Abstract
The spherical alga Volvox swims by means of flagella on thousands of surface somatic cells. This geometry and its large size make it a model organism for studying the fluid dynamics of multicellularity. Remarkably, when two nearby Volvox colonies swim close to a solid surface, they attract one another and can form stable bound states in which they “waltz” or “minuet” around each other. A surface-mediated hydrodynamic attraction combined with lubrication forces between spinning, bottom-heavy Volvox explains the formation, stability, and dynamics of the bound states. These phenomena are suggested to underlie observed clustering of Volvox at surfaces.
Long after he made his great contributions to microscopy and started a revolution in biology, Antony van Leeuwenhoek peered into a drop of pond water and discovered one of nature’s geometrical marvels [1]. This was the freshwater alga which, years later, in the very last entry of his great work on biological taxonomy, Linnaeus named Volvox [2] for its characteristic spinning motion about a fixed body axis. Volvox is a spherical colonial green alga (Fig. 1), with thousands of biflagellated cells anchored in a transparent extracellular matrix (ECM) and daughter colonies inside the ECM. Since the work of Weismann [3], Volvox has been seen as a model organism in the study of the evolution of multicellularity [4–6].
FIG. 1.
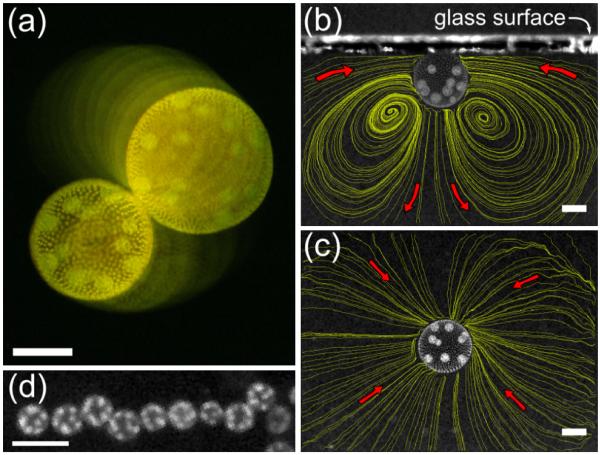
(color online). Waltzing of V. carteri. (a) Top view. Superimposed images taken 4 s apart, graded in intensity. (b) Side, and (c) top views of a colony swimming against a cover slip, with fluid streamlines. Scales are 200 μm. (d) A linear Volvox cluster viewed from above (scale is 1 mm).
Because it is spherical, Volvox is an ideal organism for studies of biological fluid dynamics, being an approximate realization of Lighthill’s “squirmer” model [7] of self-propelled bodies having a specified surface velocity. Such models have elucidated nutrient uptake at high Péclet numbers [6,8] by single organisms, and pairwise hydrodynamic interactions between them [9]. Volvocine algae may also be used to study collective dynamics of self-propelled objects [10], complementary to bacterial suspensions (E. coli, B. subtilis) exhibiting large-scale coherence in thin films [11] and bulk [12].
While investigating Volvox suspensions in glass-topped chambers, we observed stable bound states, in which pairs of colonies orbit each other near the chamber walls. Volvox is “bottom-heavy” due to clustering of daughter colonies in the posterior, so an isolated colony swims upward with its axis vertical, rotating clockwise (viewed from above) at an angular frequency ω ~ 1 rad/s for a radius R ~ 150 μm. When approaching the chamber ceiling, two Volvox colonies are drawn together, nearly touching while spinning, and they “waltz” about each other clockwise [Fig. 1(a)] at an angular frequency Ω ~ 0:1 rad/s. When Volvox have become too heavy to maintain upswimming, two colonies hover above one another near the chamber bottom, oscillating laterally out of phase in a “minuet” dance. Both dances can last many tens of minutes or even several hours. Although the orbiting component of the waltzing is reminiscent of vortex pairs in inviscid fluids, the attraction and the minuet are not, and as the Reynolds number is ~0:03, inertia is negligible.
While one might imagine that signalling and chemotaxis could result in these bound states, a combination of experiment, theory, and numerical computations is used here to show that they arise instead from the interplay of shortrange lubrication forces between spinning colonies and surface-mediated hydrodynamic interactions [13], known to be important for colloidal particles [14,15] and bacteria [16]. We conjecture that flows driving Volvox clustering at surfaces enhance the probability of fertilization during the sexual phase of their life cycle.
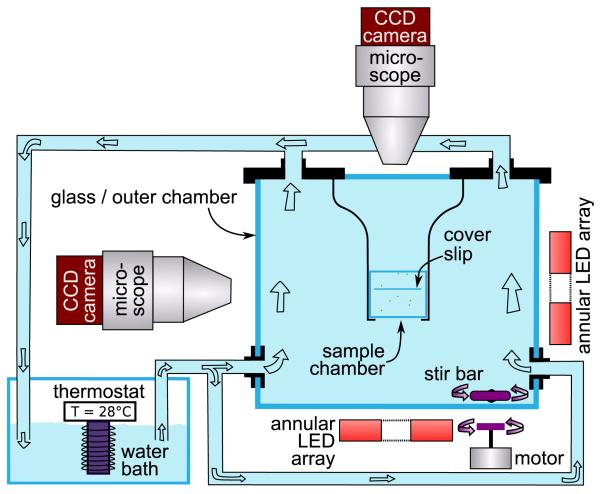
Volvox carteri f. nagariensis EVE strain (a subclone of HK10) were grown axenically in SVM [6,17] in diurnal growth chambers with sterile air bubbling, in a daily cycle of 16 h in cool white light (~4000 lux) at 28 °C and 8 h in the dark at 26 °C. Swimming was studied in a dual-view system (Fig. 2) [18], consisting of two identical assemblies, each a CCD camera (Pike 145B, Allied Vision Technologies, Germany) and a long-working distance microscope (InfiniVar CMS-2/S, Infinity Photo-Optical, Colorado). Dark-field illumination used 102 mm diameter circular LED arrays (LFR-100-R, CCS Inc., Kyoto) with narrow bandwidth emission at 655 nm, to which Volvox is insensitive [19]. Thermal convection induced by the illumination was minimized by placing the 2 × 2 × 2 cm sample chamber, made from microscope slides held to-gether with UV-curing glue (Norland), within a stirred, temperature-controlled water bath. A glass cover slip glued into the chamber provided a clean surface [Fig. 1(b)] to induce bound states. Particle imaging velocimetry (PIV) studies (Dantec Dynamics, Skovelund, Denmark) showed that the r.m.s convective velocity within the sample chamber was ≲5 μm/s.
FIG. 2.
(color online). Dual-view apparatus.
Four aspects of Volvox swimming are important in the formation of bound states, each associated, in the far field, with a distinct singularity of Stokes flow: (i) negative buoyancy (Stokeslet), (ii) self-propulsion (stresslet), (iii) bottom-heaviness (rotlet), and spinning (rotlet doublet). During the 48 h life cycle, the number of somatic cells is constant; only their spacing increases as new ECM is added to increase the colony radius. This slowly changes the speeds of sinking, swimming, self-righting, and spinning, allowing exploration of a range of behaviors. The upswimming velocity U was measured with side views in the dual-view apparatus. The Volvox density was determined by arresting self-propulsion through transient deflagellation with a pH shock [6,20], and measuring sedimentation. The settling velocity V = 2ΔρgR2∕9η, with g the acceleration of gravity and η the fluid viscosity, yields the density offset Δρ = ρc – ρ between the colony and water. Bottom-heaviness implies a distance l between the centers of gravity and geometry, measured by allowing Volvox to roll off a guide in the chamber and monitoring the axis inclination angle θ with the vertical. This angle obeys sinθ, where ζr = 8πηR3 is the rotational drag coefficient, leading to a relaxation time τ = 6η/ρcgl [21]. The rotational frequencies ω0 of free-swimming colonies were obtained from movies, using daughter colonies as markers.
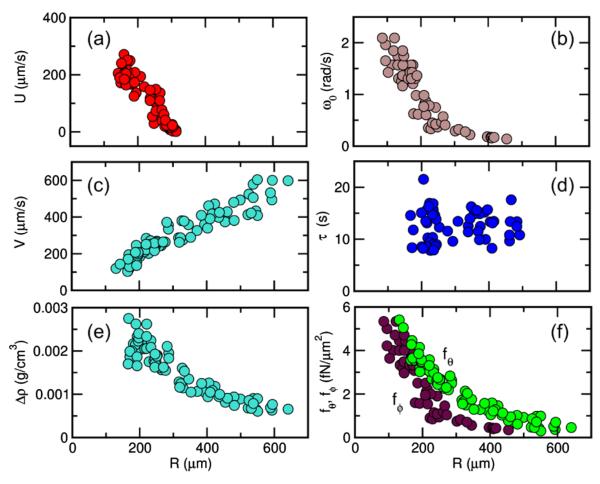
Figure 3 shows the four measured quantities (U, V, ω0, τ) and the deduced density offset Δρ. In the simplest model [6], locomotion derives from a uniform force per unit area f = (fθ, fϕ) exerted by flagella tangential to the colony surface. Balancing the net force against the Stokes drag and negative buoyancy yields fθ = 6η(U + V) ∕ πR. Balancing the flagellar torque against viscous rotational torque 8πηR3ω0 yields fϕ = 8ηω0/π. These components are shown in Fig. 3(f), where we used a linear parameterization of the upswimming data [Fig. 3(a)] to obtain an estimate of U over the entire radius range. The typical force density fθ corresponds to several pN per flagellar pair [6], while the relative smallness of fϕ is a consequence of the ~15° tilt of the flagellar beating plane with respect to the colonial axis [22,23].
FIG. 3.
(color online). Swimming properties of V. carteri as a function of radius. (a) upswimming speed, (b) rotational frequency, (c) sedimentation speed, (d) bottom-heaviness reorientation time, (e) density offset, and (f) components of average flagellar force density.
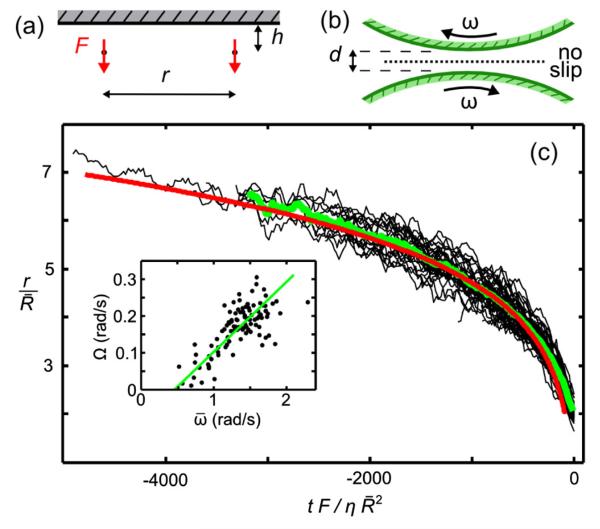
Using the measured parameters, it is possible to characterize both bound states. Figure 4(c) shows data from measured tracks of 60 pairs of Volvox, as they fall together to form the waltzing bound state. The data collapse when the intercolony separation r, normalized by , the mean of the two participating colonies’ radii, is plotted as a function of rescaled time from contact. The waltzing frequency Ω is linear in the mean spinning frequency of the pair . These two ingredients of the waltzing bound state, “infalling” and orbiting, can be understood, respectively, by far-field features of mutually advected singularities and near-field effects given by lubrication theory, which will now be considered in turn.
FIG. 4.
(color online). Waltzing dynamics. Geometry of (a) two interacting Stokeslets (side view) and (b) nearby spinning colonies (top view). (c) Radial separation r, normalized by mean colony radius, as a function of rescaled time for 60 events (black). Running average (green) compares well with predictions of the singularity model (red). Inset shows orbiting frequency Ω as a function of mean spinning frequency , and linear fit.
Infalling
When swimming against an upper surface, the net thrust induced by the flagellar beating is not balanced by the viscous drag on the colony, as the colony is at rest, resulting in a net downwards force on the fluid. The fluid response to such a force may be modeled as a Stokeslet normal to and at a distance h from a no-slip surface [13], forcing fluid in along the surface [Fig. 1(c)] and out below the colony, with a toroidal recirculation. Seen in cross section with PIV, the velocity field of a single colony has precisely this appearance [Fig. 1(b)]. This flow produces the attractive interaction between colonies; Squires has proposed a similar scenario in the context of electrophoretic levitation [24].
The motion of a pointlike object at xi, with axis orientation pi and net velocity vi from self-propulsion and buoyancy, due to the fluid velocity u and vorticity ▿ × u generated by the other self-propelled objects, obeys
| (1) |
Assuming that for the infalling, , and that u(xi) are due to Stokeslets of strength F = 6πηR(U + V), Eq. (1) may be reduced, in rescaled coordinates and with to [24]
| (2) |
Integration of (2) shows good parameter-free agreement with the experimental trajectories of nearby pairs [Fig. 4(c)]. Large perturbations to a waltzing pair by a third nearby colony can disrupt it by strongly tilting the colony axes, suggesting that bottom-heaviness confers stability. This is confirmed by a linear stability analysis [23].
Orbiting
As Volvox colonies move together under the influence of the wall-induced attractive flows [Fig. 1(b)], orbiting becomes noticeable only when their separation d is ≲ 30 μm; their spinning frequencies also decrease very strongly with decreasing separation. This arises from viscous torques associated with the thin fluid layer between two colonies [Fig. 4(b)]. We assume that in the thin fluid layer, the spinning Volvox colonies can be modeled as rigid spheres, ignoring the details of the overlapping flagella layers. For two identical colonies, ignoring the anterior-posterior “downwash,” and considering only the region where the fluid layer is thin, the plane perpendicular to the line connecting their centers is a locus of zero velocity, as with a no-slip wall. Appealing to known asymptotic results [25], we obtain the torque and a lateral force on the sphere, where ω < ω0 is the spinning frequency of a colony in the bound state. The rotational slowing of the self-propelled colony has an effect on the fluid that may be approximated by a rotlet of strength at its center. From the flow field of a rotlet perpendicular to a horizontal no-slip wall [13] and the lateral force , we then deduce the orbiting frequency
| (3) |
Typical values of d and R give a slope of ≃ 0:14–0:19 for the line, consistent with the experimental fit of 0:19 ± 0:05 [Fig. 4(c)]. The nonzero intercept is likely due to lubrication friction against the ceiling [23].
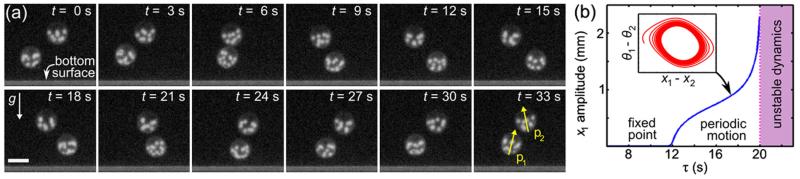
A second and more complex type of bound state, the “minuet,” is found when the upswimming just balances the settling [at R ≃ 300 μm, see Fig. 3(a)], and Volvox colonies hover at a fixed distance above the chamber bottom. In this mode (Fig. 5) colonies stacked one above the other oscillate back and forth about a vertical axis. The mechanism of oscillation is the instability of the perfectly aligned state due to the vorticity from one colony rotating the other, whose swimming brings it back, with the restoring torques from bottom-heaviness conferring stability. Studies of the coupled dynamics of xi and pi show that when the orientational relaxation time τ is below a threshold, the stacked arrangement is stable, while for τ larger, there is a Hopf bifurcation to limit-cycle dynamics [Fig. 5(b)]. In these studies, lubrication effects were ignored, was restricted to be in one horizontal dimension only, and xi were at fixed heights hi above the wall. The flow u was taken to be due to vertically oriented Stokeslets at xi, of magnitude F, equal to the gravitational force on the Volvox.
FIG. 5.
(color online). “Minuet” bound state. (a) Side views 3 s apart of two colonies near the chamber bottom. Arrows indicate the anterior-posterior axes pi at angles θi to vertical. Scale bar is 600 μm. (b) Bifurcation diagram, and phase portrait (inset), showing a limit cycle, with realistic model parameters F = 6πηRV, R = 300 μm, h1 = 450 μm, h2 = 1050 μm.
Hydrodynamic bound states, such as those described here, may have biological significance. When environmental conditions deteriorate, Volvox colonies enter a sexual phase of spore production to overwinter. Field studies show that bulk Volvox concentrations n are <1 cm−3 [26], with male/female ratio of ~1/10, and these ~100 sperm packets/male. Under conditions, the mean encounter time for females and sperm packets is a substantial fraction of the life cycle. The kinetic theory mean-free path , with R = 150 μm for females, and Rsp = 15 μm for sperm sp packets, is λ ~ 1 m, implying a mean encounter time >2 h [27]. This suggests that another mechanism for fertilization could be at work, with previous studies having excluded chemoattraction in this system [28]. At naturally occurring concentrations, more than one Volvox may partake in the waltzing bound state, leading to large arrays [Fig. 1(d)]. In such clusters, formed at the air-water interface, the recirculating flows would decrease the encounter times to seconds or minutes, clearly increasing the chance of sperm packets finding their target. Studies are underway to examine this possibility.
Acknowledgments
We thank D. Vella, S. Alben, and C. A. Solari for key observations, A. M. Nedelcu for algae, and support from the BBSRC, DOE, and the Schlumberger Chair Fund.
Footnotes
PACS numbers: 47.63.Gd, 87.17.Jj, 87.18.Ed
References
- [1].van Leeuwenhoek A. Phil. Trans. R. Soc. London. 1700;22:509. [Google Scholar]
- [2].Linnaeus C. Systema Naturae. 10th ed Impensis Laurentii Salvii; Stockholm: 1758. p. 820. [Google Scholar]
- [3].Weismann A. Essays Upon Heredity and Kindred Biological Problems. Clarendon Press; Oxford: 1891. [Google Scholar]
- [4].Kirk DL. Volvox: Molecular-genetic Origins of Multicellularity and Cellular Differentiation. Cambridge University Press; Cambridge: 1998. [Google Scholar]
- [5].Kirk DL. BioEssays. 2005;27:299. doi: 10.1002/bies.20197. [DOI] [PubMed] [Google Scholar]
- [6].Solari CA, et al. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1353. doi: 10.1073/pnas.0503810103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Short MB, et al. Proc. Natl. Acad. Sci. U.S.A. 2006;103:8315. doi: 10.1073/pnas.0600566103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Solari CA, Kessler JO, Michod RE. Am. Nat. 2006;167:537. doi: 10.1086/501031. [DOI] [PubMed] [Google Scholar]
- [7].Lighthill MJ. Commun. Pure Appl. Math. 1952;5:109. [Google Scholar]
- [8].Magar V, Goto T, Pedley TJ. Q. J. Mech. Appl. Math. 2003;56:65. [Google Scholar]
- [9].Ishikawa T, Hota M. J. Exp. Biol. 2006;209:4452. doi: 10.1242/jeb.02537. [DOI] [PubMed] [Google Scholar]; Ishikawa T, Simmonds MP, Pedley TJ. J. Fluid Mech. 2006;568:119. [Google Scholar]
- [10].Ishikawa T, Pedley TJ. Phys. Rev. Lett. 2008;100:088103. doi: 10.1103/PhysRevLett.100.088103. [DOI] [PubMed] [Google Scholar]; Ishikawa T, Locsei JT, Pedley TJ. J. Fluid Mech. 2008;615:401. [Google Scholar]
- [11].Wu X-L, Libchaber A. Phys. Rev. Lett. 2000;84:3017. doi: 10.1103/PhysRevLett.84.3017. [DOI] [PubMed] [Google Scholar]; Sokolov A, et al. Phys. Rev. Lett. 2007;98:158102. doi: 10.1103/PhysRevLett.98.158102. [DOI] [PubMed] [Google Scholar]
- [12].Dombrowski C, et al. Phys. Rev. Lett. 2004;93:098103. doi: 10.1103/PhysRevLett.93.098103. [DOI] [PubMed] [Google Scholar]
- [13].Blake JR. Proc. Cambridge Philos. Soc. 1971;70:303. [Google Scholar]; Blake JR, Chwang AT. J. Eng. Math. 1974;8:23. [Google Scholar]
- [14].Keh HJ, Anderson JL. J. Fluid Mech. 1985;153:417. [Google Scholar]
- [15].Dufresne ER, et al. Phys. Rev. Lett. 2000;85:3317. doi: 10.1103/PhysRevLett.85.3317. [DOI] [PubMed] [Google Scholar]
- [16].Berke AP, et al. Phys. Rev. Lett. 2008;101:038102. doi: 10.1103/PhysRevLett.101.038102. [DOI] [PubMed] [Google Scholar]
- [17].Kirk DL, Kirk MM. Dev. Biol. 1983;96:493. doi: 10.1016/0012-1606(83)90186-0. [DOI] [PubMed] [Google Scholar]
- [18].Drescher K, Leptos K, Goldstein RE. Rev. Sci. Instrum. 2009;80:014301. doi: 10.1063/1.3053242. [DOI] [PubMed] [Google Scholar]
- [19].Sakaguchi H, Iwasa K. Plant Cell Physiol. 1979;20:909. [Google Scholar]
- [20].Witman GB, et al. J. Cell Biol. 1972;54:507. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pedley TJ, Kessler JO. Annu. Rev. Fluid Mech. 1992;24:313. [Google Scholar]
- [22].Hoops HJ. Protoplasma. 1997;199:99. [Google Scholar]
- [23].Drescher K, et al. (to be published)
- [24].Squires T. J. Fluid Mech. 2001;443:403. doi: 10.1017/jfm.2020.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim S, Karrila SJ. Microhydrodynamics: Principles and Selected Applications. Dover; New York: 2005. [Google Scholar]
- [26].DeNoyelles F., Jr. Ph.D. thesis. Cornell Univ.; 1971. [Google Scholar]
- [27].Ishikawa T, Pedley TJ. J. Fluid Mech. 2007;588:437. [Google Scholar]
- [28].Cogging SJ, et al. Journal of Phycology. 1979;15:247. [Google Scholar]