Abstract
A promising strategy in tumor immunotherapy is the use of activated dendritic cells as vehicles for tumor vaccines with the goal of activating anti-tumor T cell responses. Current formulations for dendritic cell-based immunotherapies have limited effects on patient survival, providing motivation for further investigation of ways to enhance dendritic cell priming of anti-tumor T cell responses. Using a brief in vitro priming model, we have found that B7-H1 expressed by activated dendritic cells is integrated during priming of naïve CD8+ T cells and functions to limit the differentiation of effector T cell responses. CD8+ T cells primed by B7-H1-deficient dendritic cells exhibit increased production of IFN-γ, enhanced target cell killing, and improved anti-tumor activity. Additionally, enhanced memory populations arise from CD8+ T cells primed by B7-H1-deficient dendritic cells. Based on these findings, we suggest that early blockade of B7-H1 signaling should be investigated as a strategy to improve dendritic cell-based anti-tumor immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1563-6) contains supplementary material, which is available to authorized users.
Keywords: T cell priming, Co-stimulatory signaling, Effector CD8+ T cells, Memory CD8+ T cells
Introduction
CD8+ T cell responses are initiated in secondary lymphoid tissues by signals that are encountered by naïve CD8+ T cells during a brief interaction with dendritic cells. Priming signals are translated into molecular changes that have long-term impacts on the resulting effector and memory CD8+ T cell populations, a process known as programming [1]. In the case of stimulatory dendritic cells, these priming signals include antigenic peptide: MHC complexes, co-stimulatory molecules, and pro-inflammatory cytokines. The outcome of CD8+ T cell programming is dependent on the particular combination of priming signals encountered by naïve CD8+ T cells [2]. Factors that are integrated into programming include the duration and strength of TCR signaling, the identity and abundance of cytokines present in the local milieu, and the presence of various positive and negative co-stimulatory molecules [3–5]. CD80 (B7-1) and CD86 (B7-2) are expressed at high levels by activated dendritic cells and signal through CD28 expressed by CD8+ T cells [6]. Signaling downstream of CD28 ligation leads to the recruitment of molecules that enhance TCR signaling [7, 8]. In addition to positive co-stimulatory molecules, dendritic cells also express negative co-stimulatory molecules, such as B7-H1 (PD-L1), in order to fine-tune the priming of naïve CD8+ T cells.
B7-H1, originally characterized by our laboratory in 1998, is a transmembrane protein expressed on the surface of multiple cell types [9]. B7-H1 is known to interact with both PD-1 and CD80 on CD8+ T cells, and signaling downstream of both receptors for B7-H1 has been shown in several models to negatively regulate TCR signaling [10–13]. Expression of B7-H1 by dendritic cells is up-regulated upon TLR-dependent activation, thus tempering the stimulatory capability of activated dendritic cells [14]. Much is known about the influence of B7-H1 signaling through PD-1 during the effector phase of an immune response for the induction of an exhausted phenotype in activated CD8+ T cells [15]. Relatively, fewer studies have focused on the role of B7-H1 signaling during the priming stage of CD8+ T cell responses.
The goal of many anti-tumor immunotherapy approaches is the generation of tumor antigen-specific effector and memory CD8+ T cell responses. Promising advances have been made recently in the development of dendritic cell-based anti-tumor immunotherapies, yet it is necessary to continue investigating means to improve patient outcomes [16]. In theory, an effective dendritic cell-based anti-tumor immunotherapy will be able to prime a robust anti-tumor CD8+ T cell response. In the process of conditioning dendritic cells to have stimulatory capacities for immunotherapy, expression of B7-H1 is likely induced, thus limiting the immunostimulatory capacity of the therapy. After taking into consideration the challenges of antigen selection and vaccine delivery, the limited success of patient outcomes in dendritic cell-based immunotherapy trials could be attributed in part to high expression of B7-H1 by the therapeutic dendritic cells. Thus, we sought to investigate B7-H1 signaling encountered during CD8+ T cell priming and the impact on resulting CD8+ T cell effector populations. In this study using a brief in vitro priming model for CD8+ T cell programming, we have demonstrated that B7-H1 signaling is integrated into priming signals that determine CD8+ T cell programming and influence the resulting CD8+ effector T cell population.
Materials and methods
Mice, cell lines, and reagents
Female wild-type (WT) CD45.2+ C57BL/6 (B6) mice were purchased from Taconic Farms (Germantown, NY, USA) and CD45.1+ congenic C57BL/6-Ly5.1 mice were purchased from National Cancer Institute. OT-1 TCR (Thy 1.1+) transgenic mice were provided by T. Tian (Harvard University, Boston, MA, USA). B7-H1 knockout (KO) C57BL/6 mice were provided by L. Chen (Yale University, New Haven, CT, USA). Act-mOVA transgenic mice were purchased from the Jackson Laboratory (Bar Harbor, MA, USA). B7-H1 KO mice were used to produce B7-H1 KO Act-mOVA transgenic mice in our animal facility. Mice were maintained under pathogen-free conditions and used at 8–12 weeks of age. B16-OVA murine melanoma cells were provided by R. Vile (Mayo Clinic, Rochester, MN, USA) and were cultured in RPMI 1640 medium (Cellgro, Hendon, VA, USA) with 10 % FBS (Life Technologies, Carlsbad, CA, USA), 1 U/ml penicillin, 1 μg/ml streptomycin, and 20 mM HEPES buffer (all from Mediatech, Manassas, VA, USA). Recombinant mouse GM-CSF and IL-4 were purchased from R&D Systems (Minneapolis, MN, USA). Hamster anti-mouse B7-H1 mAb (10B5) and PD-1 (G4) was obtained from hybridoma cells provided by L. Chen. Hamster anti-mouse B7-H1 mAb (43H12) was provided by K. Tamada (John Hopkins University, Baltimore, MD, USA). Studies were conducted in accordance with the National Institutes of Health guidelines for the proper use of animals in research and with local Institutional Animal Care and Use Committee approval.
Flow cytometry analysis
Fluorochrome-conjugated Abs against B7-H1, CXCR3, CD8, CD43, CD44, CD45.2, CD62L, CD80, CD86, CD90.1 (Thy 1.1), CD90.2 (Thy 1.2), CD107a, CD127, IFN-γ, KLRG-1, OVAp/Kb (25-D1.16), and PD-1 were purchased from BD Biosciences (Mountain View, CA, USA), BioLegend (San Diego, CA, USA), or eBiosciences (San Diego, CA, USA). To detect intracellular cytokine levels, cells were incubated with GolgiPlug (BD Biosciences) for 4 h prior to analysis. Cells were stained for surface antigens and then incubated in fixation buffer (BioLegend) for 20 min at room temperature, followed by permeabilization in permeabilization wash buffer (BioLegend). Fixed and permeabilized cells were then stained with Abs for 20 min at room temperature. Abs to Akt, Bim, Blimp-1, Eomes, and T-bet and fluorochrome-conjugated secondary Abs were purchased from Cell Signaling (Danvers, MA, USA). To detect the intracellular levels of Akt, Bim, Blimp-1, Eomes, and T-bet, T cells were first stained for surface antigens, then fixed with 2 % paraformaldehyde for 10 min at 37 °C, followed by permeabilization with ice-cold methanol for 30 min. After blocking with 15 % rat serum for 15 min, cells were stained with Abs for 1 h at room temperature. After staining, cells were washed three times with incubation buffer before analysis. At least 100,000 viable cells were live gated on FACScan or FACSCailbur (BD Biosciences, USA) instrumentation. Flow cytometry analysis was performed using FlowJo software (Tree Star, Ashland, OR, USA).
Bone marrow dendritic cell culture
Bone marrow (BM) was extracted from wild-type (WT) Act-mOVA or B7-H1 KO Act-mOVA mice, and red blood cells were removed. Cells were cultured in Petri dishes at 2 × 106/ml containing 10 ng/ml GM-CSF and 1 ng/ml of IL-4 (R&D systems) for 5–6 days. The resulting cell populations consisted of 50–80 % CD11c+ cells. Poly (I:C) (Sigma) was then added at 10 µg/ml for 24 h.
In vitro T cell priming by dendritic cells
CD8+ T cells were purified (EasySep CD8+ T cell negative selection kit, Stem Cell Technologies, Vancouver, British Columbia) from OT-1 mice and incubated with activated BM-dendritic cells isolated form WT Act-mOVA or B7-H1 KO Act-mOVA mice. After 20 or 4 h of co-culture, CD11c+ dendritic cells were removed from culture by a magnetized MACS column (Miltenyi Biotec). The primed CD8+ T cells were cultured in fresh medium in a new culture plate for additional 40 h before functional assay or transfer.
Analysis of primed CD8+ T cells and in vivo cytotoxic T lymphocyte (CTL) assay
Lymphocytes from lung or spleen were incubated in digestion buffer (RPMI medium containing 5 % fetal bovine serum, 0.02 % collagenase IV, 0.002 % DNase I and 10 U/ml of heparin) for 40 min before use in phenotypic and functional assays. Primed OT-1 CD8+ T cells were isolated and re-stimulated with OVA peptide (SIINFEKL, Sigma, 0.2 µg/ml) in the presence of 1 µl/ml of GolgiPlug™ (BD Bioscience) for 4 h ex vivo. Following incubation, cell surface staining was performed followed by fixation, permeabilization, and intracellular staining. For the in vivo CTL assay, OVA peptide-pulsed or control peptide-pulsed spleen cells (as target cells) from syngeneic mice were labeled with a high dose of CFSE (5 μM) or low dose of CFSE (0.5 μM), mixed at 1:1 (2.5 × 106 of each) before injection. Target cells were intravenously injected into immunized mice on day 4 after re-challenge with cognate antigen protein. The CTL activity was determined 4 h after target cell transfer. Specific lysis was calculated using the following formulas: ratio = (% CFSEhigh/% CFSElow), % specific lysis = [1 − (ratio primed/ratio unprimed)] × 100 %.
Tumor studies
Mice were inoculated intravenously with 5 × 105 B16-OVA tumor cells. On day 7 post-tumor injection mice were intravenously injected with primed OT-1 CD8+ T cells (1 × 105). On day 14 after T cell transfer, mice were sacrificed, and the number of tumor foci on the lung tissue was counted.
Statistical analysis
All statistical analyses were performed using GraphPad Prism software 5.0 (GraphPad Software, Inc., San Diego, CA, USA). A two-sided, unpaired or paired Student’s t test was used to assess statistical differences in experimental groups. A p value <0.05 was considered statistically significant.
Results
B7-H1 signaling during the priming phase influences the differentiation of effector CD8+ T cells in vitro
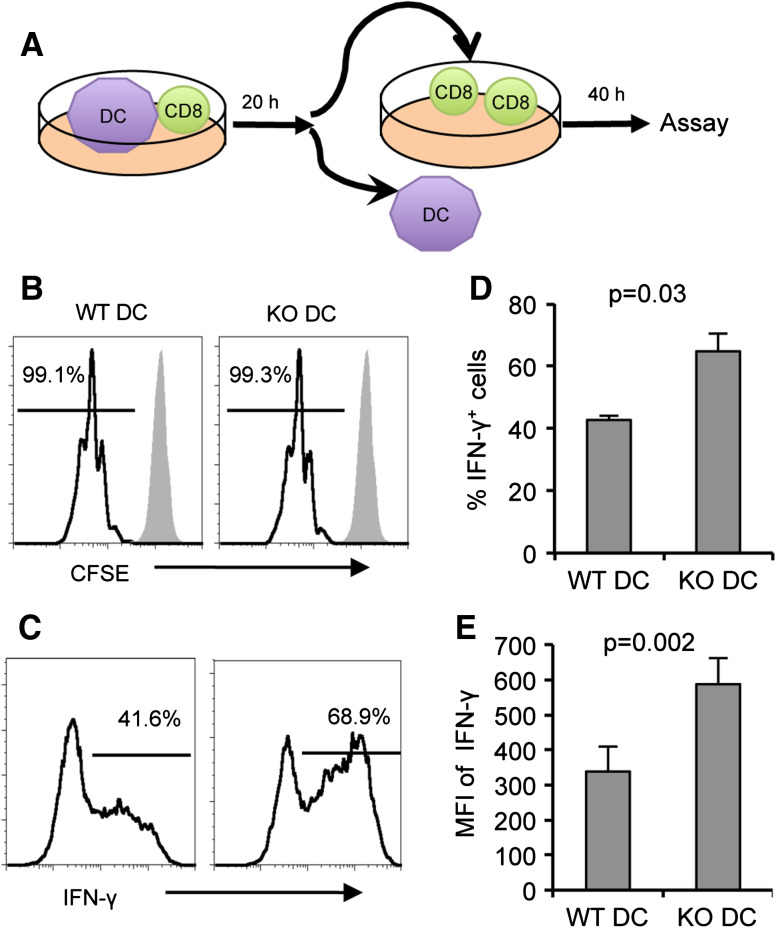
In previous studies, our laboratory has demonstrated that immunization with B7-H1-deficient dendritic cells or immunization with dendritic cells in combination with B7-H1 blockade induced strong CD8+ T cell responses capable of rejecting established tumors [14]. These results suggest that in the absence of B7-H1 signaling, dendritic cells are able to prime an enhanced CD8+ T cell response. We were interested in further investigating the influence of B7-H1 signaling on the priming of naïve CD8+ T cells by dendritic cells. Using a brief in vitro T cell priming model [17–19], we investigated the influence of B7-H1 expressed by dendritic cells on CD8+ T cell proliferation and acquisition of effector functions. CD8+ T cells were isolated from spleens of naïve OT-1 mice and co-cultured with poly I:C-activated bone marrow-derived dendritic cells from WT Act-mOVA or B7-H1 KO Act-mOVA transgenic mice (mice that express membrane-bound class I-restricted OVA on the surface of all nucleated cells) [20]. After 20 h of co-culture, the OT-1 CD8+ T cells were re-isolated from the Act-mOVA dendritic cells using a CD11c negative selection protocol, ensuring that all CD11c+ DCs were removed from the T cell population. The primed CD8+ T cells were then maintained in culture for an additional 40 h, followed by a brief re-stimulation with OVA peptide to assay proliferation and effector functions (Fig. 1a). The level of proliferation, as indicated by CFSE dilution, was similar for CD8+ T cells primed by WT Act-mOVA or B7-H1 KO Act-mOVA dendritic cells (Fig. 1b). However, an increased percentage of CD8+ T cells primed by B7-H1 KO dendritic cells produced IFN-γ in response to OVA peptide re-stimulation as compared to CD8+ T cells primed by WT dendritic cells (p < 0.05, Fig. 1c, d). CD8+ T cells primed by B7-H1 KO dendritic cells also produced more IFN-γ on a per-cell level as compared to CD8+ T cells primed by WT dendritic cells (p < 0.01, Fig. 1e). CD8+ T cells cultured under these conditions up-regulated surface expression of both receptors for B7-H1, PD-1 and CD80, within the 20 h time frame that the CD8+ T cells were co-cultured with the dendritic cells (Supplementary Fig. 1). We went on to test whether a shorter co-culture period would produce similar results. As shown in Supplementary Fig. 2B, after a 4 h co-culture period, OT-1 CD8+ T cells primed by either WT Act-mOVA or B7-H1 KO Act-mOVA dendritic cells underwent similar levels of proliferation, as indicated by CFSE dilution. CD8+ T cells primed by B7-H1 KO dendritic cells for 4 h produced more IFN-γ as compared to CD8+ T cells primed by WT dendritic cells (Supplementary Fig. 2C–E). Together, our data indicate that B7-H1 signaling is integrated into the programming of naïve CD8+ T cells by activated dendritic cells, influencing the acquisition of effector functions by primed CD8+ T cells.
Fig. 1.
CD8+ T cells programmed in vitro for 20 h with B7-H1 KO dendritic cells produce more IFN-γ. CD8+ T cells were purified from the spleens of naïve OT-1 mice and co-cultured for 20 h with activated dendritic cells derived from the bone marrow of WT or B7-H1 KO Act-mOVA mice. CD8+ T cells were then re-isolated from the dendritic cells and maintained in culture for 40 h. a Experimental design. b Proliferation (CFSE dilution) and c IFN-γ production by primed CD8+ T cells was assayed by flow cytometry after a 4 h re-stimulation with OVA peptide. Numbers are percentages. d Bar graphs show the average percent of IFN-γ+ CD8+ T cells and e levels (MFI) of IFN-γ production by CD8+ T cells (mean ± SD, n = 3). One of three independent experiments is shown
We next wanted to confirm that the results obtained using B7-H1 KO DC could be recapitulated by using antibodies that block B7-H1 signaling. Using the same brief in vitro priming model as described above, we co-cultured naïve CD8+ T cells from OT-1 mice with poly I:C-activated bone marrow-derived dendritic cells from WT Act-mOVA mice for 20 h. The co-culture was done in the presence of various blocking antibodies that either inhibit the interaction between B7-H1 and both PD-1 and CD80 (10B5 antibody), or selectively inhibit the interaction between B7-H1 and PD-1 (G4 antibody) or between B7-H1 and CD80 (43H12 antibody). As shown in Supplementary Fig. 3, there was no significant difference in the proliferation of CD8+ T cells primed by WT Act-mOVA DC in the presence of the various blocking antibodies. This result is in accordance with the data obtained by priming CD8+ T cells with WT or B7-H1 KO DC. We went on to look at IFN-γ production by CD8+ T cells primed by WT DC in the presence of the same blocking antibodies. As shown in Supplementary Fig. 4, when B7-H1 signaling is blocked during CD8+ T cell priming, the resulting CD8+ T cells produce increased levels of IFN-γ, in accordance with the data obtained by priming with B7-H1 KO DC. We found that IFN-γ production is enhanced when the interaction between B7-H1 and both PD-1 and CD80 is blocked and when the interaction between either B7-H1 and PD-1 or B7-H1 and CD80 is selectively blocked. We also investigated the expression levels of CD80 and CD86 by WT Act-mOVA and B7-H1 KO Act-mOVA activated DC, as shown in Supplementary Fig. 5. There are similar expressions of CD80 and CD86 as well as antigen presentation (OVAp/Kb, detected by 25-D1.16 antibody) between B7-H1 KO Act-mOVA DC and WT Act-mOVA DC. Thus, we believe that the differences in T cell priming are due to B7-H1 expressed by DCs.
CD8+ T cells primed by B7-H1 KO dendritic cells have increased CTL activity and anti-tumor function
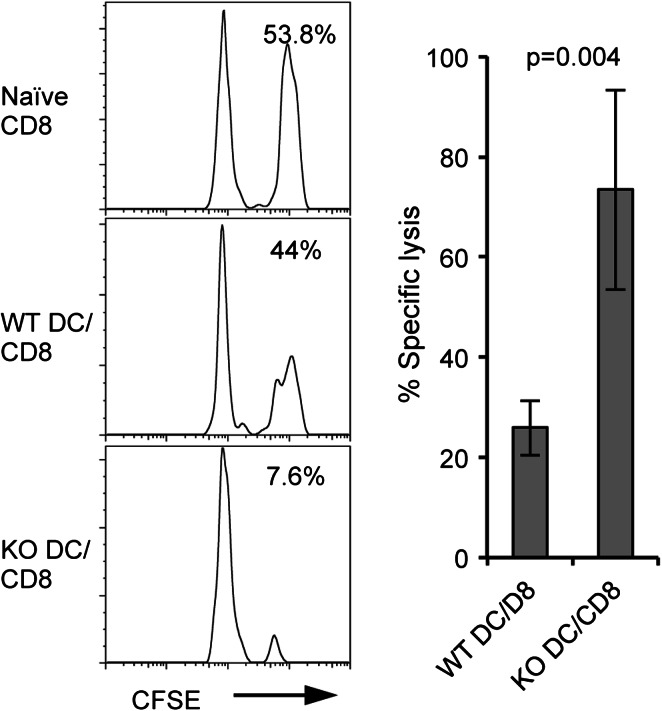
We next asked whether CD8+ T cells primed in our in vitro system could undergo further differentiation into effector cells in vivo. To test in vivo CTL function of the in vitro-primed CD8+ T cells, we transferred into WT B6 hosts 1 × 105 OT-1 CD8+ T cells that had been primed by WT Act-mOVA or B7-H1 KO Act-mOVA dendritic cells for 20 h in vitro. Five days after T cell transfer, we injected OVA peptide-pulsed (labeled with a high dose of CFSE) or control peptide-pulsed (labeled with a low dose of CFSE) B6 splenocytes into host mice as target cells for an in vivo cytotoxicity assay. Four hours after target cell injection, spleens of the host mice were harvested and analyzed for remaining CFSE-labeled target cells. As shown in Fig. 2, fewer CFSEhigh OVA peptide-pulsed target cells remained in the spleens of host mice that had received OT-1 CD8+ T cells primed by B7-H1 KO dendritic cells as compared to host mice that had received OT-1 CD8+ T cells primed by WT dendritic cells, indicating increased cytotoxicity of CD8+ T cells primed by B7-H1 KO dendritic cells as compared to WT dendritic cells (p < 0.01). This suggests that CD8+ T cells primed in vitro for 20 h, upon transfer into host mice, are able to differentiate into effector CTLs, and CD8+ T cells primed in vitro by B7-H1 KO dendritic cells as compared to those primed by WT dendritic cells differentiate into a more potent effector population.
Fig. 2.
CD8+ T cells primed in vitro by B7-H1 KO dendritic cells have increased CTL function in vivo. Naïve OT-1 CD8+ T cells were primed in vitro as described above. After the 20 h co-culture 1 × 105 primed CD8+ T cells were transferred i.v. into naïve B6 hosts. OVA peptide or control peptide-pulsed target cells (syngeneic splenocytes) were labeled with high or low dose CFSE (5 μM for OVA peptide-pulsed cells; 0.5 μM for control peptide-pulsed cells) and mixed 1:1 (2.5 × 106 of each) and injected i.v. into host mice on day 5 after transfer of primed CD8+ T cells. Histogram plot shows the percentage of remaining target cells in the spleen of host mice 4 h after target cell transfer. Bar graph shows percentage of specific lysis in the spleen (mean ± SD, n = 3)
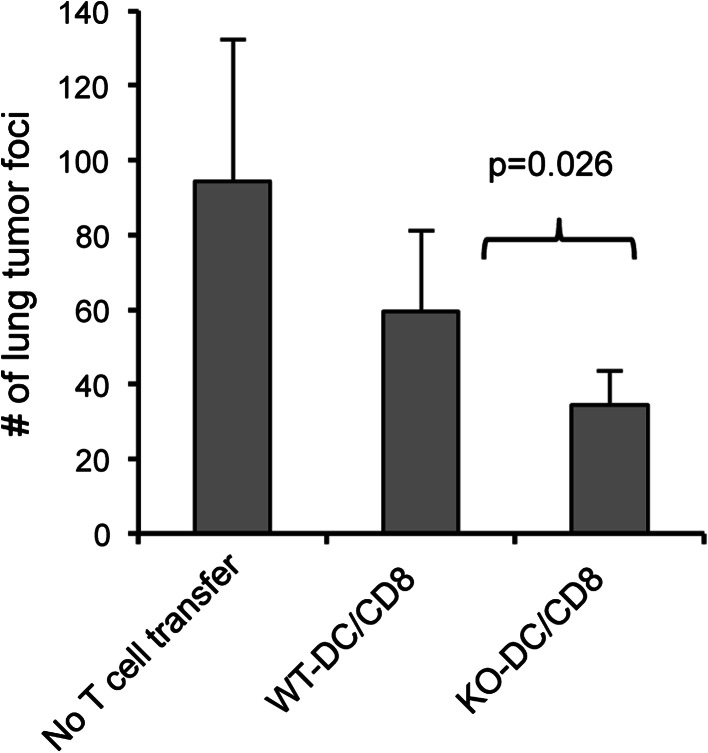
We went on to investigate whether the more potent effector population of CD8+ T cells primed by B7-H1 KO dendritic cells could mediate enhanced anti-tumor activity. WT B6 mice were injected intravenously with 1 × 106 B16-OVA tumor cells (engineered to express ovalbumin as a surrogate tumor antigen). Seven days after tumor cell injection, mice were treated with 1 × 105 OT-1 CD8+ T cells that had been primed in vitro for 20 h by WT Act-mOVA or B7-H1 KO Act-mOVA dendritic cells. Fourteen days after transfer of the primed OT-1 CD8+ T cells, tumor foci formation in the lung tissue was compared for mice treated with OT-1 CD8+ T cells primed by WT dendritic cells or B7-H1 KO dendritic cells. We found that OT-1 CD8+ T cells primed by B7-H1 KO dendritic cells were more protective against tumor foci formation than OT-1 CD8+ T cells primed by WT dendritic cells (p < 0.05, Fig. 3).
Fig. 3.
CD8+ T cells primed in vitro by B7-H1 KO dendritic cells have stronger anti-tumor activity. Naïve OT-1 CD8+ T cells were primed in vitro as described above. After the 20 h co-culture, 1 × 105 primed CD8+ T cells were injected into B6 mice that had received an i.v. injection of B16-OVA tumor cells (1 × 106) 7 days earlier. On day 21 after tumor cell injection or day 14 after T cell transfer, the number of tumor foci was determined by counting the surface metastases under a dissecting microscope. Bar graph shows the numbers of tumor foci (mean ± SD, n = 3) in the lung on day 21 after tumor i.v. injection. *p < 0.01 compared to control (no treatment)
Enhanced memory population arises from CD8+ T cells primed by B7-H1 KO dendritic cells
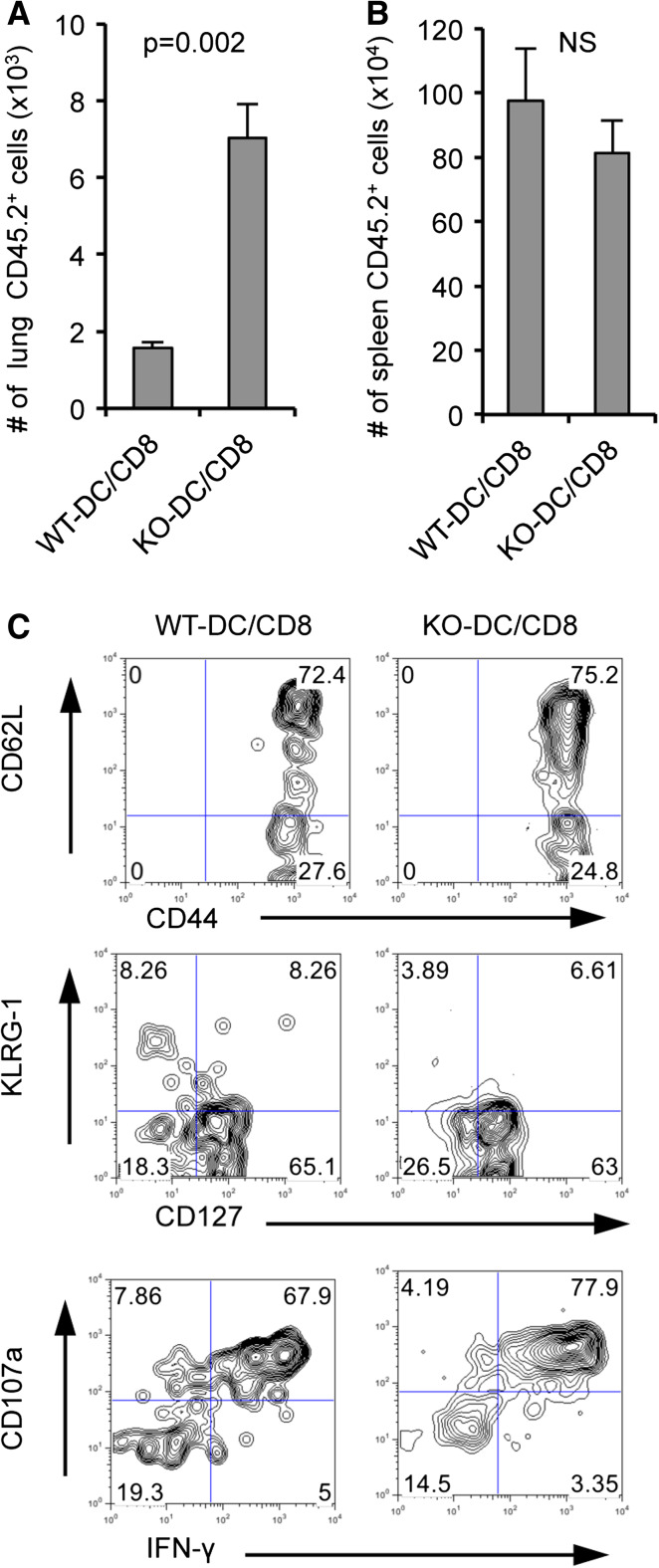
We next asked whether the integration of B7-H1 signaling during a brief 20 h priming period has any effect on the generation of memory CD8+ T cells. Naïve OT-1 CD8+ T cells (CD45.2+) were co-cultured in vitro with WT Act-mOVA or B7-H1 KO Act-mOVA dendritic cells for 20 h, then re-isolated, and transferred into naïve WT hosts (CD45.1+). On day 33 after T cell transfer, spleens and lungs were harvested and the remaining transferred cells were analyzed. In the lung tissue, there were a significantly higher number of CD8+ T cells primed by KO dendritic cells remaining after transfer as compared to the number CD8+ T cells primed by WT dendritic cells that were remaining (p < 0.01, Fig. 4a). In contrast, there were equivalent numbers of CD8+ T cells primed by WT or KO dendritic cells in the spleen on day 33 after cell transfer (p = 0.136, Fig. 4b). We investigated the phenotype and function of the in vitro-primed cells recovered from the host spleens by performing a brief ex vivo re-stimulation with OVA peptide. CD8+ T cells primed by either WT or B7-H1 KO dendritic cells exhibited a central memory phenotype (CD62L+, CD44+, CD127+, KLRG1−, Fig. 4c). CD8+ T cells primed by B7-H1 KO dendritic cells exhibited a slight increase in effector functions as compared to CD8+ T cells primed by WT dendritic cells, as indicated by CD107a expression (degranulation) and IFN-γ secretion (Fig. 4c). These data indicate that B7-H1 KO dendritic cells prime an enhanced memory CD8+ T cell population based on the increased number and peripheral tissue localization of CD8+ T cells primed by B7-H1 KO dendritic cells.
Fig. 4.
Enhanced memory population of CD8+ T cells primed in vitro by B7-H1 KO dendritic cells. Naïve OT-1 CD8 T cells (CD45.2+) were primed in vitro as described above. After the 20 h co-culture 1 × 105 primed CD8+ T cells were injected into naïve B6 hosts (CD45.1+). Host mice were killed on day 33 after T cell transfer. Bar graph shows absolute cell numbers of CD45.2+ cells in lung (a) and spleen (b) of host mice, determined by flow cytometry. c Splenocytes were re-stimulated ex vivo for 4 h with OVA peptide, followed by flow cytometry analysis of surface phenotype, degranulation (CD107a expression), and IFN-γ secretion (mean ± SD, three mice per group)
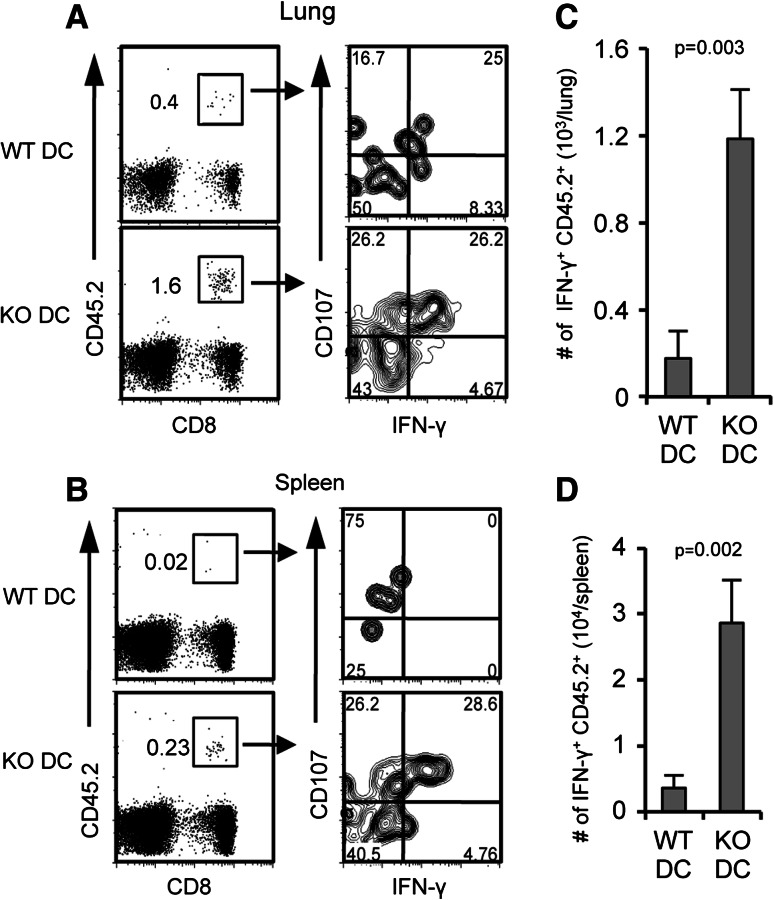
More memory CD8+ T cells generated in mice immunized with B7-H1 KO dendritic cells
Our results using an in vitro priming model indicate that B7-H1 signaling during a brief priming period has long-term impacts on the resulting memory CD8+ T cell population. We wanted to further characterize the impact of B7-H1 signaling received during priming on the generation of durable memory responses. To do so, naïve OT-1 CD8+ T cells (CD45.2+) were transferred into WT hosts, followed by transfer of WT Act-mOVA or B7-H1 KO Act-mOVA-activated dendritic cells to achieve in vivo priming of the transferred T cells. On day 30 after transfer of the activated dendritic cells, the CD45.2+ population of in vivo primed OT-1 CD8+ T cells was analyzed in the lung and spleen of host mice. In both lung and spleen, we detected an increased frequency of CD45.2+ CD8+ T cells in mice that received B7-H1 KO dendritic cells as compared to WT dendritic cells (Fig. 5a, b), corresponding to our in vitro priming data. These memory cells are functional as indicated by IFN-γ production and degranulation (CD107a expression) after a 4 h ex vivo re-stimulation with OVA peptide (Fig. 5a, b). The absolute cell numbers of CD45.2+ CD8+ T cells that produced IFN-γ was significantly increased in both lung and spleen of mice that received B7-H1 KO dendritic cells as compared to WT dendritic cells (Fig. 5c, d), again corresponding with our in vitro priming data. The phenotypes of the memory CD8+ T cells generated by WT or B7-H1 KO dendritic cells were comparable; they were all CD44high and 40–50 % were CD62Lhigh (data not shown).
Fig. 5.
More memory CD8+ T cells induced by B7-H1 KO dendritic cells. Naïve OT-1 CD8+ T cells (CD45.2+) were transferred into naïve B6 hosts, followed by immunization of host mice with 1 × 105 of activated WT or B7-H1 KO Act-mOVA dendritic cells. Numbers are percentages of functional memory CD45.2+ OT-1 CD8+ T cells in the lung (a) and spleen (b) on day 30 after dendritic cell immunization. Bar graphs show the numbers of IFN-γ+ CD45.2+ CD8+ T cells in the lung (c) and spleen (d) (mean ± SD, three mice per group)
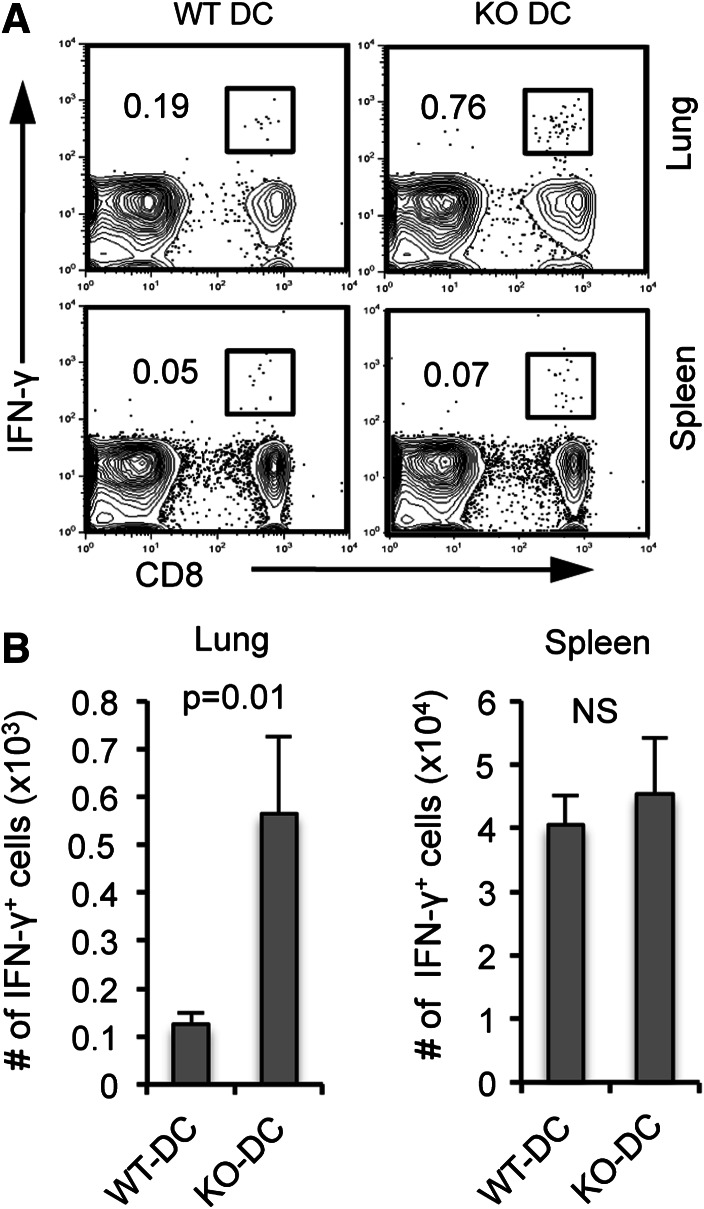
We found that CD8+ T cells primed in vitro by B7-H1 KO dendritic cells had enhanced anti-tumor activity (Fig. 3), so we next investigated the recall response to tumor antigen of in vivo primed memory CD8+ T cells. Mice were immunized with activated WT Act-mOVA or B7-H1 KO Act-mOVA dendritic cells, and on day 30, following immunization was challenged with B16-OVA tumor cells (intravenous injection). Four days after tumor cell injection, lymphocytes were isolated from lung and spleen of immunized mice and re-stimulated ex vivo with OVA peptide. As shown in Fig. 6, there was an increase in the frequency and number of IFN-γ-producing memory CD8+ T cells in the lungs of mice immunized with B7-H1 KO dendritic cells compared to WT dendritic cells (p < 0.01). We detected a comparable expansion of IFN-γ+ memory CD8+ T cells in the spleens of mice immunized with either WT or B7-H1 KO dendritic cells (Fig. 6a, b). Altogether, indicating that priming by B7-H1 KO dendritic cells leads to the generation of memory CD8+ T cells with preferred localization to peripheral tissues and enhanced recall responses to surrogate tumor antigens.
Fig. 6.
B7-H1 KO dendritic cell immunization leads to an increased recall response in the lung. B16-OVA tumor cells were injected (i.v.) into mice that have been immunized with poly I:C-activated WT or KO Act-mOVA dendritic cells (1 × 106, i.v.) 25 days earlier. On day 4 post-tumor cell injection, spleen and lung cells were isolated and analyzed for IFN-γ production by intracellular staining following a brief 4 h re-stimulation with OVA peptide. a Numbers are percentages of IFN-γ+ CD8+ T cells in the lung and spleen. b Bar graphs show the numbers of IFN-γ+ CD8+ T cells in the lung and spleen (mean ± SD, three mice per group)
Discussion
The concept of T cell programming predicts that a brief encounter with antigen-presenting cells triggers a cellular program leading to autonomous expansion and subsequent differentiation of T cells [1]. Our study demonstrates that during a brief interaction between dendritic cells and naïve CD8+ T cells, regulatory signaling from B7-H1 expressed by dendritic cells is integrated into T cell programming to restrain the differentiation of effector T cells. When naïve CD8+ T cells were primed by B7-H1 KO dendritic cells in vitro, they were able to acquire enhanced CTL activity and displayed increased anti-tumor activity as compared to effector cells primed by WT dendritic cells. In addition, the memory population arising from in vitro priming of naïve CD8+ T cells by B7-H1 KO dendritic cells was enhanced in both frequency and function as compared to the population resulting from priming by WT dendritic cells. Mice immunized with B7-H1 KO dendritic cells also exhibited enhanced anti-tumor memory CD8+ T cell responses as compared to mice immunized with WT dendritic cells.
In recent years, a series of studies have investigated the influence of B7-H1 on T cell activation. Using an in vitro activation model in which T cells and dendritic cells are co-cultured for 3–5 days, multiple investigators have reported that B7-H1 expressed by dendritic cells limits the proliferation and cytokine production of the activated T cells [21–23]. Goldberg et al. [24] demonstrated in vivo that B7-H1/PD-1 interactions regulate early-fate decisions of CD8+ T cells during the induction of tolerance by transferring naïve HA-transgenic CD8+ T cells into HA-expressing hosts, then analyzing the resulting CD8+ effector T cells on day 4 after transfer. Benedict et al. [25] demonstrated that CMV-infected dendritic cells have decreased expression of MHC and positive co-stimulatory molecules, but selectively maintain high expression of B7-H1. As a result of this phenotype, CMV-infected dendritic cells were unable to prime effective CD8+ T cell responses in a 70 h in vitro priming model; however, the addition of anti-B7-H1-blocking antibody to the co-cultures restored the stimulatory capacity of the infected dendritic cells, implicating a role for B7-H1 in limiting the priming of T cell responses. Our laboratory, using a dendritic cell immunization model, also reported a role for B7-H1 expressed by activated dendritic cells in limiting the expansion and proliferation of effector CD8+ T cells [14]. Our findings reported here are novel in that we used an in vitro priming model in which naïve CD8+ T cells are co-cultured with activated dendritic cells for only 20 h (Fig. 1). This model is based on a series of publications in which investigators determined that 20 h is the optimal time period that is required for CTL priming [17, 18]. According to those findings, co-culture periods of naïve T cells and activated dendritic cells lasting several days are not necessary to achieve full CTL activation. By using a brief 20 h in vitro co-culture, we can be confident that we are investigating the influence of B7-H1 signaling specifically during the priming phase of a CD8+ T cell response.
B7-H1 signaling is generally considered to be most important in restraining the effector functions of activated T cells in the periphery [13], but as described above, our group and others have characterized a role for B7-H1 signaling in restraining the activation of T cell responses. Importantly, we demonstrate here that the binding partners for B7-H1, PD-1 and CD80, are both expressed by CD8+ T cells within 4 h of initiating the co-culture of naïve CD8+ T cells with activated dendritic cells (Supplementary Fig. 1). We found that CD8+ T cells primed by B7-H1 KO dendritic cells exhibited enhanced effector functions, including increased IFN-γ production (Fig. 1), enhanced CTL killing (Fig. 2), and improved anti-tumor activity (Fig. 3). Thus, we conclude that B7-H1 signaling during a 20-h in vitro co-culture period limits the priming of effector CD8+ T cell responses. The linear differentiation model of memory generation proposes that memory CD8+ T cells arise from the effector cells that survive the contraction phase of an immune response [19]. According to this model, an enhanced effector CD8+ T cell population will lead to the formation of a superior memory CD8+ T cell population. In line with this idea, we found that priming naïve CD8+ T cells with B7-H1 KO dendritic cells led to the development of an improved memory population as demonstrated by an increase in the number and peripheral localization of memory cells arising from CD8+ T cells primed by B7-H1 KO dendritic cells (Figs. 4, 5). In addition, the memory population arising from CD8+ T cells primed by B7-H1 KO dendritic cells exhibited an enhanced recall response to antigen (Fig. 6).
We have yet to determine the mechanism by which B7-H1 signaling during priming limits the differentiation of effector CD8+ T cells. We found no differences in the expression levels of the transcription factors responsible for regulating effector and memory cell differentiation (T-bet, Eomes, and Blimp-1) in CD8+ T cells primed by WT or B7-H1 KO dendritic cells (data not shown). In another study, we have characterized a role for B7-H1 in inducing apoptosis in effector CD8+ T cells by inducing an increase in Bim protein levels [26]. Here, we found that B7-H1 signaling during the priming phase had no effect on Bim protein levels (data not shown). Since CD8+ T cell priming is influenced in part by cytokine signaling, we also investigated the cell surface expression levels of various cytokine receptors (CD25, CD127, CD122, CD212, and IFNAR) on CD8+ T cells primed by WT versus B7-H1 KO dendritic cells and found no differences (data not shown). Thus, further investigations are warranted to determine the mechanism by which B7-H1 signaling during the priming phase regulates CD8+ T cell effector differentiation.
A promising strategy for anti-tumor immunotherapy is the use of activated dendritic cells to prime anti-tumor CD8+ T cell responses. Clinical trials using this approach have proven successful, resulting in recent FDA approval for their use in the treatment of late-stage metastatic prostate cancer [16]. Current formulations for dendritic cell-based immunotherapies are capable of prolonging patient survival by only 4 months, providing motivation for further investigations of ways to enhance dendritic cell priming of anti-tumor immune responses. As B7-H1 is up-regulated during dendritic cell activation [14], the activated dendritic cells used for therapy likely express high levels of B7-H1. According to our data presented here, this B7-H1 expression could be responsible for limiting the differentiation of anti-tumor CD8+ T cells in response to dendritic cell-based therapies. Thus, we suggest that early blockade of B7-H1 signaling should be included in studies investigating the development of improved dendritic cell-based vaccination formulations.
In summary, our findings suggest that B7-H1 expressed by activated dendritic cells signals during the priming of naïve CD8+ T cells, resulting in negative regulation of the differentiation and acquisition of effector cell function, and subsequently limits the generation of effective memory CD8+ T cell populations. These results contribute to the understanding of how B7-H1 signaling influences various stages of CD8+ T cell responses, information that is clinically relevant in the design of anti-tumor immunotherapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are thankful to Tian Tian (Harvard University) for providing OT-1 Thy 1.1+ mice, Lieping Chen (Yale University) for providing B7-H1 KO mice, G4 hybridoma cells, and 10B5 hybridoma cells, Dr. Koji Tamada for anti-B7-H1 (43H12) monoclonal antibody, and Richard Vile (Mayo Clinic) for providing B16-OVA tumor cells. This work was supported by the American Cancer Society’s New Investigator Award, the Wendy Case Cancer Research Fund, and in part by the National Institutes of Health (RO1 CA134345).
Conflict of interest
Eugene. D. Kwon, Haidong Dong, and Mayo Clinic have a financial conflict of interest related to this research (Patents of B7-H1 as a cancer prognostic marker). This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic conflict of interest policies. The other authors have no financial conflicts of interest.
Abbreviations
- B6
C57BL6
- BM
Bone marrow
- CTL
Cytotoxic T lymphocyte
- KO
Knock out
- MFI
Mean fluorescence intensity
- WT
Wild type
Footnotes
Drs. Eugene D. Kwon and Haidong Dong share senior authorship.
References
- 1.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 3.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/S1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 4.Mescher MF, Curtsinger JM, Agarwal P, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen L. Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 6.Slavik JM, Hutchcroft JE, Bierer BE. CD28/CTLA-4 and CD80/CD86 families: signaling and function. Immunol Res. 1999;19:1–24. doi: 10.1007/BF02786473. [DOI] [PubMed] [Google Scholar]
- 7.Lanzavecchia A, Sallusto F. From synapses to immunological memory: the role of sustained T cell stimulation. Curr Opin Immunol. 2000;12:92–98. doi: 10.1016/S0952-7915(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 8.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 9.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 10.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong H, Strome SE, Salomao DR et al (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800. doi:10.1038/nm730 [DOI] [PubMed]
- 12.Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulko V, Liu X, Krco CJ, et al. TLR3-stimulated dendritic cells up-regulate B7-H1 expression and influence the magnitude of CD8 T cell responses to tumor vaccination. J Immunol. 2009;183:3634–3641. doi: 10.4049/jimmunol.0900974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 16.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 17.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 18.van Stipdonk MJB, Hardenberg G, Bijker MS, et al. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 19.Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol Rev. 2010;235:190–205. doi: 10.1111/j.0105-2896.2010.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 21.Brown JA, Dorfman DM, Ma F-R, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 22.Selenko-Gebauer N, Majdic O, Szekeres A, et al. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 23.Gröschel S, Piggott KD, Vaglio A, et al. TLR-mediated induction of negative regulatory ligands on dendritic cells. J Mol Med. 2008;86:443–455. doi: 10.1007/s00109-008-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg MV, Maris CH, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedict CA, Loewendorf A, Garcia Z, et al. Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J Immunol. 2008;180:4836–4847. doi: 10.4049/jimmunol.180.7.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbons RM, Liu X, Pulko V, et al. B7-H1 limits the entry of effector CD8+ T cells to the memory pool by upregulating Bim. Oncoimmunology. 2012;1(7):1061–1073. doi: 10.4161/onci.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.