Significance
Oxygenases are classified into two groups, mono- and dioxygenases, which, respectively, catalyze insertion of one and two oxygen atoms of O2 into substrates. These two modes of oxygenation proceed through distinct reaction mechanisms and require totally different active site structures. For more than 60 years, oxygenase enzymes thus far have been found to catalyze either a mono- or dioxygenation reaction. This study has broken this paradigm as it clearly demonstrates that the heme-degrading enzyme from Mycobacterium tuberculosis, MhuD, catalyzes successive mono- and dioxygenation reactions within its single active site. This unprecedented bifunctionality of the single active center rationally explains the unique heme catabolites of MhuD, which have been proposed to meet the physiological demands of the pathogenic bacterium.
Keywords: heme degradation, monooxygenation, dioxygenation, Mycobacterium tuberculosis, MhuD
Abstract
Bacterial pathogens must acquire host iron for survival and colonization. Because free iron is restricted in the host, numerous pathogens have evolved to overcome this limitation by using a family of monooxygenases that mediate the oxidative cleavage of heme into biliverdin, carbon monoxide, and iron. However, the etiological agent of tuberculosis, Mycobacterium tuberculosis, accomplishes this task without generating carbon monoxide, which potentially induces its latent state. Here we show that this unusual heme degradation reaction proceeds through sequential mono- and dioxygenation events within the single active center of MhuD, a mechanism unparalleled in enzyme catalysis. A key intermediate of the MhuD reaction is found to be meso-hydroxyheme, which reacts with O2 at an unusual position to completely suppress its monooxygenation but to allow ring cleavage through dioxygenation. This mechanistic change, possibly due to heavy steric deformation of hydroxyheme, rationally explains the unique heme catabolites of MhuD. Coexistence of mechanistically distinct functions is a previously unidentified strategy to expand the physiological outcome of enzymes, and may be applied to engineer unique biocatalysts.
Oxygenases catalyze substrate oxidation with incorporation of one or two oxygen atoms from O2. The monooxygenation and dioxygenation reactions have little in common at the mechanistic levels and require different protein scaffolds, so that no enzyme is thought to catalyze the two distinct oxygenation modes in a single active site. Monooxygenases activate O2 by using two electrons and two protons, which in total is equivalent to hydrogen peroxide (H2O2), and results in the release of one oxygen atom as a water molecule (Eq. 1). Efficient proton and electron transfer machinery is equipped within the active sites of monooxygenases including cytochrome P450, where O2 is reductively activated on the heme iron to form a high-valent oxo-species responsible for substrate monooxygenation (1, 2). In contrast, dioxygenases directly add two oxygen atoms of O2 into substrates without consuming electrons and protons (Eq. 2). Dioxygenase active sites are generally hydrophobic as observed for tryptophan and indoleamine 2,3-dioxygenases, where O2 binds ferrous heme iron akin to cytochrome P450, but perform distinct chemistry (3–5). Prostaglandin-endoperoxide synthase, also known as cyclooxygenase (COX), is another type of dioxygenase where a nonactivated O2 molecule binds to a fatty acid radical generated by initial hydrogen abstraction (6, 7). Whereas COX as well as linoleate diol synthase further convert the dioxygenated product with peroxidase or isomerase activity, respectively (6, 8), each dioxygenase has another active site designed for the latter reaction. Contrary to the conventional one active site, one reaction paradigm, here we report that MhuD, a heme-degrading enzyme from Mycobacterium tuberculosis, catalyzes a sequential monooxygenation and dioxygenation in its single active site.
| [1] |
| [2] |
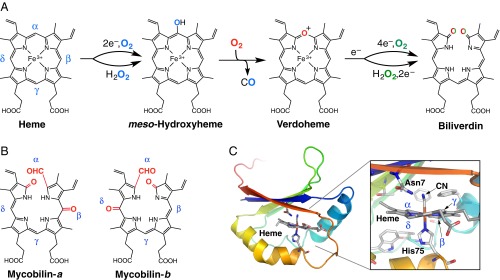
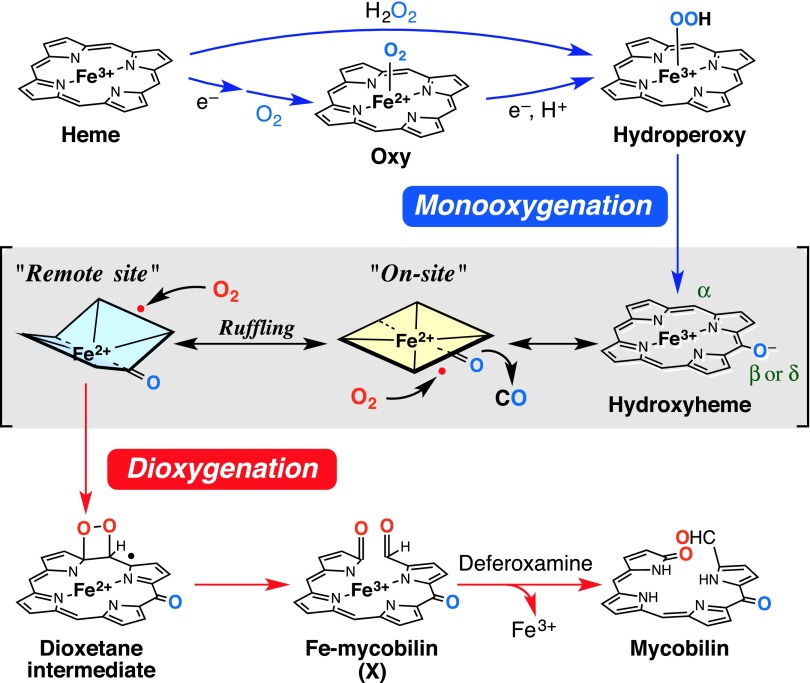
Biological heme degradation proceeds through a unique self-oxidation mechanism where the substrate heme activates O2 molecules. The canonical enzyme, heme oxygenase (HO), degrades heme into ferrous iron, carbon monoxide (CO), and biliverdin by three successive monooxygenation reactions (Fig. 1A) (9, 10). This mechanism, which passes through both hydroxyheme and verdoheme intermediates, has been considered the “gold standard” of heme degradation even for recently identified noncanonical HO-type enzymes (11–13). We have discovered, however, that MhuD cleaves the porphyrin ring without releasing CO, which is a clear indication of a distinct mechanism, without producing the verdoheme intermediate (14). MhuD products termed “mycobilins” (mycobilin-a and -b in Fig. 1B) retain the α-meso carbon atom at the cleavage site as a formyl group with an extra oxidation at either the β- or δ-meso position. MhuD has a single active site where heme is bound in an unusually distorted conformation, best described as ruffled (Fig. 1C and Fig. S1) (15, 16). The ruffling is expected to drastically modulate the heme-dependent O2 activation to initiate this unique mechanism (14). Similar heme ruffling is observed for IsdG and IsdI (heme-degrading components of the iron regulated surface determinant system), MhuD homologs in Staphylococcus aureus, which liberate formaldehyde upon heme degradation instead of CO (17, 18).
Fig. 1.
HO- and MhuD-type heme degrading enzymes. (A) Heme degradation mechanism of canonical HO enzymes with alternative H2O2 pathways. (B) Structures of mycobilin isomers. (C) Crystal structure of cyanide-bound ferric heme-MhuD [Protein Data Bank (PDB) ID code 4NL5].
Fig. S1.
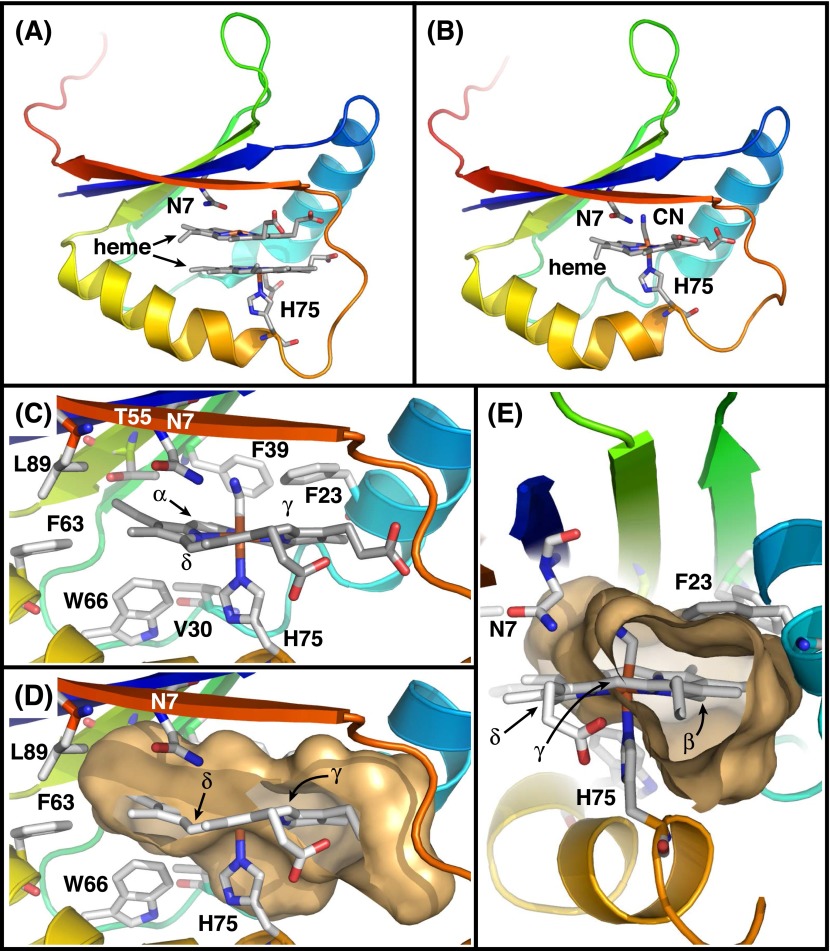
Structures of heme-MhuD complexes. (A and B) Overall structures of diheme- and monoheme-complexes of MhuD (PDB ID code 3HX9 and 4NL5, respectively). MhuD can bind up to two molecules of heme in the same active site, and the second heme binding is known to inhibit MhuD (15). No other heme binding site is observed for MhuD. (C) Residues surrounding heme except for Asn7 are mostly hydrophobic in the monoheme-MhuD complex. (D and E) Cavity surface created by MhuD. γ- and δ-meso carbon atoms are exposed to solvent. A large space is found under the β-meso carbon. Thus, these three meso-carbon atoms appear to be more accessible than the α-meso carbon to be dioxygenated.
Results
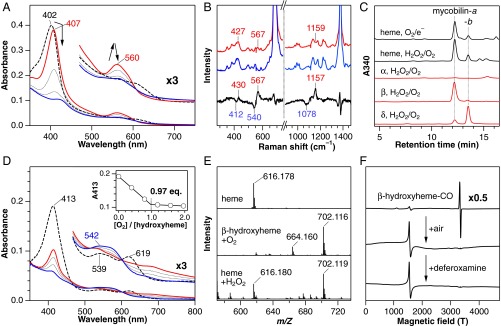
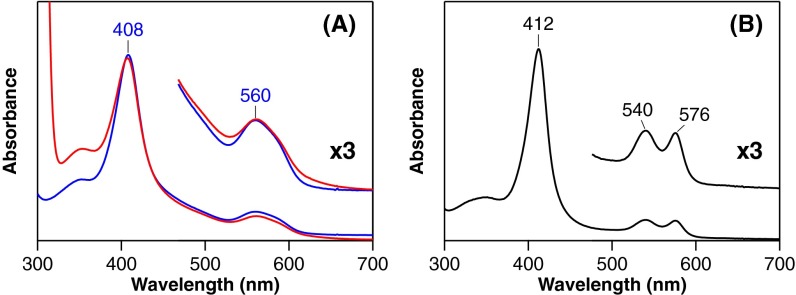
First, we demonstrate that MhuD catalysis is initiated by reductive O2 activation (O2/e–), suggesting HO-type self-monooxygenation. Reduction of ferric heme-MhuD in the presence of O2 accumulates an intermediate with Soret and visible peaks at 407 and 560 nm, respectively (Fig. 2A). The same intermediate can be prepared by the reaction of ferrous heme-MhuD with O2 (Fig. S2). Resonance Raman spectroscopy detects three 18O2-sensitive signals, suggesting that this metastable intermediate is oxy-ferrous heme-MhuD (Fig. 2B and Table S1). Oxy-MhuD is further reduced to produce the mycobilin isomers in the presence of deferoxamine, a high-affinity iron chelator (Figs. 1B and 2C). We next find that H2O2 can substitute for the first O2 activation (O2/e–) step, as expected for monooxygenation (19). The reaction of heme-MhuD and H2O2 in the absence of a reductant results in mycobilin formation with a similar isomer ratio (Fig. 2C and Fig. S3A). For H2O2-dependent mycobilin formation, there is a requirement for O2 (Fig. S3B), suggesting the transient formation of meso-hydroxyheme, which is similar to its conversion in the HO second step that also strictly requires O2 (Fig. 1A) (19, 20).
Fig. 2.
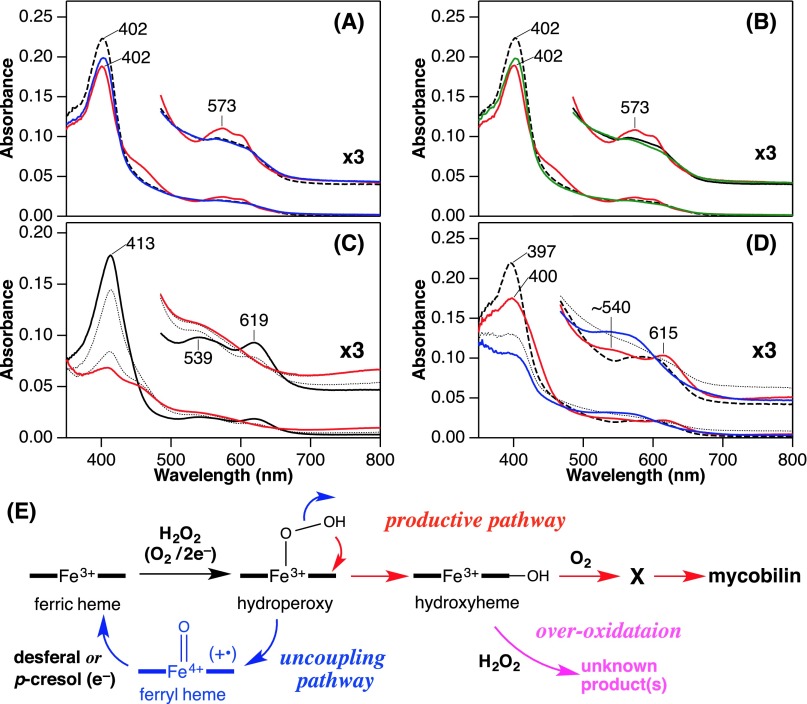
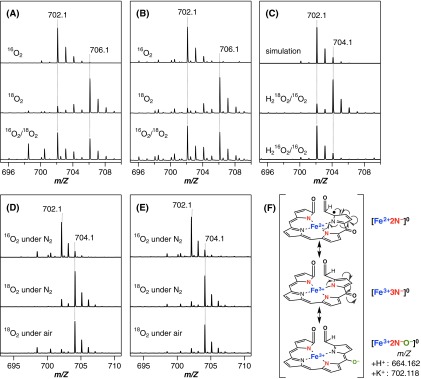
Heme degradation by MhuD. (A) Absorption spectral change for reduction of ferric heme-MhuD (dashed) by sodium ascorbate in the presence of O2 and deferoxamine at pH 6.0 and 37 °C. Spectra were recorded at 2 (red), 10 and 20 (dotted), and 30 min (blue) after the ascorbate addition. (B) Resonance Raman spectra of ferrous oxy-MhuD at pH 7.0 obtained with 16O2 (red), 18O2 (blue), and their difference (black). (C) HPLC chromatograms for degradation products of heme (black) and indicated isomers of meso-hydroxyheme (red) in the O2/e–- or H2O2-dependent reactions. (D) Absorption spectral change of β-hydroxyheme-MhuD (dashed) in conversion to mycobilin at pH 7.0 and 20 °C. Spectra were recorded at 1 (red), 6, and 15 (dotted), and 40 min (blue) after addition of H2O2/O2 in the presence of deferoxamine. (Inset) Absorbance change at 413 nm upon titration with air-saturated buffer. (E) ESI-MS spectra of heme-MhuD (Top) and the intermediate X prepared by adding O2 to β-hydroxyheme-MhuD under N2 (Middle) or H2O2 to heme-MhuD under air (Bottom). (F) EPR spectra of CO-bound β-hydroxyheme-MhuD (Top) and its reaction products with O2 (Middle) and with O2/deferoxamine (Bottom) at 10 K.
Fig. S2.
Comparison of absorption spectra of ferrous oxy-hemes. (A) Oxy-MhuD complexes accumulated during heme degradation in 0.1 M Mes, pH 6.0 (red) and prepared by adding O2 to anaerobically reduced heme-MhuD in 0.1 M potassium phosphate, pH 7.0 (blue). These exhibit essentially the same absorption spectra having one broad peak at 560 nm with a shoulder around 580 nm in the visible region. (B) Rat oxy-HO-1 at pH 7.0 shows a distinct absorption spectrum compared with that of MhuD despite their same His axial ligands. Two sharp visible peaks found for oxy-HO-1 are characteristic of canonical oxy-hemeproteins with a His axial ligand. The unique spectrum of oxy-MhuD is similar to that reported for an oxy-form of myoglobin reconstituted with α-ethyl-2,4-dimethyldeuteroheme, which is suggested to be highly distorted (38). The unique spectrum of oxy-MhuD could be attributed to its substantial heme ruffling, although this requires confirmation.
Table S1.
Vibrational frequencies of oxy forms of MhuD and heme proteins (cm–1)
| Protein | δ(FeOO) | ν(Fe–O) | ν(O–O) | Source |
| MhuD | ∼427* | 567 | 1,157 | This study |
| Hemoglobin | 425 | 568 | 1,130 | 39 |
| Rat HO-1 | 414 | 565 | ∼1,134† | 40 |
| P450cam | 402 | 540 | 1,139 | 41 |
Approximate value due to the weak signal.
Tentative assignment based on the shift of a heme mode by O2 isotope substitution.
Fig. S3.
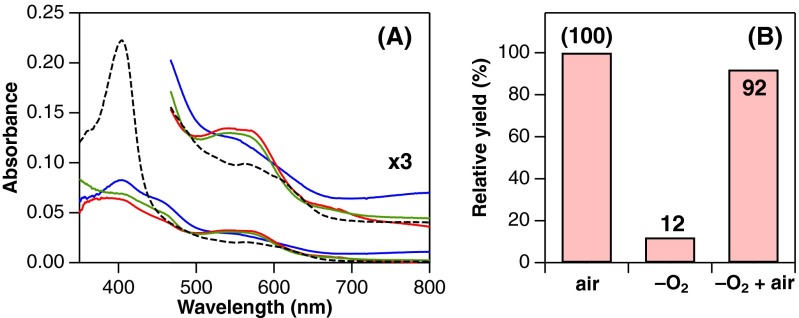
Product analysis of reactions between ferric heme-MhuD and H2O2 in the presence and absence of O2. (A) Absorption spectra before (dashed) and after reaction with 100 µM H2O2 in 0.1 M potassium phosphate, pH 7.0 containing 2.5 mM deferoxamine at 20 °C. The reaction under air results in an absorbance increase around 540 nm, typical for mycobilin production (green, air). Similar reactions were also performed in an anaerobic glove box with an O2-free phosphate buffer (blue, –O2) and with the same buffer (800 μL) bubbled with 100 µL air (red, –O2 + air). Although the product spectrum with the air-bubbled buffer is very similar to that obtained under air, the O2-free reaction exhibits a distinct product spectrum. (B) Mycobilin yields from 2.3 µM heme-MhuD determined by HPLC analysis. The values are relative to the yield obtained under air. The low yield in the O2-free reaction clearly indicates the requirement of O2 in the H2O2-dependent mycobilin formation by MhuD.
Formation of hydroxyheme from ferric heme-MhuD with H2O2 was examined under anaerobic conditions. The primary product exhibited its Soret peak at 402 nm with a visible peak at 573 nm (Fig. S4A). This absorption spectrum is distinct from that of MhuD complexed with chemically synthesized hydroxyheme (Fig. S4C). This species has no reactivity with O2 and is likely to be ferryl heme-MhuD because addition of p-cresol, a typical substrate for one-electron oxidation, mostly regenerates the starting ferric heme complex (Fig. S4A). The dead-end ferryl heme may be generated by an uncoupling reaction of a putative hydroperoxy intermediate (FeOOH, Fig. S4E) (21). Because of this uncoupling pathway, heme-MhuD exhibits weak peroxidation activity of guaiacol (0.3 min–1 at pH 7.0), which is far lower than those of native peroxidases and is comparable to that of myoglobin (22). Reduction of ferryl heme, which is also achieved in the presence of deferoxamine (Fig. S4B), allows the regenerated ferric heme to cycle through another H2O2 reaction. If the H2O2 reaction affords a small amount of hydroxyheme via this pathway (Fig. S4E), its accumulation should become detectable upon multiple turnovers. In fact, a reaction of ferric heme-MhuD with a fourfold excess of H2O2 in the presence of deferoxamine results in the emergence of characteristic visible bands around 540 and 615 nm (Fig. S4D), indicating partial accumulation of hydroxyheme. The observed intermediate exhibits a similar reactivity as the hydroxyheme complex of MhuD as described below. Thus, the initial step of MhuD heme degradation is the inefficient conventional self-monooxygenation of heme to hydroxyheme through oxy-heme and possibly FeOOH-heme intermediates (Fig. 3) (20, 23, 24).
Fig. S4.
Absorption spectral changes and possible scheme of reactions between ferric heme-MhuD and H2O2. (A) Formation of putative ferryl heme (red) in reaction between ferric heme-MhuD (dashed) and 50 µM H2O2 in 0.1 M potassium phosphate, pH 7.0 at 20 °C. This species appears to be reduced back to the ferric state by 100 µM p-cresol (blue). Incomplete recovery of Soret absorption suggests formation of a small amount of hydroxyheme. (B) Putative ferryl heme (red) prepared as in A was also reduced to the ferric state by 2.5 mM deferoxamine (green). (C) β-Hydroxyheme-MhuD (solid black) prepared by anaerobic reconstitution of MhuD with the synthetic hydroxyheme isomer in phosphate buffer, pH 7.0 at 20 °C. Overoxidation with 100 µM H2O2 were monitored at 1 and 15 (dotted), and 40 min (red) after mixing. (D) Partial accumulation of hydroxyheme in 0.1 M Tris⋅HCl, pH 8.0 at 20 °C. Anaerobic reaction of 2.5 µM ferric heme-MhuD (dashed) with 10 µM H2O2 in the presence of 2.5 mM deferoxamine yielded an intermediate (red) with a similar spectrum, especially its visible region, to that of β-hydroxyheme-MhuD. Discrepancy in the Soret peak position could be attributed to the intense absorption of residual ferric heme-MhuD. At higher H2O2 concentrations the reaction resulted in diminishment of the 540/615-nm absorption characteristic for hydroxyheme probably due to its overoxidation as shown in C. The accumulated intermediate rapidly reacted with O2 to mainly afford the intermediate X (dotted), and then, mycobilin (blue) as observed for β-hydroxyheme-MhuD. (E) Reaction scheme for MhuD including uncoupling pathway of the initial monooxygenation (blue) and the overoxidation of hydroxyheme (magenta).
Fig. 3.
Heme degradation mechanism of MhuD.
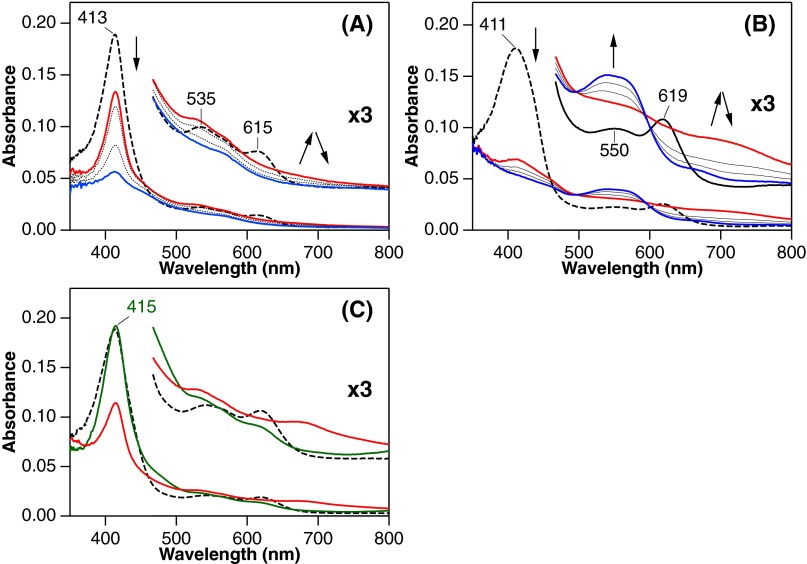
The hydroxyheme oxidation leading to ring cleavage has been reported to occur only at the hydroxylated site (on-site oxidation in Fig. 3) (25, 26). Despite the α-meso cleavage in both mycobilin isomers (Fig. 1B), α-hydroxyheme-MhuD does not afford mycobilin even in the presence of H2O2 and O2 (Fig. 2C and Fig. S5A). Instead, β-hydroxyheme-MhuD produces mycobilin-a with the additional oxidation at the β-meso carbon atom, whereas mycobilin-b with the δ-meso oxidation is generated from δ-hydroxyheme-MhuD (Figs. 1B and 2C). β-Hydroxyheme-MhuD immediately exhibits a significant decrease in Soret absorbance and concomitant increase around 700 nm upon H2O2/O2 addition, and this intermediate termed “X” is further converted into mycobilin by deferoxamine (Fig. 2D). These results indicate different reaction sites for primary hydroxylation (β- or δ-meso carbon atom) followed by oxidative ring cleavage at the α-meso position. The unusual “remote-site oxidation” in MhuD gives a rationale for the lack of CO produced because the conventional “on-site oxidation” would be a prerequisite to extrude the vicinal C–O moiety as CO (Fig. 3).
Fig. S5.
Absorption spectral changes of hydroxyheme complexes of MhuD. (A) α-Hydroxyheme-MhuD in the reaction with H2O2 and O2 in 0.1 M potassium phosphate, pH 7.0 containing 2.5 mM deferoxamine at 20 °C. Spectra were taken before reaction (dashed), and at 1 (red), 3 and 15 (dotted), and 40 min (blue) after the H2O2/O2 addition. Directions of spectral changes are indicated by arrows. (B) The same H2O2/O2 reaction for δ-hydroxyheme-MhuD at 30 °C with spectral measurements before reaction (dashed), and at 1 (red), 5 and 10 (dotted), and 25 min (blue) after the H2O2/O2 addition. δ-Hydroxyheme exhibited an absorbance increase around 540 nm typical for mycobilin formation whereas the α-isomer showed an absorbance decrease in this region. (C) β-Hydroxyheme-MhuD in 0.1 M potassium phosphate, pH 7.0 (dashed) was bubbled with CO gas at 20 °C (green). Further addition of O2 affords the intermediate X within 15 s (red) as observed in the absence of CO.
Quantitative analysis of the hydroxyheme conversion reveals that O2 and deferoxamine, but not H2O2, are required to yield mycobilin (Table S2). Titration experiments indicate that one molar equivalent of O2 converts β-hydroxyheme-MhuD to X (Fig. 2D). The mycobilin formation from β-hydroxyheme also requires an equimolar amount of O2 (Table S2). Electrospray ionization-mass spectrometry (ESI-MS) analysis of X shows positive-ion signals at 664.160 and 702.116 (Fig. 2E), which, respectively, correspond to H+ and K+ adducts of [FeC34H31N4O7] (calculated: 664.162 and 702.118). The same K+ adduct of X is also detected in the reaction of heme-MhuD with H2O2 in the presence of O2 (Fig. 2E). The formula of X suggests addition of two oxygen atoms into β-hydroxyheme. The 18O2-labeling experiments on X results in a mass increase by 4 (Fig. S6A), further corroborating the incorporation of two oxygen atoms. The two β-hydroxyheme incorporated oxygen atoms originate from the same O2 molecule, because no isotope scrambling is observed for X produced with a mixture of 16O2 and 18O2 (Fig. S6A). Very similar results are obtained for δ-hydroxyheme-MhuD (Figs. S5B and S6B and Table S2). These findings unambiguously indicate incorporation of two oxygen atoms from one O2 molecule; i.e., dioxygenation of hydroxyheme.
Table S2.
Relative yields of mycobilin produced from β- and δ-hydroxyheme-MhuD
| Run | Hydroxyheme | Condition | Relative yield, %* |
| 1 | β | O2/H2O2/deferoxamine | 90 ± 3 |
| 2 | β | H2O2/deferoxamine | 10 ± 1 |
| 3 | β | O2/deferoxamine | (100 ± 3) |
| 4 | β | O2 (1.2 eq.)/deferoxamine | 99 ± 1 |
| 5 | β | O2 | 4 ± 1 |
| 6 | δ | O2/H2O2/deferoxamine | 102 ± 2 |
| 7 | δ | H2O2/deferoxamine | 12 ± 1 |
| 8 | δ | O2/deferoxamine | (100 ± 2) |
| 9 | δ | O2 (1.2 eq.)/deferoxamine | 99 ± 4 |
| 10 | δ | O2 | 2 ± 1 |
Mycobilin yields from β- and δ-hydroxyheme MhuD were determined relative to the values obtained in runs 3 and 8, respectively.
Fig. S6.
ESI-MS analysis of the intermediate X. (A and B) The intermediate X prepared in O2 reactions of β- and δ-hydroxyheme-MhuD, respectively. Immediately after addition of O2, the reaction solutions were flash-frozen and stored at –80 °C to minimize the oxygen exchange with water. Mass increase by four in reactions using 18O2 reveal incorporation of two oxygen atoms from O2. A mixture gas of 16O2 and 18O2 does not afford an intense signal at 704.1, indicating no isotope scrambling. (C) The intermediate X prepared from the reaction of heme-MhuD with labeled and nonlabeled H2O2 in the presence of O2. Mass increase by 2 reveals incorporation of one oxygen atom from H2O2. A simulated spectrum for [FeC34H31N4O7+K]+ is also presented. (D and E) The intermediate X prepared from β- and δ-hydroxyheme-MhuD, respectively, after 12-h incubation in the presence and absence of O2, results in a mass decrease by 2 only for X prepared with 18O2 regardless of the presence of O2. The decrease is not due to decomposition of X as judged from negligible changes in MS spectra of X prepared with 16O2. These findings reveal exchange of one oxygen, probably a formyl oxygen, with water. (F) Resonance structures of the iron–mycobilin complex with charged atoms indicated. Porphyrin substituents are omitted for clarity.
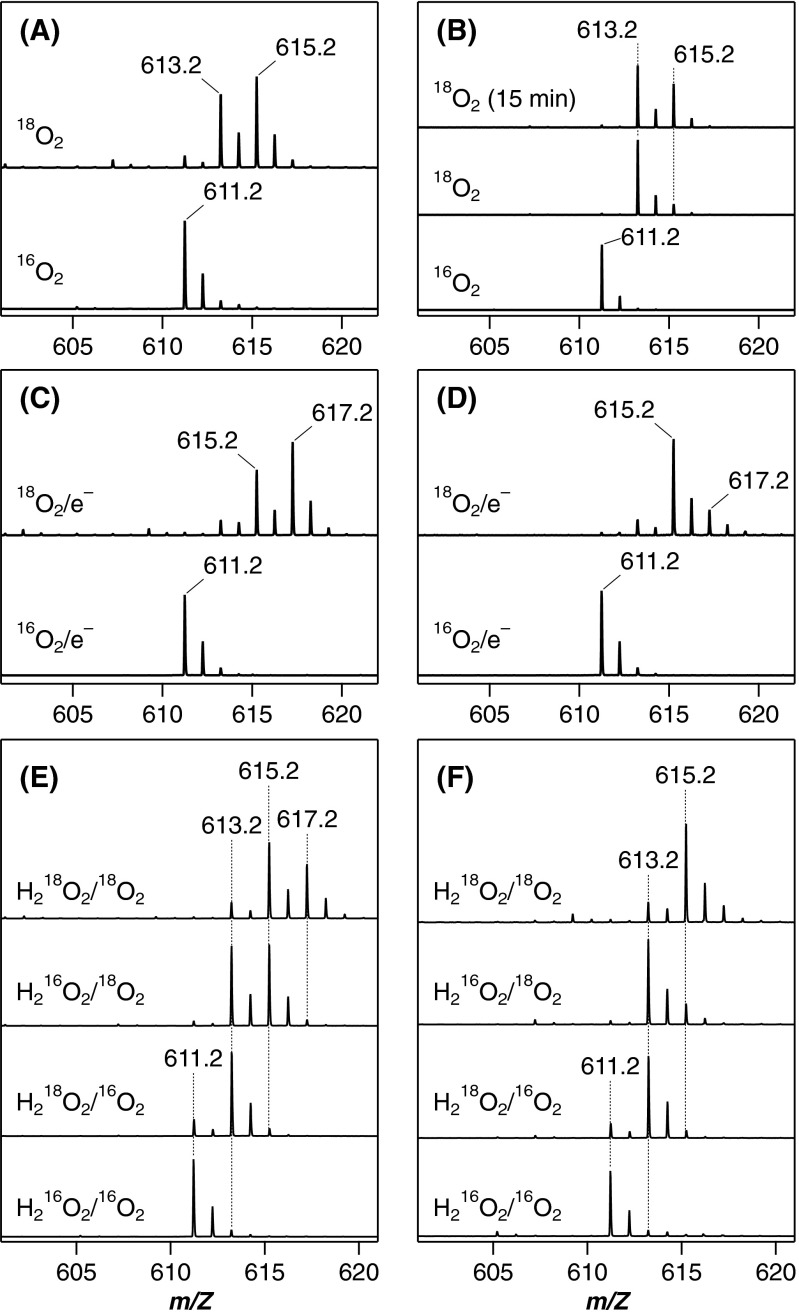
The hydroxyheme dioxygenation is also supported by following the 18O2 labeling through to the mycobilin products. Optimization of the reaction and analysis conditions is required due to the problematic exchange of the formyl oxygen with water over a longer reaction time of 30 min for mycobilin instead of a few seconds for X. Once optimized, we detect a significant amount of doubly labeled mycobilin in the 18O2 reactions of β- and δ-hydroxyheme-MhuD (Fig. S7 A and B). Moreover, MS analyses of these 18O-labeling experiments initiated from heme-MhuD are consistent with the sequential mono/dioxygenation mechanism. In the reductive O2 activation, the mycobilin isomers appear to accommodate three oxygen atoms from O2, one of which is exchangeable with water (Fig. S7 C and D). In the H2O2/O2 reaction, one nonexchangeable oxygen is inserted from H2O2 into X and mycobilin (Figs. S6C and S7 E and F), identifying the initial H2O2 reaction as a monooxygenation process. At the latter stage, insertion of two oxygen atoms from O2 appears to occur, one of which is again exchangeable with water (Fig. S7 E and F).
Fig. S7.
ESI-MS analysis of mycobilins. Mycobilin-a and -b were prepared by the reaction of β- and δ-hydroxyheme-MhuD with O2 (A and B), by the reductive O2 activation of heme-MhuD (C and D), and by the H2O2 reaction of heme-MhuD in the presence of O2 (E and F), respectively, with indicated isotopes of O2 and H2O2. The trace at the top of B labeled “18O2 (15 min)” represents the MS spectrum of mycobilin-b prepared with shorter reaction time (approximately half compared with the normal reaction, Middle) to suppress the oxygen exchange of the formyl group.
The high reactivity of ferric hydroxyheme with O2 is attributed to its resonance with a ferrous porphyrin radical form (Fig. 3). As reported for HO (23, 24), “ferric” hydroxyheme-MhuD binds CO, a typical ferrous iron ligand, to exhibit an intense radical EPR signal (Fig. 2F). CO binding does not interfere with the hydroxyheme-MhuD reaction (Fig. S5C), suggesting O2 binding to the heme periphery rather than on the iron (23). Similar radical addition of O2 is observed for dioxygenases including COX and lipoxygenase (6, 7), both of which initially oxidize their substrate by one electron. Lipoxygenase as well as MhuD uses a ferric iron to generate the substrate radical, whereas the intermolecular electron transfer is not required for MhuD owing to the intrinsic radical properties of hydroxyheme. To produce mycobilin, O2 is destined to covalently bond with both the meso and pyrrole carbon atoms, probably to yield a dioxetane intermediate (Fig. 3) (27). Following O–O bond cleavage, a ferric–mycobilin complex would be produced, whose iron is finally removed by deferoxamine. Stepwise dioxygenation-like Criegee rearrangement is improbable because it would require temporary holding of one oxygen atom by iron (27, 28).
The mass signals of the intermediate X (Fig. 2E) are consistent both with the ferric–mycobilin and dioxetane forms having the same mass number (the open and closed ring structures, Fig. 3). Nevertheless, the intermediate X prepared either from β- or δ-hydroxyheme-MhuD with 18O2 shows a mass decrease by 2 after incubation under either N2 or normal air (Fig. S6 D and E, respectively). This finding clearly indicates exchange of one oxygen atom with water but not with O2, strongly suggesting the ferric–mycobilin structure for X. This assignment is consistent with the fact that deferoxamine facilitates mycobilin formation from X presumably by capturing iron released from the open ring intermediate (Fig. 2D). Moreover, an EPR signal of X observed at g ∼ 4.3 (Fig. 2F) suggests a highly rhombic environment for iron, as observed for ferric–biliverdin complexes of HO (29) and myoglobin (26). One may speculate that the rhombic signal arises from free iron released upon unexpected decomposition of X during the EPR measurement, especially because the spectra of X and the ferric iron–deferoxamine complex closely resemble each other (Fig. 2F). However, the EPR sample of X exhibited a distinct color change to purple, characteristic of mycobilin, upon addition of deferoxamine, inferring that the majority of iron in the EPR sample of X is still complexed with the tetrapyrrole ligand.
Discussion
Our study identifies MhuD as the first enzyme, to our knowledge, that performs both monooxygenation and dioxygenation in a single active site (Fig. 3). This enzyme appears to be designed primarily for dioxygenation with minimum adaptation to monooxygenation. Inefficiency of monooxygenation is evident both in the H2O2- (Fig. S4) and O2/e– reactions of MhuD (14), probably due to its predominantly hydrophobic heme environment lacking a proton-donating candidate to control the reactive FeOOH intermediate (16, 30). Such a structural defect for monooxygenation can be a trade-off for an efficient dioxygenation reaction. The hydroxyheme intermediate is dioxygenated with a stoichiometric amount of O2 (Fig. 2D) while almost completely suppressing the monooxygenation pathway as evident from a negligible formation of CO (14). The high hydrophobicity of the dioxygenase active sites is expected to increase O2 affinity and suppress undesired reactions through nonregulated protonation, although its exact roles are yet to be clarified. Moreover, in the MhuD active site, the hydrophobic residues forming noncovalent bonds with heme might also be critical for its ruffling (Fig. S1C).
The most important determinant to enable MhuD hydroxyheme dioxygenation should be the remote-site oxidation, i.e., the regiospecific oxygenation of the nonhydroxylated meso-carbon (Fig. 3). Because all of the three successive oxygenations by canonical HO take place at the same position to cleave the heme ring, the initial hydroxylation by MhuD was believed to occur on the α-meso carbon atom. In this study, however, MhuD is shown to primarily hydroxylate the β- or δ-meso carbon atoms, and then to dioxygenate the α-meso position. The β/δ preference in the first hydroxylation is not due to a result of an approximation effect accompanied by the heme ruffling. The β- and δ-meso carbon atoms in MhuD deviate from the heme plane toward the heme proximal side (Fig. 1C), while the reactive FeOOH species should be generated in the heme distal pocket. It is more likely that a hydrogen bond interaction directs the terminal OH moiety of FeOOH to Asn7, which is the only polar residue within the MhuD active site located above the δ-meso carbon atom. Normally, heme can be rotated 180° along the α-γ axis to position Asn7 in close proximity of the β-meso carbon atom as well.
In the following dioxygenation, O2 is not activated on the central iron but is expected to directly attack the porphyrin ring. This mechanistic difference of the first and second oxygenation allows exhibition of their different regioselectivity even though the normal on-site oxidation of hydroxyheme results in the same regioselectivity. Studies on COX and lipoxygenase have concluded that their regioselectivity for the radical addition of O2 is controlled cooperatively by several factors including steric shielding and radical localization (31). Because the β-, γ-, and δ-meso heme carbons appear to be accessible (Fig. S1 D and E), a major requirement in MhuD product formation seems to be radical localization on the α-meso carbon (Fig. 3), which may be induced by ruffling of hydroxyheme. Further, ruffling of heme has been suggested to promote the initial monooxygenation by changing its electronic structure (16, 32). Thus, the unique substrate deformation appears to enhance two different modes of oxygenation at different stages of catalysis to achieve the unprecedented dual function of MhuD. This further suggests that the MhuD homologs in S. aureus, IsdG and IsdI, also perform both modes of oxygenation due to similarities in their single hydrophobic active sites that bind highly ruffled heme (17, 33). Cramming two mechanistically distinct functions into one enzyme active site must have required high evolutionary pressure to yield a structure that promotes this unique bifunctional reaction. Unraveling this “cramming” method could provide a blueprint for the biomimetic design of artificial enzymes with extended functions.
Materials and Methods
Materials.
A stable isotope labeled oxygen gas (18O2) was obtained from Taiyo Nippon Sanso. H218O2 and hydroxyheme were synthesized as reported earlier (23, 34). The 18O content of the H218O2 solution was estimated to be ∼80% by analyzing the H218O2-dependent biliverdin formation by rat HO-1. Concentration of H2O2 was determined by horseradish peroxidase-assisted iodide oxidation using ε353 (triiodide) = 2.6 × 104 M–1⋅cm–1 (35). Other chemicals obtained from Wako and Aldrich were used without further purification. Catalase and superoxide dismutase were purchased from Sigma. The MhuD protein was purified as a heme-free form as reported earlier (14). Preparation of a heme complex of rat HO-1 was performed according to a method described previously (36).
Reconstitution of MhuD with Heme or Hydroxyheme.
MhuD can bind up to two molecules of heme in its catalytic center while the active form is the 1:1 complex (Fig. S1 A and B) (15). The monoheme–MhuD complex was selectively prepared by incubating the purified protein in 20 mM Tris⋅HCl, pH 8.0 containing 10 mM NaCl with 1.0 molar equivalent of hemin at 4 °C for 12 h. The crude complex was loaded on an anion exchange column (DE52, Whatman) equilibrated with 0.1 M potassium phosphate, pH 7.0 and the monoheme complex was eluted with the same buffer containing 350 mM NaCl. Anaerobic reconstitution of heme-free MhuD with the hydroxyheme isomers was performed as reported for rat HO-1 (23). Hydroxyheme dissolved in 0.1 M KOH was added to approximately twofold excess of heme-free MhuD in 0.1 M potassium phosphate, pH 7.0 at 4 °C for 8 h in an anaerobic glove box (MBraun, UNIlab). The hydroxyheme complex was purified by gel filtration chromatography (Sephadex G-25, GE Healthcare). Absorption coefficients of β- and δ-hydroxyheme-MhuD in 0.1 M potassium phosphate, pH 7.0 at 20 °C were determined by anaerobic titration with sodium dithionite to be 80 and 65 mM–1⋅cm–1 at 413 and 410 nm, respectively. Alternate titrations of hydroxyheme-MhuD and horse myoglobin were performed to standardize the dithionite solution. UV-vis absorption spectra were recorded on Agilent 8453 and Shimadzu UV1500 diode array spectrophotometers for reactions in the presence and absence of O2, respectively.
Heme Degradation.
Heme degradation by reductive O2 activation was performed in 0.1 M Mes, pH 6.0 at 37 °C containing ∼4 µM heme-MhuD, 25 mM sodium ascorbate, 2.5 mM deferoxamine, 50 U/mL superoxide dismutase, and 4.5 kU/mL catalase. Oxy-MhuD accumulated in the weakly acidic condition. Heme degradation with H2O2 was typically examined in 0.1 M potassium phosphate, pH 7.0 at 20 °C with 100 µM H2O2 and 2.5 mM deferoxamine. All of the reactions under anaerobic condition, reactions with 18O2, 16O2/18O2 and controlled amounts of O2, and reactions of hydroxyheme-MhuD, were performed in the anaerobic glove box. Reactions of α- and β-hydroxyheme were examined in 0.1 M potassium phosphate, pH 7.0 at 20 °C while the temperature was increased to 30 °C for the less reactive δ-isomer. O2 was injected with a gas-tight syringe or added as air-saturated water. Absorption spectral changes during the reactions were monitored by the diode array spectrophotometers without UV light illumination to avoid severe photoreaction. For quantitative analysis of mycobilin, 5 µM propiophenone was added as an internal standard after completion of the reaction. Solid-phase extraction of the reaction products was performed with Supelclean LC-18 columns (100 mg, Supelco). The sample bound to the column was washed with 1 mL 20% methanol/80% water (vol/vol) and eluted with 150 µL methanol. The effluents were analyzed on a Shimadzu LC-10 HPLC system equipped with a Tosoh ODS-80Tm reverse-phase column (4.6 × 150 mm) using a linear gradient from 45% 0.1 M ammonium acetate/55% methanol (vol/vol) to 70% methanol (vol/vol) over 15 min at a flow rate of 1 mL/min. The eluate was monitored using a Shimadzu photodiode array detector (SPD-M20A).
Resonance Raman Spectra.
Resonance Raman spectra were obtained by excitation using the 405-nm line of a solid-state laser (Omicron-Laserage Laserprodukte GmbH, Bluephoton LDM405.120.CWA.L.WS). The scattered light at 90° was dispersed with a single polychrometer (Princeton Instruments, Acton SpectraPro SP-2500) and detected by a cooled CCD detector (Roper Scientific, CCD-1340/400-EM). The laser power on the sample was ∼20 mW with the accumulation time of 30 min. The sample cell was cooled by a cryostream of N2 at ∼4 °C and rotated at 800 rpm. The Raman shifts were calibrated with indene. Oxy-ferrous heme-MhuD in 0.1 M potassium phosphate, pH 7.0 was prepared by adding either 16O2 or 18O2 to ferrous heme-MhuD just before the measurements. The ferrous heme-MhuD solution was prepared in the anaerobic glove box by reducing the ferric heme-MhuD with excess sodium dithionite, which was removed by gel filtration chromatography using a G-25 column.
Peroxidation Assay.
Peroxidation activity of guaiacol was measured with 2 µM heme-MhuD, 100 µM H2O2, and 1.0 mM guaiacol in 0.1 M potassium phosphate, pH 7.0 at 20 °C. Guaiacol oxidation was monitored at 470 nm by using ε470 = 26.6 mM–1⋅cm–1 (37). The initial rate was calculated from the absorbance increase at 1 min after the H2O2 addition. Significant deactivation was observed for heme-MhuD probably due to simultaneous heme degradation.
EPR Spectra.
EPR spectra were obtained at 10 K by a Bruker ELEXSYS E580 spectrometer in the continuous wave mode operating at 9.38 GHz with an incident microwave power of 0.2 mW and 5-G field modulation at 100 kHz. An Oxford liquid helium flow cryostat with a Mercury iTC temperature controller was used for cryogenic measurements. The microwave frequency was monitored by a frequency counter (Bruker SuperX-FT bridge), and the magnetic field was determined by a teslameter (Bruker ER 036TM). A 100 µM solution of β-hydroxyheme-MhuD in 0.1 M potassium phosphate, pH 7.0 was bubbled with 100 µL CO and sealed in an EPR tube. After data acquisition, air was introduced in the tube to prepare the intermediate X. Further reaction with deferoxamine was performed at 37 °C for 1 h. The mycobilin formation was confirmed by color change of the reaction solution to purple.
ESI-MS Analysis.
ESI-MS spectra in positive ion mode were measured on a Bruker micrOTOF-Q-II mass spectrometer calibrated with sodium formate. Reaction intermediates of MhuD were analyzed by direct infusion at 5 µL/min with the following optimized settings: end plate offset, –500 V; capillary, –4,500 V; nebulizer gas, 0.4 bar; dry gas, 4.0 L/min; dry gas temperature, 180 °C; in-source collision induced dissociation, 140 eV. The mycobilin products were solid phase extracted as noted above with an extra wash step with 10 mL of 30% methanol/70% water/0.1% acetic acid (vol/vol) to remove deferoxamine. The concentrated samples were analyzed by LC-MS by using an Agilent 1260 HPLC system with higher nebulizer gas pressure (1.6 bar) and a dry gas flow rate (8.0 L/min). LC separation with an Agilent Extend-C18 reverse-phase column (2.1 × 150 mm) was performed using a linear gradient from 50% 10 mM ammonium acetate/50% methanol (vol/vol) to 70% methanol (vol/vol) over 15 min at a flow rate of 0.2 mL/min.
Acknowledgments
We thank Dr. C. S. Raman for helpful comments. This work has been supported by Grants-in-Aid for Scientific Research (2412006 and 24350081 to M.I.-S.; 23550186, 25109504, 15K05555, and 15H00912 to T.M.) from Japan Society for the Promotion of Science and The Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan; by Strategic Alliance Project for the Creation of Nano-Materials, Nano-devices, and Nano-systems from MEXT, Japan; and by National Institutes of Health Grant AI081161 (to C.W.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523333113/-/DCSupplemental.
References
- 1.Denisov IG, Makris TM, Sligar SG, Schlichting I. Structure and chemistry of cytochrome P450. Chem Rev. 2005;105(6):2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 2.Rittle J, Green MT. Cytochrome P450 compound I: Capture, characterization, and C-H bond activation kinetics. Science. 2010;330(6006):933–937. doi: 10.1126/science.1193478. [DOI] [PubMed] [Google Scholar]
- 3.Sugimoto H, et al. Crystal structure of human indoleamine 2,3-dioxygenase: Catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc Natl Acad Sci USA. 2006;103(8):2611–2616. doi: 10.1073/pnas.0508996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forouhar F, et al. Molecular insights into substrate recognition and catalysis by tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA. 2007;104(2):473–478. doi: 10.1073/pnas.0610007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millett ES, et al. Heme-containing dioxygenases involved in tryptophan oxidation. Curr Opin Chem Biol. 2012;16(1-2):60–66. doi: 10.1016/j.cbpa.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Rouzer CA, Marnett LJ. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem Rev. 2003;103(6):2239–2304. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 7.Tsai AL, Kulmacz RJ. Prostaglandin H synthase: Resolved and unresolved mechanistic issues. Arch Biochem Biophys. 2010;493(1):103–124. doi: 10.1016/j.abb.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su C, Sahlin M, Oliw EH. A protein radical and ferryl intermediates are generated by linoleate diol synthase, a ferric hemeprotein with dioxygenase and hydroperoxide isomerase activities. J Biol Chem. 1998;273(33):20744–20751. doi: 10.1074/jbc.273.33.20744. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz de Montellano PR. Heme oxygenase mechanism - Evidence for an electrophilic, ferric peroxide species. Acc Chem Res. 1998;31(9):543–549. [Google Scholar]
- 10.Matsui T, Unno M, Ikeda-Saito M. Heme oxygenase reveals its strategy for catalyzing three successive oxygenation reactions. Acc Chem Res. 2010;43(2):240–247. doi: 10.1021/ar9001685. [DOI] [PubMed] [Google Scholar]
- 11.Suits MD, et al. Identification of an Escherichia coli O157:H7 heme oxygenase with tandem functional repeats. Proc Natl Acad Sci USA. 2005;102(47):16955–16960. doi: 10.1073/pnas.0504289102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilks A, Burkhard KA. Heme and virulence: How bacterial pathogens regulate, transport and utilize heme. Nat Prod Rep. 2007;24(3):511–522. doi: 10.1039/b604193k. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, et al. Functional identification of HugZ, a heme oxygenase from Helicobacter pylori. BMC Microbiol. 2008;8:226. doi: 10.1186/1471-2180-8-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nambu S, Matsui T, Goulding CW, Takahashi S, Ikeda-Saito M. A new way to degrade heme: The Mycobacterium tuberculosis enzyme MhuD catalyzes heme degradation without generating CO. J Biol Chem. 2013;288(14):10101–10109. doi: 10.1074/jbc.M112.448399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chim N, Iniguez A, Nguyen TQ, Goulding CW. Unusual diheme conformation of the heme-degrading protein from Mycobacterium tuberculosis. J Mol Biol. 2010;395(3):595–608. doi: 10.1016/j.jmb.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graves AB, et al. Crystallographic and spectroscopic insights into heme degradation by Mycobacterium tuberculosis MhuD. Inorg Chem. 2014;53(12):5931–5940. doi: 10.1021/ic500033b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee WC, Reniere ML, Skaar EP, Murphy ME. Ruffling of metalloporphyrins bound to IsdG and IsdI, two heme-degrading enzymes in Staphylococcus aureus. J Biol Chem. 2008;283(45):30957–30963. doi: 10.1074/jbc.M709486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui T, et al. Heme degradation by Staphylococcus aureus IsdG and IsdI liberates formaldehyde rather than carbon monoxide. Biochemistry. 2013;52(18):3025–3027. doi: 10.1021/bi400382p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilks A, Ortiz de Montellano PR. Rat liver heme oxygenase. High level expression of a truncated soluble form and nature of the meso-hydroxylating species. J Biol Chem. 1993;268(30):22357–22362. [PubMed] [Google Scholar]
- 20.Matsui T, et al. O2- and H2O2-dependent verdoheme degradation by heme oxygenase: Reaction mechanisms and potential physiological roles of the dual pathway degradation. J Biol Chem. 2005;280(44):36833–36840. doi: 10.1074/jbc.M503529200. [DOI] [PubMed] [Google Scholar]
- 21.Matsui T, Furukawa M, Unno M, Tomita T, Ikeda-Saito M. Roles of distal Asp in heme oxygenase from Corynebacterium diphtheriae, HmuO: A water-driven oxygen activation mechanism. J Biol Chem. 2005;280(4):2981–2989. doi: 10.1074/jbc.M410263200. [DOI] [PubMed] [Google Scholar]
- 22.Sievers G, Rönnberg M. Study of the pseudoperoxidatic activity of soybean leghemoglobin and sperm whale myoglobin. Biochim Biophys Acta. 1978;533(2):293–301. doi: 10.1016/0005-2795(78)90376-8. [DOI] [PubMed] [Google Scholar]
- 23.Matera KM, et al. Oxygen and one reducing equivalent are both required for the conversion of α-hydroxyhemin to verdoheme in heme oxygenase. J Biol Chem. 1996;271(12):6618–6624. doi: 10.1074/jbc.271.12.6618. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Moënne-Loccoz P, Loehr TM, Ortiz de Montellano PR. Heme oxygenase-1, intermediates in verdoheme formation and the requirement for reduction equivalents. J Biol Chem. 1997;272(11):6909–6917. doi: 10.1074/jbc.272.11.6909. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, et al. Stereoselectivity of each of the three steps of the heme oxygenase reaction: Hemin to meso-hydroxyhemin, meso-hydroxyhemin to verdoheme, and verdoheme to biliverdin. Biochemistry. 2003;42(24):7418–7426. doi: 10.1021/bi027173g. [DOI] [PubMed] [Google Scholar]
- 26.Sano S, Sano T, Morishima I, Shiro Y, Maeda Y. On the mechanism of the chemical and enzymic oxygenations of α-oxyprotohemin IX to Fe.biliverdin IX α. Proc Natl Acad Sci USA. 1986;83(3):531–535. doi: 10.1073/pnas.83.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sono M, Roach MP, Coulter ED, Dawson JH. Heme-containing oxygenases. Chem Rev. 1996;96(7):2841–2888. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- 28.Knoot CJ, Purpero VM, Lipscomb JD. Crystal structures of alkylperoxo and anhydride intermediates in an intradiol ring-cleaving dioxygenase. Proc Natl Acad Sci USA. 2015;112(2):388–393. doi: 10.1073/pnas.1419118112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda-Saito M, Fujii H. EPR characterization of the heme oxygenase reaction intermediates and its implication for the catalytic mechanism. In: Tesler J, editor. Paramagnetic Resonance of Metallobiomolecules (ACS Symposium) Vol 858. American Chemical Society; Washington, DC: 2003. pp. 97–112. [Google Scholar]
- 30.Unno M, Matsui T, Ikeda-Saito M. Structure and catalytic mechanism of heme oxygenase. Nat Prod Rep. 2007;24(3):553–570. doi: 10.1039/b604180a. [DOI] [PubMed] [Google Scholar]
- 31.Schneider C, Pratt DA, Porter NA, Brash AR. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem Biol. 2007;14(5):473–488. doi: 10.1016/j.chembiol.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takayama SJ, Ukpabi G, Murphy ME, Mauk AG. Electronic properties of the highly ruffled heme bound to the heme degrading enzyme IsdI. Proc Natl Acad Sci USA. 2011;108(32):13071–13076. doi: 10.1073/pnas.1101459108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reniere ML, et al. The IsdG-family of haem oxygenases degrades haem to a novel chromophore. Mol Microbiol. 2010;75(6):1529–1538. doi: 10.1111/j.1365-2958.2010.07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawaki Y, Foote CS. Acyclic mechanism in the cleavage of benzils with alkaline hydrogen peroxide. J Am Chem Soc. 1979;101(21):6292–6296. [Google Scholar]
- 35.Ramette RW, Sandford RWJ. Thermodynamics of iodine solubility and triiodide ion formation in water and in deuterium oxide. J Am Chem Soc. 1965;87(22):5001–5005. [Google Scholar]
- 36.Matera KM, et al. Histidine-132 does not stabilize a distal water ligand and is not an important residue for the enzyme activity in heme oxygenase-1. Biochemistry. 1997;36(16):4909–4915. doi: 10.1021/bi962321m. [DOI] [PubMed] [Google Scholar]
- 37.Maehly AC, Chance B. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- 38.Neya S, et al. Molecular insight into intrinsic heme distortion in ligand binding in hemoprotein. Biochemistry. 2010;49(27):5642–5650. doi: 10.1021/bi1003553. [DOI] [PubMed] [Google Scholar]
- 39.Hirota S, et al. Observation of a new oxygen-isotope-sensitive Raman band for oxyhemoproteins and its implications in heme pocket structures. J Am Chem Soc. 1994;116(23):10564–10570. [Google Scholar]
- 40.Takahashi S, et al. Oxygen-bound heme-heme oxygenase complex - Evidence for a highly bent structure of the coordinated oxygen. J Am Chem Soc. 1995;117(22):6002–6006. [Google Scholar]
- 41.Macdonald IDG, Sligar SG, Christian JF, Unno M, Champion PM. Identification of the Fe-O-O bending mode in oxycytochrome P450cam by resonance Raman spectroscopy. J Am Chem Soc. 1999;121(2):376–380. [Google Scholar]