Significance
Similar to changes in DNA sequence, induced or naturally occurring variation in cytosine methylation can impact gene expression. How distinct methylation states of genes and transposons, called epialleles, emerge is not well understood. Here, we report that combining identical genomes with drastically different DNA methylation patterns in the same individual results in an epigenomic shock that is characterized by widespread changes in DNA methylation and gene expression. Many novel epialleles not found in the parents are formed at genes whereas transposons often experience decreased DNA methylation associated with transcriptional activation. Our work provides a scenario for the rapid and broad-scale emergence of epigenetic variation and may have implications for transposon dynamics within populations.
Keywords: DNA methylation, transcription, transposable elements, gene silencing, Arabidopsis
Abstract
Genes and transposons can exist in variable DNA methylation states, with potentially differential transcription. How these epialleles emerge is poorly understood. Here, we show that crossing an Arabidopsis thaliana plant with a hypomethylated genome and a normally methylated WT individual results, already in the F1 generation, in widespread changes in DNA methylation and transcription patterns. Novel nonparental and heritable epialleles arise at many genic loci, including a locus that itself controls DNA methylation patterns, but with most of the changes affecting pericentromeric transposons. Although a subset of transposons show immediate resilencing, a large number display decreased DNA methylation, which is associated with de novo or enhanced transcriptional activation and can translate into transposon mobilization in the progeny. Our findings reveal that the combination of distinct epigenomes can be viewed as an epigenomic shock, which is characterized by a round of epigenetic variation creating novel patterns of gene and TE regulation.
In eukaryotic genomes, cytosine methylation represents an epigenetic mark involved in the silencing of transposable elements (TEs), genes, and transgenes (1, 2). In plant genomes, TEs are typically silent and associated with dense DNA methylation in the three cytosine contexts CG, CHG, and CHH (where H is any base but G). Repression of gene transcription by DNA methylation often correlates with methylation of promoter sequences whereas transcriptionally active protein-coding genes tend to be methylated exclusively at CG positions in their bodies (3–6).
In the plant Arabidopsis thaliana (Arabidopsis), faithful propagation of CG methylation patterns upon de novo DNA synthesis during DNA replication is safeguarded by the DNA methyltransferase METHYLTRANSFERASE 1 (MET1), the plant homolog of human DNA methyltransferase 1, such that symmetrical CG sites in the genome are usually either fully methylated or not at all (7, 8). Maintenance of non-CG methylation is more complex and involves the partially redundant activities of the DNA methyltransferases DOMAINS REARRANGED METHYLTRANSFERASE 2 (DRM2), CHROMOMETHYLASE 2 (CMT2), and CHROMOMETHYLASE 3 (CMT3). The three proteins act in self-reinforcing methylation and silencing loops that also rely on histone H3 methylation at lysine 9 (H3K9me) and small RNAs of 24 nt in length (9–12). The interplay between chromatin and methylation is also apparent from the activity of the DECREASE IN DNA METHYLATION 1 (DDM1) chromatin remodeler, which seems to control access of methyltransferases to their H1-containing heterochromatic DNA targets (10).
Similar to changes in DNA sequence, differences in DNA methylation, either of natural, spontaneous origin or experimentally induced, can impact genome stability, gene expression, and phenotypic variation. Deficiencies in DDM1 induce drastic hypomethylation of heterochromatin at all cytosine contexts, resulting in transcriptional up-regulation of some heterochromatic genes, a massive activation of TE transcription, and mobilization of various TEs (10, 13–18). Elimination of MET1 in null met1 mutants causes a near-complete loss of CG methylation at genes and TEs, which is associated with misexpression of numerous protein-coding genes and broad transcriptional activation of TEs (3–5, 8). However, although transcriptionally activated, some autonomous TEs remain immobile, owing to other silencing mechanisms; for example, transposition of the Evadé (EVD) retrotransposon occurs only after several generations of inbreeding or in combinations of met1 with mutations in other silencing factors (2, 19, 20).
In addition to the loss of CG methylation, met1 mutants display substantial ectopic CHG and CHH hypermethylation at thousands of genes and TEs (4, 21, 22). Depending on the target, this aberrant methylation has been linked to erratic or mistargeting of RNA-directed DNA methylation (RdDM), transcriptional silencing of the gene encoding the REPRESSOR OF SILENCING 1 (ROS1) DNA demethylase, and misregulation of the INCREASE IN BONSAI METHYLATION 1 (IBM1) gene, which encodes an H3K9me2 demethylase that specifically targets protein-coding genes (22–24). IBM1 contains a large intron with a heterochromatin-like segment that is methylated at CG and non-CG sites. Lack of either type of methylation in this region disrupts the heterochromatin structure and is associated with impaired production of the long IBM1 transcript IBM1-L, which encodes the functional form of IBM1 (23, 25). In addition to the ROS1- and IBM1-mediated defects in met1 mutants, genes premarked with H3K27me3 tend to gain ectopic H3K9me2 and to be depleted in H3K27me3 in met1 whereas heterochromatic loci show depletion in H3K9me2 and ectopic H3K27me3 upon CG methylation loss (26, 27).
In ddm1 and met1 mutants, changes in DNA methylation generate epialleles without changes in DNA sequence. In a few examples, such epialleles have been associated with heritable differential transcription (7, 28–31). It has been long established that some ddm1- and met1-induced hypomethylated epialleles can be stably transmitted through several generations after outcrossing the causative mutation (13, 32). Similarly, hypermethylated epialleles can be transmitted across generations although they seem to be less stable than hypomethylated ones (29, 30, 33). To further analyze transgenerational epigenetic inheritance, segregation of allelic DNA methylation variants, or epialleles, was induced in epigenetic recombinant inbred lines (epiRILs) that were created by outcrossing either a met1 or a ddm1 hypomethylated parent plant to an isogenic WT plant (34, 35). Studies of the met1- or ddm1-derived epiRIL populations have confirmed overall stable inheritance of parental DNA methylation patterns but also have revealed some degree of epigenetic instability of parental epialleles. In ddm1-epiRILs, several TEs and single copy sequences inherited from the mutant parent undergo progressive DNA remethylation in a process requiring sRNA biogenesis (36) whereas, in later generations of some met1-epiRIL lines, nonparental methylation patterns can be detected, revealing the creation of further epigenetic novelty in these plants (34). Additionally, although EVD and CACTA TEs are immobile in both parents, proliferation of the two TEs was detected in a fraction of met1-derived epiRILs (19, 34), reinforcing the notion that parental epigenetic regulatory circuits have been altered in these lines.
Here, we examined the immediate consequences of combining the contrasting WT and met1 methylomes in F1 plants. Using whole genome DNA methylation analysis in combination with sequencing of mRNA and small RNA transcriptomes, we reveal widespread nonadditive variation in DNA methylation, TE silencing, and gene expression. These effects translate into the rapid emergence of novel heritable epigenetic variants and changes in silencing control and efficiency.
Results
A Novel Nonparental Heritable Hypomethylated IBM1 Epiallele in F1 Epihybrids.
We previously reported that proper IBM1 transcription requires the concomitant presence of CG and CHG methylation in the largest intron of the gene (23). To determine the role of MET1 in methylation of IBM1 intron sequences, we crossed a met1-3 mutant (hereafter referred to as met1) in a Col-0 background, which has reduced DNA methylation in this intron, to a Ler-0 MET1 WT (MET1+) plant. Not only was CG methylation of the met1-derived IBM1 allele not restored in F1 plants (23) (Fig. 1A), but there was a further, drastic loss of intronic CHG methylation (Fig. 1A). This hypomethylation was not due to parental effects of met1 because a drastic loss of CHG methylation was not seen upon selfing of met1 plants over several generations (Fig. 1B). These results indicated that the drastic loss of CHG methylation was a specific property of interaction between met1 and MET1+ genomes in F1 hybrid plants.
Fig. 1.
A nonparental IBM1 epiallele in F1 epihybrids. (A) DNA methylation at the large IBM1 intron, as determined by Sanger sequencing of bisulfite-treated DNA. Filled and empty circles indicate methylated and unmethylated cytosines, respectively; the color denotes sequence context (red, CG; blue, CHG; green, CHH). (B) CHG methylation of the large IBM1 intron, as determined by Sanger sequencing of bisulfite-treated DNA. Parental origin of IBM1 alleles in C×m and m×C F1 epihybrid plants was assigned based on presence/absence of CG methylation. (C) DNA methylation at the large IBM1 intron, as determined by CHG methylation-sensitive AluI digestion followed by PCR. For each genotype, PCR amplification was performed on digested (+) and undigested DNA (−). A region unmethylated in the WT and a region deprived of the AluI site were amplified as controls of digestion efficiency and DNA loading, respectively. (D) Quantitative RT-PCR analysis of the accumulation of IBM1 full-length mRNA (IBM1-L) in the indicated genotypes.
To eliminate effects caused by background genetic differences between the Col-0 met1 and the Ler-0 MET1+ parents, we outcrossed a met1 plant to an isogenic Col-0 MET1+ plant. Because the genomes of these parents are nearly identical but differ drastically in DNA methylation patterns, we named the resulting F1 plants “epihybrids.” MET1+ plants of the Col-0 and Ler-0 accessions showed similar DNA methylation patterns at the IBM1 intronic region (Fig. 1 A and B). Independent of the orientation of the cross [Col-0 × met1 (C×m) or met1 × Col-0 (m×C)], we again observed the emergence of a nonparental DNA methylation pattern at DNA sequences that were assigned to the met1-derived IBM1 allele based on the absence of CG methylation (Fig. 1 A and B). Similar results were obtained when we crossed the met1-7 allele in the Col-0 background to a MET1+ plant of the Van-0 accession (SI Appendix, Fig. S1A). Together, these results suggest that the appearance of the nonparental hypomethylated IBM1 epiallele in F1 epihybrids is linked to the difference in DNA methylation patterns between parental plants. The mechanism involved in CHG methylation loss remains to be determined, but it seems to be independent of IBM1 and ROS1 (SI Appendix, Fig. S2).
To assess heritability of the novel IBM1 epiallele, we determined IBM1 intron methylation in F2 progeny of a self-fertilized m×C F1 individual. As expected for Mendelian segregation, one quarter of the MET1+ F2 plants were homozygous for the hypomethylated IBM1 epiallele and accumulated only little IBM1-L transcript (Fig. 1 C and D). Additionally, absence of intronic methylation was inherited in F3 progeny of a self-fertilized MET1+ F2 individual homozygous for the hypomethylated IBM1 epiallele (SI Appendix, Fig. S1B). Therefore, outcrossing a hypomethylated met1 parent to a MET1+ plant induces the generation of a novel, nonparental hypomethylated IBM1 epiallele in F1 epihybrid progeny. The novel epiallele was stable through at least two generations, independently of the presence of the met1 mutation.
DNA Methylation Variation in F1 Epihybrids Mainly Occurs at Pericentromeric TEs.
Our observation at the IBM1 locus prompted us to examine the extent of DNA methylation variation in F1 epihybrid plants on a genome-wide scale. We performed whole-genome bisulfite sequencing in Col-0 MET1+ and met1 parents and in pools of both reciprocal F1 epihybrids. We combined the parental datasets to reconstruct a synthetic F1 sample (hereafter named rF1) where each genomic cytosine is methylated at the midparent value (MPV). Overall DNA methylation levels were similar in the two reciprocal F1 epihybrids and showed a substantial increase in CG methylation, decreased CHG methylation, and localized changes in CHH methylation compared with rF1 (Fig. 2A). Gain of CG methylation was prominent at pericentromeric regions and peaked at centromeres, where CHG and CHH methylation also showed the strongest deviation from rF1 levels (Fig. 2B). Consistently, TEs globally displayed increased CG methylation in F1 epihybrids relative to rF1 whereas CG gene-body methylation was at the expected rF1 level (SI Appendix, Fig. S3 A and B). These results indicate that most of the variation from the expected rF1 DNA methylation level occurs at pericentromeric regions of F1 epihybrids.
Fig. 2.
Global DNA methylation changes in F1 epihybrids. (A) Kernel density plots of DNA methylation differences between C×m or m×C epihybrids and the reconstructed F1 (rF1) at CG, CHG, and CHH contexts. (B) Mean methylation difference between C×m and rF1 in 100-kb tiles along chromosome 1 (Chr1). The “m” prefix denotes methyl.
By comparing the two reciprocal F1 epihybrids to rF1, we determined differentially methylated regions (DMRs) at any of the three cytosine sequence contexts significantly deviating from the mere 1:1 addition of parental methylomes. We also determined DMRs between the MET1+ and met1 parents. We defined a total of 9,892 F1 hypomethylated DMRs (hypo-DMRs), where the F1 epihybrids had lost DNA methylation, and 8,892 F1 hypermethylated DMRs (hyper-DMRs), where the F1 epihybrids had gained methylation compared with the synthetic rF1 (SI Appendix, Fig. S3C). Independent of their type, the vast majority of DMRs matched TE loci, and gains in CG methylation and losses in CHG methylation were the most abundant types of DMRs. In each case, more than 60% of the DMRs were common to reciprocal F1 epihybrids, indicating that most DNA methylation changes in epihybrids occur independently of cross orientation, similar to the creation of the hypomethylated IBM1 epiallele.
We previously showed that methylation patterns are unstable upon met1 inbreeding (22). To rule out the possibility that methylation changes in F1 epihybrids merely reflect the continuation of alterations present in the met1 parent used, we also profiled DNA methylation in progeny of the self-fertilized met1 parent. The vast majority of the F1 DMRs did not differ between the two successive met1 generations (SI Appendix, Fig. S4), indicating specificity in the methylation changes occurring in F1 epihybrids. To further account for interindividual variation, we profiled DNA methylation in five m×C F1 individual plants. Most of the F1 DMRs were also detected in these plants (SI Appendix, Fig. S4). Finally, we determined DNA methylation patterns in met1/+ F2 progeny of self-fertilized m×C F1 epihybrids. F1 and F2 showed comparable methylation at a large proportion of F1 non-CG DMRs (SI Appendix, Fig. S5A), indicating that methylation changes triggered in F1 epihybrids can be inherited in the next generation. In agreement with the requirement of MET1 for maintaining CG methylation during gametogenesis (8), CG methylation was reduced at protein-coding genes and TEs in F2 compared with F1 epihybrids (SI Appendix, Fig. S5B). Noticeably, we observed gene-body CHG hypermethylation at IBM1 target genes in the F2 (SI Appendix, Fig. S5 B and C), further confirming segregation of the lowly expressed hypomethylated IBM1 epiallele in F2 plants.
Ectopic CHG Methylation at Genes in F1 Epihybrids.
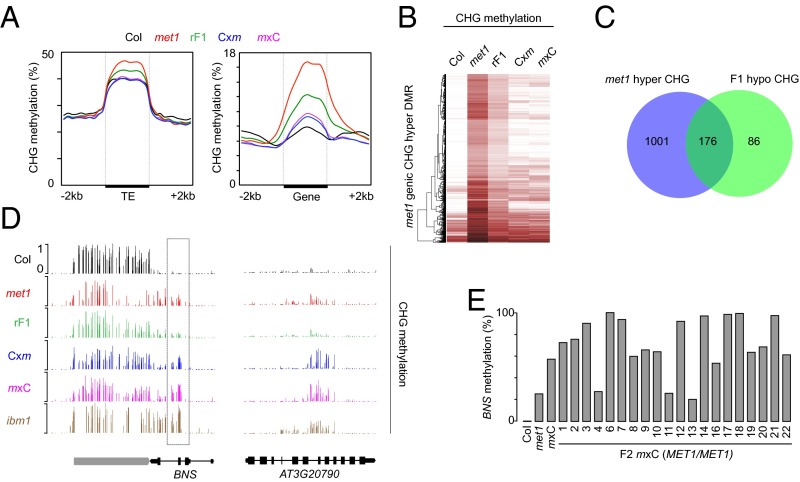
Thousands of genomic loci are ectopically hypermethylated at CHG positions in met1 mutants, in part due to misregulation of IBM1 and ROS1 (4, 22, 23, 26). Overall, genes, but not TEs, that are CHG-hypermethylated in met1 continued to have a higher CHG methylation level in F1 epihybrids compared with MET1+ plants (Fig. 3 A–C). Using published data (21), we defined genes ectopically methylated at CHG positions in ibm1 and in the ros1 dml2 dml3 (rdd) triple mutant, which lacks all three main DNA demethylases expressed in vegetative tissue. There was limited overlap between genes that are hypomethylated in F1, but hypermethylated in met1 and ibm1 or in met1 and rdd, indicating that normal methylation at IBM1 targets and RDD targets was mostly not restored in the F1 (SI Appendix, Fig. S6A). We previously demonstrated that restoring proper levels of functional IBM1 transcript accumulation in met1 resulted in removal of ectopic CHG methylation and H3K9me2 at selected genes (23). Also, we found that IBM1 and ROS1 were transcribed at a near MPV in F1 epihybrids (Fig. 1D and SI Appendix, Fig. S6B). Together, these data suggest that the amount of functional IBM1 produced in F1 epihybrids is insufficient to immediately restore normal methylation, thus contributing to persistent ectopic CHG methylation in F1. Furthermore, about 80% of the genes CHG-hypermethylated in met1, and not in either ibm1 or rdd, retained CHG methylation in F1 epihybrids (SI Appendix, Fig. S6A). Thus, whatever the cause of ectopic genic CHG hypermethylation in met1, this methylation largely persists in F1 plants.
Fig. 3.
Genic CHG methylation in epihybrids. (A) Average CHG methylation over TEs and genes containing at least one met1 CHG hypermethylation DMR. (B) Heat-map of CHG methylation levels within met1 CHG hypermethylation DMRs. Rows represent the DMRs, and the columns genotypes. (C) Venn diagram of the overlap between genes containing at least one CHG hypermethylation DMR in met1 and genes containing at least one CHG hypomethylation DMR in F1 epihybrids. (D) Genome-browser views of CHG methylation at the indicated loci. (E) The DNA methylation level at the BNS locus in indicated genotypes was determined by McrBC digestion followed by qPCR. The individual m×C F2 plants analyzed include the ones analyzed in Fig. 1 C and D.
We identified about 100 genes with CHG hypermethylation in each F1 epihybrid compared with either parent (SI Appendix, Fig. S7A). Although, at a small subset of genes, CHG hypermethylation seemed to be initiated specifically in F1 plants (SI Appendix, Fig. S7 A and B), most transgressive CHG hyper-DMRs already displayed a certain level of ectopic CHG methylation in the met1 parent (Fig. 3D and SI Appendix, Fig. S7A). About 60% of these genes were also CHG hypermethylated in ibm1 (Fig. 3D and SI Appendix, Fig. S7C), supporting the notion that insufficient IBM1 activity is a major contributor to transgressive CHG hypermethylation in F1 epihybrids. For example, BONSAI (BNS), the first identified IBM1 target (24), gained CHG methylation at the same positions in ibm1 and, to a lesser extent, in met1, and became further hypermethylated in F1 epihybrids (Fig. 3D). To determine which BNS allele was hypermethylated in F1 epihybrids, we self-pollinated m×C F1 plants, and assessed the BNS methylation state in MET1+ F2 individuals. We did not recover MET1+ F2 plants with MET1+-like BNS methylation levels. In about three-fourths of the F2 progeny, the methylation level was similar or higher than in the F1 epihybrid parent whereas the other quarter showed a met1-like level of methylation (Fig. 3E). These results suggest that the MET1+-derived BNS allele acquired CHG hypermethylation in F1 epihybrids, creating novel nonparental BNS epialleles. Intriguingly, some F2 individuals with high BNS CHG methylation levels had normal IBM1-L expression (Figs. 1D and 3E), suggesting that, unlike the original BNS allele in the MET1+ background, the novel CHG hypermethylated BNS epiallele formed in F1 epihybrids is largely insensitive to IBM1 activity. Similarly, ddm1-induced hypermethylated BNS epialleles are inherited after restoration of the DDM1 function (29).
Immediate CG Remethylation at TEs in F1 Epihybrids.
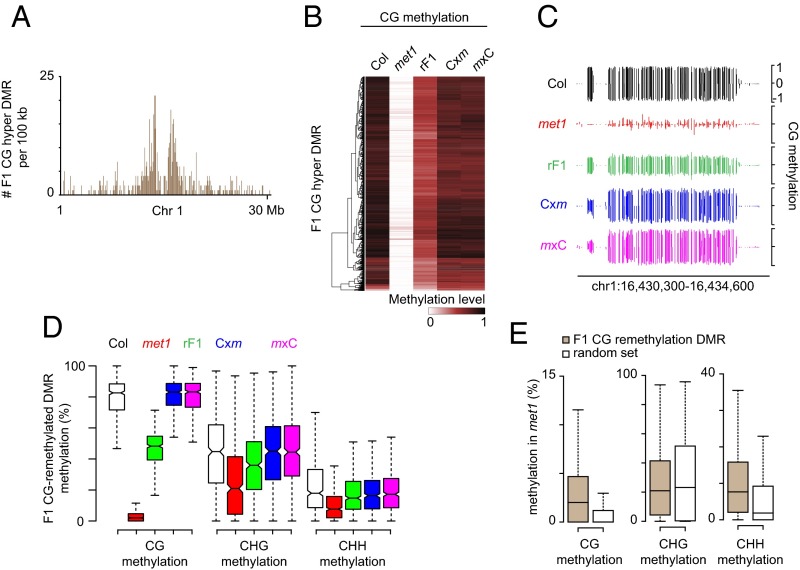
F1 CG hyper-DMRs mostly overlapped with TEs (2,138 TEs) (SI Appendix, Fig. S3C) preferentially located in pericentromeric regions (Fig. 4A). These genomic loci, which were highly CG-methylated in the MET1+ plants, had lost most of their CG methylation in met1 (Fig. 4B), indicating that F1 CG hyper-DMRs mostly reflect remethylation of the corresponding met1-derived TE epialleles. At about 25% of remethylated loci, CG methylation was fully restored to the MET1+ level in F1 epihybrids (Fig. 4 C and D). The relative distribution of TEs overlapping with fully remethylated F1 CG hyper-DMRs closely mirrored the distribution of all TEs in the Arabidopsis genome (SI Appendix, Fig. S8A), showing that CG remethylation in F1 epihybrids affects all types of pericentromeric TEs. Notably, compared with an equivalent set of random TE regions, CG-remethylated loci retained more CHH methylation and a substantial CG methylation level in the null met1-3 mutant (Fig. 4E), indicating that part of CG methylation at these TE sequences is independent of MET1 activity. Full restoration of CG methylation in F1 epihybrids was globally accompanied by reestablishment of CHG methylation whereas CHH methylation did not significantly deviate from the rF1 level (Fig. 4D).
Fig. 4.
CG remethylation in epihybrids. (A) Cumulative number of CG hypermethylation DMRs in F1 epihybrids in 100-kb tiles along chromosome 1. (B) Heat-map of CG methylation levels within CG hypermethylation DMRs in F1 epihybrids. (C) Genome-browser view of CG methylation at a representative genomic region displaying CG remethylation in F1 epihybrids. (D) Box plot of CG, CHG, and CHH methylation levels in F1 CG-remethylated DMRs. Values were extracted from one replicate per genotype. (E) Box plot of CG, CHG, and CHH methylation levels in F1 CG-remethylated DMRs and in a random set of sequences of equivalent type and length.
We also observed CHH hyper-DMRs in F1 epihybrids although these DMRs were less common than CG hyper-DMRs (SI Appendix, Fig. S3C). CHH hyper-DMRs were enriched at the edges of pericentromeric LTR/Gypsy retroelements and were associated with DNA hypermethylation in all cytosine contexts, with transgressive CHH and CHG methylation where the F1 had higher methylation than either parent (SI Appendix, Fig. S8). This hypermethylation is likely mediated by DRM2, which preferentially targets the edges of long retrotransposons (10).
Transdemethylation of TEs in F1 Epihybrids.
Hypomethylation in F1 epihybrids primarily affected TEs and was mostly due to changes at CHG and CHH positions, which often occurred concurrently (SI Appendix, Figs. S3C and S9A). VANDAL transposons were overrepresented among TEs with non-CG hypomethylation in F1 (SI Appendix, Fig. S9 B and C), which were characterized by very low DNA methylation in met1 mutants (Fig. 5A), indicating that DNA hypomethylation in F1 epihybrids affects TE copies inherited from the MET1+ parent.
Fig. 5.
Hypomethylation at TEs in epihybrids. (A) Average distribution of CG, CHG, and CHH methylation in indicated genotypes over VANDAL elements containing at least one F1 CHH and CHG hypo-DMR. (B) Distribution of CG hypomethylation DMR density over DNA/En-Spm elements in F1 epihybrids. (C) Genome-browser view of CG and CHG methylation at CAC1. Col and met1 parental methylation patterns are shown together with the rF1 profile subtracted from the C×m or the m×C F1 profiles. The dashed boxes show regions with CG and CHG hypomethylation in epihybrids. (D) PCR assay of CAC1 excision in indicated genotypes. Amplification of the “absence” band reflects mobilization of the TE. (E) CHG methylation levels at CHG hypomethylation DMRs that show transgressive behavior in F1 epihybrids. Values were extracted from one replicate per genotype. (F) CHG hypomethylation DMR density over LTR/Gypsy elements in F1 epihybrids.
A specific VANDAL21 element, Hi, transposes in ddm1 and met1 mutants (16, 18). The new Hi copies induce transdemethylation, mediated by the Hi-encoded VANC protein, having a high specificity for VANDAL21 family elements and being more pronounced at non-CG sites (18). In our material, Hi was hypomethylated in met1 at all cytosine contexts and associated with strong non-CG hypomethylation in F1 epihybrids (SI Appendix, Fig. S10A). We detected new Hi copies in met1 and in F1 plants (SI Appendix, Fig. S10B), supporting that transposed Hi copies may induce transdemethylation of VANDAL21 elements in F1 epihybrids (SI Appendix, Fig. S9C). Because many other elements from other VANDAL families were also strongly hypomethylated in F1 epihybrids (SI Appendix, Fig. S9C), we sought to determine whether this hypomethylation was due to the same type of transposition-dependent transdemethylation mechanism. We estimated TE genome-wide copy numbers by comparing the methyl-C DNA sequencing coverage of met1 and F1 epihybrid plants with that of the MET1+ parents. Validating this approach, we detected an increase in EVD and Hi copy number in the second generation of the met1 homozygous line (SI Appendix, Fig. S10C). Additionally, we detected more DNA copies of the ATCOPIA21 element AT5TE65370 in met1, previously found to be active only in advanced generations of ddm1 mutants (16) (SI Appendix, Fig. S10C). Importantly, this analysis did not reveal mobilization of other VANDAL elements in either met1 or F1 epihybrids (SI Appendix, Fig. S10D), suggesting that loss of DNA methylation occurring at MET1+-derived VANDAL elements in F1 epihybrids is neither necessarily caused by, nor associated with, transposition of TE members from the corresponding hypomethylated families. Considering the previously reported high specificity for Hi-induced transdemethylation, we conclude that production of the VANC anti-silencing factor from transcriptionally activated VANDAL elements in met1 is sufficient to induce transdemethylation of elements of the same family in F1 without requirement for transposition.
Transdemethylation Is Associated with TE Mobilization.
The CG hypomethylation of VANDAL elements was more pronounced in their 3′ halves, which contain the ORF encoding VANC (Fig. 5A). F1 CG hypo-DMRs also overlapped with DNA/En-Spm DNA transposons (SI Appendix, Fig. S9B), and CG hypomethylation was preferentially located near the borders of these elements (Fig. 5B). Studies of the maize Spm transposon have revealed that the transposon-encoded TnpA protein can induce DNA hypomethylation at TnpA binding sites that are located at the element ends, and promote its transcription (37, 38). The distribution of F1 CG hypo-DMRs suggests that a similar mechanism may operate at ATENSPM elements in F1 epihybrids. The En-Spm elements CACTA1 (CAC1) and CAC2 transpose in ddm1 mutants and ddm1-epiRILs (14, 16, 35). CACTA movement has also been detected in met1-epiRILs although the mobilized elements were not assigned to a specific element (34). CAC1 is mobilized in met1 cmt3 but not in met1, indicating that both CG and CHG methylation cooperate to repress CAC1 activity (20). In F1 epihybrids, CAC1 edges were hypomethylated at CG sites and also displayed transgressive CHG hypomethylation (Fig. 5C). Because CG methylation was completely lost in the met1 parent, this result indicates hypomethylation of the MET1+-derived CAC1 allele in the epihybrids. The hypomethylation of CAC1 at both CG and CHG sites in F1 epihybrids correlated with mobilization of this element in the F2 progeny (Fig. 5D). Together, these data show that DNA hypomethylation induced in F1 epihybrids, potentially involving the TE-encoded TnpA protein, can result in mobilization of elements that did not transpose in either parent.
Transgressive Hypomethylation of LTR/Gypsy Elements in F1 Epihybrids.
Transgressive CHG hypomethylation was specifically initiated in F1 epihybrids at one thousand genomic regions, which were highly methylated in both MET1+ and met1 parents (Fig. 5E and SI Appendix, Fig. S11A) and were overrepresented for long LTR/Gypsy elements (mean length of 6.4 kb) (SI Appendix, Fig. S11 B and C). Transgressive CHG hypomethylation preferentially occurred in internal coding regions of LTR/Gypsy TEs, with a bias toward the 3′ half of the elements (Fig. 5F), and it was accompanied by decreased CHH methylation within TE bodies, with little change at TE edges (SI Appendix, Fig. S11D). CMT2 targets the body of long TEs for CHH methylation, and to a lesser extent for CHG methylation (9, 10). CMT2 transcripts were reduced threefold in met1, potentially contributing to decreased non-CG methylation at long TEs whereas CMT2 transcripts accumulated at a midparental level in F1 plants (SI Appendix, Fig. S11E). Reduced CMT2 expression relative to MET1+ plants may be at the origin of CHH hypomethylation at internal sequences of long TEs in F1 epihybrids. Collectively, our results reveal widespread DNA hypomethylation at both DNA transposons and retroelements in F1 epihybrids.
Transcription Dynamics in F1 Epihybrids.
It is generally accepted that DNA methylation can influence RNA expression although the mechanisms are often unclear. Because of the profound modification of DNA methylation patterns occurring in F1 epihybrids, we determined how DNA methylation correlated with genome-wide mRNA profiles of F1 epihybrids and their parents. At the chromosomal level, the strongly increased transcript levels in pericentromeric regions were largely unchanged in F1 epihybrids (SI Appendix, Fig. S12A), extending previous reports of inheritance of met1-induced release of silencing at selected loci (7, 15, 34). Additional changes in transcript profiles indicated, however, that the silencing/desilencing states of many loci are not necessarily stably inherited by the F1. Overall gene transcript levels varied only modestly between parents and F1 epihybrids, independent of DNA methylation changes in F1 (Fig. 6A and SI Appendix, S12B). In contrast, TEs that were strongly activated in met1 were expressed at levels above the MPV in F1 epihybrids, and both TEs overlapping with F1 CG and CHG hypo-DMRs and F1 CHH hyper-DMRs showed increased expression relative to the MPV (Fig. 6A and SI Appendix, S12B). These data indicate that changes in DNA methylation in epihybrids are mostly associated with further derepression of TE silencing.
Fig. 6.
RNA expression in F1 epihybrids. (A) RNA expression at loci overlapping with different DMR types in F1 epihybrids relative to MPV. (B) Misregulated genes and TEs in met1 grouped according to their transcription level in epihybrids. Inter., intermediate. Only genes with similar expression in both reciprocal F1 epihybrids are shown. (C) Average distribution of CHG and CHH methylation over TEs activated in met1 and transcribed at a met1-like or higher level in F1 epihybrids. (D) Average number of different DMRs overlapping with TEs grouped according to their transcript level in epihybrids. Inter., intermediate. (E) Genome browser view of mRNA accumulation at ATCOPIA48 (AT1TE66380) in indicated genotypes. RNA levels in cmt3 and suvh4/5/6 are from ref. 45. (F) DNA methylation levels at the 5′ LTR of ATCOPIA48. (G) Genome-browser view of mRNA accumulation at EVD (AT5TE20395) and ATCOPIA21 (AT5TE65370) elements in indicated genotypes. (H) DNA methylation levels at the 5′ LTRs of EVD and ATCOPIA21 elements.
Combining two complementary approaches to determine differential expression between samples, we identified 3,606 up-regulated and 2,169 down-regulated loci in met1 compared with the MET1+ parent (SI Appendix, Fig. S12C), substantially adding to the number of met1-misexpressed loci previously identified (4–6, 39). The large majority (more than 85%) of loci misregulated in met1 behaved in a similar manner in both F1 epihybrids (Fig. 6B). Of the 1,576 protein-coding gene transcripts up-regulated in met1, about 40% accumulated at an intermediate level in epihybrids, indicating stable inheritance of met1-induced transcriptional derepression in epihybrids. This set of genes includes, and behaves similarly to, the archetype FWA gene, which remains transcriptionally derepressed and hypomethylated upon restoration of the MET1 function (7). Expression of another 40% of met1-derepressed genes was corrected in epihybrids (Fig. 6B), revealing efficient reestablishment of transcriptional repression. The remaining genes, about 240, continued to be up-regulated in F1 epihybrids at met1-like levels. Independently of their behavior in F1 epihybrids, genes up-regulated in met1 were rarely remethylated in F1 epihybrids (SI Appendix, Fig. S13A), indicating that restoration of normal expression levels does not necessarily depend on DNA methylation. There were two notable exceptions: SDC, a well-known target of DNA methylation-dependent silencing (40), and KELP (AT4G10920), which encodes a target of tobamoviruses (41), were efficiently resilenced in F1 epihybrids. Resilencing correlated with DNA remethylation of tandem repeats located in the promoter regions of both genes (SI Appendix, Fig. S14).
Similar to what we had observed for IBM1 and ROS1, expression of most met1–down-regulated genes was not restored to MET1+ levels in F1 epihybrids (Fig. 6B). Thus, similar to transcriptional activation, met1-induced transcriptional repression can be inherited. Transcriptional down-regulation in met1 was not associated with hypermethylation, and the F1 epihybrid methylation pattern at these genes was similar to the expected rF1, independent of their F1 epihybrid expression level (SI Appendix, Fig. S13B). These results indicate that transcriptional changes at these genes in F1 epihybrids mostly result from indirect effects rather than alterations of their DNA methylation patterns.
That resilencing of about 40% of genes up-regulated in met1 is independent of remethylation in F1 epihybrids indicates that the mechanism preventing formation of stable epialleles at these genes does not rely on DNA methylation. For the remaining genes that are up-regulated in met1, and for most met1-repressed genes, the met1 transcriptional state is stably inherited or exacerbated in F1 epihybrids. Reexamining published data (42), we found that only genes that were up-regulated in met1 but that were expressed at an intermediate or met1-like level in F1 epihybrids were enriched in H2A.Z in met1 (SI Appendix, Fig. S15), even though all genes equally lost CG methylation in met1 (SI Appendix, Fig. S13). This observation suggests that H2A.Z enrichment caused by CG methylation depletion (42, 43) possibly plays a role in stabilizing transcriptional derepression upon restoration of MET1 function in epihybrid plants. Additionally, because H2A.Z enrichment correlates with responsiveness of gene expression to environmental or developmental factors (43), these transcriptionally activated epialleles may acquire new sensitivity to such stimuli.
Further Release of TE Silencing in F1 Epihybrids.
In sharp contrast with genes, very few TEs derepressed in met1 were resilenced in F1 epihybrids: only 55 out of 1,921 (Fig. 6B). Unexpectedly, more than half of met1-activated TEs accumulated to met1-like or even higher levels in F1 epihybrids whereas only a third were expressed at MPV, reflecting stable inheritance of their altered transcriptional state in F1 epihybrids (Fig. 6B). For instance, the MET1+-derived, hypomethylated copies of Hi and CAC1 (Fig. 5C and SI Appendix, Fig. S10A) were expressed at met1-like levels in F1 epihybrids (SI Appendix, Fig. S16A), suggesting release of silencing at the corresponding MET1+-derived alleles in the F1 epihybrids.
Overall, TEs hyperactivated in F1 epihybrids were enriched for LTR retroelements, in particular pericentromeric LTR/Gypsy ATHILA retroelements (SI Appendix, Fig. S16B), which showed CHH hypomethylation in their body and strong transgressive CHG hypomethylation (Fig. 6 C and D). Transcript accumulation in F1 epihybrids was especially high for the 3′ half of LTR/Gypsy elements (SI Appendix, Fig. S16C), a region known as Transcriptionally Silent Information (TSI) at some ATHILA elements (44), which also displayed preferential transgressive CHG hypomethylation in F1 epihybrids (Fig. 5F). Besides LTR/Gypsy retroelements, several LTR/Copia retrotransposons, including ATCOPIA48 (AT1TE66380), were transgressively up-regulated in F1 epihybrids (Fig. 6E and SI Appendix, S16B). ATCOPIA48 LTRs are rich in CG and CHG sites, with average MET1+ methylation levels of 82% and 46% at the 11 CG and 17 CHG sites (Fig. 6F). ATCOPIA48 was expressed at a very low level in met1 despite loss of CG methylation, indicating that release of silencing in F1 epihybrids likely reflects alteration in an additional repressive mark. CHG methylation was reduced at the ATCOPIA48 5′ LTR in F1 epihybrids (Fig. 6F). Using published data (45), we found that ATCOPIA48 is strongly activated in cmt3 and suvh4/5/6 mutants (Fig. 6E), revealing that its silencing state depends on CHG methylation. Therefore, derepression of ATCOPIA48 in F1 epihybrids likely results from transgressive CHG hypomethylation. Collectively, our results show that silencing is further released at numerous TEs in F1 epihybrids in association with transgressive CHG hypomethylation.
Immediate Resilencing at TEs in F1 Epihybrids.
The few TEs with MET1+-like expression in F1 epihybrids were enriched for DMRs that were CG-hypermethylated relative to the rF1 (Fig. 6D), suggesting that resilencing at these elements relies on CG remethylation. Silencing of the EVD and ATCOPIA21 retroelements, which are expressed and mobilized in met1, was efficiently reestablished in F1 epihybrids (Fig. 6G and SI Appendix, Fig. S10C) although the EVD 5′ LTR, which is almost exclusively methylated at CG sites (19), was not remethylated in F1 epihybrids (Fig. 6H). ATCOPIA21 LTRs, which lack CG and CHG sites, have around 25% CHH methylation in MET1+, which is nearly lost in met1 mutants. Similar to EVD, ATCOPIA21 5′ LTRs were not remethylated in either reciprocal F1 epihybrid (Fig. 6H). Thus, these results reveal that resilencing of active autonomous TEs originally repressed by DNA methylation, and of their neocopies, can occur rapidly and independently of DNA remethylation. Future work needs to determine the molecular mechanism by which this resilencing occurs.
Changes in 24-nt sRNA Populations and DNA Methylation Changes in F1 Epihybrids.
In plants, small RNAs (sRNAs) are involved in the establishment of DNA methylation and part of its maintenance in asymmetric context. We analyzed small RNA populations, and monitored the activity of loci with differential 24-nt sRNA accumulation between parents in the F1 epihybrids. In met1, there were about twice as many regions of 24-nt sRNA over-accumulation (13,871) as of regions of decreased 24-nt sRNA abundance (6,582) (SI Appendix, Fig. S17A), with the magnitude of loss, on average 99-fold, being much more severe than the magnitude of gain, 13-fold, accounting for the reduction in 24-nt sRNAs in met1 (SI Appendix, Fig. S17B). The levels of 24-nt sRNAs deviated at a few regions from the MPV; regions with reduced 24-nt sRNA accumulation were enriched for MuDR TEs, and regions with increased accumulation for LTR/Gypsy TEs (SI Appendix, Fig. S17 A and C).
DNA hypermethylation in F1 epihybrids was associated with increased, often transgressive 24-nt sRNA levels in epihybrids (SI Appendix, Fig. S18A). Conversely, DMRs that were hypomethylated in F1 epihybrids accumulated few 24-nt sRNAs in both met1 and in F1 epihybrids, suggesting that 24-nt sRNA production becomes impaired at the corresponding MET1+-derived alleles in F1 epihybrids (SI Appendix, Fig. S18B). For instance, VANDAL elements that are hypomethylated in F1 epihybrids featured drastically reduced levels of 24-nt sRNA in epihybrid plants (SI Appendix, Fig. S18D). These results show that DNA methylation changes at TEs in F1 epihybrids are often associated with changes in 24-nt local sRNA accumulation.
In contrast to TE hypermethylation, CHG hypermethylation at genes in F1 epihybrids, including IBM1 targets BNS and AT3G20790, was not associated with accumulation of sRNAs, either in the parents or in F1 epihybrids (SI Appendix, Fig. S19). Therefore, hypermethylation at these genes is likely caused by impaired IBM1 activity and/or H3K9me2 enrichment, rather than by an sRNA-mediated process, although sRNAs might be transitorily produced and required for DNA methylation during an earlier developmental window. Similarly, ddm1-induced BNS DNA hypermethylation depends on H3K9me2 and not on sRNAs (46).
Differential Inheritance of met1-Induced easiRNA Production in F1 Epihybrids.
When transcriptionally activated, a number of TEs generate 21-nt epigenetically activated siRNAs, or easiRNAs (4, 47–49). met1 leaves, similar to flower buds (4), were characterized by a global overaccumulation of 21-nt sRNAs matching TEs, suggesting that production of easiRNAs also takes place in the met1 sporophyte (SI Appendix, Figs. S17B and S20A). Regions of increased abundance of 21-nt sRNAs in met1 leaves mostly overlapped with TEs from the LTR/Gypsy and En-Spm superfamilies (SI Appendix, Fig. S20B). Only a few 21-nt sRNA loci that deviated significantly from the MPV were identified in F1 epihybrids (SI Appendix, Fig. S20A), suggesting that easiRNA production from met1-derived TE alleles is generally inherited in F1 plants. However, out of the 42 En-Spm elements producing easiRNA in met1, easiRNA accumulation in reciprocal F1 epihybrids was reduced at 32 elements, half of which being ATENSPM5 elements (SI Appendix, Fig. S20 A and C).
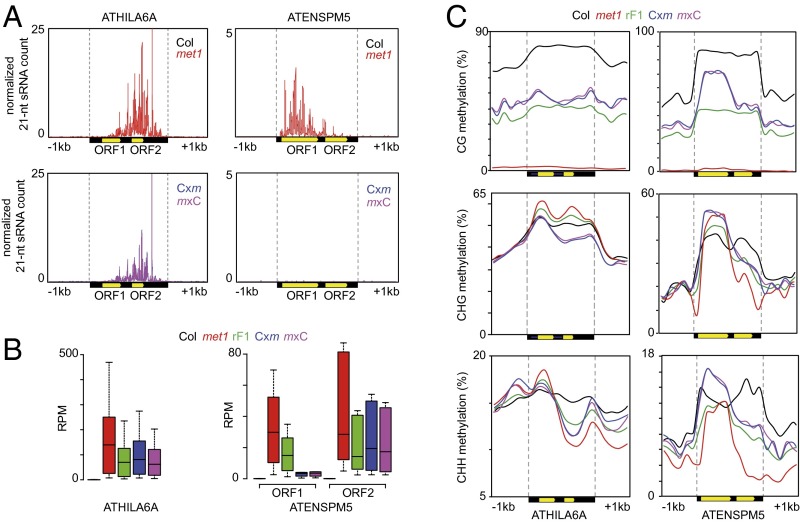
Similar to ddm1 inflorescences (50), the ATHILA6A and ATHILA2 LTR-retrotransposon families made up the bulk of easiRNAs in met1 leaves whereas ATENSPM5 was the top DNA TE family (SI Appendix, Fig. S20C). Accumulation of easiRNAs was biased toward the 3′ TSI part of ATHILA6A elements and toward the transposase-encoding ORF1 of ATENSPM5 elements (Fig. 7A). Production of easiRNAs from these TEs was, however, radically different in F1 epihybrids. Although the overall ATHILA6A easiRNA levels were near the MPV, ATENSPM5 elements accumulated virtually no easiRNAs in F1 epihybrids (Fig. 7A). The loss of easiRNA was associated with efficient resilencing of the ATENSPM5 ORF1 in F1 epihybrids whereas the second ORF, which produced no or only very few easiRNA in met1, remained transcriptionally active (Fig. 7 A and B). At ATHILA6A elements, the 3′ TSI region was strongly derepressed in met1 and remained transcribed at an MPV in F1 epihybrids (Fig. 7B). Resilencing of ATENSPM5 ORF1 in F1 epihybrids correlated with DNA hypermethylation in all cytosine contexts and with increased 24-nt sRNA accumulation (Fig. 7C and SI Appendix, Fig. S20D). ATHILA6A elements, which accumulated high levels of 24-nt sRNA in met1 and F1 epihybrids, were instead hypomethylated at CHG sites in epihybrids (Fig. 7C and SI Appendix, Fig. S20D). EasiRNAs have previously been implicated in reestablishment of silencing at TEs after ddm1-induced transcriptional activation and restoration of functional DDM1 protein by outcrossing (50). Together, our results demonstrate a complex relationship between easiRNA and/or 24-nt sRNAs produced from different TEs activated in met1, DNA methylation, and resilencing in F1 epihybrids. At the transposase-encoding ORF1 of ATENSPM5, easiRNAs can likely efficiently initiate DNA hypermethylation early during development of F1 epihybrids. Upon reestablishment of silencing, DNA hypermethylation leads to high 24-nt sRNA production, which then takes over to ensure the maintenance of silencing throughout development. We note that ATHILA elements, which are not resilenced in F1 epihybrids, are nonautonomous retrotransposons whereas most easiRNA-producing ATENSPM5 elements are long intact DNA TEs.
Fig. 7.
The 21-nt sRNA accumulation, mRNA, and DNA methylation levels at ATHILA6A and ATENSPM5 elements. (A) Accumulation of 21-nt sRNAs along ATHILA6A and ATENSPM5 elements in parent (Top) and F1 epihybrids (Bottom). The two ORFs in both TEs are depicted as yellow arrows. (B) Box plots of mRNA accumulation at ATHILA6A (Left) and the two ATENSPM5 ORFs (Right) in indicated genotypes. (C) Average distribution of CG, CHG, and CHH methylation in indicated genotypes over ATHILA6A and ATENSPM5 elements producing 21-nt sRNAs in met1.
Discussion
When normally methylated WT chromosomes and extensively demethylated met1 chromosomes meet in F1 epihybrids, an epigenomic shock is triggered that is characterized by transgressive DNA methylation and gene expression. Most of the deviation from MPV in F1 epihybrids is independent of the direction of the cross. The epigenomic shock results in the creation of new nonparental epialleles at protein-coding genes, which can have reduced or increased non-CG methylation and expression. Particularly notable is the formation of a new nonparental hypomethylated, stable IBM1 epiallele with low expression. A quarter of the previously described met1-epiRIL lines, which are MET1+ progeny of self-pollinated F1 epihybrids (34), should be homozygous for this IBM1 epiallele. Because IBM1 protects several thousand genes from H3K9me2 and associated CHG methylation, the nonparental IBM1 epiallele likely contributes to the appearance of nonparental genome-wide methylation patterns that have been observed in these lines.
Most of the deviation from MPV in F1 epihybrids occurs at TEs. Many pericentromeric elements, including many Helitrons (SI Appendix, Fig. S8A), experience immediate CG remethylation in F1 epihybrids. At these elements, DNA methylation, including CG methylation, is likely tightly dependent on sRNAs because these loci are associated with high levels of 24-nt sRNAs and retain a certain level of CG methylation even in the met1-3 null mutant. However, CG remethylation is not observed at all loci associated with high levels of 24-nt sRNAs, suggesting that remethylated loci must have specific properties. Immediate CG remethylation in epihybrids, potentially involving sRNA-directed MET1-dependent de novo methylation (51), may be stimulated by remaining CG methylation at the met1-derived alleles and/or by specific sequence motifs or chromatin features. Either way, immediate CG remethylation in epihybrids prevents persistence of stable hypomethylated epialleles at these loci. Previous analyses of met1-derived epiRILs in the F9 generation have suggested active remethylation of centromeric DNA at CG and/or CHG sites (34). Our results support this conclusion and further reveal that pericentromeric regions are efficiently targeted for rapid CG remethylation as soon as MET1 activity is restored in the F1 epihybrids. In contrast, DNA remethylation of TEs located throughout the genome occurred only over several generations in ddm1-epiRILs (36), indicating that remethylation after outcrossing of met1 or ddm1 mutations involves at least partly distinct mechanisms. That parental CG methylation patterns within gene bodies are overall faithfully inherited in F1 epihybrids (SI Appendix, Fig. S3B) suggests the absence of a corrective mechanism that can reestablish gene-body CG methylation. It is possible that remethylation eventually occurs, but only after multiple generations.
Despite DNA remethylation, only few met1-activated TEs are resilenced in F1 epihybrids, and the two LTR retrotransposons EVD and ATCOPIA21, which are active in the met1 parent, are transcriptionally resilenced in F1 epihybrids independently of DNA remethylation of their LTRs. Therefore, the epigenomic shock also triggers the activity of an enigmatic silencing mechanism that was not active in the parents. Whether EVD and ATCOPIA21 acquire the same silencing state in F1 epihybrids and what the molecular nature of this state is remain to be determined. Restoration of silencing at EVD in epihybrids is surprising, given that EVD remains mobile in late generations of some met1 epiRIL lines (19), suggesting that the silenced state established in F1 epihybrids can be easily reversed.
In F1 epihybrids, DNA hypomethylation at numerous TEs is associated with enhanced release of silencing or transcriptional activation. At some, including VANDAL and CACTA superfamily members, hypomethylation in F1 epihybrids likely initiates through transdemethylation of MET1+-derived TEs, mediated by TE-encoded factors produced from the active met1-inherited elements. We also detected transgressive CHG hypomethylation at numerous TEs in F1 epihybrids, especially at many LTR/Gypsy ATHILA elements, which are highly CHG-methylated in both MET1+ and met1 parents. It has been previously shown that only when TEs are transcriptionally activated can they become targets of IBM1-mediated demethylation of H3K9me2, resulting in CHG hypomethylation and further transcriptional activation (52). Accordingly, forced IBM1 expression in met1 leads to transcriptional overactivation of some TEs (23). IBM1 expression is compromised in met1, and, despite high H3K9me2 and CHG methylation levels, these TEs are active. We propose that, in F1 epihybrids, active transcription of these TEs may preferentially recruit the limited amount of IBM1 available from the MET1+-derived IBM1 allele, resulting in H3K9me2 and CHG demethylation and thus further transcriptional derepression. Supporting this view, TEs expressed at or above met1-levels in F1 epihybrids, which are overrepresented for ATHILA elements, had a higher-than-average density of CHG sites (SI Appendix, Fig. S16D), a feature known to affect sensitivity to ibm1 mutations (52). Long TEs are also CHH-hypomethylated in F1 epihybrids, especially in their center, which may be caused by reduced CMT2 activity. Because CMT2 mediates CHH methylation through binding to H3K9me2 (9), CMT2 recruitment to these TEs may be impaired due to the initial reduction in H3K9me2 and CHG methylation. Additionally, because CHH methylation is strongly required for H3K9me2 at CMT2-target sites (9), defective CMT2 targeting may contribute to the transgressive reduction in CHG methylation at long TEs in F1 epihybrids. Our data support a scenario in which transcriptional up-regulation of ATHILA TEs with transgressive CHG hypomethylation in epihybrids would occur at already transcriptionally active TE copies inherited from the met1 parent. Transcriptional activation of ATHILA elements has been observed in interspecific Arabidopsis thaliana × arenosa hybrids (53). Supporting our hypothesis, only ATHILA elements contributed by the Arabidopsis arenosa parent, which already show a low level of expression in this species, are up-regulated in these hybrids (53).
Intraspecific F1 hybrids derived from crosses between the A. thaliana accessions C24 and Ler deviate from MPV DNA methylation at numerous loci, along with a global decrease in 24-nt sRNA accumulation (54, 55). In these hybrids, mostly genic and gene-proximal regions are affected, in sharp contrast with the situation in our F1 epihybrids, in which the majority of DNA methylation effects are seen at TEs. Transgressive DNA methylation was, however, not found in C24 × Ler F1 hybrids, suggesting that the epigenomic shock is triggered only when parental chromosomes differ markedly in DNA methylation level, and especially at TEs. TE methylation is likely similar between C24 and Ler because previous studies have pointed out that methylation of TE sequences is highly stable, both across generations and between A. thaliana accessions (56–59).
It has been suggested that both genetic and epigenetic differences between parents contribute to hybrid vigor, or heterosis. Although met1 and MET1+ plants differ drastically in DNA methylation, sRNA accumulation, and gene expression, the F1 epihybrids are not obviously superior in biomass or rosette diameter (SI Appendix, Fig. S21). We have not yet measured other traits, such as pathogen or stress tolerance, but this observation already calls into question simplistic models for the contribution of epigenetic differences to heterosis.
Various stresses or environmental conditions can modulate patterns of DNA methylation and/or activate TE transcription (39, 60). Further, mutations altering the function of DNA methylation regulators may spontaneously arise in natural populations, generating individuals with partially hypomethylated genomes and even activated TEs (61–63). Although A. thaliana is a mainly self-fertilizing species, its effective outcrossing rate varies considerably between different stands and can be higher than 10% (64). Thus, it is plausible that epigenomic shocks, although likely less drastic than the one we report here, may occur in the wild and generate novel heritable epigenetic diversity and associated regulatory patterns. Given their impact on TEs, such shocks may also have important implications for genome-wide TE dynamics.
Materials and Methods
Second generation met1-3 mutant plants were used in this study (8). All experiments were performed starting from 3-wk-old leaf material. An extended description of materials and methods, including poly(A)-RNA sequencing, whole-genome bisulfite sequencing, small RNA sequencing, RT-PCR analysis, and locus-specific DNA methylation analysis is provided in SI Appendix, SI Materials and Methods. Sequencing data generated in this study have been deposited with the European Nucleotide Archive (ENA, www.ebi.ac.uk/ena) under accession number PRJEB9919.
Supplementary Material
Acknowledgments
This work was supported by the Max Planck Society and Deutsche Forschungsgemeinschaft SFB 1101 (to D.W.), the Centre National de la Recherche Scientifique (O.M.), the European Community’s Seventh Framework Programme through a Starting Independent Research Grant from the European Research Council (I2ST, 260742) (to O.M.), and a Young Researcher Grant from the Auvergne Regional Council (to O.M.). O.M. is a young investigator of the European Molecular Biology Organization (EMBO).
Footnotes
The authors declare no conflict of interest.
Data deposition: Sequencing data generated in this study have been deposited with the European Nucleotide Archive (ENA), www.ebi.ac.uk/ena (accession no. PRJEB9919).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600672113/-/DCSupplemental.
References
- 1.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11(3):204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rigal M, Mathieu O. A “mille-feuille” of silencing: Epigenetic control of transposable elements. Biochim Biophys Acta. 2011;1809(8):452–458. doi: 10.1016/j.bbagrm.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Cokus SJ, et al. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452(7184):215–219. doi: 10.1038/nature06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lister R, et al. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133(3):523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in arabidopsis. Cell. 2006;126(6):1189–1201. doi: 10.1016/j.cell.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Zilberman D, Gehring M, Tran RK, Ballinger T, Henikoff S. Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet. 2007;39(1):61–69. doi: 10.1038/ng1929. [DOI] [PubMed] [Google Scholar]
- 7.Kankel MW, et al. Arabidopsis MET1 cytosine methyltransferase mutants. Genetics. 2003;163(3):1109–1122. doi: 10.1093/genetics/163.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saze H, Mittelsten Scheid O, Paszkowski J. Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat Genet. 2003;34(1):65–69. doi: 10.1038/ng1138. [DOI] [PubMed] [Google Scholar]
- 9.Stroud H, et al. Non-CG methylation patterns shape the epigenetic landscape in Arabidopsis. Nat Struct Mol Biol. 2014;21(1):64–72. doi: 10.1038/nsmb.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zemach A, et al. The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell. 2013;153(1):193–205. doi: 10.1016/j.cell.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du J, et al. Mechanism of DNA methylation-directed histone methylation by KRYPTONITE. Mol Cell. 2014;55(3):495–504. doi: 10.1016/j.molcel.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du J, et al. Dual binding of chromomethylase domains to H3K9me2-containing nucleosomes directs DNA methylation in plants. Cell. 2012;151(1):167–180. doi: 10.1016/j.cell.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vongs A, Kakutani T, Martienssen RA, Richards EJ. Arabidopsis thaliana DNA methylation mutants. Science. 1993;260(5116):1926–1928. doi: 10.1126/science.8316832. [DOI] [PubMed] [Google Scholar]
- 14.Miura A, et al. Mobilization of transposons by a mutation abolishing full DNA methylation in Arabidopsis. Nature. 2001;411(6834):212–214. doi: 10.1038/35075612. [DOI] [PubMed] [Google Scholar]
- 15.Lippman Z, May B, Yordan C, Singer T, Martienssen R. Distinct mechanisms determine transposon inheritance and methylation via small interfering RNA and histone modification. PLoS Biol. 2003;1(3):E67. doi: 10.1371/journal.pbio.0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsukahara S, et al. Bursts of retrotransposition reproduced in Arabidopsis. Nature. 2009;461(7262):423–426. doi: 10.1038/nature08351. [DOI] [PubMed] [Google Scholar]
- 17.Singer T, Yordan C, Martienssen RA. Robertson’s Mutator transposons in A. thaliana are regulated by the chromatin-remodeling gene Decrease in DNA Methylation (DDM1) Genes Dev. 2001;15(5):591–602. doi: 10.1101/gad.193701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y, et al. Mobilization of a plant transposon by expression of the transposon-encoded anti-silencing factor. EMBO J. 2013;32(17):2407–2417. doi: 10.1038/emboj.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirouze M, et al. Selective epigenetic control of retrotransposition in Arabidopsis. Nature. 2009;461(7262):427–430. doi: 10.1038/nature08328. [DOI] [PubMed] [Google Scholar]
- 20.Kato M, Miura A, Bender J, Jacobsen SE, Kakutani T. Role of CG and non-CG methylation in immobilization of transposons in Arabidopsis. Curr Biol. 2003;13(5):421–426. doi: 10.1016/s0960-9822(03)00106-4. [DOI] [PubMed] [Google Scholar]
- 21.Stroud H, Greenberg MV, Feng S, Bernatavichute YV, Jacobsen SE. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell. 2013;152(1-2):352–364. doi: 10.1016/j.cell.2012.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathieu O, Reinders J, Caikovski M, Smathajitt C, Paszkowski J. Transgenerational stability of the Arabidopsis epigenome is coordinated by CG methylation. Cell. 2007;130(5):851–862. doi: 10.1016/j.cell.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Rigal M, Kevei Z, Pélissier T, Mathieu O. DNA methylation in an intron of the IBM1 histone demethylase gene stabilizes chromatin modification patterns. EMBO J. 2012;31(13):2981–2993. doi: 10.1038/emboj.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saze H, Shiraishi A, Miura A, Kakutani T. Control of genic DNA methylation by a jmjC domain-containing protein in Arabidopsis thaliana. Science. 2008;319(5862):462–465. doi: 10.1126/science.1150987. [DOI] [PubMed] [Google Scholar]
- 25.Saze H, et al. Mechanism for full-length RNA processing of Arabidopsis genes containing intragenic heterochromatin. Nat Commun. 2013;4:2301. doi: 10.1038/ncomms3301. [DOI] [PubMed] [Google Scholar]
- 26.Deleris A, et al. Loss of the DNA methyltransferase MET1 Induces H3K9 hypermethylation at PcG target genes and redistribution of H3K27 trimethylation to transposons in Arabidopsis thaliana. PLoS Genet. 2012;8(11):e1003062. doi: 10.1371/journal.pgen.1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathieu O, Probst AV, Paszkowski J. Distinct regulation of histone H3 methylation at lysines 27 and 9 by CpG methylation in Arabidopsis. EMBO J. 2005;24(15):2783–2791. doi: 10.1038/sj.emboj.7600743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stokes TL, Kunkel BN, Richards EJ. Epigenetic variation in Arabidopsis disease resistance. Genes Dev. 2002;16(2):171–182. doi: 10.1101/gad.952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saze H, Kakutani T. Heritable epigenetic mutation of a transposon-flanked Arabidopsis gene due to lack of the chromatin-remodeling factor DDM1. EMBO J. 2007;26(15):3641–3652. doi: 10.1038/sj.emboj.7601788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobsen SE, Meyerowitz EM. Hypermethylated SUPERMAN epigenetic alleles in arabidopsis. Science. 1997;277(5329):1100–1103. doi: 10.1126/science.277.5329.1100. [DOI] [PubMed] [Google Scholar]
- 31.Soppe WJ, et al. The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol Cell. 2000;6(4):791–802. doi: 10.1016/s1097-2765(05)00090-0. [DOI] [PubMed] [Google Scholar]
- 32.Kakutani T, Munakata K, Richards EJ, Hirochika H. Meiotically and mitotically stable inheritance of DNA hypomethylation induced by ddm1 mutation of Arabidopsis thaliana. Genetics. 1999;151(2):831–838. doi: 10.1093/genetics/151.2.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobsen SE, Sakai H, Finnegan EJ, Cao X, Meyerowitz EM. Ectopic hypermethylation of flower-specific genes in Arabidopsis. Curr Biol. 2000;10(4):179–186. doi: 10.1016/s0960-9822(00)00324-9. [DOI] [PubMed] [Google Scholar]
- 34.Reinders J, et al. Compromised stability of DNA methylation and transposon immobilization in mosaic Arabidopsis epigenomes. Genes Dev. 2009;23(8):939–950. doi: 10.1101/gad.524609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johannes F, et al. Assessing the impact of transgenerational epigenetic variation on complex traits. PLoS Genet. 2009;5(6):e1000530. doi: 10.1371/journal.pgen.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teixeira FK, et al. A role for RNAi in the selective correction of DNA methylation defects. Science. 2009;323(5921):1600–1604. doi: 10.1126/science.1165313. [DOI] [PubMed] [Google Scholar]
- 37.Cui H, Fedoroff NV. Inducible DNA demethylation mediated by the maize Suppressor-mutator transposon-encoded TnpA protein. Plant Cell. 2002;14(11):2883–2899. doi: 10.1105/tpc.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schläppi M, Raina R, Fedoroff N. Epigenetic regulation of the maize Spm transposable element: Novel activation of a methylated promoter by TnpA. Cell. 1994;77(3):427–437. doi: 10.1016/0092-8674(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 39.Dowen RH, et al. Widespread dynamic DNA methylation in response to biotic stress. Proc Natl Acad Sci USA. 2012;109(32):E2183–E2191. doi: 10.1073/pnas.1209329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson IR, Jacobsen SE. Tandem repeats upstream of the Arabidopsis endogene SDC recruit non-CG DNA methylation and initiate siRNA spreading. Genes Dev. 2008;22(12):1597–1606. doi: 10.1101/gad.1667808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsushita Y, Deguchi M, Youda M, Nishiguchi M, Nyunoya H. The tomato mosaic tobamovirus movement protein interacts with a putative transcriptional coactivator KELP. Mol Cells. 2001;12(1):57–66. [PubMed] [Google Scholar]
- 42.Zilberman D, Coleman-Derr D, Ballinger T, Henikoff S. Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature. 2008;456(7218):125–129. doi: 10.1038/nature07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coleman-Derr D, Zilberman D. Deposition of histone variant H2A.Z within gene bodies regulates responsive genes. PLoS Genet. 2012;8(10):e1002988. doi: 10.1371/journal.pgen.1002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steimer A, et al. Endogenous targets of transcriptional gene silencing in Arabidopsis. Plant Cell. 2000;12(7):1165–1178. doi: 10.1105/tpc.12.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stroud H, et al. DNA methyltransferases are required to induce heterochromatic re-replication in Arabidopsis. PLoS Genet. 2012;8(7):e1002808. doi: 10.1371/journal.pgen.1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki T, Kobayashi A, Saze H, Kakutani T. RNAi-independent de novo DNA methylation revealed in Arabidopsis mutants of chromatin remodeling gene DDM1. Plant J. 2012;70(5):750–758. doi: 10.1111/j.1365-313X.2012.04911.x. [DOI] [PubMed] [Google Scholar]
- 47.Slotkin RK, et al. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell. 2009;136(3):461–472. doi: 10.1016/j.cell.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanurdzic M, et al. Epigenomic consequences of immortalized plant cell suspension culture. PLoS Biol. 2008;6(12):2880–2895. doi: 10.1371/journal.pbio.0060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Creasey KM, et al. miRNAs trigger widespread epigenetically activated siRNAs from transposons in Arabidopsis. Nature. 2014;508(7496):411–415. doi: 10.1038/nature13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nuthikattu S, et al. The initiation of epigenetic silencing of active transposable elements is triggered by RDR6 and 21-22 nucleotide small interfering RNAs. Plant Physiol. 2013;162(1):116–131. doi: 10.1104/pp.113.216481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aufsatz W, Mette MF, Matzke AJ, Matzke M. The role of MET1 in RNA-directed de novo and maintenance methylation of CG dinucleotides. Plant Mol Biol. 2004;54(6):793–804. doi: 10.1007/s11103-004-0179-1. [DOI] [PubMed] [Google Scholar]
- 52.Inagaki S, et al. Autocatalytic differentiation of epigenetic modifications within the Arabidopsis genome. EMBO J. 2010;29(20):3496–3506. doi: 10.1038/emboj.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Josefsson C, Dilkes B, Comai L. Parent-dependent loss of gene silencing during interspecies hybridization. Curr Biol. 2006;16(13):1322–1328. doi: 10.1016/j.cub.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 54.Groszmann M, et al. Changes in 24-nt siRNA levels in Arabidopsis hybrids suggest an epigenetic contribution to hybrid vigor. Proc Natl Acad Sci USA. 2011;108(6):2617–2622. doi: 10.1073/pnas.1019217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greaves IK, et al. Trans chromosomal methylation in Arabidopsis hybrids. Proc Natl Acad Sci USA. 2012;109(9):3570–3575. doi: 10.1073/pnas.1201043109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becker C, et al. Spontaneous epigenetic variation in the Arabidopsis thaliana methylome. Nature. 2011;480(7376):245–249. doi: 10.1038/nature10555. [DOI] [PubMed] [Google Scholar]
- 57.Schmitz RJ, et al. Transgenerational epigenetic instability is a source of novel methylation variants. Science. 2011;334(6054):369–373. doi: 10.1126/science.1212959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmitz RJ, et al. Patterns of population epigenomic diversity. Nature. 2013;495(7440):193–198. doi: 10.1038/nature11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaughn MW, et al. Epigenetic natural variation in Arabidopsis thaliana. PLoS Biol. 2007;5(7):e174. doi: 10.1371/journal.pbio.0050174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tittel-Elmer M, et al. Stress-induced activation of heterochromatic transcription. PLoS Genet. 2010;6(10):e1001175. doi: 10.1371/journal.pgen.1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dubin MJ, et al. DNA methylation in Arabidopsis has a genetic basis and shows evidence of local adaptation. eLife. 2015;4:e05255. doi: 10.7554/eLife.05255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen X, et al. Natural CMT2 variation is associated with genome-wide methylation changes and temperature seasonality. PLoS Genet. 2014;10(12):e1004842. doi: 10.1371/journal.pgen.1004842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woo HR, Pontes O, Pikaard CS, Richards EJ. VIM1, a methylcytosine-binding protein required for centromeric heterochromatinization. Genes Dev. 2007;21(3):267–277. doi: 10.1101/gad.1512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bomblies K, et al. Local-scale patterns of genetic variability, outcrossing, and spatial structure in natural stands of Arabidopsis thaliana. PLoS Genet. 2010;6(3):e1000890. doi: 10.1371/journal.pgen.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.