Abstract
Background
People who inject drugs (PWID) constitute 10 million people globally with hepatitis C virus, including many opioid agonist treatment patients. Little data exist describing clinical outcomes for patients receiving HCV treatment with direct-acting antiviral agents (DAAs) in opioid agonist treatment settings.
Methods
In this retrospective observational study, we describe clinical outcomes for 50 genotype-1 patients receiving HCV treatment with triple therapy: telaprevir (n = 42) or boceprevir (n = 8) in combination with pegylated interferon and ribavirin on-site in an opioid agonist treatment program.
Results
Overall, 70% achieved an end of treatment response (ETR) and 62% achieved a sustained virological response (SVR). These treatment outcomes are nearly equivalent to previously published HCV outcomes shown in registration trials, despite high percentages of recent drug use prior to treatment (52%), ongoing drug use during treatment (45%) and psychiatric comorbidity (86%). Only 12% (n=6) discontinued antiviral treatment early for non-virological reasons. Four patients received a blood transfusion, and one discontinued telaprevir due to severe rash.
Conclusions
These data demonstrate that on-site HCV treatment with direct-acting antiviral agents is effective in opioid agonist treatment patients including patients who are actively using drugs. Future interferon-free regimens will likely be even more effective. Opioid agonist treatment programs represent an opportunity to safely and effectively treat chronic hepatitis C, and PWID should have unrestricted access to DAAs.
Keywords: HCV, PWID, PWUD, IDU, injection drug users, direct-acting antiviral agents
Introduction
Hepatitis C virus (HCV) infection affects over 10 million people worldwide and is a major cause of morbidity, mortality, and healthcare expenditure (Nelson et al, 2011; Wong et al, 2000). Although people who inject drugs (PWID) have high HCV infection rates and are likely to transmit HCV by sharing drug paraphernalia, few active or recent PWID have received treatment for HCV (Grebely, 2009; Iversen, 2014; Alavi, 2014). Some of the physician reluctance to treat HCV in PWID can be attributed to concerns about poor treatment adherence associated with ongoing substance abuse or comorbid psychiatric disorders, lack of urgency to address HCV, or pessimism regarding HCV treatment tolerability or effectiveness (Davis & Rodrigue, 2001; Edlin et al, 2001). Despite these concerns, several systematic reviews provide support for using interferon and ribavirin to treat HCV in patients with active substance abuse disorders (Aspinall et al, 2012; Dimova et al, 2013; Hellard et al, 2009). These studies show that PWID respond to HCV treatment as well as non-drug using patients.
All of these studies followed PWID who initiated treatment with pegylated interferon and ribavirin. First-generation direct-acting antiviral agents (telaprevir and boceprevir) when used in combination with pegylated interferon and ribavirin (triple therapy) are associated with significantly increased proportions of sustained virological response for both treatment-naïve and treatment-experienced patients in large registration trials (Bacon et al, 2011; Jacobson et al, 2011; Poordad et al, 2011; Zeuzem et al, 2011). However, triple HCV regimens are associated with increased dosing frequency (three times daily), pill burden (up to 20 pills daily), co-administration of specific dietary requirements (e.g. 20 grams fat with telaprevir), as well as additional significant additive and novel side effects (anemia, nausea, severe rash, anal discomfort, and dysgeusia). Indeed, there have been no published real-world reports of HCV treatment outcomes in people who use drugs or opioid agonist treatment patients treated with direct-acting antiviral agents. We have previously demonstrated high percentages of SVR (40% in genotype 1) in opioid agonist treatment patients initiating treatment with pegylated interferon and ribavirin at a comprehensive on-site opioid agonist treatment program. (Litwin et al, 2009). We now describe treatment outcomes of the first fifty patients initiating treatment with triple therapy on-site within the same opioid agonist treatment program.
Methods
Treatment setting
The Division of Substance Abuse of the Department of Psychiatry and Behavioral Sciences at Albert Einstein College of Medicine operates three large methadone maintenance treatment clinics in three Bronx communities, serving approximately 3200 adults with opioid dependence. In addition to comprehensive substance abuse treatment, clinics offer medical and psychiatric care to Medicaid-insured patients choosing on-site care. Approximately 65% of all patients are HCV-antibody positive, and 50% have chronic hepatitis C.
In October 1, 2010, New York State Departent of Health funded Albert Einstein College of Medicine to continue to provide on-site hepatitis C medical, care coordination, treatment and supportive services for hepatitis C mono-infected persons at two of our Einstein clinics (n=2000). HCV evaluation and treatment were provided by internists and physician assistants with expertise in both HCV and addiction medicine, using a standardized protocol. Patient with chronic hepatitis C were referred by medical and non-medical providers, HCV program staff (coordinator or health educator), and self-referred. HCV medical care was supervised by an experienced HCV provider (AL). Of the 2000 patients, 850 patients were eligible to receive medical care on-site due to having appropriate Medicaid managed care insurance coverage, and approximately 425 patients had chronic hepatitis C. Most patients with current psychiatric comorbidities were eligible for HCV treatment. Formal psychiatric criteria were used to determine treatment ineligibility (e.g. active suicidal ideation, any psychiatric condition significantly disrupting activities of daily living, and nonadherence to psychotropic medications). HIV/HCV coinfected patients also received on-site HIV-related primary care, including highly active antiretroviral treatment when appropriate.
Staging was performed either by Fibrosure or liver biopsy. The treatment program is explained in detail in an earlier publication (Litwin et al, 2005).
All patients were treated on-site at the opioid agonist treatment program and received weekly directly administered pegylated interferon injections. Many patients were treated within a group model of treatment (Stein et al, 2012), and some were treated with modified directly observed treatment (oral medications taken at the methadone window). All patients initiating treatment with telaprevir-based regimens were provided with monthly food bags containing fatty snacks (20 grams of fat).
HCV treatment eligibility criteria
Patients with active drug or alcohol use, HIV/HCV coinfection, current psychiatric illness, and compensated cirrhosis were all eligible for HCV treatment.
Treatment
Our standardized protocol for genotype-1 infected patients called for treatment with either telaprevir or boceprevir plus once-weekly pegylated interferon in combination with twice-daily ribavirin for either 24 or 48 weeks (Ghany et al, 2011). Genotype-1 infected patients received treatment with these regimens because it was endorsed by AASLD/IDSA, was the standard of HCV care in the United States, and covered by Medicaid. Pegylated interferon alfa-2a was dosed subcutaneously at 180 ucg weekly. The dose of ribavirin was weight-based and taken with food: 1000 mg if ≤75 kg or 1200 mg if > 75 kg. Patients received telaprevir for 12 weeks - 750 mg three times daily with high-fat food (20 grams of fat). After results of the Optimize trial were available, patients received telaprevir at a dose of 1125 mg twice daily (Buti et al, 2014). Patients starting boceprevir-based treatment received 800 mg threes time daily taken with food after a four week lead-in period of pegylated interferon alfa-2a in combination with ribavirin. Standard futility rules were applied, and patients without cirrhosis were eligible for response guided treatment if they achieved an extended rapid virological response (Ghany et al, 2011).
Key Definitions
Rapid virological response (RVR) is an undetectable viral load at week 4 after initiating either telaprevir or boceprevir.
Extended rapid virological response (eRVR) is an undetectable viral load at weeks 4 and 12 after initiating either telaprevir or boceprevir.
End of treatment response (ETR) is an undetectable viral load at the end of treatment.
Sustained virological response (SVR) is an undetectable viral load 24 weeks after completion of treatment.
Psychiatric diagnoses (depression, anxiety, psychosis, bipolar disorder, and post traumatic stress disorder) were determined by either psychiatrists or internists. Internists determined psychiatric diagnoses by structured interviews, screening tools (e.g. PHQ-9), and patient self-report. Psychiatrists determined psychiatric diagnoses by structured interviews.
Medical comorbidities included diabetes mellitus, hypertension, asthma, chronic obstructive pulmonary disease, renal disease, seizure disorder, thyroid disease, congestive heart failure, coronary artery disease, or HIV.
Recent drug use was defined as at least one positive monthly urine toxicologies (either opioids, cocaine, or benzodiazepines) in the 6 months preceding HCV treatment initiation. Patients with prescriptions in the chart for either opioids or benzodiazepines were not considered to be using drugs if toxicologies were positive for opioids and benzodiazepines, respectively.
Active drug use was defined as any positive urine toxicology within 1 month of HCV treatment initiation.
Drug use during treatment was defined as any positive urine toxicology during the period of HCV treatment.
Alcohol abuse or dependence was determined through review of most recent Addiction Severity Index-lite (administered by substance abuse counselor on intake and annually), problem list and most recent annual physical exam.
Tobacco use was determined through review of most recent annual physical exam.
Cirrhosis was defined by either liver biopsy (Ishak Stage 5 – 6) or Fibrosure (≥ 0.75).
Homelessness was determined through review of psychosocial assessment form (administered by substance abuse counselor annually).
Employment status was determined through review of psychosocial assessment form.
Adherence to both DAA (telaprevir or boceprevir) and ribavirin was assessed monthly through self-report using Visual Analogue Scale (e.g. “How much of your telaprevir or Incivek have you taken in the past 4 weeks?”) (Kalichman et al, 2009).
Data Collection and analysis
We conducted a retrospective review of medical charts using a standardized chart review instrument for all genotype-1 patients undergoing on-site HCV treatment with DAAs between January 21, 2011 and April 2, 2013. All charts were reviewed systematically including review of all laboratory data and monthly urine toxicologies. We hypothesized that the following factors may be associated with decreased proportions of SVR: IL28B (TC or TT vs. CC); cirrhosis (present vs. absent), prior treatment history (partial or non-responder vs. naïve or prior relapser); recent drug use (used within 6 months of treatment vs. used more than 6 months prior to treatment); depression (present vs. absent); anxiety (present vs. absent); drug use during treatment (any vs. none); and adherence to DAA (< 90% vs. ≥ 90%). Factors associated with SVR were identified using chi square and Fisher exact tests. Effect sizes were quantified using odds-ratio (OR). In separate multivariate models, we examined the associations of recent drug use and drug use during treatment with SVR, after adjusting for depression, IL28B, cirrhosis, and prior treatment history. This study was approved by the Albert Einstein College of Medicine Committee on Clinical Investigations.
Results
Fifty genotype-1 infected patients initiated antiviral treatment with DAAs for HCV between January 21, 2011 and April 2, 2013. Patient characteristics are summarized in Tables 1 and 2. Almost all (97%) had Medicaid-insurance, were minorities - Latinos (68%) and African Americans (28%), and had psychiatric illness (86%). The majority received telaprevir (84%) and 16% received boceprevir. Eleven patients had been previously treated unsuccessfully.
Table 1.
Pre-treatment Characteristics of 50 HCV-infected Patients Treated On-site with Triple Therapy at Opiate Agonist Treatment Program
| Characteristic | N (%) or Mean (± SD) |
|---|---|
|
|
|
| Sex | |
| Male | 31 (62) |
| Female | 19 (38) |
|
| |
| Race/Ethnicity | |
| Latino | 34 (68) |
| African American | 14 (28) |
| Caucasian | 2 (4) |
|
| |
| Age (years) | 49.4 ± 8.7 |
| ≥ 40 | 41 (82) |
| < 40 | 9 (18) |
|
| |
| Injection drug use | |
| Injection drug use | 45 (90) |
| No injection drug use | 5 (10) |
|
| |
| Opiate agonist treatment | |
| Methadone | 39 (78) |
| Buprenorphine | 7 (14) |
| None | 4 (8) |
|
| |
| Recent drug use | |
| Used within 6 months | 26 (52) |
| Used more than 6 months ago | 24 (48) |
|
| |
| Active drug use + | |
| Used within 1 month | 7/49 (14) |
| Used more than 1 month ago | 42/49 (86) |
|
| |
| Tobacco | |
| Current smoker | 41 (82) |
| Not current smoker | 9 (18) |
|
| |
| Psychiatric comorbidity | |
| Current psychiatric illness | 43 (86) |
| No current psychiatric illness | 7 (14) |
| Depression | |
| Current depression | 30 (60) |
| No current depression | 20 (40) |
| Anxiety disorder | |
| Current anxiety disorder | 17 (34) |
| No current anxiety disorder | 33 (66) |
| Psychotic disorder | |
| Current psychotic disorder | 4 (8) |
| No current psychotic disorder | 46 (92) |
| Bipolar disorder | |
| Current bipolar disorder | 10 (20) |
| No current bipolar disorder | 40 (80) |
|
| |
| Medical comorbidity | |
| Current medical cormbidity | 33 (66) |
| No medical comorbidity | 17 (34) |
| Hypertension | |
| Current hypertension | 21 (42) |
| No hypertension | 29 (58) |
| Diabetes | |
| Current diabetes | 13 (26) |
| No diabetes | 37 (74) |
| Asthma/COPD | |
| Current asthma/COPD | 11 (22) |
| No asthma/COPD | 39 (78) |
| HIV | |
| HIV+ | 3 (6) |
| HIV− | 47 (94) |
|
| |
| Body Mass Index (BMI) | 31.2 ± 6.2 |
| Normal (20 – 24.9) | 5 (10) |
| Overweight (25 – 29.9) | 19 (38) |
| Obese (≥ 30) | 26 (52) |
|
| |
| HCV viral load | |
| ≥ 800,000 IU/ml | 26 (52) |
| < 800,000 IU/ml | 24 (48) |
|
| |
| HCV subtype + | |
| 1a | 24 (75) |
| 1b | 8 (25) |
|
| |
| IL28B + | |
| CC | 11 (22) |
| TC or TT | 38 (78) |
|
| |
| Prior treatment + | |
| Naïve or prior relapser | 45 (92) |
| Partial or non-responder | 4 (8) |
|
| |
| Cirrhosis | |
| Cirrhosis | 18 (36) |
| No cirrhosis | 32 (64) |
|
| |
| Protease inhibitor | |
| Telaprevir | 42 (84) |
| Boceprevir | 8 (16) |
|
| |
| Dosing frequency | |
| Three times daily | 41 (82) |
| Two times daily | 9 (18) |
|
| |
| Employment + | |
| Employed | 7 (16) |
| Unemployed | 37 (84) |
|
| |
| Housing Status + | |
| Stable Housing | 34 (77) |
| Unstable Housing | 10 (23) |
missing data
Table 2.
On-treatment Characteristics of 50 HCV-infected Patients Treated On-site with Triple Therapy at Opiate Agonist Treatment Program
| Characteristic | N (%) or Mean (± SD) |
|---|---|
|
| |
| Active drug use during treatment + | |
| Used during treatment | 22/49 (45) |
| No use during treatment | 27/49 (55) |
|
| |
| Model of care | |
| Group treatment | 38 (76) |
| Individual treatment | 12 (24) |
|
| |
| Directly observed treatment | |
| Pegylated interferon only | 44 (88) |
| Pegylated interferon + oral meds | 6 (12) |
|
| |
| DAA Adherence+ | 90.1 ± 0.15 |
| < 90% | 13 (29) |
| ≥ 90% | 32 (71) |
|
| |
| Ribavirin Adherence+ | 91.8 ± 0.15 |
| < 90% | 10 (21) |
| ≥ 90% | 37 (79) |
missing data
Five patients discontinued treatment during the first 12 weeks due to virologic nonresponse, and six additional patients discontinued treatment due to non-virological reasons including depression (n=1), anxiety (n=1), debilitating fatigue (n=2), incarceration (n=1), and inability to care for sick family member (n=1). Fifty-four percent of patients required erythropoietin due to anemia, and 4 patients required a blood transfusion. One patient had a severe telaprevir-related rash during the second week of treatment, but achieved an SVR after completion with pegylated interferon and ribavirin alone.
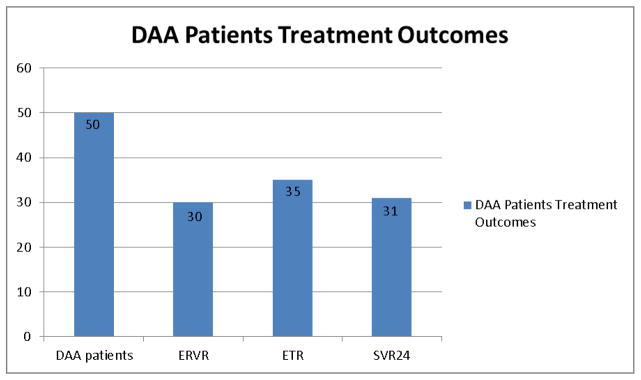
The majority (82%) of patients had a viral load less than 43 IU/ml at 4 weeks after initiation of DAA, and 68% had a rapid virological response (RVR). Sixty percent achieved an extended rapid virological response (eRVR), 70% an end of treatment response (ETR), and 62% a sustained virological response (SVR) (Figure 1). Patients with depression, anxiety disorder, and unfavorable IL28b genotype (TC or TT versus CC) were significantly less likely to achieve SVR (Table 3). Sixty-nine percent of patients without cirrhosis (22 out of 32) and 50% of patients with cirrhosis (9 out of 18) achieved SVR. Fifty-two percent of patients used drugs within six months of initiating antiviral treatment, and 45% used drugs during HCV treatment. There was no association between recent drug use and SVR, or between drug use during treatment and SVR (Table 3). We found similar results when examining the association between each specific type of drug (opioids, cocaine, or benzodiazepines) and SVR. During HCV treatment, 27% used opioids (13 out of 49), 27% cocaine (13 out of 49), and 16% benzodiazepenes (8 out of 49). In the multivariate analysis after adjusting for depression, IL28B, cirrhosis, and prior treatment history, neither recent drug use (p=0.204) nor drug use during treatment (p=0.646) was significantly associated with SVR. Two out of the four patients who were not taking opioid agonist treatment during antiviral treatment returned to methadone maintenance treatment during the follow-up period, and both achieved an SVR. Mean adherence was high to both DAA – telaprevir or boceprevir (mean=90.1%, SD=0.15) and ribavirin (mean=91.8%, SD=0.15). There was no association between low adherence (<90%) and SVR (Table 3). No patients died during treatment or during the 6-month follow-up period.
Figure 1.
Table 3.
SVR rates of 50 HCV-infected Patients Treated On-site with Triple Therapy at Opiate Agonist Treatment Program
| Characteristic | N (%) or Mean (± SD) | SVR (n=50) | OR (95% CI) for SVR |
|---|---|---|---|
|
| |||
| Recent drug use | |||
| Used within 6 months | 26 (52) | 15/26 (58) | Ref |
| Used more than 6 months ago | 24 (58) | 16/24 (67) | 1.47 (0.46 , 4.64) |
|
| |||
| Active drug use + | |||
| Used within 1 month | 7/49 (14) | 4/7 (57) | Ref |
| Used more than 1 month ago | 42/49 (86) | 26/42 (62) | 1.22 (0.24 , 6.17) |
|
| |||
| Depression | |||
| Current depression | 30 (60) | 15/30 (50)# | Ref |
| No current depression | 20 (40) | 16/20 (80)# | 4.00# (1.08 , 14.81) |
|
| |||
| Anxiety disorder | |||
| Current anxiety disorder | 17 (34) | 7/17 (41)# | Ref |
| No current anxiety disorder | 33 (66) | 24/33 (73)# | 3.81# (1.11 , 13.07) |
|
| |||
| IL28B + | |||
| TC or TT | 38 (78) | 20/38 (67)# | Ref |
| CC | 11 (22) | 10/11 (91)# | 9.00# (1.05 , 77.4) |
|
| |||
| Prior treatment + | |||
| Naïve or prior relapser | 4 (8) | 1/4 (25) | Ref |
| Partial or non-responder | 45 (92) | 29/45 (64) | 5.44 (0.52 , 56.7) |
|
| |||
| Cirrhosis | |||
| Cirrhosis | 18 (36) | 9/18 (50) | Ref |
| No cirrhosis | 32 (64) | 22/32 (69) | 1.96 (0.58 , 6.56) |
|
| |||
| Active drug use during treatment + | |||
| Used during treatment | 22/49 (45) | 13/22 (59) | Ref |
| No use during treatment | 27/49 (55) | 17/27 (63) | 1.18 (0.37 , 3.73) |
|
| |||
| DAA Adherence+ | 90.1 ± 0.15 | ||
| < 90% | 13 (29) | 8/13 (62) | Ref |
| ≥ 90% | 32 (71) | 21/32 (66) | 1.19 (0.31 , 4.53) |
missing data
Discussion
To our knowledge, this is the first comprehensive description of hepatitis C triple therapy treatment delivered on-site at an opioid agonist treatment program. Treatment outcomes were nearly equivalent to outcomes in HCV monoinfected patients treated in large registration trials (Bacon et al, 2011; Jacobson et al, 2011; Poordad et al, 2011; Zeuzem et al, 2011). These results are equivalent to real world results in a retrospective study from Hawaii - SVR=66%. (Akiyama et al, 2013), and higher than results from a multi-site study from New York City and Baltimore – SVR =44% (Martel-Laferriere et al, 2013). These results are equivalent to the national TARGET study that followed over 2000 patients treated with either telaprevir (SVR=60% without cirrhosis; 46% with cirrhosis) or boceprevir (SVR=50% without cirrhosis; 31% with cirrhosis). In our study, 69% of patients without cirrhosis and 50% of patients with cirrhosis achieved SVR. It’s noteworthy that these three other real-world studies did not report on patients who were actively using drugs. Our results demonstrate that PWID including those actively using drugs can be effectively treated for HCV with a model of co-located substance abuse and primary medical care. Integrated HCV care allows successful HCV treatment with complex regimens with high pill budren and significant side effects. It’s important to note that current depression and anxiety were associated with decreased proportions of SVR. It’s possible that the additional side effects related to protease inhibitors may have led to decreased effectiveness in these psychiatrically comorbid populations. Alternatively, more intensive psychiatric care may have led to improved HCV outcomes in these patients.
Opioid agonist treatment programs, which require regular contact with patients, may promote adherence to antiviral treatment. As in the correctional setting (Moorjani et al, 2015), methadone treatment programs may also facilitate directly administered once-weekly interferon injections. HCV treatment completion is associated with improved virological outcomes (McHutchison, 2002).
There are several limitations to our study. The study is from a single institution, retrospective, and has a modest sample size. There was also a potential for selection bias given the small percentage of infected patients treated, psychiatric diagnoses were not standardized, and adherence was determined by self-report instead of pill counts or electronic monitors. We were unable to distinguish between non-injecting and injecting drug use.
With the increased costs of antiviral therapy, payers within the United States have restricted access to DAAs among people who use drugs (Barua et al, 2015). The exclusion of PWID from treatment for HCV has unjustly shaped the new era of direct-acting antiviral therapies, despite significant evidence that PWID can be successfully treated with interferon-based regimens and endorsement of the HCV treatment of PWID by multiple national and international guidelines - AASLD/IDSA, EASL, INHSU, WHO – (Davis, 2015; EASL, 2015; Grebely, 2015; WHO, 2014). We can expect that interferon-free all-oral regimens that are available now (including sofosbuvir and ribavirin; sofosbuvir and simeprevir; sofosbuvir and ledipasvir; and ombitsavir, paritaprevir, ritonavir, and dasabuvir with or without ribavirin) will be similarly associated with high percentages of SVR in PWID and opioid agonist treatment patients. Our study provides strong evidence that treatment should not be withheld from PWID and opioid agonist treatment patients despite their exclusion from large registration trials and by payers.
Highlights.
HCV triple therapy delivered on-site at an opiate agonist treatment program
SVR rates nearly equivalent to outcomes of patients treated in registration trials
SVR rates equivalent to other real-world studies which did not enroll PWUDs
There was no association between illicit drug use and SVR
People who use drugs must have unrestricted access to direct-acting antiviral agents
Acknowledgments
The authors thank Sarah Church, Valerie Bartlett, Melissa Stein, Jordan Wong, and Division of Substance Abuse HCV Peer Educators for their expertise and/or support. We would also like to thank other Division providers, staff and patients who participated in this treatment program. The Division of Substance Abuse HCV program is funded by the New York State Department of Health AIDS Institute. This study was supported by NIH K23 DA022454 and R01 DA034086 awarded to the Albert Einstein College of Medicine of Yeshiva University from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama MJ, Piotrowski JI, Roytman MM, et al. New triple therapy for chronic hepatitis C: real life clinical experience in a community setting. Hawaii Journal of Medicine and Public Health. 2013;72(9) Suppl 4:6–13. [PMC free article] [PubMed] [Google Scholar]
- Alavi M, Raffa JD, Deans GD, et al. Continued low uptake of treatment for hepatitis C virus infection in a large community-based cohort of inner city residents. Liver International. 2014;34(8):1198–1206. doi: 10.1111/liv.12370. [DOI] [PubMed] [Google Scholar]
- Aspinall E, Corson S, Doyle J, Grebely J, Hutchinson SJ, Dore GJ, et al. Peginterferon and ribavirin treatment for chronic hepatitis C in people who inject drugs: a systematic review and meta-analysis. Clin Infect Dis. 2012 doi: 10.1093/cid/cit306. [DOI] [PubMed] [Google Scholar]
- Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua S, Greenwald R, Yekta S, Swan T, Taylor L. Sofosbuvir and Medicaid: getting it right. JAMA. 2015 [Google Scholar]
- Buti M, Agarwal K, Horsmans Y, et al. Telaprevir twice daily is noninferior to telaprevir every 8 hours for patients with chronic hepatitis C. Gastro. 2014;146(3):744–753. doi: 10.1053/j.gastro.2013.11.047. [DOI] [PubMed] [Google Scholar]
- Davis GL, Rodrigue JR. Treatment of chronic hepatitis C in active drug users. New England Journal of Medicine. 2001;345:215–217. doi: 10.1056/NEJM200107193450312. [DOI] [PubMed] [Google Scholar]
- Davis G, et al. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015 doi: 10.1002/hep.27950. in press. [DOI] [PubMed] [Google Scholar]
- Dimova RB, Zeremski M, Jacobson IM, Hagan H, Des Jarlais DC, Talal AH. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis. 2013;56(6):806–816. doi: 10.1093/cid/cis1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Edlin BR, Seal KH, Lorvick J, et al. Is it justifiable to withhold treatment from hepatitis C from illicit drug-users? N Engl J Med. 2001;345:211–214. doi: 10.1056/NEJM200107193450311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(4):433–444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Raffa JD, Lai C. Low uptake of treatment of hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepatitis. 2009;16:352–358. doi: 10.1111/j.1365-2893.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- Grebely J, Rabaeys G, Bruggmann P. Recommendations for the management of hepatitis C virus infection among people who inject drugs. International J Drug Policy. 2015 doi: 10.1016/j.drugpo.2015.07.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis. 2009;49(4):561–573. doi: 10.1086/600304. [DOI] [PubMed] [Google Scholar]
- Iversen J, Grebely J, Topp L, Wand H, Dore G, Maher L. Uptake of hepatitis C treatment among people who inject drugs attending Needle and Syringe Programs in Australia 1999–2011. J Viral Hepatitis. 21(3):198–207. doi: 10.1111/jvh.12129. [DOI] [PubMed] [Google Scholar]
- Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C infection. N Engl J Med. 2011;364(25):2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Amaral CM, Swetzes C, et al. A simple single-item rating scale to measure medication adherence: further evidence for convergent validity. J Int Assoc Physicians AIDS Care. 2009;8(6):367–74. doi: 10.1177/1545109709352884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin AH, Soloway I, Gourevitch MN. Integrating services for injection drug users infected with hepatitis C virus with methadone maintenance treatment: challenges and opportunities. Clinical Infectious Diseases. 2005;40:S339–345. doi: 10.1086/427450. [DOI] [PubMed] [Google Scholar]
- Litwin AH, Harris KA, Jr, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Substance Abuse Treatment. 2009;37(1):32–40. doi: 10.1016/j.jsat.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Laferriere V, Brinkley S, Bichoupan K, et al. On-treatment and sustained virologic response rates of telaprevir-based HCV treatments do not differ between HIV/HCV co-infected and HCV mono-infected patients. Program and abstracts of the International Conference on Viral Hepatitis; 2013; March 25–26, 2013; New York, New York. p. Abstract 31. http://iapac.org/icvh/presentations/ICVH2013_OA31.pdf. [Google Scholar]
- McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1 infected patients with chronic hepatitis C. Gastroenterology. 2002;123(4):1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- Moorjani H, Koenigsmann C, Kim MJ, Spaulding AC. Prisoners treated for hepatitis C with protease inhibitor. Emerg Infect Dis. 2015 doi: 10.3201/eid2101.141025. [cited February 17, 2015] http://dx.doi.org/10.3201/eid2101.141025. [DOI] [PMC free article] [PubMed]
- Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH. Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva, Switzerland: World Health Organization; 2014. [PubMed] [Google Scholar]
- Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaeys G, Grebely J, Mauss S, Bruggmann P, Moussalli J, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Clin Infect Dis. 2013;57(Suppl 2):S129–137. doi: 10.1093/cid/cit302. [DOI] [PubMed] [Google Scholar]
- Stein MR, Soloway IJ, Jefferson KS, Roose RJ, Arnsten JH, Litwin AH. Concurrent group treatment for hepatitis C: implementation and outcomes in a methadone maintenance treatment program. Journal of Substance Abuse Treatment. 2012;43(4):424–432. doi: 10.1016/j.jsat.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling RK, Kuo A, Rustgi VK. Virological outcomes and treatment algorithms utilization in observational study of patients with chronic hepatitis C treated with boceprevir and telaprevir. Alimentary Pharmacology and Therapeutics. 2015 doi: 10.1111/apt.13095. [cited February 17, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. 2014 [PubMed] [Google Scholar]
- Wong JB, McQuillan GM, McHutchison JG, et al. Estimating future hepatitis C morbidity, mortality, and cost in the United States. AJPH. 2000;90(10):1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S, Andreone P, Pol S, Lawitz E, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364(25):2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]