Significance
The secretion of aqueous humor from the ciliary body is regulated by osmotic gradients, yet the mechanism through which these cells sense these gradients is still under debate. We have identified the calcium-permeable transient receptor potential vanilloid isoform 4 (TRPV4) ion channel as critical for translating hypotonic stimuli into intracellular signals and linked the activation of this channel to a known proinflammatory lipid signaling pathway. The channel was confined to nonpigmented cells that secrete aqueous fluid and regulate intraocular pressure. Thus, activation of TRPV4 may contribute to vision through metabolic support of anterior eye tissues and regulation of osmotic and tensile homeostasis within the eye.
Keywords: TRPV4, ciliary body, intraocular pressure, aqueous humor, glaucoma
Abstract
Fluid secretion by the ciliary body plays a critical and irreplaceable function in vertebrate vision by providing nutritive support to the cornea and lens, and by maintaining intraocular pressure. Here, we identify TRPV4 (transient receptor potential vanilloid isoform 4) channels as key osmosensors in nonpigmented epithelial (NPE) cells of the mouse ciliary body. Hypotonic swelling and the selective agonist GSK1016790A (EC50 ∼33 nM) induced sustained transmembrane cation currents and cytosolic elevations in dissociated and intact NPE cells. Swelling had no effect on levels in pigment epithelial (PE) cells, whereas depolarization evoked elevations in both NPE and PE cells. Swelling-evoked signals were inhibited by the TRPV4 antagonist HC067047 (IC50 ∼0.9 μM) and were absent in Trpv4−/− NPE. In NPE, but not PE, swelling-induced signals required phospholipase A2 activation. TRPV4 localization to NPE was confirmed with immunolocalization and excitation mapping approaches, whereas in vivo MRI analysis confirmed TRPV4-mediated signals in the intact mouse ciliary body. Trpv2 and Trpv4 were the most abundant vanilloid transcripts in CB. Overall, our results support a model whereby TRPV4 differentially regulates cell volume, lipid, and calcium signals in NPE and PE cell types and therefore represents a potential target for antiglaucoma medications.
Formation of aqueous humor in the vertebrate eye takes place within the ciliary body (CB), a highly folded tissue consisting of pigmented epithelial (PE) cells, nonpigmented epithelial (NPE) cells, and the ciliary muscle (1, 2). Together, PE cells, which face the vascularized stroma and represent a forward continuation of the retinal pigment epithelium (RPE), and NPE cells, which face the posterior chamber (lumen) of the eye and extend the neuronal retina, form the blood–aqueous barrier and regulate the production and secretion of aqueous humor. The aqueous fluid supplies nutrients and oxygen to nonvascularized tissues (lens, cornea, and trabecular meshwork) and is ultimately drained through the ciliary muscle and the trabecular meshwork in the anterior chamber of the eye. Aqueous secretion is subserved by the unidirectional transport of ions and water through gap junctions between PE cells and NPE cells (3, 4) and is driven by the osmotic gradient generated by Na+/K+ exchange across basolateral NPE membranes (2–5). Despite the critical dependence of aqueous humor secretion on osmotic pressure (1, 4, 6), the molecular mechanism through which NPE and PE cells sense and regulate changes in volume is not well understood.
In addition to osmotic shifts, CB cells experience mechanical forces associated with mean and time-varying aspects of intraocular pressure (IOP), a phenomenon that reflects balanced regulation of fluid secretion from NPE cells and its drainage from the anterior eye. Excessive IOP elevations represent the primary, and major, risk factor for contracting glaucoma (6, 7), an optic neuropathy that represents the second leading cause of blindness in the world. Therefore, aqueous secretion is often targeted by antiglaucoma medications that include β-adrenergic receptor antagonists, carbonic anhydrase inhibitors, α2-adrenergic agonists, and muscarinic cholinergic agonists (7). A key question, however, is whether CB cells themselves are able to sense force mediated by membrane stretch induced by hydrostatic pressure or swelling, and what such mechanisms might be.
Here, we identify a key osmosensor in CB as transient receptor potential channel vanilloid isoform 4 (TRPV4), a polymodal nonselective cation-permeable channel that has been implicated in mechanotransduction (8, 9) as well as regulation of paracellular permeability in multiple epithelial tissues (10–15). Intriguingly, we found that TRPV4 is selectively distributed across CB by being confined to the NPE and excluded from PE cells. We characterized the functional role of TRPV4 as the predominant NPE swelling sensor and determined its contribution to swelling-dependent intracellular second messenger signaling mediated through calcium ions and long-chain, polyunsaturated lipids associated with the phospholipase A2 (PLA2) pathway. By elucidating the molecular mechanisms that underlie differential volume regulation in the two CB constituent cell types, and characterizing their susceptibility to lipid messenger modulation, our findings may provide new insight into the mechanism of aqueous fluid secretion and IOP modulation. A preliminary account of this work has been recently given (16).
Results
TRPV4 Immunolocalization Within the CB.
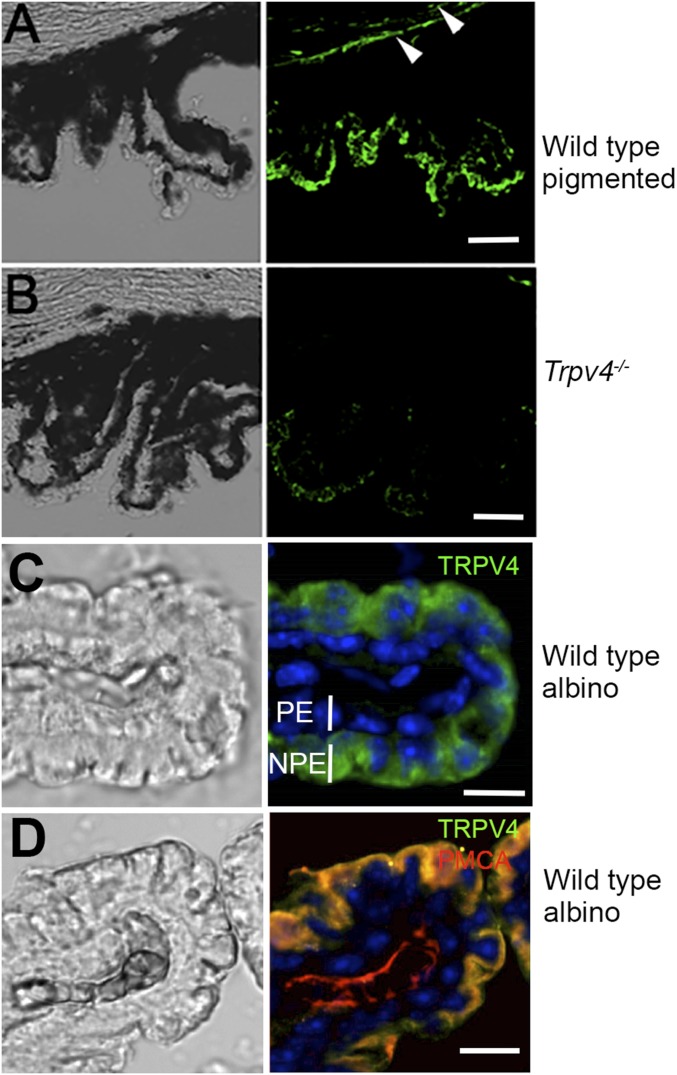
We sought to determine the identity of the osmotransducer that links hypotonic stimuli to Ca2+ homeostasis in CB cells by focusing on TRPV4, a nonselective cation channel that was originally identified by its sensitivity to hypotonic challenge (8, 17) but was also recently suggested to regulate melatonin release from the CB (18). Immunocytochemistry with a validated antibody (19, 20), showed clear TRPV4 immunoreactivity (ir) across the mouse CB. The fluorescent signal was predominantly concentrated in the ciliary processes of the pars plicata (arrows in Fig. 1A, iv), whereas stroma showed minimal immunoreactivity (arrowheads in Fig. 1A, iv). Staining was also observed in the corneal epithelium that anchors the CB (arrowhead in Fig. 1A, ii). The specificity of the signals was confirmed by concomitant staining of Trpv4−/− tissue, which showed markedly lower fluorescence compared with the WT CB and by labeling CB tissue from nonpigmented mice (Fig. S1).
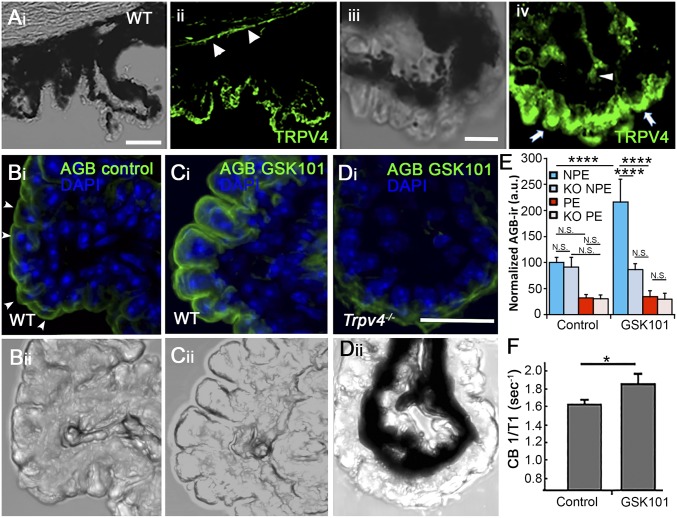
Fig. 1.
Localization and functional expression of TRPV4 in the mouse CB. (A, i and ii) Vertical cryosections of WT mouse retinas immunolabeled for TRPV4 show preferential localization to the basolateral layer corresponding to NPE, with additional signals in the limbal corneal epithelium (arrowheads). (i) Transmitted image of the CB and the supporting cornea; (ii) TRPV4 immunoreactivity. (Scale bar, 100 μm.) (iii and iv) Close-up of the CB epithelium, showing TRPV4-ir in putative NPE cells (arrows). Modest immunofluorescence is detected in the putative stromal area (arrowhead). (Scale bar, 20 μm.) (B–E) Excitation mapping shows TRPV4-evoked cation influx into CB ex vivo. CBs were fixed, stained with an anti-AGB antibody (FITC), and counterstained with DAPI (blue). ROIs of equal size were placed around DAPI-positive cells in the NPE layer and PE layer, respectively. (B, i and ii) Transmitted + fluorescence image of isolated unstimulated (PBS-treated) nonpigmented CB tissue. (C, i and ii) Transmitted + fluorescence image of nonpigmented CB tissue stimulated with GSK101 (100 nM) and tested for AGB-ir + DAPI. (D, i and ii) Transmitted + fluorescence image of Trpv4−/− CB tissue stimulated with GSK101 simultaneously with preparations in B and C. (Scale bar, 20 μm.) (E) Quantification of fluorescence from GSK101-treated vs. PBS-treated WT and KO sections. P < 0.005. (F) In vivo CB signal evaluated using MEMRI. Quantification of average CB 1/T1 from vehicle (n = 5) and GSK101-treated (100 μM; n = 5) eyes. Values are presented as means ± SEM; *P = 0.02, ***P < 0.005, ****P < 0.0001.
Fig. S1.
(A and B) Vertical sections of concomitantly labeled pigmented WT and Trpv4−/− ciliary bodies (see also Fig. 1A, ii and iv). (Scale bars, 100 μm.) The KO tissue exhibits little TRPV4-ir. Arrowheads in A point at TRPV4-ir in the corneal endothelium. (C) Nonpigmented CB tissue labeled for TRPV4 and counterstained for DAPI shows immunoreactivity predominantly in the NPE layer. (Scale bar, 20 μm.) (D) Nonpigmented CB. Double labeling with the anti-TRPV4 and anti-PMCA antibodies shows colocalization between TRPV4 and the calcium pump within the NPE plasma membrane. PMCA is also expressed in the stroma. (Scale bar, 20 μm.)
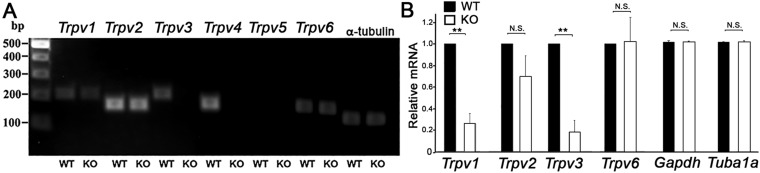
Because vanilloid TRP isoforms show a predilection toward heteromerization (21, 22), we evaluated the overall expression pattern of Trpv4 and cognate vanilloid mRNAs in the mouse CB tissue. Primers targeting TRP isoforms (Table S1) showed strong expression for Trpv2 and Trpv4 transcripts in every sample (n = 3). Messenger RNAs produced by Trpv1, Trpv3, and Trpv6 genes were also detected (Fig. S2), but their expression levels were markedly lower compared with isoforms 2 and 4. Down-regulation of Trpv1 and Trpv3, but not Trpv2, gene expression in Trpv4−/− CB suggests that, as observed recently in the retina (23), TRPV4 activation might be linked to the transcription apparatus.
Table S1.
Primers used for detection of mTrpv, mGapdh, and mTuba1a sequences in CB
| Name | NCBI reference | Forward primer (5′-3′) | Reverse primer (5′-3′) |
| mTrpv1 | NM_001001445.2 | AGGGTGGATGAGGTGAACTGGACT | GCTGGGTGCTATGCCTATCTCG |
| mTrpv2 | NM_011706.2 | GTTGGCCTACGTCCTCCTCACCTA | TGCACCACCAGTAACCATTCTCC |
| mTrpv3 | NM_145099.2 | CTCACCTTCGTCCTCCTCCTCAAC | CAGCCGGAAGTCCTCATCTGCTA |
| mTrpv4 | NM_022017.3 | TCCTGAGGCCGAGAAGTACA | TCCCCCTCAAACAGATTGGC |
| mTrpv5 | NM_001007572.2 | GCCACACTGCTCATGCTCAACTT | AGGCCGTATTCGCAACCACAGAT |
| mTrpv6 | NM 022413.4 | GACTCTGTGGTCCGTGCCTCAT | CAGTGTTCTCCATCCGTCGTCTG |
| mGapdh | NM_001289726.1 | GGTTGTCTCCTGCGACTTCA | TAGGGCCTCTCTTCCTCAGT |
| mTuba1a | NM_001653.2 | AAGCAGCAACCATGCGTGA | CCTCCCCCAATGGTCTTGTC |
Fig. S2.
(A) The CB expresses mRNAs coding for multiple vanilloid TRP isoforms. Analysis of Trpv transcripts shows prominent signals for Trpv2 and Trpv4 primers and weaker signals for Trpv1, TRpv3, and Trpv6 primers (n = 3). (B) Semiquantitative real-time PCR analysis of mRNA levels in WT and Trpv4−/− CB tissue shows significant decreases in the relative abundance of Trpv1 and Trpv3 transcripts but no changes in the expression of housekeeping Gapdh and α-tubulin (Tuba1a) genes (n = 5).
Functional TRPV4 Expression in CB in Vivo and ex Vivo.
To investigate functional TRPV4 expression at higher spatial resolution, we performed excitation mapping in intact CB sheets from WT and KO tissue. To eliminate the potentially confounding fluorescence screening by the pigment, the WT experiments were additionally performed in isolated from nonpigmented [B6.Cg-Tg(Thy1-YFP)HJrs/J] mouse eyes. Tissue was perfused with AGB+, an immunogenic cation that has been used previously to mark activity-dependent cation permeability in vertebrate tissues, including the retina (20, 24), and double-labeled for DAPI to localize NPE vs. PE nuclei. The anti-AGB antibody showed a modest degree of ir at the basolateral membrane in unstimulated cells preincubated with the immunogen (arrowheads in Fig. 1B, i), suggesting unstimulated NPE experiences steady-state cation influx. Despite the steady-state AGB+ signal in the basolateral NPE (Fig. 1B), comparison of fluorescence averaged across regions of interest (ROIs) positioned over NPE vs. PE layers showed them not to be significantly different (Fig. 1E). The selective agonist GSK1016790A (GSK101) (100 nM) profoundly enhanced AGB-ir at the basolateral edge of the NPE layer (Fig. 1 C, i and E), which expresses the highest density of TRPV4 channels (Fig. 1A and Fig. S1). The normalized AGB-ir in WT CB was 2.16 ± 0.34-fold higher in GSK101-exposed NPE cells compared with vehicle-treated controls (P < 0.0001; n = 3). The small (and nonsignificant; P > 0.05) increase in ir observed in the PE layer may be attributed to cation influx through gap junctions between the two epithelial layers and/or potential spillover of fluorescence from the NPE layer. AGB-ir in Trpv4−/− tissue (from pigmented animals) did not change following exposure to the TRPV4 agonist (Fig. 1D). Rather, the steady-state cation entry in GSK-treated and unstimulated CB KO cells was similar to signals observed in unstimulated WT cells (Fig. 1 B–E). These data demonstrate that functional TRPV4 signaling is mainly confined to the NPE layer with negligible cation influx into the PE layer or stroma. Interestingly, these data show that the basolateral NPE membrane supports steady-state, non-TRPV4-mediated cation influx that is counterbalanced by plasmalemmal clearance mechanisms that may include high-affinity calcium ATPases (PMCAs; Fig. S1D).
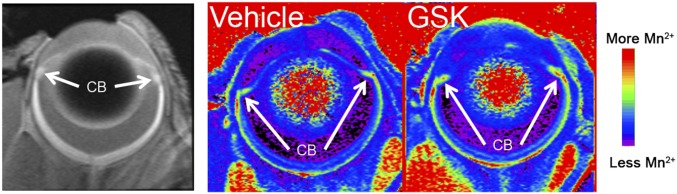
We took advantage of manganese-enhanced MRI (MEMRI) to evaluate the functional consequence of TRPV4 activation in the intact mouse eye. MEMRI, based on the activity-dependent Mn2+ influx as a contrast agent that readily permeates cation channels but is much more slowly removed through intracellular clearance mechanisms, represents the imaging modality of choice for noninvasively mapping ion influx into ocular cells in vivo (25–28). In vehicle-treated eyes, systemic (i.p.) injection of MnCl2 resulted in a 51% increase over baseline CB 1/T1 values. Intravitreal injection of the GSK101 evoked an 86% increase in the CB over baseline that was statistically significant (P < 0.05) compared with eyes injected with the vehicle (PBS) (Fig. 1F and Fig. S3). These data are consistent with functional TRPV4 signals in the mouse CB.
Fig. S3.
TRPV4 activation mediates cation influx into CB in vivo. MEMRI. (Left) Representative data of a control eye in vivo indicating the CB ROI. (Middle and Right) Representative 1/T1 maps from a control (Middle) and treated (Right) eye illustrating the increased uptake in the treated eye. The same color scheme was applied to both images.
Swelling Triggers TRPV4-Mediated Increase in NPE, but Not PE, Cells.
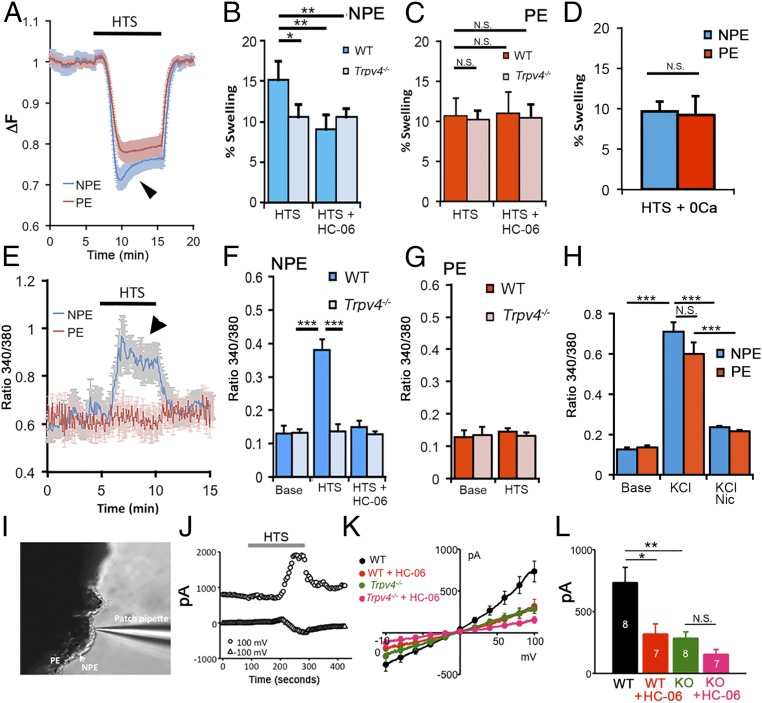
Although osmotic gradients are likely to play a key role in fluid ultrafiltration and aqueous secretion in the CB (2, 4), it is not yet known how CB cells sense the swelling impelled by hypotonic environments. Fig. 2 shows that the volume of both CB cell types is highly sensitive to a decrease in extracellular tonicity. A 37% decrease in tonicity at constant ionic strength, achieved by switching to hypotonic stimulation (HTS; 190 mOsm) from isotonic saline (300 mOsm), reversibly decreased fluorescence intensity in dissociated PE and NPE cells (Fig. 2 A–C), indicating an increase in cell volume. It was immediately apparent that NPE cells exhibit a greater tendency to swell compared with PE (Fig. 2A). Furthermore, in contrast to PE cells, fluorescent signals in NPE cells gradually recovered in the continued presence of HTS, indicative of regulatory volume decrease (RVD). Following termination of the hypotonic challenge, both NPE and PE cells rapidly recovered the initial volume.
Fig. 2.
Hypotonic challenge (190 mOsm) differentially regulates cell swelling and calcium homeostasis in dissociated NPE and PE cells. (A) Cell volume changes in concomitantly recorded NPE (blue) and PE (red) cells. The 340/380 Fura-2 ratio ΔF, adjusted to yield calcium-insensitive fluorescence, shows dose-dependent decreases as cell volume increases in the presence of hypotonic saline. Both volume increase and RVD (arrowhead) are more pronounced in NPE cells. (B) Percent swelling in NPE cells. WT cells (dark blue) swell more compared with Trpv4−/− cells (light blue); the TRPV4 antagonist HC-06 (1 μM) suppresses volume increase in WT (n = 33) but not KO cells (n = 29). (C) WT PE cells (dark red; n = 30) and Trpv4−/− cells (light red; n = 31). Percent swelling is unaffected by the loss of the Trpv4 gene or TRPV4 antagonism. (D) NPE and PE cells swell by comparable amounts in the absence of extracellular Ca2+ (n = 23 and 27, respectively). (E–G) HTS elevates in NPE (n = 57), but not PE (n = 42), cells. (F) HTS-evoked signals are antagonized by HC-06 and absent in Trpv4−/− cells. (G) [Ca2+]i levels in WT (n = 46) and KO (n = 53) PE cells are unaffected by HTS. (H) Thirty millimolar KCl. Depolarization evokes comparable elevations in NPE (blue; n = 23) and PE (red, n = 21) cells. High KCl-induced signals were antagonized by the L-type channel blocker nicardipine. (I–L) Whole-cell patch clamp experiments. Hypotonicity activates TRPV4-mediated currents in NPE cells. (I) Experimental configuration with NPE recording in ex vivo CB tissue. (J) Representative time course of the whole-cell current in a NPE cell at the holding potentials of −100 mV and 100 mV. I-V curves taken immediately before the application of HTS (baseline) were subtracted from preceding and following traces. (K) Averaged current–voltage relationship curves of HTS-induced transmembrane currents in untreated control, Trpv4−/−, and HC-06–treated NPE cells. The antagonist (5 μM) was administered at least 10 min before applying HTS. HTS-induced currents were calculated by subtracting I-V curves taken before the application of HTS from the peak response. (L) Cumulative data for GSK101-induced current amplitudes at +100 mV. The numbers in bars indicate the number of cells studied per each condition. Results are represented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.005.
To test whether HTS-induced swelling in CB cells is facilitated by TRPV4 activation, we exposed them to HTS in the presence of the selective antagonist HC067047 (HC-06). As illustrated in Fig. 2 B and C, HC-06 significantly suppressed the extent of HTS-induced increase in NPE cell volume but had little effect on swelling of (melanin-containing) PE cells. Consistent with this observation, the extent of cell swelling was inhibited in NPE cells isolated from Trpv4−/− tissues, whereas Trpv4−/− PE cells exhibited no differences from controls (Fig. 2 B and C). These findings suggest that TRPV4 channels differentially regulate swelling within the CB.
Given that TRPV4 channels are permeable to Ca2+ (PCa/PNa ∼6) (8, 9, 11), which regulates numerous epithelial functions that include secretion (12–15, 29, 30), we tested whether HTS affects Ca2+ homeostasis in PE and NPE cells. Analysis of fluorescent signals from cells loaded with the Ca2+ indicator dye Fura-2 AM showed cell swelling to be associated with significant elevations in (Fig. 2 E and F). Interestingly, this effect was confined to NPE cells, in which exposure to 190 mOsm saline increased the average 340/380 ratio by 65.55 ± 7.56% from the baseline of 0.13 ± 0.021 (n = 57; P < 0.0001) (Fig. 2F). During continued HTS, levels returned to a sustained plateau that mirrored RVD dynamics observed during volume measurements (Fig. 2 A and E, arrowheads). HTS-evoked [Ca2+]i responses were not observed in the absence of extracellular Ca2+ (Fig. 2D), indicating that that Ca2+ influx across the plasma membrane is required for swelling-induced Ca2+ homeostasis. The selective TRPV4 antagonist HC-06 reduced the amplitude of HTS-evoked elevations by 82.21 ± 9.55% to 0.1486 ± 0.02 (n = 46; P < 0.05) (Fig. 2F). To determine whether TRPV4 is necessary for mediating the osmoresponse in the hypotonic direction, we challenged CBs from Trpv4−/− tissue with HTS. levels in KO NPE cells were unaffected by the exposure to HTS (n = 53), demonstrating that TRPV4 mediates hypotonically evoked calcium signals in NPE. Interestingly, PE cells did not respond to HTS with elevations (Fig. 2G). These observations indicate that NPE and PE cells exhibit fundamental differences in terms of their osmotransduction and Ca2+ homeostasis.
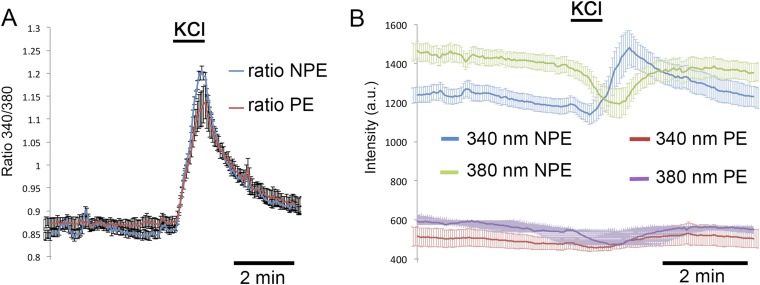
Given the fundamental difference in TRPV4 sensitivity, we asked whether NPE and PE cells differ in their response to depolarization, known to induce regenerative calcium signals in rabbit CB (31). Mouse CB cells responded to depolarization evoked by increased extracellular KCl (30 mM) with elevations. Unlike the responses evoked by GSK101 and despite the fluorescence screening by PE pigment, the amplitudes of depolarization-evoked signals denoted by 340/380 ratios were comparable in NPE and PE cells, and were antagonized by nicardipine (5 μM) (Fig. 2H and Fig. S4). Thus, depolarization-evoked responses in both epithelial layers of the CB are mediated by high-threshold L-type channels.
Fig. S4.
Dissociated CB cells. (A) Fluorescence 340/380 nm ratios and (B) emission intensities evoked by 340-, 380-nm excitation of Fura-2 in NPE and PE cells observed during high K stimulation (30 mM KCl). Although the absolute emission intensities are twice as strong in NPE cells (green and blue traces in B) compared to PE cells (red and purple traces in B), the 340/380 ratio magnitudes for NPE vs. PE cells (blue vs. red traces shown in A) are similar.
We next addressed the functional connection linking TRPV4-mediated Ca2+ signaling and cell swelling by directly recording transmembrane ion fluxes in NPE cells. These experiments were performed in the presence of carbenoxolone (100 µM) and mefloquine (10 µM) to suppress potential leakage across gap junctions and hemichannels, and/or possible ATP release (16, 32, 33). As illustrated in Fig. 2 I–L, HTS evoked reversible increases in the amplitude of the transmembrane current. HTS-induced currents had outwardly rectifying current–voltage relationship, with amplitudes of −381.8 ± 77.0 pA and 732.9 ± 124.7 pA at the holding potentials −100 mV and 100 mV, respectively (n = 8 cells). HTS-evoked currents were significantly attenuated by HC-06 to −185.5 ± 63.0 pA and 317.0 ± 83.7 pA at −100 mV and 100 mV, respectively (n = 7 cells, P < 0.05; Fig. 2 K and L). Thus, during the NPE swelling response, TRPV4 mediates a significant portion of the membrane conductance.
TRPV4 Activation Mediates Increases in NPE, but Not PE, Cells.
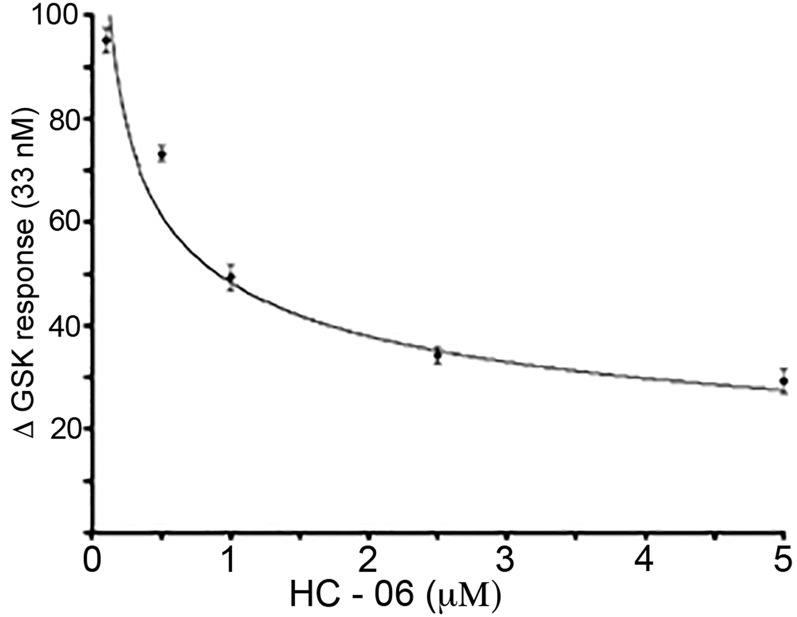
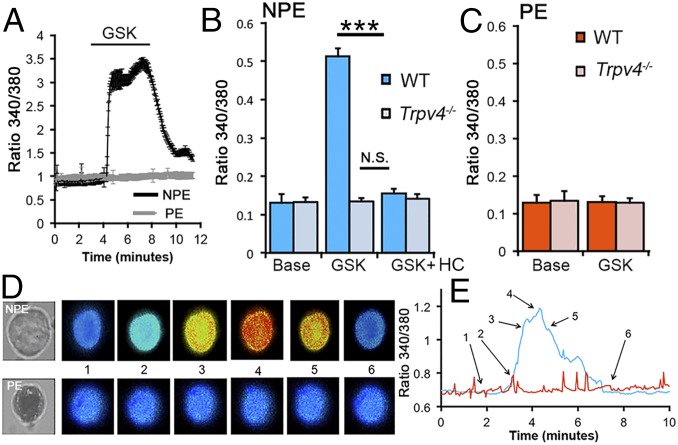
To directly evaluate the relationship between TRPV4 activation and , we stimulated CB cells with the selective agonist GSK101. Stimulation with different GSK101 concentrations established an EC50 of ∼33 nM (Fig. S5), comparable to the half-maximal response observed in retinal neurons and glia (20, 23). Consistent with the data obtained in HTS-stimulated cells, GSK101 (25 nM) evoked large, sustained and reversible elevations in NPE cells, whereas PE cells were unresponsive (Fig. 3). Altogether, a significant 74.395 ± 8.3% increase in ΔR/R over the baseline of 0.128 ± 0.022 was measured (n = 62 NPE cells; n = 4 animals; P < 0.005), compared with the baseline level of 0.131 ± 0.015 in PE cells (n = 46 cells; n = 4 animals; P not significant). Demonstrating specificity, agonist-induced responses in NPE were blocked by HC-06 (10 μM) (P < 0.005) and were not detectable in Trpv4−/− cells (Fig. 3B). The antagonist had an IC50 of ∼900 nM (Fig. S5). These data confirm that TRPV4 is functionally localized to NPE, but not PE, cells of the CB. The blockade of HTS-induced elevations by HC-06 demonstrates that the channel represents a primary transducer of NPE cell swelling.
Fig. S5.
NPE cells stimulated with 33 nM GSK101 in the presence of 50 nM, 100 nM, 2.50 μM, and 5 μM HC-06. The IC50, calculated from the exponential fit, is ∼900 nM.
Fig. 3.
TRPV4 is functionally expressed in NPE, but not PE, cells. (A–C) Dissociated cells. (A) The selective agonist GSK101 (25 nM) evokes signals in NPE (black trace; n = 15), but not PE (gray trace; n = 14), cells. (B) NPE cells. GSK101-evoked responses were observed in WT (n = 62) but not Trpv4−/− cells (n = 41) and were antagonized by the selective blocker HC-06 (1 μM). (C) PE cells were unresponsive to GSK101 (WT, n = 54; KO, n = 34). (D and E) Representative time course of the calcium response in dissociated NPE (Top) and PE cell (Bottom) stimulated with GSK101. Note the melanin pigment in the PE cell. The timing of each calcium response in D is shown on the intensity time course in E (blue, NPE; red, PE). ***P < 0.005.
TRPV4 Signaling in NPE Cells Requires Activation of the Canonical Lipid Messenger Pathway.
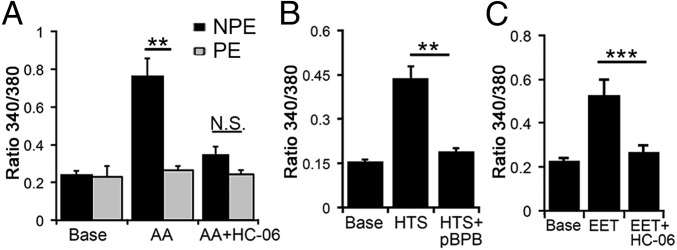
Cell swelling in numerous cell types activates the cytosolic isoforms of PLA2 (cPLA2), which is required for TRPV4 activation in some, but not all, cell types (10, 20, 23). According to a prevailing schema (20, 34, 35), the primary PLA2 product arachidonic acid (AA), a long-chain polyunsaturated ω6 fatty acid metabolite, is the precursor for epoxyeicosatrienoic acids (EETs), which function as endogenous final activators of TRPV4. To simulate this transduction pathway, we exposed CB cells to AA (50 μM). AA elevated in NPE cells but had no effect on levels in PE cells (P < 0.01) (Fig. 4A) and was markedly curtailed in KO NPE (Fig. S6). HC-06 inhibited AA-evoked elevations in NPE cells (n = 35; P = 0.023), indicating that AA was stimulating Ca2+ increases primarily through TRPV4.
Fig. 4.
TRPV4 activation in NPE cells requires activation of the canonical PLA2 pathway. Dissociated cells. (A) AA-evoked elevations in NPE cells (n = 30) were antagonized by HC-06. AA had little effect on levels (n = 28). (B) NPE cells. The PLA2 antagonist pBPB inhibits HTS-evoked signals (n = 25). (C) NPE cells. 11′,12′-EET induces elevations that are antagonized by HC-06 (n = 32).
Fig. S6.
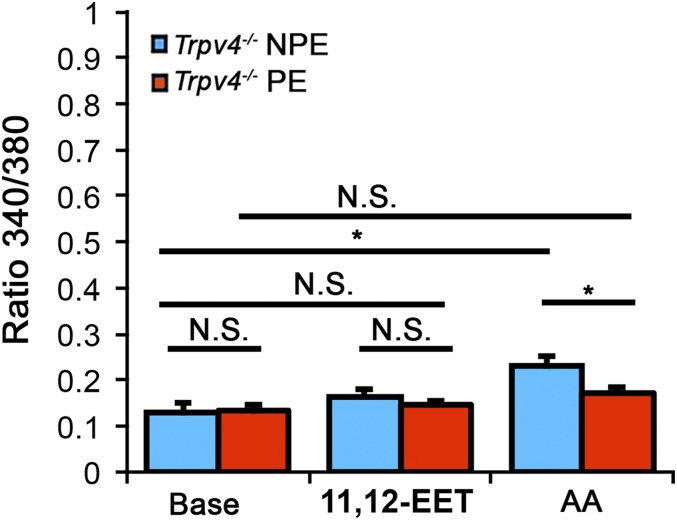
Dissociated cells NPE and PE cells from Trpv4−/− CBs. Compared with WT (e.g., Fig. 4), both AA- (100 μM) and 11′,12′-EET- (5 μM) induced responses are curtailed in KO NPE cells. A residual AA-sensitive increase (P < 0.05) in NPE cells suggests a possible, auxiliary AA-sensitive Ca2+ pathway (46).
To test whether this pathway is activated by osmotic stress, we challenged NPE cells with HTS in the presence of the PLA2 blocker 4-bromophenacyl bromide (pBPB; 100 µM). In the presence of pBPB, HTS-evoked elevations in NPE cells were blocked (n = 29; P < 0.01; Fig. 4B). We also observed that pBPB does not affect GSK101-evoked responses (n = 23) (P > 0.05), suggesting that, as reported for HEK 293 cells heterologously overexpressing TRPV4 (35), cell swelling and agonists activate NPE TRPV4 channels through different transduction pathways. Further consistent with this canonical pathway, the eicosanoid 11,12-EET (5 µM) induced increases (ΔR/R = 0.52 ± 0.076) that were also inhibited by HC-06 (ΔR/R = 0.26 ± 0.035) (Fig. 4C). AA- and 11′,12′-EET-evoked increases were reduced or absent in Trpv4−/− NPE cells (Fig. S6). We conclude that TRPV4 activation in NPE cells involves the requisite PLA2-CYP450 step, whereas swelling does not stimulate arachidonic acid metabolism in PE cells.
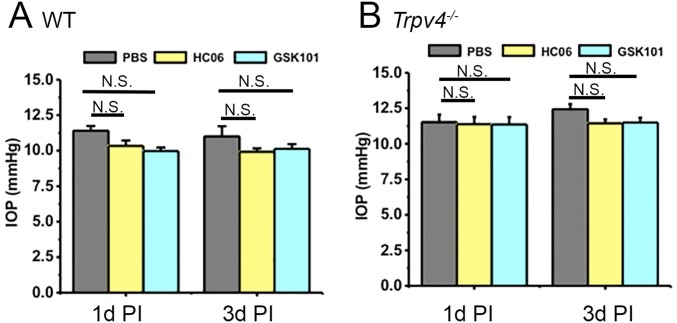
It has been suggested that TRPV4 channels in anterior eye tissues such as the CB and/or trabecular meshwork might regulate IOP (18, 36). Specifically, if TRPV4 channels control the “inflow” pathway by regulating steady-state release of aqueous fluid then activation, suppression, or ablation of the channel might be expected to affect steady-state IOP. However, intravitreal injection of the antagonist (HC-06, 10 µM) or the agonist (GSK101; 75 nM) had no effect on IOP levels in WT or KO tissue (Fig. S7). Moreover, IOP levels in WT eyes were comparable to those in KOs. This suggests that TRPV4 channels are not likely to control steady-state IOP levels in healthy mouse eyes.
Fig. S7.
Tonometric IOP measurements from WT (A) and KO (B) eyes show no effect of genetic ablation or intravitreal injection of the agonist (GSK101; 75 nM) or antagonist (HC-06; 10 μM) on IOP levels at 1 d (1d PI) or 3 d (3d PI) postinjection. IOP in WT eyes was not significantly different from the IOP in KO eyes, suggesting that TRPV4 is not required for the maintenance of steady-state IOP in healthy mouse eyes.
Discussion
We report that the polymodal TRPV4 channel is essential for osmoregulation and Ca2+ homeostasis in the mammalian CB. Our findings include (i) identification of the NPE osmosensor as TRPV4, (ii) discovery that calcium ions potentiate NPE swelling, (iii) observation of differential osmoregulation and Ca2+ homeostasis mechanisms in NPE vs. PE cells, and (iv) previously unidentified role for lipid metabolism in calcium signaling and osmoregulation within the CB. By showing differential contributions to volume regulation and Ca2+ signaling in a tightly coupled epithelial syncytium that plays an essential function in mammalian vision, these findings expand the known key role for TRPV4 in epithelial gene expression, intracellular signaling, protein transport, and secretion (11, 13–15, 30, 37, 38) and place it within the context of ocular volume regulation (39).
The expression of the TRPV4 gene and protein in CB was determined with transcript analyses and immunostaining and confirmed with functional studies that included excitation mapping, electrophysiology, and optical imaging in dissociated cells, ex vivo tissue, and intact mouse eyes. MRI imaging provided in vivo confirmation that TRPV4 activation induces cation influx into the CB. Antibody staining and functional analyses showed no obvious spatiotemporal differences in TRPV4 distribution, suggesting that CB regions, which vary in morphology and function (40, 41), universally use the channel to regulate their response to osmotic stress. To our knowledge, few if any studies have concurrently investigated Ca2+ signaling mechanisms in NPE and PE cells. Excitation mapping showed that TRPV4-induced cation influx takes place in the NPE layer but is absent from the PE layer. This is consistent with critical importance of calcium homeostasis and volume regulation in these aqueous fluid-producing cells (2, 4, 5, 32, 33, 37, 41–44). The confinement of the agmatine signal to the vicinity of the plasma membrane suggests that potent local cation clearance mechanisms (Na/K ATPases and PMCAs) limit the diffusion of Ca2+ signals and their spread across the NPE–PE syncytium. The absence of HTS-induced signals in Ca2+-free saline, in cells with deleted Trpv4 gene, and the effectiveness of pharmacological TRPV4 blockers suggest that TRPV4 is obligatory for transducing increases in NPE cell volume into elevated . Swelling-induced TRPV4-mediated responses were sustained, indicating continuous activation of PLA2 (20, 34). It remains to be determined whether TRPV4-mediated Ca2+ signaling in NPE is modulated by downstream Ca2+/calmodulin binding and/or heteromerization with other TRP subunits (e.g., refs. 21, 22, and 45).
Recent investigations have shown that TRPV4 is ubiquitously expressed in secretory and absorptive epithelia, where it regulates Ca2+ signaling, volume changes, cytoskeletal remodeling, and responses to shear flow and mechanical stress (11–15, 29, 39). We hypothesize that the channel influences the transepithelial CB resistance, NPE volume regulation, and fluid secretion through Ca2+-dependent modulation of Na/K ATPases, PMCAs, Cl− channels, aquaporin channels, and/or ATP release (23, 29, 32, 33, 37, 43). Multiple lines of evidence indicate that TRPV4 signaling in NPE cells requires activation of the PLA2 pathway. Thus, HTS-evoked increases were inhibited by PLA2 and CYP450 antagonists. Furthermore, AA and 11,12-EET, two endogenous activators of the channel, evoked elevations that were suppressed by TRPV4 blockers and channel ablation. While modulation of TRPV4 resulted in significant changes in NPE cell volume and [Ca2+]i it had very little effect on PE cells. signals, induced by relatively low AA concentrations (50 μM), were inhibited by HC-06, indicating that this abundant polyunsaturated fatty acid, often present in phospholipids, selectively stimulated TRPV4. Higher concentrations (>100 μM) of AA may modulate additional intracellular signaling mechanisms (possibly including Orai/ARC, Cl−, and/or TREK1 channels) (46). If Ca2+ influx through TRPV4 stimulates the cPLA2 C2 domain when the enzyme is phosphorylated at serine-505, it would activate a positive feedback loop mediated through CYP450 (34, 35) whereas Ca/CaM could contribute an inactivation component through the C terminus (45).
Sensing and regulation of cell volume is of critical importance for CB cells, which continuously sustain the inflow pathway. It is therefore noteworthy that NPE and PE layers differ in volume sensing, regulation, and the expression and function of volume-activated Cl− channels, K+ channels, PMCAs, Na/K ATPases, and NKCC transporters (5, 33, 43, 44). The distinctiveness of volume regulatory and Ca2+ homeostatic apparati is consistent with the strikingly different functions in generation and secretion of aqueous humor (2, 4). Interestingly, we found that NPE and PE cells both respond to depolarization almost exclusively through high-threshold L-type voltage-gated channels. It is likely that volume-sensitive TRPV4-mediated Ca2+ elevations in these cells modulate Ca2+-dependent Cl− and K+ channels (38), Ca2+-CaM kinase II, volume-sensitive Cl− currents, and/or AQP-mediated water transport (23, 32, 37, 38) that modulate RVD (23, 37), exocytosis (18), and/or secretion (32). Expression of transcripts encoding other vanilloid TRP isoforms suggests additional homeostatic complexity that might involve TRPV1-3 and TRPV6 isoforms together with metabotropic mechanisms associated with muscarinic AcH, α2-norepinephrine, somatostatin, endothelin, bradykinin, and P2Y1/P2Y2 receptor activation (2, 4, 47–49) within NPE/PE layers and stroma.
In summary, we present evidence that links swelling-induced Ca2+ signals in CB NPE cells to an identified molecular channel, TRPV4. Given that Ca2+ regulates epithelial secretory activity (15, 30), might regulate ATP release from NPE (as shown for lens and airway epithelia; refs. 29, 39, and 47) and modulate secretion of ions, water transport, pigment production, and release of nitric oxide (18, 23, 24, 43, 48), it is not inconceivable that volume-sensitive, PLA2-dependent TRPV4 signals affect Ca2+-dependent activity of Na/K ATPases, the primary determinant of aqueous humor secretion (2, 5, 32, 39). Consistent with this conjecture, topical application of Ca2+ ionophores elevates IOP without significantly changing the outflow facility (50). Our finding that genetic ablation and intraocular injection of GSK101/HC-06 have no effect on IOP argues against a role for the channel in steady-state maintenance of IOP (e.g., ref. 36). However, its possible sensitivity to mechanical stimuli (10, 35) suggests that TRPV4 might be overactivated during ocular hypertension. Mutations in a CB CYP450 isoform (CYP1B1) represent the predominant cause of primary congenital glaucoma (2), suggestive of possible links between elevated IOP, anomalous aqueous secretion, and biosynthesis of eicosanoid TRPV4 activators. The differential localization and function of the volume-sensitive TRPV4 channel suggests a new complexity in volume regulation of cell types forming epithelial syncytia.
Materials and Methods
Animals.
Pigmented C57BL/6J, nonpigmented B6.Cg-Tg(Thy1-YFP)HJrs/J and pan-null Trpv4−/− mice with excised transmembrane domains 5 and 6 encoded by exon 12 of the TRPV4 gene (17) were maintained in a 12-h light/dark cycle with free access to food and water. Trpv4−/− mice were back-crossed with C57BL/6J mice for 10 generations so that C57BL/6J animals could be used as controls. The experiments adhered to the NIH Guide for the Care and Use of Laboratory Animals (51) and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committees at the University of Utah.
CB Preparation.
More detail is available in Supporting Information. CB tissue was dissected out of the eye and plated onto Con A-coated coverslips. The NPE layer was distinguished from the PE layer by absence of melanin, DAPI staining, and outward location in the tissue. Cells were dissociated from CB in papain using published protocols (19, 20, 52) and identified based on size and melanin content (Fig. S2).
Immunofluorescence.
Antibody staining in WT and KO tissue was conducted as described preciously (19, 53, 55) using rabbit anti-TRPV4 and anti-agmatine antibodies.
Superfusion of CB Tissue and Cell Swelling Assay.
Changes in cell volume were measured following the Fura-2-based protocol (23, 53).
Calcium imaging was conducted following routine protocols (20, 52, 54, 55). was detected using the ratiometric protocol based on the fluorescent dye Fura-2. In each experiment, ΔR/R (peak F340/F380 ratio – baseline/baseline) quantification was obtained for multiple cells in the same visual field. Results represent cell responses from at least three animals, averaged across multiple cells and several slides per eye.
Electrophysiology.
Whole-cell transmembrane currents in NPE cells were performed using standard whole-cell recording (20, 23, 54). Data acquired were sampled at 10 kHz and filtered at 2 kHz.
High-Resolution MRI.
The general MEMRI procedure in mice has been described previously (25–27). For GSK or vehicle injection, 2 μL of either 100 μM GSK101 or vehicle was injected into the anterior chamber over 45 s.
Statistical Analysis.
Means are shown ± SEM. Unless specified, an unpaired t test was used to compare two means and an ANOVA along with Tukey’s multiple comparisons test was used to compare three or more means.
SI Materials and Methods
Animals.
Mice from CB57BL/6J (pigmented; JAX) and B6.Cg-Tg(Thy1-YFP)HJrs/J (albino; a gift from Dr. Ning Tian, University of Utah, Salt Lake City, UT) strains were used. Mice were genotyped using tail DNA and PCR primers described in our previous reports (20, 23). Data from male and female mice were pooled.
CB Preparation.
For intact tissue, mice were killed, eyes were removed, and CB tissue was dissected out of the eye under stereoscopic guidance, in cold Leibovitz 15 (L15) medium (Life Technologies) containing 11 mg/mL L15 powder, 20 mM d-glucose, 10 mM Na-Hepes, 2 mM Na-pyruvate, 0.3 mM Na-ascorbate, and 1 mM glutathione. For experiments, CB pieces were plated onto Con A- (1 mg/mL) coated coverslips, loaded with Fura-2 AM (5–10 µM; Life Technologies) for 30–40 min, and washed for 10–20 min. NPE cells were clearly distinguished from PE cells by their absence of melanin and outward location in the tissue. For dissociated cells, CBs were dissected in L15 and dissociated following published protocols (2) in L15 containing papain (7 U/mL; Worthington) for 1 h at room temperature. CBs were subsequently triturated using cut 100-µL pipette tips and plated on Con A-coated (0.2 mg/mL; Sigma) coverslips. NPE cells were distinguished from PE cells based on the lack of melanin granules. Under our experimental conditions, CB cells maintained homeostasis for many hours at room temperature without substantial shifts in baseline or the amplitude of responses to agonists.
Reagents.
The TRPV4 antagonist HC-06 was purchased from Calbiochem or Sigma. Arachidonic acid and PLA2 antagonists were purchased from Cayman Chemicals. Other reagents and salts were purchased from Sigma, except where noted otherwise. GSK101 (1–10 mM) and HC-06 (4 mM) stocks in DMSO were diluted 1:1,000 in extracellular saline just before use and placed into reservoirs connected to gravity-fed perfusion systems (Warner Instruments).
Semiquantitative Real-Time PCR.
Retinas were collected from four mice per group (WT and Trpv4-/−). RNA was isolated with the Arcturus PicoPure RNA isolation kit (Applied Biosystems or Life Technologies); total RNA concentrations (500 μg) were converted to cDNA using the XLT cDNA super mix kit (Quanta) and real-time PCR was performed using the Cyber Green PCR master mix (Applied Biosystems). Amplification was run on the CFX Connect Real-Time PCR System (Bio-Rad) following the manufacturer’s protocols (Takara; 95 °C for 15 s, 95 °C for 5 s, 60 °C for 30 s, 40 cycles). The ratio of gene-of-interest mRNA to the housekeeping gene, α-tubulin, was calculated for every sample. The primers are listed in Table S1.
Immunofluorescence.
Dissected CBs were fixed with 4% (vol/vol) paraformaldehyde at room temperature and cryoprotected by incubation in 15% (vol/vol) sucrose for 45 min at room temperature and in 30% (vol/vol) sucrose overnight at 4 °C (1–4, 6). The tissue was transferred to optimum cutting temperature compound (Electron Microscope Sciences), frozen over dry ice and stored at −80 °C until ready to cut. Tissue was sliced with a cryostat into 16-µm sections, and mounted on slides. After washing, CB sections were placed for 30 minutes in blocking buffer [0.3% Triton-X, 3% FBS in PBS (vol/vol)]. Primary antibodies were diluted in antibody dilution buffer [0.3% Triton-X (vol/vol), 1% BSA (wt/vol) in PBS] and applied overnight at 4 °C. After rinsing, CB tissue from WT or Trpv4−/− eyes was incubated with the secondary antibody diluted to 1:1,000. Unbound antibodies were rinsed away and conjugated fluorophores were protected with Fluoromount-G (Southern Biotech) before mounting coverslips and confocal imaging. Primary antibodies were obtained from Lifespan Biosciences or Alomone (TRPV4, 1:1,000) and Sigma (pan-PMCA 5F10, 1:100). Goat anti-rabbit IgG (H+L) secondary antibody was conjugated to fluorophores (Alexa 488 or Alexa 594; Life Technologies). Some experiments included a nuclear stain (DAPI; Invitrogen). The secondary antibodies did not label retinal tissue when primary antibodies were omitted. Immunostaining was imaged with an upright confocal microscope (Olympus CV1200) using argon (488 nm) and helium/neon (543 nm) lasers, suitable emission filters, and 20×/1.0 N.A. or 40×/0.9 N.A. water objectives.
Excitation Mapping.
Ex vivo anterior chamber tissue (containing the cornea, CB, and lens) dissected from the eye from WT and KO mice was incubated in saline to which 10 mM AGB was added. Following previously described protocols (20, 24), tissues were incubated for 5 min with the vehicle (PBS) or GSK101 (100 nM) and fixed for 30 min with 4% paraformaldehyde + 0.5 glutaraldehyde, followed by immersion in 15% sucrose (45 min) and 30% sucrose (overnight). Sections at 12-mm thickness were collected on positively charged slides, with WT (pigmented and albino) and Trpv4−/− sections positioned side by side. The indirect immunofluorescence was performed using rabbit anti-agmatine (1:2,000; Signature Immunologics; a generous gift from Robert Marc, Signature Immunologics, Salt Lake City, UT); specificity was confirmed with dot blot immunoassays that showed no cross-reactivity with other amino acids (24). The secondary antibodies were goat anti-rabbit Alexa-Fluor 488 (1:500), coapplied with DAPI (1-h incubation at room temperature). Quantification of AGB-labeled cells was as described (20). Briefly, ROIs were outlined around DAPI-labeled nuclei in the NPE and PE layers excluding areas showing obvious anatomical disruptions or layer misfolding. The fluorescence (approximately five to eight ROIs per slide) was averaged across slides and then animals (three to five slides per animal). The data are presented as final averages across animal cohorts (n = 3), with ± SEM and statistical significance determined using ANOVA.
Superfusion of CB Tissue and Cell Swelling Assay.
The swelling assays were performed as reported (23). Briefly, imaging experiments were performed with Ringer’s saline perfusion that was gravity-fed using an eight-reservoir system (Warner Instruments) that converged toward a manifold tube inserted into the experimental chamber. The superfusate contained, in millimolar, NaCl 57, KCl 2.5, NaH2PO4 1.5, MgCl2 1.5, CaCl2 2, glucose 10, Hepes hemisodium salt 10, pyruvic acid 1, lactic acid 1, l-glutamine 0.5, glutathione 0.5, and Na-ascorbate 0.3. pH was set at 7.4 and osmolarity at 300 mOsm. Extracellular osmolarity was set by addition or removal of mannitol, a procedure that minimally disrupts the ionic gradients during solution exchanges. Osmolarity was checked using a vapor pressure osmometer (Wescor). Cell volume was measured by calcein fluorescence and by adapting a protocol developed by the Putney laboratory (53). Fluorescence emissions from 340 and 380 nm stimuli were normalized to baseline fluorescence and algebraically summed (Fvol = F340 + F380/x, where x = 1–3, a value empirically derived for each preparation to equalize the magnitude of the Ca2+-dependent and opposing changes in F340 and F380). At the appropriate x value, the intensity of the summed fluorescence was insensitive to calcium (i.e., the summed trace showed no response to GSK101 or HTS). The x–y cross-sectional area was determined offline using NIS-Elements AR 3.2 or Olympus image analysis software. We confirmed with confocal z-stacks over time that swelling occurs uniformly in all directions (20), indicating that cell volume is proportional to the square root of area3.
Calcium Imaging.
Inverted or upright Nikon microscopes with 20× (0.75 N.A. oil), 40× (1.3 N.A. oil and 0.8 N.A. water), and 60× (1.0 N.A. water) objectives were used to obtain epifluorescent images (1–3). Lambda DG-4 (Sutter Instruments) Xe arc lamps were used to deliver excitation light. Emissions were detected with 14-bit Photometrics HQ2 cameras and analyzed using NIS-Elements AR 3.2 and MS Excel. was detected using the previously described ratiometric protocol based on the fluorescent dye Fura-2 (23, 52, 54). In each experiment, ΔR/R (peak F340/F380 ratio – baseline/baseline) quantification was obtained for multiple cells in the same visual field. Results represent cell responses from at least three animals, averaged across multiple cells and several slides per eye.
Electrophysiology.
Whole-cell transmembrane currents in NPE cells in dissected CB were recorded using a Multiclamp 700B (Molecular Devices) amplifier and Digidata 1440A acquisition board (Molecular Devices) on the stage of an Eclipse E600N upright microscope, as described (20, 23, 52). CBs placed onto a recording chamber were superfused continually with oxygenated recording solution that contained, in millimolar, 57 NaCl, 2.5 KCl, 0.63 Na2HPO4, 0.5 sodium pyruvate, 5 lactic acid, 0.75 MgCl2, 2 CaCl2, 5 Hepes hemisodium salt, 0.5 glutathione, 0.3 sodium ascorbate, 10 d-glucose, and 0.5 l-glutamine. Osmolarity was adjusted to 300 mOsm with mannitol. The extracellular HTS was adjusted to 190 mOsm by removing mannitol. The pipette solution contained, in millimolar, 135 K-gluconate, 10 KCl, 10 Hepes, 1 MgCl2, 4 Mg-ATP, 0.6 mM Na-GTP, and 10 mM EGTA, 296 mOsm, adjusted with sucrose. Data acquired with pClamp 10 software (Molecular Devices) were sampled at 10 kHz and filtered at 2 kHz. Currents were elicited by RAMP pulses ascending from −100 mV to +100 mV from the holding potential of −50 mV. RAMP pulses (1-s duration) were applied at 0.2 Hz. Data were analyzed and plotted using Clampfit (Molecular Devices). Patch pipettes were pulled from borosilicate glass capillaries (World Precision Instruments) and had final open-tip resistances between 8 and 10 MΩ when filled with the intracellular (pipette) solution. All experiments were performed at room temperature (22–23 °C).
Mouse IOP Measurements.
A rebound TonoLab tonometer (Tiolat) was used to measure IOP in awake mice between 10:00 AM and 12:00 PM; 0.5% proparacaine hydrochloride was applied before measurements. IOP was determined from the mean of 10–20 tonometer readings.
High-Resolution MRI.
GSK or vehicle injection.
C57BL/6 mice (The Jackson Laboratory) were anesthetized with ketamine/xylazine (100 mg/kg:10 mg/kg) and placed under a microscope. Eyes were dilated with 1% Tropicamide and the topical anesthetic Proparacaine hydrochloride 0.5% was applied. Two microliters of either 100 μM GSK or vehicle was injected into the anterior chamber over 45 s. The general MEMRI procedure in mice has been described previously (25–28). Briefly, C57BL/6 mice were maintained in darkness for 16–20 h. Mice were brought into the light ∼15 min before ocular injection (discussed above). Ten minutes following the injection, MnCl2 was administered as an i.p. injection (66 mg MnCl2⋅4H2O/kg) on the right side of awake mice. After this injection, animals were maintained in light for another 4 h. Immediately before the MRI experiment, animals were anesthetized with urethane (36% solution i.p.; 0.083 mL per 20 g animal weight, prepared fresh daily). MRI data were acquired on a 7T system (Clinscan; Bruker). Retinal partial saturation T1 data were acquired using a dual coil mode on a 7T BrukerClinscan system. Several single spin-echo (time to echo 13 ms, 7 × 7 mm2, matrix size 160 × 320, slice thickness 600 μm) images were acquired at different repetition times (TRs) in the following order (number per time between repetitions in parentheses): TR 0.15 s (6), 3.50 s (1), 1.00 s (2), 1.90 s (1), 0.35 s (4), 2.70 s (1), 0.25 s (5), and 0.50 s (3). To compensate for reduced signal-to-noise ratios at shorter TRs, progressively more images were collected as the TR decreased.
MRI analysis.
Single images acquired with the same TR were first registered (rigid body) and then averaged. These averaged images were then registered across TRs. The same ROIs as above were analyzed by calculating 1/T1 maps by first fitting to a three-parameter T1 equation [y = a + b*(exp(−c*TR), where a, b, and c are fitted parameters] on a pixel-by-pixel basis using R scripts developed in-house and the minpack.lm package. The reciprocal (1/T1) values are proportional to manganese levels. Values from the superior and inferior ciliary bodies were averaged. Note that only those animals that took up manganese above baseline (i.e., ∼0.65 s−1) were included in the final analysis. A generalized estimating equation (GEE) approach was used to adjust for correlations between inferior and superior 1/T1 values. GEE performs a general linear regression analysis using contiguous locations in each subject and accounts for the within-subject correlation. In all cases, P ≤ 0.05 was considered statistically significant.
Statistical Analysis.
GraphPad Prism 6.0 was used for statistics in non-MRI experiments. Means are shown ± SEM. Unless specified, an unpaired t test was used to compare two means and an ANOVA along with Tukey’s multiple comparisons test was used to compare three or more means. Significance was defined as a corrected P value of <0.05. Comparisons of MEMRI retinal signal intensities were performed with a GEE approach (SAS/STAT). The data are presented as mean ± SEM. P > 0.05 is not significant, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Acknowledgments
We thank Drs. Wolfgang Liedtke (Duke University) for Trpv4−/− mice, Ning Tian for albino mice, and Daniel Ryskamp (University of Texas Southwestern Medical Center) for help with the initial experiments. This work was supported by the University of Utah Undergraduate Research Opportunity Program (A.O.J.); National Institutes of Health Grants EY021619 (to B.A.B.), EY022076, and P30EY014800 (to D.K.); Department of Defense Grant W81XWH-12-1-0244; Willard L. Foundation; State of Utah Technology Commercialization and Innovation Program; the University of Utah Neuroscience Initiative; and unrestricted support from Research to Prevent Blindness to Moran Eye Institute and the Kresge Eye Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1515895113/-/DCSupplemental.
References
- 1.Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): A study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15(1):15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- 2.Coca-Prados M, Escribano J. New perspectives in aqueous humor secretion and in glaucoma: The ciliary body as a multifunctional neuroendocrine gland. Prog Retin Eye Res. 2007;26(3):239–262. doi: 10.1016/j.preteyeres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin CW, Zellhuber-McMillan S, Macknight AD, Civan MM. Electron microprobe analysis of rabbit ciliary epithelium indicates enhanced secretion posteriorly and enhanced absorption anteriorly. Am J Physiol Cell Physiol. 2007;293(5):C1455–C1466. doi: 10.1152/ajpcell.00205.2007. [DOI] [PubMed] [Google Scholar]
- 4.Delamere NA. Ciliary body and ciliary epithelium. Adv Organ Biol. 2005;10:127–148. doi: 10.1016/S1569-2590(05)10005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farahbakhsh NA, Fain GL. Volume regulation of non-pigmented cells from ciliary epithelium. Invest Ophthalmol Vis Sci. 1987;28(6):934–944. [PubMed] [Google Scholar]
- 6.Miglior S, Bertuzzi F. Relationship between intraocular pressure and glaucoma onset and progression. Curr Opin Pharmacol. 2013;13(1):32–35. doi: 10.1016/j.coph.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Woodward DF, Gil DW. The inflow and outflow of anti-glaucoma drugs. Trends Pharmacol Sci. 2004;25(5):238–241. doi: 10.1016/j.tips.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2(10):695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe H, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277(16):13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 10.Loukin S, Zhou X, Su Z, Saimi Y, Kung C. Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J Biol Chem. 2010;285(35):27176–27181. doi: 10.1074/jbc.M110.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harteneck C, Reiter B. TRP channels activated by extracellular hypo-osmoticity in epithelia. Biochem Soc Trans. 2007;35(Pt 1):91–95. doi: 10.1042/BST0350091. [DOI] [PubMed] [Google Scholar]
- 12.Sokabe T, Fukumi-Tominaga T, Yonemura S, Mizuno A, Tominaga M. The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J Biol Chem. 2010;285(24):18749–18758. doi: 10.1074/jbc.M110.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrade YN, et al. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol. 2005;168(6):869–874. doi: 10.1083/jcb.200409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamenko M, Zaika O, Boukelmoune N, O’Neil RG, Pochynyuk O. Deciphering physiological role of the mechanosensitive TRPV4 channel in the distal nephron. Am J Physiol Renal Physiol. 2015;308(4):F275–F286. doi: 10.1152/ajprenal.00485.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narita K, Sasamoto S, Koizumi S, Okazaki S, Nakamura H, Inoue T, Takeda S. TRPV4 regulates the integrity of the blood-cerebrospinal fluid barrier and modulates transepithelial protein transport. FASEB J. 2015;29(6):2247–2259. doi: 10.1096/fj.14-261396. [DOI] [PubMed] [Google Scholar]
- 16.Jo A, Ryskamp DA, Frye AN, Berkowitz BA, Krizaj D. TRPV4 channels regulate the inflow pathway in the anterior eye. Invest Ophthalmol Vis Sci. 2015;56:1298. [Google Scholar]
- 17.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci USA. 2003;100(23):13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alkozi HA, Pintor J. TRPV4 activation triggers the release of melatonin from human non-pigmented ciliary epithelial cells. Exp Eye Res. 2015;136:34–37. doi: 10.1016/j.exer.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Ryskamp DA, et al. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci. 2011;31(19):7089–7101. doi: 10.1523/JNEUROSCI.0359-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryskamp DA, et al. Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J Neurosci. 2014;34(47):15689–15700. doi: 10.1523/JNEUROSCI.2540-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer M. Homo- and heteromeric assembly of TRP channel subunits. Pflugers Arch. 2005;451(1):35–42. doi: 10.1007/s00424-005-1467-6. [DOI] [PubMed] [Google Scholar]
- 22.Ma X, et al. Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler Thromb Vasc Biol. 2010;30(4):851–858. doi: 10.1161/ATVBAHA.109.196584. [DOI] [PubMed] [Google Scholar]
- 23.Jo AO, et al. TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal Müller glia. J Neurosci. 2015;35(39):13525–13537. doi: 10.1523/JNEUROSCI.1987-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marc RE. Mapping glutamatergic drive in the vertebrate retina with a channel-permeant organic cation. J Comp Neurol. 1999;407(1):47–64. doi: 10.1002/(sici)1096-9861(19990428)407:1<47::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Berkowitz BA, et al. Quantitative mapping of ion channel regulation by visual cycle activity in rodent photoreceptors in vivo. Invest Ophthalmol Vis Sci. 2009;50(4):1880–1885. doi: 10.1167/iovs.08-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkowitz BA, Grady EM, Roberts R. Confirming a prediction of the calcium hypothesis of photoreceptor aging in mice. Neurobiol Aging. 2014;35(8):1883–1891. doi: 10.1016/j.neurobiolaging.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Calkins DJ, Horner PJ, Roberts R, Gradianu M, Berkowitz BA. Manganese-enhanced MRI of the DBA/2J mouse model of hereditary glaucoma. Invest Ophthalmol Vis Sci. 2008;49(11):5083–5088. doi: 10.1167/iovs.08-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos de Carvalho JE, Verbraak FD, Aalders MC, van Noorden CJ, Schlingemann RO. Recent advances in ophthalmic molecular imaging. Surv Ophthalmol. 2014;59(4):393–413. doi: 10.1016/j.survophthal.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Seminario-Vidal L, et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286(30):26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashby MC, Tepikin AV. Polarized calcium and calmodulin signaling in secretory epithelia. Physiol Rev. 2002;82(3):701–734. doi: 10.1152/physrev.00006.2002. [DOI] [PubMed] [Google Scholar]
- 31.Farahbakhsh NA, Cilluffo MC, Chronis C, Fain GL. Dihydropyridine-sensitive Ca2+ spikes and Ca2+ currents in rabbit ciliary body epithelial cells. Exp Eye Res. 1994;58(2):197–205. doi: 10.1006/exer.1994.1008. [DOI] [PubMed] [Google Scholar]
- 32.Mito T, Delamere NA, Coca-Prados M. Calcium-dependent regulation of cation transport in cultured human nonpigmented ciliary epithelial cells. Am J Physiol. 1993;264(3 Pt 1):C519–C526. doi: 10.1152/ajpcell.1993.264.3.C519. [DOI] [PubMed] [Google Scholar]
- 33.Yantorno RE, Carré DA, Coca-Prados M, Krupin T, Civan MM. Whole cell patch clamping of ciliary epithelial cells during anisosmotic swelling. Am J Physiol. 1992;262(2 Pt 1):C501–C509. doi: 10.1152/ajpcell.1992.262.2.C501. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe H, et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 35.Vriens J, et al. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA. 2004;101(1):396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo N, et al. Primary cilia signaling mediates intraocular pressure sensation. Proc Natl Acad Sci USA. 2014;111(35):12871–12876. doi: 10.1073/pnas.1323292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Botchkin LM, Matthews G. Swelling activates chloride current and increases internal calcium in nonpigmented epithelial cells from the rabbit ciliary body. J Cell Physiol. 1995;164(2):286–294. doi: 10.1002/jcp.1041640209. [DOI] [PubMed] [Google Scholar]
- 38.Shahidullah M, Mandal A, Delamere NA. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. Am J Physiol Cell Physiol. 2012;302(12):C1751–C1761. doi: 10.1152/ajpcell.00010.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krizaj D, et al. From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr Eye Res. 2014;39:105–119. doi: 10.3109/02713683.2013.836541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hara K, Lütjen-Drecoll E, Prestele H, Rohen JW. Structural differences between regions of the ciliary body in primates. Invest Ophthalmol Vis Sci. 1977;16(10):912–924. [PubMed] [Google Scholar]
- 41.Ghosh S, Freitag AC, Martin-Vasallo P, Coca-Prados M. Cellular distribution and differential gene expression of the three alpha subunit isoforms of the Na,K-ATPase in the ocular ciliary epithelium. J Biol Chem. 1990;265(5):2935–2940. [PubMed] [Google Scholar]
- 42.Jacob TJ, Civan MM. Role of ion channels in aqueous humor formation. Am J Physiol. 1996;271(3 Pt 1):C703–C720. doi: 10.1152/ajpcell.1996.271.3.C703. [DOI] [PubMed] [Google Scholar]
- 43.Civan MM, Coca-Prados M, Peterson-Yantorno K. Pathways signaling the regulatory volume decrease of cultured nonpigmented ciliary epithelial cells. Invest Ophthalmol Vis Sci. 1994;35(6):2876–2886. [PubMed] [Google Scholar]
- 44.Zhang JJ, Jacob TJ. Three different Cl- channels in the bovine ciliary epithelium activated by hypotonic stress. J Physiol. 1997;499(Pt 2):379–389. doi: 10.1113/jphysiol.1997.sp021935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe H, et al. Modulation of TRPV4 gating by intra- and extracellular Ca2+ Cell Calcium. 2003;33(5-6):489–495. doi: 10.1016/s0143-4160(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 46.Meves H. Arachidonic acid and ion channels: An update. Br J Pharmacol. 2008;155(1):4–16. doi: 10.1038/bjp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farahbakhsh NA. Purinergic signaling in the rabbit ciliary body epithelium. J Exp Zoolog A Comp Exp Biol. 2003;300(1):14–24. doi: 10.1002/jez.a.10304. [DOI] [PubMed] [Google Scholar]
- 48.Sharif NA, et al. Human non-pigmented ciliary epithelium bradykinin B2-receptors: Receptor localization, pharmacological characterization of intracellular Ca(2+) mobilization, and prostaglandin secretion. Curr Eye Res. 2014;39(4):378–389. doi: 10.3109/02713683.2013.816324. [DOI] [PubMed] [Google Scholar]
- 49.Martin-Gil A, et al. Silencing of P2Y(2) receptors reduces intraocular pressure in New Zealand rabbits. Br J Pharmacol. 2012;165(4b):1163–1172. doi: 10.1111/j.1476-5381.2011.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Podos SM. The effect of cation ionophores on intraocular pressure. Invest Ophthalmol. 1976;15(10):851–854. [PubMed] [Google Scholar]
- 51.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies; Washington, DC: 2011. [Google Scholar]
- 52.Szikra T, et al. Calcium homeostasis and cone signaling are regulated by interactions between calcium stores and plasma membrane ion channels. PLoS One. 2009;4(8):e6723. doi: 10.1371/journal.pone.0006723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiavaroli C, Bird G, Putney JW., Jr Delayed “all-or-none” activation of inositol 1,4,5-trisphosphate-dependent calcium signaling in single rat hepatocytes. J Biol Chem. 1994;269(41):25570–25575. [PubMed] [Google Scholar]
- 54.Molnar T, et al. Store-operated channels regulate intracellular calcium in mammalian rods. J Physiol. 2012;590(15):3465–3481. doi: 10.1113/jphysiol.2012.234641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Witkovsky P, Gábriel R, Križaj D. Anatomical and neurochemical characterization of dopaminergic interplexiform processes in mouse and rat retinas. J Comp Neurol. 2008;510(2):158–174. doi: 10.1002/cne.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]