Significance
Applying well-established psychological paradigms to our closest relatives represents a promising approach for providing insight into similarities and differences between humans and apes. Numerous articles have been published on the dot-probe task, showing that humans have an attentional bias toward emotions, especially when threatening. For social species like primates, efficiently responding to others’ emotions has great survival value. Observational research has shown that, compared with humans and chimpanzees, bonobos excel in regulating their own and others’ emotions, thereby preventing conflicts from escalating. The present study is an initial effort to apply a psychological test to the bonobo, and demonstrates that they, like humans, have heightened attention to emotional—compared with neutral—conspecifics, but are mostly drawn toward protective and affiliative emotions.
Keywords: bonobo, ape, affect, empathy, attention
Abstract
In social animals, the fast detection of group members’ emotional expressions promotes swift and adequate responses, which is crucial for the maintenance of social bonds and ultimately for group survival. The dot-probe task is a well-established paradigm in psychology, measuring emotional attention through reaction times. Humans tend to be biased toward emotional images, especially when the emotion is of a threatening nature. Bonobos have rich, social emotional lives and are known for their soft and friendly character. In the present study, we investigated (i) whether bonobos, similar to humans, have an attentional bias toward emotional scenes compared with conspecifics showing a neutral expression, and (ii) which emotional behaviors attract their attention the most. As predicted, results consistently showed that bonobos’ attention was biased toward the location of the emotional versus neutral scene. Interestingly, their attention was grabbed most by images showing conspecifics such as sexual behavior, yawning, or grooming, and not as much—as is often observed in humans—by signs of distress or aggression. The results suggest that protective and affiliative behaviors are pivotal in bonobo society and therefore attract immediate attention in this species.
Efficiently responding to others’ emotions has great survival value, especially for social species, such as primates, who establish close, long-term bonds with group members (1–3). Previous research in humans has shown that one component of this strong sensitivity to others’ emotions is heightened attention to their affective states (4–6). Together with the chimpanzee, the bonobo is the closest living relative of humans. Studying components of their emotional behavior, such as emotional attention, may help us not only to understand this rarely studied species better, but also provide insight into (human) emotions and their evolutionary past (7–9). In the present study, we investigated whether bonobos, like humans, show increased attention to scenes depicting conspecifics showing an emotion or are involved in emotion regulatory behaviors, compared with scenes where conspecifics are in a neutral state.
In natural environments, attention is preferentially sustained by stimuli that have affective significance, in contrast to routine, emotionally neutral events (6, 10). This attentional bias reflects a functional mechanism where fast and automatic attention allocation to emotional information can aid humans in threatening situations by fostering fast actions (11, 12). Experimentally, this has, for example, been demonstrated with the dot-probe task. Previous dot-probe studies have shown that emotional signals induce a bias in spatial attention, in that participants respond faster to a presented dot (the target, henceforth, “probe”) when it appears at the location of a previously presented emotion compared with neutral stimulus (13–17). Although in humans a bias toward threatening compared with neutral stimuli is most commonly observed, some studies also report increased attention toward positive versus neutral stimuli (18–21). Other research has shown that there are marked individual differences in biases and that these are, for example, modulated by mood (22, 23). From an evolutionary perspective, it is most adaptive to be able to quickly attend to relevant stimuli, whether those are threats in the environment or an affiliative signal from an individual who could provide support and care (24, 25).
Most primates spend their lives in social groups. To prevent conflicts, they keep close track of others’ behaviors, emotions, and social debts. For example, chimpanzees remember who groomed whom for long periods of time (26). In the chimpanzee, but also in the rarely studied bonobo, grooming is a major social activity and a means by which animals living in proximity may bond and reinforce social structures. It is also used as a form of reconciliation and a means of conflict resolution. Despite their general similarities and close phylogenetic relationship, bonobos, chimpanzees, and humans show clear differences in their social behavior. Although chimpanzee and human societies are dominated by males and encounters between different groups frequently result in violence and sometimes the killing of conspecifics (27–29), bonobo society is controlled by females and aggression is usually prevented through nonconceptive sexual behavior, grooming, and play (30–33). Compared with chimpanzees, bonobos live in more predictable environments with low seasonality and stable food availability, which is why they have a lower degree of fission-fusion and which, next to the peace-keeping role of the females, may also explain the lower rates of competition (31).
Bonobos have rich personalities and share most of their traits with humans and chimpanzees, suggesting these were also present in their common ancestor (34). From early age on, bonobos are sensitive to others’ emotions, which is demonstrated by their ability to provide appropriate consolatory behaviors after conflicts (35), a skill individuals with more effective self-regulation capabilities excel in particularly (35), but which is also demonstrated by more automatic behavior, including yawn contagion, an index of empathy (36, 37).
Bonobos’ personality characteristics and complex social emotional behavior have rarely been studied experimentally, but the conclusions that can be drawn from the few studies available are in line with the conclusions from observational research, described above. It has for example been demonstrated that great apes, bonobos included, can reliably recognize human emotions (38) and experiments with chimpanzees show that they are also sensitive to the emotions of conspecifics (39–42). By using an experimental cofeeding set-up, it has been shown that bonobos are more tolerant than chimpanzees (43; but see ref. 44). In addition, an eye-tracking study demonstrated that bonobos make more eye contact than chimpanzees (45). Finally, in a risky decision game, bonobos showed more risk-aversion than chimpanzees (46, 47).
The present study investigates emotional attention in the bonobo. [Although the usage of the adjective “emotion” is debated when it concerns animals, this term is used in dot-probe studies in humans. To be able to portray commonalities and differences with the human literature, we feel that using the same adjective is appropriate. We are also aware that at this point we cannot tell what bonobo-emotions are, and we therefore have to rely on observational work and on research into human and chimpanzee expressions. The term “emotional attention” is therefore used when attention is directed toward an emotional expression, but also when directed toward scenes showing emotion regulatory behaviors (Methods).] For this purpose, four bonobos completed a dot-probe task during which emotional and neutral pictures of unfamiliar bonobos and control animals were being presented on a touch-screen (Fig. 1). Per individual, the task was spread out over 13 sessions with 25 trials each (Methods and Table S1). Given their highly social nature, we predicted that bonobos would show heightened attention toward the pictures showing emotional compared with neutral bonobos. In addition, as bonobos are known to be less aggressive than chimpanzees and humans, and spend a lot of time on positive social behaviors—such as play, sex, and grooming—we predicted seeing this reflected in a specific attentional bias toward these more affiliative or protective behaviors than to signals of distress.
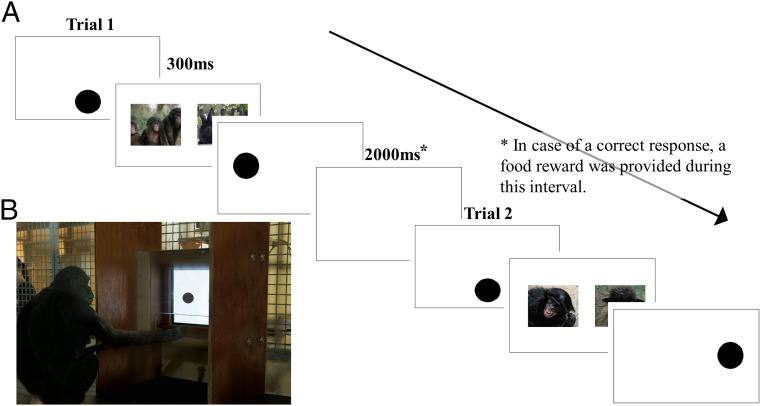
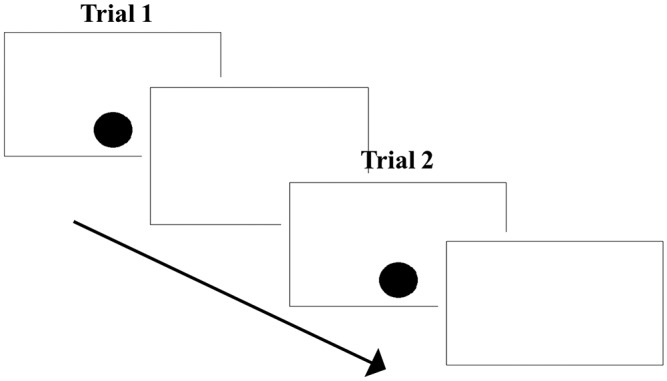
Fig. 1.
(A) Trial outline. A trial started with the presentation of a dot that was shown until touched. Next, two images emerged for 300 ms, followed by the probe. (B) Experimental setting. Kumbuka conducts the dot-probe task.
Table S1.
Stimulus material
| Picture category | No. of pictures | Description | No. of individuals per picture |
| Distress | 28 | Aggressive displays, such as long and short charges, mutual and direct displays; submissive behaviors, such as, grin faces, fleeing and crouching | M = 1.429, SE = 0.112 |
| Sex | 34 | Mating, genitogenital rubbing, masturbation, prominent full swelling, penile erection | M = 1.765, SE = 0.152 |
| Play | 16 | Together or alone, with a relaxed open mouth, without an object | M = 1.625, SE = 0.155 |
| Food | 18 | Eating, with visible food item | M = 1.778, SE = 0.222 |
| Groom | 19 | Two or more individuals grooming | M = 2.526, SE = 0.193 |
| Yawn | 9 | Open mouth, canines mostly visible | M = 1.111, SE = 0.111 |
| Pant hoot | 14 | Without clear distress; pursed lips | M = 1.429, SE = 0.227 |
| Total emotional bonobo | 139 | M = 1.712, SE = 0.175 | |
| Neutral bonobo | 159 | Walking, laying down, sitting, sleeping, bathing | M = 1.878, SE = 0.163 |
| Total bonobo | 309 | M = 1.800, SE = 0.112 | |
| Total control animal | 329 | Walking, laying down, sitting, sleeping, bathing, yawning, mating, jumping, running, stretching | M = 1.891, SE = 0.125 |
Results
Reaction Times.
Data were analyzed in a generalized mixed multilevel model with picture category [emotional bonobo, neutral bonobo, or control animal (coded as 1, 0, and −1)] as fixed factor and random intercepts for each individual and individual-by-session. The multilevel structure was defined by the different trials, nested within sessions, nested within individuals. As the reaction time data were skewed, a γ-probability distribution was selected with a log-link function (Methods).
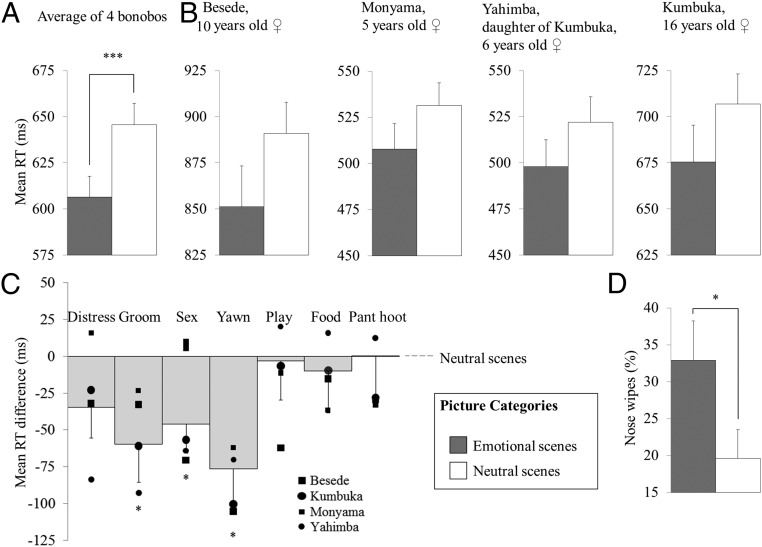
As predicted, a main effect of picture category [F(2, 1.171) = 5.135, P = 0.006] showed that bonobos were faster in tapping on the probe that replaced a picture of an emotional bonobo (mean reaction time = 611.240, SE = 78.819) compared with a neutral bonobo [mean reaction time = 645.927, SE = 83.176, t(1.171) = 2.669, P = 0.008] (Fig. 2A and Table S2) (details on all the statistical tests described in the main text are provided in tables in the SI Results). This pattern was visible in all subjects (Fig. 2B).
Fig. 2.
(A) Mean reaction times (RT) following scenes with emotional and neutral bonobos. (B) Mean reaction times per individual. (C) Contrast estimate for emotional versus neutral bonobo. The individual data represented by the squares and circles are the mean reaction times per individual. (D) Percentage of nose wipes in one individual. Error bars represent the SEM. ***P < 0.005; *P < 0.05.
Table S2.
Fixed effects: Reaction times differed between the picture categories preceding the probe
| Source | F | df1 | df2 | P value | ||
| Picture category (emotional, neutral, control animal) | 5.328 | 2 | 1.190 | 0.005 | ||
| Random effects | Estimate | SE | Z | P value | 95% confidence interval | |
| Variance | 0.054 | 0.002 | 24.331 | 0.000 | 0.050 | 0.059 |
| Intercept (ID) | 0.066 | 0.054 | 1.218 | 0.223 | 0.013 | 0.328 |
| Intercept (ID × Session) | 0.000 | 0.001 | 0.420 | 0.675 | 0.000 | 0.024 |
Reaction times (dependent variable) were analyzed in a generalized mixed multilevel model with trials (25 trials) nested in sessions (13 sessions), nested in individuals (4 individuals). Probability distribution = gamma; link function = log; covariance structure of the random effects = variance components. Emotion was coded as “1,” neutral as “0,” and control animal as “−1.”
Although luminance or contrast could not explain the differences in reaction times following the emotional versus neutral category (SI Results), it is possible that other unforeseen differences between the emotional and neutral category influenced reaction times. To rule this out, we tested whether subjects’ attention was grabbed more, the more emotionally intense a stimulus was rated by human experts. To that extent, we replaced the factor “picture category” by the “picture emotional intensity score” (the average of the four expert ratings) and left out the control animals. As predicted, a significant effect was observed [F(1, 581) = 3.885, P = 0.049], indicating that the more emotionally intense a picture was rated, the faster bonobos’ responses were when the probe replaced these pictures (Table S3; but see also Table S4).
Table S3.
Fixed effects: Reaction times were faster as the emotional intensity score of the picture preceding the probe was higher
| Source | F | df1 | df2 | P value | ||
| Picture emotional intensity score | 3.885 | 1 | 581 | 0.049 | ||
| Random effects | Estimate | SE | Z | P value | 95% confidence interval | |
| Variance | 20.934 | 1.232 | 16.986 | 0.000 | 18.653 | 23.495 |
| Intercept (ID) | 27.561 | 22.949 | 1.201 | 0.230 | 5.389 | 140.947 |
| Intercept (ID × Session) | 49.960 | 709.174 | 0.681 | 0.496 | 27.165 | 8.586 |
Reaction times (dependent variable) were analyzed in a generalized mixed multilevel model with trials (25 trials) nested in sessions (13 sessions), nested in individuals (4 individuals). Probability distribution = gamma; link function = log; covariance structure of the random effects = variance components. Emotional Intensity scores were provided for each picture by four raters and ranged from 1 (not intense) to 7 (very intense).
Table S4.
Fixed effects and emotional intensity related to Table S2
| Source | F | df1 | df2 | P value | ||
| Picture emotional intensity score | 0.081 | 1 | 583 | 0.777 | ||
| Picture category (emotional, neutral, control animal) | 4.703 | 1 | 583 | 0.031 | ||
| Random effects | Estimate | SE | Z | P value | 95% confidence interval | |
| Variance | 0.054 | 0.003 | 16.956 | 0.000 | 0.048 | 0.060 |
| Intercept (ID) | 0.063 | 0.053 | 1.185 | 0.236 | 0.012 | 0.327 |
| Intercept (ID × Session) | 0.002 | 0.003 | 0.776 | 0.438 | 0.000 | 0.027 |
The table shows that if the factor emotional intensity score is added to the model shown in Table S2, then the P value of picture category drops from 0.006 to 0.031. This shows that by controlling for emotional intensity, the remaining effect of emotion is reduced, providing evidence for our thesis that the attentional bias toward emotions we observed is of truly emotional nature. Reaction times (dependent variable) were analyzed in a generalized mixed multilevel model with trials (25 trials), nested in sessions (13 sessions), nested in individuals (4 individuals). Probability distribution = gamma; link function = log; covariance structure of the random effects = variance components. Emotional Intensity scores were provided for each picture by four raters and ranged from 1 (not intense) to 7 (very intense). Emotion was coded as “1,” neutral as “0,” and control animal as “−1.”
To test our second hypothesis, that the attentional bias toward emotions would be stronger for pictures showing affiliative or protective behaviors than for pictures showing distress, we repeated the analysis with the positive-negative ratings of the experts included as a fixed factor. Results show that valence was no significant predictor of reaction times: F(1, 581) = 3.885, P = 0.855. However, although valence as a dimension did not predict attention, it is still possible that certain picture categories grabbed more attention than others. A similar analysis was therefore conducted, but this time with the eight different emotion categories included as a fixed factor. Results showed that some emotion categories indeed attracted attention more than other emotion categories: F(7, 581) = 2.249, P = 0.029 (Table S5). In line with our prediction, the categories sex and grooming but also yawning yielded faster reaction times than neutral stimuli [sex: t(581) = 2.293, P = 0.022; groom: t(581) = 2.323, P = 0.021; yawn: t(581) = 2.458, P = 0.014]. Pictures of playing bonobos did not attract more attention than neutral scenes: t(581) = 0.101, P = 0.920. Interestingly, distressed bonobos did not attract more attention than pictures of neutral bonobos either: t(581) = 1.625, P = 0.105. It must be noted, however, that reaction times in this condition were also not significantly different from the conditions sex, grooming, and yawning (Ps ≥ 0.229) (Fig. 2C).
Table S5.
Fixed effects: Reaction times predicted by the picture category preceding the probe
| Source | F | df1 | df2 | P value | ||
| Picture category (distress, groom, sex, yawn, play, food, pant hoot, neutral) | 2.249 | 7 | 581 | 0.029 | ||
| Random effects | Estimate | SE | Z | P value | 95% confidence interval | |
| Variance | 0.053 | 0.003 | 16.919 | 0.000 | 0.047 | 0.059 |
| Intercept (ID) | 0.063 | 0.053 | 1.184 | 0.237 | 0.012 | 0.329 |
| Intercept (ID × Session) | 0.002 | 0.003 | 0.777 | 0.437 | 0.000 | 0.029 |
Reaction times (dependent variable) were analyzed in a generalized mixed multilevel model with trials (25 trials) nested in sessions (13 sessions), nested in individuals (4 individuals). Probability distribution = gamma; link function = log; covariance structure of the random effects = variance components. Emotional Intensity scores were provided for each picture by four raters and ranged from 1 (not intense) to 7 (very intense).
Nose Wipes.
Nose wipes represent an expression of negative emotion (Methods) (48, 49) and occurred relatively frequently (Kumbuka, 84 trials; Yahimba, 23 trials, Monyama, 10 trials; and Beselde, 7 trials). Kumbuka had a sufficient number of trials to test this behavior statistically. To test whether nose wipes occurred more frequently following trials where emotional versus neutral bonobos or control animals were shown, L.J. and M.E.K. coded trials where nose wipes occurred as “1” and trials where this behavior was absent as “0.” The correlation between the two raters was significant (R = 0.701, P < 0.001), as also demonstrated by a high level of agreement, κ = 0.688, P < 0.001. Data were analyzed in a generalized mixed multilevel model with picture category [emotional or neutral bonobo (coded as 1 and −1)] as a fixed factor and random intercepts for each session. The multilevel structure was defined by the different trials, nested within sessions. The distribution was binomial with a logit log-link function.
A main effect for picture category was observed [F(1,184) = 4.194, P = 0.042] showing that Kumbuka touched her nose more frequently after seeing an emotional compared with a neutral bonobo being replaced by the probe. To investigate whether it was seeing the emotional scene that caused this behavior and possibly inhibited her response, or whether it was the approach movement that she had to make toward that location on the screen, we repeated the analysis, but instead of including the probe-picture category as the predictor, we included the distracting picture as the predictor. This analysis yielded no significant effect [F(1, 200) = 0.620, P = 0.603], from which we can conclude that the inhibition of her response following an emotion was a result of the movement she had to make toward that location, which resulted in edginess, motivational ambivalence, or frustration (48, 49) (Fig. 2D).
SI Results
The following section provides all of the statistical models described in the main text, in tables. This section also contains additional statistical analyses to rule out alternative interpretations of the data.
Comparison of Emotional Bonobo with Control Animals.
The difference in reaction times following pictures of emotional bonobos (mean reaction time = 611.103, SE = 78.852) and the control category black sheep and rabbits (mean reaction time = 617.040, SE = 79.326) was not significant: t(1, 1.190) = 0.556, P = 0.578). Reaction times were similar for the sheep (mean reaction time = 629.11, SE = 83.80) and rabbit pictures (mean reaction time = 610.72, SE = 81.36) [F(1, 584) = 2.438, P = 0.119], suggesting that body shape did not play a role here, but possibly that reaction times were also relatively fast following control animals, because these animals were novel to the bonobos.
Luminance and Contrast.
To check whether luminance or contrast could have impacted our results, we measured these values in each picture, and conducted independent simple t tests between the different stimulus categories. The results are listed in Table S7.
Table S7.
Luminance and contrast
| Animal | Luminance | Contrast | ||||
| Mean | SD | t test | Mean | SD | t test | |
| Control animal > | 0.361 | 0.098 | t(626) = 6.711, P < 0.001 | 0.213 | 0.049 | t(626) = 6.711, P < 0.001 |
| Bonobo | 0.411 | 0.091 | 0.23 | 0.053 | ||
| Bonobo scenes | ||||||
| Distress | 0.346 | 0.102 | P = 0.525 | 0.223 | 0.053 | P = 0.054 |
| Groom | 0.334 | 0.057 | P = 0.114* | 0.214 | 0.048 | P = 0.418 |
| Sex | 0.361 | 0.114 | P = 0.910 | 0.198 | 0.054 | P = 0.423* |
| Yawn | 0.402 | 0.075 | P = 0.206 | 0.258 | 0.063 | t(166) = 3.395, P = 0.001 |
| Play | 0.343 | 0.078 | P = 0.533 | 0.213 | 0.034 | P = 0.514 |
| Food | 0.410 | 0.103 | t(175) = 2.039, P = 0.043 | 0.252 | 0.043 | t(175) = 4.208, P < 0.001 |
| Pant hoot | 0.386 | 0.095 | P = 0.321 | 0.239 | 0.058 | P = 0.054* |
| > | ||||||
| Neutral | 0.359 | 0.1 | 0.206 | 0.044 | ||
The t tests are not corrected for multiple comparisons (14 tests comparing the emotional conditions with the neutral condition).
A Levene's Test of Equality of Variances showed a significant effect, so this P value is corrected for that (equal variances not assumed).
The animal pictures were somewhat higher in luminance [t(626) = 6.711, P < 0.001] and contrast [t(626) = 4.091, P < 0.001] than the bonobo images. This result does not, however, cause a major problem in the interpretation of our results because the key comparison is between the emotional and the neutral category of the bonobo pictures. The major result of our study is that bonobos showed heightened attention toward emotional compared with neutral bonobos. This difference was significant in the categories groom, sex, and yawn. Comparing these categories on luminance and contrast with the neutral scenes showed that only the images with yawns were higher in contrast than the neutral ones [t(166) = 3.395, P = 0.001]. However, the important question is whether pictures high in contrast or luminance attracted attention more than pictures low in contrast or luminance. In testing this theory, we used the exact same model as described in the main text and in Table S2, but with “luminance” rather than “picture category” included as fixed factor. This analysis showed that the luminosity of the picture preceding the probe did not impact on the reaction times on the probe: F(1, 1.191) = 0.619, P = 0.431. The same analysis was conducted for “contrast.” The results show that contrast did not impact on reaction times either: F(1, 1.191) = 0.347, P = 0.556. Moreover, adding either “contrast” or “luminance” as a covariate did not change main findings whatsoever. For example, after adding “contrast” as a covariate to the statistical model where emotion conditions are compared with neutral ones (i.e., the model shown in Table S4), the difference between the “yawn” and “neutral” category is still significant: t(580) = 2.695, P = 0.007 (without the covariate it was P = 0.014, so in fact, it became slightly more significant because even though “contrast” did not explain reaction times significantly, it did explain some of the variance). To conclude, neither luminance nor contrast explained the attentional bias towards emotions in our study.
Action Versus Attention.
It is possible that the mere presentation of an emotional stimulus triggered faster actions and greater goal-directed action rather than enhanced attention per se. If true, we would expect a similar effect of emotion when the distracting picture category is included as a predictor: that is, the picture that appeared on the other side of the screen than the probe, rather than the picture that appeared at the location of the probe. If, however, the emotional stimuli sped up reaction times compared with neutral stimuli because of heightened attention, as is assumed in the emotional dot-probe task, we would expect to find no effect or even an opposite effect for the distracting picture. Results of this analysis showed no effect of the distracting picture category: F(2, 1.171) = 0.737, P = 0.479. This finding suggests that, in line with the theory of the emotional dot-probe paradigm, attention was attracted toward the emotional stimulus, and if the probe was presented on that location, this facilitated and thereby sped up the action significantly.
Discussion
Bonobos spend their lives in large social groups and for their survival have to rely on that group and its members. Observational research has shown that bonobos are very social and can adequately regulate their own and group members’ emotions, thereby often preventing conflicts from arising or solving them quickly. The present study is, to our knowledge, the first to experimentally demonstrate that bonobos, like humans, have heightened attention for emotional compared with neutral signals of conspecifics. With aid of the dot-probe task, a paradigm designed for testing attentional biases in humans, we here demonstrate that bonobos have an attentional bias toward the emotions of others. Most interestingly, bonobos were particularly drawn toward scenes showing other bonobos that were yawning, mating, or grooming, but not toward scenes depicting distressed bonobos, bonobos pant-hooting, playing, or handling food compared with neutral scenes. In addition to an attentional bias towards emotions, Kumbuka showed more nose wipes—a behavior indicating edginess, motivational ambivalence, or frustration (48, 49)—on trials where the probe was located on the side of the emotional compared with the neutral picture. We will now discuss the most attended emotional behaviors in more detail, and the putative drivers of their saliency.
The bonobos that were tested in the present study showed the strongest attentional bias toward pictures of yawning bonobos. Yawning is an evolutionarily old behavior that is widespread among vertebrates. Although there is a scientific debate about the exact function, different studies have demonstrated a social signaling function. There is convincing evidence that yawning serves a thermoregulatory function (i.e., it cools the brain back to homeostasis). It is thought that by cooling the brain, yawning induces vigilance, which is why it is evolutionary adaptive for the whole group to pick up or mimic this behavior (50). Yawning indeed is very contagious in many social species, including bonobos, and its effect is even stronger when the observed yawners are kin or friends or of higher rank (36, 37, 51). Research has shown the involvement of the mirror neuron system during contagious yawning and supports the premise of a connection between this system and higher cognitive empathic functions, including mentalizing. Hence, it has been suggested that contagious yawning is based on a functional substrate of empathy (52).
It is possible that as a sign of threat, bonobos’ attention was caught by the display of canines (which are less impressive compared with the chimpanzee). However, after close inspection of the different pictures and their associated reaction times, we can conclude that this is unlikely. First, the pictures where the canines were most visible did not attract more attention than the pictures where they were less visible. Second, the canines were also visible during fear grimaces, which did not attract attention more than did neutral images.
Touch is a powerful tool for communicating positive emotions. Human and nonhuman primates use social touch for maintenance and reinforcement of social structures (53). Most primate species communicate affection and reduce group tension by means of so-called grooming, the act of tidying or cleaning one another's body or appearance. Grooming is regarded as a service given by one individual that confers benefits to the recipient in terms of hygiene and has possible calming effects (54). Grooming is a recipe for social support and releases oxytocin in both the groomer and groomed (55). Bonobos keep close track of who groomed whom, reciprocate grooming, and distribute their grooming according to the rank of the receivers (56). Given that grooming plays such a prominent role in bonobo society, it is not surprising that their attention was drawn toward scenes showing this behavior.
The third category that attracted bonobos’ attention were pictures showing scenes with bonobos involved in sexual behavior. In humans, sexual frequency has been found to be a strong positive predictor of general wellbeing and of the quality of the social relationship (57). Sexual activity plays an even bigger role in bonobo society. In bonobos, sex is being used as a form of greeting, a means of forming social bonds, conflict resolution, and postconflict reconciliation. Bonobos are the only nonhuman animal to have been observed engaging in face-to-face genital sex, tongue kissing, and oral sex (58). Bonobos do not form permanent monogamous sexual relationships with individual partners and do not discriminate in their sexual behavior by sex or age (with the possible exception of abstaining from sexual activity between mothers and their adult sons). When bonobos find a new food source or feeding ground, the increased excitement will usually lead to communal sexual activity, presumably decreasing tension and encouraging peaceful feeding (58). The attentional bias for sexual images as observed in our study reflects the high frequency and importance of this behavior in their daily lives.
Despite their great genetic similarity, bonobos’ behavior is strikingly different from that of chimpanzees. Whereas males are the dominant sex in the chimpanzee, bonobo society is female-dominant. Remarkably, like humans, chimpanzees make war with rivaling groups and do not take kindly to strangers. In stark contrast, bonobos live in friendly and tolerant societies and, although they sometimes hunt smaller animals for consumption, never kill one of their own (9, 31, 32). Intriguingly, bonobos prefer to share food and mate with strangers than with acquaintances (33). The social and emotional differences between the two species of Pan are reflected in the anatomy of the brain. A neuroimaging study comparing both species showed that bonobos not only have more gray matter in the amygdala and insula, regions involved in perceiving emotions in self and others, they also have a larger pathway linking the amygdala with the anterior cingulate cortex, which is implicated in top-down control of aggressive impulses (59). The results are in line with earlier work showing that bonobos have more Von Economo neurons, which are involved in social cognition, in the anterior cingulate cortex than chimpanzees, and showed a pattern that closely resembled that seen in humans (59–61).
The present study showed that bonobos have heightened attention toward conspecifics’ yawns, grooming, and sexual behaviors. Based on the results of the present study, can these behaviors be interpreted as emotional behaviors? Heightened attention as measured via shorter reaction times in the dot-probe task has in the human literature been widely interpreted as an emotional bias. Can we then draw the same conclusion for bonobos? For the following reasons, we indeed think we can. First, reaction times of all four subjects reflected an attentional bias toward conspecifics’ emotional behaviors. Second, the scenes that were rated by four experts as being emotionally intense for bonobos, especially grabbed bonobo’s attention most. Third, one subject showed more nose wipes following trials where she had to approach emotional compared with neutral scenes. A nose wipe is considered a sign of edginess, motivational ambivalence, or frustration (48, 49).
To conclude, bonobos’ attention is quickly captured by the emotional expressions of others. Interestingly, this attentional bias was strongest for affiliative behaviors (grooming and mating) and behaviors that are highly contagious (yawning) and not significant for scenes depicting distress. The results suggest that protective and affiliative behaviors are pivotal in bonobo society and therefore prioritized.
Methods
Subjects.
Four female bonobos participated in this study: Besede (10 y old), Monyama (5 y old), Kumbuka (16 y old and mother of Yahimba), and Yahimba (6 y old). The bonobos live within a social group of 12 individuals in an enriched environment with a 2,812-m2 outdoor compound and an attached indoor residence of 158 m2 that is illuminated during daytime at Apenheul Primate Park, Apeldoorn, The Netherlands. The outdoor compound is equipped with climbing frames, ropes, small streams, and various species of trees. Access to the outdoor compound is available to the bonobos when the temperature outside exceeds 15 °C. The group composition varies per day but is always divided into two subgroups because of frequent conflicts between the alpha-female and a lower ranking male, two individuals that did not take part in this study.
Daily meals include a wide variety of fruits, vegetables, branches and leaves, and occasionally chicken, fed four to five times a day, supplemented with nutritionally balanced biscuits (fed twice daily) and water available ad libitum. The bonobos that took part in the current study have been familiar with humans since birth and interact with them on a daily basis. The three-weekly experimental sessions took part in the indoor enclosure. The bonobos were called one by one to the computer screen and were not separated from the rest of the group. One testing session took ∼10 min per individual (SI Methods).
Ethics.
The care and use of the bonobos adhered to the guidelines of the European Endangered Species Program, formulated by the European Association of Zoos and Aquaria. Bonobos were never forced to take part in the experiment, were never separated from their group, were only positively reinforced, never negatively, and were on average rewarded equally as the bonobos that were also present in the testing room but involved in another training task with the keeper. All testing was conducted as part of the bonobo’s enrichment program of Apenheul. The research activities were fully integrated into the daily routine and required no manipulation of individuals. Consequently, the bonobos were never deprived of water and food at any stage. Therefore, this study was conducted in compliance with all relevant Dutch laws and in agreement with international and scientific standards and guidelines. Furthermore, because of the noninvasive character of the study and absence of any potential discomfort, our study did not meet the definition of an animal experiment as mentioned in Article 1 of the Dutch “Experiments on Animals Act.” Consequently, the Ethics Committee of Apenheul waived the need for approval.
Apparatus.
Stimuli were presented via Presentation software (NeuroBehavioral Systems) on a touch-screen of the model Iiyama T1931SR-B1 (19 inch, 1,280 × 1,024 pixels, ISO 5 ms). A correct response (a tap on the probe) resulted in the activation of the feeder and the distribution of a small piece of apple or a raisin that fell into a funnel. The funnel was attached to the back of the apparatus and to a connecting acryl tube that ended right below the touch-screen where the bonobo could easily catch the reward with her right hand. Wooden side panels were attached on each side of the touch-screen apparatus so that the bonobo in front of the screen could complete the task without too much distraction (Fig. 1).
Stimulus Material.
Stimulus material consisted of a set of pictures (sized 330 × 400 or 400 × 330 pixels) of bonobos (n = 298) and control animals (n = 330; rabbits and hare n = 167; sheep and goat n = 159). The pictures of bonobos showed neutral (n = 159) and emotional scenes (n = 138). The emotional pictures included scenes where bonobos showed clear signs of distress or were playing, but also scenes that can be associated with stress, such as when there is food at play, or sex, which can be used as a means of (re)establishing social bonds, as is grooming. Furthermore, we included pictures where bonobos were calling each other (pant hoot) or were yawning. Yawning is a highly contagious behavior and its mimicry has been linked to empathy and increases in social affinity (36, 37). The pant hoot is used to express excitement but mostly to maintain spatial contact with other individuals during traveling. Chimpanzees can easily match pictures showing this expression with the correct vocal sound (40).
The number of individuals shown in a picture was matched across the emotional versus neutral category, and an ANOVA confirmed that indeed there were no differences: F(1, 295) = 0.488, P = 0.485. The number of depicted individuals was also similar for the pictures showing bonobos compared with control animals: F(1, 624) = 0.270, P = 0.603. The pictures were selected from the Internet by M.E.K. and divided into different categories: distress, sex, play, food, groom, yawn, pant hoot (Table S1).
Four different experts from de Apenheul rated the pictures on emotionality. Two of them were caregivers of the bonobos with 6 and 19 y of almost daily exposure to them (Keetie de Koeiier and Jacqueline Ruys). T.B. has worked in de Apenheul for 3 y and also sees them on almost a daily basis. L.J. has conducted an observational study with this group of bonobos and has observed them for 4 m for 5 to 6 full days a week. The experts were shown the pictures of bonobos and subsequently asked how negative or positive they thought a bonobo would experience each picture (1 = very negative; 7 = very positive) and how emotionally intense each picture would be for a bonobo (1 = not intense; 7 = very intense). All correlations between the negative-positive ratings of the experts were significant: mean R = 0.390, P < 0.001. The lowest correlation was R = 0.138, P = 0.016. The mean correlation for the intensity ratings was R = 0.3178, P < 0.001. Except for one nonsignificant correlation between two experts (P = 0.445), all other correlations on this rating scale were significant P ≤ 0.036.
A linear mixed-model analysis with the bonobo pictures nested within experts, and “emotion” as a fixed factor (emotion coded as 1; neutral coded as −1), showed that the experts predicted that the pictures showing emotional bonobos would indeed be perceived as more emotionally intense by bonobo subjects than the neutral pictures: F(1, 1.222) = 6.373, P = 0.012. A second analysis with “category” as a fixed predictor showed that they believed this to be the case for the conditions distress: t(1.216) = 4.069, P < 0.001; sex t(1.216) = 3.197, P = 0.001; play t(1.216) = 2.380, P = 0.017; and yawn t(1.216) = 2.246, P = 0.025; but not for the conditions pant hoot t(1.216) = 1.594, P = 0.111; grooming t(1.216) = 1.475, P = 0.141; and food t(1.216) = 0.485, P = 0.628.
Procedure.
For the bonobos, it was the first time to take part in a touch-screen experiment. Therefore, after the screen was installed, a week of habituation was implemented to make sure the bonobos were accustomed to the apparatus before training started. The first phase of training consisted of calling the bonobos by their name, while standing next to the screen. Vocal appraisal was used as soon as they approached the screen. The next step was to train them on using the screen and to sit and remain seated right in front of it. Because individuals were not separated from the group during testing, we also had to train the other individuals not to disturb the individual that was tested (see SI Methods for more details and Figs. S1–S3).
Fig. S1.
Dot shown at center of the bottom half of the screen.
Fig. S3.
Training on the emotional dot-probe task.
We managed to train four individuals on the experimental task (subjects). They were called to the screen by L.J. If the bonobo would not come to the screen after three calls, the next individual would be called and the previous bonobo would be called again later. At the same time, the other bonobos, who were also present in the indoor enclosure, took part in a body-part target-training by one of the caregivers. During this common-practice training, a body part was called (e.g., “hand”) and if the bonobo put her hand against the meshwork toward the caregiver, she would receive a food reward. Similar amounts of food rewards were distributed during the experiment as during the body-part training.
During the experiment, the bonobos sat at a distance of ∼60 cm from the screen. A trial started with the presentation of a black dot that was presented in the lower, middle part of the screen. After tapping on the dot, two pictures appeared, one on the left and one on the right side of the screen for 300 ms. Next, the probe (a similar dot) appeared either on the left or on the right side that stayed on the screen until touched, after which a food reward was provided through a funnel that ended just below the screen. The next trial started after a delay of 2,000 ms (Fig. 1). By the end of each individual session, the bonobo was given a bonus food reward (a pellet with three raisins). For each individual we made a pseudorandomized trial list with 25 or 26 trials divided over 13 sessions. In half of the trials, an animal picture was paired with an emotional bonobo, and in the other half with a neutral bonobo. The location of the probe was counterbalanced. The average number of trials per emotion category (distress, groom, sex, yawn, play, food, pant hoot) ranged from 10 to 22 trials per individual.
Already during testing, L.J. noted that some individuals frequently wiped their nose, sometimes halfway through a trial. All sessions were videotaped without interruption; therefore, this behavior was coded afterward by M.E.K. and L.J. Trials with nose wipes were not included in the analysis of reaction times. In addition, some trials had to be excluded because of the interference of another individual. All trials were thus watched back on video to identify those that based on these criteria should be excluded from the reaction-time analysis. The correlation between M.E.K. and L.J. on the trials that should be excluded based on these criteria was R = 0.704, P < 0.001. Trials with reaction times faster than 200 ms and reaction times that were three SDs above the individual mean were also excluded from the statistical analysis. These restrictions resulted in the exclusion of 9.31% of the trials; 40.67% of the excluded trials were excluded because of nose wipes. Because this behavior is a sign of edginess, motivational ambivalence, or frustration and is in itself potentially interesting (48, 49), nose wipes—along with other behaviors, including scratching, yawning, making a grin face, vocalizing, bending backward, and wiping over the head—were coded by M.E.K. and L.J. (Table S6).
Table S6.
Occurrence of certain behaviors that could be defined as stress behavior
| Occurrence of behavior (%) | Picture category | ||||||||
| Control animal | Distress | Groom | Sex | Yawn | Play | Food | Pant hoot | Neutral | |
| Nose wipe | 8.989 | 9.091 | 16.667 | 16.667 | 9.524 | 11.765 | 13.333 | 19.231 | 9.706 |
| Gentle autoscratch | 3.828 | 1.695 | 2.439 | 5.479 | 0.000 | 0.000 | 6.250 | 0.000 | 4.190 |
| Rough autoscratch | 0.744 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 3.333 | 0.539 |
| Vocalize | 0.594 | 1.695 | 0.000 | 1.316 | 4.545 | 0.000 | 0.000 | 3.333 | 0.811 |
| Bending backward | 4.314 | 3.448 | 2.439 | 0.000 | 0.000 | 5.556 | 6.250 | 6.897 | 2.473 |
| Head wipe | 0.148 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Statistical Analyses.
Except when explicitly stated otherwise, all statistical analyses were generalized mixed multilevel models, implemented in SPSS, v20. In studies with small sample sizes, it is especially recommended to avoid averaging data (as is common in a simple ANOVA) and to include all valid datapoints into the analysis. Because datapoints within individuals or sessions are likely to be correlated, our data had a nested structure where covariance was taken into account. The multilevel structure was defined by the different trials (25 or 26 trials), nested within sessions (13 sessions), nested within subjects (4 subjects). By default, all statistical models included a random intercept for each individual and for each individual by session. All variables were centered (for a similar procedure, see ref. 39). Reaction-times analyses only included the correct trials and as these data were skewed, a γ-probability distribution was selected with a log-link function. For the analysis of nose wipes, the distribution was binomial with a logit log-link function (there was a nose wipe, “1,” or there was not, “0”). The SI Methods and SI Results provide tables with all of the statistical models described in the main text; see Table S7 for luminance and contrast results.
SI Methods
Training.
M.E.K., T.B., and L.J., in collaboration with two keepers, designed a training protocol with the aim to train bonobos on an emotional dot-probe task implemented on a touch-screen. In total, the bonobos were trained on 34 d over a period of 15 wk. Training was initially started with the seven bonobos older than 7 y (Jill, 30-y-old female; Kumbuka, 16-y-old female; Hortense, 37-y-old female; Zuani, 25-y-old female; Besede, 10-y-old female; Zamba, 17-y-old male; and Bolombo, 17-y-old male) because we expected that Yahimba (6-y-old female), Monyama (5-y-old female), and Makasi (6-y-old male) would not sit behind the screen independently, as they were still at the age where they followed their mothers. The training consisted of several steps, which are outlined in detail below.
Step 1.
After the installation of the touchscreen, 1 wk of habituation to the screen was implemented before training started so that the bonobos could explore the new set-up at their own pace. The screen was turned off during this first week.
Step 2.
The first phase of training consisted of calling the bonobos by their name while standing next to the screen behind mesh wire. Small rewards were provided through the funnel together with vocal appraisal as soon as they approached the screen. A black dot counting 200 pixels was presented against a white background in the center of the bottom half of the computer screen (Fig. S1). The bonobos received a reward (small piece of apple) if they touched the screen, irrespective of whether they tapped on the dot or on another location of the screen. The experimenter handed these rewards manually and also started a new trial manually. After 3 wk of training a “feeder” apparatus was installed above the touchscreen behind the mesh wire. The feeder consisted of a large disk grooved with 50 furrows. Each furrow was just large enough for the reward to fit in. When the dot on the screen was tapped, a small brush on the feeder would push the reward out of the furrow, making it fall into the funnel that led toward the bottom of the touch screen and was accessible to the bonobos. When the reward was pushed out of the furrow, the disk rotated until the next furrow was placed underneath the brush for the next reward to be given at the following trial.
It was noted that two young female bonobos aged 5 and 6 y were very much interested in touching the screen, even though this behavior was not rewarded. These youngsters were observing adult bonobos (including their mothers) during their training and seemed very curious about the screen. It was therefore decided to include them in the training because they spontaneously started touching the dot, which was rewarded at the second day of training.
The alpha female, Jill, showed interest in the screen and responded to our calls. However, she was sitting next to the screen and looked at it without touching it. To get her to touch the screen, we followed a suggestion of one of the keepers, and smeared some apple syrup on the screen, which worked immediately. When Jill touched the screen to remove the syrup, we rewarded her and continued to reward her each time she touched the screen. We repeated this once more on the same day and then rewarded her whenever she touched the screen, after which we continued with the protocol. The apple-syrup procedure was also tried with Zamba, unfortunately without success. After 9 wk and between 18 and 20 sessions of training, we decided to stop the training of three bonobos because they did not respond to calls for training (Hortense) or had never touched the screen (Zamba and Zuani). Besede, Bolombo, Kumbuka, Yahimba, and Monyama were able to complete this step within the first week of training.
Step 3.
After the bonobos had touched the screen, irrespective of the location for a minimum of 10 times in total, with a reward after each touch, they only received a reward when they touched the black dot, except for Monyama and Yahimba, who started receiving rewards after the first session of training after it was decided that the juveniles should be trained as well. Four bonobos (Bolombo, Kumbuka, Yahimba, and Monyama) learned to touch the dot during the first week of training. At this point, we also added an error sound, which was played when another part of the screen was touched, then the dot. However, after hearing the first error sound all of the bonobos in the enclosure were startled, and for at least 10 min after the sound not a single individual would approach the screen after being called. It was therefore decided not to use the error sound anymore and work with positive rewards only. We reasoned that it would most likely cost more time to habituate them to the sound than to teach them to touch the dot without it.
Step 4.
After the bonobos had touched the dot in the center of the bottom half of the screen 20 times in total, the central dot was followed by a similar dot, but then presented on either the left or the right side of the screen, in randomized order, still controlled by the experimenter (Fig. S2).
Fig. S2.
Dot, followed by a second dot, shown on the left or right side of the screen.
After 2 wk of training and five sessions of training, four bonobos (Bolombo, Kumbuka, Monyama, and Yahimba) could reliably touch the dot on the center, left, and right side of the screen. After the third week and seven sessions of training, bonobo Besede was also able to do this and was ready for the next step as well. After 9 wk and 20 sessions of training, Jill was also ready for step 5. To motivate her and the others a bit more, we started to give somewhat larger rewards at the end of each individual training session (three raisins and one chunk of mashed up vegetables and vitamins).
Step 5.
After the bonobos successfully touched the dot on either side of the screen at least 15 times, they were only rewarded for tapping on the second dot. Thus, to receive a reward (a piece of apple or a raisin via the feeder), the bonobo first had to touch the dot in the center of the screen and then a second dot on either the left or right side of the screen. Six bonobos (Bolombo, Kumbuka, Yahimba, Besede, Monyama, and Jill) successfully completed step 5 within 1 wk of training.
Step 6.
Six bonobos (Bolombo, Kumbuka, Yahimba, Besede, Monyama, and Jill) were trained on the trial outline of the emotional dot-probe task. A trial started with a centrally presented dot (that stayed on the screen until tapped), followed by the presentation of two different pictures (300 ms), followed by a dot on the left or right side of the screen. The next trial started 2,000 ms after the second dot was touched. Pictures were presented in color and showed 159 different pictures of various animals (elephants, birds, dogs, horses, mice, cats, chimpanzees, and rabbits). The bonobos were trained on this task for 6 wk (number of sessions ranged from 5 to 13 and the total number of trials ranged from 50 to 400) (Fig. S3).
Step 7.
We noted that not all bonobos (Bolombo, Besede, and Kumbuka in particular) had the fine motor skills to reliably tap right on the dot and sometimes just missed it by a few millimeters and were still performing at chance level. We therefore decided to increase the size of the dot to 252 pixels. To keep the bonobos motivated, we also changed the pictures. We included 184 unique and new color pictures of animals, shown in a random order. The size of the pictures was equal to the size of the pictures that were used in the final task.
After 1 wk of training in step 7, we noticed that four (Jill, Monyama, Besede, and Bolombo) of six bonobos were not improving. To get more insight into the reasons behind this, we installed a monitor at the side of the trainer that was connected to a camera showing the bonobos’ live performance. In hindsight, this should have been done at step 2. Now the trainer could see why the four bonobos had such a high error rate. It was discovered that the four bonobos touched the pictures and kept tapping until the second dot appeared.
Step 8.
To unlearn tapping on the photos, a new training task was designed showing a dot and a picture simultaneously. Bonobos were rewarded after tapping on the dot and were not rewarded when tapping on the picture. The start of a trial was controlled by the experimenter. This step was repeated until the bonobos were able to successfully touch the dot 20 times. Bolombo succeeded in completing step 8 in 3 sessions and 60 trials, Besede succeeded in 5 sessions and 105 trials, Jill succeeded in 6 sessions and 190 trials, and Monyama succeeded in 4 sessions and 90 trials.
Back to Step 7.
After tapping on the picture was unlearned, the six bonobos (Kumbuka, Yahimba, Jill, Monyama, Besede, and Bolombo) continued with step 8 and we verified that this time they indeed touched the second dot, and not the pictures that were presented prior to the appearance of the second dot. After 3 mo of training, four bonobos (Kumbuka, Yahimba, Monyama, and Besede) passed step 8 with a success rate of 80%. Unfortunately, there was not enough time to continue the training with Jill and Bolombo.
Acknowledgments
We thank the Kyoto University Primate Research Institute, in particular Prof. Tetsuro Matsuzawa and Prof. Masaki Tomonaga for providing us with a feeding device, and Coos Hakvoort and Bert Molenkamp for implementing it in the set-up; Jacqueline Ruys, Keetie de Koeiier, Carolijn de Jong, Grietje Grootenhuis, and Iris Schapelhouman for their assistance during testing; and Toinny Lukken and Chantal Kapteijn for their advice with regard to the training. Research was supported by the Netherlands Science Foundation Grant VENI 016-155-082, and The Royal Netherlands Academy of Arts and Sciences Dobberke Foundation for Comparative Psychology Grant UPS/BP/4387 2014-3 (to M.E.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522060113/-/DCSupplemental.
References
- 1.Darwin CR. The Expression of the Emotions in Man and Animals. John Murray; London: 1872. [Google Scholar]
- 2.Spoor JR, Kelly JR. The evolutionary significance of affect in groups: Communication and group bonding. Group Process Intergroup Relat. 2004;7(4):398–412. [Google Scholar]
- 3.de Waal FB. Putting the altruism back into altruism: The evolution of empathy. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 4.Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol Sci. 2006;17(4):292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schupp HT, Junghöfer M, Weike AI, Hamm AO. Attention and emotion: An ERP analysis of facilitated emotional stimulus processing. Neuroreport. 2003;14(8):1107–1110. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- 6.Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Panksepp J. Affective Neuroscience: The Foundation of Human and Animal Emotions. Oxford Univ Press; New York: 1998. [Google Scholar]
- 8.Anderson DJ, Adolphs R. A framework for studying emotions across species. Cell. 2014;157(1):187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Waal FBM. The Bonobo and the Atheist: In Search of Humanism Among the Primates. Norton & Company; New York: 2014. [Google Scholar]
- 10.Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biol Psychiatry. 1998;44(12):1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- 11.Frijda NH. Impulsive action and motivation. Biol Psychol. 2010;84(3):570–579. doi: 10.1016/j.biopsycho.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 12.LeDoux JE. Emotion: Clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- 13.Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120(1):3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Bradley B, et al. Attentional biases for emotional faces. Cogn Emotion. 1997;11(1):25–42. [Google Scholar]
- 15.Carlson JM, Reinke KS. Masked fearful faces modulate the orienting of covert spatial attention. Emotion. 2008;8(4):522–529. doi: 10.1037/a0012653. [DOI] [PubMed] [Google Scholar]
- 16.Holmes A, Green S, Vuilleumier P. The involvement of distinct visual channels in rapid attention towards fearful facial expressions. Cogn Emotion. 2005;19(6):899–922. [Google Scholar]
- 17.de Valk JM, Wijnen JG, Kret ME. Anger fosters action. Fast responses in a motor task involving approach movements toward angry faces and bodies. Front Psychol. 2015;6:1240. doi: 10.3389/fpsyg.2015.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuilleumier P, Schwartz S. Emotional facial expressions capture attention. Neurology. 2001;56(2):153–158. doi: 10.1212/wnl.56.2.153. [DOI] [PubMed] [Google Scholar]
- 19.Tamietto M, et al. Effects of emotional face cueing on line bisection in neglect: A single case study. Neurocase. 2005;11(6):399–404. doi: 10.1080/13554790500259717. [DOI] [PubMed] [Google Scholar]
- 20.Williams MA, Mattingley JB. Unconscious perception of non-threatening facial emotion in parietal extinction. Exp Brain Res. 2004;154(4):403–406. doi: 10.1007/s00221-003-1740-x. [DOI] [PubMed] [Google Scholar]
- 21.Flaisch T, Schupp HT, Renner B, Junghöfer M. Neural systems of visual attention responding to emotional gestures. Neuroimage. 2009;45(4):1339–1346. doi: 10.1016/j.neuroimage.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 22.Rowe G, Hirsh JB, Anderson AK. Positive affect increases the breadth of attentional selection. Proc Natl Acad Sci USA. 2007;104(1):383–388. doi: 10.1073/pnas.0605198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derryberry D, Tucker DM. In: The Heart’s Eye: Emotional Influences in Perception and Attention. Niedenthal PM, Kitayama S, editors. Academic; San Diego: 1994. pp. 167–196. [Google Scholar]
- 24.Todd RM, Cunningham WA, Anderson AK, Thompson E. Affect-biased attention as emotion regulation. Trends Cogn Sci. 2012;16(7):365–372. doi: 10.1016/j.tics.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proc Natl Acad Sci USA. 2011;108(25):10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes CM, Mundry R, Boesch C. Long-term reciprocation of grooming in wild West African chimpanzees. Proc Biol Sci. 2009;276(1657):699–706. doi: 10.1098/rspb.2008.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson ML, et al. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature. 2014;513(7518):414–417. doi: 10.1038/nature13727. [DOI] [PubMed] [Google Scholar]
- 28.Mitani JC, Watts DP, Amsler SJ. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr Biol. 2010;20(12):R507–R508. doi: 10.1016/j.cub.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Bingham PM. Human evolution and human history: A complete theory. Evol Anthropol. 2000;9(6):248–257. [Google Scholar]
- 30.de Waal FBM. Sociosexual behavior used for tension regulation in all age and sex combinations among bonobos. In: Fierman JR, editor. Pedophilia. Springer; New York: 1990. pp. 378–393. [Google Scholar]
- 31.Furuichi T. Female contributions to the peaceful nature of bonobo society. Evol Anthropol. 2011;20(4):131–142. doi: 10.1002/evan.20308. [DOI] [PubMed] [Google Scholar]
- 32.Hare B, Wobber V, Wrangham R. The self-domestication hypothesis: Evolution of bonobo psychology is due to selection against aggression. Anim Behav. 2012;83(3):573–585. [Google Scholar]
- 33.Tan J, Hare B. Bonobos share with strangers. PLoS One. 2013;8(1):e51922. doi: 10.1371/journal.pone.0051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss A, et al. Personality in bonobos. Psychol Sci. 2015;26(9):1430–1439. doi: 10.1177/0956797615589933. [DOI] [PubMed] [Google Scholar]
- 35.Clay Z, de Waal FB. Bonobos respond to distress in others: Consolation across the age spectrum. PLoS One. 2013;8(1):e55206. doi: 10.1371/journal.pone.0055206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palagi E, Norscia I, Demuru E. Yawn contagion in humans and bonobos: Emotional affinity matters more than species. PeerJ. 2014;2:e519. doi: 10.7717/peerj.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demuru E, Palagi E. In bonobos yawn contagion is higher among kin and friends. PLoS One. 2012;7(11):e49613. doi: 10.1371/journal.pone.0049613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buttelmann D, Call J, Tomasello M. Do great apes use emotional expressions to infer desires? Dev Sci. 2009;12(5):688–698. doi: 10.1111/j.1467-7687.2008.00802.x. [DOI] [PubMed] [Google Scholar]
- 39.Kret ME, Tomonaga M, Matsuzawa T. Chimpanzees and humans mimic pupil-size of conspecifics. PLoS One. 2014;9(8):e104886. doi: 10.1371/journal.pone.0104886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izumi A, Kojima S. Matching vocalizations to vocalizing faces in a chimpanzee (Pan troglodytes) Anim Cogn. 2004;7(3):179–184. doi: 10.1007/s10071-004-0212-4. [DOI] [PubMed] [Google Scholar]
- 41.Parr LA. The discrimination of faces and their emotional content by chimpanzees (Pan troglodytes) Ann N Y Acad Sci. 2003;1000:56–78. doi: 10.1196/annals.1280.005. [DOI] [PubMed] [Google Scholar]
- 42.Kano F, Tanaka M, Tomonaga M. Enhanced recognition of emotional stimuli in the chimpanzee (Pan troglodytes) Anim Cogn. 2008;11(3):517–524. doi: 10.1007/s10071-008-0142-7. [DOI] [PubMed] [Google Scholar]
- 43.Hare B, Melis AP, Woods V, Hastings S, Wrangham R. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr Biol. 2007;17(7):619–623. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann E, Tomasello M. Apes’ and children’s understanding of cooperative and competitive motives in a communicative situation. Dev Sci. 2006;9(5):518–529. doi: 10.1111/j.1467-7687.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- 45.Adachi I, Kuwahata H, Fujita K, Tomonaga M, Matsuzawa T. Plasticity of ability to form cross-modal representations in infant Japanese macaques. Dev Sci. 2009;12(3):446–452. doi: 10.1111/j.1467-7687.2008.00780.x. [DOI] [PubMed] [Google Scholar]
- 46.Haun DB, Nawroth C, Call J. Great apes’ risk-taking strategies in a decision making task. PLoS One. 2011;6(12):e28801. doi: 10.1371/journal.pone.0028801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosati AG, Hare B. Chimpanzees and bonobos exhibit emotional responses to decision outcomes. PLoS One. 2013;8(5):e63058. doi: 10.1371/journal.pone.0063058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aureli F, de Waal FB. Inhibition of social behavior in chimpanzees under high-density conditions. Am J Primatol. 1997;41(3):213–228. doi: 10.1002/(SICI)1098-2345(1997)41:3<213::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Leavens DA, Aureli F, Hopkins WD. Behavioral evidence for the cutaneous expression of emotion in a chimpanzee (Pan troglodytes) Behav Brain Sci. 2004;141(8):979–997. [Google Scholar]
- 50.Gallup AC. Why do we yawn? Primitive versus derived features. Neurosci Biobehav Rev. 2011;35(3):765–769. doi: 10.1016/j.neubiorev.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Massen JJM, Vermunt DA, Sterck EHM. Male yawning is more contagious than female yawning among chimpanzees (Pan troglodytes) PLoS One. 2012;7(7):e40697. doi: 10.1371/journal.pone.0040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haker H, Kawohl W, Herwig U, Rössler W. Mirror neuron activity during contagious yawning—An fMRI study. Brain Imaging Behav. 2013;7(1):28–34. doi: 10.1007/s11682-012-9189-9. [DOI] [PubMed] [Google Scholar]
- 53.Suvilehto JT, Glerean E, Dunbar RIM, Hari R, Nummenmaa L. Topography of social touching depends on emotional bonds between humans. Proc Natl Acad Sci USA. 2015;112(45):13811–13816. doi: 10.1073/pnas.1519231112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Waal FB. The Chimpanzee’s service economy: Food for grooming. Evol Hum Behav. 1997;18(6):375–386. [Google Scholar]
- 55.Crockford C, et al. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc Biol Sci. 2013;280(1755):20122765. doi: 10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vervaecke H, de Vries H, van Elsacker L. The pivotal role of rank in grooming and support behavior in a captive group of bonobos (Pan paniscus) Behaviour. 2000;137(11):1463–1485. [Google Scholar]
- 57.Heiman JR, et al. Sexual satisfaction and relationship happiness in midlife and older couples in five countries. Arch Sex Behav. 2011;40(4):741–753. doi: 10.1007/s10508-010-9703-3. [DOI] [PubMed] [Google Scholar]
- 58.Manson JH, Perry S, Parish AR. Nonconceptive sexual behavior in bonobos and capuchins. Int J Primatol. 1997;18(5):767–786. [Google Scholar]
- 59.Rilling JK, et al. Differences between chimpanzees and bonobos in neural systems supporting social cognition. Soc Cogn Affect Neurosci. 2011;7(4):369–379. doi: 10.1093/scan/nsr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nimchinsky EA, et al. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA. 1999;96(9):5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parr LA, Waller BM, Fugate J. Emotional communication in primates: implications for neurobiology. Curr Opin Neurobiol. 2005;15(6):716–720. doi: 10.1016/j.conb.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]