Significance
A description of the structural and functional connections in the human brain is necessary for the understanding of both normal and abnormal brain functioning. Although it has become clear in recent years that stable patterns of functional connectivity can be observed during the resting state, to date, it remains unclear what the dominant patterns of information flow are in this functional connectome and how these relate to the integration of brain function. Our results are the first to describe the large-scale frequency-specific patterns of information flow in the human brain, showing that different subsystems form a loop through which information “reverberates” or “circulates.” These results could be extended to give insights into how such flow optimizes integrative cognitive processing.
Keywords: information flow, phase transfer entropy, resting-state networks, magnetoencephalography, atlas-based beamforming
Abstract
Normal brain function requires interactions between spatially separated, and functionally specialized, macroscopic regions, yet the directionality of these interactions in large-scale functional networks is unknown. Magnetoencephalography was used to determine the directionality of these interactions, where directionality was inferred from time series of beamformer-reconstructed estimates of neuronal activation, using a recently proposed measure of phase transfer entropy. We observed well-organized posterior-to-anterior patterns of information flow in the higher-frequency bands (alpha1, alpha2, and beta band), dominated by regions in the visual cortex and posterior default mode network. Opposite patterns of anterior-to-posterior flow were found in the theta band, involving mainly regions in the frontal lobe that were sending information to a more distributed network. Many strong information senders in the theta band were also frequent receivers in the alpha2 band, and vice versa. Our results provide evidence that large-scale resting-state patterns of information flow in the human brain form frequency-dependent reentry loops that are dominated by flow from parieto-occipital cortex to integrative frontal areas in the higher-frequency bands, which is mirrored by a theta band anterior-to-posterior flow.
The brain is an extremely complex system (1–3) containing, at the macroscopic scale, interconnected functional units (4) with more-or-less specific information processing capabilities (5). However, cognitive functions require the coordinated activity of these spatially separated units, where the oscillatory nature of neuronal activity may provide a possible mechanism (6–9). A complete description of these interactions, in terms of both strength and directionality, is therefore necessary for the understanding of both normal and abnormal brain functioning.
Functional interactions may be inferred from statistical dependencies between the time series of neuronal activity at different sites, so-called functional connectivity (10). Indeed, interactions in large-scale functional networks have been observed using Electroencephalography, Magnetoencephalography (EEG/MEG) and functional Magnetic Resonance Imaging (fMRI) (e.g., refs. 11–14). However, as yet, little is known about the directionality of these interactions in large-scale functional networks during the resting state. Estimating directionality from fMRI is challenging due to its limited temporal resolution and indirect relation to neuronal activity (15, 16). In contrast, EEG studies in healthy controls have revealed a front-to-back pattern of directed connectivity, particularly in the alpha band (17–22), consistent with modeling studies that have shown that such patterns may arise due to differences in the number of anatomical connections (the degree) of anterior and posterior regions (22, 23). However, modeled patterns of information flow depend on the assumed strength of the underlying structural connections (22–24), and the observed EEG patterns strongly depend on the choice of reference (25), which may explain why, controversially, the reverse back-to-front pattern has also been observed in EEG (26–28). An important advantage of MEG over EEG in this context is that it is reference-free. Moreover, the large number of sensors (several hundreds) in modern whole-head MEG systems allow for sophisticated spatial filtering approaches to accurately reconstruct time series of neuronal activation across the cortex (29, 30). The directed functional connectome can subsequently be reconstructed by estimating information flow between these time series, using either model-based or data-driven approaches (31–34). Here, we used a recently introduced, sensitive, yet computationally efficient, data-driven measure of information flow, the phase transfer entropy (PTE) (35), to test the hypothesis that resting-state MEG data are characterized by a dominant front-to-back pattern in the alpha band. PTE was applied to MEG source-reconstructed eyes-closed resting-state data in a cohort of healthy subjects to characterize frequency-specific patterns of information flow in the human brain.
Results
Time series of neuronal activity were reconstructed by applying an atlas-based beamforming approach to eyes-closed resting-state MEG data from a cohort of 67 healthy subjects. The preferred direction of information flow between the 78 cortical regions in the automated anatomical labeling (AAL) atlas was estimated using the directed PTE (dPTE). The average information flow between a region and all other regions was first computed, resulting in a single estimate of preferred direction of information flow (outgoing or incoming) for each region (Fig. 1). Based on this pattern, the posterior−anterior index (PAx) was computed to establish if there was consistent information flow in the posterior−anterior direction. The flow between pairs of regions was subsequently examined in more detail (Fig. 2).
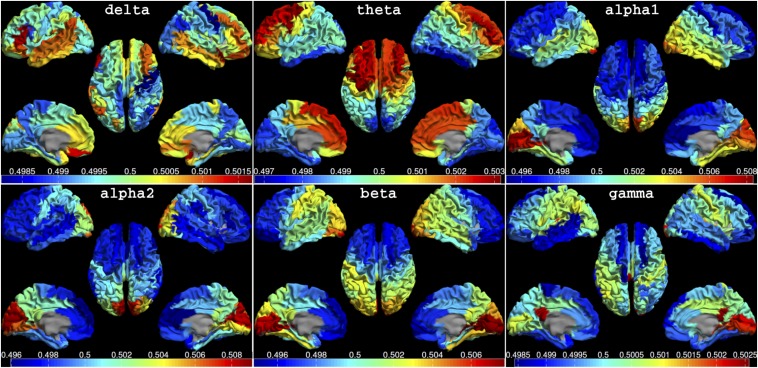
Fig. 1.
Mean dPTE for each ROI displayed as a color-coded map on the parcellated template brain, viewed from, in clockwise order, the left, top, right, right midline, and left midline. Note the smooth global patterns of preferential information flow in the higher-frequency bands (alpha1 to beta), consisting of posterior regions that are leading anterior regions. The opposite pattern was observed in the theta band. Hot and cold colors indicate information outflow and inflow, respectively. See Fig. S1A for the connectivity matrices.
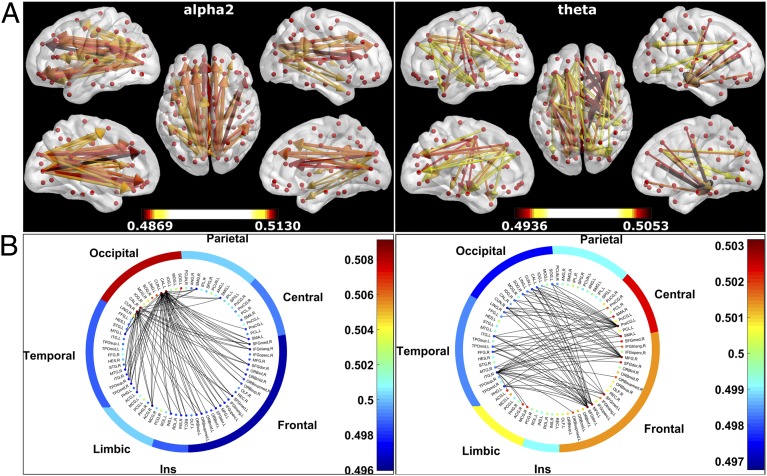
Fig. 2.
Preferred direction of information flow between regions. (A) Preferential information flow for the alpha2 and theta band displayed on the template brain using BrainNet Viewer (version 1.5), viewed from, in clockwise order, the left, top, right, right midline, and left midline. Colors and line thickness indicate the dPTE values (lower and upper thresholds: [0.4892, 0.5108] and [0.4955, 0.5045] for the alpha2 and theta bands, respectively), and arrows indicate the preferred direction of information flow. Thresholds were chosen to highlight the dominant patterns formed by the information streams between regions; for statistically thresholded images, we refer to Fig. S1B. (B) Preferential information flow for the alpha2 and theta bands displayed as circular plots (after ref. 22). The color of each node indicates the mean dPTE value for that ROI. The full names of the ROIs are given in Table S1. The nodes were grouped as frontal lobe, central regions, parietal lobe, occipital lobe, temporal lobe, limbic region, and Insula (Ins), with the color of each group indicating the mean dPTE value for the group. The interior shows the connections between nodes (threshold as in A). See also Tables S2−S5.
Fig. 1 reveals smooth global patterns of preferential information flow in the alpha1 (8–10 Hz), alpha2 (10–13 Hz), and beta (13–30 Hz) bands, consisting of posterior regions that are leading anterior regions [PAx = 0.39, 0.42, and 0.55 (P < 0.001), respectively]. The opposite pattern was observed in the theta (4–8 Hz) band (PAx = −0.50, P < 0.001). The patterns in the gamma (30–48 Hz) and delta (0.5–4 Hz) bands were more dispersed [although still significant (P < 0.001); PAx = 0.26 and −0.29, respectively]. We describe the results for the alpha2 and theta band in more detail below, as the patterns for these bands (i) were most pronounced and (ii) resulted in high PAx values.
It can be seen in Fig. 2 and Tables S2 and S4 that, in the alpha2 band, the strongest information flow was from posterior regions, including the (primary) visual areas and posterior parts of the default mode network (DMN), to anterior cingulate, frontal, and temporal regions. The 500 strongest outgoing connections all started in precuneus, superior, middle, and inferior occipital gyrus, calcarine fissure, cuneus, lingual gyrus, and posterior cingulate; the areas that received most of these connections were, in rank order, left medial superior frontal gyrus; right inferior frontal gyrus and right anterior cingulate; right superior frontal gyrus (dorsal and medial); left superior frontal gyrus (dorsal); and left superior frontal gyrus (medial orbital), left anterior cingulate, right supramarginal gyrus, right temporal pole (superior temporal gyrus), and the right insula. For the theta band (Fig. 2 and Tables S3 and S4), the pattern was more dispersed, with the strongest and most frequent connections between frontal and temporal regions, but also with many other regions showing (only) a few strong connections. Out of the 500 strongest outgoing connections, the outgoing connections started most frequently in (rank-ordered) left precentral gyrus; left superior frontal gyrus (dorsolateral); right middle frontal gyrus; left middle frontal gyrus; left superior frontal gyrus (medial) and left supplementary motor area; right supplementary motor area; left anterior cingulate and right superior frontal gyrus (dorsolateral); and right anterior cingulate and the right median cingulate. The most frequent receiving areas were right inferior temporal gyrus; right cuneus; left cuneus, left parahippocampal gyrus, and right fusiform gyrus; left calcarine fissure; left inferior temporal gyrus; left temporal pole (middle temporal gyrus); and left superior occipital gyrus and the right inferior occipital gyrus. Note that some of the most frequent sending regions in the alpha2 band tended to be frequent receivers in the theta band (Table S5). A similar effect, although less pronounced, was seen for the most frequent sending regions in the theta band: These were also often frequent receivers in the alpha2 band. Indeed, there was a significant negative correlation between the upper triangles of the dPTE matrices (Fig. S1A) in the alpha2 and theta band [r(3,001) = −0.57, P < 0.001].
Table S2.
Frequency table of the 500 strongest dPTE connections in the alpha2 band
| ROI | Number of links | Frequency, % |
| ROI_out | ||
| CUN_L | 67 | 13.4 |
| CAL_L | 66 | 13.2 |
| CAL_R | 63 | 12.6 |
| CUN_R | 62 | 12.4 |
| SOG_L | 58 | 11.6 |
| LING_L | 50 | 10.0 |
| SOG_R | 49 | 9.8 |
| MOG_R | 27 | 5.4 |
| LING_R | 26 | 5.2 |
| IOG_R | 10 | 2.0 |
| PCG_R | 7 | 1.4 |
| PCG_L | 4 | 0.8 |
| PCUN_L | 3 | 0.6 |
| MOG_L | 3 | 0.6 |
| IOG_L | 3 | 0.6 |
| PCUN_R | 2 | 0.4 |
| ROI_in | ||
| SFGmed_L | 16 | 3.2 |
| IFGtriang_R | 15 | 3.0 |
| ACG_R | 15 | 3.0 |
| SFGdor_R | 12 | 2.4 |
| SFGmed_R | 12 | 2.4 |
| SFGdor_L | 11 | 2.2 |
| ORBsupmed_L | 10 | 2.0 |
| ACG_L | 10 | 2.0 |
| SMG_R | 10 | 2.0 |
| TPOsup_R | 10 | 2.0 |
| INS_R | 10 | 2.0 |
| REC_L | 9 | 1.8 |
| ORBsup_L | 9 | 1.8 |
| ORBmid_L | 9 | 1.8 |
| IFGoperc_L | 9 | 1.8 |
| IFGtriang_L | 9 | 1.8 |
| SMA_L | 9 | 1.8 |
| PreCG_L | 9 | 1.8 |
| SMG_L | 9 | 1.8 |
| MTG_L | 9 | 1.8 |
| INS_L | 9 | 1.8 |
| ORBsup_R | 9 | 1.8 |
| ORBmid_R | 9 | 1.8 |
| MTG_R | 9 | 1.8 |
| TPOmid_R | 9 | 1.8 |
| MFG_L | 8 | 1.6 |
| SMA_R | 8 | 1.6 |
| ITG_R | 8 | 1.6 |
| OLF_L | 7 | 1.4 |
| ORBinf_L | 7 | 1.4 |
| PCL_L | 7 | 1.4 |
| HES_L | 7 | 1.4 |
| STG_L | 7 | 1.4 |
| ITG_L | 7 | 1.4 |
| TPOsup_L | 7 | 1.4 |
| PHG_L | 7 | 1.4 |
| REC_R | 7 | 1.4 |
| OLF_R | 7 | 1.4 |
| ORBsupmed_R | 7 | 1.4 |
| ORBinf_R | 7 | 1.4 |
| MFG_R | 7 | 1.4 |
| IFGoperc_R | 7 | 1.4 |
| PreCG_R | 7 | 1.4 |
| ROL_R | 7 | 1.4 |
| ANG_R | 7 | 1.4 |
| STG_R | 7 | 1.4 |
| PHG_R | 7 | 1.4 |
| FFG_L | 6 | 1.2 |
| TPOmid_L | 6 | 1.2 |
| SPG_R | 6 | 1.2 |
| HES_R | 6 | 1.2 |
| DCG_R | 6 | 1.2 |
| ROL_L | 5 | 1.0 |
| SPG_L | 5 | 1.0 |
| IPL_L | 5 | 1.0 |
| PoCG_R | 5 | 1.0 |
| IPL_R | 5 | 1.0 |
| FFG_R | 5 | 1.0 |
| PoCG_L | 4 | 0.8 |
| ANG_L | 4 | 0.8 |
| DCG_L | 4 | 0.8 |
| PCL_R | 4 | 0.8 |
| PCUN_R | 3 | 0.6 |
| PCUN_L | 2 | 0.4 |
| MOG_L | 2 | 0.4 |
| IOG_L | 2 | 0.4 |
| PCG_L | 1 | 0.2 |
Note that there are a few posterior regions with preferential information outflow, and that there is a more distributed network, mainly frontal, temporal, and in cingulate cortex, with preferential information inflow. L, left; R, right; See Table S1 for abbreviations.
Table S4.
The 100 strongest connections between pairs of regions in the alpha2 and theta band, in rank order
| alpha2 | theta | ||
| Source | Target | Source | Target |
| CAL_L | IFGtriang_R | PreCG_L | ITG_R |
| CUN_L | ACG_R | MFG_R | ITG_R |
| CAL_L | SFGmed_L | SFGdor_L | ITG_R |
| CAL_L | ACG_R | MFG_L | ITG_R |
| CUN_L | IFGtriang_R | SFGmed_L | ITG_R |
| CUN_R | IFGtriang_R | PreCG_L | CUN_R |
| CUN_R | ACG_R | SMA_L | ITG_R |
| CUN_L | SFGmed_R | PreCG_L | CUN_L |
| CAL_R | SFGmed_L | SFGmed_R | ITG_R |
| CUN_L | SFGmed_L | SMA_R | ITG_R |
| CUN_R | SFGmed_L | PreCG_L | PHG_L |
| CAL_L | SFGmed_R | SFGdor_R | ITG_R |
| CAL_R | ACG_R | PreCG_L | ITG_L |
| CAL_L | ORBsupmed_L | PreCG_L | CAL_L |
| CAL_L | SFGdor_R | DCG_R | ITG_R |
| CUN_L | SFGdor_R | ACG_L | ITG_R |
| CAL_L | SFGdor_L | ACG_R | ITG_R |
| CAL_L | INS_R | SFGdor_L | CUN_R |
| CUN_L | SMG_R | SFGdor_L | CUN_L |
| CAL_R | IFGtriang_R | PreCG_L | TPOmid_L |
| CAL_L | TPOsup_R | PreCG_L | SOG_L |
| CAL_L | SMG_L | ORBsupmed_R | ITG_R |
| CAL_L | ORBsup_L | PreCG_L | FFG_R |
| CAL_R | SFGmed_R | SFGdor_L | PHG_L |
| CUN_R | SFGdor_L | MFG_R | CUN_R |
| CAL_L | TPOmid_R | MFG_L | CUN_L |
| CUN_L | SFGdor_L | MFG_R | CUN_L |
| CUN_L | MTG_L | SFGdor_L | ITG_L |
| CUN_R | SFGmed_R | MFG_R | FFG_R |
| CUN_L | TPOsup_R | MFG_R | PHG_L |
| CUN_L | ORBsupmed_L | PreCG_R | ITG_R |
| CAL_L | SMG_R | PreCG_L | LING_L |
| CAL_L | MTG_L | SFGmed_L | CUN_R |
| CAL_L | ACG_L | SFGdor_L | FFG_R |
| CAL_L | ORBmid_R | PreCG_L | IOG_R |
| CUN_R | ORBsupmed_L | PreCG_L | SOG_R |
| CAL_L | ITG_R | MFG_R | CAL_L |
| CUN_L | ORBsup_L | SFGdor_L | CAL_L |
| CAL_L | MTG_R | MFG_R | TPOmid_L |
| CUN_R | SFGdor_R | MFG_R | ITG_L |
| CUN_L | IFGoperc_L | ORBsupmed_L | ITG_R |
| CUN_L | TPOmid_R | IFGtriang_R | ITG_R |
| CAL_L | ORBmid_L | SFGdor_L | SOG_R |
| CUN_R | SMG_R | SFGdor_L | SOG_L |
| CUN_R | MTG_L | PreCG_L | FFG_L |
| CUN_L | MFG_L | PreCG_L | LING_R |
| CAL_L | MFG_L | MFG_R | SOG_L |
| CUN_R | INS_R | PreCG_L | MTG_R |
| CAL_L | ORBsupmed_R | MFG_R | LING_R |
| CUN_L | REC_L | MFG_L | PHG_L |
| CAL_L | IFGoperc_L | MFG_R | MTG_R |
| CUN_L | SMG_L | MFG_L | CUN_R |
| CAL_L | ORBsup_R | SFGdor_L | IOG_R |
| CAL_R | SMG_R | MFG_R | IOG_R |
| CUN_L | ORBmid_R | SFGdor_L | LING_R |
| CUN_R | TPOsup_R | MFG_R | SOG_R |
| CAL_L | SMA_L | IFGtriang_L | ITG_R |
| CAL_R | SFGdor_R | SMA_L | CUN_R |
| CUN_R | MTG_R | PreCG_L | IOG_L |
| CUN_L | INS_L | MFG_L | FFG_R |
| CUN_R | TPOmid_R | SFGmed_L | PHG_L |
| CAL_R | TPOmid_R | SMA_L | FFG_R |
| CAL_R | TPOsup_R | MFG_L | CAL_L |
| CAL_L | INS_L | SFGdor_L | MTG_R |
| CAL_L | IFGtriang_L | SFGdor_L | TPOmid_L |
| CAL_L | REC_L | MFG_L | TPOmid_L |
| CAL_R | MTG_L | MFG_L | SOG_R |
| CUN_L | PreCG_L | MFG_L | ITG_L |
| CUN_L | INS_R | SMA_L | CUN_L |
| CUN_R | ORBsup_L | SFGmed_L | ITG_L |
| CAL_R | MTG_R | SFGmed_L | CAL_L |
| SOG_L | ACG_R | MFG_R | LING_L |
| CUN_R | SMG_L | SFGdor_R | CUN_R |
| CUN_L | ORBsup_R | SFGdor_L | LING_L |
| CUN_L | ACG_L | ACG_R | CUN_R |
| CUN_L | SMA_L | PreCG_L | PreCUN_R |
| CAL_R | ACG_L | SMA_L | PHG_L |
| CUN_R | INS_L | PreCG_L | CAL_R |
| CUN_R | REC_L | MFG_R | FFG_L |
| CUN_L | MTG_R | MFG_L | IOG_R |
| LING_L | SFGmed_L | DCG_L | ITG_R |
| CUN_L | ORBsupmed_R | SFGdor_L | FFG_L |
| CAL_R | ORBsupmed_L | ORBmid_L | ITG_R |
| CUN_R | ORBmid_R | SMA_R | CUN_R |
| CAL_R | SFGdor_L | SMA_L | ITG_L |
| CAL_L | PreCG_L | SFGdor_R | PHG_L |
| SOG_L | SFGmed_R | ORBmid_R | ITG_R |
| CUN_L | ORBinf_L | SFGdor_L | PreCUN_R |
| CAL_L | ORBinf_L | SFGmed_L | FFG_R |
| CUN_R | ACG_L | ACG_R | FFG_R |
| CAL_R | INS_L | MFG_R | PreCUN_R |
| CAL_R | REC_L | PreCG_L | TPOsup_R |
| CAL_R | ORBsup_L | DCG_R | CUN_R |
| CUN_L | IFGtriang_L | ACG_L | CUN_R |
| CAL_L | REC_R | SFGmed_R | PHG_L |
| SOG_L | SFGmed_L | SMA_L | SOG_L |
| CUN_R | SMA_L | SMA_L | IOG_R |
| CAL_L | SMA_R | SFGmed_L | CUN_L |
| SOG_L | IFGtriang_R | DCG_R | PHG_L |
| CUN_R | MFG_L | SFGmed_R | CUN_R |
Table S3.
Frequency table of the 500 strongest dPTE connections in the theta band
| ROI | Number of links | Frequency, % |
| ROI_out | ||
| PreCG_L | 45 | 9.0 |
| SFGdor_L | 42 | 8.4 |
| MFG_R | 40 | 8.0 |
| MFG_L | 32 | 6.4 |
| SFGmed_L | 28 | 5.6 |
| SMA_L | 28 | 5.6 |
| SMA_R | 25 | 5.0 |
| ACG_L | 23 | 4.6 |
| SFGdor_R | 23 | 4.6 |
| ACG_R | 22 | 4.4 |
| DCG_R | 22 | 4.4 |
| SFGmed_R | 21 | 4.2 |
| ORBsupmed_L | 19 | 3.8 |
| ORBsupmed_R | 17 | 3.4 |
| IFGtriang_R | 17 | 3.4 |
| PreCG_R | 17 | 3.4 |
| IFGtriang_L | 14 | 2.8 |
| DCG_L | 11 | 2.2 |
| ORBmid_L | 10 | 2.0 |
| ORBinf_L | 8 | 1.6 |
| ORBmid_R | 7 | 1.4 |
| ORBsup_L | 5 | 1.0 |
| Postcentral_R | 5 | 1.0 |
| ORBsup_R | 3 | 0.6 |
| REC_R | 2 | 0.4 |
| REC_L | 1 | 0.2 |
| OLF_L | 1 | 0.2 |
| IFGoperc_L | 1 | 0.2 |
| PCL_L | 1 | 0.2 |
| PoCG_L | 1 | 0.2 |
| HES_L | 1 | 0.2 |
| OLF_R | 1 | 0.2 |
| ORBinf_R | 1 | 0.2 |
| IFGoperc_R | 1 | 0.2 |
| PCL_R | 1 | 0.2 |
| ROL_R | 1 | 0.2 |
| SPG_R | 1 | 0.2 |
| IPL_R | 1 | 0.2 |
| PCG_R | 1 | 0.2 |
| ROI_in | ||
| ITG_R | 39 | 7.8 |
| CUN_R | 25 | 5.0 |
| CUN_L | 23 | 4.6 |
| PHG_L | 23 | 4.6 |
| FFG_R | 23 | 4.6 |
| CAL_L | 22 | 4.4 |
| ITG_L | 21 | 4.2 |
| TPOmid_L | 20 | 4.0 |
| SOG_L | 19 | 3.8 |
| IOG_R | 19 | 3.8 |
| SOG_R | 18 | 3.6 |
| LING_L | 17 | 3.4 |
| LING_R | 17 | 3.4 |
| MTG_R | 17 | 3.4 |
| FFG_L | 16 | 3.2 |
| PreCUN_R | 16 | 3.2 |
| CAL_R | 16 | 3.2 |
| IOG_L | 13 | 2.6 |
| TPOsup_R | 13 | 2.6 |
| PreCUN_L | 12 | 2.4 |
| STG_L | 12 | 2.4 |
| PCG_L | 10 | 2.0 |
| TPOmid_R | 9 | 1.8 |
| INS_L | 7 | 1.4 |
| HES_R | 7 | 1.4 |
| SPG_L | 6 | 1.2 |
| MOG_R | 6 | 1.2 |
| IPL_L | 5 | 1.0 |
| ANG_L | 5 | 1.0 |
| INS_R | 5 | 1.0 |
| ROL_L | 4 | 0.8 |
| TPOsup_L | 4 | 0.8 |
| SMG_L | 3 | 0.6 |
| MOG_L | 3 | 0.6 |
| MTG_L | 3 | 0.6 |
| SMG_R | 3 | 0.6 |
| ANG_R | 3 | 0.6 |
| STG_R | 3 | 0.6 |
| PHG_R | 3 | 0.6 |
| PCG_R | 3 | 0.6 |
| ORBinf_R | 2 | 0.4 |
| IFGoperc_R | 2 | 0.4 |
| HES_L | 1 | 0.2 |
| ROL_R | 1 | 0.2 |
| SPG_R | 1 | 0.2 |
Table S5.
Reentry of information
| ROI | Joint frequency |
| α2out, θin | |
| CUN_L | 1,541 |
| CAL_L | 1,452 |
| CAL_R | 1,008 |
| CUN_R | 1,550 |
| SOG_L | 1,102 |
| LING_L | 850 |
| SOG_R | 882 |
| MOG_R | 162 |
| LING_R | 442 |
| IOG_R | 190 |
| PCG_R | 21 |
| PCG_L | 40 |
| PreCUN_L | 36 |
| MOG_L | 9 |
| IOG_L | 39 |
| PreCUN_R | 32 |
| θout, α2in | |
| PreCG_L | 405 |
| SFGdor_L | 462 |
| MFG_R | 280 |
| MFG_L | 256 |
| SFGmed_L | 448 |
| SMA_L | 252 |
| SMA_R | 200 |
| ACG_L | 230 |
| SFGdor_R | 276 |
| ACG_R | 330 |
| DCG_R | 132 |
| SFGmed_R | 252 |
| ORBsupmed_L | 190 |
| ORBsupmed_R | 119 |
| IFGtriang_R | 255 |
| PreCG_R | 119 |
| IFGtriang_L | 126 |
| DCG_L | 44 |
| ORBmid_L | 90 |
| ORBinf_L | 56 |
| ORBmid_R | 63 |
| ORBsup_L | 45 |
| PoCG_R | 25 |
| ORBsup_R | 27 |
| REC_R | 14 |
| REC_L | 9 |
| OLF_L | 7 |
| IFGoperc_L | 9 |
| PCL_L | 7 |
| PoCG_L | 4 |
| HES_L | 7 |
| OLF_R | 7 |
| ORBinf_R | 7 |
| IFGoperc_R | 7 |
| PCL_R | 4 |
| ROL_R | 7 |
| SPG_R | 6 |
| IPL_R | 5 |
Regions with many strong outgoing connections in one frequency band (α2out or θout) were often a frequent target in another frequency band (θin or α2in). Joint frequency is computed as the number of outgoing connections (among the 500 strongest connections) in one frequency band multiplied with the number of incoming connections (among the 500 strongest connections) in another frequency band.
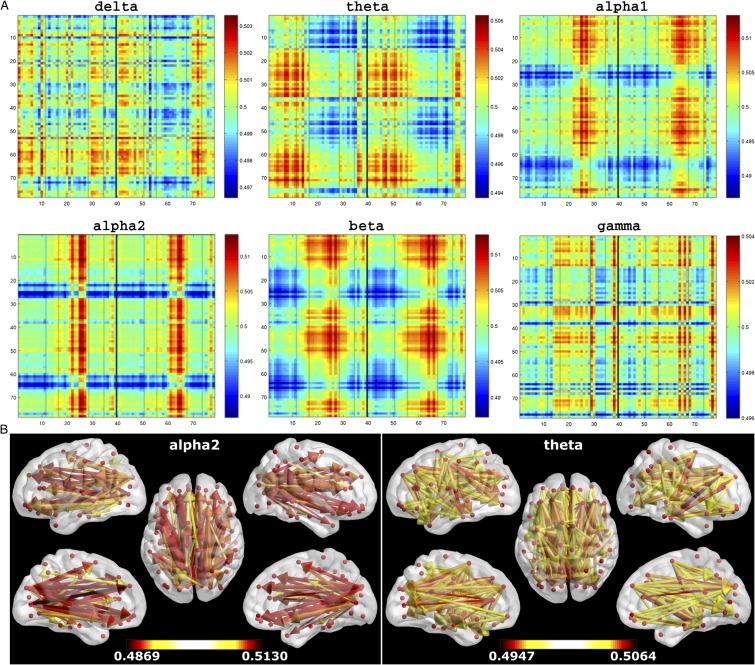
Fig. S1.
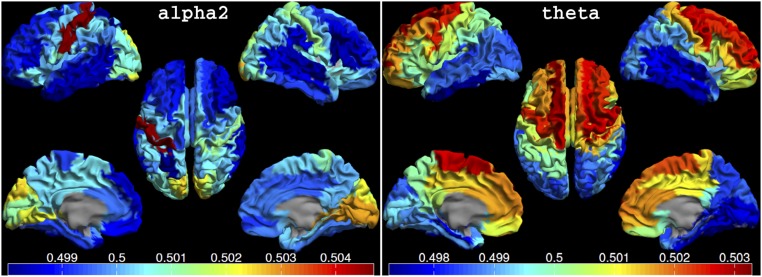
(A) Mean dPTE for all frequency bands represented as color-coded connectivity matrices. Hot and cold colors indicate information outflow and inflow, respectively. A column represents the connection from an ROI to all other ROIs. The axes labels indicate the ROI indices in the atlas (Table S1). The solid vertical line separates the left and right hemisphere, and the dotted lines separate, from left to right, frontal, central, parietal/occipital, temporal, and remaining regions. (B) Preferred direction of information flow between regions for the alpha2 and theta bands displayed on the template brain, viewed from, in clockwise order, the left, top, right, right midline, and left midline. Colors and line thickness indicate the (significant) dPTE values, and arrows indicate the preferred direction of information flow.
To aid the interpretation of our results, we performed several extra analyses, which are described in Supporting Information: We compared results obtained for the eyes-closed condition to those obtained for the eyes-open condition, revealing similar patterns of information flow (Fig. S2). Similarly, these patterns, albeit with lower spatial resolution, were also found at the sensor level (Fig. S3), confirming that the observed patterns were not an artifact of the source reconstruction approach. In addition, we compared dPTE to another directional measure, the directed Phase Lag Index (dPLI) (23), which is based on phase differences rather than information flow (Fig. S4). Importantly, we also showed that these patterns of phase differences in a toy system of coupled Rössler oscillators depend on the global strength of the underlying structural connections, whereas dPTE revealed the correct patterns of information flow, independent of the coupling strength (Fig. S5). Finally, we estimated the relation between relative power and dPTE, as well as the effect of the choice of beamformer approach (Fig. S6).
Fig. S2.
Mean dPTE for the eyes-open condition. The mean dPTE for each ROI is displayed as a color-coded map on the parcellated template brain, viewed from, in clockwise order, the left, top, right, right midline, and left midline. Note the global posterior-to-anterior pattern of preferential information flow in the alpha2 band, consisting of posterior regions that are leading anterior regions. The opposite pattern was observed in the theta band. Hot and cold colors indicate information outflow and inflow, respectively.
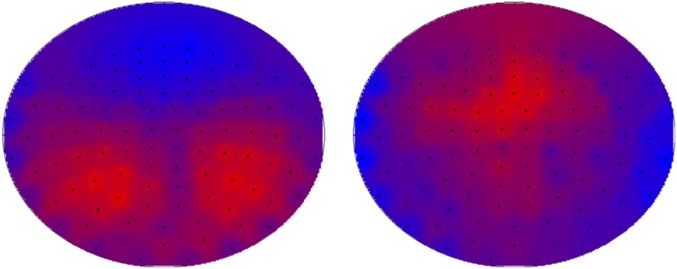
Fig. S3.
Mean dPTE for 67 subjects at the sensor level (102 magnetometers) for the alpha2 (Left) and theta (Right) bands. The panels show a schematic representation of the head, viewed from above; black dots indicate the sensor locations (projected to 2D). Hot and cold colors indicate information outflow and inflow, respectively.
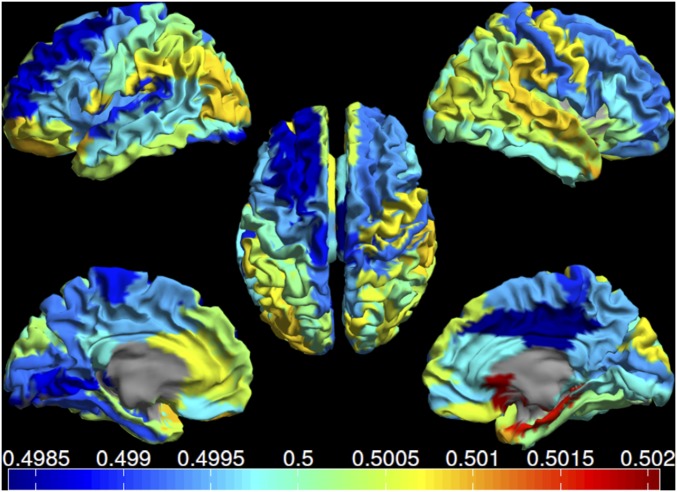
Fig. S4.
Mean alpha2 dPLI for each ROI displayed as a color-coded map on the parcellated template brain, viewed from, in clockwise order, the left, top, right, right midline, and left midline. Note the absence of a clear posterior-to-anterior pattern (compare with Fig. 1).
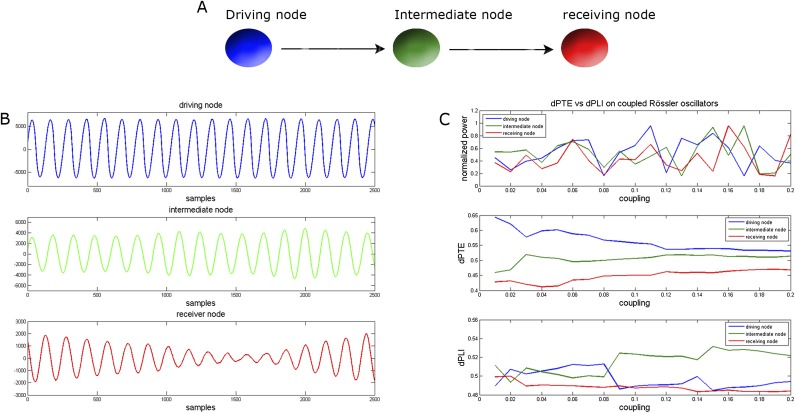
Fig. S5.
Rössler simulations. (A) Layout of the three coupled Rössler oscillators. The Rössler oscillators (nodes) were unidirectionally coupled, where the first driving oscillator was influencing the second, intermediate, oscillator, and the latter driving the third, receiving, oscillator. (B) Part of the time series generated by the system of three coupled Rössler oscillators. (C) Mean dPTE and dPLI for each node, together with the power modulations, for different global coupling strengths. In contrast to the dPLI, the ordering of dPTE values does not flip when the global coupling strength increases. Also note that neither the dPLI nor dPTE have a simple one-to-one relationship with the power modulations.
Fig. S6.
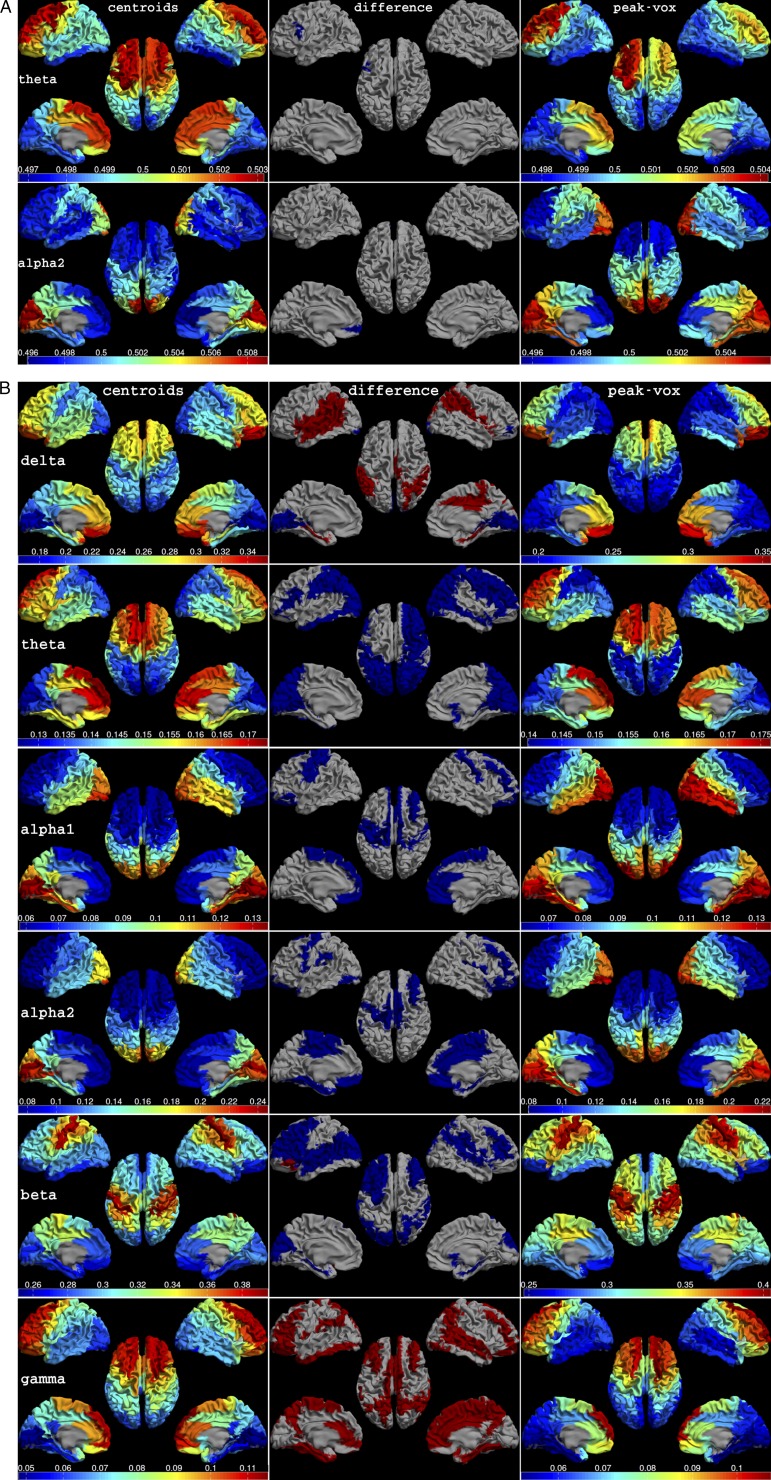
(A) Mean dPTE for each ROI displayed as a color-coded map on the parcellated template brain, viewed from, in clockwise order, the left, top, right, right midline, and left midline. Left and Right show the results obtained with the centroids beamformer and the peak voxel beamformer, respectively. Middle shows the comparison between the two approaches, with red and blue indicating a significant increase and decrease, respectively (permutation testing, P < 0.05, corrected). Top and Bottom show the results for the theta and alpha2 bands, respectively. Note that there were no consistent differences between the two approaches. (B) Mean relative power for each ROI for, from top to bottom, delta, theta, alpha1, alpha2, beta, and gamma bands. Note that the patterns of relative power in the different frequency bands were similar across beamformer approaches, although some regions showed a significant difference in relative power (see Table S6).
Discussion
We have demonstrated, for the higher-frequency bands (8−30 Hz), dominant posterior-to-anterior patterns of information flow in an eyes-closed resting state from regions in the parieto-occipital lobe toward frontal areas. In contrast, a pattern of anterior-to-posterior flow was found in the theta band, involving mainly regions in the frontal lobe that were sending information to a more distributed network. Interestingly, strong senders of information in the alpha2 band were also often receivers of information in the theta band, and vice versa, suggesting a frequency-specific loop of information flow in the human brain.
Posterior regions of the DMN were found to be strong senders of information in the higher-frequency bands (8−30 Hz), and frequent receivers in the theta band. The DMN is strongly activated during the resting state (see also Fig. S6B), and has putatively been linked to spontaneous cognition (internal mentation) and/or general unconscious low-level attention to the external world (36, 37). At least two distinct interacting subsystems play a role in the DMN: one temporal system involved in memory processes, and a fronto-parietal system involved in self-relevant mental simulations (36). Our observation of a dominant posterior-to-anterior pattern of information flow in the higher-frequency bands between parieto-occipital regions and frontal regions, as well as an anterior-to-posterior pattern from frontal regions to temporal and posterior regions in the theta band, suggests that these subsystems form a loop (Table S5), or cell assembly (38, 39), through which information “reverberates” or “circulates.” The observation that the theta band is important for memory processes in frontal and hippocampal areas further supports this idea (40, 41). A counterargument against such interpretations of our results is our observation that not only the posterior part of the DMN is involved in the posterior-to-anterior flow, but also, and most strongly, regions in the visual cortex. Therefore, the interpretation of the current findings can also be placed in a broader context, superseding the sole role of the DMN with respect to the observed patterns. The observation of a mirrored flow of information is reminiscent of reentry in neural systems as a mechanism for integration of brain function (42).
Recent hypotheses have stressed the importance of both alpha and theta connectivity in attention, where theta band connectivity from the medial frontal cortex to various regions plays a key role in inducing control from higher association areas over lower-level, perceptual processes, but also over the DMN (43). Our theta band findings seem to be in line with this theory, as it may explain why we found distributed theta band information flow from frontal areas to various regions, including the DMN. At the same time, this model predicts that anterior-to-posterior alpha connectivity provides a gating mechanism for attention, because top-down modulation by alpha could inhibit irrelevant activity (43, 44). This may seem to be in disagreement with our findings of a posterior-to-anterior dominant pattern of information flow in the alpha band, although it is possible that the enhanced bottom-up signaling in the alpha band is in itself a consequence of enhanced top-down signaling in the theta band (45). Moreover, one should take into consideration that our recordings were resting state and not task-based, and that neuronal signatures of different forms of attention (46) are assumed to be related but can have different spatiotemporal and frequency contents. The observed posterior-to-anterior information flow during the resting state is likely to be a signature for internal attention rather than for attention to external stimuli [also supported by our finding that opening the eyes during the resting state did not alter the dominant patterns of information flow (Fig. S2)]. These ideas could be tested in future task-based studies, where the exact role and direction of fronto-posterior connections could be analyzed for different forms of attention.
Alternatively, the oppositely directed propagated electrophysiological activity in different frequency bands could play a role in contexts other than attention (47), such as memory consolidation (48). Our results (Fig. 2) are, for example, consistent with animal recordings that have shown cortico-hippocampal propagation at low frequencies and cortico-cortical propagation at higher frequencies (49). Similarly, bottom-up and top-down influences through distinct fast- and slow-frequency channels have also been reported for the visual system (45, 50). Although these observations from task-based invasive animal recordings suggest that similar mechanisms may play a role during noninvasive resting-state recordings in humans, more work is needed to further elucidate the functional role of resting-state information flow through distinct frequency channels.
An alternative explanation for the observed stable patterns of information flow may come from the topological properties of the underlying structural network, as recent modeling work has suggested. In particular, highly connected regions in the network, so-called hubs, become phase-lagging with respect to nonhub regions (22, 23). Brain networks have their strongest hubs in posterior regions (36, 51), which would hence lead to a front-to-back pattern of phase differences. However, simply changing the global strength of the underlying structural connections may reverse this pattern (Fig. S5), indicating that patterns of phase differences should not be confused with patterns of directed connectivity or, indeed, information flow. Instead, one could hypothesize that the net outflow of information from posterior regions is due to an increase in encoded information at higher firing rates (52), as simulations have shown that hubs also possess the highest levels of neuronal activity in the network, that is, the highest firing rates (53) and the highest power (22, 53).
Another factor that may have contributed to the discrepancy between our observed posterior-to-anterior pattern in the alpha band and the reverse pattern that was hypothesized on the basis of previous EEG studies in healthy subjects (refs. 17–22, but see refs. 26–28) is that the patterns of directionality in EEG strongly depend on the choice of reference (25). MEG, in contrast, is reference-free. Interestingly, we did not observe any consistent patterns of phase differences between anterior and posterior regions in any of the frequency bands in our source-reconstructed MEG data (Fig. S4). Finally, the artifact caused by the electrocardiogram (ECG) can cause consistent patterns of phase differences at the sensor level (54), because the artifact can be modeled as a rotating source (55). Application of beamforming not only allows for the reconstruction of information flow at the cortical level but also removes the ECG artifact (55), especially in combination with temporal extension of Signal Space Separation (tSSS) (56).
We observed a strong positive correlation between the patterns of dPTE and those of relative power [dPTE Versus Relative Power (Centroid Beamformer)], particularly for the alpha bands. It is therefore possible that the observed patterns of information flow are a direct consequence of differences in signal-to-noise ratio (SNR) between anterior and posterior regions, although the strong negative correlation between dPTE and relative power in the gamma band shows, at least, that this would not always be the case. Moreover, PTE is based on phase information and should therefore be relatively insensitive to differences in power or SNR (ref. 35, but see ref. 57). In addition, in our Rössler simulations (Fig. S5), there was a consistent pattern of information flow that did not depend on power differences. Furthermore, patterns of information flow observed in the experimental data for the eyes-open condition, i.e., when occipital alpha has low power, were similar to those obtained for the eyes-closed condition. Finally, modeling work suggests that the relationship between power and directionality may not simply be caused by differences in SNR but may be due to a neuronal mechanism that drives this correlation (52). Akam and Kullmann (52) showed that the amount of synchronous activity in a sending neuronal population, reflected in the amplitude of oscillations, is strongly related to the amount of information available to the receiving neuronal population. At the same time, interneuron motifs within the receiving neuronal population can act as bandpass filters to selectively gate incoming signals. This selective signal-gating mechanism could explain the strong positive correlation between amplitude and dPTE in our study, but it also explains why this correlation is not perfect, as the amplitude of the sending population itself is not the only prerequisite for routing information: There is also the sensitivity of receiving populations. However, given the mesoscopic level at which the analysis was performed and the infancy of this field, future modeling approaches need to include the influence of global structural topology (22–24) and inhomogeneity of neuronal populations to systematically study how patterns of information flow depend on frequency, power, SNR, and the role of regions within the network. Moreover, experimental studies should investigate if, and how, these patterns of information flow optimize integrative cognitive processing. An interesting hypothesis is, for example, that interruptions in the alpha−theta circular flow of information lead to poorer cognition.
Our results give direction to future studies on information flow in the human brain. Here, we analyzed the different frequency bands separately, and found posterior-to-anterior flow of information in the higher-frequency bands (alpha1, alpha2, and beta band), and anterior-to-posterior flow in the theta band, where some regions were frequent senders in one frequency band and frequent receivers in other frequency bands. Cross-frequency interactions, i.e., information flow across different frequency bands, were not studied directly, and our interpretation of the results in terms of a reentry loop of information flow should be accepted provisionally. Such cross-frequency interactions could be studied by applying the PTE to data filtered in different frequency bands for the senders and receivers (35). In addition, we only estimated information flow for relatively long time windows, such that we could not establish whether a single region switched from a sending to receiving state on short time scales or whether a region was simultaneously a sender in one frequency band and a receiver in another, consistent with the idea of oscillatory multiplexing for selective communication (58). This could be addressed by developing a dynamic dPTE approach.
Our approach, a combination of an atlas-based beamformer and PTE, revealed stable frequency-specific patterns of information flow in the healthy human brain. Future work should investigate how these patterns are disrupted in neurological disease, and how these disruptions relate to disease severity and cognitive performance.
Methods
Participants and Recording Protocol.
Data from 67 healthy controls were analyzed. These data formed part of two cohorts in studies on Multiple Sclerosis (MS) at the VU University Medical Center (VUmc) MS Center (59–61), for which approval was obtained from the Medical Ethical Review Committee of VUmc, whose ethics review criteria conformed to the Helsinki declaration. All subjects gave written informed consent before participation.
Anatomical images of the head were obtained on a 3.0T whole-body MRI scanner (GE Signa HDxt), using a 3D T1-weighted fast spoiled gradient echo sequence [repetition time (TR) 7.8 ms, echo time (TE) 3 ms, inversion time (TI) 450 ms, 12° flip angle (FA), sagittal 1.0-mm-thick slices, 0.94 × 0.94 mm in-plane resolution].
MEG data were recorded using a 306-channel whole-head system (Elekta Neuromag Oy) while participants were in a supine position in a magnetically shielded room (Vacuumschmelze). Data were recorded during no-task eyes-open (3 min) and eyes-closed (5 min) conditions, using a sample frequency of 1,250 Hz and online anti-aliasing (410 Hz) and high-pass (0.1 Hz) filters. The head position relative to the MEG sensors was recorded continuously using the signals from four head localization coils. The head localization coil positions were digitized, as was the outline of the participants scalp (∼500 points), using a 3D digitizer (Fastrak; Polhemus).
Channels that were malfunctioning, for example due to excessive noise, were identified by visual inspection of the data (mean number of excluded channels was six, range 2–11), and removed before applying the tSSS in MaxFilter software (version 2.2.15; Elekta Neuromag Oy) (56). The tSSS filter was used to remove artifacts that SSS without temporal extension would fail to discard, typically from noise sources near the head, using a subspace correlation limit of 0.9 and a sliding window of 10 s.
The scalp surfaces of all subjects were coregistered to their structural MRIs using a surface-matching procedure, with an estimated resulting accuracy of 4 mm (62). A single sphere was fitted to the outline of the scalp as obtained from the coregistered MRI, which was used as a volume conductor model for the beamformer approach described in Beamforming.
Beamforming.
An atlas-based beamformer approach was adopted to project MEG data from sensor level to source space (14). First, the coregistered MRI was spatially normalized to a template MRI using the New Segment toolbox in SPM8 (63). The AAL atlas was used to label the voxels in a subject’s normalized coregistered MRI (64). Subcortical structures were removed, as MEG is most sensitive to cortical regions (65), and the voxels in the remaining 78 cortical regions of interest (ROIs) were used for further analysis (66), after inverse transformation to the patient’s coregistered MRI. Next, neuronal activity in the labeled voxels was reconstructed using a scalar beamformer implementation (beamformer, version 2.1.28; Elekta Neuromag Oy) similar to Synthetic Aperture Magnetometry (67).
This beamformer sequentially reconstructs the activity for each voxel in a predefined grid covering the entire brain (spacing 2 mm) by selectively weighting the contribution from each MEG sensor to a voxel's time series. The beamformer weights are based on the data covariance matrix and the forward solution (lead field) of a dipolar source at the voxel location (30, 67, 68). A time window of, on average, 287 s (range 139–394 s) was used to compute the data covariance matrix. Singular value truncation was used when inverting the data covariance matrix to deal with the rank deficiency of the data after SSS (∼70 components).
Each ROI in the atlas contains many voxels, and the number of voxels per ROI differs. To obtain a single time series for an ROI, we used each ROI’s centroid as representative for that ROI, with the centroid defined here as the voxel within the ROI that is nearest, in terms of Euclidean distance, to all other voxels in the ROI [see Fig. S6 and Table S6 for comparison with an approach based on selection of the voxel with maximum activation (14)].
Table S6.
Comparison of results for the centroid beamformer and the peak voxel beamformer
| Frequency band | Relative power | PLI | dPTE |
| δ | ROL (L, R)↑; IPL (L, R)↑ | SMG (R)↑ | n.s. |
| SMG (L, R)↑; ANG (L, R)↑ | |||
| CAL (L, R)↓; LING (L, R)↓ | |||
| HES (L)↑; STG (L)↑ | |||
| MTG (L)↑; PHG (L, R)↑ | |||
| INS (L, R)↑; ORBmid (R)↓ | |||
| PCL (R)↑; SPG (R)↑ | |||
| SOG (R)↑; DCG (R)↑ | |||
| θ | ORBmid (L)↓; IFGoperc (L, R)↓ | n.s. | IFGoperc (L)↓ |
| IFGtriang (L, R)↓; PoCG (L, R)↓ | |||
| SPG (L, R)↓; IPL (L, R)↓ | |||
| SMG (L)↓; ANG (L, R)↓ | |||
| PCUN (L, R)↓; SOG (L, R)↓ | |||
| MOG (L, R)↓; IOG (L, R)↓ | |||
| CAL (L, R)↓; CUN (L, R)↓ | |||
| LING (L, R)↓; ITG (L)↓ | |||
| OLF (R)↓; ORBinf (R)↓ | |||
| SFGdor (R)↓; MFG (R)↓ | |||
| PreCG (R)↓; MTG (R)↓ | |||
| TPOsup (R)↓ | |||
| α1 | REC (L, R)↓; ORBsup (L, R)↓ | ORBsup (R)↓ | n.s. |
| ORBsupmed (L, R)↓; ORBinf (L)↓ | CAL (R)↓ | ||
| SFGmed (L, R)↓; SMA (L, R)↓ | |||
| PCL (L, R)↓; PreCG (L)↓ | |||
| PoCG (L, R)↓; MFG (R)↓ | |||
| ACG (R)↓ | |||
| α2 | REC (L,R)↓; ORBsup (L, R)↓ | n.s. | ORBsupmed (L)↓ |
| ORBsupmed (L, R)↓; SMA (L, R)↓ | |||
| PCL (L, R)↓; PreCG (L)↓ | |||
| ROL (L, R)↓; SMG (L)↓ | |||
| FFG (L)↓; TPOmid (L)↓ | |||
| PHG (L, R)↓; DCG (L, R)↓ | |||
| ORBinf (R)↓; MFG (R)↓ | |||
| TPOsup (R)↓; ACG (R)↓ | |||
| β | ORBinf (L)↑; SFGdor (L)↓ | ORBsup (L)↓ | OLF (L, R)↑ |
| MFG (L, R)↓; IFGoperc (L)↓ | ROL (L, R)↓ | CAL (R)↑ | |
| IFGtriang (L, R)↓; PreCG (L)↓ | MOG (L, R)↓ | LING (R)↑ | |
| ROL (L, R)↓; ANG (L, R)↓ | HES (L)↓ | ITG (R)↓ | |
| SOG (L, R)↓; MOG (L)↓ | TPOsup (L)↓ | PHG (R)↓ | |
| CAL (L)↓; CUN (L, R)↓ | DCG (L, R)↓ | ||
| HES (L)↓; STG (L, R)↓ | ORBsupmed (R)↓ SMA (R)↓ | ||
| MTG (L)↓; PHG (L, R)↓ | PoCG (R)↓ | ||
| INS (L, R)↓; OLF (R)↓ | STG (R)↓ | ||
| SPG (R)↓; SMG (R)↓ | TPOmid (R)↓ | ||
| ACG (R)↓ | |||
| INS (R)↓ | |||
| γ | ORBsup (L, R)↑ | ANG (L)↓ | ORBmid (L)↑ PCG (L, R)↑ |
| ORBsupmed (L, R)↑ | MTG (L)↓ | ||
| ORBmid (L, R)↑; ORBinf (L)↑ | ORBsup (R)↓ | ||
| MFG (L, R)↑; IFGtriang (L)↑ | |||
| SMA (L, R)↑; PLC (L, R)↑ | |||
| ROL (L)↑; SPG (L, R)↑ | |||
| SMG (L)↑; ANG (L, R)↑ | |||
| PCUN (L, R)↑; SOG (L)↑ | |||
| IOG (L, R)↑; FFG (L, R)↑ | |||
| ITG (L)↑; TPOmid (L)↑ | |||
| PHG (L, R)↑; ACG (L, R)↑ | |||
| SFGmed (R)↑; IPL (R)↑ | |||
| STG (R)↑; MTG (R)↑ | |||
| TPOsup (R)↑; DCG (R)↑ |
For each frequency band, the regions for which there was a significant difference between the centroid beamformer and the peak voxel beamformer (P < 0.05, corrected) are listed for different metrics; n.s., not significant; L, left; R, right. See Table S1 for abbreviations. Arrow up or down indicates whether the metric had a higher or lower value, respectively, for the centroid beamformer. Note that there were no significant differences for the dPLI (not shown).
The broadband (0.5–48 Hz) time series were projected through the normalized (69) broadband beamformer weights for each target voxel (i.e., centroid) to obtain a time series for an ROI. From these time series, the first 20 artifact-free epochs, containing 4,096 samples (3.2768 s), were selected to obtain stable results (70). These time series were then filtered in classical EEG/MEG frequency bands [delta (0.5–4 Hz), theta (4–8 Hz), alpha1 (8–10 Hz), alpha2 (10–13 Hz), beta (13–30 Hz), and lower gamma (30–48 Hz)], using an offline discrete fast Fourier transform filter that does not distort the phases (BrainWave, version 0.9.150.6; home.kpn.nl/stam7883/brainwave.html). Subsequently, the instantaneous phase for each time series was computed by taking the argument of the analytic signal as computed using the Hilbert transform (see e.g., ref. 71 for details).
PTE.
The information flow between ROIs was estimated using the PTE, which has recently been introduced by Lobier et al. (35). PTE is based on the same principle as Wiener−Granger Causality, namely that a source signal has a causal influence on a target signal if knowing the past of both signals improves the ability to predict the target's future compared with knowing only the target's past (72, 73). In the framework of Information Theory, this can best be understood in terms of uncertainty: A source signal has a causal influence on a target signal if the uncertainty of the target signal conditioned on both its own past and that of the source signals is smaller than the uncertainty of the target signal conditioned only on its own past. If the uncertainty of a target signal Y at a delay δ is expressed in terms of Shannon Entropy (74), then the Transfer Entropy (TE) from source signal X to target signal Y can be expressed as (75)
| [1] |
where the definition for Shannon Entropy (74), , was used, and the sum runs over all discrete time steps t.
For observed data, estimation of the probabilities in Eq. 1 is time-consuming and requires fine-tuning of several parameters (76). To solve these problems, Staniek and Lehnertz proposed to estimate transfer entropy by converting observed time series into sequences of symbols (77). In the same spirit, time series can be described in terms of their amplitudes and instantaneous phases (78), following which transfer entropy can be estimated from the time series of the instantaneous phases (PTE), at low computational cost (35). Dropping the subscript t for clarity, and using the fact that p(Yδ,Y) = p(Yδ) p(Y), the PTE becomes
| [2] |
where the probabilities are obtained by building histograms of occurrences of single, pairs or triplets of phase estimates in an epoch (35). The number of bins in the histograms was set as (78), and the prediction delay δ was set as (Ns x Nch)/N±, with Ns and Nch the number of samples and channels (ROIs), respectively, and N± the number of times the phase changes sign across time and channels.
Finally, because the PTE does not have a meaningful upper bound (35), and to reduce biases, i.e., the effect of having (small) nonzero PTE values in situations when there is no actual information flow, we normalized the PTE,
| [3] |
The value of dPTExy ranges between 0 and 1. When information flows preferentially from time series X to time series Y, 0.5 < dPTExy ≤ 1. When information flows preferentially toward X from Y, 0 ≤ dPTExy < 0.5. In the case of no preferential direction of information flow, dPTExy = 0.5.
It should be noted that our implementation differs slightly from Lobier et al. (35) in the way the number of bins, the prediction delay, and the normalization were computed. However, Lobier et al. showed that dPTE is robust against the a priori choice of the prediction delay, or the method used to construct the (conditional) probability distributions, and these differences in implementation should therefore not have affected our results.
Statistical Analysis.
For each frequency band and subject separately, the dPTE matrices were averaged over the 20 epochs, yielding one matrix per subject. These were then averaged over subjects. The average dPTE value was subsequently computed for each ROI; that is, the average preferred direction of information flow for a region was also computed.
To establish if there was a consistent pattern of information flow, a PAx was computed as follows:
| [4] |
where the dPTE was averaged over a set of posterior and anterior regions, respectively (see Table S1). A positive PAx indicates preferential flow from posterior regions toward anterior regions, and negative PAx preferential flow from anterior regions toward posterior regions. PAx was normalized by the absolute maximum PAx value that could have been obtained with the dPTE values for these individual ROIs. Significance of the PAx was assessed using randomization testing, where the average dPTE values were permuted across the ROIs, after which the PAx was computed. This was repeated 5,000 times to build a distribution of surrogate PAx values against which the observed PAx was tested (P < 0.05).
Table S1.
Abbreviations for the cortical regions used in this study (66)
| Cortical regions | Abbreviations |
| Gyrus Rectus | REC |
| Olfactory Cortex | OLF |
| Superior frontal gyrus, orbital part | ORBsup |
| Superior frontal gyrus, medial orbital | ORBsupmed |
| Middle frontal gyrus orbital part | ORBmid |
| Inferior frontal gyrus, orbital part | ORBinf |
| Superior frontal gyrus, dorsolateral | SFGdor |
| Middle frontal gyrus | MFG |
| Inferior frontal gyrus, opercular part | IFGoperc |
| Inferior frontal gyrus, triangular part | IFGtriang |
| Superior frontal gyrus, medial | SFGmed |
| Supplementary motor area | SMA |
| Paracentral lobule | PCL |
| Precentral gyrus | PreCG |
| Rolandic operculum | ROL |
| Postcentral gyrus | PoCG |
| Superior parietal gyrus | SPG |
| Inferior parietal, but supramarginal and angular gyri | IPL |
| Supramarginal gyrus | SMG |
| Angular gyrus | ANG |
| Precuneus | PCUN |
| Superior occipital gyrus | SOG |
| Middle occipital gyrus | MOG |
| Inferior occipital gyrus | IOG |
| Calcarine fissure and surrounding cortex | CAL |
| Cuneus | CUN |
| Lingual gyrus | LING |
| Fusiform gyrus | FFG |
| Heschl gyrus | HES |
| Superior temporal gyrus | STG |
| Middle temporal gyrus | MTG |
| Inferior temporal gyrus | ITG |
| Temporal pole: superior temporal gyrus | TPOsup |
| Temporal pole: middle temporal gyrus | TPOmid |
| Parahippocampal gyrus | PHG |
| Anterior cingulate and paracingulate gyri | ACG |
| Median cingulate and paracingulate gyri | DCG |
| Posterior cingulate gyrus | PCG |
| Insula | INS |
Regions in boldface formed the set of anterior regions [Montreal Neurological Institute (MNI) coordinate Y > 16 for the centroid of these regions + IFGoperc], and the regions in italics formed the set of posterior regions (MNI Y < −40 for the centroid of these regions + SMG). These posterior and anterior regions were used in the computation of the PAx, which establishes whether there is a consistent posterior–anterior pattern, or vice versa (see Eq. 4).
dPTE for Eyes-Closed Data
The main results for the eyes-closed data are given in Figs. 1 and 2. Fig. S1 provides more detailed information, with, in Fig. S1A, the group-averaged connectivity matrices for all frequency bands and, in Fig. S1B, the statistically significant information flow between pairs of regions. Statistical significance was assessed using randomization testing, where the dPTE matrix was transposed (i.e., information inflow was swapped for information outflow, and vice versa) for a random number of randomly selected subjects, after which the average dPTE matrix was computed. This was repeated 5,000 times to build a distribution of surrogate dPTE matrices against which the observed averaged dPTE matrix was tested (P < 0.05).
Tables S2−S5 highlight the regions that have many outgoing and incoming connections in the alpha2 (Table S2) and theta (Table S3) band, as well as the strongest connections between pairs of regions in these bands (Table S4). Table S5 provides reentry information, i.e., it shows which regions with many outgoing connections in one frequency band were also frequent targets in another frequency band.
dPTE for Eyes-Open Data
To exclude the possibility that the observed patterns of preferential information flow, particularly in the alpha2 band, were primarily due to the dominant alpha rhythm in posterior regions during the eyes-closed resting state, we also applied our analysis pipeline to the data that were recorded for the eyes-open condition (Fig. S2). The eyes-open data for one subject was not usable due to excessive artifacts.
The posterior-to-anterior pattern in the alpha2 band, and the reverse pattern in the theta band, as found for the eyes-closed data (Fig. 1) could also be discerned for the eyes-open data. This result provides further support for the notion that the observed patterns are due to patterns of information flow, and that they are not simply due to patterns of oscillatory power. It was further noted that areas around the left central sulcus showed high dPTE values in the alpha2 band during the eyes-open condition (and not during the eyes-closed condition). This is consistent with the well-known antagonistic behavior of the occipital alpha rhythm and the central mu rhythm during eyes-closed and eyes-open conditions.
dPTE at Sensor Level
We reconstructed patterns of information flow at the source level using an atlas-based beamforming approach. To verify that the observed patterns were not introduced by this approach to solve the inverse problem, we also performed a sensor-level analysis on the same 20 time segments that were used for the source-level analysis.
The posterior-to-anterior pattern in the alpha2 band, and the reverse pattern in the theta band, as found in source space (Fig. 1) can also be discerned at the sensor level (Fig. S3), albeit with lower spatial resolution and with reduced interpretability in terms of the anatomical regions that are involved.
dPTE Versus dPLI
In the main text, we estimated the preferred direction of information flow using the PTE, which was recently introduced by Lobier et al. (35). They thoroughly tested its performance using simulated data from coupled neural mass models and showed that dPTE is sensitive to information flow in both broadband and narrowband data, even in the presence of noise and linear mixing, and with limited amount of data.
Volume conduction and field spread, also referred to as “signal leakage” in source-reconstructed data, leads to a common signal in two time series. When two time series contain the same signal, then there is no information flow between them, and, consequently, the PTE should be equal to zero (or dPTE = 0.5). Using the same argument, PTE should also be insensitive to the effects of reference choice in EEG data. Although the immunity to linear mixing has also been claimed for the directed Transfer Function (79), other measures based on Granger causality are affected (80).
PTE does not suffer from systematic biases or increased risk for false positives, yet it is computationally straightforward (35). Many alternative model-based or data-driven approaches (31–34) are computationally more demanding. With Dynamic Causal Modeling (81), different models that capture the causal interactions between regions may be compared in order to test different hypotheses about directionality. However, this approach requires strong prior information and hypotheses, making it less suitable for exploratory analyses (82) and whole-brain studies (but see ref. 83). Similarly, several techniques that have extended bivariate Wiener−Granger Causality to multivariate signals (26, 84, 85) depend on the estimation of many parameters, as well as the model order, of a multivariate autoregressive model. Model estimation is not straightforward, especially for narrowband signals (33).
In previous work, we (20, 21, 23) and others (19, 22), have revealed an anterior-to-posterior pattern in simulations and EEG data of healthy controls (particularly in the alpha band) using the dPLI (23). This measure quantifies directionality on the basis of phase leading and lagging between time series. It assumes that the phase differences remain bounded, and that the sign of the phase differences does not depend on the strength of coupling. Here, we compared the patterns of directed connectivity as obtained using the dPLI with those obtained with the dPTE.
The dPLI estimates which one of two time series is leading or lagging in phase. It is an extension of a popular metric for phase synchronization, the PLI, which quantifies the consistency of a phase relationship between two signals, while ignoring zero-lag phase differences. The PLI is therefore a conservative measure that is insensitive to spurious interactions caused by the effects of volume conduction and/or field spread (14, 71). First, the instantaneous phase for each time series is computed by taking the argument of the analytical signal (71) as computed using the Hilbert transform. The PLI then calculates the asymmetry of the distribution of instantaneous phase differences between two time series,
| [S1] |
where the phase difference Δϕ is defined in the interval [−π, π], <> denotes the mean value, sign stands for signum function, || indicates the absolute value, and t corresponds to time samples 1,...,Ns, where Ns is the number of samples. The PLI ranges between 0 [completely symmetric phase distribution, i.e., no phase synchrony, or coupling with a phase difference centered around zero (modulus π)] and 1 (completely asymmetric phase distribution, i.e., a consistent, nonzero phase difference). As field spread and volume conduction causes a zero phase lag (modulus π) between two time series, this hardly influences the PLI (71). The direction of the interaction is reflected in the asymmetry of the phase distribution, i.e., it can be quantified as the probability that the phase differences are larger than zero (23). This can be computed as
| [S2] |
where θ is the Heaviside step function. The dPLI, like the PLI, is bounded by 0 and 1. When time series X is consistently phase leading compared with Y, 0.5 < dPLIxy ≤ 1. When time series X is phase lagging compared with Y, 0 ≤ dPLIxy < 0.5. In the case neither X or Y is leading or lagging on average: dPLIxy = 0.5.
Both the PLI and dPLI assume that the phase differences remain bounded; phase-based measures become ambiguous when phase differences can take on values larger than π (i.e., a signal that is leading with a large phase difference becomes lagging with a small phase difference, and vice versa), unless the phase difference is unwrapped and its evolution over time is carefully tracked (78). Moreover, it is implicitly assumed that the sign of the phase differences does not vary with the strength of the underlying structural coupling, which may not be the case in nonlinear systems of coupled oscillators (see Rössler Simulation).
In contrast to the dPTE (Fig. 1), the dPLI did not reveal clear patterns (Fig. S4); the PAx (see Methods) was not significant for any of the frequency bands.
Rössler Simulation: Effect of Structural Coupling Strength on dPTE and dPLI
Previous modeling studies have shown that patterns of phase differences may reverse when the global strength of the underlying structural connections changes (23, 24). Here, we simulated the behavior of a system of three coupled Rössler oscillators, and examined the effect of the global connectivity strength on the estimated pattern of directed connectivity when using either dPTE or dPLI. The three Rössler oscillators were coupled unidirectionally, with the first oscillator driving the second intermediate oscillator, which in turn drove the last receiving oscillator. The coupled differential equations for the first oscillator (i = 1) were (78)
| [S3] |
and the intermediate node (j = 2) and receiving node (j = 3) were coupled as follows:
| [S4] |
where i = j − 1, and the frequencies of the oscillators ωi,j were set to ωi = 2π, ωj = ωi+δ, with δ = 0.2, similar to ref. 86. The noise terms ξi,j are described by Gaussian delta-correlated noise terms. The system was simulated by Euler’s forward technique with time step 10−3. The global coupling strength, ε, between the oscillators was varied from 0 to 0.2. To avoid effects of initial conditions, we simulated 2 × 105 samples, and used the time series of the final 4,096 samples for further analyses using the dPTE or dPLI. The dPTE and dPLI were computed between the time series of a node and the two other nodes, and subsequently averaged to obtain one dPTE or dPLI value per node.
Fig. S5 shows the results of the simulations, where the dPTE and dPLI for the three oscillators are plotted as a function of global coupling strength. For the dPTE, the driving oscillator was identified as such, having the highest dPTE over the whole range of global coupling values. The other two oscillators were also identified in the correct order, with the receiving oscillator having the lowest dPTE values and the intermediate oscillator always having a value in between that of the driving and receiving oscillator, for all values of global coupling strength. This behavior was not observed for the dPLI, where the order of dPLI values for the three nodes flipped as global coupling strength increased. For coupling strengths larger than 0.08, the driving oscillator was, incorrectly, identified as lagging the intermediate oscillator. Lastly, we observed that the behavior of both the dPLI and the dPTE was not a one-to-one reflection of the behavior of the power of the Rössler oscillators.
In conclusion, the observed phase differences in our system of coupled Rössler oscillators depend on the global strength of the underlying structural connections, whereas dPTE reveals the correct pattern of information flow, independent of the coupling strength.
Centroid Beamformer Versus Peak Voxel Beamformer
Our analysis pipeline is based upon the use of a standard atlas, which facilitates the comparison of beamformer results across individuals, cohorts, studies, and different modalities (e.g., ref. 61). Each ROI in the atlas contains many voxels, and different strategies may be used to select a representative time series for a ROI (14). In the main text, we selected the centroid in each ROI (centroid beamformer). To demonstrate the effect of this choice on our results, we compared the results for the centroid beamformer to those obtained with the computationally more demanding peak voxel approach described previously (14) (peak voxel beamformer). For the latter approach, the voxel with maximum pseudo-Z value (87) in a frequency band is chosen as representative for an ROI (a unity matrix was used as estimate for the noise covariance matrix). For each frequency band, the time series from the corresponding peak voxels were used for the computation of the relative power, PLI, dPLI, or dPTE. For each subject and for each ROI, the relative power values, computed by normalizing the power in each frequency band by the overall power in the 0.5–48-Hz band, were averaged across the 20 epochs, and, subsequently, a group average was computed. Similarly, for each frequency band separately, for the PLI, dPLI, and dPTE analyses, we averaged, for each subject, the 20 epochs, yielding one matrix per subject. These were then averaged over subjects, and the average PLI, dPLI, or dPTE value was then computed for each ROI.
The results obtained with the two beamformer approaches, i.e., centroid beamformer versus peak voxel beamformer, were compared using permutation testing (random effects, 5,000 permutations, P < 0.05), where the maximum statistic (paired t test) across the ROIs was used to correct for multiple testing (88).
The results obtained with the centroid beamformer were very similar to those obtained with the peak voxel beamformer. Table S6 lists the regions that had a significantly different relative power, PLI, dPLI, or dPTE. It is clear that, for the connectivity measures, there were no consistent differences (see also Fig. S6A, which shows the consistency, across beamformer approaches, of the theta and alpha2 band dPTE patterns). These findings are in line with a recent study by Zobay et al., where three alternative approaches were compared with the peak voxel approach (89).
The patterns of relative power in the different frequency bands were also similar across beamformer approaches (Fig. S6B), although some regions showed a significant difference in relative power. There was a very weak, yet significant, relation between the difference in the relative power between beamformer approaches and the relative power as determined with the centroid beamformer [R2 = 0.088, F(1, 76) = 7.30, P < 0.01]. This can be explained by the finding that the resolution of beamformer reconstructions increases as SNR increases (90), i.e., the amplitude of the reconstructed signal drops more rapidly when moving away from the peak voxel when the SNR is higher. Choosing a voxel other than the peak voxel will therefore have a larger effect for regions with strong activations than for regions with weak activations. The maximum distance between the peak voxels and centroids was limited due to the use of an atlas. The fact that we only found a weak correlation between power and the difference in reconstructed power for both approaches indicates that the resolution of our MEG source reconstructions closely matched that of the AAL atlas.
A significant advantage of the centroid-based approach is that it does not require the computation of the power for all voxels within an ROI, and is therefore computationally much more efficient, enabling, for example, the use of advanced MEG analyses for routine clinical work in our memory clinic.
dPTE Versus Relative Power (Centroid Beamformer)
The patterns of relative power itself were similar to those obtained for the dPTE (compare Fig. S6B with Fig. 1), particularly for the alpha1 and alpha2 bands [r(76) = 0.91 and 0.88, respectively; P < 0.001]. However, for the beta band, the correlation between these patterns was smaller [r(76) = 0.24, P = 0.03], with most relative power in sensorimotor cortex, and the dPTE showing the marked posterior-to-anterior pattern. For the gamma band, there was a negative correlation between dPTE and power [r(76) = −0.55, P < 0.001]. For the delta and theta band, the correlation was 0.61 and 0.68, respectively (P < 0.001).
Acknowledgments
We thank Nico Akemann, Ndedi Sijsma, Karin Plugge, Marlous van den Hoek, and Peter-Jan Ris for the MEG acquisitions, as well as the three anonymous reviewers for their constructive comments during the preparation of this manuscript. This work was supported by a private sponsorship to the VUmc MS Center Amsterdam. The MS Center is sponsored through a program grant by the Dutch MS Research Foundation (Grant 09-358d).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1515657113/-/DCSupplemental.
References
- 1.Tononi G, Edelman GM, Sporns O. Complexity and coherency: Integrating information in the brain. Trends Cogn Sci. 1998;2(12):474–484. doi: 10.1016/s1364-6613(98)01259-5. [DOI] [PubMed] [Google Scholar]
- 2.Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13(5):336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 3.Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci. 2014;15(10):683–695. doi: 10.1038/nrn3801. [DOI] [PubMed] [Google Scholar]
- 4.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120(Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- 5.Kanwisher N. Functional specificity in the human brain: A window into the functional architecture of the mind. Proc Natl Acad Sci USA. 2010;107(25):11163–11170. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Varela F, Lachaux J-P, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2(4):229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 8.Singer W. Neuronal synchrony: A versatile code for the definition of relations? Neuron. 1999;24(1):49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 9.Canolty RT, et al. Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies. Proc Natl Acad Sci USA. 2010;107(40):17356–17361. doi: 10.1073/pnas.1008306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- 11.Brookes MJ, et al. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci USA. 2011;108(40):16783–16788. doi: 10.1073/pnas.1112685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA. 2007;104(32):13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillebrand A, Barnes GR, Bosboom JL, Berendse HW, Stam CJ. Frequency-dependent functional connectivity within resting-state networks: An atlas-based MEG beamformer solution. Neuroimage. 2012;59(4):3909–3921. doi: 10.1016/j.neuroimage.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb JT, Ferguson MA, Nielsen JA, Anderson JS. BOLD Granger causality reflects vascular anatomy. PLoS One. 2013;8(12):e84279. doi: 10.1371/journal.pone.0084279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsey JD, et al. Six problems for causal inference from fMRI. Neuroimage. 2010;49(2):1545–1558. doi: 10.1016/j.neuroimage.2009.08.065. [DOI] [PubMed] [Google Scholar]
- 17.Ito J, Nikolaev AR, van Leeuwen C. Spatial and temporal structure of phase synchronization of spontaneous alpha EEG activity. Biol Cybern. 2005;92(1):54–60. doi: 10.1007/s00422-004-0533-z. [DOI] [PubMed] [Google Scholar]
- 18.Nolte G, et al. Robustly estimating the flow direction of information in complex physical systems. Phys Rev Lett. 2008;100(23):234101. doi: 10.1103/PhysRevLett.100.234101. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Mashour GA, Noh GJ, Kim S, Lee U. Reconfiguration of network hub structure after propofol-induced unconsciousness. Anesthesiology. 2013;119(6):1347–1359. doi: 10.1097/ALN.0b013e3182a8ec8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Dellen E, et al. Decreased functional connectivity and disturbed directionality of information flow in the electroencephalography of intensive care unit patients with delirium after cardiac surgery. Anesthesiology. 2014;121(2):328–335. doi: 10.1097/ALN.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 21.van Straaten EC, et al. Disturbed phase relations in white matter hyperintensity based vascular dementia: an EEG directed connectivity study. Clin Neurophysiol. 2015;126(3):497–504. doi: 10.1016/j.clinph.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Moon JY, Lee U, Blain-Moraes S, Mashour GA. General relationship of global topology, local dynamics, and directionality in large-scale brain networks. PLOS Comput Biol. 2015;11(4):e1004225. doi: 10.1371/journal.pcbi.1004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stam CJ, van Straaten EC. Go with the flow: Use of a directed phase lag index (dPLI) to characterize patterns of phase relations in a large-scale model of brain dynamics. Neuroimage. 2012;62(3):1415–1428. doi: 10.1016/j.neuroimage.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 24.Osterhage H, Mormann F, Wagner T, Lehnertz K. Detecting directional coupling in the human epileptic brain: Limitations and potential pitfalls. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;77(1 Pt 1):011914. doi: 10.1103/PhysRevE.77.011914. [DOI] [PubMed] [Google Scholar]
- 25.Guevara R, et al. Phase synchronization measurements using electroencephalographic recordings: What can we really say about neuronal synchrony? Neuroinformatics. 2005;3(4):301–314. doi: 10.1385/NI:3:4:301. [DOI] [PubMed] [Google Scholar]
- 26.Pascual-Marqui RD, et al. Assessing direct paths of intracortical causal information flow of oscillatory activity with the isolated effective coherence (iCoh) Front Hum Neurosci. 2014;8:448. doi: 10.3389/fnhum.2014.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babiloni C, et al. White matter vascular lesions are related to parietal-to-frontal coupling of EEG rhythms in mild cognitive impairment. Hum Brain Mapp. 2008;29(12):1355–1367. doi: 10.1002/hbm.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blinowska KJ, Kuś R, Kamiński M. Granger causality and information flow in multivariate processes. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70(5 Pt 1):050902. doi: 10.1103/PhysRevE.70.050902. [DOI] [PubMed] [Google Scholar]
- 29.Baillet S, Mosher JC, Leahy RM. Electromagnetic brain mapping. IEEE Signal Process Mag. 2001;18(6):14–30. [Google Scholar]
- 30.Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp. 2005;25(2):199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereda E, Quiroga RQ, Bhattacharya J. Nonlinear multivariate analysis of neurophysiological signals. Prog Neurobiol. 2005;77(1-2):1–37. doi: 10.1016/j.pneurobio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Blinowska KJ. Review of the methods of determination of directed connectivity from multichannel data. Med Biol Eng Comput. 2011;49(5):521–529. doi: 10.1007/s11517-011-0739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenblatt RE, Pflieger ME, Ossadtchi AE. Connectivity measures applied to human brain electrophysiological data. J Neurosci Methods. 2012;207(1):1–16. doi: 10.1016/j.jneumeth.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakkalis V. Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput Biol Med. 2011;41(12):1110–1117. doi: 10.1016/j.compbiomed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Lobier M, Siebenhühner F, Palva S, Palva JM. Phase transfer entropy: A novel phase-based measure for directed connectivity in networks coupled by oscillatory interactions. Neuroimage. 2014;85(Pt 2):853–872. doi: 10.1016/j.neuroimage.2013.08.056. [DOI] [PubMed] [Google Scholar]
- 36.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 37.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 38.Buzsáki G. Neural syntax: Cell assemblies, synapsembles, and readers. Neuron. 2010;68(3):362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hebb DO. The Organization of Behavior. Wiley; New York: 1949. [DOI] [PubMed] [Google Scholar]
- 40.Tóth B, et al. Frontal midline theta connectivity is related to efficiency of WM maintenance and is affected by aging. Neurobiol Learn Mem. 2014;114:58–69. doi: 10.1016/j.nlm.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 41.von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38(3):301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 42.Edelman GM, Gally JA. Reentry: A key mechanism for integration of brain function. Front Integr Neurosci. 2013;7:63. doi: 10.3389/fnint.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clayton MS, Yeung N, Cohen Kadosh R. The roles of cortical oscillations in sustained attention. Trends Cogn Sci. 2015;19(4):188–195. doi: 10.1016/j.tics.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30(4):150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Bastos AM, et al. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron. 2015;85(2):390–401. doi: 10.1016/j.neuron.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 46.Frey JN, Ruhnau P, Weisz N. Not so different after all: The same oscillatory processes support different types of attention. Brain Res. 2015;1626:183–197. doi: 10.1016/j.brainres.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 47.Zheng C, Colgin LL. Beta and gamma rhythms go with the flow. Neuron. 2015;85(2):236–237. doi: 10.1016/j.neuron.2014.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirota A, et al. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60(4):683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: A gateway to memory and attention. Curr Opin Neurobiol. 2011;21(3):475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 50.van Kerkoerle T, et al. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci USA. 2014;111(40):14332–14341. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomasi D, Volkow ND. Association between functional connectivity hubs and brain networks. Cereb Cortex. 2011;21(9):2003–2013. doi: 10.1093/cercor/bhq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akam T, Kullmann DM. Oscillations and filtering networks support flexible routing of information. Neuron. 2010;67(2):308–320. doi: 10.1016/j.neuron.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Haan W, Mott K, van Straaten EC, Scheltens P, Stam CJ. Activity dependent degeneration explains hub vulnerability in Alzheimer’s disease. PLOS Comput Biol. 2012;8(8):e1002582. doi: 10.1371/journal.pcbi.1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schoffelen J-M, Gross J. Studying dynamic neural interactions with MEG. In: Supek S, Aine CJ, editors. Magnetoencephalography: From Signals to Dynamic Cortical Networks. Springer, New York; 2014. pp. 405–427. [Google Scholar]
- 55.Litvak V, et al. Optimized beamforming for simultaneous MEG and intracranial local field potential recordings in deep brain stimulation patients. Neuroimage. 2010;50(4):1578–1588. doi: 10.1016/j.neuroimage.2009.12.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 2006;51(7):1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- 57.Muthukumaraswamy SD, Singh KD. A cautionary note on the interpretation of phase-locking estimates with concurrent changes in power. Clin Neurophysiol. 2011;122(11):2324–2325. doi: 10.1016/j.clinph.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Akam T, Kullmann DM. Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nat Rev Neurosci. 2014;15(2):111–122. doi: 10.1038/nrn3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tewarie P, et al. Disruption of structural and functional networks in long-standing multiple sclerosis. Hum Brain Mapp. 2014;35(12):5946–5961. doi: 10.1002/hbm.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tewarie P, et al. Structural degree predicts functional network connectivity: A multimodal resting-state fMRI and MEG study. Neuroimage. 2014;97:296–307. doi: 10.1016/j.neuroimage.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 61.Tewarie P, et al. Functional brain networks: Linking thalamic atrophy to clinical disability in multiple sclerosis, a multimodal fMRI and MEG study. Hum Brain Mapp. 2015;36(2):603–618. doi: 10.1002/hbm.22650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whalen C, Maclin EL, Fabiani M, Gratton G. Validation of a method for coregistering scalp recording locations with 3D structural MR images. Hum Brain Mapp. 2008;29(11):1288–1301. doi: 10.1002/hbm.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiskopf N, et al. Unified segmentation based correction of R1 brain maps for RF transmit field inhomogeneities (UNICORT) Neuroimage. 2011;54(3):2116–2124. doi: 10.1016/j.neuroimage.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 65.Hillebrand A, Barnes GR. A quantitative assessment of the sensitivity of whole-head MEG to activity in the adult human cortex. Neuroimage. 2002;16(3 Pt 1):638–650. doi: 10.1006/nimg.2002.1102. [DOI] [PubMed] [Google Scholar]
- 66.Gong G, et al. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 2009;19(3):524–536. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson SE, Vrba J. Functional neuroimaging by Synthetic Aperture Magnetometry (SAM) In: Yoshimoto T, Kotani M, Kuriki S, Karibe H, Nakasato N, editors. Recent Advances in Biomagnetism. Tohoku Univ Press; Sendai, Japan: 1999. pp. 302–305. [Google Scholar]
- 68.Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng. 1997;44(9):867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- 69.Cheyne D, Bostan AC, Gaetz W, Pang EW. Event-related beamforming: A robust method for presurgical functional mapping using MEG. Clin Neurophysiol. 2007;118(8):1691–1704. doi: 10.1016/j.clinph.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 70.van Diessen E, et al. Opportunities and methodological challenges in EEG and MEG resting state functional brain network research. Clin Neurophysiol. 2015;126(8):1468–1481. doi: 10.1016/j.clinph.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 71.Stam CJ, Nolte G, Daffertshofer A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum Brain Mapp. 2007;28(11):1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiener N. The theory of prediction. In: Bechenbach E, editor. Modern Mathematics for the Engineer: First Series. McGraw-Hill; New York: 1956. pp. 165–190. [Google Scholar]
- 73.Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37(3):424–438. [Google Scholar]
- 74.Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27(3):379–423. [Google Scholar]
- 75.Schreiber T. Measuring information transfer. Phys Rev Lett. 2000;85(2):461–464. doi: 10.1103/PhysRevLett.85.461. [DOI] [PubMed] [Google Scholar]
- 76.Wibral M, et al. Transfer entropy in magnetoencephalographic data: quantifying information flow in cortical and cerebellar networks. Prog Biophys Mol Biol. 2011;105(1-2):80–97. doi: 10.1016/j.pbiomolbio.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 77.Staniek M, Lehnertz K. Symbolic transfer entropy. Phys Rev Lett. 2008;100(15):158101. doi: 10.1103/PhysRevLett.100.158101. [DOI] [PubMed] [Google Scholar]
- 78.Rosenblum MG, Pikovsky AS, Kurths J, Schäfer C, Tass P. 2001. Phase synchronization: From theory to data analysis. Neuro-Informatics and Neural Modeling, Handbook of Biological Physics, ed Hoff AJ (Elsevier, New York), Vol 4, pp 279–321. [DOI] [PubMed]
- 79.Kaminski M, Blinowska KJ. Directed Transfer Function is not influenced by volume conduction-inexpedient pre-processing should be avoided. Front Comput Neurosci. 2014;8:61. doi: 10.3389/fncom.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vinck M, et al. How to detect the Granger-causal flow direction in the presence of additive noise? Neuroimage. 2015;108:301–318. doi: 10.1016/j.neuroimage.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 81.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 82.Daunizeau J, David O, Stephan KE. Dynamic causal modelling: A critical review of the biophysical and statistical foundations. Neuroimage. 2011;58(2):312–322. doi: 10.1016/j.neuroimage.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 83.Friston KJ, Li B, Daunizeau J, Stephan KE. Network discovery with DCM. Neuroimage. 2011;56(3):1202–1221. doi: 10.1016/j.neuroimage.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamiński MJ, Blinowska KJ. A new method of the description of the information flow in the brain structures. Biol Cybern. 1991;65(3):203–210. doi: 10.1007/BF00198091. [DOI] [PubMed] [Google Scholar]
- 85.Baccalá LA, Sameshima K. Partial directed coherence: A new concept in neural structure determination. Biol Cybern. 2001;84(6):463–474. doi: 10.1007/PL00007990. [DOI] [PubMed] [Google Scholar]
- 86.Hadjipapas A, et al. Can we observe collective neuronal activity from macroscopic aggregate signals? Neuroimage. 2009;44(4):1290–1303. doi: 10.1016/j.neuroimage.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 87.Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25(2):249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- 88.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zobay O, Palmer AR, Hall DA, Sereda M, Adjamian P. Source space estimation of oscillatory power and brain connectivity in tinnitus. PLoS One. 2015;10(3):e0120123. doi: 10.1371/journal.pone.0120123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barnes GR, Hillebrand A. Statistical flattening of MEG beamformer images. Hum Brain Mapp. 2003;18(1):1–12. doi: 10.1002/hbm.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]