Abstract
We examined the moderating effect of personality on the association between leisure activities and executive control in healthy community-dwelling older adults. We found two distinct personality typologies: individuals with a Resilient personality were characterized by emotional stability and self-confidence; whereas, those who resembled an Overcontrolled personality tended to be introverted, but also low on neuroticism. Resilient individuals were more likely than Overcontrolled individuals to demonstrate higher executive function and attention as a result of participation in mental activities. These results suggest that personality might be important to include in studies that test the efficacy of activity interventions for improving cognition.
Keywords: Personality, Leisure Activity, Cognition
Introduction
Even among healthy individuals, cognitive function is known to decline with age (Park, O’Connell, & Thomson, 2003). Although there is great inter-individual variability in patterns of cognitive decline, domains related to executive control, the cognitive processes needed for planning and performance, are often among the first abilities to demonstrate decline (Park & Reuter-Lorenz, 2009; Salthouse, 2010). Accumulated evidence suggests that even subtle changes in these abilities can lead to dependence in basic and instrumental activities of daily living (Brown, Devanand, Liu, & Caccappolo, 2011; Marshall et al., 2011; Vaughan & Giovanello, 2010). There are also data demonstrating the huge public health and cost implications of cognitive decline among older adults (e.g., Gaugler et al., 2013; Zhu et al., 2013).
Participation in mental (Stern & Munn, 2010; Valenzuela & Sachdev, 2006; Wilson et al., 2013), physical (Ratey & Loehr, 2011), and social activities (Fratiglioni, Paillard-Borg, & Winblad, 2004; Seeman & Crimmins, 2001) have demonstrated effectiveness in slowing cognitive decline in older adults, particularly in the area of executive control (Saczynski et al., 2008). However, there are vast differences in leisure activity participation among older adults, and a number of factors are likely to contribute to these differences. In this exploratory study, we examine the moderating effects of one such factor, personality, on the association between participation in leisure activities and executive control in a sample of cognitively intact community-dwelling older adults. Identifying individuals who may benefit most from leisure activities will support the future design and targeting of activity interventions for optimal effect in maintaining independence.
Review of Literature
Executive Control
Executive control represents the ability to control cognitive processes necessary for the execution of complex activities and performance of self-regulation behaviors (Royall et al., 2002). This ability is dependent upon three cognitive domains: executive function, attention, and speed of processing. Executive function involves the processes necessary for goal-oriented activity including planning, initiation, sequencing, and monitoring. Attention is a prerequisite for executive function and includes the mental states and operations needed to detect stimuli, select stimuli over ‘noise,’ and manage resources for the detection and processing of competing stimuli (Robertson, 1996). Speed of processing is the efficiency in understanding and manipulating information processed during goal-oriented activity (Lin, Chen, Vance, & Mapstone, 2013).
Leisure Activities and Cognitive Function
An engaged lifestyle consisting of physical, social, and mentally stimulating leisure activity is associated with better cognitive functioning well into adulthood (Hertzog, Kramer, Wilson, & Lindenberger, 2009). Physical activity, and aerobic exercise in particular (Barnes, Yaffe, Satariano, & Tager, 2003), improves cognitive function overall and executive control specifically. The cognitive benefits of mentally stimulating activities (Wilson et al., 2003; Wilson et al., 2013), and meaningful social engagement (Barnes, Mendes de Leon, Wilson, Bienias, & Evans, 2004; Fratiglioni et al., 2004) are evident when participation in these leisure activities is a lifetime pattern as well as when they are initiated for the first time in late life (Leung et al., 2011). The potentially protective effects of late-life leisure activity may provide an opportunity to improve executive control and promote independence among older adults. In our previous work, routine mentally stimulating activities (e.g., doing crossword puzzles, playing computer games) significantly alleviated the impact of cardiac risk factors on executive function (Lin, Heffner, Mapstone, Chen, & Porsteisson, 2013), while regular physical activity moderated the effect of cardiac risk factors on episodic memory (Lin, Friedman, Quinn, Chen, & Mapstone, 2012). An important next step is to identify individuals who are most responsive to the executive control effects of leisure activity so that these interventions can be targeted more precisely.
Leisure Activities and Personality
Personality is an individual’s relatively stable intrinsic structure of thinking, feeling, and behaving (Roberts & Mroczek, 2008). The predominant framework for the study of personality is the Five Factor Model (FFM) which includes five distinct personality traits: neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness (Costa, 1991). There is a large and long-standing body of literature that underscores the utility of these traits for predicting important health behaviors and outcomes such as leisure engagement and cognitive performance. However, these relationships are likely complex and much is yet to be understood regarding the role of personality in activity participation. For example, some evidence suggests high neuroticism predicts low participation in leisure activities (Furnham, 1990; Kirkcaldy & Furnham, 1991), while other research has found no significant relationship between neuroticism and leisure activity (Lu & Hu, 2005; Sale, Guppy, & El-Sayed, 2000). Consistent with this latter point, Parisi (2007) found that although level of neuroticism did not predict extent of participation, individuals higher in neuroticism reported lower levels of competence and enjoyment when participating in daily activities. Openness to experience, agreeableness, and conscientiousness are also predictive of participation in specific types of leisure activities (Jopp & Hertzog, 2010), such as the performing arts (Barnett, 2006).
Personality and Cognitive Function
Individual personality traits have also been associated with cognitive performance in older adults. Higher neuroticism has been associated with lower overall cognitive function and an increased rate of decline over time (Chapman et al., 2012; Hock et al., 2013). The cognitive protective effects of higher conscientiousness have been found in several studies (Chapman et al., 2012; Hock et al., 2013; Wilson, Schneider, Arnold, Bienias, & Bennett, 2007), including beneficial effects for the cognitive domain of executive function (Booth et al., 2006; Schaie, Willis, & Caskie, 2004).
The Moderating Effect of Personality
Individuals select or are drawn toward certain environments, situations, and relationships based, at least in part, on personality tendencies (Buss, 1987; Caspi, Roberts, & Shiner, 2005; Friedman, 2000, 2008). Furthermore, personality not only influences the selection of situations which influence health, but also reactions to these situations and resultant health-related outcomes (e.g., Taylor, 2007). Multiple studies have demonstrated the moderating influence of personality on health, i.e., the effect of personality traits on the direction or magnitude of relationships between predictors and outcomes (Baron & Kenny, 1986). For example, the FFM has been investigated for the moderating role of personality traits on the relationship between medical burden and health-related quality of life in older adults (Chapman, Duberstein, & Lyness, 2007). Primary care patients aged 65 or older completed assessments of cumulative illness, health-related quality of life, mental disorders, IADLs, physical self-maintenance, and personality. Higher neuroticism moderated the relationship between medical burden and impairment in ADLs (p=.001) and decreased health-related quality of life (p< .001). Our previous work among older adults at high risk for cognitive decline found that individuals who were less extraverted were more likely to have improved executive function as a result of a personally-tailored cognitively stimulating activity intervention delivered in one-on-one sessions (Hill, Kolanowski, Fick, Chinchilli, & Jablonski, 2014). Although personality has a demonstrated influence on health and treatment outcomes across populations and conditions, as well as a moderating effect on health outcomes including cognition, much is yet unknown regarding its influence on the relationship between activity participation and cognitive performance. This study addresses one of these gaps: the moderating effect of personality on the relationship between leisure activity and executive control.
Personality Types
Recently, there has been interest in using a “person-centered” approach in contrast to the earlier “variable-centered” approach as an appropriate level for assessing the effect of personality on health outcomes (Cloninger, 2013). This perspective focuses on the configuration of personality traits within the person and defines distinct, but common, “personality types.” It is meant to complement the variable approach by identifying aspects of heterogeneity that are different across subpopulations. Characterizing individuals according to personality types has gained wider acceptance in the field of personality research and has demonstrated utility as a conceptual framework for examining health-related outcomes across populations (e.g., Chapman & Goldberg, 2011; Howard & Hughes, 2012; Sava & Popa, 2011).
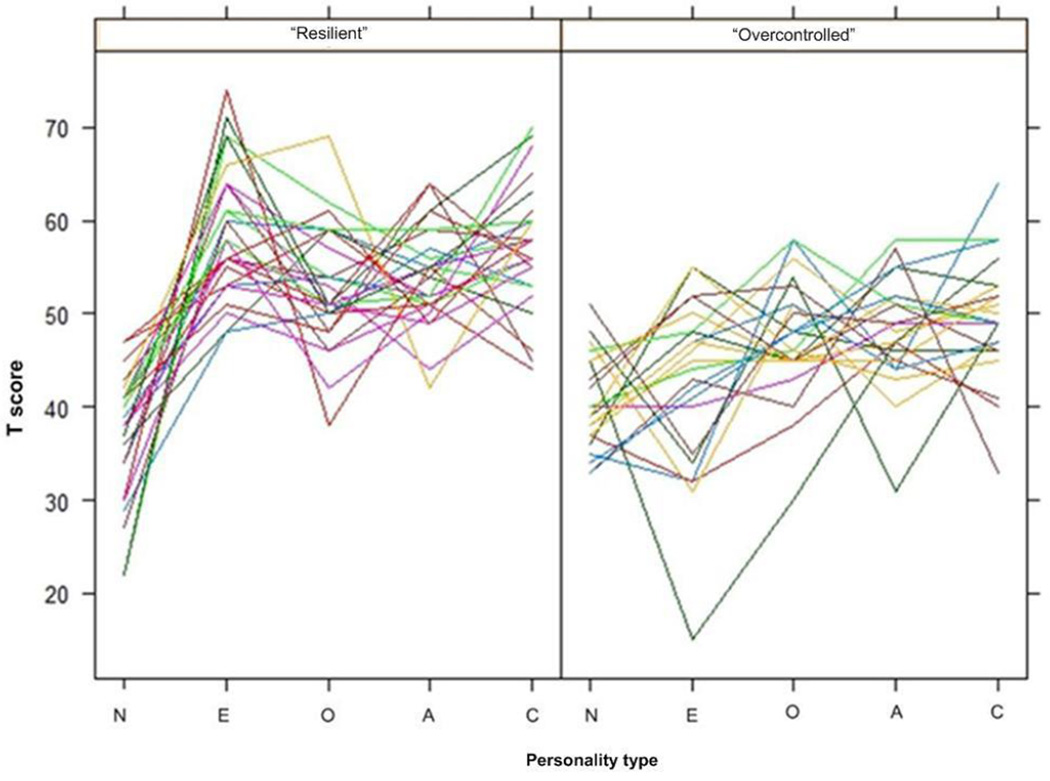
Based on earlier “person-centered” approaches (e.g., cluster analyses) using the Five-Factor model, three empirically replicable personality types have been identified: Resilient, Overcontrolled and Undercontrolled personality types. The external validity of these types has been demonstrated by their relationships to various measures of functioning and the prediction of developmental outcomes (See Donnellan & Robins, 2010 for a more complete review). The Resilient personality type is generally characterized by low neuroticism and average or above-average scores on all other traits. Resilient individuals are generally well-adjusted, i.e., emotionally stable, sociable, curious, cooperative, and self-disciplined (McCrae & Costa, 2010). The Resilient type is associated with higher IQ as well as success in academic and social pursuits. The Overcontrolled personality type is generally characterized by high neuroticism, low extraversion, and either low or average scores on the other FFM traits. Overcontrolled individuals typically display maladaptive tendencies: they tend to be reserved, pragmatic, competitive, unreliable, and more susceptible to psychological distress. They are also more prone to internalizing problems, shyness, and loneliness. The Undercontrolled personality type is generally characterized by low agreeableness, low conscientiousness, as well as average neuroticism, extraversion, and openness to experience. Undercontrolled individuals are often antagonistic, uncooperative, unreliable, and tend to lack discipline.
The relationships among personality types, leisure activities, and executive control are likely complex and intertwined in ways which are not yet understood, especially in older adults. One possibility is that personality moderates the associations between leisure activity and executive control. As such, the benefits of leisure activity on cognitive performance in later adulthood may represent individual differences in stable dispositional tendencies to engage in leisure pursuits across the life course. Further, studies have additionally found that combinations of personality traits may better explain activity pursuits (e.g., Lochbaum, Bixby, & Wang, 2007) and cognitive performance (e.g., Crowe, Andel, Pedersen, Fratiglioni, & Gatz, 2006) through their interactions with one another, underscoring the importance of considering personality types as opposed to single traits when examining health outcomes (Friedman & Booth-Kewley, 1987).
Given the clinical and cost outcomes associated with age-related cognitive decline, there is a need to identify moderating factors that influence the efficacy of interventions, such as leisure activities, for preventing the loss of executive control, and ultimately independence. Using a person-centered approach to the study of personality types may help to more clearly identify best responders to leisure activity and ultimately reduce the inefficiency and high costs of poorly targeted interventions. As we better understand these personality effects, principles for targeting activity interventions can be developed, such as identifying important traits or groups of traits to consider, designing messages and protocols appropriate for specific personality types, and tailoring intervention content for the greatest benefit (Hagger-Johnson & Pollard, 2008). Determining the moderating effects of personality on the association between leisure activities and cognitive abilities crucial to everyday function, such as executive control, is an important step toward reaching these goals. To our knowledge, no study has examined these relationships in a cognitively intact sample of older adults.
Study Purpose
In line with the existing literature and our previous work, we conducted an exploratory study to examine the moderating effects of personality type on the association between leisure activities and executive control among healthy community-dwelling older adults.
Methods
Design and Participants
The present study was approved by the University-affiliated Institutional Review Board. An exploratory cross-sectional study was conducted by recruiting participants enrolled in a regional cohort study (Mapstone et al., 2014). This cohort study was designed to identify blood-based predictors for incident dementia. Community-dwelling older adults aged 75 years or older were invited to participate in a series of neuropsychological, functional, and neuropsychiatric tests conducted by a group of geriatricians, neurologists, and neuropsychologists. Individuals with mild cognitive impairment (MCI; Albert et al., 2011), dementia due to Alzheimer’s disease (McKhann et al., 2011), or who were cognitively healthy were eligible for participation in the cohort study. Only individuals who were cognitively healthy, as defined by having no MCI, dementia, or active relevant treatment (i.e., donepezil, galatamine, rivastigmine, or memantine) per cohort study protocol, were referred to the present study within one month of their annual cohort study visit. Additionally, participants were required to be English speaking and to have adequate auditory and visual acuity for testing, determined by asking “Do you feel you have a hearing loss that interferes with your ability to participate in daily conversations even when wearing your hearing aid?” and “How much difficulty do you have reading ordinary print in the newspaper (with your glasses or contact lenses)?,” respectively. Participants who responded “No difficulty” to these questions were included. Participants were excluded if they had a self-reported history of stroke (not including transient ischemic attack), sleep disorder, or major depression (based on self-report of medication use), within the past 10 years.
A power analysis was conducted to determine the needed sample size for the study. In our previous study, engagement in leisure activities had a small to medium effect on executive control (Lin, Friedman, Quinn, Chen, & Mapstone, 2012). Using Gpower (Faul, Erdfelder, Lang, & Buchner, 2007), applying the number of predictors as six (three covariates , two main predictors, and one interactive predictor), an effect size as 0.20, and α as 0.05, a sample size of 42 participants yields a power of 0.80.
A total of 71 individuals from the cohort study were referred to the present study: seven declined to participate, 13 were found to be ineligible, and two cancelled the lab appointment for unknown reasons. Written consent was obtained from the 49 eligible participants and these individuals then completed an interview and neuropsychological testing.
Procedure
The study was conducted in the CogT Laboratory at the University of Rochester. Data were collected by either a licensed speech pathologist or a registered nurse trained in both the study procedure as well as neuropsychological testing. Participants were individually scheduled for testing. On the testing day, each participant was interviewed and asked to complete a series of leisure activity questionnaires, cognitive tests, and a personality inventory. Participants also provided demographic data and a health history.
Measurements
Executive control
A series of cognitive tests were conducted, including the Trail making test, Part A and B (Reitan, 1958), Stroop word and color test (Golden, 1978), and Digit span forward and backward (Guarch, Marcos, Salamero, & Blesa, 2004). These tests are commonly used for examining different aspects of cognitive processes in clinical settings (Bossers, van der Woude, Boersma, Scherder, & van Heuvelen, 2012) and took approximately 10 minutes to administer. Seven performance scores were calculated using the following tests: Trail Making Test A, Trail Making Test B, Stroop Word (total words read), Stroop Color (total colors named), Stroop Interference (total colors named), Digit Span Forward (span length), and Digit Span Backward (span length). Each individual performance score was standardized to a Z score. Three composite scores (cf. Lin, Chen, Vance, Ball, & Mapstone, 2013) were calculated to represent the three domains of executive control by averaging the Z scores from individual tasks: executive function (Trail making test B, Stroop interference, and Digit span backward-longest span), attention (Stroop word and Digit span forward-longest span), and speed of processing (Trail Making Test A and Stroop Color). Finally, a summary score for executive control was calculated by averaging the three composite scores.
Leisure activity scores included frequency and duration for three activity domains (mental, social, and physical activities), measured by separate questionnaires developed in previous studies (Bennett et al., 2005; Lachman et al., 2010; McPhillips, Pellettera, Barrett-Connor, Wingard, & Criqui, 1989). The Mental activities questionnaire included six items: reading, doing word games, playing cards, attending lectures, writing, and using a computer (Lachman et al., 2010). The Social activities questionnaire included six items: going to a restaurant or sporting event, going on trips, visiting relatives/friends, doing volunteer work, participating in groups, and attending church (Bennett et al., 2005). Frequency of doing each mental and social activity was measured by asking how often the participant engaged in each activity over the past year. Participants responded using a six-point rating scale from “Never” to “Daily.” We weighted these responses by recoding “never” to 0, “once a month” to 1, “several times a month” to 2.5, “once a week” to 4, “several times a week” to 10, and “daily” to 30 (cf. Carlson et al., 2012). The duration of each activity was measured by asking how long the participant spent on the activity (in minutes) on each occasion, on average, over the past year. The Physical activities questionnaire included five items: walking for exercise, gardening or yard work, calisthenics or general exercise, bicycle riding, and swimming or water exercise (McPhillips, Pellettera, Barrett-Connor, Wingard, & Criqui, 1989). In terms of frequency and duration, participants were asked how many times during the last 2 weeks they participated in the activity, ranging from 0 to 14 times, and how many minutes were spent engaged in that specific activity on each occasion, on average.
All three questionnaires have been used in previous longitudinal cohort studies and demonstrated correlations with cognitive abilities (James et al., 2012; James et al., 2011; Lin et al., 2012). To develop composite scores for each domain of leisure activity, we first computed the total time spent on each specific activity within each domain (i.e., duration × frequency). We then summed the total time spent on each item for each activity domain (mental, social, and physical; cf. Lin, Friedman, Quinn, Chen, & Mapstone, 2012).
Personality was measured with the NEO-Five Factor Inventory (NEO-FFI), a short form of the revised NEO Personality Inventory (McCrae & Costa, 2010). Based on the FFM, the 60-item NEO-FFI is a self-report measure of neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness. Each item is rated on a five-point scale: strongly disagree, disagree, neutral, agree, and strongly agree. Raw scores were converted to T scores with a mean of 50 and standard deviation of 10 using adult normative data. This was done because NEO-FFI scores are considered meaningful only when compared to appropriate norms. Cronbach’s α for the five personality subscales ranged between 0.72 and 0.85 in the present study.
Other demographic characteristics and health variables
Age, gender, and years of education were collected via self-report. Depressive symptoms were measured by the 15-item Geriatric Depression Scale (GDS; Sheikh & Yesavage, 1986). Participants responded “yes” or “no” to questions related to their depressive symptoms during the past week and a total depressive symptom score was calculated. The GDS is a well-established instrument for use with cognitively intact older adults, and has demonstrated a Cronbach’s α of 0.749 for the total scale as well as construct and criterion validity (Friedman, Heisel, & Delavan, 2005). Additionally, the Charlson Co-morbidity Index (van Doorn et al., 2001), a weighted index that takes into account the number and seriousness of co-morbid diseases, was used to further characterize the study sample. The Charlson Index is the most extensively studied index of co-morbidity with established concurrent, predictive, and construct validity as well as moderate to good test-retest and interrater reliability (de Groot, Beckerman, Lankhorst, & Bouter, 2003).
Data Analysis
Analyses were conducted using IBM SPSS 19.0 (Chicago, IL). Distributions for each main variable (leisure activities, personality type, and executive control) were examined. Descriptive statistics for main variables and other demographic and health characteristics were calculated. Correlational analysis was conducted. Spearman’s rho was used for non-parametric analysis; Pearson’s correlation was used for parametric analysis.
Cluster analysis was conducted to group the individual personality traits into clusters representative of personality types. First, a Hierarchical Cluster Analysis using Ward’s Method identified the number of homogenous clusters. The dendrogram plot was examined to determine the number of clusters (two clusters in this study). Second, using the number of clusters identified in step 1, a K-means Cluster Analysis of the five personality trait variables was performed. After these two steps, each of the 49 participants was classified into one of two clusters of personality traits. This methodology has been validated in small samples (Clatworthy, Hankins, Buick, Weinman, & Horne, 2007).
To examine whether the clusters of personality traits moderated the association between each domain of leisure activities and executive control (as well as its individual domains), Generalized Linear Model (GLM) was applied. The equation was: Y executive function or attention = ϐ0 + ϐ1covariate1 … + ϐn covariate n + ϐ(n+1)personality cluster + ϐ(n+2)leisure activities + ϐ(n+3)personality cluster × leisure activities + ε. We controlled for age, gender, and education because they are the most common confounding factors in examinations of cognitive function. The p-value was set at 0.05.
Results
Sample Characteristics
Table 1 displays the sample characteristics. The mean age of this sample was 83 years and participants were primarily female. Depressive symptoms were infrequent. On average, subjects had a relatively low co-morbidity burden and were college educated.
Table 1.
Sample Characteristics (n = 49)
| Mean (SD) or N (%) | Range | |
|---|---|---|
| Age | 82.73 (3.03) | 78 – 93 |
| Year of education | 15.69 (2.23) | 12 – 20 |
| Female (N, %) | 27 (54.1%) | |
| Charlson Comorbidity Index | 2.12 (1.86) | 0 – 7 |
| Geriatric Depression Scale | 0.86 (1.24) | 0 – 6 |
| Personality (T score) | ||
| Neuroticism | 38.55 (6.17) | 22 – 55 |
| Openness | 50.35 (7.01) | 30 – 69 |
| Conscientiousness | 53.69 (7.61) | 33 – 70 |
| Extraversion | 51.55 (11.61) | 15 – 74 |
| Agreeableness | 51.31 (6.34) | 31 – 64 |
| Leisure activities | ||
| Mental activities | 5703.01 (4166.85) | 750 – 21870 |
| Social activities | 3637.24 (4640.74) | 240 – 29910 |
| Physical activities | 542.63 (522.96) | 0 – 2410 |
| Executive Control † | ||
| Executive function | −0.03 (0.66) | −1.76 – 1.13 |
| Trail making test B‡ | 215.35 (35.98) | 55 – 259 |
| Stroop interference § | 45.44 (6.19) | 31 – 58 |
| Digit span backward-longest span | 4.61 (1.00) | 3 – 7 |
| Attention † | 0.004 (0.72) | −1.49 – 1.65 |
| Stroop word § | 43.21 (10.21) | 21 – 68 |
| Digit span forward-longest span | 6.57 (1.56) | 4 – 8 |
| Speed of Processing | −0.06 (0.80) | −2.88 – 1.30 |
| Stroop color | 37.12 (10.37) | 8 – 58 |
| Trail making test A‡ | 264.10 (10.87) | 235 – 281 |
Composite scores; otherwise, raw data
Reversed coded
One participant’s data was missing due to color blindness
Data on leisure activities, each personality trait, executive function, attention, and speed of processing were normally distributed. Since individual personality trait scores were standardized T scores, a score of 50 on one of the five traits indicates a score equivalent to the mean of an age-matched normative sample. Mean personality trait T scores were within 4 points of a score of 50, indicating a similar distribution to the normative sample, with the exception of neuroticism which had a mean of 38.55. Individual cognitive performance data were comparable to the age- and education-adjusted normative data from the literature (Tombaugh, 2004; Van der Elst, Van Boxtel, Van Breukelen, & Jolles, 2006).
Correlations between Executive Control and Other Sample Characteristics
Table 2 displays the correlations between executive control, its individual cognitive domains, and the sample characteristics. Executive function, attention, and speed of processing were all moderately correlated (r = .37 – .40, p = .005 – .009). Older age was significantly related to lower executive function (r = −.47, p = .001) and speed of processing (r = −.46, p = .001). Higher agreeableness was related to lower speed of processing (r = −.30, p = .04). Higher levels of mental (r= .30, p = .04) and physical (r = .32, p = .02) activities were related to higher executive function. Similar to its individual domains, a lower composite score of executive control was significantly related to older age and lower levels of mental and physical activities. Other correlations were not significant.
Table 2.
Correlation between Individual Domains of Executive Control and Other Sample Characteristics (n = 49)
| Executive control |
Executive function |
Attention | Speed of processing |
|
|---|---|---|---|---|
| Age | −.40 (.004) | −.47 (.001) | .01 (.96) | −.46 (.001) |
| Years of education | −.03 (.87) | .08 (.57) | −.08 (.61) | −.05 (.72) |
| Charlson Comorbidity Index | .04 (.77) | −.22 (.12) | .06 (.67) | .17 (.24) |
| Geriatric Depression Scale | .19 (.20) | .21 (.15) | .15 (.31) | .08 (.56) |
| GDS† | −.07 (.64) | −.22 (.13) | .08 (.61) | −.01 (.94) |
| Personality | ||||
| Neuroticism | .03 (.84) | −.11 (.46) | .06 (.69) | −.03 (.85) |
| Openness | .08 (.58) | .13 (.27) | .08 (.60) | −.11 (.44) |
| Conscientiousness | −.13 (.39) | −.21 (.15) | −.03 (.87) | −.07 (.65) |
| Extraversion | .05 (.73) | .28 (.06) | −.004 (.98) | −.14 (.35) |
| Agreeableness | −.24 (.09) | −.06 (.70) | −.20 (.18) | −.30 (.04) |
| Leisure activities | ||||
| Mental activities§ | .32 (.03) | .30 (.04) | .15 (.30) | .27 (.06) |
| Social activities§ | .03 (.86) | −.08(.58) | .06 (.67) | .06 (.66) |
| Physical activities§ | .33 (.02) | .32 (.02) | .19 (.19) | .25 (.08) |
Spearman’s rho; otherwise, Pearson’s r;
log-transformed; for Physical activities, the equation for transformation was “log (raw data + 1)” due to zero value in raw data.
Personality Types Identified
Cluster analysis of the five personality traits generated two clusters, or personality types (see Figure 1). Table 3 shows the comparison of the two types with other study variables. The first cluster was based on very low levels of neuroticism but above-average levels of other traits (“Resilient” type), and the second cluster had low levels of extraversion and neuroticism, and moderate levels of other traits (“Overcontrolled” type). The two clusters were significantly different in all traits with the exception of neuroticism for which there was a trend toward significance. Individuals with the Overcontrolled personality type had significantly more depressive symptoms and more female members than the Resilient type. The two personality types did not differ in other demographic and health characteristics, executive control, or leisure activities.
Figure 1.
Resilient and Overcontrolled Personality Typologies (Note. N = Neuroticism, E = Extraversion, O = Openness, A = Agreeableness, C= Conscientiousness)
Table 3.
Comparisons of Personality Types
| Resilient (n = 27) |
Overcontrolled (n = 22) |
t-test or χ2 (p value) |
|
|---|---|---|---|
| Personality | |||
| Neuroticism | 37.22 (6.74) | 40.18 (5.07) | −1.70 (.10) |
| Openness | 52.74 (6.53) | 47.41 (6.57) | 2.84 (.007) |
| Conscientiousness | 57.15 (6.60) | 49.45 (6.65) | 4.04 (< .001) |
| Extraversion | 58.96 (6.94) | 42.45 (9.56) | 7.00 (< .001) |
| Agreeableness | 54.00 (5.28) | 48.00 (6.04) | 3.71 (.001) |
| Age | 82.26 (3.55) | 83.32 (2.19) | −1.22 (.23) |
| Year of education | 15.70 (2.25) | 15.68 (2.26) | 0.03 (.97) |
| Male | 16 (59.3%) | 6 (27.3%) | 5.01 (.025) |
| Comorbidity | 2.22 (1.72) | 2.00 (2.05) | 0.41 (.68) |
| GDS | 0.41 (0.64) | 1.41 (1.56) | −2.82 (.009) |
| Executive control | −0.12 (0.60) | 0.08 (0.49) | −1.27 (.21) |
| Executive Function | 0.01 (0.67) | −0.08 (0.67) | 0.51 (.62) |
| Attention | −0.12 (0.72) | 0.15 (0.69) | −1.31 (.20) |
| Speed of Processing | −0.25 (0.82) | 0.18 (0.72) | −1.93 (.06) |
| Mental activities § | 8.34 (0.65) | 8.56 (0.67) | −1.17 (.25) |
| Social activities § | 7.77 (0.89) | 7.68 (1.13) | 0.31 (.76) |
| Physical activities § | 5.56 (1.92) | 5.25 (2.28) | 0.52 (.61) |
Note: # indicates using χ2; Bold indicates p value < .05.
log-transformed; for Physical activities, the equation for transformation was “log (raw data + 1)” due to zero value in raw data.
Moderating Effect of Personality Types on the Association between Leisure Activities and Executive Control
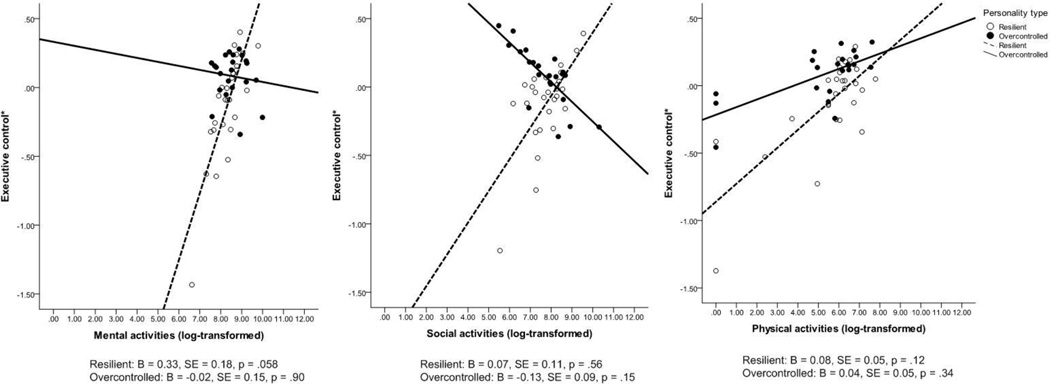
Table 4 shows the moderating effect of personality type on the association between each leisure activity category and the composite executive control score, followed by the three individual components of executive control. Compared to the Overcontrolled personality type, the Resilient personality type was associated with lower levels of executive control (composite score) as well as the individual domains of executive function and attention. However, there were significant interaction effects between the Resilient personality type and mental activities for executive control and the two individual domains (executive function and attention). Figure 2 shows the plots for stratifying the relationship between three categories of leisure activities and executive control by personality type. Among individuals with a Resilient personality type, engaging in more mental activities was marginally significantly associated with higher levels of executive control (B = 0.33, SE = 0.18, p = .058); while among those with the Overcontrolled personally type, there was no association between engaging in mental activities and executive control.
Table 4.
Moderating Effect of Personality Type on Leisure Activities and Executive Control
| Executive Control (df1 = 1, df2 = 40) |
Executive Function (df1 = 1, df2 = 40) |
Attention (df1 = 1, df2 = 40) |
Speed of Processing (df1 = 1, df2 = 40) |
|||||
|---|---|---|---|---|---|---|---|---|
| B (SE) | p | B (SE) | p | B (SE) | p | B (SE) | p | |
| Personality type† | −4.24 (1.92) | .027 | −4.45 (2.23) | .046 | −7.16 (2.73) | .009 | −1.12 (2.66) | .68 |
| Mental activities (MA)§ | −0.03 (0.15) | .86 | 0.05 (0.17) | .80 | −0.19 (0.22) | .36 | 0.07 (0.21) | .75 |
| Personality type† × MA§ | 0.47 (0.23) | .036 | 0.52 (0.26) | .046 | 0.82 (0.32) | .011 | 0.08 (0.31) | .81 |
| Personality type† | −2.06 (1.16) | .077 | −1.69 (1.37) | .22 | −1.90 (1.69) | .26 | −2.58 (1.52) | .09 |
| Social activities (SA)§ | −0.10 (0.09) | .26 | −0.16 (0.11) | .13 | −0.03 (0.14) | .81 | −0.11 (0.12) | .36 |
| Personality type† × SA§ | 0.23 (0.15) | .12 | 0.21 (0.18) | .22 | 0.21 (0.22) | .33 | 0.27 (0.20) | .17 |
| Personality type† | −0.49 (0.40) | .21 | 0.19 (0.48) | .69 | −0.84 (0.56) | .13 | −0.82 (0.52) | .12 |
| Physical activities (PA)§ | 0.05 (0.07) | .28 | 0.07 (0.06) | .22 | 0.04 (0.07) | .50 | 0.04 (0.06) | .56 |
| Personality type† × PA§ | 0.04 (0.07) | .52 | −0.04 (0.08) | .60 | 0.11 (0.10) | .24 | 0.06 (0.09) | .50 |
Note: Controlled for age, gender, education; Bold indicates p value < .05.
Taking Overcontrolled as the reference.
log-transformed; for Physical activities, the equation for transformation was “log (raw data + 1)” due to zero value in raw data.
Figure 2.
Association between Leisure Activities and Executive Control by Personality Type. Note: Models were adjusted for age, gender, and education.
Discussion
We found that a personality type characterized by very low neuroticism and above-average extraversion, openness, agreeableness, and conscientiousness was associated with greater executive control as a result of mental activity participation compared to a personality type characterized by low levels of extraversion and neuroticism and moderate levels of other traits. The two clusters identified in this study are generally consistent with Resilient and Overcontrolled personality types found in many, but not all, studies across age groups (Asendorpf, Borkenau, Ostendorf, & Van Aken, 2001) and cultures (Alessandri et al., 2013). However, the distribution of neuroticism in this sample, which was skewed toward lower scores, likely contributed to the lack of a significant difference in neuroticism between cluster groups. In other studies, neuroticism has been identified as a key difference between Resilient and Overcontrolled personality types; therefore, our categorization of these types in this exploratory study was based on the other personality traits and the observed trend in neuroticism scores. Large studies that have classified personality typologies have typically also identified a third personality type, Undercontrolled, which is characterized by low agreeableness, low conscientiousness, as well as average neuroticism, extraversion, and openness to experience (Donnellan & Robins, 2010). We did not find an Undercontrolled cluster in this exploratory study, possibly due to a small sample size or to our convenience sample which was recruited from a parent longitudinal study in which individuals participate in five years of follow-up assessments. The hallmark characteristics of the Undercontrolled personality type are associated with antagonism, a lack of cooperation, unreliability, and a lack of discipline. Individuals with this personality type may have self-selected out of the parent study due to these tendencies.
In their investigation of personality typology and successful aging, Steca, Alessandri, and Caprara (2010) found that Resilient older adults had higher life satisfaction, trust in others, positive affect, and activity participation compared to Overcontrolled older adults. In our sample, there were no significant differences in activity participation between the two personality types, yet Resilient individuals were more likely to demonstrate higher executive function and attention as a result of their participation in mental activities specifically. These results suggest that participation in mental activities by itself may not lead to cognitive benefit. Instead, the tendencies that Resilient older adults may exhibit within the context of activities (e.g., sociability, cooperation, curiosity) may be essential for improving cognitive domains relevant to executive control. Individual preferences for mental activity participation may affect the depth and meaning of these experiences, leading to different cognitive outcomes among individuals with the same exposure (Parisi, Stine-Morrow, Noh, & Morrow, 2009). That said, practitioners need to monitor preferences for everyday living when prescribing activities (Van Haitsma, Curyto, Spector, Towsley, Kleban, Carpenter, et al., 2013) keeping in mind that an individual’s personality type may determine that their cognitive health is better served by interventions other than mental activities. When used, it is also incumbent upon practitioners to tailor the activities they prescribe by individual preferences so they are engaging and meaningful (Kolanowski, Buettner, Costa & Litaker, 2001).
It is important to note that significant moderating effects were not found for social and physical leisure activities. We may have failed to detect these effects due to our small sample size or the relatively small degree of variability in the executive control measures. However, there may be other factors, such as the quality of social networks or physical function, which are stronger moderators of the associations between social/physical activities and executive control than personality type.
The results regarding personality type and mental activities have implications for both future personality investigations in older adults as well as the design of interventions. As relatively few studies have explored moderating effects of individual characteristics on activity-cognition relations, it remains unclear as to who is most likely to engage with and benefit from activity participation. In particular, understanding how personality impacts the association between activity and cognition allows us to move beyond a “one size fits all model” and think through innovative tailored approaches for participating in leisure activity.
Hagger-Johnson & Pollard Whiteman (2008) proposed a typology to direct future personality-health research, the Five T’s: targeting groups of individuals based on personality preferences; tailoring interventions based on individual assessments; training to modify personality traits themselves in order to improve treatment outcomes; pharmacological treatment to improve psychological functioning, a more controversial approach; and, observation of personality transformation throughout a disease course such as dementia. Given our findings, future investigations in this area should consider targeting mental activity interventions to Resilient older adults, exploring methods of increasing motivation for participation among non-Resilient older adults, determining intervention characteristics most suitable to other personality types, or exploring interventions which may positively impact the traits associated with Resilient individuals, namely decreasing neuroticism and increasing scores on the other four traits. Due to the substantial negative health effects of high neuroticism (Lahey, 2009), considerable research with other populations has targeted high neuroticism and associated temperaments with some promising results (see Barlow, Sauer-Zavala, Carl, Bullis, & Ellard, 2014 for a review).
Our findings are limited by the small convenience sample that was generally healthy, well-educated, and cognitive intact for their age, and was not representative of all personality types found in other studies. Larger sample sizes are needed in future research in order better characterize the general population as well as examine reliability of the leisure measures. In addition, this was a cross-sectional study; therefore, we are not able to draw conclusions regarding causality or reciprocal relationships between lifestyle activities and cognitive performance. Our leisure activity measures relied on self-report of individuals who may have varying ability to recall their extent of participation in leisure activities. Nevertheless, all participants were screened for cognitive impairments by a team of geriatricians, neurologists, and neuropsychologists and none had MCI or dementia.
Despite these limitations, the findings from this exploratory study provide a new perspective of the moderating effect of personality on the association between leisure activity participation and executive control in older adults. While some research has examined personality as a moderator of leisure activity-related outcomes, such as the relationship between intention, conscientiousness, and exercise behavior among young adults (Conner, Rodgers, & Murray, 2007), these relationships have not been adequately explored among older adults or with a specific emphasis on cognitive performance crucial to everyday function, such as executive control. Our results indicate that personality types may be useful moderator variables to inform our understanding of why individuals respond differently to the same stimuli, such as mental activity, in late life.
Conclusion
In our sample of older adults at risk for cognitive decline, we found that personality type moderated the association between mental activity and executive control, such that individuals with a Resilient personality type benefited more from mental activity than those with an Overcontrolled personality type. This held even though the amount of time engaged in mental activity did not differ between the groups. Although preliminary, these findings provide new insight into the complex relationships among mental activities in late life, personality, and cognition in a group of individuals vulnerable to cognitive decline. While participation in leisure activities in late life has been repeatedly associated with better cognitive outcomes, our results suggest that personality type may be of particular importance in future investigations as well as intervention development.
Acknowledgments
Data collection of the present project was supported by Sigma Theta Tau International Small Grants to F. Lin. Manuscript preparation was supported by the National Hartford Centers of Gerontological Nursing Excellence Claire M. Fagin Fellow Award Program to N. Hill and the University of Rochester CTSA award number KL2 TR000095 from the National Center for Advancing Translational Sciences of the National Institutes of Health to F. Lin. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri G, Vecchione M, Donnellan BM, Eisenberg N, Caprara GV, Cieciuch J. On the cross-cultural replicability of the resilient, undercontrolled, and overcontrolled personality types. Journal of Personality. 2013 doi: 10.1111/jopy.12065. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Asendorpf JB, Borkenau P, Ostendorf F, van Aken MA. Carving personality description at its joints: Confirmation of three replicable personality prototypes for both children and adults. European Journal of Personality. 2001;15(3):169–198. [Google Scholar]
- Barlow DH, Sauer-Zavala S, Carl JR, Bullis JR, Ellard KK. The nature, diagnosis, and treatment of neuroticism: Back to the future. Clinical Psychological Science. 2014 doi: 10.1177/1745691614544528. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. Journal of the American Geriatrics Society. 2003;51(4):459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- Barnett LA. Accounting for Leisure Preferences from Within: The Relative Contributions of Gender, Race or Ethnicity, Personality, Affective Style, and Motivational Orientation. Journal of Leisure Research. 2006;38(4):445–474. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: Study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Booth JE, Schinka JA, Brown LM, Mortimer JA, Borenstein AR. Five-factor personality dimensions, mood states, and cognitive performance in older adults. Journal of Clinical and Experimental Neuropsychology. 2006;28(5):676–683. doi: 10.1080/13803390590954209. [DOI] [PubMed] [Google Scholar]
- Bossers WJ, van der Woude LH, Boersma F, Scherder EJ, van Heuvelen MJ. Recommended measures for the assessment of cognitive and physical performance in older patients with dementia: A systematic review. Dementia and Geriatric Cognitive Disorders EXTRA. 2012;2(1):589–609. doi: 10.1159/000345038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PJ, Devanand DP, Liu X, Caccappolo E Alzheimer’s Disease Neuroimaging Initiative. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Archives of General Psychiatry. 2011;68(6):617–626. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss DM. Selection, evocation, and manipulation. Journal of Personality and Social Psychology. 1987;53(6):1214–1221. doi: 10.1037//0022-3514.53.6.1214. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Parisi JM, Xia J, Xue Q-L, Rebok GW, Bandeen-Roche K, Fried LP. Lifestyle activities and memory: Variety may be the spice of life. The Women’s Health and Aging Study II. Journal of the International Neuropsychological Society. 2012;18:286–294. doi: 10.1017/S135561771100169X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Roberts B, Shiner RL. Personality development: Stability and change. Annual Review of Psychology. 2005;56:453–484. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Chapman B, Duberstein P, Lyness JM. Personality traits, education, and health-related quality of life among older adult primary care patients. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2007;62B(6):343–352. doi: 10.1093/geronb/62.6.p343. [DOI] [PubMed] [Google Scholar]
- Chapman B, Duberstein P, Tindle HA, Sink KM, Robbins J, Tancredi DJ, et al. Personality predicts cognitive function over 7 years in older persons. American Journal of Geriatric Psychiatry. 2012;20(7):612–621. doi: 10.1097/JGP.0b013e31822cc9cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Goldberg LR. Replicability and 40-year predictive power of childhood ARC types. Journal of Personality and Social Psychology. 2011;101(3):593–606. doi: 10.1037/a0024289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy J, Hankins M, Buick D, Weinman J, Horne R. Cluster analysis in illness perception research: A Monte Carlo study to identify the most appropriate method. Psychology & Health. 2007;22(2):123–142. [Google Scholar]
- Cloninger CR. Person-centered health promotion in chronic disease. The International Journal of Person Centered Medicine. 2013;3(1):5–12. doi: 10.5750/ijpcm.v3i1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner M, Rodgers W, Murray T. Conscientiousness and the intention-behavior relationship: predicting exercise behavior. Journal of Sport & Exercise Psychology. 2007;29(4):518–533. doi: 10.1123/jsep.29.4.518. [DOI] [PubMed] [Google Scholar]
- Costa PT., Jr Clinical use of the five-factor model: An introduction. Journal of Personality Assessment. 1991;57(3):393–398. doi: 10.1207/s15327752jpa5703_1. [DOI] [PubMed] [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Fratiglioni L, Gatz M. Personality and risk of cognitive impairment 25 years later. Psychology and Aging. 2006;21(3):573–580. doi: 10.1037/0882-7974.21.3.573. [DOI] [PubMed] [Google Scholar]
- de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: A critical review of available methods. Journal of Clinical Epidemiology. 2003;56(3):221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- Donnellan MB, Robins RW. Resilient, overcontrolled, and undercontrolled personality types: Issues and controversies. Social and Personality Psychology Compass. 2010;4(11):1070–1083. [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15-item geriatric depression scale in functionally impaired, cognitively intact, community-dwelling elderly primary care patients. Journal of the American Geriatrics Society. 2005;53(9):1570–1576. doi: 10.1111/j.1532-5415.2005.53461.x. [DOI] [PubMed] [Google Scholar]
- Friedman HS. Long-term relations of personality and health: Dynamisms, mechanisms, tropisms. Journal of Personality. 2000;68(6):1089–1107. doi: 10.1111/1467-6494.00127. [DOI] [PubMed] [Google Scholar]
- Friedman HS. The multiple linkages of personality and disease. Brain, Behavior, and Immunity. 2008;22(5):668–675. doi: 10.1016/j.bbi.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HS, Booth-Kewley S. The “disease-prone personality”: A meta-analytic view of the construct. American Psychologist. 1987;42:539–555. doi: 10.1037//0003-066x.42.6.539. [DOI] [PubMed] [Google Scholar]
- Furnham A. Personality and demographic determinants of leisure and sports preference and performance. International Journal of Sport Psychology. 1990;21(3):218–236. [Google Scholar]
- Gaugler JE, Hovater M, Roth DL, Johnston JA, Kane RL, Sarsour K. Analysis of cognitive, functional, health service use, and cost trajectories prior to and following memory loss. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2013;68(4):562–567. doi: 10.1093/geronb/gbs078. [DOI] [PubMed] [Google Scholar]
- Golden CJ. The Stroop color and word test: A manual for clinical and experimental uses. Chicago, IL: Stoelting Co.; 1978. [Google Scholar]
- Guarch J, Marcos T, Salamero M, Blesa R. Neuropsychological markers of dementia in patients with memory complaints. International Journal of Geriatric Psychiatry. 2004;19(4):352–358. doi: 10.1002/gps.1074. [DOI] [PubMed] [Google Scholar]
- Hagger-Johnson GE, Pollard MC. Personality and Health - So what? The Psychologist. 2008;21(7):594–597. [Google Scholar]
- Hertzog C, Kramer A, Wilson R, Lindenberger U. Enrichment effects on adult cognitive development. Psychological Science and the Public Interest. 2009;9(1):1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Hill NL, Kolanowski AM, Fick D, Chinchilli VM, Jablonski RA. Personality as a moderator of cognitive stimulation in older adults at high risk for cognitive decline. Research in Gerontological Nursing. 2014 doi: 10.3928/19404921-20140311-01. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock RS, Lee HB, Bienvenu OJ, Nestadt G, Samuels JF, Parisi JM, et al. Personality and cognitive decline in the Baltimore Epidemiologic Catchment Area follow-up study. American Journal of Geriatric Psychiatry. 2013 doi: 10.1016/j.jagp.2012.12.217. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S, Hughes BM. Construct, concurrent and discriminant validity of Type D personality in the general population: Associations with anxiety, depression, stress and cardiac output. Psychology and Health. 2012;27(2):242–258. doi: 10.1080/08870446.2011.603423. [DOI] [PubMed] [Google Scholar]
- James BD, Boyle PA, Bennett DA, Buchman AS. Total daily activity measured with actigraphy and motor function in community-dwelling older persons with and without dementia. Alzheimer Disease and Associated Disorders. 2012;26(3):238–245. doi: 10.1097/WAD.0b013e31822fc3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James BD, Wilson RS, Barnes LL, Bennett DA. Late-life social activity and cognitive decline in old age. Journal of the International Neuropsychological Society. 2011;17(6):998–1005. doi: 10.1017/S1355617711000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopp DS, Hertzog C. Assessing adult leisure activities: An extension of a self-report activity questionnaire. Psychological Assessment. 2010;22(1):108–120. doi: 10.1037/a0017662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkcaldy B, Furnham A. Extraversion, neuroticism, psychoticism and recreational choice. Personality and Individual Differences. 1991;12(7):737–745. [Google Scholar]
- Kolanowski AM, Buettner L, Costa PT, Jr, Litaker MS. Capturing interests: Therapeutic recreation activities for persons with dementia. Therapeutic Recreation Journal. 2001;35(3):220–235. [Google Scholar]
- Lachman ME, Agrigoroaei S, Murphy C, Tun PA. Frequent cognitive activity compensates for education differences in episodic memory. American Journal of Geriatric Psychiatry. 2010;18(1):4–10. doi: 10.1097/JGP.0b013e3181ab8b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB. Public health significance of neuroticism. American Psychologist. 2009;64(4):241–256. doi: 10.1037/a0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung GT, Fung AW, Tam CW, Lui VW, Chiu HF, Chan WM, Lam LC. Examining the association between late-life leisure activity participation and global cognitive decline in community-dwelling elderly Chinese in Hong Kong. International Journal of Geriatric Psychiatry. 2011;26(1):39–47. doi: 10.1002/gps.2478. [DOI] [PubMed] [Google Scholar]
- Lin F, Friedman E, Quinn J, Chen DG, Mapstone M. Effect of leisure activities on inflammation and cognitive function in an aging sample. Archives of Gerontology and Geriatrics. 2012;54(3):e398–e404. doi: 10.1016/j.archger.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Heffner K, Mapstone M, Chen DG, Porsteisson A. Frequency of mentally stimulating activities modifies the relationship between cardiovascular reactivity and executive function in old age. American Journal of Geriatric Psychiatry. 2013 doi: 10.1016/j.jagp.2013.04.002. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Chen DG, Vance D, Mapstone M. Trajectories of combined laboratory- and real world-based speed of processing in community-dwelling older adults. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2013;68(3):364–373. doi: 10.1093/geronb/gbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Chen DG, Vance DE, Ball KK, Mapstone M. Longitudinal relationships between subjective fatigue, cognitive function, and everyday functioning in old age. International Psychogeriatrics. 2013;25(2):275–285. doi: 10.1017/S1041610212001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochbaum MR, Bixby WR, Wang CKJ. Achievement goal profiles for self-report physical activity participation: Differences in personality. Journal of Sport Behavior. 2007;30(4):471–490. [Google Scholar]
- Lu L, Hu C-H. Personality, leisure experiences and happiness. Journal of Happiness Studies. 2005;6(3):325–342. [Google Scholar]
- Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, Macarthur LH, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nature Medicine. 2014;20(4):415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GA, Rentz DM, Frey MT, Locascio JJ, Johnson KA, Sperling RA Alzheimer’s Disease Neuroimaging Initiative. Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):300–308. doi: 10.1016/j.jalz.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr . NEO Inventories for the NEO Personality Inventory-3 (NEO-PI-3), NEO Five-Factor Inventory-3 (NEO-FFI-3), NEO Personality Inventory-Revised (NEO PI-R): Professional Manual. Lutz, FL: PAR, Inc.; 2010. [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. American Journal of Preventive Medicine. 1989;5(2):65–72. [PubMed] [Google Scholar]
- Parisi JM. Determinants and effects of engagement in adulthood (Publication No. AAT 3301209) Dissertation Abstracts International. 2007;69B:155. [Google Scholar]
- Parisi JM, Stine-Morrow EA, Noh SR, Morrow DG. Predispositional engagement, activity engagement, and cognition among older adults. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition. 2009;16(4):485–504. doi: 10.1080/13825580902866653. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, O’Connell J, Thomson R. A systematic review of cognitive decline in the general elderly population. International Journal of Geriatric Psychiatry. 2003;18(12):1121–1134. doi: 10.1002/gps.1023. [DOI] [PubMed] [Google Scholar]
- Ratey JJ, Loehr JE. The positive impact of physical activity on cognition during adulthood: A review of underlying mechanisms, evidence and recommendations. Reviews in the Neurosciences. 2011;22(2):171–185. doi: 10.1515/RNS.2011.017. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8(3):271–276. [Google Scholar]
- Roberts B, Mroczek D. Personality trait change in adulthood. Current Directions in Psychological Science. 2008;17(1):31–35. doi: 10.1111/j.1467-8721.2008.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IH. The structure of normal human attention: The Test of Everyday Attention. Journal of the International Neuropsychological Society. 1996;2(6):525–534. doi: 10.1017/s1355617700001697. [DOI] [PubMed] [Google Scholar]
- Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, et al. Executive control function: A review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. The Journal of Neuropsychiatry & Clinical Neurosciences. 2002;14(4):377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Jonsdottir MK, Sigurdsson S, Eiriksdottir G, Jonsson PV, Garcia ME, et al. White matter lesions and cognitive performance: the role of cognitively complex leisure activity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63(8):848–854. doi: 10.1093/gerona/63.8.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale C, Guppy A, El-Sayed M. Individual differences, exercise and leisure activity in predicting affective well-being in young adults. Ergonomics. 2000;43(10):1689–1697. doi: 10.1080/001401300750004096. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Selective review of cognitive aging. Journal of the International Neuropsychological Society. 2010;16(5):754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sava FA, Popa RI. Personality types based on the Big Five model. A cluster analysis over the Romanian population. Cognition, Brain, Behavior: An Interdisciplinary Journal. 2011;15(3):359–384. [Google Scholar]
- Schaie KW, Willis SL, Caskie GI. The Seattle longitudinal study: Relationship between personality and cognition. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition. 2004;11(2–3):304–324. doi: 10.1080/13825580490511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E. Social environment effects on health and aging: integrating epidemiologic and demographic approaches and perspectives. Annals of the New York Academy of Sciences. 2001;954:88–117. doi: 10.1111/j.1749-6632.2001.tb02749.x. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist. 1986;5(1–2):165–173. [Google Scholar]
- Steca P, Alessandri G, Caprara GV. The utility of a well-known personality typology in studying successful aging: Resilients, undercontrollers, and overcontrollers in old age. Personality and Individual Differences. 2010;48(4):442–446. [Google Scholar]
- Stern C, Munn Z. Cognitive leisure activities and their role in preventing dementia: A systematic review. International Journal of Evidence-Based Healthcare. 2010;8(1):2–17. doi: 10.1111/j.1744-1609.2010.00150.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Social support. In: Friedman HS, Silva RC, editors. Foundation of Health Psychology. New York: Oxford University Press; 2007. pp. 145–171. [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Valenzuela M, Sachdev P. Brain reserve and dementia: A systematic review. Psychological Medicine. 2006;36(4):441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J. The Stroop color-word test: Influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment. 2006;13(1):62–79. doi: 10.1177/1073191105283427. [DOI] [PubMed] [Google Scholar]
- van Doorn C, Bogardus ST, Williams CS, Concato J, Towle VR, Inouye SK. Risk adjustment for older hospitalized persons: A comparison of two methods of data collection for the Charlson index. Journal of Clinical Epidemiology. 2001;54(7):694–701. doi: 10.1016/s0895-4356(00)00367-x. [DOI] [PubMed] [Google Scholar]
- Van Haitsma K, Curyto K, Spector A, Towsley G, Kleban M, Carpenter B, et al. The preferences for everyday living inventory: Scale development and description of psychosocial preferences responses in community-dwelling elders. The Gerontologist. 2013;53(4):582–595. doi: 10.1093/geront/gns102. [DOI] [PubMed] [Google Scholar]
- Vaughan L, Giovanello K. Executive function in daily life: Age-related influences of executive processes on instrumental activities of daily living. Psychology and Aging. 2010;25(2):343–355. doi: 10.1037/a0017729. [DOI] [PubMed] [Google Scholar]
- Wilson R, Schneider J, Arnold S, Bienias J, Bennett D. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Archives of General Psychiatry. 2007;64(10):1204–1212. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61(6):812–816. doi: 10.1212/01.wnl.0000083989.44027.05. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81(4):314–321. doi: 10.1212/WNL.0b013e31829c5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CW, Sano M, Ferris SH, Whitehouse PJ, Patterson MB, Aisen PS. Health-related resource use and costs in elderly adults with and without mild cognitive impairment. Journal of the American Geriatrics Society. 2013;61(3):396–402. doi: 10.1111/jgs.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]