Significance
Apart from the lysosomal integral membrane protein type-2 (LIMP-2)–dependent trafficking of β-glucocerebrosidase (GC) to lysosomes, little is known about the interaction of LIMP-2 and GC on the molecular level. The structural and biochemical characterization of LIMP-2/GC interaction sites has potential importance for the design of GC-activating compounds. We show that a LIMP-2–derived helical peptide can be used for efficient purification and activation of recombinant and endogenous GC. These results provide a molecular framework for the design of GC activators as potential treatments in Parkinson’s disease and related synucleinopathies.
Keywords: LIMP-2, Gaucher's disease, β-glucocerebrosidase, GC activators, Parkinson's disease
Abstract
The lysosomal integral membrane protein type-2 (LIMP-2) plays a pivotal role in the delivery of β-glucocerebrosidase (GC) to lysosomes. Mutations in GC result in Gaucher's disease (GD) and are the major genetic risk factor for the development of Parkinson's disease (PD). Variants in the LIMP-2 gene cause action myoclonus-renal failure syndrome and also have been linked to PD. Given the importance of GC and LIMP-2 in disease pathogenesis, we studied their interaction sites in more detail. Our previous data demonstrated that the crystal structure of LIMP-2 displays a hydrophobic three-helix bundle composed of helices 4, 5, and 7, of which helix 5 and 7 are important for ligand binding. Here, we identified a similar helical motif in GC through surface potential analysis. Coimmunoprecipitation and immunofluorescence studies revealed a triple-helical interface region within GC as critical for LIMP-2 binding and lysosomal transport. Based on these findings, we generated a LIMP-2 helix 5-derived peptide that precipitated and activated recombinant wild-type and GD-associated N370S mutant GC in vitro. The helix 5 peptide fused to a cell-penetrating peptide also activated endogenous lysosomal GC and reduced α-synuclein levels, suggesting that LIMP-2–derived peptides can be used to activate endogenous as well as recombinant wild-type or mutant GC efficiently. Our data also provide a structural model of the LIMP-2/GC complex that will facilitate the development of GC chaperones and activators as potential therapeutics for GD, PD, and related synucleinopathies.
The lysosomal glucosidase β-glucocerebrosidase (GC) is required for hydrolysis of glucosylceramide and is targeted to lysosomes in a mannose-6 phosphate–independent manner by the lysosomal integral membrane protein type-2 (LIMP-2) (1, 2). Interaction of the two proteins occurs in the endoplasmic reticulum (ER) (1, 3), followed by trafficking of the LIMP-2/GC complex to lysosomes. Mutations in LIMP-2 cause action myoclonus-renal failure (AMRF) (4). LIMP-2 mutants linked to AMRF localize to the ER (3), causing missorting and lysosomal depletion of GC, highlighting the importance of functional LIMP-2 for correct targeting of GC. Reduced lysosomal activity of GC also is a hallmark of Gaucher`s disease (GD), which is caused by mutations in GC. Although only a few AMRF-causing mutations are known for LIMP-2, more than 300 mutations within GC, affecting the activity, stability, and/or the intracellular distribution of the enzyme, have been described (5). Patients carrying mutations in GC have an increased risk of developing synucleinopathies including Parkinson`s disease (PD) and dementia with Lewy bodies (6, 7). Importantly, a reduction in GC activity also is found in patients with sporadic PD (8). We recently showed reduced neuronal GC activity and increased α-synuclein in LIMP-2–deficient mice that also exhibited severe neurological deficits (9). These findings are supported by a significant association of genetic variations in the LIMP-2 locus with dementia with Lewy bodies (10) and emphasize the involvement of the LIMP-2–mediated lysosomal transport of GC in the pathogenesis of synucleinopathies. Augmentation of GC activity in murine brain of GD and PD mouse models led to a reduction of α-synuclein accumulation and amelioration of neuronal pathology (11, 12). Several hypotheses suggest a link between mutated GC and dysregulated α-synuclein homeostasis (13). For example, the GC substrate glucosylceramide has been proposed to promote α-synuclein accumulation by exerting a stabilizing effect on toxic oligomeric forms of α-synuclein (14). A feedback loop in which accumulated α-synuclein partially blocks the ER-to-Golgi transport of GC was suggested to increase this pathological cascade further (14).
The recently solved crystal structure of the LIMP-2 ectodomain revealed an exposed three-helix bundle, which is formed by helices 4, 5, and 7; helices 5 and 7 likely serve as a GC-binding domain (15). Because the secondary structure and hydrophobicity of this region are important for binding and intracellular transport of GC (1), we hypothesized that GC might harbor a similar motif necessary for LIMP-2 binding. Here, we describe the identification and characterization of a hydrophobic helical interface within GC, mediating binding to LIMP-2. We therefore suggest a model of LIMP-2/GC interaction that may be important for the design of small-molecule GC activators. Furthermore, we generated a LIMP-2–derived helical peptide that can be used to purify, activate, and stabilize GC in vitro as well as in cell-based assays. Our data also suggest that this chaperone-like activity of LIMP-2 could increase lysosomal targeting of wild-type or mutant forms of GC, thereby decreasing intralysosomal accumulation of glucosylceramide in synucleinopathies.
Results
LIMP-2 and GC Interaction Is Mediated by Hydrophobic Helical Interfaces on both Proteins.
Previous mutagenesis studies, guided by the crystal structure of the LIMP-2 ectodomain, indicated that the hydrophobic helices 5 and 7 are critical for an interaction with GC (15). Consistent with these findings, we show here that mutations within helix 5 and 7 of LIMP-2 that reduced the hydrophobicity of this region (Fig. S1 A and B) impaired the ability to rescue reduced GC activity in a LIMP-2–deficient cell system (Fig. S1C). Thus we confirm in a cellular model that the hydrophobicity of the helical bundle in LIMP-2 is critical for binding and intracellular transport of GC. Because the interaction domain within the GC protein is unknown, we used the available crystal structure of GC (16, 17) and surface potential analysis to identify potential GC/LIMP-2 interaction sites in silico (Fig. 1A). A potential interaction region in GC, which consisted of a hydrophobic helical interface (white area in Fig. 1A) and is composed of three helices, 1a, 1b, and 2 (Fig. 1B), was identified by its similarity to helices 5 and 7 of LIMP-2.
Fig. S1.
Structural and molecular studies of LIMP-2 and GC interaction. (A) Crystal structure of LIMP-2 (PDB ID code 4F7B). The helical bundle—helix 5, helix 7, and helix 4—is shown in red. Hydrophobic amino acids are indicated in yellow. The box shows a magnification of the helical bundle with the mutated amino acids visualized. The D400K control mutant located outside the helical bundle is shown in green. (B) Human and murine amino acid sequences of the LIMP-2–binding region with introduced point mutations. Hydrophobic amino acids within the helical regions (red) of helix 5 and helix 7 are highlighted in yellow. (C) GC activity after the transfection of LIMP-2 point mutants into LIMP-2–deficient MEFs. Only mutants capable of binding and transporting GC to the lysosome can rescue GC activity as seen in wild-type LIMP-2. Enzymatic activity is normalized to wild-type LIMP-2 and subtracted from background values (n = 4–7). (D) Triple-IF staining of GC-deficient MEF cells transfected with GC mutants (α-hGC, green) and stained for the endogenous lysosomal markers LAMP-2 (red) and LIMP-2 (blue). Purple color indicates a colocalization of the two lysosomal markers. A white signal (Upper Row) points to an additional overlay of GC highlighting lysosomal localization of the respective GC construct. (E) Analysis of signal overlay of stained GC mutants with the lysosomal marker LAMP-2 shown as Pearson’s index (n = 2 or 3). (F) IF of binding-impaired GC mutants in GC-deficient MEF cells stained for GC (α-hGC, red) and the ER marker protein disulfide isomerase (PDI) (green). A yellow signal indicates ER localization of GC mutants. (G) Pearson’s index of colocalization of GC and PDI (n = 3–6). (H) IF staining of wild-type GC and mutants L91E, L94E, and L156E (α-hGC; red) coexpressed with LIMP-2 (α-myc; green) in GC-deficient cells. The areas magnified in the lower row are indicated by the white boxes in the upper row. (I) Pearson‘s index as a measure of the degree of LIMP-2–GC colocalization (n = 3–9). (J) Immunoblot of EndoH and PNGaseF digests of GC mutants (α-hGC) with and without coexpression of LIMP-2 (anti-myc) in N2a cells with actin as loading control. Protein fractions resistant to EndoH digestion indicate post-ER localization. Dotted lines separate individual blots. (K) Densitometry of EndoH digests showing the post-ER/ER ratio normalized to wild-type GC (n = 3–6). (L) Enzyme activity (normalized to mock control) of GC mutants overexpressed in N2a cells with or without LIMP-2 coexpression. The dotted line indicates background activity; n = 4. A one-way ANOVA together with a subsequent Tukey–Kramer post hoc test was used for results shown in C, E, G, and I. A two-sided Student’s t test was used for analyses shown in K and L. *P < 0.05; **P < 0.01; ***P < 0.001 when comparing indicated mutants with wild-type GC/LIMP-2.
Fig. 1.
Identification of the LIMP-2 interaction site in GC by structural and molecular analyses. (A) Illustration of surface charges in LIMP-2 [Protein Data Bank (PDB) ID code 4F7B] and GC (PDB ID code 2J25). Hydrophobic areas are shown in white. (B, Upper) Protein structure of GC (PDB ID code 2J25) with a hydrophobic patch (shown in red) revealing three helices: helix 1a, helix 1b, and helix 2. (Lower) Magnification of the helical region. Hydrophobic amino acids are indicated in yellow. (C) Sequence alignment of multiple GC species (red boxes: helix1a/b and 2; yellow: hydrophobic residues). (D) Co-IP of overexpressed GC helix mutants L91E, L94E, and L156E and wild-type and control R211E mutant in N2a cells using a LIMP-2– (immunoprecipitated) and a human GC-specific antibody (α-hGC). #, bands from denatured antibody used for IP; AB ctrl, antibody control. The dotted line indicates different exposure times of the same immunoblot. (E) Densitometric quantification of bound GC protein normalized to precipitated LIMP-2 (n = 4–12). (F) IF of GC-deficient cells transfected with the GC helical motif mutants (L91E, L94E, and L156E) and control R211E mutant (α-hGC; red), costained for endogenous LIMP-2 (green). The areas of magnification in the lower row are outlined by white boxes in the upper row. (G) Colocalization of GC and LIMP-2 was determined using the Pearson‘s index (n = 4–10). (H and I) Immunoblot (H) and densitometric quantification (I) (post-ER/ER ratio normalized to wild-type GC; n = 2 or 3) of EndoH- or PNGaseF-treated cell extracts of GC-deficient cells expressing GC mutants L91E, L94E, and L156E and wild-type GC (α-hGC) with or without myc-tagged LIMP-2 (α-myc). Actin was used as loading control. EndoH resistance of proteins indicates their post-ER localization. Dotted lines in H separate individual blots. **P < 0.01; ***P < 0.001. See also Fig. S1.
To determine if these helices are important for binding to LIMP-2, we substituted single amino acids within this helical motif by replacing conserved hydrophobic leucines with negatively charged glutamic acids (Fig. 1 B and C). This substitution resulted in the three GC mutants L91E (helix 1a), L94E (helix 1a), and L156E (helix 2). A R211E GC mutant served as a control because this mutation is located outside the identified hydrophobic helical motif (Fig. 1B). The GC mutants were expressed in cells and assayed for their ability to bind LIMP-2 by coimmunoprecipitation (co-IP). In contrast to wild-type GC and the control mutant R211E, the three point mutations within the helical motif of GC impaired co-IP with LIMP-2 (Fig. 1 D and E). Immunofluorescence (IF) studies demonstrated the colocalization of wild-type GC and the R211E mutant with endogenous LIMP-2 (Fig. 1 F and G) and lysosome-associated membrane glycoprotein 2 (LAMP-2) (Fig. S1 D and E) in lysosomes, whereas the GC helical motif mutants L91E, L94E, and L156E remained in the ER (Fig. S1 F and G). Furthermore a colocalization of wild-type GC with overexpressed LIMP-2 also was found in lysosomes but was reduced significantly upon expression of the GC mutants L91E and L156E (Fig. S1 H and I). To evaluate the cellular fate of the GC helical motif mutants further, we used GC-deficient mouse embryonic fibroblasts (MEFs) (Fig. 1 H and I) and murine neuroblastoma (N2a) cells (Fig. S1 J and K) for endoglycosidase H (EndoH) and peptide-N-glycosidase F (PNGaseF) treatment of cellular extracts. Although PNGaseF removes all N-linked glycans from GC and served as a control to detect unglycosylated GC, EndoH discriminates between mature (EndoH-insensitive) and immature (EndoH-sensitive) N-linked glycans. Thus, complete EndoH-sensitive bands indicate the ER localization of GC. Overexpression of wild-type GC in GC-deficient MEFs and N2a cells resulted in a small fraction of post-ER forms of GC (Fig. 1H and Fig. S1J; see the EndoH-treated sample in the second lane). Coexpression of LIMP-2 caused a 10-fold increase in the post-ER form of wild-type GC (Fig. 1 H, lane 5, and I, and Fig. S1 J, lane 5, and K). In contrast, LIMP-2 overexpression did not alter the post-ER levels of the GC helical motif mutants L91E and L156E and altered the post-ER levels of the L94E GC mutant only to a minor degree (Fig. 1 H and I and Fig. S1 J and K). This result indicates some residual interaction of LIMP-2 with the L94E mutant under more native cellular conditions. The residual interaction found here was not detected by the previous co-IP experiments (Fig. 1 D and E), possibly because of the stringency of the applied co-IP buffer. We then evaluated whether the observed increase in GC maturation also leads to changes in its enzymatic activity. Upon coexpression of wild-type GC and LIMP-2, we observed a significant increase in GC activity, which was not evident for the three GC helical motif mutants L91E, L94E, and L156E (Fig. S1L). Overall our data suggest that the hydrophobicity of a three-helix motif within GC is critical for proper LIMP-2 binding. Furthermore, LIMP-2 expression appears to be a limiting factor for ER exit and post-ER trafficking of GC.
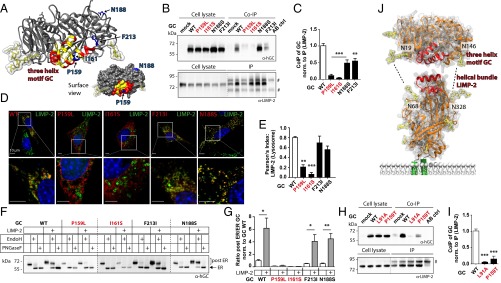
Identification of GD-Causing Mutations Within the Three-Helix Motif of GC and Their Interference with LIMP-2 Binding.
To evaluate if GD-associated mutations within the potential binding motif of GC might interfere with LIMP-2 interactions, we analyzed two GD mutants, P159L and I161S. Both mutations are located within helix 2 of the hydrophobic helical interface (Figs. 1C and 2A) (5, 18). As a control, we used two additional mutants, obtained from GD patients, F213I and N188S (5, 19), which reside outside the hydrophobic helical motif of GC and thus should not interfere with LIMP-2 interactions (Fig. 2A). We first analyzed LIMP-2 binding of these mutants and then the LIMP-2–dependent intracellular transport of GC. Co-IP experiments revealed impaired binding of the GC mutants P159L and I161S to LIMP-2 as compared with wild-type GC (Fig. 2 B and C). The two control mutants F213I and N188S still bound to LIMP-2, albeit to a reduced extent compared with wild-type GC (Fig. 2 B and C). IF microscopy in GC-deficient cells demonstrated decreased lysosomal transport of the hydrophobic helix mutants P159L and I161S (Fig. 2 D and E and Fig. S2 A and B). In contrast the two control mutants F213I and N188S still colocalized with LIMP-2 (Fig. 2 D and E) and LAMP-2 (Fig. S2 A and B), indicating their lysosomal localization. Furthermore, colocalization with protein disulfide-isomerase (PDI) revealed increased ER localization of the two GD mutants P159L and I161S compared with wild-type GC or the control mutant N188S (Fig. S2 C and D). In addition the two GD-associated helix mutants P159L and I161S showed significantly reduced colocalization with overexpressed LIMP-2, but the control mutants F213I and N188S did not (Fig. S2 E and F). An EndoH digest confirmed the retention of the clinical mutants P159L and I161S within the ER, whereas the GC mutants F213I and N188S were found in post-ER fractions (Fig. 2 F and G). In addition, overexpression of LIMP-2 did not increase the post-ER transport of the P159L and I161S, mutants as observed for the F123 and N188S mutants (Fig. 2 F and G), strengthening our hypothesis that the P159L and I161S mutants are incapable of binding to LIMP-2 via their hydrophobic helical motif, thus resulting in decreased ER exit and lysosomal transport.
Fig. 2.
Analysis of the LIMP-2 interaction site in GC mutants obtained from GD patients. (A) Structure of GC (PDB ID code 2J25); helical motif and hydrophobic amino acids are highlighted in red and yellow, respectively. The clinically relevant GC mutants obtained from GD patients, P159L and I161S (located in helix 2) and F213I and N188S (outside the helical motif), are depicted in blue. A surface view shows surface exposure of indicated amino acids. (B) Co-IP of the GC mutants found in GD patients, P159L, I161S, F213I, and N188S (α-hGC), expressed in N2a cells. A LIMP-2 antibody was used for IP. #, bands from denatured antibody used for IP; AB ctrl, antibody control. The dotted line indicates different exposure times of the same immunoblot. (C) Densitometry of co-IP studies (normalized to LIMP-2) (n = 4–11). (D) IF costaining of GC mutants (α-hGC; red) expressed in GC-deficient cells with endogenous LIMP-2 (green). The areas of magnification in the lower row are indicated by white boxes in the upper row. (E) Pearson‘s index give the degree of GC–LIMP-2 colocalization (n = 3–10). (F and G) Immunoblot (F) and densitometric quantification (G) of GC mutants found in GD patients (α-hGC) with and without coexpression of LIMP-2 in GC-deficient cells treated with EndoH or PNGaseF. The post-ER/ER ratio is normalized to wild-type GC (n = 3). In F, the upper EndoH-resistant band of GC indicates the post-ER location of the protein; the lower band corresponds to ER residence. (H) Co-IP of L91A and GC mutant P159T (found in a GD patient) overexpressed in N2a cells (α-hGC). The dotted line indicates different exposure time of same immunoblot. (I) Quantification of bound GC protein normalized to precipitated LIMP-2 (n = 4–5). (J) Binding model of LIMP-2 and GC with potential stabilizing interaction of carbohydrate chains of both proteins (dotted lines). *P < 0.05; **P < 0.01; ***P < 0.001. See also Fig. S2.
Fig. S2.
Analysis of GC mutants obtained from GD patients and their LIMP-2–binding behavior. (A) Triple-IF staining of GC-deficient cells transfected with GC mutants obtained from GD patients (α-hGC, green) and endogenous lysosomal markers LAMP-2 (red) and LIMP-2 (blue). A purple color indicates a colocalization of LAMP-2 with LIMP-2, and a white signal shows an additional overlay with GC indicating lysosomal localization of the respective GC construct. (B) Pearson’s index of colocalization of the GC mutants I161S and N188S obtained from GD patients with LAMP-2 (n = 2 or 3). The respective GC wild-type control can be found in Fig. S1D. (C) IF of GC-deficient cells transfected with GC mutants obtained from GD patients (α-hGC; red) and costained for the endogenous ER marker PDI (green). The areas of magnification in the lower row are indicated by white boxes in the upper row. (D) Analysis of colocalization of GC mutants with PDI shown as Pearson’s index (n = 2–6). (E) IF staining of GC mutants (α-hGC, red) coexpressed with LIMP-2 (α-myc; green) in GC-deficient cells. Areas of magnification in the lower row are indicated by white boxes in the upper row. (F) Colocalization of GC and LIMP-2 was analyzed by Pearson’s index (n = 3–7). (G) Expression level of all analyzed GC mutants in N2a cells normalized to wild-type GC. (H) Interaction model of LIMP-2 (PDB ID codes 4F7B and 4Q4F) and GC (PDB ID code 2J25) showing pH-dependent structural differences between pH 7.5 and pH 5.5. Apical hydrophobic amino acids F151, I155, A158, and A162 are indicated in blue. A one-way ANOVA and a subsequent Tukey–Kramer post hoc test were used for statistical analyses. **P < 0.01; ***P < 0.001 compared with wild-type GC.
To characterize the LIMP-2–binding domain further, we analyzed two additional GC mutants, the GD-associated point mutation P159T, which carries a polar threonine at position 159, and the L91A mutant carrying an alanine at position 91, which represents a hydrophobic amino acid but has a less bulky side chain than the original leucine. Both mutations resulted in impaired binding of mutated GC to LIMP-2 as revealed by co-IP studies (Fig. 2 H and I), further indicating the importance of single amino acids for LIMP-2 binding in this highly conserved region. Importantly, all GC mutants analyzed so far in this study exhibited expression levels comparable with that of the wild-type enzyme (Figs. 1D and 2B, Upper, and Fig. S2G).
In summary, our findings suggest that the LIMP-2–binding region in GC is located in a helical interface formed by helix 1a (residues T86–L96), helix 1b (residues P99–S110), and helix 2 (P150–A168), displaying a hydrophobic patch similar to that found in LIMP-2. Therefore, we propose a model in which GC and LIMP-2 interact via two hydrophobic helical interfaces (Fig. 2J, Fig. S2H, and Movie S1). Consistent with this model, a crystal structure of LIMP-2 solved at pH 5.5 (20) shows a large conformational change in the identified binding site of helix 5; this change likely is responsible for the dissociation of GC at low lysosomal pH (Fig. S2H).
A Synthetic LIMP-2–Derived Peptide Is Sufficient to Interact with GC and Increases the Enzymatic Activity.
We then asked if helix 5, the most apically exposed helix of LIMP-2, is sufficient for binding to GC. To this end, we generated a LIMP-2–derived helix 5 peptide together with a control peptide with two isoleucine and one leucine residues replaced by three aspartates (3×D) (Fig. 3A). We have shown previously that a LIMP-2 mutant containing these three aspartates failed to bind GC (3). Circular dichroism spectroscopy confirmed the helical structure of the helix 5 peptide, but the control peptide was nonhelical (Fig. S3A). Both peptides were N-terminally tagged with biotin and used for GC pulldown experiments at neutral pH. After the peptides were incubated with either recombinant GC (Fig. 3 B and C) or cellular lysates (Fig. 3D), only the wild-type helix 5 peptide specifically coprecipitated recombinant as well as endogenous GC. In addition, no interaction of the helix 5 peptide with recombinant α-mannosidase (LAMAN), a lysosomal hydrolase, or albumin (BSA) could be detected (Fig. S3 B and C), demonstrating the specific interaction of this helix 5 peptide with GC.
Fig. 3.
Structural characteristics of LIMP-2–derived peptide comprised of helix 5 and its effect on GC function. (A) Protein structure of LIMP-2 (PDB ID code 4F7B) with the helix 5 peptide sequence (L152–E175) highlighted. Amino acids in red [two isoleucines (I) and one leucine (L)] were replaced by aspartic acid (3×D). (B and C) Pulldown (B) and densitometry (C) of recombinant GC bound to peptides (helix 5 or 3×D) relative to the bound protein fraction of buffer control (n = 4–5). Proteins were visualized by Coomassie staining (CBB). (D) Immunoblot of endogenous GC in Cos 7 cells (α-hGC) after pulldown with the helix 5 peptide. (E) Assay of the GC activity of recombinant GC incubated with helix 5 or 3×D peptide (n = 4). (F) Stabilization assay of GC mixed with buffer, helix 5, or 3×D peptide incubated at 37 °C for 270 h (n = 3). (G) Lysosomal GC activity of living H4 cells measured in vivo after incubation with uptake-optimized TAT peptides (helix 5 and 3×D). GC activity was normalized to cell volume and is shown relative to buffer control (n = 4). (H and I) TAT peptide uptake (helix 5 and 3×D) in H4 cells, stably overexpressing α-synuclein under a tetracycline-inducible promoter in conjunction with doxycycline to stop de novo synthesis of α-synuclein (α-Syn). Immunoblot (H) and densitometry analysis (I) of the α-synuclein level (normalized to loading control) after 74 h of incubation with helix 5 TAT peptide. The value at incubation time t0 was set as 1 (n = 6). (J) Pulldown of recombinant wild-type GC and the GC N370S mutant with TAT peptides (helix 5 and 3×D). (K) Activity assay of recombinant mutant N370S GC after incubation with TAT peptides (helix 5 and 3×D). As a reference value, wild-type GC activity is shown on the right. GC activity was normalized to buffer control (n = 3–5). See also Fig. S3.
Fig. S3.
Characterization of a helical LIMP-2–derived peptide and its effect on GC activity. (A) CD spectra (250–200 nm) exhibiting a helical fold for the helix 5 peptide that is absent in the control 3×D helix peptide. (B) In vitro peptide pulldown assay: Helix 5 peptides were incubated with recombinant LAMAN and BSA. Input and bound fractions were loaded onto SDS/PAGE, and proteins were visualized by Coomassie staining (CBB). (C) Densitometry of LAMAN and BSA protein bound to peptide, normalized to the protein fraction bound only to beads (buffer; n = 2–3). (D and E) Activity assay of recombinant GC (D) and LAMAN (E) incubated with different excess molarities (1×–10×) of helix 5 peptide (n = 3–5) and LIMP-2 ectodomain (n = 4). GC activities were normalized and statistically analyzed compared with 0 input of BSA/helix 5 peptide/LIMP-2 ectodomain. (F) GC activity assay in cell lysates of mock- or GC-transfected N2a cells incubated with helix 5 or 3×D peptide. Enzyme activity was normalized to buffer control (n = 2–8). (G) Activity assay of recombinant GC incubated with TAT-modified peptides (helix 5 and 3×D). GC activity was normalized to buffer control (n = 4). (H) IF picture of H4 cells incubated with biotin-labeled helix 5 TAT peptide, stained with a fluorescently labeled streptavidin (green), and costained for LIMP-2 (red). (I) Live-cell lysosomal GC activity of H4 cells after uptake of helix 5 and 3×D TAT peptides. GC activity was measured every 30 min up to 3.5 h. Cells were treated with DMSO (red trace) or the lysosomal inhibitor bafilomycin A1 (black trace). The area under the DMSO curve shows whole-cell activity. Lysosomal activity is calculated by subtracting the area under the curve (AUC) of bafilomycin A1-treated samples from the AUC of DMSO-treated samples (gray area; n = 4). (J) Whole-cell GC activity of H4 cell lysate after the uptake of helix 5 peptide and the control 3xD TAT peptide as shown in the assay in Fig. 3H. GC activity was measured for 16–74 h and is shown in milliunits per milligram (n = 3). (K) Interaction model of GC (PDB ID code 2J25) with the LIMP-2–derived helix 5 peptide (yellow). The hydrophobic three-helix motif on GC indicated in red serves as a proposed binding site. The asparagine on amino acid position 370 (N370) is shown in blue, indicating its localization outside the proposed LIMP-2/helix 5 interaction site on GC. A one-way ANOVA and a subsequent Tukey–Kramer post hoc test were applied in I, and a two-sided Student’s t test was used for analysis in D (comparing GC activity with the 0-value) and G. *P < 0.05; **P < 0.01; ***P < 0.001.
To address the functional impact of the observed interaction between the helix 5 peptide and recombinant GC, we measured GC activity in the presence of a one- to 10-fold molar excess of the helix 5 peptide (Fig. S3D). Enzyme activity was increased five times in the presence of a 10-fold molar excess of the helix 5 peptide, whereas the 3×D control peptide did not increase the GC activity (Fig. 3E). A random helical control peptide consisting of 24 amino acids (21) also was unable to increase GC activity, further supporting the specificity of the helix 5 peptide (Fig. 3E). The purified luminal domain of LIMP-2 had a similar effect on GC activity (Fig. S3D), suggesting that the activating effect of LIMP-2 on GC is mediated mainly by helix 5. No effect on LAMAN enzymatic activity was detected after incubation with the helix 5 peptide or the LIMP-2 ectodomain, further emphasizing the specificity of the helix 5 peptide on GC activity (Fig. S3E). The enzymatic activity of endogenous and overexpressed GC in cell lysates also was increased after incubation with the helix 5 peptide (Fig. S3F). To analyze if the increase in GC activity is caused by the stabilization of the enzyme, recombinant GC was incubated with the helix 5 and the control peptide at 37 °C, and the activity of GC was measured at regular intervals (Fig. 3F). Incubation of GC with buffer alone or the control 3×D peptide led to a complete loss of enzymatic activity within 72 h, whereas GC still displayed significant enzymatic activity in presence of the helix 5 peptide (helix 5: t1/2 = 48 h; 3×D: t1/2 = 24 h) (Fig. 3F).
We also analyzed if the helix 5 peptide-mediated increase in GC activity measured in vitro could be detected in lysosomes of living cells. To facilitate cellular uptake of the peptide, we used a cell-penetrating helix 5 and a control peptide (3×D) that were C-terminally linked with a HIV-derived TAT motif (22). We also added the chaperone-mediated autophagy-targeting motif KFERQ to support lysosomal import of these peptides (23). This effect of helix 5 peptide, as well as the control (3×D) TAT peptide, on recombinant GC activity was comparable to the effect observed for the unmodified peptides (Fig. S3G). Using IF, we detected the helix 5 TAT peptide in vesicular structures that partly colocalized with LIMP-2 in H4 human neuroglioma cells, indicating lysosomal localization (Fig. S3H). Next, using a compartment-specific activity assay (24), we confirmed that the helix 5 TAT peptide could elevate GC activity directly within lysosomes of living cells by ∼18% (Fig. 3G and Fig. S3I). Because it has been demonstrated previously that elevated GC activity reduces α-synuclein levels (9, 11, 12), we investigated the effect of the helix 5 TAT peptide on the clearance of α-synuclein in H4 cells stably overexpressing wild-type α-synuclein under a tetracycline-inducible promoter. These cells were incubated with the helix 5 or the control (3×D) TAT peptide and were treated with doxycycline to suppress de novo α-synuclein synthesis. Cells were harvested at 0 and 74 h after doxycycline addition, and the remaining α-synuclein levels were analyzed by Western blot. Enhancement of GC activity persisted for the 74-h time course of the assay with the helix 5 TAT peptide but not with the control 3×D TAT peptide (Fig. S3J). A significant reduction in α-synuclein levels was observed 74 h after incubation with the helix 5 TAT peptide, as compared with the 3×D control peptide (Fig. 3 H and I). To evaluate the therapeutic potential of the LIMP-2–derived helix 5 peptide further, we assessed its effect on the recombinant GC mutant N370S, which represents one of the most prevalent GD-causing mutations with low catalytic activity (25, 26). Using a cell-free system, we found that recombinant N370S mutant GC could be precipitated by the helix 5 TAT peptide as efficiently as recombinant wild-type GC (Fig. 3J); this finding is consistent with the localization of the N370S mutation outside the three helical LIMP-2–binding motif (Fig. S3K). Furthermore, similar to its effect on wild-type GC (Fig. 3E), the helix 5 TAT peptide led to a fourfold increase in the activity of the N370S mutant (Fig. 3K).
Our data provide evidence that the interaction site of LIMP-2 and GC consists of two hydrophobic helical interfaces. The integrity of these helical motifs on both proteins is critical for LIMP-2–mediated lysosomal transport of GC. Additionally, a LIMP-2–derived helix 5 peptide is sufficient for binding and activating both wild-type and mutant GC in vitro and in cell-based assays. We propose binding of the LIMP-2–derived helix 5 peptide to the hydrophobic three-helix motif found on GC as described for LIMP-2 (Fig. S3K and Movie S1). The characterization of this interaction site on GC might have important implications for the future drug design of GC activators.
Discussion
The determination of the crystal structures of LIMP-2 (15) and GC (16) and their respective binding sites revealed here provides a deeper understanding of how this receptor/ligand protein complex triggers transport of GC to the lysosomal compartment. Our data suggest that LIMP-2 and GC interact via two helical interfaces in a 1:1 stoichiometry, as is consistent with our previous crosslinking experiments (1). The described helical interfaces on LIMP-2 and GC expose mainly hydrophobic side chains, indicating a hydrophobic interaction. This notion is supported by our findings that introduction of negatively charged amino acids in either helical interface impaired LIMP-2 binding to GC. The two clinically relevant GC mutations in helix 2 support this mode of interaction, because the I161S mutation decreases the hydrophobicity and the P159L mutant interferes with the secondary structure of the helical motif of GC or neighboring protein structures. Interestingly, the hydrophobic helical motif is found opposite the catalytic cavity and also opposite the proposed saposin C-binding site (27, 28), suggesting that LIMP-2/GC interaction does not interfere with the binding of saposin C. Furthermore, in agreement with our previous findings of a glycosylation-independent LIMP-2/GC interaction (1, 3), the LIMP-2/GC interaction site does not harbor glycosylation sites. Our data propose a model in which sugar chains of both proteins come in close contact upon complex formation (Fig. 2J), potentially exerting a stabilizing effect on the LIMP-2/GC protein complex and thereby assisting in the lysosomal transport of the enzyme. Interestingly, very few GD-causing mutations in GC have been reported within this interface region so far (5). It is possible that such mutations do not affect the catalytic activity of GC but rather diminish its binding to LIMP-2, leading to the secretion and recapture of a still functional enzyme via endocytosis. The amount of GC reaching lysosomes through this indirect pathway could be sufficient for several cell types to degrade sphingolipids (e.g., macrophages), as demonstrated by the successful application of exogenous recombinant GC in enzyme-replacement therapy (29–31).
Recently, Liou et al. (32) proposed that the LIMP-2–binding motif in GC consists of an 11-amino acid stretch that forms a surface-accessible loop in the close vicinity of the helical interface reported here. However, most of the residues within this loop that are mutated in this study point toward the core of GC, suggesting they have a secondary effect on the helical motif rather than affecting binding directly.
We found that a LIMP-2–derived helix 5 peptide sufficiently binds to the helical motif of GC leading to a fivefold increase of recombinant GC activity. The use of this helix 5-derived peptide could offer a previously unidentified strategy to purify GC efficiently from cell-culture medium or cell lysates. Moreover, this helix 5-derived LIMP-2 peptide could be exploited as an activator of wild-type and even mutant GC. The underlying mechanism of the helix 5 peptide-mediated GC activation remains to be established, but our in vitro assays already indicate that the peptide has a stabilizing effect on the enzyme. We propose the helix 5 peptide binds to the same hydrophobic interface of GC as described for LIMP-2 in this study. Most of the recently described chaperones of GC are inhibitors of the enzyme (33, 34). In contrast, we propose here that the binding site of the helix 5 peptide resides outside the catalytic cavity of GC. Thus, we assume that the bound helix 5 peptide has an allosteric, noninhibitory effect on GC activity.
In summary, our study describes a helix motif in GC responsible for the interaction with LIMP-2 and presents a model of the receptor/ligand complex. It also reveals an activating effect of a small LIMP-2–derived peptide on GC. Identification of the peptide binding at this particular region on GC further opens the possibility of designing small molecules to target this domain. Understanding the LIMP-2 interaction site in GC may further elucidate the molecular aspects of GD and AMRF and help optimize therapeutic strategies for patients. Preserving or enhancing LIMP-2/GC interaction will be important in therapeutic efforts geared toward the development of activators and chaperones of LIMP-2 or GC.
Experimental Procedures
Expression plasmids of LIMP-2 and human GC constructs (Table S1) were generated as described previously (1). For the cell lines used, please refer to SI Experimental Procedures and Table S2. For Western blotting nitrocellulose or PVDF membranes were used. EndoH/PNGaseF digests were performed according to the manufacturer’s instructions (New England Biolabs). For co-IP experiments magnetic agarose G beads (Thermo Fisher Scientific) were used. For more information refer to SI Experimental Procedures. IF studies were performed in cells as previously described (1, 3). Cellular colocalization of two proteins was determined by Pearson’s index (35) (also see SI Experimental Procedures). Enzyme activity assays of cell lysates or recombinant protein were measured at acid pH using absorbent and fluorescent artificial substrates; for further information see SI Experimental Procedures. For peptide studies, peptides were N-terminally tagged with biotin. If not stated otherwise, recombinant enzyme was incubated with a 10-fold higher molarity of peptides. Conditions for pulldown experiments were kept at neutral pH. More information can be found in SI Experimental Procedures. Protein modeling, molecular analyses, graphics, and animations were performed with the University of California, San Francisco Chimera package (www.cgl.ucsf.edu/chimera) supported by National Institute of General Medical Sciences Grant P41-GM103311.
Table S1.
Expression constructs used
| Name and description: Vector name-construct-tag | Resistance in E. coli |
| pFrog-mLIMP2-WT-myc | Ampicillin |
| pFrog-mLIMP2-L155D-myc | Ampicillin |
| pFrog-mLIMP2-I156D-myc | Ampicillin |
| pFrog-mLIMP2-M159D-myc | Ampicillin |
| pFrog-mLIMP2-L160D-myc | Ampicillin |
| pFrog-mLIMP2-Y163D-myc | Ampicillin |
| pFrog-mLIMP2-I184D-myc | Ampicillin |
| pFrog-mLIMP2-L187D-myc | Ampicillin |
| pFrog-mLIMP2-F191D-myc | Ampicillin |
| pFrog-mLIMP2-D400K-myc | Ampicillin |
| pFrog-hGC-WT | Ampicillin |
| pFrog-hGC-L91A | Ampicillin |
| pFrog-hGC-L91E | Ampicillin |
| pFrog-hGC-L94E | Ampicillin |
| pFrog-hGC-L156E | Ampicillin |
| pFrog-hGC-L211E | Ampicillin |
| pFrog-hGC-P159L | Ampicillin |
| pFrog-hGC-P159T | Ampicillin |
| pFrog-hGC-I161S | Ampicillin |
| pFrog-hGC-N188S | Ampicillin |
| pFrog-hGC-F213E | Ampicillin |
All constructs were generated in house.
Table S2.
Cell culture
| Name | Growth medium | Source |
| GC-deficient MEFs | High-glucose DMEM (4.5 g/mL) (GE Healthcare); additives: 10% FCS (PAA Laboratories), 1% penicillin/streptomycin (PAA Laboratories) | Ellen Sidransky, Section on Molecular Neurogenetics, Clinical Neuroscience Branch, National Institute of Mental Health, Bethesda |
| LIMP-2–deficient MEFs | DMEM | Generated in-house |
| H4 cells overexpressing α-synuclein under the control of a tetracycline-inducible promoter (“tet-off”) | Opti-MEM medium (Thermo Fisher Scientific); additives: 5% FCS, 1% penicillin/streptomycin, 200 μg/mL G418, 200 μg/mL hygromycin (both from Thermo Fisher Scientific) | Pamela J. McLean, Mayo Clinic, Jacksonville, FL |
| N2a (murine neuroblastoma) cells | DMEM | Deutsche Sammlung von Mikroorganismen und Zellkulturen (DMSZ) |
| Cos 7 cells | DMEM | DMSZ |
Primers used for cloning and site-directed mutagenesis are given in Table S3; antibodies used are listed in Table S4; peptide sequences are provided in Table S5; buffers, solutions, and recombinant proteins are described in Table S6; and the settings for CD spectroscopy are detailed in Table S7.
Table S3.
Primers used for cloning and site-directed mutagenesis
| Name | Sequence, 5′→3′ |
| pFrog forward | gagaacccactgcttactgg |
| pFrog reverse | gagactccattcgggtgttct |
| hGC_L91A forward | gacagatgctgctgctGCGaacatccttgccctg |
| hGC_L91A reverse | cagggcaaggatgttCGCagcagcagcatctgtc |
| hGC_L91E forward | gacagatgctgctgctGAGaacatccttgccctg |
| hGC_L91E reverse | cagggcaaggatgttCTCagcagcagcatctgtc |
| hGC_L94E forward | gctgctctcaacatcGAGgccctgtcaccccct |
| hGC_L94E reverse | agggggtgacagggcCTCgatgttgagagcagc |
| hGC_L156E forward | gaggaagataccaagGAGaagatacccctgattc |
| hGC_L156E reverse | gaatcaggggtatcttCTCcttggtatcttcctc |
| hGC_N188S forward | ctcaagaccaGtggagcggtg |
| hGC_N188S reverse | caccgctccaCtggtcttgag |
| hGC_R211E forward | accagacctgggccGAAtactttgtgaagttcctg |
| hGC_R211E reverse | caggaacttcacaaagtaTTCggcccaggtctggt |
| hGC_P159L forward | ataccaagctcaagataCTTctgattcaccgagc |
| hGC_P159L reverse | gctcggtgaatcagAAGtatcttgagcttggtat |
| hGC_P159T forward | ataccaagctcaagataACTctgattcaccgagc |
| hGC_P159T reverse | gctcggtgaatcagAGTtatcttgagcttggtat |
| hGC_I161S forward | agctcaagatacccctgTCGcaccgagccctgcag |
| hGC_I161S reverse | ctgcagggctcggtgCGAcaggggtatcttgagct |
| hGC_R213E forward | gacctgggccagatacGAAgtgaagttcctgga |
| hGC_R213E reverse | tccaggaacttcacTTCgtatctggcccaggtc |
Table S4.
Antibodies used
| Name | Host | Western blot dilution | IF dilution | Source |
| Anti-actin | Rabbit | 1:1,000 | — | Sigma Aldrich |
| Anti-GAPDH | Mouse | 1:2,000 | — | EMD Millipore, |
| Anti-hGC | Mouse | 1:500 | 1:250 | Johannes Aerts, Leiden University, Leiden, The Netherlands |
| Anti–LAMP-2 (Abl 93) | Rat | 1:2,000 | 1:200 | Developmental Studies Hybridoma Bank |
| Anti–LIMP-2 (L2T2) | Rabbit | 1:1,000 | 1:250 | Custom-made |
| Anti–myc-GTX | Goat | 1:1,000 | 1:250 | Gentex |
| Anti-NSE | Rabbit | 1:2,000 | — | Polysciences, Inc. |
| Anti-PDI (A6) | Rabbit | — | 1:750 | Abcam |
| Anti–α-Synuclein (C-20) | Rabbit | 1:1,000 | — | Santa Cruz Biotechnology |
| Alexa Fluor 488 streptavidin | — | — | 1:300 | Thermo Fisher Scientific |
Table S5.
Peptide sequences
| Peptide name | Sequence, N terminus→C terminus |
| Helix 5 | Biotin-Ttds-LREIIEAMLKAYQQKLFVTHTVDE (acid) |
| M = 3,404 g/mol | |
| 3×D helix 5 | Biotin-Ttds-LREDDEAMDKAYQQKLFVTHTVDE (acid) |
| M = 3,409 g/mol | |
| Helix 5 TAT | Biotin-Ttds-KFERQLREIIEAMLKAYQQKLFVTHTVDEYGRKKRRQRRR (amide) |
| M = 5,107 g/mol | |
| 3×D helix 5 TAT | Biotin-Ttds-KFERQLREDDEAMDKAYQQKLFVTHTVDEYGRKKRRQRRR (amide) |
| M = 5,113 g/mol | |
| Helical control peptide: ADAM 17 Conserved ADAM-seventeen Dynamic Interaction Sequence (CANDIS) domain (21) | N–KRVQDVIERFWDFIDQLSINTFGK–C |
| M = 2,955 g/mol |
The chaperone-mediated autophagy-targeting motif KFERQ is indicated in italic, whereas the HIV-derived TAT-sequence is highlighted in bold. M, molecular weight.
Table S6.
Buffers, solutions, and recombinant proteins
| Name | Composition or source |
| Laemmli loading buffer | 500 mM Tris/HCl, pH 6.8 |
| 4% SDS | |
| 40% (vol/vol) glycerol | |
| 0.02% bromophenol blue | |
| 400 mM DTT | |
| EBC buffer (cell lysis buffer for co-IP experiments) | 50 mM Tris |
| 120 mM NaCl | |
| 0.5% Nonidet P-40 | |
| pH 7.4 (HCl) | |
| One tablet Complete (Roche) | |
| LIMP-2 ectodomain | Luminal domain of LIMP-2 with C-terminal human IgG-tag (R&D Systems) |
| LAMAN | Zymenex |
| GC (Cerezyme) | Genzyme Therapeutics |
| GC-N370S | Custom-made |
| Mounting solution for IF experiments | 1 mL Mowiol solution [17% Mowiol/33% (vol/vol) glycerol in PBS; pH 6–7] |
| 100 µL DABCO (200 mg/mL diazobicyclooctane; end concentration 50 mg/mL) | |
| 1 µL DAPI solution (end concentration 1 µg/mL) |
Table S7.
Settings for CD spectroscopy
| Setting | |
| Data pitch | 1 nm |
| Scanning mode | Continuous |
| Speed | 5 nm/min |
| Response | 8 s |
| Bandwidth | 2.0 nm |
| Accumulation | Three measurements |
| Wavelength | 250–200 nm |
| Width of cuvette | 0.05 cm |
For statistical analyses, all values are expressed as the mean ± SEM and were analyzed via a two-sided, unpaired Student’s t test or one-way ANOVA followed by a Tukey–Kramer multiple comparison test using GraphPad Instat 3 software when multiple samples were analyzed. In all analyses the null hypothesis was rejected at P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001). If not indicated otherwise, significant differences in the graphs show GC/LIMP-2 mutants compared with each respective wild-type or buffer/control peptides compared with the helix 5 peptide.
SI Experimental Procedures
Cell Culture.
The cell lines used in this study and their sources can be found in Table S2.
Expression Vectors and Transfection of Cells.
Murine and human wild-type/mutant LIMP-2 and wild-type/mutant GC cDNAs were cloned into the pFrog vector (a derivative of pcDNA3.1) using the HindIII and EcoRI restriction sites, according to refs. 1, 3, and 15 and were verified by sequencing (GATC Biotech AG). A summary of all expression vectors used can be found in Table S1. LIMP-2 and GC mutants were generated by site-directed mutagenesis. To insert a point mutation within a DNA sequence, the PCR protocol shown below was performed using a pfu DNA polymerase (Thermo Fisher Scientific). Oligonucleotides carrying the desired point mutations were purchased from Sigma Aldrich (Table S3). All LIMP-2 constructs were C-terminally tagged with an myc sequence (EQKLISEEDL). Cells were transiently transfected with TurboFect (Thermo Fisher Scientific) according to the manufacturer's instructions. In brief, plasmid DNA (3 μg for a 10-cm dish and 1 μg for a 6-cm dish) was incubated with twice the amount of transfection reagent for 20 min in 100–500 μL DMEM high-glucose medium without the addition of FCS (PAA Laboratories) or penicillin/streptomycin (PAA Laboratories; GE Healthcare Life Sciences) before the transfection sample was added to the cells. The transfection reagent was removed ∼6 h after transfection, and the cells were harvested 1–3 d after transfection. The following PCR sequence was used:
| Step | Temperature | Time, min | Cycles |
| 1. | 95 °C | 2:00 | |
| 2. | 95 °C | 0:30 | 35–40 |
| 52–58 °C | 0:30 | ||
| 72 °C | 1:00 | ||
| 3. | 72 °C | 2 min/1,000 bp DNA | |
| 4. | 4 °C | ∞ |
SDS/PAGE and Western Blotting.
Cells were harvested by scraping them off the cell-culture dishes, pelleted (1,500 × g; 4 °C), and lysed by sonification. As a standard lysis buffer, PBS (pH 7.4) including protease inhibitors (Complete; Roche) and 1% of the detergent Triton X-100 was used. For co-IP experiments the cells were lysed in EBC buffer (Table S6). Depending on the size of the cell pellets, 20–150 μL lysis buffer was applied, and the samples were sonicated 2 × 10 s, incubated on ice for 30 min, and sonicated again for 2 × 20 s. Lysates then were centrifuged at 17,000 × g for 10 min at 4 °C. The lysed cell sample (supernatant) was transferred to a clean tube and used for protein concentration by using a BCA kit (Pierce, Thermo Fisher Scientific) according to the manufacturer’s manual.
For Western blotting 20–40 μg of protein was loaded on a 10% Tris-SDS (made in house) or 4–12% Bis-Tris/NuPAGE Novex gel system (Thermo Fisher Scientific), subjected to electrophoresis, and blotted on nitrocellulose or PVDF membranes [2 h at 4 °C and 0.85 ampere (A) constant]. Membranes were blocked in 5% (wt/vol) milk Tris-buffered saline (TBS-T, pH 7.4, 0.1% Tween-20), and primary antibodies were incubated overnight (for more details on antibody dilutions see Table S4). PVDF membranes for α-synuclein detection were postfixed in 0.4% paraformaldehyde (PFA) (Polysciences Inc.) for 20 min after blotting. Signals were normalized to the respective loading controls [actin, neuronal-specific enolase (NSE), or GAPDH] (Table S4). Primary antibodies were incubated overnight at 4 °C. After three washing steps in TBS-T the membranes were incubated for 1 h with the respective secondary antibody at room temperature. After the membrane was washed again three times with TBS-T, the signal of the antibody was detected using a chemiluminescence detection system (LAS4000; GE Healthcare Life Sciences) or by scanning on an infrared imager (Odyssey; LI-COR Biosciences).
Antibodies.
Table S4 gives detailed information about the primary antibodies used. Secondary antibodies used included Alexa Fluor 488 and 594 (Life Technologies) and peroxidase conjugates (Dianova). For visualization of peroxidase-conjugated secondary antibodies, signals were detected by chemiluminescence (SuperSignal West; Pierce) with densitometric analyses performed using Image J (Wayne Rasband, NIH).
Immunoblots for α-synuclein (C-20) were developed using the LI-COR imaging system. Alexa Fluor-labeled secondary antibodies were used, and signal intensities were analyzed with Image Studio software (LI-COR Biosciences).
Deglycosylation of Proteins/Molecular Shift Assay.
To study the subcellular localization and transport of the various GC mutants (ER and post-ER localization), EndoH and PNGaseF digestions were performed. For both reactions 20 μg of protein was used, and the experimental procedure was performed according to the manufacturer’s handbook (New England Biolabs). A positive digestion resulted in a shift in molecular size after the protein was subjected to SDS/PAGE. α-hGC (Table S4) was used to detect the different forms of GC. The ratio of the post-ER (70–74 kDa)/ER (55 kDa) forms of GC was determined and used as a measurement of GC protein transport.
Co-IP.
For co-IP studies cells were lysed in EBC buffer (Table S6), and 500–1,000 μg of protein lysate were incubated with the LIMP-2 antibody (Table S4) overnight at 4 °C. Blocked (1% BSA) magnetic agarose G beads (Thermo Fisher Scientific, Life Technologies) were added to the lysates. Antibody precipitation was performed for 30 min at room temperature. The beads were washed four times with EBC buffer. After the last washing, the supernatant was discarded carefully, and the beads were incubated with Laemmli buffer (Table S6) at 60 °C for 15 min and subsequently were analyzed by SDS/PAGE and immunoblotting. Coprecipitated GC was visualized using α-hGC (Table S4).
Peptides and Recombinant Proteins.
All peptides were purchased from JPT Peptide Technologies (Table S5). Peptides and recombinant proteins (GC: Cerezyme, Genzyme Therapeutics; GC N370S: made in house; LAMAN: Zymenex; and LIMP-2 ectodomain: R&D Systems) (Table S6) were dissolved in a NaPi buffer (50 mM sodium phosphate, 150 mM NaCl, pH 7). For peptide studies including pulldown, activity, and uptake assays, recombinant GC was incubated with a 10-fold higher molarity of the peptides unless otherwise stated. The concentration of the peptides and the recombinant proteins was determined spectroscopically on a Nanodrop 2000c spectrophotometer (Thermo Fisher Scientific).
Peptide Pulldowns.
For the pulldown of recombinant protein using LIMP-2–derived peptides, 2 nmol of protein (GC, LAMAN, and BSA) were incubated overnight at 4 °C with the 10-fold molecular amount (20 nmol) of biotinylated LIMP-2–derived peptides (helix 5, 3×D, and TAT peptides; see Table S5) in NaPi buffer. The total incubation volume was 250 μL. High-capacity streptavidin beads (Thermo Fisher Scientific) were blocked with 1% BSA solution and equilibrated with the NaPi buffer. For each pulldown 50 μL of beads were incubated with 200 μL of the enzyme/peptide sample for 30 min at room temperature. Fifty microliters of the initial enzyme/peptide mixture were prepared for SDS/PAGE (input fraction). After the beads were incubated with the enzyme/peptide mixture, the samples were centrifuged for 1 min at 1,500 × g at 4 °C. The supernatant was removed and stored at 4 °C (unbound fraction). Subsequently, the beads were washed three times with NaPi buffer followed by centrifugation at 1,500 × g at room temperature. The washing buffer was removed, and the beads were incubated with 30 μL Laemmli loading buffer (Table S6) at 60 °C for 20 min, resulting in the release of the precipitated enzymes and peptides (bound fraction). The input and bound fractions of the pulldown experiments were loaded on a NuPAGE Novex 4–12% Bis-Tris gels (Thermo Fisher Scientific), subjected to electrophoresis, and stained with Coomassie Brilliant Blue (R-250; Bio-Rad). The gels were scanned after destaining.
For pulldown of endogenous and overexpressed GC from Cos7 cell lysates, 20 nmol of LIMP-2–derived peptide (helix 5 and 3×D) were incubated with 50 μL of cell lysate at 4 °C overnight. The cells were harvested in EBC buffer (Table S6). After the streptavidin beads were blocked and equilibrated with EBC buffer (see above), the cell lysate/peptide mixtures were added to the beads for 30 min at room temperature. Then the supernatant was removed. After the beads were extensively washed with EBC buffer, the bound peptides and proteins were released from the beads by incubation with Laemmli buffer at 60 °C for 20 min (see above). After centrifugation for 2 min at 17,000 × g the supernatant was removed (bound fraction) and subjected to SDS/PAGE and Western blotting. α-hGC was used for detection. For details see Table S4.
Uptake Assay of LIMP-2–Derived TAT Peptides in H4 Cells.
The α-synuclein turnover rate in the presence of the LIMP-2–derived peptides was assessed in H4 cells stably overexpressing α-synuclein (Table S2). To stop de novo synthesis of α-synuclein, cells were treated with 2 μg/mL doxycycline at the start of the assay and were incubated with 2 nmol of uptake-optimized TAT peptides (helix 5 and 3×D; see Table S5) in 2 mL cell medium in a 6-cm culture dish. For prolonged treatment of cells, new TAT peptides were applied to the cell medium every 24 h.
Enzyme Activity Assays.
Whole-cell/recombinant protein activity assay.
To determine the enzymatic activity of the recombinant enzymes (Table S6), lyophilized enzymes were reconstituted in the NaPi buffer described above. The lyophilized samples of wild-type GC (Cerezyme) and the custom-made recombinant N307S mutant enzyme contain 0.01% polysorbate 80 (Tween 80) (see prescription information for Cerezyme at https://www.cerezyme.com/). As indicated in our experiments, the concentration of the detergent is sufficient to support active GC enzyme and to perform the in vitro GC activity assays without adding further detergents to the reaction mix. For enzyme activity assays of recombinant proteins, 0.1–0.2 nmol of GC (Cerezyme; N370S) and LAMAN were used in the absence or presence of 10× the molar amount of LIMP-2–derived peptides (Table S5) or LIMP-2 ectodomain (1–2 nmol) (Table S6).
Whole-cell or recombinant enzyme activity of GC and LAMAN was measured using 4-nitrophenyl β-d-glucopyranoside (Fig. 3E and Fig. S3 D, F, and G) or 4-Methylumbelliferyl β-d-glucopyranoside (4 MU) (Fig. 3K) and 10 mM 4-nitrophenyl-N-acetyl-β-d-glucosaminide (Fig. S3E) (all substrates were purchased from Sigma-Aldrich). The artificial substrates were dissolved in sodium citrate buffer (0.2 M Na-citrate, 0.4% BSA, pH 4.6). All activity assays were performed at acid pH.
Cell lysate protein (20–200 μg) or recombinant protein (0.2 nmol GC/N370S or LAMAN) was incubated with 100 μL of 10 mM artificial absorbent substrate (4-nitrophenyl β-d-glucopyranoside or 4-nitrophenyl-N-acetyl-β-d-glucosaminide). The samples were incubated at 37 °C for 2 h to measure GC activity or for 5 h to assess LAMAN activity. The reaction was stopped by applying 500–1,000 μL of stop solution (0.4 M glycine, pH 10.4), and the absorbance was measured in a clear 96-well plate at 405 nm in a plate reader (Synergy HT; BioTek). Enzyme activities of cellular lysates were normalized to protein concentration (expressed in milliunits per milligram) and are shown relative to buffer control. Enzyme activities of recombinant proteins are shown as milliunits per milligram or are stated relative to buffer control.
Using the fluorescent substrate 4-MU (Fig. 3K), 10 μL of the recombinant enzyme/peptide mixture (0.1 nmol GC/N370S previously incubated with 1 nmol helix 5 TAT peptide in 50 mM NaPi buffer; total incubation volume 40 μL) were incubated directly with 60 μL of 0.2 M sodium citrate buffer and 10 μL of the substrate (5 mM dissolved in 0.2 M sodium citrate buffer) in a black-bottomed 96-well dish (Nunc no. 446473; Thermo Fisher Scientific). After the mixture was incubated at 37 °C for 30 min, 90 μL of stop solution (see above) was added. The fluorescence was assessed at an excitation wavelength of 365 nm and an emission wavelength of 445 nm in a SpectraMax i3 plate reader (Molecular Devices). GC activity is presented relative to buffer control.
Live-cell lysosomal activity assay.
Lysosomal GC activity in living cells was assessed by applying a drug-response assay in the presence and absence of a lysosomal inhibitor (bafilomycin A1; Invivogen) dissolved in DMSO (24). TAT peptides (10 μM) were added to the cell medium for 1 h; then H4 cells (see Table S2) were incubated with 100 μg/mL cell-permeable artificial substrate 5-(pentafluorobenzoylamino) fluorescein di-β-d-glucopyranoside (PFB-FDGlu) (Life Technologies, Thermo Fisher Scientific) for another hour. Cells were washed with warm medium; then the medium was replaced with phenol red-free neurobasal medium (Life Technologies, Thermo Fisher Scientific). The fluorescence intensity was recorded every 30 min for 3–4 h in a SpectraMax i3 plate reader (Molecular Devices) (PFB-FDGlu: excitation = 485 nm, emission = 530 nm). After the final reading, cells were fixed in 4% formaldehyde/PBS and were stained with CellTag 700 (LI-COR Biosciences) according to the manufacturer’s instructions to measure cell volume. The plate was scanned on an Odyssey infrared imager (LI-COR Biosciences). Fluorescence intensities were normalized to cell volume and graphed versus time. Whole-cell activity was obtained by calculating the area below the DMSO curve. Nonlysosomal activity corresponds to the area under the bafilomycin A1 curve. Lysosomal activity was obtained by subtracting both areas (Fig. S3H).
IF and Pearson’s Index.
IF studies were performed as previously described (1, 15). Cells were grown in six-well dishes on glass coverslips. If necessary, the cells were treated or transfected according to established protocols. When cells reached a confluency of ∼80%, they were fixed with 4% PFA (Polysciences, Inc.) in PBS for 20 min at room temperature. Then they were washed three times with PBS and were permeabilized for 5 min in 0.2% saponin (Sigma Aldrich) in PBS and for 10 min in 0.2% saponin (Sigma Aldrich)/0.12% glycine (Sigma Aldrich) in PBS at room temperature. To reduce unspecific binding of the antibodies, the cells were incubated for 20 min in 0.2% saponin/10% (wt/vol) FCS (PAA Laboratories) in PBS. The primary as well as the secondary antibody was diluted in this blocking solution [0.2% saponin/10% (wt/vol) FCS/PBS; see Table S4 for antibody dilutions]. The primary antibody was incubated for 1 h at room temperature or overnight at 4 °C in a wet chamber. Before incubation in secondary antibody, the coverslips were washed four times in 0.2% Saponin/PBS. The secondary antibody exhibits a fluorophore-labeling (Alexa Fluor 488 nm, 594 nm or 647 nm; Invitrogen, Thermo Fisher Scientific) and was applied in a concentration of 1:500 for 1 h at room temperature. After the coverslips were washed three times in 0.2% saponin/PBS and once in ddH2O, they were embedded on microscope slides with a mixture of DAPI/DABCO (both from Sigma Aldrich)/Mowiol (Calbiochem) (Table S6). The next day the samples were analyzed by confocal laser microscopy (FluoView 1000R; Olympus). The pictures were taken in the sequential mode to prevent an overlay of the different color channels. Cells were visualized at a magnification of 60–100× using oil objectives. The Pearson’s correlation coefficient (PCC) was used to determine the colocalization of two proteins using the FV1000-ASW 3.0 Viewer-Software (Olympus). The PCC is a mathematical description of the degree of colocalization between two fluorophores (35).
For studies visualizing cellular peptide uptake, H4 cells (Table S2) were incubated for 8 h with 10 μM of helix 5 TAT peptide. The presence of the peptide was demonstrated after Alexa Fluor 488 streptavidin (1:300; Thermo Fisher Scientific) binding to the biotin tag of the peptide; then cells were costained for LIMP-2 (for antibody details, see Table S4).
CD Spectroscopy.
The CD measurements were carried out with a Jasco-J-720-CD spectropolarimeter (Japan Spectroscopic Company) at 20 °C. The LIMP-2–derived peptides (helix 5, 3×D helix 5) were dissolved in 50 mM Na phosphate buffer (plus 10 mM NaCl, pH 7) in a concentration of 0.2 μg/μL in a total volume of 300 μL and were measured with the settings given in Table S7.
Supplementary Material
Acknowledgments
We thank Ellen Sidransky and Pamela McLean for the β-glucocerebrosidase (GC)-deficient mouse embryonic fibroblasts and H4 cells, respectively, and Johannes Aerts (Leiden University) for the GC antibody and for critically reading the manuscript. This work was supported by a Böhringer Ingelheim Fonds Fellowship (to F.Z.), a Deutsche Forschungsgemeinschaft (DFG) Heisenberg fellowship (to M.S.), and DFG Grants GRK1459 (to M.S. and J.B.), R01NS076054 (to D.K.), and R01NS092823 (to J.R.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514005113/-/DCSupplemental.
References
- 1.Reczek D, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131(4):770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Blanz J, et al. Mannose 6-phosphate-independent Lysosomal Sorting of LIMP-2. Traffic. 2015;16(10):1127–1136. doi: 10.1111/tra.12313. [DOI] [PubMed] [Google Scholar]
- 3.Blanz J, et al. Disease-causing mutations within the lysosomal integral membrane protein type 2 (LIMP-2) reveal the nature of binding to its ligand beta-glucocerebrosidase. Hum Mol Genet. 2010;19(4):563–572. doi: 10.1093/hmg/ddp523. [DOI] [PubMed] [Google Scholar]
- 4.Berkovic SF, et al. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am J Hum Genet. 2008;82(3):673–684. doi: 10.1016/j.ajhg.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: Mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Hum Mutat. 2008;29(5):567–583. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 6.Nalls MA, et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 2013;70(6):727–735. doi: 10.1001/jamaneurol.2013.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westbroek W, Gustafson AM, Sidransky E. Exploring the link between glucocerebrosidase mutations and parkinsonism. Trends Mol Med. 2011;17(9):485–493. doi: 10.1016/j.molmed.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gegg ME, et al. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol. 2012;72(3):455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothaug M, et al. LIMP-2 expression is critical for β-glucocerebrosidase activity and α-synuclein clearance. Proc Natl Acad Sci USA. 2014;111(43):15573–15578. doi: 10.1073/pnas.1405700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bras J, et al. Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum Mol Genet. 2014;23(23):6139–6146. doi: 10.1093/hmg/ddu334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sardi SP, et al. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci USA. 2011;108(29):12101–12106. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sardi SP, et al. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc Natl Acad Sci USA. 2013;110(9):3537–3542. doi: 10.1073/pnas.1220464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siebert M, Sidransky E, Westbroek W. Glucocerebrosidase is shaking up the synucleinopathies. Brain. 2014;137(Pt 5):1304–1322. doi: 10.1093/brain/awu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzulli JR, et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146(1):37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neculai D, et al. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature. 2013;504(7478):172–176. doi: 10.1038/nature12684. [DOI] [PubMed] [Google Scholar]
- 16.Brumshtein B, Wormald MR, Silman I, Futerman AH, Sussman JL. Structural comparison of differently glycosylated forms of acid-beta-glucosidase, the defective enzyme in Gaucher disease. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 12):1458–1465. doi: 10.1107/S0907444906038303. [DOI] [PubMed] [Google Scholar]
- 17.Dvir H, et al. X-ray structure of human acid-beta-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep. 2003;4(7):704–709. doi: 10.1038/sj.embor.embor873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cormand B, et al. Mutation analysis of Gaucher disease patients from Argentina: High prevalence of the RecNciI mutation. Am J Med Genet. 1998;80(4):343–351. doi: 10.1002/(sici)1096-8628(19981204)80:4<343::aid-ajmg8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Kawame H, Eto Y. A new glucocerebrosidase-gene missense mutation responsible for neuronopathic Gaucher disease in Japanese patients. Am J Hum Genet. 1991;49(6):1378–1380. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Ren J, Padilla-Parra S, Fry EE, Stuart DI. Lysosome sorting of β-glucocerebrosidase by LIMP-2 is targeted by the mannose 6-phosphate receptor. Nat Commun. 2014;5:4321. doi: 10.1038/ncomms5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Düsterhöft S, et al. Extracellular juxtamembrane segment of ADAM17 interacts with membranes and is essential for its shedding activity. Biochemistry. 2015;54(38):5791–5801. doi: 10.1021/acs.biochem.5b00497. [DOI] [PubMed] [Google Scholar]
- 22.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 23.Horst M, Knecht EC, Schu PV. Import into and degradation of cytosolic proteins by isolated yeast vacuoles. Mol Biol Cell. 1999;10(9):2879–2889. doi: 10.1091/mbc.10.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. Alpha-synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci USA. 2016;113(7):1931–1936. doi: 10.1073/pnas.1520335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liou B, et al. Analyses of variant acid beta-glucosidases: Effects of Gaucher disease mutations. J Biol Chem. 2006;281(7):4242–4253. doi: 10.1074/jbc.M511110200. [DOI] [PubMed] [Google Scholar]
- 26.Grace ME, Graves PN, Smith FI, Grabowski GA. Analyses of catalytic activity and inhibitor binding of human acid beta-glucosidase by site-directed mutagenesis. Identification of residues critical to catalysis and evidence for causality of two Ashkenazi Jewish Gaucher disease type 1 mutations. J Biol Chem. 1990;265(12):6827–6835. [PubMed] [Google Scholar]
- 27.Atrian S, et al. An evolutionary and structure-based docking model for glucocerebrosidase-saposin C and glucocerebrosidase-substrate interactions - relevance for Gaucher disease. Proteins. 2008;70(3):882–891. doi: 10.1002/prot.21554. [DOI] [PubMed] [Google Scholar]
- 28.Lieberman RL. A guided tour of the structural biology of Gaucher disease: Acid-β-glucosidase and saposin C. Enzyme Res. 2011;2011:973231. doi: 10.4061/2011/973231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sly WS, Kaplan A, Achord DT, Brot FE, Bell CE. Receptor-mediated uptake of lysosomal enzymes. Prog Clin Biol Res. 1978;23:547–551. [PubMed] [Google Scholar]
- 30.Stahl PD, Rodman JS, Miller MJ, Schlesinger PH. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci USA. 1978;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pastores GM, et al. Therapeutic goals in the treatment of Gaucher disease. Semin Hematol. 2004;41(4) Suppl 5:4–14. doi: 10.1053/j.seminhematol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Liou B, Haffey WD, Greis KD, Grabowski GA. The LIMP-2/SCARB2 binding motif on acid β-glucosidase: Basic and applied implications for Gaucher disease and associated neurodegenerative diseases. J Biol Chem. 2014;289(43):30063–30074. doi: 10.1074/jbc.M114.593616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benito JM, García Fernández JM, Ortiz Mellet C. Pharmacological chaperone therapy for Gaucher disease: A patent review. Expert Opin Ther Pat. 2011;21(6):885–903. doi: 10.1517/13543776.2011.569162. [DOI] [PubMed] [Google Scholar]
- 34.Patnaik S, et al. Discovery, structure-activity relationship, and biological evaluation of noninhibitory small molecule chaperones of glucocerebrosidase. J Med Chem. 2012;55(12):5734–5748. doi: 10.1021/jm300063b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson K. Determination of the Coefficient of Correlation. Science. 1909;30(757):23–25. doi: 10.1126/science.30.757.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.