Significance
A major concern for terrestrial biosphere models is accounting for the temperature response of leaf respiration at regional/global scales. Most biosphere models incorrectly assume that respiration increases exponentially with rising temperature, with profound effects for predicted ecosystem carbon exchange. Based on a study of 231 species in 7 biomes, we find that the rise in respiration with temperature can be generalized across biomes and plant types, with temperature sensitivity declining as leaves warm. This finding indicates universally conserved controls on the temperature sensitivity of leaf metabolism. Accounting for the temperature function markedly lowers simulated respiration rates in cold biomes, which has important consequences for estimates of carbon storage in vegetation, predicted concentrations of atmospheric carbon dioxide, and future surface temperatures.
Keywords: temperature sensitivity, climate models, carbon exchange, Q10, thermal response
Abstract
Plant respiration constitutes a massive carbon flux to the atmosphere, and a major control on the evolution of the global carbon cycle. It therefore has the potential to modulate levels of climate change due to the human burning of fossil fuels. Neither current physiological nor terrestrial biosphere models adequately describe its short-term temperature response, and even minor differences in the shape of the response curve can significantly impact estimates of ecosystem carbon release and/or storage. Given this, it is critical to establish whether there are predictable patterns in the shape of the respiration–temperature response curve, and thus in the intrinsic temperature sensitivity of respiration across the globe. Analyzing measurements in a comprehensive database for 231 species spanning 7 biomes, we demonstrate that temperature-dependent increases in leaf respiration do not follow a commonly used exponential function. Instead, we find a decelerating function as leaves warm, reflecting a declining sensitivity to higher temperatures that is remarkably uniform across all biomes and plant functional types. Such convergence in the temperature sensitivity of leaf respiration suggests that there are universally applicable controls on the temperature response of plant energy metabolism, such that a single new function can predict the temperature dependence of leaf respiration for global vegetation. This simple function enables straightforward description of plant respiration in the land-surface components of coupled earth system models. Our cross-biome analyses shows significant implications for such fluxes in cold climates, generally projecting lower values compared with previous estimates.
Plant respiration provides continuous metabolic support for growth and maintenance of all tissues and contributes ∼60 Pg C y−1 to the atmosphere (1, 2), with ∼50% of the carbon (C) released by whole-plant respiration from leaves (3). As rates of leaf respiration (R) vary substantially with changes in temperature (T) (4, 5), even slight increases in ambient T can lead to increases in the flux of carbon dioxide (CO2) from leaves to the atmosphere. This has the potential to create concomitant decreases in net primary productivity, and affect the implications of fossil fuel burning by contributing additionally to atmospheric CO2 levels due to any imposed surface-level global warming. Hence, quantification of the T response of leaf R, and how this response may vary across diverse ecosystems and plant species, is critical to current estimations and future projections of the global carbon cycle (6–8). Evaluating how leaf R relates to T in terrestrial plants will clarify fundamental controls on energy metabolism and enable more accurate parameterization, as leaf R, in addition to photosynthesis (9, 10), has been identified as a major source of uncertainty in models of the global carbon cycle (8, 11). The response of leaf R to T differs in both magnitude and mechanism with time scale (5); herein, we address how the fundamental short-term response (minutes to hours) varies among plant species and biomes globally.
The short-term T response of leaf R is strongly regulated by the T dependence of the reaction rates of enzymes involved in a variety of respiratory pathways in the cytosol and mitochondria within plant cells (5, 12). Given that these many processes influence the realized rates of leaf R across broad ranges in T, the T dependence of R might be expected to vary widely among contrasting thermal regimes and environments, or among species that differ in metabolic capacity or life span. For example, R–T relations could vary predictably, according to plant functional types (PFTs; groupings of plant species by life history attributes, growth strategies and/or geographic location), or with variation corresponding with types that differ in rates of net photosynthetic CO2 uptake and potential growth rates (e.g., fast-growing herbs versus slower-growing trees). A key issue, therefore, is whether the T dependence of leaf R has spatially invariant features across the earth’s surface, or instead varies as a consequence of genotypic and multiple environmental factors. This is critically important, as the global estimation of leaf R is a significant uncertainty in terrestrial biosphere models (TBMs) and associated land-surface components of earth system models (ESMs). The latter quantify the global carbon cycle now and project it into the future (8, 11), including feedbacks as a consequence of anthropogenic emissions of CO2 on climate.
Although it has been known for over a century that the near-instantaneous increase in plant R with rising T is nonlinear (13, 14), there has been uncertainty whether a single general form for the leaf R–T relationship applies both phylogenetically and biogeographically (15–17). A widely adopted physiological model framework (18, 19) assumes that R exhibits an exponential response to T, with R roughly doubling with every 10 °C rise in T (corresponding to a fixed “Q10-type” formulation, with Q10 ∼ 2.0). However, it has long been recognized that the Q10 is often not constant nor close to 2.0 except over a limited T range (14, 20), and this pattern is consistent when also considering ecosystem respiration (21). For this reason, alternative models have been developed, including modified Arrhenius formulations, universal temperature dependence (UTD), and T-dependent Q10 functions (15–17, 22). All of these models attempt to address the shortcomings of an exponential model that provides a fixed T-sensitivity term across a wide range of temperatures. Here, we evaluate a comprehensive set of empirical, thermally high-resolution T-response curves for multiple taxa and environments. Doing so enables a full assessment of the suitability of these quantitative physiological models in accurately representing the variation in the observed short-term R–T relationship, and implications of the short-term response in different seasons. We aim to significantly improve how the short-term R–T response is represented, and recognize this is one element of a complex and dynamic process. As leaf R is also impacted by acclimation to sustained changes in growth T, future modeling work will determine the effect of a more accurate short-term T response applied in concert with recent advances in modeling basal rates of leaf R (23) and longer-term (weeks to months) acclimation of R to changing growth Ts (24, 25).
Physiological model representations of leaf respiratory T responses vary in complexity and in their ability to account for observed biological patterns, such as decreases in the T sensitivity of R over increasing Ts (5, 17) (see Supporting Information for model descriptions and Figs. S1 and S2). Modification of the T sensitivity of leaf R (based on ref. 16) in TBMs and the associated land-surface component of ESMs results in significant alterations to modeled carbon fluxes (8, 26), demonstrating the high sensitivity of the carbon cycle simulations to the R–T function, and thus the need to improve our understanding and quantification of this relationship. The evidence for apparent complexity in the leaf R–T response (16, 27) and consequences for carbon cycling indicates both the need for, and, opportunity to improve quantification of the leaf R–T relationship in globally widespread, but thermally contrasting, biomes. Here, we report on filling that critical knowledge gap.
Fig. S1.
An example temperature (T) response curve of respiration (R) from 10 to 45 °C, normalized to the rate of R at 25 °C (solid black line), displayed with commonly applied functional models of the T response (also normalized to 25 °C) that vary in their characterization of R. (A) Functional models that do not account for the temperature-dependent T sensitivity of the R–T response [exponential-fixed Q10, Arrhenius/UTD (15)] are represented with dashed lines, and models that do account for this sensitivity [Lloyd & Taylor (17), variable Q10 (12, 16), and polynomial (27)] are shown with solid lines. Differences between the functional models are more pronounced at Ts below 20 °C (B) and above 40 °C (C).
Fig. S2.
Mean relativized residuals (percent error in prediction) of estimates of commonly applied models based on all replicate R–T response curves. All replicates (n = 673 leaves) across 10–45 °C (A) highlight the significance of T-dependent parameter inclusion, as seen in the variable-Q10 and polynomial fits (solid lines) in contrast to the fixed-T sensitivity models (broken lines). The global mean response of R to T across all species measured in this study (B, Inset, n = 231) are bracketed by 95% CI (dashed lines).
The goals of our study are threefold: (i) to quantify the T response of leaf R through use of a new and comprehensive set of thermally high-resolution field measurements of leaf R across large T ranges for each leaf; (ii) to assess the shape of T-response curves in leaves of species representing diverse environments and PFTs; and (iii) to assess the implications of altered T sensitivity of R for simulated carbon fluxes using the land-surface component of a leading ESM (28). Using methods (27) that enabled high-resolution measurement of the T dependence of leaf R in leaves, we present results from 673 short-term T-response curves of 231 species collected in situ across 18 sites representing contrasting biomes, geographical locations, and PFTs (Table S1). Based on this unprecedented dataset of standardized physiological measurements, we provide evidence of a global, fundamental T response of leaf R in terrestrial plants and thus a mathematical model that outperforms alternative representations of how leaf R responds to T. We also show that in cross-biome analyses, application of this mathematical model significantly alters simulated carbon fluxes, particularly in cold climate ecosystems.
Table S1.
Geographic, climatic, and sampling information of field sites from which leaves were sampled for measurement
| Biome | Dates of measurement | Lat. (oN) | Long.(oE) | Elevation (m.a.s.l.) | MAT (°C) | TWQ (°C) | Annual precip. (mm) | Aridity index | PFTs represented | No. Species | No. total reps | Fig. 3A site code |
| Tundra | ||||||||||||
| Toolik Lake, AK | June 2010 | 68.63 | −149.6 | 720 | −11.3 | 8.2 | 225 | 0.61 | C3H, BlDcTmp, SEv, NlEv | 20 | 79 | USA-1 |
| Boreal forest | ||||||||||||
| Umea, Sweden | Aug. 2013 | 63.821 | 20.311 | 29 | 2.5 | 14.3 | 579 | 1.13 | BlDcTmp, NlEv | 10 | 37 | Swed |
| Ely, MN | July 2013 | 47.956 | −91.75 | 420 | 3.2 | 17.6 | 703 | 0.9 | BlDcTmp, NlEv | 15 | 59 | USA-2 |
| Temperate deciduous forest | ||||||||||||
| Black Rock Forest, NY | June 2013 | 41.408 | −74.012 | 335 | 7.43 | 19.52 | 1103 | 1.17 | BlDcTmp | 10 | 38 | USA-3 |
| Temperate woodland | ||||||||||||
| Aranda, ACT | Sept. 2011 | −35.275 | 149.079 | 580 | 12.7 | 19.5 | 682 | 0.55 | BlEvTmp | 10 | 33 | AUS-1 |
| ANU campus, ACT | March 2012 | −35.279 | 149.108 | 571 | 13.1 | 19.8 | 637 | 0.51 | BlDcTmp | 4 | 15 | AUS-2 |
| Calperum, SA | March 2013 | −34.037 | 140.674 | 35 | 17.25 | 23.6 | 255 | 0.17 | SEv, BlEvTmp, NlEv | 16 | 34 | AUS-3 |
| College Station, TX | Oct. 2010 | 30.6 | −96.400 | 103 | 20 | 28.5 | 995 | 0.68 | BlDcTmp, NlEv | 2 | 8 | USA-4 |
| Great Western Woodlands, WA | April 2013 | −30.264 | 120.692 | 459 | 18.5 | 25.6 | 273 | 0.18 | SEv, BlEvTmp, NlEv, C3H | 16 | 41 | AUS-4 |
| Jurien Bay, WA | Nov. 2011 | −30.241 | 115.071 | 23 | 18.87 | 23.83 | 558 | 0.39 | BlDcTmp, SEv, C3H, BlEvTmp | 15 | 56 | AUS-5 |
| Alice Mulga, NT, AUS | Feb. 2012 | −22.283 | 133.249 | 607 | 22.4 | 28.9 | 321 | 0.17 | BlEvTmp, SEv | 4 | 6 | AUS-6 |
| Temperate rain forest | ||||||||||||
| Warra, TAS, AUS | March 2012 | −43.095 | 146.724 | 86 | 10.78 | 14.43 | 1380 | 1.69 | BlEvTmp, SEv | 12 | 45 | AUS-7 |
| Tropical rainforest (high altitude) | ||||||||||||
| Wayquecha, Peru | Sept. 2011 | −13.19 | −71.587 | 3000 | 13.4 | 14.5 | 335 | 0.23 | BlEvTrp | 16 | 17 | PERU-1 |
| Tropical rainforest (low altitude) | ||||||||||||
| San Isidro, Costa Rica | July 2011 | 10.38 | −84.620 | 479 | 24 | 25 | 4045 | 2.61 | BlEvTrp, SEv | 5 | 16 | CoRi |
| Atherton, QLD | Aug. 2012 | −17.12 | 145.632 | 728 | 21 | 23.8 | 2140 | 1.47 | BlEvTrp | 16 | 58 | AUS-8 |
| Cape Tribulation, FNQ | Sept. 2010 | −16.28 | 145.480 | 90 | 25.2 | 27.5 | 2087 | 1.39 | BlEvTrp | 12 | 35 | AUS-9 |
| Paracou, French Guiana | Oct. 2010 | 5.27 | −52.920 | 21 | 25.8 | 26.2 | 2824 | 1.88 | BlEvTrp, BlDcTrp | 32 | 76 | FrGu |
| Iquitos, Peru | Sept. 2011 | −3.949 | −73.434 | 114 | 25.3 | 26.8 | 2769 | 1.64 | BlEvTrp | 16 | 16 | PERU-2 |
The aridity index is the quotient of mean annual precipitation divided by mean annual evapotranspiration (46).
Results
Evaluating Temperature Response Models.
Our data of high-resolution measurement of the T response of leaf R enabled a comparison of commonly applied quantitative physiological models to determine which offered the best fit for replicate response curves across the entire 10–45 °C range. A comparison of residuals from model estimates for all individual leaf response curves for five models (exponential fixed-Q10, Arrhenius, Lloyd & Taylor, variable-Q10, and second-order log-polynomial function; Supporting Information) demonstrates that a second-order log-polynomial model best characterized the T response of R (Fig. S2A). This selection is made on the basis that the polynomial model had the best projections of leaf R against data from over the entire T range, has a straightforward application, and is independent from biological assumptions about activation energies; we applied this approach to all measured response curves that collectively comprise the total mean response (Fig. S2B). Accordingly, to best represent our high-resolution leaf R measurements quantitatively, all individual leaf T-response curve data were natural-log–transformed (ln) and to those values, a second-order polynomial model was fitted as:
| [1] |
where R is the rate at a given leaf T, and a, b, and c are coefficients that provided the fit that minimized residuals.
The application of a polynomial model fit to high-resolution ln R–T response curves provides a three-parameter description of leaf R across the T range. The a parameter, which indicates ln R at 0 °C, determines a reference value offset of the response curve. The b parameter—the slope of ln R vs. T plot at 0 °C—and the c parameter, which represents any quadratic nonlinearity in ln R vs. T slope with increasing measuring T, are both key to describing the fundamental shape of the short-term T response of leaf R. To assess the influence of site environment and plant form, we analyzed the variation in values of each model parameter, a, b, and c for diverse biomes and PFTs based on individual leaf sample curves. We calculated this variation for both the entire measured T range (10–45 °C), as well as for shorter, discrete segments (i.e., 15–25 °C) of the entire measured T range, to evaluate potential influence of measurement T range on these parameters. No difference was found between the parameters calculated from shorter, discrete T ranges and the entire measurement T range, (Tables S2 and S3, Fig. S3), further justifying the applicability of the polynomial function for this response. Together, mean values of a, b, and c parameters create data-derived equations for leaf R that clearly mirror observed mean respiratory responses aggregated for discrete levels of the two corresponding factors (i.e., biome or PFT; Fig. 1). This approach can also fully capture the deceleration of rates of R observed as Ts increase (Figs. 1 and S1), clearly demonstrating the utility of the polynomial formulation for creating realistic models of leaf R.
Table S2.
Mean values (±S.D.) of coefficients (a, b, and c) of polynomial models of the ln R–T response, calculated for four 20 °C segmented intervals across the full-measurement range of the response for all replicate curves (n = 673)
| Segmented interval range | n | a | SD | b | SD | c | SD |
| 10–30 °C | 346 | −2.1001 | 1.2962a | 0.0969 | 0.0944a | −0.00044 | 0.00210a |
| 15–35 °C | 523 | −2.1539 | 1.3440ab | 0.0979 | 0.0896a | −0.00045 | 0.00169a |
| 20–40 °C | 623 | −2.1203 | 1.8446a | 0.0964 | 0.1168a | −0.00042 | 0.00189a |
| 25–45 °C | 599 | −2.3610 | 1.4938b | 0.1163 | 0.0764b | −0.00076 | 0.00108b |
| Complete available T range | 673 | −2.2003 | 1.3559ab | 0.1034 | 0.0715ab | −0.00055 | 0.00110ab |
Values of a, b, and c parameters were statistically compared individually using a mixed model with the segmented interval range as a fixed effect, and nests the random effects of Biome and PFT for each replicate. This approach accommodates the unbalanced dataset across the interval ranges. Significant variation between parameters by segment range is marked with unshared letters. Parameter values calculated from ln R–T curves that include all available data are not significantly different from any parameter values calculated from individual 20 °C segmented intervals, justifying our use of all available data for the calculation of coefficient values.
Table S3.
Ecologically relevant mean a, b, and c parameter values and 95% confidence intervals (CI) of biomes and PFTs across all species
| Biome/PFT | a | 95% CI | b | 95% CI | c | 95% CI | n |
| Biome | |||||||
| Tu | −1.6297ab | [-2.1322, -1.1272] | 0.1257a | [0.0869, 0.1645] | −0.00095a | [-0.0018, -0.0001] | 20 |
| BF | −1.9455ab | [-2.3502, -1.5409] | 0.0836a | [0.0488, 0.1184] | −0.00025a | [-0.0010, 0.0004] | 25 |
| TeDF | −1.8827ab | [-2.2722, -1.4931] | 0.0423a | [0.0162, 0.0683] | 0.00080a | [0.0002, 0.0014] | 10 |
| TeW | −1.5478a | [-2.1334, -0.9622] | 0.0743a | [0.0357, 0.1130] | 0.000002a | [-0.0006, 0.0006] | 66 |
| TeRF | −2.0273ab | [-2.4007, -1.6540] | 0.0986a | [0.0625, 0.1347] | −0.00051a | [-0.0014, 0.0003] | 13 |
| TrRF_hi | −1.9061ab | [-2.4132, -1.3990] | 0.0961a | [0.0704, 0.1218] | −0.00056a | [-0.0011, -0.00003] | 16 |
| TrRF_lw | −2.7370b | [-3.1060, -2.3679] | 0.1070a | [0.0837, 0.1302] | −0.00038a | [-0.0008, 0.00004] | 81 |
| PFT | |||||||
| BlDcTmp | −1.9553ab | [-2.2335, -1.6770] | 0.0800a | [0.0578, 0.1022] | −0.00013a | [-0.0006, 0.0003] | 40 |
| BlDcTrp | −3.1352ab | [-4.3860, -1.8843] | 0.1526a | [0.0821, 0.2230] | −0.00165a | [-0.0038, 0.0005] | 4 |
| BlEvTmp | −1.2877a | [-1.9003, -0.6751] | 0.0518a | [0.0127, 0.0909] | 0.00047a | [-0.0002, 0.0011] | 34 |
| BlEvTrp | −2.5695b | [-2.9071, -2.2318] | 0.0962a | [0.0756, 0.1168] | −0.00037a | [-0.0007, -0.000001] | 92 |
| C3H | −1.6821ab | [-2.1694, -1.1948] | 0.1272a | [0.0928, 0.1615] | −0.00103a | [-0.0017, -0.0004] | 13 |
| NlEv | −1.7876ab | [-2.6843, -0.8909] | 0.0864a | [0.0148, 0.1579] | −0.00013a | [-0.0015, 0.0005] | 13 |
| SEv | −1.8495ab | [-2.7611, -0.9379] | 0.1003a | [0.0390, 0.1616] | −0.00054a | [-0.0015, 0.0005] | 35 |
| Global mean | −2.0812 | [-2.3137, -1.8487] | 0.0897 | [0.0747, 0.1046] | −0.00027 | [-0.0005, -0.00001] | 231 |
Biomes and PFTs are listed in the text of Table 1. The parameters were calculated from a 20 °C interval of the R–T response curve that best represents Ts experienced by an individual species at the site from which it was sampled, based on the mean T of the warmest quarter (46) therefore referred to as the ecologically relevant T range. The global mean value was calculated considering all species parameter values equally. To determine the influence of Biome and PFT on the parameter values, we used a mixed model that nested random effect terms, with Species nested in Site when evaluating Biome, and with nested Species as a random effect when evaluating PFT. Significant differences across biomes and PFT groups were evaluated by a post hoc comparison of least-square means, and are indicated by unshared letters. Ecologically relevant values of these parameters are not statistically significantly different from the Full T range parameter values (Table 1), as determined by a separate mixed-model analysis, with Site nested in Biome, and Species nested in PFT.
Fig. S3.
Segmented interval approach to polynomial model analysis. Three representative leaf respiration (R) temperature response curves (A) of replicate leaves of sampled species from Toolik Lake, Alaska (AK; Alnus tenufolia), Cape Tribulation, Far North Queensland, Australia (CT; Acmena graveolens), and Great Western Woodlands, Western Australia sites (GWW; Eucalyptus transcontinentalis). To assess the effect of measurement T range variation in a, b, and c parameters calculated from the log-polynomial fit, we used a “segmented interval” approach (B). The segmented interval approach fit polynomial curves across 20 °C range intervals of replicate ln R data, specifically 10–30 °C (blue), 15–35 °C (green), 20–40 °C (orange), and 25–45 °C (light blue) as shown in B. The resulting a, b, and c parameters calculated from these segmented intervals were then statistically compared with each other, and to the a, b, and c values resulting from a polynomial fit that included the entire range of data available from the original measured R–T replicates.
Fig. 1.
Mean measured leaf respiration (natural log transformed; ±SE) of biome (A) and PFTs (C) calculated for each degree Centigrade from measured species respiration response curves of those categories, for the available temperature ranges. Polynomial models based on species’ mean values of a, b, and c (Table 1) of those biomes (B) and PFTs (D) are shown across the same T range.
Comparison Among Biomes and Plant Functional Types.
Mean species values for the polynomial model parameters (a, b, and c) at each site were statistically compared by biome and PFTs using a nested mixed-model approach (Table 1). The curves presented in Fig. 1 show that rates of leaf R at a common T were highest in the coldest biomes (i.e., higher a values for tundra and high-altitude tropical rainforests). By contrast, low-altitude tropical forests, the warmest biome included in this study (Table S1), exhibited the lowest value of parameter a and the lowest values of leaf R over the measurement ranges of T (Fig. 1 A and B). Similarly, variation in leaf R at a common T was found among PFTs (Fig. 1 C and D).
Table 1.
Biome and PFT mean values (with 95% confidence intervals, CI) of a, b, and c coefficients aggregated across all species (n = 231)
| Biome/PFT | a | 95% CI | b | 95% CI | c | 95% CI |
| Biome | ||||||
| Tu | −1.6043a | [-1.8372, -1.3713] | 0.1277a | [0.1190, 0.1364] | −0.00107a | [-0.0012, -0.0009] |
| BF | −2.0043a | [-2.2781, -1.7305] | 0.0894a | [0.0665, 0.1122] | −0.00037a | [-0.0008, 0.00003] |
| TeDF | −2.4286a | [-2.7959, -2.0612] | 0.0923a | [0.0757, 0.1089] | −0.00026a | [-0.0006, 0.00004] |
| TeW | −1.8958a | [-2.3435, -1.4481] | 0.0974a | [0.0716, 0.1232] | −0.00040a | [-0.0008, -0.00002] |
| TeRF | −2.1544a | [-2.4057, -1.9032] | 0.1014a | [0.0773, 0.1255] | −0.00046a | [-0.0008, -0.0001] |
| TrRF_hi | −2.0173a | [-2.5325, -1.5021] | 0.1154a | [0.0956, 0.1352] | −0.00071a | [-0.0010, -0.0004] |
| TrRF_lw | −2.7493a | [-2.9831, -2.5155] | 0.0998a | [0.0879, 0.1117] | −0.00047a | [-0.0007, -0.0003] |
| PFT | ||||||
| BlDcTmp | −2.2264ab | [-2.4829, -1.9699] | 0.0993a | [0.0829, 0.1158] | −0.00050a | [-0.0008, -0.0002] |
| BlDcTrp | −2.7270ab | [-3.6757, -1.7782] | 0.1125a | [0.0961, 0.1288] | −0.00058a | [-0.0008, -0.0003] |
| BlEvTmp | −1.8106a | [-2.3349, -1.2864] | 0.0896a | [0.0577, 0.1215] | −0.00021a | [-0.0007, 0.0003] |
| BlEvTrp | −2.6105b | [-2.8366, -2.3844] | 0.1022a | [0.0912, 0.1132] | −0.00052a | [-0.0007, -0.0003] |
| C3H | −1.7507ab | [-2.0680, -1.4334] | 0.1271a | [0.1169, 0.1374] | −0.00110a | [-0.0013, -0.0009] |
| NlEv | −2.0464ab | [-2.5569, -1.5358] | 0.1125a | [0.0934, 0.1316] | −0.00063a | [-0.0009, -0.0004] |
| Sev | −1.8150a | [-2.4609, -1.1691] | 0.0971a | [0.0593, 0.1349] | −0.00047a | [-0.0006, -0.0004] |
| Global mean | −2.2276 | [-2.3966, -2.0586] | 0.1012 | [0.0921, 0.1104] | −0.00050 | [-0.0006,-0.0004] |
Biomes include tundra (Tu; n = 20), boreal forest (BF; n = 25), temperate deciduous forest (TeDF; n = 10), temperate woodland (TeW; n = 67), temperate rainforest (TeRF; n = 12), high-elevation tropical rainforest (TrRF_hi; n = 16), and low-elevation tropical rainforest (TrRF_lw; n = 81); PFTs include broadleaf deciduous temperate (BlDcTmp; n = 40), broadleaf deciduous tropical (BlDcTrp; n = 4), broadleaf evergreen temperate (BlEvTmp; n = 38), broadleaf evergreen tropical (BlEvTrp; n = 88), C3 herbaceous (C3H; n = 13), needle-leaf evergreen (NlEv; n = 13), and evergreen shrubs (SEv; n = 35). Mean values and confidence intervals were calculated from natural-log–transformed rates of leaf respiration R–T curve data available from the ∼10–45 °C curve range. The global mean value was calculated from all individual species parameter values. To determine the effect Biome and PFT groups, we used a mixed model that nested random effects, with Species nested in Site when evaluating Biome, and Species as a single random effect to evaluate the fixed effect of PFT. Post hoc comparisons of least-square means determined differences between Biome and PFT groups (denoted by unshared letters).
In strong contrast to large differences across biomes and PFTs in leaf R at a common measurement T, we found that the rise in R with T as leaves warm follows a remarkably consistent function, suggesting more universal values of parameters b and c. Fig. 1 illustrates the common shape of the response curve to leaf T that is almost invariant across plants, despite representing highly diverse growth environments and functional groups. This low variation across species means of both b and c parameters is present when grouped by either biome or PFT (Table 1).
Based on our observation of a near-universal shared response shape of leaf R to T, we determined the parameters for our global polynomial R–T model (GPM) of Eq. 1. The mean polynomial model parameter values for all species included in our study were: b = 0.1012 and c = −0.0005, which generate the GPM:
| [2] |
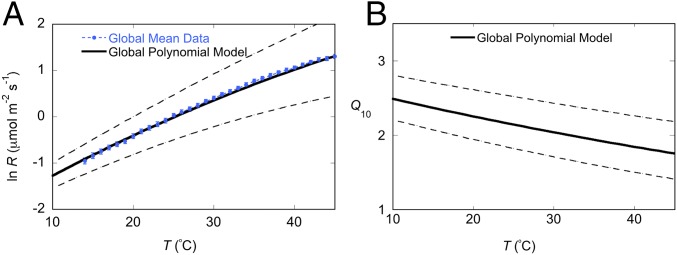
where ln R and a are as defined for Eq. 1. This equation is an empirically based mathematical model of the instantaneous T response of leaf R (Fig. 2A). Average leaf R for all study species across the 10–45° T range (within 1 °C temperature bins; untransformed global mean response in Fig. S2B)—the “global mean data”—can be effectively summarized by the GPM (Fig. 2A). Values of a do, however, vary significantly across PFTs, shifting the curve of Eq. 2; thus, the a parameter value should be appropriately assigned in the GPM to fit the model’s application, using a rate measured at a known T or values from our global survey (Dataset S1).
Fig. 2.
Global mean data reflected by modeled R–T and corresponding declining Q10 responses. The mean T response of (A) natural-log–transformed rates of leaf respiration (ln R ± SE, Global Mean Data, shown with blue symbols with error bars) for all measured species (n = 231) across all biomes and PFTs, overlaid on the GPM of ln R (solid black line, bracketed by dashed lines representing 95% confidence intervals), calculated from the species values of a, b, and c parameters of the polynomial model. The GPM is defined as ln R = −2.2276 + 0.1012* T − 0.0005*T2. The T response of Q10 values (B) based on GPM b and c coefficients as calculated by Q10 = e10*(0.1012+(2*0.0005T)), shown with 95% confidence intervals (dashed lines).
The input of a known value of leaf R (RTref in the below equation), measured at a T (Tref in the below equation) with the universal b and c response curve parameters can be applied to a derivation of our GPM to predict values of leaf R (RT) at a desired T, according to:
| [3] |
where RTref = exp (a + 0.1012Tref − 0.0005Tref2). This equation incorporates the common intrinsic T sensitivity of respiration (i.e., response curve shape) observed from our field measurements, and when combined with measured or assumed rates of R at Tref, enables prediction of R at various Ts.
The T sensitivity of the GPM (Fig. 2B), here calculated for illustrative purposes using Q10 values, shows decreasing sensitivity of leaf R with increases in T. Up to 35 °C, the decline has similarities to (and a steeper slope than) that reported from more limited data by Tjoelker et al. (16). Moreover, our GPM demonstrates that leaf R remains more T sensitive at higher leaf Ts (e.g., near 45 °C) than assessed by Tjoelker et al. (16).
Impacts on Simulated Annual Respiration.
The consequence of using our GPM in existing global models that exclude acclimation responses to sustained changes in growth T is illustrated in Fig. 3, which shows annually averaged rates of leaf R for our 18 field sites, comparing Joint U.K. Land Environmental Simulator (JULES) estimates modeled with a Q10 = 2 with those from our GPM derivation Eq. 3.
Fig. 3.
Impact of two T functions on annual average of modeled instantaneous leaf respiration rates (R) using the JULES coupled climate carbon model to extrapolate respiration measurements (42, 43). A shows annual average of leaf R (averaged over the five years of 2010–2014 inclusive) at 18 globally distributed field sites (Table S1), with annual rates of R calculated assuming a fixed Q10 of 2.0 (43) or our GPM (Eq. 3). Annual averages of leaf T (same period) in the upper canopy is shown as green dots. Sites are ordered by temperature, with site codes as shown in Table S1; B shows percentage changes in annual averages of rates of leaf R that result from switching from a fixed Q10 to our GPM, plotted against annual averages of leaf T—the dashed line shows a parabolic curve fit i.e., with three degrees of freedom; C shows seasonal variation in rates leaf R (expressed on an LAI basis) for three thermally contrasting sites [Toolik Lake (tundra), Alaska; Great Western Woodlands (temperate woodland), Western Australia; and Paracou (tropical rainforest), French Guiana]. Site-averaged leaf R values at 25 °C, measured in the field, were used for the calculations.
As a sensitivity study, we replaced the derivation of the GPM (Eq. 3) with the commonly applied fixed Q10 formulation, setting Q10 = 2, and compared the two. The difference between annual rates of leaf R calculated using either the derived GPM (Eq. 3) or a fixed Q10 equation where Q10 = 2 had almost no impact on at the warm tropical sites (Fig. 3 A and B); similarly, there was no effect of the GPM on seasonal variations in leaf R at the tropical sites (Fig. 3C). By contrast, at colder sites, estimates of annual leaf R were markedly lower when calculated using the GPM derivation (e.g., 28% lower in Toolik Lake, Alaska, and 10–20% lower in the temperate sites) compared with the fixed Q10 function (Fig. 3B), although recognizing these changes are for generally lower R values. At temperate woodland sites with evergreen, long-lived foliage, replacement of a fixed Q10 of 2.0 model with the GPM had its greatest absolute and proportional effect during the cold months of winter, but negligible effect during summer months when leaf T values were near 25 °C. For sites where winters are characterized by winter freezing (and thus where metabolic activity is minimal), use of the GPM reduced estimates of leaf R across the entire growing season (Fig. 3C).
Discussion
Universality of Temperature Response.
Despite the huge diversity in plant growth form and local environment represented in our comprehensive dataset, we find remarkable convergence in the functional form of the response of leaf R to T. Basal rates of R vary widely among biomes and PFTs (Fig. 1), and are known to be related to differences in growth T, site aridity, and leaf functional traits (23, 29, 30). That R at a given T is highest in leaves of arctic tundra plants and lowest in leaves of plants from low-elevation tropical forests (Fig. 1A) agrees with the concept that leaf R (when measured at a common T) is higher in plants grown in colder environments (12), and this pattern can be consistently modeled based on known growth Ts (23). There is significant variation in the curve offset between PFTs; C3 herbs exhibit the highest rates of leaf R across the 10–45 °C range (Fig. 1C), which is also associated with high rates of leaf R at a common leaf nitrogen compared with other PFT groups (23, 29). However, here we show the overall shape of the response curve, and thus intrinsic T sensitivity of R, does not significantly vary; the only variation is an overall offset of the curve. The consistency in the response of leaf R to T strongly suggests its universality among C3 plants and that the T dependencies of underlying enzymatic controls of multiple metabolic pathways are widely conserved, even among the most thermally contrasting biomes on earth. Further, a global, fundamental T response can be described in a simple, empirically driven log-polynomial equation, available for incorporating into the land-surface component of ESMs and ready to replace current imperfect representations of the short-term T response of leaf R. Notably, when implemented in a leading TBM (28) for different geographical regions, this equation significantly reduces annual rates of leaf-level respiration in cold climates. We believe this global short-term leaf R–T response, when applied in conjunction with data-based models of basal leaf R (23) and the acclimation response to longer-term growth Ts (24), will have important consequences for predicted rates of ecosystem and global carbon exchange, estimates of future carbon storage in vegetation, predicted concentrations of atmospheric CO2, and impacts of future surface temperatures.
Utility for Predictive Simulation Models.
Our finding of a universal T response provides an opportunity for leaf R to be better represented in ecosystem models, TBMs, and associated land-surface components of ESMs. It is well known that the use of a fixed-Q10 or Arrhenius activation energy leads to inaccuracies in estimations of respiratory efflux, especially at relatively high and low Ts (5). In particular, Arrhenius-derived functions may overestimate rates at low Ts and underestimate the decline in T sensitivity of R (22) (Fig. S1A). To date, there has been no consensus or consistent assessment based on comprehensive datasets on how to represent the T response of R in simulation models (31). Our GPM (Eq. 1) and its parameterization (Eqs. 2 and 3) against a massive dataset for R is comprised of only three and two coefficients, respectively, and offers a simple, yet robust, approach to calculating the T response of R in leaves. Importantly, our GPM demonstrates that leaf R remains T sensitive at high leaf Ts (e.g., near 45 °C; seen in our Fig. S1A compared with variable Q10 model; ref. 12), which will have important consequences for predicted rates of respiratory CO2 efflux at high Ts, particularly as extreme heat-wave events are predicted to increase in frequency and duration (2).
Application of the GPM requires knowledge of basal rates of leaf R, designated by the a parameter (Eq. 2) or measured/assumed rates of R at a standard measurement T = TRef (Eq. 3). In cases where the basal rate of R is unknown, we suggest application of specific a parameter values representing appropriate PFTs and/or biomes (Table 1) or species (Dataset S1). Alternatively, rates of leaf R at common TRef (25 °C) reported in a recent global compilation (23) can be used. We believe future integration of the recent global leaf R dataset (23) with the short-term R–T response model defined by our GPM and climatically variable estimates of longer-term T response of R through acclimation will result in a vastly improved representation of leaf R across scales.
Consequences for Terrestrial C Exchange.
Our sensitivity study (Fig. 3) showed that although replacing a fixed Q10 of 2 with the GPM will have little impact on calculated rates of leaf R in lowland tropical forests, impacts are significant for temperate, boreal, and arctic/alpine ecosystems. In such ecosystems, reliance on a fixed Q10 greatly overestimates annual leaf R, which in turn will result in underestimates of net primary productivity (NPP), as generally TBMs estimate NPP by subtraction of total canopy leaf R from modeled estimates of gross primary productivity (GPP). Though future model implementations that consider the extent to which leaf R acclimates to long-term changes in air T across the globe (24, 25) will likely further improve how leaf R is represented in TBMs, our findings point to lower rates of modeled respiratory CO2 release—and thus possible higher rates of simulated NPP—at sites further away from the equator, compared with current model scenarios. As replacement of a fixed Q10 formulation with our GPM is likely to have profound effects on estimates of global plant R and calculations of NPP, its adoption in ESMs will adjust projections of both contemporary and future carbon storage in vegetation. This includes estimates of PFT composition in TBMs that also calculate biome extent through NPP-dependent competition rules. Furthermore, via influence on atmospheric CO2 levels, the GPM will affect estimates of what constitutes “permissible” fossil fuel emissions needed to stay below any warming thresholds that society determines as unsafe to cross. This might include the presently much-debated limit of 2° warming since the preindustrial era (32, 33).
Finally, a priority for environmental science remains the building and operating of ESMs with robust parameterizations, allowing trustworthy forward projections of carbon cycle evolution and assessment of the influence of fossil fuel burning on that cycle and associated implications for future climate change. Plant respiration, and any adjustment to that in response to global warming, places a strong control on earth’s carbon cycle and may modulate human influence on future atmospheric CO2 concentrations. The urgency to estimate climate change implies ESMs must be operated routinely, both now and in the future. Computational constraints, combined with limited available data, force a compromise in ESMs where numerical code “lumps” features of terrestrial ecosystems into low numbers of PFTs and relatively general parameterizations. Our study across a massive dataset of leaf R measurements, and subsequent testing and fitting to a model of T response, shows a remarkable level of invariance between geographical sites and biomes. This provides great encouragement that, for leaf R at least, the generality of ESMs can be viewed as a neutral, or perhaps, positive feature.
Methods
Field Sites and Species.
Details on the 18 field sites used in our study are provided in Supporting Information and Table S1, and a full list of all 231 species included in this study can be found, grouped by site and biome, in Dataset S1.
High-Resolution Measurements of the Temperature Response of Leaf Respiration.
At each field site, replicate branches of sunlit leaves were used to generate high-resolution R–T curves (see Supporting Information for details). In brief, whole replicate leaves from these branches, or shoot segments for conifers and small-leaved species, were placed in a T-controlled, well-mixed cuvette, and allowed to adapt to darkness for 30 min. Leaf cuvettes were T controlled via a thermostatically controlled circulating water bath as in O’Sullivan et al. (27) and Heskel et al. (34), or via a Peltier system (3010-GWK1 Gas-Exchange Chamber; Walz, Heinz Walz GmbH). After the 30-min dark adaption period, the cuvette chamber was cooled to 10 °C. Thereafter, the cuvette chamber was heated continuously at a rate of 1 °C min−1 until a maximum rate of respiration was reached (generally leaf T between 55 and 70 °C), although only data up to T = 45 °C was used in our model. The net release of CO2 from leaves was recorded at 30-s intervals. Postmeasurement, each replicate leaf was removed from the cuvette, placed in a drying oven at ∼60 °C for a minimum of 2 d, and weighed afterward, so that rates could be expressed on a dry-mass basis (nmol CO2 g−1 s−1).
Quantification of R–T curves and Model Comparison.
The 673 R–T curves collected by the methods described above required thorough quantification for comparison across replicates, species, sites, biomes, and plant functional types. For each replicate R–T response curves, we assessed the fits commonly applied R–T models, including: (i) an exponential model with a fixed-Q10 across the entire T range (though not specifically a fixed Q10 of 2, as is applied in some biosphere models of R); (ii) an Arrhenius model; (iii) a model of R responding to the UTD as defined by Gillooly et al. (15), which contains an activation energy parameter and uses Boltzmann’s constant; (iv) a model presented by Lloyd and Taylor (17) to describe the response of soil R to T that includes a temperature-sensitive activation energy; (v) a model that incorporates a variable-Q10 response across the T range as described by two parameters; and (vi) a simple second-order polynomial model. Equations for these models are shown in Supporting Information. To compare how these models fit to data, we fitted each of the aforementioned models to all replicate R–T response curves in JMP (Version 11; SAS Institute), with parameters calculation controlled by the minimal residuals produced from each individual fit for each model. In cases where model convergence was not possible via the curve-fitting software, those replicate curves were not included to calculate mean residuals for the model fit over all replicates. Further, to evaluate the impact of different measurement temperature span (i.e., 10–45 °C vs. 20–45 °C) on model fits, we compared fit coefficients across all replicate curves at different segmented intervals of the response curve (Table S2, Fig. S3, and Supporting Information). Using these data, we also compared model fit coefficients from the approximate 20 °C T range that best represents the climate of that species (the “ecologically relevant” T range; Table S3 and Supporting Information) to the fit coefficients calculated from all available data from the entire measurement T range.
Global Polynomial Model Calculation.
After polynomial curve fit analysis, each replicate curve could be defined by specific a, b, and c parameters. The mean value of replicates for individual species at given sites were calculated for a, b, and c, resulting in a total of 231 species-site means of these parameters used for our study. To create a “global model” of the T response of R, we calculated the mean of all 231 species-site mean values of the a, b, and c parameters.
Modeling Site-Based Leaf R with JULES.
For our 18 field sites, we incorporated our derived global T response (Eq. 3), with local values of RTref, into an offline version of Joint U.K. Land Environmental Simulator (JULES) to investigate the potential impacts of altered T sensitivity of R. JULES is the land-surface model of the U.K. Hadley Centre HadGEM (Hadley Centre Global Environment Model) family of global circulation models (28, 35). In its current form, JULES assumes that leaf R doubles for every 10 °C rise in T (i.e., Q10 = 2); other TBM frameworks have also assumed fixed Q10 [e.g., BIOME-BGC (36), PnET-CN (37) CLM4 (38), TEM (39)], or modified Q10 [e.g., BETHY (40)] functions. This is done using both the fixed Q10 and GPM formulations, and with JULES adopting the site-mean values leaf R at RTref = 25 °C derived from our short-term T response curves. The Q10 value is set as 2.0 for all 18 sites, and similarly for the GPM model, the b and c parameters are invariant, taking their cross-site means (Table 1 and Eq. 3).
Here we use a version of JULES driven with the WATCH (water and global change) Forcing Data ERA-interim (WFDEI) surface climatology (41) for each of the 18 sites and for the period 2010–2014 inclusive. Each site uses the WFDEI gridded data values from its 0.5′ × 0.5′ grid resolution nearest to site location; and in time is therefore a subset of the WFDEI data, presently covering 1979–2014. The DGVM (Dynamic Global Vegetation Model) component of JULES is kept switched off, and therefore known local values of leaf area index (LAI) are prescribed. Four JULES PFTs were adopted (broadleaf trees, needleleaf trees, shrubs, and C3 grasses/herbs). With the DGVM off, then the main difference between these PFTs is the inclusion of deciduous phenology (where observed, affecting the prescribed LAI), and slightly different response curves for stomatal opening.
Our runs are made for each site, weighed by known fractional covers of the four PFTs above (predominantly broadleaf trees). The actual JULES model diagnostic presented (Fig. 3) is the canopy-top-level R value [μmol CO2 (m−2 of leaf cover)−1 s−1), representing those fluxes that might be observed in fully sun-exposed leaves at the canopy crown, if fluxes from lower leaves were ignored.
Materials and Methods
Field Sites and Species.
Our 18 field sites (Table S1) cover extensive variation in climate and species diversity across four continents. The seven biomes represented across these sites are: arctic tundra (Tu), boreal forest (BF), temperate deciduous forest (TeDF), temperate woodland (TeW), temperate rainforest (TeRF), high-altitude tropical rainforest (TrRF_hi), and low-altitude tropical rainforest (TrRF_lw). At each site, a survey of representative woody tree and shrub (and in the Arctic tundra, herbaceous forb) species were selected for measurement. For comparison, these species were classified into the following broad plant functional groups that represent current classification groups in JULES: broadleaved deciduous temperate (BlDcTmp), broadleaved deciduous tropical (BlDcTrp), broadleaved evergreen temperate (BlEvTmp), broadleaved evergreen tropical (BlEvTrp), C3 herbaceous (C3H), needle-leaved evergreen (NlEv), and broadleaved evergreen shrubs (SEv). A full list of all 231 species included in this study can be found, grouped by site and biome, in Dataset S1.
High-Resolution Measurements of the Temperature Response of Leaf Respiration.
At each field site, replicate branches of sunlit leaves were cut from plant species and either recut under water or placed in plastic bags containing moistened paper towels to minimize desiccation. Postsampling, all branches were recut again and kept in a water-filled bucket; all measurements occurred on the same day as branch sampling. For individual measurements, whole replicate leaves from these branches, or ∼10-cm shoot segments for conifers and small-leaved species, were placed in a T-controlled, well-mixed cuvette, and allowed to adapt to darkness for 30 min. Leaf cuvettes were T controlled via a thermostatically controlled circulating water bath (model F32-HL; JULABO Labortechnik GmbH) as in O’Sullivan et al. (27) and Heskel et al. (34), or via a Peltier system (3010-GWK1 Gas-Exchange Chamber, Walz, Heinz Walz GmbH). O’Sullivan et al. (27) used the same approach to measurement of R–T curves, found no differences between attached and detached leaves, and to allow for higher replication and species sampling, detached leaves were used for this study.
The exiting air stream from the cuvette was fed to the “sample” gas line and infrared gas analyzer of a portable gas exchange system (LI-6400xt; Li-Cor Inc.), allowing for instantaneous, continuous rates of CO2 efflux from the darkened leaves across the measurement T range. Rates of net exchange were calculated by comparing the “sample,” cuvette-based rates to those of the “reference” gas line. [CO2] (set to the prevailing ambient concentration) and flow rate (700 μmol s−1) of the air entering the cuvette chamber were controlled by the LI-6400XT console flow meter and 6400–01 CO2 mixer. Before entering the cuvette chamber, air was routed through the LI-6400XT desiccant column to control relative humidity inside the chamber.
After the 30-min dark adaption period, the cuvette chamber was cooled to 10 °C. Thereafter, the cuvette chamber was heated continuously at a rate of 1 °C min−1 until a maximum rate of respiration was reached (generally leaf T between 55 and 70 °C), although only data up to T = 45 °C was used in our model. Throughout the warming period, leaf T was continuously measured with a small-gauge wire chromel-constantan thermocouple pressed to the lower leaf surface in the cuvette chamber and attached to a LI-6400 external thermocouple adaptor (LI6400-13, Li-Cor Inc.), allowing for leaf T to be recorded by the LI-6400XT portable gas exchange system. Over the 10–45 °C range, leaves typically heated at a rate of 1 °C min−1 (i.e., matching the rate at which air T increased); however, at higher leaf T, the rate at which leaf T increased often slowed, reflecting an increase in evaporative loss of water from leaf surfaces. The net release of CO2 from leaves, as determined from the instantaneous difference between sample and reference lines, was recorded at 30s intervals, allowing for ∼ two measurements of R per 1 °C increase in T, resulting in a continuous, high-resolution T response of R.
Postmeasurement, each replicate leaf was removed from the cuvette, placed in a drying oven at ∼60 °C for a minimum of 2 d, and weighed afterward, so that rates could be expressed on a dry-mass basis (nmol CO2 g−1 s−1). Because the measured replicate leaf often became highly desiccated to accurately measure leaf area, to determine area-based fluxes (μmol CO2 m−2 s−1), a leaf of similar size and shape and adjacent to the measured leaf was digitally scanned (or determined with a leaf area meter; LI-3100 LiCor Inc.), dried, and weighed. The resulting leaf mass per unit area (LMA) of this adjacent leaf could then be used to calculate the area of the measured leaf (assuming a similar LMA) and the area-based R fluxes.
Quantification of R–T Curves and Model Comparison.
The main objective of this study was to assess how leaf R responds to T experienced across their current environmental range within the growing season. For this reason, we limited the T range of replicate curves evaluated in this study to 10–45 °C. Though it is possible that T experienced by leaves may exceed this range, especially in arctic tundra and hot, arid woodland ecosystems, 10–45 °C approximately spans the mean T of the warmest quarter (i.e., warmest 3-mo period) for all sites presented in this study (Table S1).
Before analyzing T responses of R across biomes and plant functional types, we needed to determine which model would best describe the nuances of this response. Physiological model representations of plant respiratory T response can vary in their complexity and ability to account for observed biological patterns, such as decreases in the T sensitivity of R over increasing Ts. For example, Arrhenius and fixed-Q10 exponential equations, which are widely used in many TBMs (6, 7) and feature little or no T sensitivity of the R–T response across biologically relevant T ranges. Thus, these models, and the UTD model (15) (which provides a nearly identical response as the Arrhenius) tend to overpredict R rates at low and high Ts compared with observed R data (Dataset S1; Fig. 1). The Lloyd & Taylor (17) model contains a modified activation energy parameter to improve the representation of R in Arrhenius-based physiological models by allowing for a T-variable response. An R–T model presented by Tjoelker et al. integrates the T dependence of R more explicitly, which accounts for a predictable T-variable Q10 shared among species representing several diverse environments (16). To date, data available to rigorously test alternative empirical model fits were typically constrained by low resolution and a narrow range of measurement Ts, and were further limited by species sample sizes when testing for biomes and PFTs differences. Generally, the inclusion of a T-variable Q10 to model the T response of R substantially improves predicted estimates of R (Fig. S1) compared with models that do not include this parameter (i.e., Arrhenius, UTD, exponential fixed-Q10) and to models whose T-variable parameter effect is less pronounced (i.e., Lloyd & Taylor). Recent high-resolution T-response curves for a single species (27) were consistent with the general shape of the T-variable Q10 (5).
Exponential fixed-Q10.
where RTref is the rate of R at chosen reference T (Tref, in degrees Centigrade) and Q10 is a fixed value.
Arrhenius.
where RTref is the rate of R at chosen reference T (Tref, in K), Ea is an activation energy and r is the gas law constant, 8.314 J mol−1 K−1.
UTD.
where R0 is the rate of R at 273K (T0), Ei is an activation energy, and k is Boltzmann’s constant, 8.61733 × 10−5 eV K−1.
Lloyd & Taylor.
where R Tref is the rate of R at chosen reference T (Tref), Eo is an activation energy, and T0, which is a temperature between T and 0K.
Variable-Q10.
where R Tref is the rate of R at chosen reference T (Tref), and x and y are constants that describe the temperature dependence of Q10. Finally, the polynomial model (Eq. 1), where a, b, and c are fit coefficients from the second-order polynomial applied to ln-transformed R.
Over all of the replicates available, we assessed the mean residuals produced from each model at each T, from 10 to 45 °C (Fig. S2A). The Arrhenius model and UTD models produced identical fits, due to their similar structure and use of a single activation energy value; for this reason, we treat their response as identical for comparisons (Fig. S2A). We found a pronounced difference between models that included a T-dependent parameter or allowed for T sensitivity of the T response (variable-Q10, polynomial, and to a lesser degree Lloyd & Taylor), and models that did not (exponential fixed-Q10, Arrhenius/UTD), mainly in their ability to fit R at low Ts. Overall, the models that allowed for the most T sensitivity—the variable-Q10 and the polynomial—provided the lowest mean residuals considering all Ts. These results were also seen when fitting all models to the mean R response of individual biomes and plant functional type groups, as well as with the mean R response of all species. Between the variable-Q10 and polynomial models, the polynomial model is further removed from the dependence on the concept of Q10 formulation, which can be problematic in applying in larger biosphere models, and further, it does not rely on biologically based assumptions of activation energies. For these reasons we selected to use the polynomial model when comparing the global database of R–T response curves.
Thus, based on the results of model comparison between the commonly applied R–T model functions on all replicates, we confirmed results found in O’Sullivan et al. (27) that a second-order polynomial can best represent how R (here, log transformed) responds to T between 10 and 45 °C. The polynomial fit of the replicate T response curves (Eq. 1) provides three coefficients: a, the y axis intercept; b, the value of the slope when T = 0 °C; and c, which determines the decline in the slope (i.e., curvature) with increasing measuring T. Thus, each replicate fitted T-response curve provides a specific a, b, and c value.
Tests for Normality and Outlier Removal.
The total number of T response curves of R originally collected across all field campaigns was 787, though ∼40 measured replicate curves were not included in initial analysis due to measurement error caused by instability of the measurement equipment under hot conditions. Replicate measurements were removed from the remaining dataset before analysis when values of R at 25 °C (area and mass based), and values of Q10 at 25 and 10 °C were found to be greater or less than 2 times the interquartile range of all values (values were log transformed for normality when necessary). Following that filter for outliers, replicates where values of b and c exceeded more than 2 times the interquartile range of all remaining values were removed. The final dataset consisted of 673 replicate measured R temperature-response curves resulting in a total of 231 individual species-site means, which were used for data analysis.
Segmented Interval Analysis.
Our study aimed to compare the T response of R, measured at high resolution between 10 and 45 °C across species representing diverse ecosystems and plant forms and functional types. Collecting these response curves under field conditions can sometimes restrict the minimum T reached before curve measurement initiation due to limitations in the ability of the Peltier cooling system of the leaf cuvette to reach 10 °C, especially in hot climates. Although the rate of warming and reaching of high temperatures were not restricted by the field-site environmental conditions, the starting T was often ∼5 °C above 10 °C for measurements made at the hotter sites. For this reason, there is some variability in the low, starting T of replicate curves.
The variation in starting T values between curves posed a potential issue when comparing curves of different ranges (i.e., 10–45 °C, 17–45 °C, 24–45 °C, etc.), and their resulting a, b, and c parameters. To address this issue, we performed a segmented interval analysis, wherein each replicate curve was divided into 20 °C length segments (10–30 °C, 15–35 oC, 20–40 °C, and 25–45 °C) and a polynomial fit was applied to each segment (Fig. S3). The values of a, b, and c derived from each segmented interval where then compared with each other and the a, b, and c values derived from the original, full-length, nonsegmented curve that included the maximum amount of data (Table S2). A mixed-model analysis, which accounted for the unbalanced dataset and potential random effects of Biome and PFT, indicated that none of the parameter values derived from the distinct, 20 °C segments differed significantly from the parameter values from response curves that contained all data available (Table S2). Although there was some variation between distinct segmented intervals, the lack of significance between any segment and the full length curve supported our use of the full curves, as they provided the most information for a given replicate without compromising comparisons between curves of different lengths.
Ecologically Relevant Parameters.
In addition to the full measured T range (10–45 °C), we also calculated polynomial parameters a, b, and c, for an ecologically relevant T range—a 20 °C span centered around the mean T value of the warmest quarter at the sampling site, which represents an approximation of growing season T range. The parameters for the ecologically relevant T range (Table S3) follow similar patterns in variation among intercept values (a) as those calculated using the full T range, and maintain no difference in b and c between biome or PFT groups, suggesting the fundamental response curve shape is unaffected by measurement T range. Thus, despite differences among biomes and PFTs in the offset, the shared shape and curvature of the response of R to T, as defined by the b and c model parameters, did not differ significantly, whether over the full T range or the ecologically relevant T range (Tables 1 and S3).
Parameterizing JULES for Modeling Leaf R.
The JULES model is the land-surface description for the current U.K. Hadley Centre HadGEM family of global circulation models (28). Two key requirements placed on the model are to determine the split of surface available energy into sensible and latent heat fluxes, and to calculate terrestrial carbon cycling and thus the role of ecosystems in the changing global carbon cycle. The two calculations are coupled, as in one configuration JULES can operate with a dynamic global vegetation model (DGVM) component; top-down representation of interactive foliage and flora including dynamics (TRIFFID) (28, 44). Climatically induced changes to leaf components such as stomatal opening can alter net primary productivity, which in turn can feedback on energy partitioning via DGVM projections of altered LAI.
The JULES model is also available independent of a GCM, and as a fully offline description of terrestrial response. Our descriptions of leaf R could be modeled completely independent of any land-surface model, if leaf-level temperature is known throughout our years of interest. In general this quantity is unavailable, and so the main purpose of our JULES simulations is to generate leaf T values resulting from the WFDEI-based estimated mean screen-level meteorological conditions (41). Leaf level T is a diagnostic from the JULES solution to the surface energy balance, a consequence of solution to a form of the Penman–Monteith equation (45). This value will depend on parameters set, including LAI (28). In our configuration, as LAI is known at each site, this value is prescribed although it is allowed to change as the model is extrapolated to other seasons to capture phenology on leaf cover—hence the “dynamic” component of TRIFFID is overridden.
Further, in other applications of JULES, leaf R varies through the canopy, and then the energy balance will create different leaf T values through the canopy due to changing light levels. As we are interested in leaf-level response of fully sun exposed leaves, for this run we ignore intracanopy variability in the resulting R values. That is, a “tree” of LAI of unity, and with no self-shading. The exception to this is inclusion of phenology, where we normalized leaf R by LAI(t)/LAIM, where t is time, LAI(t) is modeled LAI based only on phenological changes, and LAIM is maximum, prescribed LAI.
Supplementary Material
Acknowledgments
This work was funded by the Australian Research Council Grants/Fellowships DP0986823, DP130101252, CE140100008, and FT0991448 (to O.K.A.), FT110100457 (to P.M.), and DP140103415 (to M.G.T.); Natural Environment Research Council (UK) Grant NE/F002149/1 (to P.M.); Award DE-FG02-07ER64456 from the US Department of Energy, Office of Science, Office of Biological and Environmental Research (to P.B.R.); and National Science Foundation International Polar Year Grant (to K.L.G.). A.M.-d.l.T., and C.H. acknowledge the Centre for Ecology and Hydrology (UK) National Capability fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520282113/-/DCSupplemental.
References
- 1.Canadell JG, et al. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc Natl Acad Sci USA. 2007;104(47):18866–18870. doi: 10.1073/pnas.0702737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. IPCC (2013) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds Stocker TF, et al. (Cambridge University Press, Cambridge, UK)
- 3.Atkin OK, Scheurwater I, Pons TL. Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. New Phytol. 2007;174(2):367–380. doi: 10.1111/j.1469-8137.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 4.Amthor JS. The McCree-de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later. Ann Bot (Lond) 2000;86(1):1–20. [Google Scholar]
- 5.Atkin OK, Tjoelker MG. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003;8(7):343–351. doi: 10.1016/S1360-1385(03)00136-5. [DOI] [PubMed] [Google Scholar]
- 6.King AW, Gunderson CA, Post WM, Weston DJ, Wullschleger SD. Atmosphere. plant respiration in a warmer world. Science. 2006;312(5773):536–537. doi: 10.1126/science.1114166. [DOI] [PubMed] [Google Scholar]
- 7.Valentini R, et al. Respiration as the main determinant of carbon balance in European forests. Nature. 2000;404(6780):861–865. doi: 10.1038/35009084. [DOI] [PubMed] [Google Scholar]
- 8.Huntingford C, et al. Simulated resilience of tropical rainforests to CO2-induced climate change. Nat Geosci. 2013;6(4):268–273. [Google Scholar]
- 9.Pappas C, Fatichi S, Leuzinger S, Wolf A, Burlando P. Sensitivity analysis of a process-based ecosystem model: Pinpointing parameterization and structural issues. J Geophys Res Biogeosci. 2013;118(2):505–528. [Google Scholar]
- 10.Booth BBB, et al. High sensitivity of future global warming to land carbon cycle processes. Environ Res Lett. 2012;7(2):024002. [Google Scholar]
- 11.Atkin OK, Meir P, Turnbull MH. Improving representation of leaf respiration in large-scale predictive climate-vegetation models. New Phytol. 2014;202(3):743–748. doi: 10.1111/nph.12686. [DOI] [PubMed] [Google Scholar]
- 12.Atkin OK, Bruhn D, Tjoelker MG. In: Response of Plant Respiration to Changes in Temperature: Mechanisms and Consequences of Variations in Q10; Values and Acclimation. Plant Respiration, Advances in Photosynthesis and Respiration. Lambers H, Ribas-Carbo M, editors. Vol 18. Springer, Dordrecht, The Netherlands; 2005. pp. 95–135. [Google Scholar]
- 13.Blackman FF, Matthaei GL. Experimental researches in vegetable assimilation and respiration. IV.–A quantitative study of carbon-dioxide assimilation and leaf-temperature in natural illumination. Proc R Soc Lond B. 1905;76(511):402–460. [Google Scholar]
- 14.Wager HG. On the respiration and carbon assimilation rates of some arctic plants as related to temperature. New Phytol. 1941;40(1):1–19. [Google Scholar]
- 15.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293(5538):2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- 16.Tjoelker MG, Oleksyn J, Reich PB. Modelling respiration of vegetation: Evidence for a general temperature-dependent Q10. Glob Change Biol. 2001;7(2):223–230. [Google Scholar]
- 17.Lloyd J, Taylor JA. On the temperature dependence of soil respiration. Funct Ecol. 1994;8:315–323. [Google Scholar]
- 18.Amthor JS. The role of maintenance respiration in plant growth. Plant Cell Environ. 1984;7(8):561–569. [Google Scholar]
- 19.Ryan MG. Effects of climate change on plant respiration. Ecol Appl. 1991;1(2):157–167. doi: 10.2307/1941808. [DOI] [PubMed] [Google Scholar]
- 20.James WO. Plant Respiration. Clarendon Press; Oxford: 1953. [Google Scholar]
- 21.Mahecha MD, et al. Global convergence in the temperature sensitivity of respiration at ecosystem level. Science. 2010;329(5993):838–840. doi: 10.1126/science.1189587. [DOI] [PubMed] [Google Scholar]
- 22.Zaragoza-Castells J, et al. Climate-dependent variations in leaf respiration in a dry-land, low productivity Mediterranean forest: The importance of acclimation in both high-light and shaded habitats. Funct Ecol. 2008;22(1):172–184. [Google Scholar]
- 23.Atkin OK, et al. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 2015;206(2):614–636. doi: 10.1111/nph.13253. [DOI] [PubMed] [Google Scholar]
- 24.Vanderwel MC, et al. Global convergence in leaf respiration from estimates of thermal acclimation across time and space. New Phytol. 2015;207(4):1026–1037. doi: 10.1111/nph.13417. [DOI] [PubMed] [Google Scholar]
- 25.Slot M, Kitajima K. General patterns of acclimation of leaf respiration to elevated temperatures across biomes and plant types. Oecologia. 2015;177(3):885–900. doi: 10.1007/s00442-014-3159-4. [DOI] [PubMed] [Google Scholar]
- 26.Wythers KR, Reich PB, Bradford JB. Incorporating temperature-sensitive Q10 and foliar respiration acclimation algorithms modifies modeled ecosystem responses to global change. J Geophys Res Biogeosci. 2013;118(1):77–90. [Google Scholar]
- 27.O’Sullivan OS, et al. High-resolution temperature responses of leaf respiration in snow gum (Eucalyptus pauciflora) reveal high-temperature limits to respiratory function. Plant Cell Environ. 2013;36(7):1268–1284. doi: 10.1111/pce.12057. [DOI] [PubMed] [Google Scholar]
- 28.Clark DB, et al. The Joint UK Land Environment Simulator (JULES), model description - Part 2: Carbon fluxes and vegetation dynamics. Geosci Mod Dev. 2011;4(3):701–722. [Google Scholar]
- 29.Reich PB, et al. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol Lett. 2008;11(8):793–801. doi: 10.1111/j.1461-0248.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 30.Reich PB, et al. Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: A test across biomes and functional groups. Oecol. 1998;114(4):471–482. doi: 10.1007/s004420050471. [DOI] [PubMed] [Google Scholar]
- 31.Smith NG, Dukes JS. Plant respiration and photosynthesis in global-scale models: Incorporating acclimation to temperature and CO2. Glob Change Biol. 2013;19(1):45–63. doi: 10.1111/j.1365-2486.2012.02797.x. [DOI] [PubMed] [Google Scholar]
- 32.Huntingford C, et al. The link between a global 2 °C warming threshold and emissions in years 2020, 2050 and beyond. Environ Res Lett. 2012;7:014039. [Google Scholar]
- 33.Rogelj J, et al. Emission pathways consistent with a 2°C global temperature limit. Nat Clim Chang. 2011;1(8):413–418. [Google Scholar]
- 34.Heskel MA, et al. Thermal acclimation of shoot respiration in an Arctic woody plant species subjected to 22 years of warming and altered nutrient supply. Glob Change Biol. 2014;20(8):2618–2630. doi: 10.1111/gcb.12544. [DOI] [PubMed] [Google Scholar]
- 35.Best MJ, et al. The Joint UK Land Environment Simulator (JULES), model description - Part 1: Energy and water fluxes. Geoscic Mod Dev. 2011;4(3):677–699. [Google Scholar]
- 36.Wang W, et al. A hierarchical analysis of terrestrial ecosystem model Biome-BGC: Equilibrium analysis and model calibration. Ecol Modell. 2009;220:2009–2023. [Google Scholar]
- 37.Ollinger SV, Aber JD, Reich PB, Freuder RJ. Interactive effects of nitrogen deposition, tropospheric ozone, elevated CO2 and land use history on the carbon dynamics of northern hardwood forests. Glob Change Biol. 2002;8(6):545–562. [Google Scholar]
- 38.Bonan GB, et al. Improving canopy processes in the Community Land Model version 4 (CLM4) using global flux fields empirically inferred from FLUXNET data. J Geophys Res Biogeosci. 2011;116(G2):G02014. [Google Scholar]
- 39.Melillo JM, et al. Global climate change and terrestrial net primary production. Nature. 1993;363(6426):234–240. [Google Scholar]
- 40.Ziehn T, Kattge J, Knorr W, Scholze M. Improving the predictability of global CO2 assimilation rates under climate change. Geophys Res Lett. 2011;38(10):L10404. [Google Scholar]
- 41.Weedon GP, et al. The WFDEI meteorological forcing data set: WATCH forcing data methodology applied to ERA-Interim reanalysis data. Water Resour Res. 2014;50(9):7505–7514. [Google Scholar]
- 42.Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature. 2000;408(6809):184–187. doi: 10.1038/35041539. [DOI] [PubMed] [Google Scholar]
- 43.Cox P. Description of the “TRIFFID” Dynamic Global Vegetation Model. Hadley Centre, Met Office; Bracknell, UK: 2001. pp. 1–16. [Google Scholar]
- 44.Huntingford C, Cox PM, Lenton TM. Contrasting responses of a simple terrestrial ecosystem model to global change. Ecol Modell. 2000;134:41–58. [Google Scholar]
- 45.Monteith JL. Evaporation and surface temperature. Q J R Meteorol Soc. 1981;107(451):1–27. [Google Scholar]
- 46.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Intern J Clim. 2005;25:1965–1978. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.