doi: 10.1371/journal.pone.0036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker AS, Doerholt K, Sharland M, Gibb DM. Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV Paediatric Study. AIDS. 2004;18:1915–1924. doi: 10.1097/00002030-200409240-00007. [DOI] [PubMed] [Google Scholar]
- 25.Kekitiinwa A, Lee KJ, Walker AS, Maganda A, Doerholt K, Kitaka SB, et al. Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr. 2008;49:384–392. doi: 10.1097/QAI.0b013e31818cdef5. [DOI] [PubMed] [Google Scholar]
- 26.Joint United Nations Programme on HIV/AIDS . Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.