Significance
The energy landscape model pictures that proteins fold through many alternative pathways. The landscape contains all possible intermediates and pathways, and folding is driven only by the downhill energy gradient, with no way to choose any particular folding step against the vast number of alternatives. On the contrary, the present experiments demonstrate that Cytochrome c folds through distinct intermediates in a reproducible pathway, adding one native-like foldon after another down a defined energy ladder. This behavior is dictated by the cooperative foldon construction of the native protein. Two other proteins are known to do the same.
Keywords: protein folding, foldons, cytochrome c
Abstract
Previous hydrogen exchange (HX) studies of the spontaneous reversible unfolding of Cytochrome c (Cyt c) under native conditions have led to the following conclusions. Native Cyt c (104 residues) is composed of five cooperative folding units, called foldons. The high-energy landscape is dominated by an energy ladder of partially folded forms that differ from each other by one cooperative foldon unit. The reversible equilibrium unfolding of native Cyt c steps up through these intermediate forms to the unfolded state in an energy-ordered sequence, one foldon unit at a time. To more directly study Cyt c intermediates and pathways during normal energetically downhill kinetic folding, the present work used HX pulse labeling analyzed by a fragment separation–mass spectrometry method. The results show that 95% or more of the Cyt c population folds by stepping down through the same set of foldon-dependent pathway intermediates as in energetically uphill equilibrium unfolding. These results add to growing evidence that proteins fold through a classical pathway sequence of native-like intermediates rather than through a vast number of undefinable intermediates and pathways. The present results also emphasize the condition-dependent nature of kinetic barriers, which, with less informative experimental methods (fluorescence, etc.), are often confused with variability in intermediates and pathways.
The protein-folding problem, one of the oldest and most difficult in all of biophysics, lies at the heart of biology. Proteins must fold to their active native state when they are initially released from the ribosome and when they unfold and refold, over and over again, during their lifetime (1). It is one reaction that all organisms—animals, plants, and microbes—absolutely require. However, 55 y after Anfinsen et al. showed that proteins can fold all by themselves without outside help (2), no generally accepted folding model has emerged.
Soon after Anfinsen’s demonstration, Levinthal noted that sizeable proteins could not find their native state within any reasonable folding time by a random search through the vast conformational space available to them (3). However, proteins can fold in milliseconds. He concluded that proteins must fold through some programmed structure formation pathway, analogous to other biochemical pathways, although one had no idea how such a pathway might look or what could guide it. A different answer to the Levinthal paradox, focused on energy landscape considerations, points out that the search is not wholly random. Refolding proteins must naturally diffuse energetically downhill stepping through a landscape that contains all possible intermediates and pathways. In the absence of more detailed direction, structural search processes at the microscopic, near single-residue level must carry protein populations heterogeneously through all of these structural forms and different tracks, chosen perhaps by each protein’s initial unfolded conformation (4–7).
The discovery that proteins are constructed of cooperative units called foldons (8–10) answers the Levinthal paradox in yet another way. The foldon construction of native proteins provides built-in instructions for assembling the native protein (10–12). Foldons are small enough, ∼20 residues, to be found quickly by random residue-level searching. Each foldon, being a cooperative unit, will tend to fold in a stepwise, all-or-none manner. Already folded native-like foldons will assist the subsequent formation of adjacent foldons by way of native-like interactions [sequential stabilization (11, 12)]. These properties will promote an ordered, stepwise, macroscopic folding pathway.
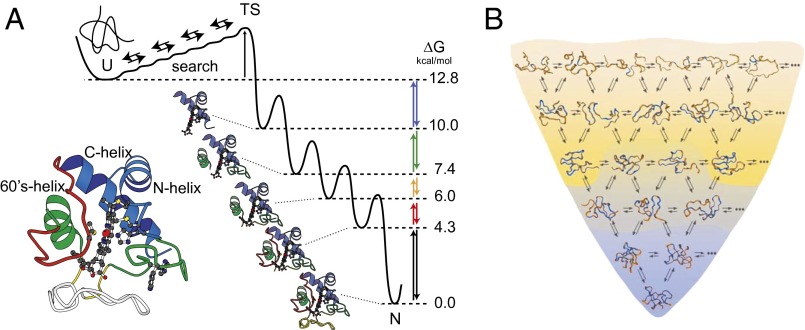
Fig. 1 compares the foldon-dependent defined pathway model (10–13) and the undirected multipath energy landscape model (5, 14–16). The different models differ strikingly in the very different folding intermediates and pathways that they predict and might be distinguished thereby, but this effort has been difficult. One is asked to discern the structures of a whole set of kinetic intermediates that are neither fully unfolded (U) nor fully native (N), that cannot be isolated for study, and that only live for milliseconds. This task may seem impossible. Our earlier experiments depended on an indirect approach, namely, investigation of the dynamic reversible unfolding reactions that carry the native protein up through the high energy landscape. The results revealed that cytochrome c (Cyt c) unfolds through a stepwise ladder of distinct foldon-dependent intermediates (Fig. 1A, Right) (10–13).
Fig. 1.
Alternative folding models. (A) The defined pathway model previously inferred from HX studies of the foldon-dependent interconversion of native Cyt c with partially unfolded forms (PUFs) in the high free energy space above the native protein (10, 11, 13). (B) A representation of the energetically downhill multiple pathway model. Redrawn from ref. 16. These models are tested in the present work during kinetic folding.
The present work applied a hydrogen exchange (HX) pulse labeling method that can provide a timed series of snapshots of the kinetic intermediates that are populated during normal kinetic folding, even when they form and dissipate on a time scale of milliseconds. Advanced HX–fragment separation–mass spectrometry (HX MS) technology (17–20) can then read out the structure and progression of the transient protein folding intermediates (21, 22). Unlike most previously used methods, which present protein-folding signals in an unresolved whole-molecule and ensemble-averaged way (fluorescence, etc.), these methods separately specify folded and unfolded populations, resolve folding events throughout the protein, and measure the time course of each one. The results show that Cyt c folds in a distinct foldon-dependent kinetic pathway sequence. As suggested in Fig. 1A, the intermediates and the pathways that mediate uphill equilibrium unfolding and downhill kinetic folding are the same.
Results
Equilibrium Foldon Unfolding by NMR.
Foldons were first defined in equilibrium native-state HX experiments with Cyt c (9), reproduced in Fig. 2A. HX rates of many individual amides were measured by 2D NMR as a function of increasing, but still very low, denaturant concentration, well below the Cyt c melting zone. Fig. 2A plots the HX rates measured for each residue amide in terms of the free energy of the opening reaction that exposes it to exchange. Amides that are exposed to exchange by small opening reactions (local fluctuations) are insensitive to denaturant and trace horizontal curves. Larger unfolding reactions, otherwise invisible, are sharply promoted by increasing denaturant to a level where they come to dominate the exchange of the amide sites that their unfolding exposes. The residues that join the HX isotherm for each large unfolding reaction define the segments that participate in that unfolding reaction. The ΔG for each unfolding reaction can be obtained by extrapolation of the sloping HX isotherm back to zero denaturant. The size of each unfolding (solvent exposure) is indicated by the slope, dΔG/d[denaturant] (23). For example, at zero denaturant, the globally unfolded state, reached by the unfolding of all the foldons including the one called blue, is at 13 kcal/mol above N. It exists in the high free energy space above N with unfolding equilibrium constant Kunf ∼ 10−10. At [GdmCl] = 1.5 M, the blue unit unfolding reaction is promoted (ΔGunf ∼ 6 kcal/mol above N) and comes to dominate the exposure and the HX of the amides in the blue foldon (Kunf ∼ 10−5).
Fig. 2.
Cooperative foldon units from unfolding experiments. (A) The native-state HX results that first defined Cyt c foldons and their cooperative unfolding. Reprinted from ref. 9. HX rates of many amide protons measured by 2D NMR at low denaturant concentrations are plotted in terms of the free energy of their determining exposure reactions. The ΔG of each exposure reaction, its size, and its identity can be calculated as described in the main text. (B) Illustrative HX MS results (complete data are provided in Fig. S1) that more clearly display the lower-lying red and infrared (black) foldon units by their equilibrium EX1 unfolding. (C) Time dependence for the equilibrium EX1 unfolding of many infrared and red peptides [pH 10, 10 °C, 0.22 M guanidinium chloride (GdmCl)]. (D) Illustrative stability labeling experiment used to determine the identity of the intermediate PUFs in terms of the foldons that are folded and unfolded in each state (26, 27). The unfolding ΔG measured as in A for each foldon unfolding is placed on a free energy ladder. The E62G mutation removes a salt link and destabilizes the yellow foldon unit by 1 kcal/mol (12). The green and blue foldon unfolded states are equally destabilized, indicating that they include the unfolded state of the yellow foldon. Lower-lying PUFs are unaffected, indicating that their unfolding does not include the unfolded yellow foldon. A set of similar experiments indicates that the identity of the PUFs is IRYGB (=N), iRYGB, irYGB, iryGB, irygB, and irygb (= U).
These results point to four cooperative foldon unfolding reactions, some more clearly than others. Seven residues through the N-terminal helix and eight through the C-terminal helix are seen to join into the same high free energy denaturant-sensitive HX isotherm (blue). One infers that a concerted transient unfolding reaction involves both terminal helices (blue). Similarly, five residues in the 60s helix and one in a nearby omega loop participate in a different concerted unfolding reaction with smaller unfolding free energy and denaturant sensitivity, which we call green. A few other residues seem to define other lower-energy unfolding units that may represent a short, two-stranded beta sheet (yellow) and an omega loop (red). Later results, confirmed below, uncover an additional lowest-energy cooperative unfolding unit, referred to as infrared, that encompasses all of the residues in the large bottom omega loop (see below and ref. 24).
The foldon units identified in this way are placed in the Cyt c model in Fig. 1. The color-coding follows, in spectral order, the placement of the unfolding reactions on a ladder of increasing unfolding free energy.
Equilibrium Foldon Unfolding by HX MS at EX1 Conditions.
The foldons that occupy the lowest rungs on the energy ladder pictured in Fig. 1A unfold and exchange too quickly to measure by NMR. A different native-state unfolding experiment (Fig. 2 B and C) takes advantage of the special ability of HX MS to display cooperative unfolding reactions by their characteristic EX1 HX signature. The reality of the red and infrared foldons was confirmed in this way (Fig. 2 B and C; see also refs. 24–26). In these experiments, fully deuterated native Cyt c was exposed to D to H exchange (pH 10, 10 °C, H2O, 6–256 ms), and timed samples were analyzed by HX MS (17–20). For analysis, labeled samples were quenched to a slow HX condition (pH 2.5, 0 °C to minimize the loss of D label during the analysis) and then injected into an online system where the protein is proteolyzed into small peptide fragments, the peptides are roughly resolved by fast HPLC, and the column eluant is continually injected by electrospray into an orbitrap mass spectrometer for further separation and analysis by mass.
Illustrative mass spectra for peptides from the infrared and red segments are shown in Fig. 2B as a function of the length of the D-to-H labeling time. The isotopic envelopes measure the remaining carried deuterium (Δmass = +1 per D) convoluted with the 13C content of each peptide (Δmass = +1, 1.1% natural abundance). If exposure reactions that allow D to H exchange reclose faster than the intrinsic HX rate, as is common for local fluctuations, exchange will require many prior opening and reclosing reactions, HX will be by an EX2 equilibrium-limited mechanism, and will be seen as a continuous slide of peptide MS envelopes to lower mass. This kind of result cannot distinguish cooperative foldon unfolding. However, if reclosing is much slower than ∼1 ms (the unprotected HX lifetime at pH 10), as may occur for sizeable unfolding reactions, HX will be by an EX1 unfolding-limited mechanism. Each unfolding reaction that occurs during the experimental exchange time will allow D to H exchange of all of the sites exposed and will produce a separate lighter peptide envelope, generating a characteristic bimodal MS signature as seen in Fig. 2B.
Fig. 2C plots the cooperative EX1 unfolding of multiple peptides from segments in the infrared and red foldons. The foldon unfolding rate is given by the increase of the unfolded MS envelope with H-exchange time. Both foldons unfold in just over 100 ms and refold more slowly than 1 ms. The least-stable infrared foldon unfolds first and the red foldon more slowly, consistent with their placement on an unfolding free energy ladder (Fig. 1A, Right). The EX1 HX of the infrared peptides (black) and their measured Δmass shows that the entire large bottom loop (Cyt c in Fig. 1A) unfolds as a unit. The peptides we were able to obtain for the red loop segment also contain several sites in the green helix. The kinetic data do not separately distinguish them, suggesting that they may both unfold at approximately the same rate under these conditions. Importantly, these results also show that the brief 10-ms pH 10 labeling pulse used in the pulse labeling experiments described below is nonperturbing. No exchange occurs within 10 ms, even for the least stable infrared foldon.
For the native-state equilibrium unfolding experiment to succeed, it is necessary to find conditions where EX1 unfolding dominates the measured HX and produces a recognizably separate MS envelope. EX1 behavior is promoted by added denaturant (faster unfolding, slower refolding) and by higher pH (faster HX in the transiently unfolded state), but higher pH also promotes competitive EX2 exchange by local fluctuations and a slide of the heavy envelope to lower mass, obscuring the desired EX1 observation. For this reason, the unfolding reactions that lead to higher-energy partially unfolded states could not be measured. Their unfolding is too slow, and their carried D is lost to EX2 exchange before they can unfold and be measured.
These results taken together with previous work establish that the 104-residue Cyt c protein is composed of five foldon units that tend to unfold in a cooperative all-or-none manner.
Pathway Nature of Equilibrium Foldon Unfolding.
Do the various foldon unfolding steps occur independently or in some interdependent way? In the globally unfolded state, all foldons are unfolded; in the native state, all are folded. Which foldons are folded and unfolded in each intermediate partially unfolded form (PUF)?
The identified Cyt c foldons can be placed on an unfolding ΔGunf ladder as in Figs. 1A, Right and 2D. An approach called stability labeling was used to define their relationships (26, 27). Stabilizing or destabilizing modifications were placed in each foldon unit, and the effect on the others was measured. For the example in Fig. 2D, a mutation eliminates a local salt link and destabilizes the yellow foldon. Its equilibrium unfolding is promoted by 1 kcal, measured by repeating the native-state HX experiment with the mutant protein. Higher-lying foldons are equally destabilized, but lower-lying ones are unaffected. This result indicates that the yellow foldon is unfolded in the higher-lying green and blue unfolded forms, but not in the lower ones, consistent with a sequential unfolding model. Analogous results occur for the other modifications tested.
Thus, the equilibrium high free energy states appear to represent a series of PUFs, with the infrared unit unfolded, then infrared + red, then infrared + red + green, and, finally, infrared + red + green + blue, the fully unfolded state. The same sequence was inferred before in terms of increasing unfolding size indicated by relative denaturant sensitivity (Fig. 2A) (9). These results are consistent with a stepwise sequential unfolding pathway up the equilibrium energy ladder (as shown in Fig. 1A, Right).
The fact that the system is at equilibrium seems to require that each up-arrow be matched by an equivalent down-arrow, apparently revealing a stepwise folding pathway. However, this inference is based on experiments that access the minimally populated high free energy states at equilibrium under native conditions. Are these equilibrium PUFs the same as the intermediate species that carry the major flux of proteins from U to N in normal kinetic folding (28) (as in Fig. 1A)? The question can be answered only by defining intermediates during kinetic folding.
Kinetic Folding by Pulse Labeling HX MS.
The development of the HX pulse labeling method made it possible to describe structure formation during protein folding (29, 30). In the present experiments, the population of folding intermediates reached after different folding times was probed by exposure to a brief high-pH D to H labeling pulse (10 ms, pH 10, 10 °C). Amide sites in not-yet-folded structural regions become fully H-labeled, whereas sites that are already folded and protected remain unlabeled. The HX labeling process was then immediately quenched by dilution of the sample to low pH, and it was analyzed by the fragmentation and HPLC-MS separation method described above. Analysis of serial samples by MS then provided a time series of snapshots that could specify the identity and measure the folding rate of segments that had become protected by structure formation before the labeling pulse.
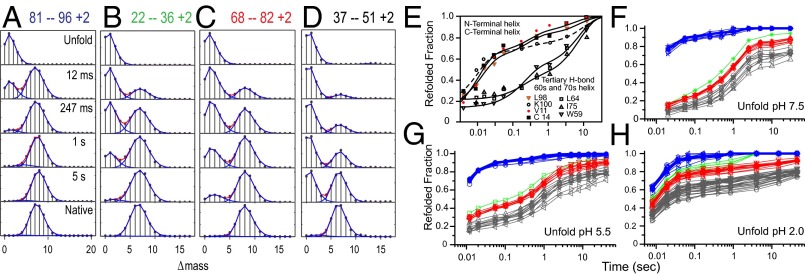
Fig. 3 A–D shows illustrative mass spectra for some Cyt c peptides (see also Fig. S2). Each peptide monitors the folding history of the protein segment that it represents. At each folding time point, the lighter isotopic envelope measures the unprotected, not-yet-folded population. Its initial D label is fully exchanged to H by the labeling pulse. The time course of folding is seen in terms of the conversion of the unprotected envelope to an envelope that is heavier by a mass increment equal to the number of deuterated sites protected by H-bonded structure. Note that, unlike the bimodal spectra in Fig. 2B, which detect unfolding in the EX1 regime, the bimodal spectra in Fig. 3 A–D measure two distinct populations. Unlike most other folding methods, which often present an ensemble population average, these data separately depict the still-unfolded and already-folded population fractions and their time-dependent conversion.
Fig. 3.
Kinetic folding of Cyt c. (A–D) Illustrative mass spectra for some peptides from HX pulse labeling experiments during Cyt c folding (more complete data are in Fig. S2). Initially unfolded fully deuterated Cyt c was diluted into folding conditions at pH 5 and probed during folding by a high-pH D to H labeling pulse. The isotopic envelope for each peptide is composed of peptide molecules that carry differing numbers of 2H atoms (variable with folding time) and 13C atoms (constant). The monoisotopic mass with no heavy isotopes is set at zero on the mass axis. As folding time proceeds in a D-to-H pulse labeling experiment, the envelopes move from a lighter mass, indicating complete D-to-H labeling in the pulse (no protection), to a heavier folded condition that protects a number of the initially deuterated sites from exchange. Each peptide monitors the folding history of the protein segment that it represents. For each segment, the protected population increases in a stepwise concerted manner with folding time. The heavy fraction and the number of sites protected by folding can be measured with high accuracy. (E) Earlier HX pulse labeling results analyzed by 2D NMR at residue resolution. (F–H) Pulse labeling results for many peptides analyzed by HX MS. Each peptide trace is colored to indicate the foldon it belongs to, as determined previously (Fig. 2 A–C). The terminal helices fold as an initial unit (blue) in the entire population, unlike E, where much higher concentration promoted aggregation. The kinetic folding pathway follows the same order as seen before in equilibrium unfolding experiments (Fig. 2). The large kinetic barrier after folding of the blue foldon is due to a His-to-heme misligation, which can be competed off by lower initial pH (indicated for each graph). The very slow phases are due to the fractional misisomerization of four trans-prolines placed in the green, red, and infrared foldons.
Different folding scenarios will produce recognizably different results. If HX protection develops in one or a few residues at a time, this result would be seen as a slide of each envelope in time from its unprotected (lighter) state to a more protected (heavier) condition. Rather, D-occupancy jumps in a concerted folding step that protects many D-labeled amides, indicating stepwise cooperative structure formation. For each peptide, the relative amplitudes of the envelopes show the increasing fraction of the protected population as a function of folding time. This parameter traces the time course for the folding of each segment and can be determined with high accuracy.
For each peptide, the number of amide NH sites that become protected in its concerted folding step is closely equal to the number protected in the native protein (bottom frame) when it is subjected to an equivalent labeling pulse. This result suggests that the structure formed in each concerted folding step is locally native-like.
If the whole protein folds in a two-state manner, all of the peptides would become protected in the same concerted step. Rather, different protein segments fold at different times, allowing their folding sequence to be determined. Any segment once folded remains folded through later folding steps elsewhere in the protein, as for a stepwise pathway.
If different molecules in the protein population fold through different pathways as in the multipath energy landscape model, any given protein segment would convert heterogeneously in fractional steps, earlier in some molecules and later in others. Rather, the data show that the entire protein population folds any given foldon on the same time scale. If any significant population fraction (≥5%), folded either faster or slower, this result would be seen as a separate protected or unprotected envelope.
In summary, HX MS results for Cyt c directly show that any given segment folds in a concerted stepwise way, and that different segments fold at different times, progressively putting cooperative native-like units into place to build the native protein. Many overlapping peptides consistently confirm each step (Fig. S2) and show that the entire refolding population (>95%) exhibits the same folding steps, each on the same time scale. These properties define a reproducible, population-wide, sequential folding pathway.
The Search for Foldons.
These same results, assembled in Fig. 3, show how the separately measured peptide fragments group into foldon units. In earlier experiments analyzed by 2D NMR (9), redrawn in Fig. 3E, sites in the N- and C-terminal helices were seen to gain structural protection (H-bonding) on a 10-ms time scale, whereas other regions folded later. However, there were anomalies. Only half of the protein population participated in this first structure formation step; the rest folded later with the slower major protein folding phase.
Fig. 3 F–H show HX MS folding results for many Cyt c peptides (Fig. S2). The curve for each peptide is color-coded according to the previous foldon assignments (Fig. 2); they are not arbitrarily colored to match the kinetic behavior observed. The increase in the protected fraction of each peptide measures the time course for folding of the segment that it represents. This measurement has high accuracy when protected and unprotected populations are well-separated. The high accuracy of the data makes it possible to determine that the various peptides fall into kinetically separable groupings, even though they are often not well separated in time. This finding is underlined by the high reproducibility found among the many peptides in each group.
The HX MS experiments measured Cyt c folding at a protein concentration much lower (1–4 µM) than the 1 mM concentration used in the earlier NMR work. All Cyt c molecules fold their terminal helices (blue) in the same early ∼10-ms step, showing that the heterogeneity previously seen was due to aggregation during folding at the high protein concentration used (31). The few peptides obtained from the green loop suggest that it folds next. No peptides were obtained that cover the short yellow strands, which are thought to be next in line. Peptides colored red fold next. Finally, the infrared loop (black) folds last.
These kinetic groupings and their folding order match the ordered stepwise pathway sequence described in various ways before (e.g., Fig. 2 and refs. 10 and 11).
Kinetic Barrier Variability.
The sequential stabilization principle implies that the first on-pathway foldon must form in an unassisted conformational search, whereas subsequent steps are assisted by prior structure and thus will have faster rate constants. This mechanism will tend to make even a multistep folding pathway appear to be kinetically two-state, rate-limited by the slow initial step. In fact, the folding steps seen for Cyt c in Fig. 3 do tend to follow each other closely. The ability to distinguish separate folding steps and their order depends on the kinetic barriers that separate them. Importantly, it can be seen that the dominant barriers that separate the Cyt c folding steps are not intrinsic to the folding process, but are condition-dependent.
Fig. 3 displays three kinds of condition-dependent barriers. At high concentration, transient reversible aggregation makes the initial folding step kinetically heterogeneous (Fig. 3E). At the much lower concentration used here (1/500), aggregation is absent, and all proteins fold the blue unit equally rapidly (Fig. 3 F–H). Subsequent steps are greatly delayed by a second kind of barrier, which depends on the tendency of peripheral histidines to ligand to the exposed heme iron in the initially unfolded state (32, 33). Both peripheral histidines are in the green loop and thus will tether that segment out of place and delay its folding. The blue unit is unaffected, as might be expected (Cyt c in Fig. 1A), but the folding of all following pathway steps is equally delayed, as expected for an obligatorily sequential pathway. When Cyt c is initially unfolded at pH 7.5, only ∼10% of the population escapes the heme misligation and folds rapidly. Decreasing the initial pH before dilution into folding conditions (Fig. 3 B and C at initial pH of 5.5 and 2.0) tends to protonate the histidine ring, which competes with heme binding, reduces the unfolded population fraction that is initially misligated, and allows a larger population fraction to fold in a fast phase.
Another kind of kinetic barrier depends on the four Cyt c proline residues, normally in the trans configuration. They are residue 30 in the green loop, 44 in the infrared loop, and 71 and 76 in the red loop. At long folding times, the different segments reach a very slow folding phase at somewhat different folding levels that is characteristic for prolines that have equilibrated to a misisomerized configuration in the initially unfolded state. To our knowledge, these results for the first time elaborate the mode of proline blockage. In the population fraction that contains a cis-proline [∼15% for each case (34)], folding of the whole foldon is retarded, and the following on-pathway foldons are blocked by the same amount plus an additional increment due to their own cis-proline content. The effect is removed in a double jump experiment that maintains the native proline isomers by minimizing the time spent in the U form. A major part of the separation that allows discrimination between the sequential Cyt c folding steps (Fig. 3) is due to the increasing offset imposed by the additive proline effects.
An appreciation of the conditional nature of kinetic barriers helps to illuminate some interpretational errors embedded in many published folding experiments. A common belief is that apparent two-state folding is inconsistent with a stepwise pathway. Instead, two-state behavior may simply depend on the absence of sizeable intervening barriers that would reveal the separate steps. Similarly, the usual interpretation of various experimental manipulations in terms of the supposed variability of intermediates and pathways may instead depend on the easy variability of kinetic barriers.
Discussion
The foldon construction of Cyt c and its role in folding was revealed in HX studies under conditions where measured HX reflects equilibrium uphill unfolding as the protein dynamically explores PUFs in the high free energy landscape. The present work, made possible by advanced HX MS methods, shows that the downhill kinetic folding of Cyt c proceeds through intermediate structures in a reproducible stepwise pathway that mirrors the previous equilibrium unfolding results.
It may seem remarkable that the intermediate forms and their ordering in downhill kinetic folding under normal solution conditions are much the same as for equilibrium unfolding up through the high free energy landscape. The reason is that folding and unfolding processes are both designed into the foldon construction of the native protein. The native foldon construction provides built-in guidance for a deliberate macroscopic pathway that assembles or disassembles the native protein one foldon unit after another. The stepwise nature of the pathway is naturally produced by the cooperative nature of the protein’s constitutive foldons, which prefer to be either fully unfolded or fully folded. An ordered pathway is naturally produced by the mutually stabilizing native-like interactions between adjacent foldons (sequential stabilization), much like the well-known folding upon binding phenomenon. It is satisfying that quantized native protein structure and the stepwise protein folding and unfolding processes are unified in this way, as represented in Fig. 1A. They all depend on the same foldon building blocks. It is important to note, however, that unfolding need not always simply reverse the delicately balanced folding process. Under aggressive stress such as high force (35), high denaturant (36), or high temperature (37), proteins may be driven to fall apart in many different ways, which should not be interpreted in terms of multiple folding pathways.
These conclusions disagree with the current paradigmatic view that protein populations fold through very many alternative intermediates and pathways. That view was founded on the understanding that protein folding depends on microscopic residue-level dynamics guided only by the downhill nature of the free energy landscape. Given that the landscape encompasses all possible protein forms and all possible folding tracks, folding directed only by the downhill landscape has no way to choose any one over the multitude of others.
Zwanzig et al. (4) calculated that, in order for folding to succeed on a realistic time scale, the system must be biased toward correct moves by >1 kcal/mol. We expect that, in competition with the innumerable available incorrect moves, this degree of bias could not be reached at the level of single residue searching. It can be obtained by the cooperative summation of many intraresidue interactions in the concerted foldon-formation steps that we see and by the summation of native-like interfoldon interactions in ordering the pathway steps.
This paper adds to accumulating evidence that revises the multiple-pathway view and the detailed energy landscape associated with it (10, 21, 22, 38–43). The cooperative nature of foldons seems to ensure that the high free energy landscape is dominated by partially unfolded protein forms that differ from each other by the unit unfolding of one more foldon. Intervening states are at higher free energy and much less represented. These considerations relegate the unguided residue-level conformational searching visualized in the multipathway view to uphill steps in the transition that carries the initial U state to the first formed on-pathway foldon. Subsequent foldon formation steps are promoted by a search that is assisted by already formed structure.
Materials and Methods
HX MS methodologies used here for data acquisition and analysis follow published approaches (17–20). They are described in some detail in SI Materials and Methods, which also shows much more complete HX MS results in Figs. S1 and S2, from which Figs. 2 and 3 were derived.
SI Materials and Methods
Materials.
Cyt c from horse heart was purchased from Sigma. Guanidinium hydrochloride (GdmCl) and guanidinium thiocyanate (GdmSCN) were from Sigma and Fisher Scientific. Deuterated GdmCl was prepared by dissolving in D2O and lyophilizing three times. GdmCl concentration was determined by refractive index measurements.
HX MS and Data Analysis.
HX MS experiments used previously described methods to maximize peptide count (21), minimize back exchange during analysis (24), and analyze data (22, 23). MS envelope population fractions were measured by using binomial and Gaussian shape fitting. We used only the peptides with the highest accuracy in terms of signal to noise and their separation into distinct, accurately fittable envelopes. Accuracy depends sharply on the degree of separation of the spectra into largely nonoverlapping envelopes and is promoted by the fact that both fractions display in the same spectrum.
Kinetic Folding and Pulse Labeling.
For HX pulse labeling during folding, the following procedures were used. Cyt c (80 or 20 μM) was initially unfolded and fully deuterated in 4.2 M deuterated guanidinium chloride at various pD in D2O buffer (10 mM sodium phosphate for pH 7.5; 20 mM sodium acetate for pH 5.5; 20 mM sodium phosphate for pH 2.0). Refolding was initiated by dilution (1:18 into H2O at pH 5, 10 mM sodium acetate, 0.22 M GdmCl) in a stopped-flow instrument at 10 °C (BioLogic Science Instruments, SFM‐400). After a given refolding time (8 ms to 45 s), a D-to-H exchange labeling pulse was imposed [10 ms, 5 volumes of 50 mM glycine to pH 10, 4% (wt/vol) D2O after mix] to probe for the development of structural protection. This pulse intensity corresponds to 25‐fold labeling of unprotected amide sites on average (unprotected amide HX lifetime is ∼0.4 ms). Amides already protected by folded H‐bonded structure remain deuterated. HX labeling was quenched by adding 5 volumes of low-pH H2O (pH 2.5, 0.6 M GdmSCN after mix). The quenched D-labeled protein was immediately injected into an online flow system where the protein was cleaved into many peptide fragments in an immobilized pepsin column or tandem pepsin + fungal protease XIII columns; the fragments were caught in a trap column, washed, roughly separated by fast HPLC, and injected by electrospray ionization into the mass spectrometer (LTQ Orbitrap XL). The resulting mass spectra were analyzed by the ExMS program to identify the many peptides and by an in-house program (HDpop) to measure the relative fractions of protected and unprotected populations and their D label.
A native control sample was prepared by passing the fully deuterated native protein through the same experimental protocol. An unfolded protein control was prepared by pulse labeling the fully deuterated, but still unfolded, protein in 4.2 M GdmCl. D-to-H back exchange during sample preparation (average 20%) was monitored by running fully deuterated protein through the same analysis, but without the labeling pulse.
Double Jump.
Fully deuterated native Cyt c was unfolded briefly (∼20 s in 4.2 M deuterated GuHCl and 20 mM sodium phosphate, pH 2.0) by manual mixing and refolded at pH 5, followed by pulse labeling and quench in a stopped-flow instrument as described above.
Native-State HX of EX1 Unfolding.
EX1 unfolding of native Cyt c was performed at 10 °C by using a stopped-flow instrument. Fully deuterated native Cyt c (100 μM, 20 mM sodium acetate, pH 5.0, D2O buffer) was diluted into a refolding buffer (1:18 into H2O at pH 5, 10 mM sodium acetate, and 0.22 M GdmCl) followed by a continuous exposure to D-to-H exchange at pH 10 [5 volumes of 50 mM glycine to pH 10, 4% (wt/vol) D2O after mix]. After different exchange times (6–256 ms), the HX reaction was quenched by adding 5 volumes of low-pH H2O (pH 2.5, 0.6 M GdmSCN after mix). The quenched D-labeled protein was processed as described above.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant 2R01GM031847-31, National Science Foundation Grant MCB-1409137, and a research grant from the Mathers Foundation (to S.W.E.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522674113/-/DCSupplemental.
References
- 1.Bai Y, Milne JS, Mayne L, Englander SW. Protein stability parameters measured by hydrogen exchange. Proteins. 1994;20(1):4–14. doi: 10.1002/prot.340200103. [DOI] [PubMed] [Google Scholar]
- 2.Anfinsen CB, Haber E, Sela M, White FH., Jr The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proc Natl Acad Sci USA. 1961;47:1309–1314. doi: 10.1073/pnas.47.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levinthal C. Are there pathways for protein folding. J Chim Phys. 1968;65:44–45. [Google Scholar]
- 4.Zwanzig R, Szabo A, Bagchi B. Levinthal’s paradox. Proc Natl Acad Sci USA. 1992;89(1):20–22. doi: 10.1073/pnas.89.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryngelson JD, Onuchic JN, Socci ND, Wolynes PG. Funnels, pathways, and the energy landscape of protein folding: A synthesis. Proteins. 1995;21(3):167–195. doi: 10.1002/prot.340210302. [DOI] [PubMed] [Google Scholar]
- 6.Sali A, Shakhnovich E, Karplus M. How does a protein fold? Nature. 1994;369(6477):248–251. doi: 10.1038/369248a0. [DOI] [PubMed] [Google Scholar]
- 7.Onuchic JN, Wolynes PG. Theory of protein folding. Curr Opin Struct Biol. 2004;14(1):70–75. doi: 10.1016/j.sbi.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Jeng MF, Englander SW. Stable submolecular folding units in a non-compact form of cytochrome c. J Mol Biol. 1991;221(3):1045–1061. doi: 10.1016/0022-2836(91)80191-v. [DOI] [PubMed] [Google Scholar]
- 9.Bai Y, Sosnick TR, Mayne L, Englander SW. Protein folding intermediates: Native-state hydrogen exchange. Science. 1995;269(5221):192–197. doi: 10.1126/science.7618079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Englander SW, Mayne L. The nature of protein folding pathways. Proc Natl Acad Sci USA. 2014;111(45):15873–15880. doi: 10.1073/pnas.1411798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englander SW, Mayne L, Krishna MM. Protein folding and misfolding: Mechanism and principles. Q Rev Biophys. 2007;40(4):287–326. doi: 10.1017/S0033583508004654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maity H, Maity M, Englander SW. How cytochrome c folds, and why: Submolecular foldon units and their stepwise sequential stabilization. J Mol Biol. 2004;343(1):223–233. doi: 10.1016/j.jmb.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Englander SW. Protein folding intermediates and pathways studied by hydrogen exchange. Annu Rev Biophys Biomol Struct. 2000;29:213–238. doi: 10.1146/annurev.biophys.29.1.213. [DOI] [PubMed] [Google Scholar]
- 14.Plotkin SS, Onuchic JN. Understanding protein folding with energy landscape theory. Part I: Basic concepts. Q Rev Biophys. 2002;35(2):111–167. doi: 10.1017/s0033583502003761. [DOI] [PubMed] [Google Scholar]
- 15.Wolynes PG, Onuchic JN, Thirumalai D. Navigating the folding routes. Science. 1995;267(5204):1619–1620. doi: 10.1126/science.7886447. [DOI] [PubMed] [Google Scholar]
- 16.Oliveberg M, Wolynes PG. The experimental survey of protein-folding energy landscapes. Q Rev Biophys. 2005;38(3):245–288. doi: 10.1017/S0033583506004185. [DOI] [PubMed] [Google Scholar]
- 17.Mayne L, et al. Many overlapping peptides for protein hydrogen exchange experiments by the fragment separation-mass spectrometry method. J Am Soc Mass Spectrom. 2011;22(11):1898–1905. doi: 10.1007/s13361-011-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kan ZY, Mayne L, Chetty PS, Englander SW. ExMS: Data analysis for HX-MS experiments. J Am Soc Mass Spectrom. 2011;22(11):1906–1915. doi: 10.1007/s13361-011-0236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kan Z-Y, Walters BT, Mayne L, Englander SW. Protein hydrogen exchange at residue resolution by proteolytic fragmentation mass spectrometry analysis. Proc Natl Acad Sci USA. 2013;110(41):16438–16443. doi: 10.1073/pnas.1315532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walters BT, Ricciuti A, Mayne L, Englander SW. Minimizing back exchange in the hydrogen exchange-mass spectrometry experiment. J Am Soc Mass Spectrom. 2012;23(12):2132–2139. doi: 10.1007/s13361-012-0476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters BT, Mayne L, Hinshaw JR, Sosnick TR, Englander SW. Folding of a large protein at high structural resolution. Proc Natl Acad Sci USA. 2013;110(47):18898–18903. doi: 10.1073/pnas.1319482110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu W, et al. Stepwise protein folding at near amino acid resolution by hydrogen exchange and mass spectrometry. Proc Natl Acad Sci USA. 2013;110(19):7684–7689. doi: 10.1073/pnas.1305887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers JK, Pace CN, Scholtz JM. Denaturant m values and heat capacity changes: Relation to changes in accessible surface areas of protein unfolding. Protein Sci. 1995;4(10):2138–2148. doi: 10.1002/pro.5560041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishna MM, Lin Y, Rumbley JN, Englander SW. Cooperative omega loops in cytochrome c: Role in folding and function. J Mol Biol. 2003;331(1):29–36. doi: 10.1016/s0022-2836(03)00697-1. [DOI] [PubMed] [Google Scholar]
- 25.Hoang L, Bedard S, Krishna MM, Lin Y, Englander SW. Cytochrome c folding pathway: Kinetic native-state hydrogen exchange. Proc Natl Acad Sci USA. 2002;99(19):12173–12178. doi: 10.1073/pnas.152439199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maity H, Englander SW. Stability labeling studies demonstrate a sequential folding/unfolding pathway in cytochrome c. Biophys J. 2005;88(1):40A–41A. [Google Scholar]
- 27.Krishna MM, Maity H, Rumbley JN, Lin Y, Englander SW. Order of steps in the cytochrome C folding pathway: Evidence for a sequential stabilization mechanism. J Mol Biol. 2006;359(5):1410–1419. doi: 10.1016/j.jmb.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 28.Clarke J, Itzhaki LS, Fersht AR. Hydrogen exchange at equilibrium: A short cut for analysing protein-folding pathways? Trends Biochem Sci. 1997;22(8):284–287. doi: 10.1016/s0968-0004(97)01087-6. [DOI] [PubMed] [Google Scholar]
- 29.Roder H, Elöve GA, Englander SW. Structural characterization of folding intermediates in cytochrome c by H-exchange labelling and proton NMR. Nature. 1988;335(6192):700–704. doi: 10.1038/335700a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udgaonkar JB, Baldwin RL. Nature of the early folding intermediate of ribonuclease A. Biochemistry. 1995;34(12):4088–4096. doi: 10.1021/bi00012a027. [DOI] [PubMed] [Google Scholar]
- 31.Nawrocki JP, Chu R-A, Pannell LK, Bai Y. Intermolecular aggregations are responsible for the slow kinetics observed in the folding of cytochrome c at neutral pH. J Mol Biol. 1999;293(5):991–995. doi: 10.1006/jmbi.1999.3226. [DOI] [PubMed] [Google Scholar]
- 32.Kurchan E, Roder H, Bowler BE. Kinetics of loop formation and breakage in the denatured state of iso-1-cytochrome c. J Mol Biol. 2005;353(3):730–743. doi: 10.1016/j.jmb.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 33.Babul J, Stellwagen E. Participation of the protein ligands in the folding of cytochrome c. Biochemistry. 1972;11(7):1195–1200. doi: 10.1021/bi00757a013. [DOI] [PubMed] [Google Scholar]
- 34.Reimer U, et al. Side-chain effects on peptidyl-prolyl cis/trans isomerisation. J Mol Biol. 1998;279(2):449–460. doi: 10.1006/jmbi.1998.1770. [DOI] [PubMed] [Google Scholar]
- 35.Zhuravlev PI, Hinczewski M, Chakrabarti S, Marqusee S, Thirumalai D. Force-dependent switch in protein unfolding pathways and transition-state movements. Proc Natl Acad Sci USA. 2016;113(6):E715–E724. doi: 10.1073/pnas.1515730113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright CF, Lindorff-Larsen K, Randles LG, Clarke J. Parallel protein-unfolding pathways revealed and mapped. Nat Struct Biol. 2003;10(8):658–662. doi: 10.1038/nsb947. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson N, Day R, Johnson CM, Daggett V, Fersht AR. Simulation and experiment at high temperatures: Ultrafast folding of a thermophilic protein by nucleation-condensation. J Mol Biol. 2005;347(4):855–870. doi: 10.1016/j.jmb.2004.12.061. [DOI] [PubMed] [Google Scholar]
- 38.Chamberlain AK, Handel TM, Marqusee S. Detection of rare partially folded molecules in equilibrium with the native conformation of RNaseH. Nat Struct Biol. 1996;3(9):782–787. doi: 10.1038/nsb0996-782. [DOI] [PubMed] [Google Scholar]
- 39.Fuentes EJ, Wand AJ. Local dynamics and stability of apocytochrome b562 examined by hydrogen exchange. Biochemistry. 1998;37(11):3687–3698. doi: 10.1021/bi972579s. [DOI] [PubMed] [Google Scholar]
- 40.Feng H, Zhou Z, Bai Y. A protein folding pathway with multiple folding intermediates at atomic resolution. Proc Natl Acad Sci USA. 2005;102(14):5026–5031. doi: 10.1073/pnas.0501372102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silverman JA, Harbury PB. The equilibrium unfolding pathway of a (beta/alpha)8 barrel. J Mol Biol. 2002;324(5):1031–1040. doi: 10.1016/s0022-2836(02)01100-2. [DOI] [PubMed] [Google Scholar]
- 42.Korzhnev DM, Neudecker P, Zarrine-Afsar A, Davidson AR, Kay LE. Abp1p and Fyn SH3 domains fold through similar low-populated intermediate states. Biochemistry. 2006;45(34):10175–10183. doi: 10.1021/bi0611560. [DOI] [PubMed] [Google Scholar]
- 43.Yan S, Kennedy SD, Koide S. Thermodynamic and kinetic exploration of the energy landscape of Borrelia burgdorferi OspA by native-state hydrogen exchange. J Mol Biol. 2002;323(2):363–375. doi: 10.1016/s0022-2836(02)00882-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.