Abstract
Dystonia is a heterogeneous neurological disorder characterized by abnormal muscle contractions for which standard medical therapy is often inadequate. For such patients, therapeutic brain stimulation is becoming increasingly utilized. Here we review the evidence and effect sizes for treating different types of dystonia with different types of brain stimulation. Strong (level B) evidence supports the use of deep brain stimulation (DBS) for the treatment of primary generalized or segmental dystonia, especially DYT-1, as well as for patients with cervical dystonia. Large effect sizes have also been reported for DBS treatment of tardive dystonia, writer’s cramp, cranial dystonia, myoclonus dystonia, and off-state dystonia associated with Parkinson’s disease. Lesser benefit is generally seen in dystonia secondary to structural brain damage. Other brain stimulation techniques including epidural cortical stimulation and noninvasive brain stimulation have been investigated, but generally report smaller effect sizes in a more limited number of patients. Recent advances relevant to patient selection, surgical approach, DBS programming, and mechanism of action are discussed.
Introduction
Dystonia is defined as a neurological disorder characterized by sustained or intermittent muscle contractions causing abnormal movements and/or postures. It is a heterogeneous group of disorders with many underlying causes and physiologies, both known and unknown. The most recent consensus guidelines classify dystonia based on clinical presentation and etiology 1. Key clinical factors include patient age at onset, extent of the body affected, temporal pattern, and whether dystonia is the only major motor finding (isolated dystonia) or one feature of a broader disorder (combined dystonia). Etiological classification relates to whether the dystonia is inherited, acquired, or due to identifiable nervous system pathology. Different types of dystonia span the spectrum of this classification scheme, from generalized childhood-onset dystonia due to genetic mutation (e.g. DYT-1) to more focal adult-onset dystonias affecting the hand (writers cramp), neck (cervical dystonia / torticollis) or face (cranial dystonia / Meige syndrome). Dystonia can arise secondary to brain insult including stroke, trauma, adverse medication effect (tardive dystonia), etc., or as a symptom of other diseases such as Parkinson’s or Wilson’s disease.
Pharmacological therapies for dystonia, which include anticholinergic agents, dopaminergics, benzodiazepines, tetrabenazine, and baclofen generally provide only modest symptomatic improvement and can cause significant side effects. A rare exception is dopa-responsive dystonia, which responds profoundly to carbidopa / levodopa. Botulinum toxin (BOTOX) injections can provide symptomatic relief of targeted muscles; however, the injections must be repeated every few months, patients can become resistant or immune to the therapy over time, side effects such as weakness are common, and the injections become both costly and impractical if a large number of muscles are affected. Surgical interventions have historically included rhizotomy for severe cervical dystonia and ablation of the thalamus (thalamotomy) and/or basal ganglia (pallidotomy) for more generalized dystonias. These ablative procedures can provide significant benefit and pallidotomy is still used in select cases. However variation in lesion location or size can yield variable results while posing a risk of irreversible adverse effects, particularly with bilateral interventions 2. Due to these limitations, deep brain stimulation (DBS) has emerged as the preferred surgical intervention for medically refractory torsion dystonia. The main advantages of DBS relative to ablation are: 1- the effects of stimulation are reversible, yielding a significant margin of safety; 2- the stimulus can be titrated to clinical effect and modified as needed over time; and 3- bilateral interventions can be performed safely.
In 2003, the US Food and Drug Administration (FDA) granted a humanitarian device exemption (HDE) for the use of DBS in primary generalized / segmental and cervical dystonia based on the relatively small number of patients thought to be surgical candidates and the robust clinical responses reported at that time, albeit in open label assessments. The HDE requires that DBS for dystonia be performed with the oversight of a local Institutional Review Board. Since 2003, the body of evidence supporting the use of DBS in dystonia has grown, including the results of two large randomized controlled trials, each performed in Europe 3,4. Here, we review the current status of DBS for the treatment of dystonia, incorporating recent evidence-based guidelines 5–8, meta-analyses 9,10, and reviews 2,11. The potential impact of investigational non-invasive brain stimulation techniques for dystonia is also discussed 12.
Selecting Patients for DBS Therapy
Deciding which dystonia patients are candidates for DBS therapy can be complex given the heterogeneous nature of the disorder, the varied responses to stimulation of different dystonia sub-types, and the potential risks of surgery. Given this complexity, DBS for dystonia is probably best performed through a dedicated multi-disciplinary movement disorder center. Regarding patient selection, one may begin with the FDA approved indications, which are supported most strongly by the available evidence. Under the current HDE, DBS is approved only for the treatment of primary segmental/generalized or cervical dystonia. The use of DBS to treat other dystonia subtypes is considered “off-label” however evidence is emerging that it may be effective.
The evaluation of dystonia patients presenting for consideration of DBS should focus on: 1- excluding conditions that would respond favorably to less invasive treatments; and 2- identifying factors predictive of a positive (eg DYT-1) or negative (eg secondary dystonia) response to stimulation. A levodopa trial should be performed in all patients with symptom onset before age 21 and considered in patients with symptom onset before age 50 to rule out levodopa-responsive dystonia. Wilson’s disease should be tested for in patients with suggestive features. Patients with focal or cervical dystonia referred for DBS due to “botox resistance” should be evaluated to ensure they would not benefit from more appropriately targeted or dosed injections. One can test for resistance by injecting a small amount of Botox into a forehead wrinkle and assessing for effect.
Patients with psychogenic dystonia may be referred for DBS, as they are often treatment refractory. Differentiating psychogenic from organic dystonia is difficult and only considered definitive if symptoms resolve with psychotherapy 13,14. Clinical features suggestive of a psychogenic etiology include inconsistency over time, incongruence with organic dystonia syndromes, sudden onset, peak severity at onset, fixed / tonic features, and comorbid psychiatric disease or other psychogenic symptoms 14. It is important to evaluate the patient for fixed skeletal deformities, spasticity, and myelopathy, all of which may mitigate the patient’s response to DBS. Similarly, the pre-operative workup should include brain MRI to exclude structural abnormalities that could indicate a secondary dystonia or impact surgical targeting. Finally, screening for pre-morbid psychiatric symptoms or cognitive dysfunction is reasonable keeping in mind that GPi DBS has been used successfully in psychiatric patients with tardive dystonia and in some patients with cognitive impairment. There is no evidence that DBS itself exacerbates these symptoms in patients with dystonia 6.
The proper timing of DBS surgery remains a controversial issue. In general, intervention should be considered once it is determined that medical therapy has failed to adequately control symptoms and prior to the development of fixed skeletal deformities or cervical myelopathy 6. As recently as 2011, the Movement Disorders Society concluded that there was insufficient evidence to recommend early surgery 6; however, accumulating data does suggest that the earlier one intervenes with DBS, the better the outcome, especially in DYT-1 15. Precisely how early one should intervene is unclear, however, as there exist scant data regarding surgical outcomes in children under the age of 7 or for symptom duration less than 2 years.
The Surgical Procedure
The DBS device is comprised of three key components: a stimulating electrode (also called a lead), an extension cable, and a programmable pulse generator (PG), which is similar to a cardiac pacemaker (Figure 1). The device is implanted in two stages. During the first stage uni- or bilateral lead(s) are implanted stereotactically into a specific therapeutic target. By far the most common target for dystonia is the GPi (Figure 2), but other targets including the subthalamic nucleus (STN) have been studied 8. During the second stage, which may be performed on the same day or later, the pulse generator(s) is implanted under the skin of the anterior chest wall or the abdomen, and connected to the lead wire(s) via subcutaneously tunneled extension cables. In most instances, bilateral implants are required; however, patients with hemi-dystonia may benefit from unilateral stimulation.
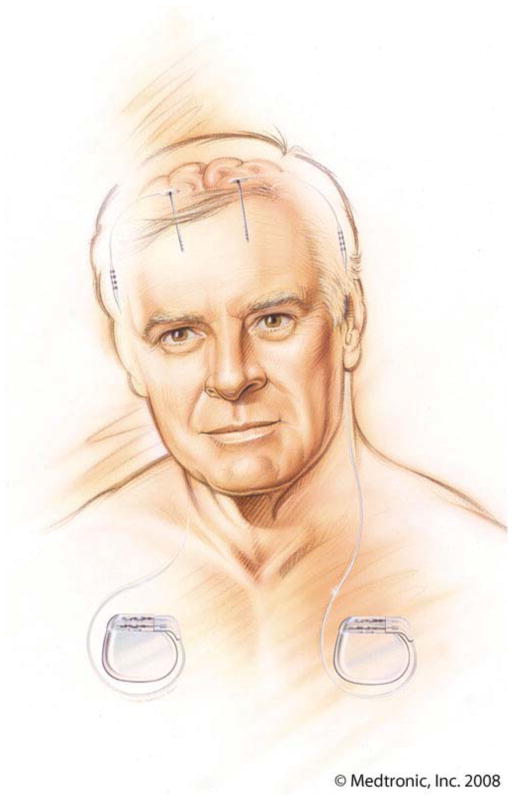
Figure 1.
Illustration of bilaterally implanted DBS devices. Each DBS device is comprised of a stimulating lead in the brain, extension cable, and programmable pulse generator, usually implanted in the chest (compliments of Medtronic, Inc.)
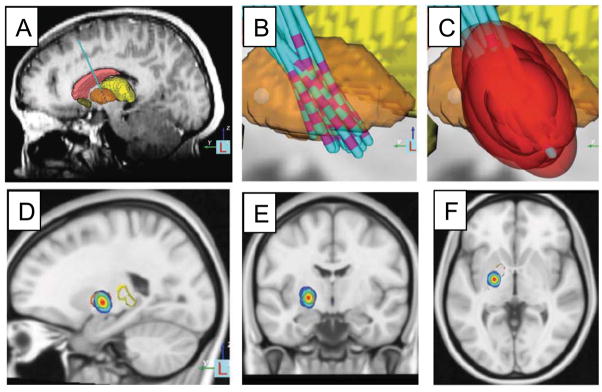
Figure 2.
Deep brain stimulation target in the globus pallidus based on retrospective analysis of the site of effective electrode contacts and modeling of stimulation fields. MRI was used to identify the location of the DBS electrode in patients with DYT-1 dystonia (A) and co-registered into a common atlas space (B). The stimulation field for the effective electrode contact in each patient was modeled (C). A probabilistic volume in the posteroventral aspect of the GPi was identified that could be used to guide future electrode placement or programming (D-F). Modified with permission from Cheung et al. 2014 Annals of Neurology.
Lead insertions may be accomplished with a variety of image- and physiologically guided techniques, though use of a cranial-mounted stereotactic headframe remains the gold-standard against which all other techniques are measured 7. Alternatives to the frame include so-called ‘frame-less’ stereotactic targeting and direct, image-guided placement within the MRI scanner itself. Regardless of the targeting technique employed, target visualization is accomplished with MRI employing techniques that provide excellent delineation of the basal ganglia 7. Intra-operatively, proper siting of the therapeutic target may be accomplished with single unit microelectrode and/or local field potential recordings 8. Traditionally, these recordings are obtained with the patient fully awake; however, acceptable recordings can be obtained in patients under anesthesia and some anesthetic agents may be better than others at not disrupting the typical patterns seen in an awake patient 8. Once proper targeting is confirmed, the lead is inserted and the acute effects of stimulation are tested. Dystonia differs from Parkinson’s disease and Essential Tremor in that days or weeks of stimulation, rather than seconds or minutes, are usually required to achieve clinical response. Therefore, an improvement in dystonia symptoms in response to intra-operative test stimulation is not expected. Instead, test stimulation is employed to assess for adverse effects, which, if present, will appear immediately and should prompt re-positioning of the lead. Once satisfactorily positioned, the lead is anchored at the skull using the surgeon’s preferred technique.
Following implantation, proper lead location can be confirmed and intracerebral hemorrhage excluded with either CT or MRI. Brain MRI can be performed safely despite the presence of the implanted leads and allows one to better assess their anatomical position. Low energy MRI sequences must be used in order to comply with FDA-mandated safety regulations, though standard (higher-energy) MRI sequences have been employed in a large number of patients without adverse effects 7.
The second stage of the DBS surgery, during which the PG(s) and extension cables are implanted and connected to the lead(s), can be performed on the same day as the lead implantation, but is often scheduled for 1–2 weeks after. This stage is performed under general anesthesia. In patients requiring bilateral stimulation, an important question is whether to implant a single PG to power both leads, or two single-channel PGs, one for each side. Although implantation of two PGs requires an extra incision and a slightly longer surgery, single channel PGs have a lower profile than dual channel PGs, last longer, and the presence of two PGs provides some protection against sudden cessation of all stimulation therapy if one battery is suddenly exhausted 16 or needs to be explanted due to infection. Though not approved for use in dystonia, the dual channel rechargeable device, which has the lowest profile of all, may minimize the risk of infection and reduce the frequency of IPG replacements that may prove particularly valuable in the treatment of children with generalized symptoms.
DBS Programming
Immediately after lead implant, some patients experience a transient improvement in their dystonia; a phenomenon termed the ‘micro lesion effect’. Though less commonly observed in dystonia than in other disorders, when it occurs, this effect often heralds a good response to stimulation. As the effect can last up to 3 weeks and complicate the assessment of stimulation response, some neurologists wait until after this period to begin programming 5. Other centers begin programming immediately or wait just 7–10 days after PG implantation, allowing time for the surgical incisions to heal. Of particular significance are patients who are implanted during a dystonic crisis, a potentially life-threatening situation in which a patient’s dystonia acutely worsens and is manifest as severe, active, painful contractions that can lead to rhabdomyolysis, respiratory compromise and other metabolic derangements. Small case series suggest that DBS may help break dystonic crises more readily than medications alone. Under these circumstances, immediate activation of stimulation is warranted.
During programming, the clinician controls four stimulus parameters: amplitude, pulse width, frequency, and the active contact(s). The goal is to find the combination of settings that improves motor function and/or reduces pain without causing adverse effects. Determining these settings is more difficult in dystonia than in essential tremor or Parkinson’s disease because the therapeutic benefit is often not apparent for weeks to months. Moreover, there is no consensus regarding the most efficient approach to arriving at optimal settings, though practical guidelines are available 5. In general, one starts by activating each contact in isolation (monopolar stimulation), slowly raising the stimulus amplitude while examining for the threshold at which adverse effects such as muscle contractions, dysarthria, or worsening dystonia occur. Some patients may also show acute beneficial effects such as a reduction in dystonic tremor, identifying a preferred contact. If no beneficial effects are seen, one can trial stimulation at each contact for longer periods of time (days to weeks) or select those contacts located in the postero-ventral GPi, as prior studies suggest that it is these contacts that are most likely to yield maximal benefit (Figure 2). Some centers select the contact at or immediately superior to that which produces phosphenes, indicating proximity to the optic tract, while others select the most ventral contact(s) that does not produce side effects at therapeutic voltage.
Unlike Parkinson’s disease, in which one usually employs the lowest effective stimulus amplitude, increasing it as needed, for dystonia many neurologists start with a relatively high voltage, one just below the adverse effect threshold and then decrease it to conserve battery power once benefit has been achieved. The most commonly used stimulation parameters (frequency: 130–180 Hz; pulse width: 60–210 _sec; amplitude: 2–5 volts) are based on published trials, though some evidence suggests that lower stimulation frequencies (60–80 Hz) may be equally effective in childhood dystonia, while prolonging battery life. Patients return for evaluation and programming adjustments every few weeks for the first few months then every 3–6 months for the first 1–2 years. Patient responses are often quantified using standardized scales such as the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS) or the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS). While useful tools for measuring improvement following DBS, these scales can be insensitive to small changes and so one must be sure also to tailor therapy to address the individual’s most disabling/bothersome symptom.
It is worth noting that this “trial and error” approach to DBS programming was developed largely on the experience of treating patients with PD and essential tremor for whom the therapeutic benefit at different electrode contacts can be rapidly assessed. Given the delayed therapeutic effects characteristic of dystonia, this process is clearly not ideal. Algorithms that allow one to identify the ideal contact and stimulation parameters based on neuroimaging, brain connectivity, and modeling of the stimulation field could represent a major advance for programming DBS in dystonia in the near future (e.g. Figure 2) 17.
Clinical Results
The results of the clinical studies of DBS for dystonia are summarized in Table 1 and Figure 3 including the magnitude of clinical benefit, number of patients studied, and level of the evidence that the intervention works better than placebo. To compile this summary we borrowed heavily from prior meta-analyses incorporating the results of numerous individual trials 9,10. This data was then updated to include more recent studies or additional indications not covered by the original meta-analyses. Although much of the efficacy data is derived from open label studies, prospective randomized trials with double-blind assessments have been completed in patients with generalized/segmental primary dystonia and cervical dystonia, both demonstrating that GPi DBS is superior to sham stimulation 3,4. Pallidal stimulation for generalized or segmental dystonia stands out as the best studied therapy with the largest number of patients and effect sizes 4,18. Larger clinical improvements have been reported in patients with the DYT-1 mutation than in patients with DYT-6 or with an unknown cause of their primary generalized dystonia, although the latter also do quite well. Improvement in severity scores following DBS for cervical dystonia is less impressive than in generalized dystonia, however other clinical features such as pain and disability may improve to a greater extent, yielding significant functional benefit 3. Similarly, in patients with myoclonus dystonia, the improvement in myoclonus can be more dramatic than the improvement in dystonia severity (73% versus 53%) 10. DBS appears very effective for alleviating dystonia associated with Parkinson’s disease, tardive dystonia, and writer’s cramp, although the later is supported by limited evidence in a finite number of patients. Patients with secondary dystonia, and particularly those with static encephalopathy, exhibit modest responses to DBS and higher complication rates as compared to patients with primary dystonia 9. Nevertheless, it is worth noting that a 24% reported average clinical improvement in dystonia severity can still result in clinically meaningful benefit 19.
Table 1.
Evidence of Efficacy for Brain Stimulation in Dystonia. For each type of brain stimulation studied in each type of dystonia, we list the percent improvement in dystonia severity, number of patients studied (N), and quality of the evidence per AAN criteria (level B = probably effective; level C = possibly effective; level U = indeterminate). DBS = deep brain stimulation, ECS = electrical ‘subdural’ cortical stimulation, TMS = transcranial magnetic stimulation, tDCS = transcranial direct current stimulation, STN = subthalamic nucleus, Gpi = globus pallidus pars interna.
| Type of Dystonia | Stimulation | % Improvement | N | Evidence | References |
|---|---|---|---|---|---|
| Generalized / Segmental | |||||
| DYT-1 | DBS-Gpi | 73 | 138 | B | (Andrews et al., 2010*; Panov et al., 2013) |
| Non DYT-1 | DBS-Gpi | 56 | 147 | B | (Andrews et al., 2010*) |
| DYT-6 | DBS-Gpi | 40 | 11 | U | (Groen et al., 2010; Panov et al., 2012) |
| Gen / Segmental | DBS-STN | 89 | 12 | U | (Sun et al., 2007) |
| Segmental | TMS | 56 | 1 | U | (Allam et al., 2007) |
| Cervical | |||||
| Cervical | DBS-Gpi | 42 | 129 | B | (Volkmann et al., 2014) |
| Cervical | DBS-STN | 54 | 15 | C | (Ostrem et al., 2011; Schjerling et al., 2013) |
| Cervical | ECS | 16 | 5 | U | (Lalli et al., 2012) |
| Focal Hand | |||||
| Writers Cramp | DBS-Gpi | 92 | 6 | U | (Andrews et al., 2010*) |
| Writers Cramp | TMS | 15 | 28 | U | (Borich et al., 2009; Murase et al., 2005; Siebner et al., 1999) |
| Focal Hand | tDCS | 12 | 26 | U | (Benninger et al., 2011; Buttkus et al., 2010; Furuya et al., 2014) |
| Cranial | |||||
| Cranial | DBS-Gpi | 64 | 17 | U | (Andrews et al., 2010*) |
| Bletharospasm | TMS | 31 | 12 | C | (Kranz et al., 2010) |
| Axial | |||||
| Camptocormia | DBS-Gpi | 62 | 9 | U | (Andrews et al., 2010*; Capelle et al., 2011) |
| Secondary | |||||
| Tardive Dystonia | DBS-Gpi | 78 | 50 | C | (Mentzel et al., 2012) |
| Cerebral Palsy | DBS-Gpi | 24 | 22 | U | (Andrews et al., 2010*) |
| Other Secondary | DBS-Gpi | 38 | 29 | U | (Andrews et al., 2010*) |
| Other Secondary | TMS | 20 | 3 | U | (Lefaucheur et al., 2004) |
| Combined | |||||
| Parkinson’s Disease | DBS-Gpi | 93 | 16 | C | (Loher et al., 2002; Volkmann et al., 1998) |
| Parkinson’s Disease | DBS-STN | 75 | 30 | C | (Detante et al., 2004; Krack et al., 1999) |
| Myoclonus Dystonia | DBS-Gpi | 60 | 30 | U | (Rughani and Lozano, 2013*) |
| PKAN | DBS-Gpi | 66 | 12 | U | (Andrews et al., 2010*) |
| Lubag (X-linked Dys-PD) | DBS-Gpi | 78 | 2 | U | (Andrews et al., 2010*) |
| Rapid Onset Dys-PD | DBS-Gpi | 13 | 2 | U | (Andrews et al., 2010*) |
| Lesch-Nyan | DBS-Gpi | 44 | 1 | U | (Andrews et al., 2010*) |
| Dystonia Deafness | DBS-Gpi | 75 | 1 | U | (Andrews et al., 2010*) |
| GM1 Gangliosides | DBS-Gpi | 20 | 1 | U | (Andrews et al., 2010*) |
| Wilsons | DBS-Gpi | 14 | 1 | U | (Sidiropoulos et al., 2013) |
Indicates meta-analyses that incorporate numerous individual studies, please refer to these articles for additional references.
References for Table 1 (can be placed in supplementary material as needed):
Allam, N., Brasil-Neto, J.P., Brandão, P., Weiler, F., Barros Filho, J.d., and Tomaz, C. (2007). Relief of primary cervical dystonia symptoms by low frequency transcranial magnetic stimulation of the premotor cortex: case report. Arquivos de neuro-psiquiatria 65, 697–699.
Andrews, C., Aviles-Olmos, I., Hariz, M., and Foltynie, T. (2010). Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. Journal of neurology, neurosurgery, and psychiatry 81, 1383–1389.
Benninger, D.H., Lomarev, M., Lopez, G., Pal, N., Luckenbaugh, D.A., and Hallett, M. (2011). Transcranial direct current stimulation for the treatment of focal hand dystonia. Movement disorders : official journal of the Movement Disorder Society 26, 1698–1702.
Borich, M., Arora, S., and Kimberley, T.J. (2009). Lasting effects of repeated rTMS application in focal hand dystonia. Restor Neurol Neurosci 27, 55–65.
Buttkus, F., Weidenmüller, M., Schneider, S., Jabusch, H.-C., Nitsche, M.A., Paulus, W., and Altenmüller, E. (2010). Failure of cathodal direct current stimulation to improve fine motor control in musician's dystonia. Movement disorders : official journal of the Movement Disorder Society 25, 389–394.
Capelle, H.-H., Schrader, C., Blahak, C., Fogel, W., Kinfe, T.M., Baezner, H., and Krauss, J.K. (2011). Deep brain stimulation for camptocormia in dystonia and Parkinson's disease. Journal of neurology 258, 96–103.
Detante, O., Vercueil, L., Krack, P., Chabardes, S., Benabid, A.L., and Pollak, P. (2004). Off-period dystonia in Parkinson's disease but not generalized dystonia is improved by high-frequency stimulation of the subthalamic nucleus. Advances in Neurology 94, 309–314.
Furuya, S., Nitsche, M.A., Paulus, W., and Altenmüller, E. (2014). Surmounting retraining limits in musician's dystonia by transcranial stimulation. Annals of Neurology 75, 700–707.
Groen, J.L., Ritz, K., Contarino, M.F., van de Warrenburg, B.P., Aramideh, M., Foncke, E.M., van Hilten, J.J., Schuurman, P.R., Speelman, J.D., Koelman, J.H., et al. (2010). DYT6 dystonia: mutation screening, phenotype, and response to deep brain stimulation. Movement disorders : official journal of the Movement Disorder Society 25, 2420–2427.
Krack, P., Pollak, P., Limousin, P., Benazzouz, A., Deuschl, G., and Benabid, A.L. (1999). From off-period dystonia to peak-dose chorea. The clinical spectrum of varying subthalamic nucleus activity. Brain : a journal of neurology 122 ( Pt 6), 1133–1146.
Kranz, G., Shamim, E.A., Lin, P.T., Kranz, G.S., and Hallett, M. (2010). Transcranial magnetic brain stimulation modulates blepharospasm: a randomized controlled study. Neurology 75, 1465–1471.
Lalli, S.S., Piacentini, S.S., Franzini, A.A., Panzacchi, A.A., Cerami, C.C., Messina, G.G., Ferré, F.F., Perani, D.D., and Albanese, A.A. (2012). Epidural premotor cortical stimulation in primary focal dystonia: clinical and 18F-fluoro deoxyglucose positron emission tomography open study. Movement disorders : official journal of the Movement Disorder Society 27, 533–538.
Lefaucheur, J.-P., Fénelon, G., Ménard-Lefaucheur, I., Wendling, S., and Nguyen, J.-P. (2004). Low-frequency repetitive TMS of premotor cortex can reduce painful axial spasms in generalized secondary dystonia: a pilot study of three patients. Neurophysiologie Clinique /Clinical Neurophysiology 34, 141–145.
Loher, T.J., Burgunder, J.-M., Weber, S., Sommerhalder, R., and Krauss, J.K. (2002). Effect of chronic pallidal deep brain stimulation on off period dystonia and sensory symptoms in advanced Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry 73, 395–399.
Mentzel, C.L., Tenback, D.E., Tijssen, M.A.J., Visser-Vandewalle, V.E.R.M., and van Harten, P.N. (2012). Efficacy and safety of deep brain stimulation in patients with medication-induced tardive dyskinesia and/or dystonia: a systematic review. The Journal of clinical psychiatry 73, 1434–1438.
Murase, N., Rothwell, J.C., Kaji, R., Urushihara, R., Nakamura, K., Murayama, N., Igasaki, T., Sakata-Igasaki, M., Mima, T., Ikeda, A., and Shibasaki, H. (2005). Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer's cramp. Brain : a journal of neurology 128, 104–115.
Ostrem, J.L., Racine, C.A., Glass, G.A., Grace, J.K., Volz, M.M., Heath, S.L., and Starr, P.A. (2011). Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology 76, 870–878.
Panov, F., Gologorsky, Y., Connors, G., Tagliati, M., Miravite, J., and Alterman, R.L. (2013). Deep Brain Stimulation in DYT1 Dystonia: A 10-Year Experience. Neurosurgery 73, 86–93.
Panov, F., Tagliati, M., Ozelius, L.J., Fuchs, T., Gologorsky, Y., Cheung, T., Avshalumov, M., Bressman, S.B., Saunders-Pullman, R., Weisz, D., and Alterman, R.L. (2012). Pallidal deep brain stimulation for DYT6 dystonia. Journal of neurology, neurosurgery, and psychiatry 83, 182–187.
Rughani, A.I., and Lozano, A.M. (2013). Surgical treatment of myoclonus dystonia syndrome. Movement disorders : official journal of the Movement Disorder Society 28, 282–287.
Schjerling, L., Hjermind, L.E., Jespersen, B., Madsen, F.F., Brennum, J., Jensen, S.R., Løkkegaard, A., and Karlsborg, M. (2013). A randomized double-blind crossover trial comparing subthalamic and pallidal deep brain stimulation for dystonia. Journal of Neurosurgery 119, 1537–1545.
Sidiropoulos, C., Hutchison, W., Mestre, T., Moro, E., Prescott, I.A., Mizrachi, A.V., Fallis, M., Rughani, A.I., Kalia, S.K., Lozano, A., and Fox, S. (2013). Bilateral pallidal stimulation for Wilson's disease. Movement disorders : official journal of the Movement Disorder Society 28, 1292–1295.
Siebner, H.R.H., Tormos, J.M.J., Ceballos-Baumann, A.O.A., Auer, C.C., Catala, M.D.M., Conrad, B.B., and Pascual-Leone, A.A. (1999). Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer's cramp. Neurology 52, 529–537.
Sun, B., Chen, S., Zhan, S., Le, W., and Krahl, S.E. (2007). Subthalamic nucleus stimulation for primary dystonia and tardive dystonia. Acta Neurochirurgica Supplement 97, 207–214.
Volkmann, J., Mueller, J., Deuschl, G., Kühn, A.A., Krauss, J.K., Poewe, W., Timmermann, L., Falk, D., Kupsch, A., Kivi, A., et al. (2014). Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet neurology 13, 875–884.
Volkmann, J., Sturm, V., Weiss, P., Kappler, J., Voges, J., Koulousakis, A., Lehrke, R., Hefter, H., and Freund, H.J. (1998). Bilateral high-frequency stimulation of the internal globus pallidus in advanced Parkinson's disease. Annals of Neurology 44, 953–961.
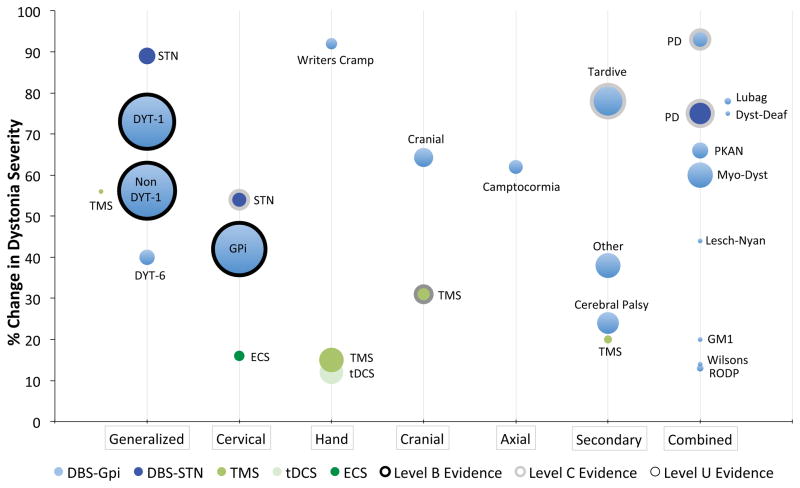
Figure 3.
Evidence of Efficacy for Brain Stimulation in Dystonia. Each bubble represents the evidence that a particular type of brain stimulation is effective for a particular type of dystonia. The position of the bubble along the y axis reflects the average improvement in dystonia severity, the size of the bubble reflects the number of patients studied, and the bubble outline reflects the quality of the evidence assessed by AAN criteria (level B = black outline, level C = grey outline, level U = no outline). Treatments with the best evidence of efficacy have larger bubbles higher on the graph and outlined by darker lines. Abbreviations refer to the conditions as listed in Table 1.
Comparing surgical targets, GPi DBS is supported by higher quality evidence in a greater number of patients and thus remains the target of choice 8. However small studies of STN DBS for dystonia have reported large effect sizes and potentially different side effect profiles, with a lower incidence of bradykinesia and higher incidence of dyskinesia relative to GPi 20. Further work directly comparing the two targets is required before any definitive conclusions can be made.
Regarding the time course of improvement, months of stimulation may be required before any clinical benefit is observed and full benefit may not be realized for a year or more. Phasic or active dystonic movements generally improve more rapidly than do fixed dystonic postures. In cervical dystonia, pain induced by muscular contractions may improve prior to and independent of any change in head position. Due to the relatively static nature of most dystonia sub-types, the response to DBS appears to be durable. Longer-term follow-up has documented continued benefit out to five and even ten years 16,21.
Risks
Deep brain stimulation poses three types of risk: surgical, stimulation related, and hardware related. For all DBS patients (including PD and tremor) the risk of intracranial hemorrhage is reported to be ~3%, the risk of permanent neurologic morbidity ~1%, and death within 30 days of surgery, 0.4%, though these rates are likely to be lower in dystonia patients who are significantly younger on average than patients with PD or ET. The rates of hardware malfunction or infection requiring re-hospitalization vary but can be as high as 10% over time 5. In the recent trial of DBS for cervical dystonia, the risk of serious adverse event was 26%, 8% of which failed to resolve 3. Adverse events related to stimulation include slurred speech and Parkinsonism, although these generally resolve with cessation of therapy or adjustments in stimulation parameters 16. Although not common, suicide has been reported in patients with DBS for dystonia, generally in patients with pre-surgical suicidal ideation 3,16. Some diseases including cerebral palsy and dystonia secondary to structural lesions may have a higher complication rate due to a greater number of medical comorbidities.
Of all the potential complications, infection is the most common and can be disheartening in patients who have enjoyed a robust treatment response. In most instances, infected components must be explanted, temporarily interrupting therapy. As most infections occur at the chest pocket, rapid removal of the PG and the extension, followed by aggressive antibiotic therapy can salvage the brain lead, thereby minimizing the risk and difficulty of re-implantation. Children under the age of 15 are particularly prone to infection; therefore, lower profile IPGs may be advantageous in this group.
Alternatives to Deep Brain Stimulation
Though highly effective in properly selected, implanted, and programmed candidates, DBS therapy involves certain risks (see above), the discomforts of surgery, and long-term limitations. Difficulties posed by the chronically implanted device include ongoing risk of infection, skin breakdown, wire breakage, device malfunction, and periodic battery replacement surgery and/or device recharging. Further, DBS currently precludes the use of body MRI under any conditions and brain MRI only under restrictive conditions. These risks/limitations are motivating research into less invasive brain stimulation alternatives such as epidural cortical stimulation (ECS), in which electrodes are implanted on the surface of the brain, and completely non-invasive techniques such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Although use of these latter techniques in dystonia remains investigational, we include them in this review to provide a comparison between modalities and because dystonia patients who are candidates for DBS increasingly express interest about these alternative technologies (Table 1, Figure 3).
Compared to DBS, noninvasive brain stimulation and epidural cortical stimulation have been studied in a far smaller number of patients and with smaller effect sizes 12. There exists significant heterogeneity in trials of noninvasive stimulation for dystonia. Targets have included the premotor cortex, primary motor cortex, and the supplementary motor area (SMA). Stimulation has been applied at rest and during specific tasks, which may prove important as evidenced in at least one study of patients with musician’s dystonia 22. The only randomized controlled trial without an accompanying conflicting report regards TMS to the anterior cingulate/SMA for blepharospasm 23. How targets used for noninvasive brain stimulation relate to those used for deep brain stimulation is a topic of active investigation 24 (Figure 4).
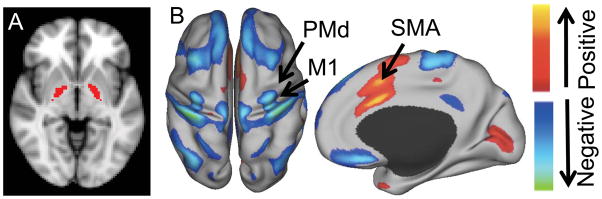
Figure 4.
Location and functional relationship between invasive and noninvasive brain stimulation sites in dystonia. The globus pallidus pars interna, the primary target of deep brain stimulation for dystonia, is shown in red (A). Resting state functional connectivity with this deep brain stimulation site identifies positive and negative correlations on the surface of the brain potentially amenable to noninvasive brain stimulation (B). Prior targets of noninvasive brain stimulation are identified including primary motor cortex (M1), dorsal premotor cortex (PMd) and supplementary motor area (SMA). Modified with permission from Fox et al. 2014 PNAS.
Mechanism and Future Directions
At present it is unclear how electrical brain stimulation yields therapeutic benefit in dystonia or any disorder, an important limiting factor in the further development of the therapy. Research directed at this question is currently hampered by restrictions imposed by the FDA regarding the performance of brain MRI in patients with implanted DBS devices and the ethical complications surrounding the use of radioligands for performing serial Positron Emission Tomography or SPECT, particularly in children. To address this gap in our knowledge, efforts are underway at multiple centers, including ours, to develop low energy functional MRI techniques in order to study the acute and chronic effects of DBS.
The fact that the clinical response to DBS is delayed for days or weeks and that some patients who have been treated with DBS for many years may not experience a return of symptoms for prolonged periods of time after stimulation ceases, suggest that in dystonia, DBS may induce neuroplastic changes that are as yet unknown. Increasingly, dystonia is conceptualized as a neural network disorder 25,26 and brain stimulation as a network-based therapy 24. As such, brain connectivity and network considerations may help us to better understand stimulation effects and guide the discovery of new therapeutic targets and the selection of optimal contacts and parameters for stimulation within a target. Recent evidence suggests that disrupting pathological oscillations within these networks plays a role in alleviating phasic dystonic movements 27. The disruption of pathological rhythms occurs rapidly with the onset of stimulation, and, consistent with this notion, phasic movements in dystonia can respond quickly to DBS. However fixed dystonic postures that take longer to respond may require brain network reorganization, a more slowly developing process.
The recent approval of the first responsive neural stimulating device for the treatment of refractory epilepsy has heightened expectations for the development of closed-looped DBS systems for the treatment of other conditions including dystonia. Analogous to on-demand cardiac pacemakers, these devices generate therapeutic impulses only when an abnormal rhythm is detected. The battery-conserving properties of such an approach could prove particularly valuable in dystonia, which often responds only to stimulation parameters that deplete batteries quickly.
Finally, despite increasing interest in noninvasive stimulation for dystonia, it is clear that significant additional research is needed before these therapies replicate or even approximate the responses currently achieved with DBS. Critical factors that may limit the ability of these non-invasive techniques to achieve significant results in dystonia include: 1- the fact that, at present, only the cortex may be targeted unlike DBS, which targets sub-cortical structures; and 2- the fact that these non-invasive techniques provide intermittent stimulation, which may be insufficient to achieve the desired clinical response. Studies of brain connectivity may allow for identification of noninvasive targets connected to deep brain structures 24 (Figure 4). Moreover, noninvasive stimulation might be combined with a particular physical activity to induce network plasticity in a shorter amount of time 22. Finally, non-invasive techniques combined with functional imaging studies may reveal cortical regions of interest that may be amenable to stimulation via chronically implanted stimulating devices.
References
- 1.Balint B, Bhatia KP. Dystonia: an update on phenomenology, classification, pathogenesis and treatment. Current opinion in neurology. 2014;27(4):468–476. doi: 10.1097/WCO.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 2.Yianni J, Green AL, Aziz TZ. Surgical treatment of dystonia. International Review of Neurobiology. 2011;98:573–589. doi: 10.1016/B978-0-12-381328-2.00021-3. [DOI] [PubMed] [Google Scholar]
- 3.Volkmann J, Mueller J, Deuschl G, et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet neurology. 2014;13(9):875–884. doi: 10.1016/S1474-4422(14)70143-7. [DOI] [PubMed] [Google Scholar]
- 4.Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355(19):1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 5.Kupsch A, Tagliati M, Vidailhet M, et al. Early postoperative management of DBS in dystonia: programming, response to stimulation, adverse events, medication changes, evaluations, and troubleshooting. Movement disorders : official journal of the Movement Disorder Society. 2011;26(Suppl 1):S37–S53. doi: 10.1002/mds.23624. [DOI] [PubMed] [Google Scholar]
- 6.Bronte-Stewart H, Taira T, Valldeoriola F, et al. Inclusion and exclusion criteria for DBS in dystonia. Movement disorders : official journal of the Movement Disorder Society. 2011;26(Suppl 1):S5–16. doi: 10.1002/mds.23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starr PA, Bejjani P, Lozano AM, Metman LV, Hariz MI. Stereotactic techniques and perioperative management of DBS in dystonia. Movement disorders : official journal of the Movement Disorder Society. 2011;26(Suppl 1):S23–30. doi: 10.1002/mds.23489. [DOI] [PubMed] [Google Scholar]
- 8.Vitek JL, DeLong MR, Starr PA, Hariz MI, Metman LV. Intraoperative neurophysiology in DBS for dystonia. Movement disorders : official journal of the Movement Disorder Society. 2011;26(Suppl 1):S31–36. doi: 10.1002/mds.23619. [DOI] [PubMed] [Google Scholar]
- 9.Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. Journal of neurology, neurosurgery, and psychiatry. 2010;81(12):1383–1389. doi: 10.1136/jnnp.2010.207993. [DOI] [PubMed] [Google Scholar]
- 10.Rughani AI, Lozano AM. Surgical treatment of myoclonus dystonia syndrome. Movement disorders : official journal of the Movement Disorder Society. 2013;28(3):282–287. doi: 10.1002/mds.25326. [DOI] [PubMed] [Google Scholar]
- 11.Moro E, Gross RE, Krauss JK. What's new in surgical treatment for dystonia? Movement disorders : official journal of the Movement Disorder Society. 2013;28(7):1013–1020. doi: 10.1002/mds.25550. [DOI] [PubMed] [Google Scholar]
- 12.Quartarone A. Transcranial magnetic stimulation in dystonia. Handbook of clinical neurology. 2013;116:543–553. doi: 10.1016/B978-0-444-53497-2.00043-7. [DOI] [PubMed] [Google Scholar]
- 13.Hallett M. Physiology of psychogenic movement disorders. Journal of Clinical Neuroscience. 2010;17(8):959–965. doi: 10.1016/j.jocn.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet neurology. 2012;11(3):250–260. doi: 10.1016/S1474-4422(11)70310-6. [DOI] [PubMed] [Google Scholar]
- 15.Panov F, Gologorsky Y, Connors G, Tagliati M, Miravite J, Alterman RL. Deep Brain Stimulation in DYT1 Dystonia: A 10-Year Experience. Neurosurgery. 2013;73(1):86–93. doi: 10.1227/01.neu.0000429841.84083.c8. [DOI] [PubMed] [Google Scholar]
- 16.Tagliati M, Krack P, Volkmann J, et al. Long-Term management of DBS in dystonia: response to stimulation, adverse events, battery changes, and special considerations. Movement disorders : official journal of the Movement Disorder Society. 2011;26(Suppl 1):S54–62. doi: 10.1002/mds.23535. [DOI] [PubMed] [Google Scholar]
- 17.Cheung T, Noecker AM, Alterman RL, McIntyre CC, Tagliati M. Defining a therapeutic target for pallidal deep brain stimulation for dystonia. Annals of Neurology. 2014;76(1):22–30. doi: 10.1002/ana.24187. [DOI] [PubMed] [Google Scholar]
- 18.Vidailhet M, Vercueil L, Houeto J-L, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352(5):459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- 19.Vidailhet M, Yelnik J, Lagrange C, et al. Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: a prospective pilot study. The Lancet Neurology. 2009;8(8):709–717. doi: 10.1016/S1474-4422(09)70151-6. [DOI] [PubMed] [Google Scholar]
- 20.Ostrem JL, Racine CA, Glass GA, et al. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology. 2011;76(10):870–878. doi: 10.1212/WNL.0b013e31820f2e4f. [DOI] [PubMed] [Google Scholar]
- 21.Volkmann J, Wolters A, Kupsch A, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. The Lancet Neurology. 2012;11(12):1029–1038. doi: 10.1016/S1474-4422(12)70257-0. [DOI] [PubMed] [Google Scholar]
- 22.Furuya S, Nitsche MA, Paulus W, Altenmüller E. Surmounting retraining limits in musician's dystonia by transcranial stimulation. Annals of Neurology. 2014;75(5):700–707. doi: 10.1002/ana.24151. [DOI] [PubMed] [Google Scholar]
- 23.Kranz G, Shamim EA, Lin PT, Kranz GS, Hallett M. Transcranial magnetic brain stimulation modulates blepharospasm: a randomized controlled study. Neurology. 2010;75(16):1465–1471. doi: 10.1212/WNL.0b013e3181f8814d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. PNAS. 2014 doi: 10.1073/pnas.1405003111. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendrix CMC, Vitek JLJ. Toward a network model of dystonia. Annals of the New York Academy of Sciences. 2012;1265:46–55. doi: 10.1111/j.1749-6632.2012.06692.x. [DOI] [PubMed] [Google Scholar]
- 26.Neychev VK, Gross RE, Lehéricy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiology of Disease. 2011;42(2):185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barow E, Neumann W-J, Brücke C, et al. Deep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movements. Brain : a journal of neurology. 2014 doi: 10.1093/brain/awu258. [DOI] [PMC free article] [PubMed] [Google Scholar]