Abstract
Background
Previous studies on male rodents found that prenatal alcohol exposure (PAE) decreases the number of serotonin immunoreactive (5-HT-ir) neurons in the brainstem. However, data on the effects of PAE in females are lacking. In light of known sex differences in responsiveness of the 5-HT system and known effects of estrogen (E2) and progesterone (P4) in the brain, we hypothesized that sex steroids will modulate the adverse effects of PAE on 5-HT neurons in adult females.
Methods
Adult females from 3 prenatal groups (Prenatal alcohol-exposed [PAE], Pair-fed [PF], and ad libitum-fed Controls [C]) were ovariectomized (OVX), with or without hormone replacement, or underwent Sham OVX. 5-HT-ir cells were examined in key brainstem areas.
Results
Our data support the hypothesis that PAE has long-term effects on the 5-HT system of females and that ovarian steroids have a modulatory role in these effects. Intact (Sham OVX) PAE females had marginally lower numbers of 5-HT-ir neurons in the dorsal raphe nucleus of the brainstem compared with PF and C females. This marginal difference became signiffcant following removal of hormones by OVX. Replacement with E2 restored the number of 5-HT-ir neurons in PAE females to control levels, while P4 reversed the effects of E2. Importantly, despite these differential responses of the 5-HT system to ovarian steroids, there were no differences in E2 and P4 levels among prenatal treatment groups.
Conclusions
These data demonstrate long-term, adverse effects of PAE on the 5-HT system of females, as well as differential sensitivity of PAE compared with control females to the modulatory effects of ovarian steroids on 5-HT neurons. Our findings have important implications for understanding sex differences in 5-HT dysfunction in depression/anxiety disorders and the higher rates of these mental health problems in individuals with fetal alcohol spectrum disorder.
Keywords: Prenatal Alcohol Exposure, Ethanol, Serotonin (5-HT), Estradiol, Progesterone
Children Prenatally Exposed to alcohol may develop a fetal alcohol spectrum disorder (FASD), which comprises a range of adverse physical, behavioral, cognitive, neurobiologial, and neuropsychological outcomes. Individuals with FASD are also at high risk for so-called secondary disabilities, including mental health problems, such as depression and anxiety (O’Connor and Kasari, 2000; Pei et al., 2011; Streissguth et al., 2004).
The serotonergic (5-HT) system, which develops in the early embryonic period, is highly vulnerable to the adverse influences of prenatal alcohol exposure (PAE; Sundstrom et al., 1993). In rats, as in other mammals, most 5-HT-containing nerve cell bodies are located in the brainstem raphe nuclei and innervate many brain regions including the hypothalamus, hippocampus, and prefrontal cortex, which play a role in the effects of alcohol and other drugs and in mental health (Azmitia, 1999; Heinz et al., 2001; Steinbusch, 1981). Growing evidence demonstrates alterations in the 5-HT system caused by PAE (e.g., Druse et al., 1991; Ohta et al., 2010; Rathbun and Druse, 1985; Sari and Zhou, 2004; Sari et al., 2001; Tajuddin and Druse, 2001; Zhou et al., 2002, 2005). Deficits in brainstem 5-HT can be seen early in the embryonic period (Druse et al., 1991; Zhou et al., 2001, 2002), with decreased numbers of 5-HT immunoreactive (5-HT-ir) neurons in the dorsal raphe (DR) and median raphe (MR) nuclei and in the B9 regions of the brainstem (Zhou et al., 2001), and reductions may persist into the preweaning period (Tajuddin and Druse, 2001), adolescence (Sari and Zhou, 2004), and adulthood (Ohta et al., 2010). PAE also compromises the development of 5-HT1A receptors (Druse et al., 1991; Maier et al., 1996; Rathbun and Druse, 1985) and 5-HT innervation and forebrain development along the 5-HT pathway (Zhou et al., 2005). Importantly, children with FASD show reduced 5-HT transporter binding in the medial frontal cortex (Riikonen et al., 2005).
The long-term effects of PAE on the 5-HT system and whether effects in females are similar to those demonstrated in males are less well documented. These are important issues as the 5-HT system plays a major role in the plasticity of the brain and is linked to a wide range of functions, behaviors, and disease processes (Azmitia, 2001, 2007). Indeed, 5-HT may serve as a regulator in brain homeostasis (for review, see Azmitia, 2007) and as an essential factor in mental health (Azmitia, 2007), including depression (Nemeroff, 2002; Newport et al., 2002). Thus, 5-HT alterations may play a role in the high prevalence of mental health problems observed in individuals with FASD (O’Connor and Kasari, 2000; Pei et al., 2011; Streissguth et al., 2004).
Interactions between the 5-HT system and the hypothalamic–pituitary–adrenal (HPA) axis have been demonstrated (Azmitia et al., 1993; Chaouloff, 1993; Lanfumey et al., 2000) and are important for the current studies, as both alcohol and corticosterone (CORT) cross the placenta and affect the developing fetal brain (Hellemans et al., 2010; Seckl, 2008). Of note, PAE animals are hyperresponsive to stressors and show central dysregulation of HPA function in a manner consistent with that observed in major depressive disorder (Hellemans et al., 2008; Weinberg et al., 2008). Furthermore, PAE induces physiological and behavioral abnormalities consistent with altered HPA and 5-HT function, including lack of response inhibition, and increased depressive-/anxiety-like behaviors (e.g., Hellemans et al., 2008; Hofmann et al., 2002, 2005; Weinberg et al., 2008).
Sex differences in 5-HT metabolism, synthesis, and release and in 5-HT effects on HPA function suggest that gonadal steroids can influence 5-HT neurotransmission (Cone et al., 1981; Di Paolo et al., 1983; Sliwowska et al., 2008). Importantly, affective disorders, in which the 5-HT system is often compromised, are more common in females than in males (Kessler, 2003), may be more common at times of low estradiol levels (Fink et al., 1996, 1998; Halbreich et al., 1992), and may be increased in relation to the menstrual cycle (e.g., premenstrual depression) and after childbirth (postpartum depression). Furthermore, ovariectomy (OVX) followed by replacement with ovarian steroids has a protective action on the 5-HT system (Bethea et al., 2009; Tokuyama et al., 2008). Understanding the interactions among the adrenal, gonadal, and 5-HT systems in the PAE model could have important implications for understanding 5-HT dysfunction in depression and anxiety disorders and the higher rates of these mental health problems in individuals with FASD, particularly, in females.
The current study had 2 goals: (i) to determine whether adverse effects of PAE on numbers of 5-HT-ir neurons reported in males are also present in adult females; and (ii) to examine the role of the sex steroids in modulating numbers of 5-HT-ir neuron in PAE females, in light of the known protective effects of estradiol and the modulatory role of progesterone in the estradiol-primed brain (Bethea and Reddy, 2010; Bethea et al., 2009; Di Paolo et al., 1983; Stein, 2011). We hypothesized that estradiol will have protective effects in adult PAE females and ameliorate or reverse deficits caused by alcohol on 5-HT neurons and that progesterone may modulate the effects of estradiol.
MATERIALS AND METHODS
Breeding and Feeding of Animals
Male and female Sprague–Dawley rats (Charles River Laboratories, St. Constant, QC, Canada) were group-housed by sex, for a 2-week adaptation period, with controlled temperature (21 to 22°C) and lighting (lights on 06:00 to 18:00 hours). Males and females were then randomly paired in cages (25 9 18 9 18 cm) with mesh front and floor and checked daily for the presence of vaginal plugs, indicating gestation day 1 (GD 1).
On GD 1, females were singly housed in polycarbonates cages (24 9 10 9 46 cm) with pine shavings and randomly assigned to 1 of 3 prenatal groups: prenatal alcohol exposed (PAE), n = 17 (liquid ethanol [EtOH] diet ad libitum, 36% EtOH-derived calories); pair-fed (PF), n = 14 (liquid control diet, maltose-dextrin isocalorically substituted for EtOH, in the amount consumed by an EtOH-treated partner [g/kg body weight/d of gestation]), which provides a control for the reduced food intake that occurs with EtOH consumption; or control (C), n = 18 (ad libitum access to laboratory chow). All rats had ad libitum access to water. Liquid diets were formulated to provide adequate nutrition to pregnant rats regardless of EtOH intake and were prepared by Dyets Inc. (Weinberg/Keiver High Protein Diet #710324; Bethlehem, PA). Diets were presented through GD 22, at which time females were given ad libitum access to standard laboratory rat chow and water.
Pregnant dams and later pups were weighed and cages changed weekly. On postnatal day 1 (PND 1), dams and pups were weighed and litters culled to 10 (5 males and 5 females, when possible). Litters from the alcohol-fed and control groups remained with their natural mothers during lactation. On PND, 22 animals were weaned and group-housed by litter and sex. All animal procedures were in accordance with NIH Guidelines and were approved by the University Animal Care Committee.
Experimental Design
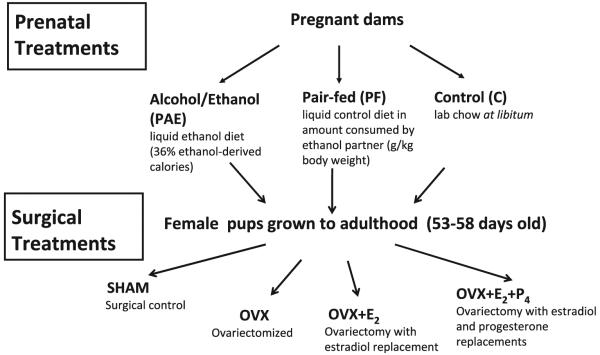
The experimental design is presented in Fig. 1. OVX with or without hormone replacement occurred in 53- to 58-day-old female offspring from C, PF, and PAE dams. OVX was performed through bilateral incisions in the dorsolateral flanks. Estrogen (E2) pellets (0.05 mg/pellet; Innovative Research of America, Sarasota, FL) were implanted subcutaneously (s.c.) immediately following OVX (OVX + E2). For replacement with physiological concentrations of both E2 and progesterone (P4; OVX + E2 + P4), both an E2 pellet (0.05 mg/pellet) and a P4 capsule (3 cm; P4 from MP Biomedicals, LLC [Aurora, OH], packed into Silastic tubing [1.57 mm ID, 3.18 mm OD; Dow Corning, Midland, MI]) were implanted s.c. immediately following OVX. E2 pellets released 17-b estradiol at doses chosen to mimic physiological levels of this hormone. Preliminary experiments determined appropriate hormone concentrations. All surgeries were carried out between 09:00 and 12:00 hours under isoflurane anesthesia. To control for litter effects, no more than 1 female from any 1 litter was used per test condition (n = 8/prenatal group/surgical treatment).
Fig. 1.
Flow chart illustrating experimental design.
Brain Perfusion
At 14 days postsurgery, animals were anesthetized and blood samples collected from the heart into tubes containing 200 µl 0.5 M EDTA. Animals were perfused (Welch Pumps; VWR, Edmonton, Alberta, Canada) transcardially with 0.9% saline for 10 minutes, followed by 4% paraformaldehyde (PFA) for 20 minutes. Brains were removed, stored in PFA for 4 hours, and transferred into a 20% sucrose solution (4°C) until saturated. Brains were sliced coronally (30 lm) in series of 4 using a microtome cryostat (HM 505E; MICROM International GmbH, Walldorf, Germany) and stored in cryopreservative solution at –20°C. Blood samples were centrifuged (2,2009g, 10 minutes at 0°C), and plasma stored at –80°C.
Vaginal Smears
Vaginal smears were taken immediately following termination to assess estrous cycle stages, within 3 to 4 hours of lights on, which is within the trough of the CORT circadian rhythm. In retrospect, this timing was not optimal for assessment of proestrus, as animals typically enter full proestrus in the late afternoon.
The estrous cycle comprises 4 stages: metestrus (diestrus I), diestrus (diestrus II), proestrus, and estrus. As ovarian hormone profiles are similar in diestrus I and diestrus II and due to the relatively low n (n = 8/prenatal treatment group/surgical treatment), we combined the 2 diestrus phases for analysis. Stages were determined by 2 independent and treatment-blind investigators (Marcondes et al., 2002; Montes and Luque, 1988).
Radioimmunoassays for E2 and P4
Plasma E2 levels were measured using the DPC Coat-A-Count Estradiol kit (KE2D; Siemens Healthcare Diagnostics Inc., Tarrytown, NY), with [125I] estradiol as the tracer. According to the manufacturer, the E2 antibody cross-reacts 100% with estradiol, 10.0% with estrone, and 4.4% with d-Equileni but does not cross-react with aldosterone or CORT. The minimum detectable E2 concentration was 8 pg/ml, and the intra- and interassay coeffcients of variation were 7.0 and 8.1%, respectively.
Surprisingly, relatively high levels of E2 were measured in OVX animals (see Results). A subset of samples was reanalyzed using the DSL Ultra-Sensitive Estradiol Radioimmunoassays (RIA) kit (DSL-4800; Beckman Coulter Inc., Brea, CA; sensitivity, 2.2 pg/ml; intra- and interassay coeffcients of variation, 8.9 and 12.2%, respectively). E2 levels were signiffcantly lower with the DSL RIA than with the DPC RIA, possibly due to differences in reaction of the immunochemical reagents to the rat serum matrix, as both kits were designed for human samples and were not optimized for use with rat plasma. Nevertheless, differences in E2 levels among groups remained proportionately the same, and statistical analyses of results were similar with the 2 assays. Here, we report values obtained with the DPC assay, as we did not have enough plasma remaining to rerun all of the samples with the DSL kit.
Plasma P4 levels were measured using the ImmuChem Double Antibody Progesterone Kit (ICN Biomedicals Inc., Costa Mesa, CA), with [125I] as the tracer. The antiserum cross-reacts 100% with progesterone, 5.41% with 20;α-dihydroprogesterone, 3.80% with desoxycorticosterone, and 0.70% with CORT. The minimum detectable P4 concentration was 0.2 ng/ml, and the intra- and interassay coeffcients of variation were 3.6 and 6.7%, respectively.
Immunohistochemistry and Quantification of 5-HT-ir Cells
All incubations were performed at room temperature, and sections were washed in Tris-buffered saline (TBS; pH = 7.4) between steps. Brainstem sections were treated with a 1% hydrogen peroxide (Sigma-Aldrich Co., St. Louis, MO), followed by incubation in 3% bovine serum albumin (Sigma-Aldrich Co.) and 0.4% Triton X-100 (Fisher Biotech, Fair Lawn, NJ) for 1 hour. Next, sections were incubated in anti-5HT primary antibody (1:5,000; horse anti-goat antibody; ImmunoStar Inc., Hudson, WI) in TBS containing 0.4% Triton X-100 and 4% normal goat serum for 48 hours. Sections were then incubated with biotinylated horse anti-goat (1:500) in TBS for 1 hour (Vector Laboratories Inc., Burlingame, CA), followed by incubation with ABC-elite kit (1:500; Vector Laboratories Inc.) in TBS for 1 hour. Sections were reacted with DAB (chromogen solution containing 0.02% 3,3′ diaminobenzidine tetrahydrochloride) and 2% nickel ammonium for 10 minutes. Finally, sections were mounted on slides and coverslipped. Controls were run omitting either the primary or secondary antibody, which resulted in the complete absence of staining for the corresponding antigen. Characterization of these 5-HT antibodies and their specificity have been previously reported (e.g., Ptak et al., 2009; Takeoka et al., 2009).
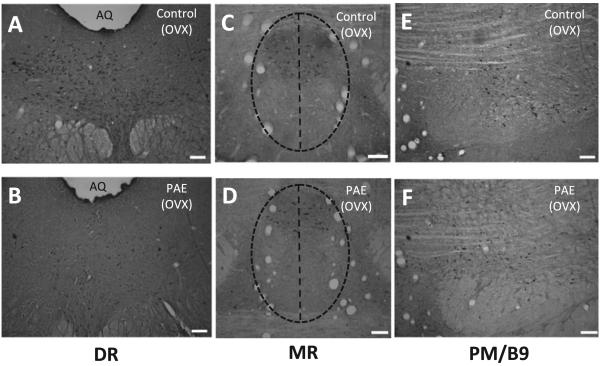
Manual counting of 5-HT-ir cells in the DR, MR, and paramedian (PM)/B9 complex (Sari and Zhou, 2004; Steinbusch, 1981) was performed under a Zeiss Axoioskop 2 Motorized Plus microscope (Zeiss, Jena, Germany), using a grid-marked eyepiece, by 2 independent and condition-blind experimenters (Fig. 3). The DR (Fig. 3A, B), MR (Fig. 3C,D), and PM/B9 complex (Fig. 3E,F) were defined according landmark described by Sari and Zhou (2004) (see figure legends). The PM raphe nuclei comprised a small number of 5-HT-ir neurons, which were outside the MR outline. As there were no landmark blood vessels that defined a border between the PM and the B9, cell counts were presented as PM/B9 (Fig. 3E,F). 5-HT-ir neurons were counted in every fourth section between the rostral end of the pons and the rostral border of the fourth ventricle at 259 magnification and verified at 409 magnification. Photomicrographs were captured with a Retiga EXi Mono 12-bit digital CCD microscope camera (Q Imaging, Burnaby, BC, Canada) using Northern Eclipse (EMPIX, Inc, Mississauga, ON, Canada). Strict criteria were established for the Quantification process. Cells with lightly stained nuclei and cells with different orientation relative to other cells of the same plane were excluded from counting.
Fig. 3.
Photomicrographs showing serotonin immunoreactive (5-HT-ir) neurons from ad libitum-fed control (C) (A, C, E) and prenatal alcohol-exposed (PAE) (B, D, F) adult ovariectomized (OVX) females. Note deficits in numbers of 5 HT-ir neurons induced by PAE in the DR nucleus (A vs. B). DR nuclei defined based on a group of landmark blood vessels, which extended vertically from the cerebral aqueduct to the level of medial longitudinal fasciculus (A, B; Sari and Zhou, 2004). MR nuclei were outlined by a group of well-defined blood vessels supplying the MR, as shown by the vertical lines in C and D. DR, dorsal raphe nuclus; MR, median raphe nucleus; PM/B9, paramedian raphe and B9 nuclei; Aq, aqueduct. Scale bar: 100 µm.
Statistical Analysis
Analyses of variance (ANOVAs; Statistica 6 software; StatSoft, Tulsa, OK) for the factors of prenatal group (C, PF, PAE) and postnatal treatment (Sham, OVX, OVX + E2, OVX + E2 + P4) were utilized to examine effects of alcohol and sex steroid replacement on number of 5-HT-ir neurons among prenatal groups. Consistent with our hypothesis that PAE would decrease the number of 5-HT-ir neurons in the brainstem, significant main effects were followed by targeted Fisher’s LSD a priori comparisons. Significance was set at p < 0.05.
RESULTS
Maternal and offspring Developmental Data
Female offspring from this study were part of larger breeding and the developmental data have been presented in detail previously (Lan et al., 2009a).
Briefly, maternal body weights were lower in PAE (355.6 ± 3.9 g) and PF (357.1 ± 4.5 g) compared with C (405.2 ± 5.4 g) females on both GD 21 (p < 0.001 for PAE vs. C, p < 0.05 for PF vs. C) and on lactation day 1 (LD 1) (PAE [285.5 ± 4.5 g], PF [291.9 ± 3.9 g], C [312.5 ± 5.8 g], ps < 0.01).
On PND 1, body weights of PAE (5.8 ± 0.1 g) and PF (5.4 ± 0.2 g) pups were lower than those of their control (6.3 ± 0.1 g) counterparts, PAE = PF < C, p < 0.01. By weaning (PND 22), there was some catch up growth in PF pups (48.5 ± 1.6 g), which were no longer different from C (51.0 ± 1.2 g). However, PAE (46.5 ± 0.9 g) females did not show this catch up in body weights (PAE < C, p < 0.05).
There Were No Effects of Prenatal Group on Hormone Levels Under Sham Conditions or Following OVX and Hormone Replacement
Sham animals were in different stages of the estrous cycle, with the majority in diestrus and only a few (in the case of PAE and PF) or none (in the case of C) in proestrus or estrus. A prenatal group 9 surgical treatment ANOVA revealed that E2 levels did not differ in Sham animals as a function of either prenatal group (p = 0.723) or stage of estrous cycle (p = 0.517). This is not surprising given the low numbers of animals in proestrus or estrus, as well as the fact that the timing of sample collection (09:00 to 12:00 hours), although optimal for obtaining basal CORT levels, was not optimal for assessment of proestrus (which occurs late afternoon). Mean E2 levels for Sham animals were 35.97 ± 4.64, 38.55 ± 5.59, and 30.76 ± 2.73 pg/ml for diestrus, proestrus, and estrus, respectively. Data were collapsed across prenatal group and stage of cycle, and a single mean is presented (Fig. 2A) for comparison with E2 levels of animal in the various hormone replacement conditions.
Fig. 2.
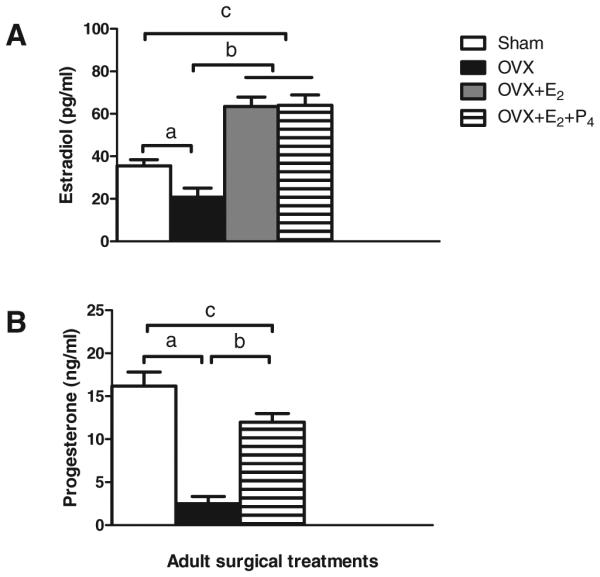
Plasma levels (mean ± SEM) of estradiol (E2) (A) and progesterone (P4) (B) in adult females, collapsed across prenatal groups. (A) aOVX < Sham, p < 0.0007; b,cOVX + E2 = OVX + E2 + P4 > Sham > OVX, ps < 0.00001; (B) aOVX < Sham, p < 0.0001; bOVX + E2 + P4 > OVX, p < 0.00004, cOVX + E2 + P4 < Sham, p < 0.031. OVX, ovariectomized.
E2 levels were lower overall in OVX than in Sham females, main effect of surgical treatment, F(1, 33) = 13.88, p < 0.0007, OVX < Sham, p < 0.003 (Fig. 2A). There were no effects of prenatal group on E2 levels in OVX animals replaced with estradiol, and data are shown collapsed across group.
As noted, surprisingly high levels of E2 were measured in OVX animals using the DPC RIA, and E2 values measured with the DSL RIA were approximately half those measured with the DPC RIA: C, 11.55 versus 20.43 pg/ml; PF, 10.96 versus 23.34 pg/ml; PAE, 7.71 versus 18.30 pg/ml, respectively. Importantly, differences in E2 levels among groups remained proportionately the same, and statistical analyses of results were similar with the 2 assays. In addition, analysis of vaginal smears taken at termination in OVX animals indicated that OVX animals were in diestrus. We conclude that measurable E2 levels in OVX animals were likely due to the parameters of the assay and not to incomplete OVX in our animals.
E2 levels in animals in the OVX + E2 and the OVX + E2 + P4 conditions did not differ from each other (p = 0.911) and were higher than levels in both OVX and Sham females (OVX + E2 = OVX + E2 + P4 > Sham > OVX, ps < 0.00001; Fig. 2A).
In agreement with the E2 data, analysis of plasma P4 levels in females in the Sham condition revealed no differences in P4 as a function of either prenatal group (p = 0.503) or stage of estrous cycle (p = 0.139). Collapsed across prenatal group, mean P4 levels for Sham animals were 17.25 ± 2.67, 16.99 ± 2.53, and 12.38 ± 2.35 ng/ml for diestrus, proestrus, and estrus, respectively. As in the case of E2, we present the data for Sham females collapsed across prenatal group and estrous cycle stage (Fig. 2B).
A main effect of surgical treatment, F(1, 33) = 40.916, p < 0.0001, indicated, as expected, lower P4 levels in OVX compared with Sham females (Fig. 2B). Furthermore, females in the OVX + E2 + P4 condition had higher P4 levels than OVX females, but lower P4 levels than Sham females (p < 0.00004, and p < 0.031, respectively; Fig. 2B). There were no differences among prenatal groups in P4 levels following OVX and hormone replacement.
Prenatal Treatment Did Not Alter the Distribution of 5-HT-ir Neurons Within the Brainstem
Distribution pattern of 5-HT-ir neurons was similar across prenatal groups and steroid replacement conditions. However, quantitative differences among groups for 5-HT-ir neuron subpopulations were observed. PAE resulted in fewer 5-HT-ir neurons in the central part of the DR compared with those in C (Fig. 3A,B) and PF (data not shown) females. In contrast, in the MR, there was no difference in the distribution patterns of 5-HT-ir neurons among C, PF, and PAE females (Fig. 3C,D; PF not shown). In the PM/B9 region, 5-HT-ir neurons were slightly smaller in size compared with those in the DR and the MR regions, but there were no differences in the distribution patterns among prenatal groups (Fig. 3E,F). Additionally, 5-HT-ir cells in the PM/B9 region were more dispersed and less densely packed compared with the 5-HT-ir neurons in the MR and DR.
PAE Differentially Altered the Effects of OVX and Sex Steroid Replacement on Numbers of 5HT-ir Neurons in the Brainstem
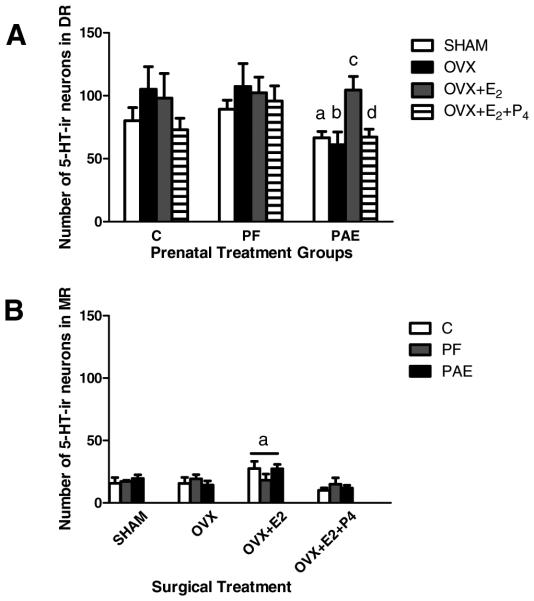
A 2-way ANOVA (prenatal group 9 surgical treatment) revealed a main effect of prenatal group, F(2, 54) = 3.2595, p = 0.046, for 5-HT-ir neuron numbers in the DR. Post hoc tests revealed a trend for lower numbers of 5-HT-ir neurons in PAE compared with both C and PF females under Sham conditions (Sham PAE < C = PF, p = 0.060 for PAE and ps > 0.174 for C and PF; Fig. 4A). Under OVX conditions, this difference became significant, with signiffcantly lower numbers of 5-HT-ir neurons in PAE than in C and PF females (PAE < C = PF; p < 0.018, p = 0.902, respectively; Figs 3A,B and 4A). Replacement with E2 (OVX + E2) or with both E2 and P4 (OVX + E2 + P4) differentially affected PAE compared with PF and C females. For both PF and C females, OVX + hormone replacement did not significantly alter 5-HT-ir neuron numbers compared with those in their SHAM and OVX counterparts. In contrast, for PAE females, replacement with E2 increased 5-HT-ir neuron numbers over that in OVX females (OVX + E2 > OVX, p < 0.029; Fig. 4A), thus rescuing the deficit, and bringing 5-HT-ir neuron numbers to the levels seen in C and PF females (ps > 0.695). However, addition of P4 (OVX + E2 + P4) countered the effects of E2 (OVX + E2 + P4 < OVX + E2, p = 0.059; Fig. 4A), such that 5-HT-ir neuron numbers decreased back to the levels of their Sham and OVX counterparts.
Fig. 4.
Total number of serotonin immunoreactive (5-HT-ir) neurons in the brainstem (A: dorsal raphe nucleus [DR] and B: median raphe nucleus [MR]) in adult female rats from ad libitum-fed control (C), pair-fed (PF), and prenatal alcohol-exposed (PAE) groups, under Sham conditions and following ovariectomized (OVX) without or with replacement with estradiol (E2) or progesterone (P4). (A) (i) for Sham: aPAE < C = PF, p = 0.060; (ii) for OVX: bPAE < C = PF, p < 0.018; (iii) for PAE only: cOVX + E2 > OVX, p < 0.029; dOVX + E2 + P4 < OVX + E2, p = 0.059, and not different from Sham; (B) aOverall, OVX + E2 > OVX = OVX + E2 + P4 = Sham, ps < 0.004.
In contrast to the DR, there were no effects of PAE on numbers of 5-HT-ir neurons in the MR, F(2, 54) = 0.00271, p = 0.917 (Fig. 4B) or the PM/B9, F(2, 54) = 0.8299, p = 0.662 (data not shown) regions. However, a main effect of surgical treatment, F(3, 54) = 4.9554, p < 0.004, revealed that for the MR overall, OVX animals replaced with E2 (OVX + E2) had higher numbers of 5-HT-ir cells compared with animals in the OVX, OVX + E2 + P4, and Sham conditions (OVX + E2 > OVX = OVX + E2 + P4 = Sham, p < 0.004; Fig. 4B).
DISCUSSION
Findings from this study demonstrate for the first time that the sex steroid hormones play a role in modulating the impact of PAE on numbers of brainstem 5-HT-ir neurons in adult female rats. PAE reduced the number of 5-HT-ir neurons in the DR of PAE females, an effect that was unmasked following removal of ovarian steroids by OVX. E2 rescued the alcohol-related loss of 5-HT-ir neurons and brought neuron numbers back to the levels observed in PF and C females. P4 attenuated the effects of E2 and reduced 5-HT-ir neuron numbers in PAE females to the levels observed in their Sham and OVX counterparts. Of note, previous studies (Ohta et al., 2010; Sari and Zhou, 2004) testing male offspring reported a decrease in numbers of 5-HT-ir neurons not only in the DR but also in the MR, which we did not see in females. Further, PAE does not appear to alter 5-HT-ir neurons in the PM/B9 nuclear groups in either males (Sari and Zhou, 2004) or females (the present study). Thus, our findings are the first to suggest the possibility that PAE may have sexually dimorphic effects on specific populations of 5-HT neurons in the brainstem.
While our data demonstrate significant modulatory effects of the sex steroid hormones on 5-HT neuron numbers in PAE females, the sex steroids appeared to have no modulatory effects on 5-HT neuron numbers in C and PF females. These latter finding are in agreement with other results showing no influence of chronic (1 month) E2 administration on 5-HT content in the DR of OVX rats (Di Paolo et al., 1983). In contrast, data suggest that ovarian steroids can modulate the 5-HT system in control animals under more acute or intermittent experimental conditions, including acute (12 or 24 hours) hormone replacement (Di Paolo et al., 1983) and weekly subcutaneous injections of estradiol benzoate alone or followed by injections of P4 (Cone et al., 1981). These paradigms parallel findings of studies conducted during the estrous cycle, where baseline 5-HT levels were shown to be higher in intact females during proestrus and estrus compared with levels in OVX rats (Felton and Auerbach, 2004).
Despite differential responses of the 5-HT system to ovarian steroids in PAE compared with control females, there were no differences in E2 and P4 levels among prenatal groups in the Sham condition, suggesting the possibility of differential sensitivity to the sex hormones in PAE compared with control females. This is supported by previous data from our laboratory (Lan et al., 2009b), where we examined the effects of estradiol on the CORT response to stress across the estrous cycle. We found that overall, across prenatal groups, the CORT stress response was greater in proestrus and estrus than in diestrus. Importantly, PAE induced an even greater enhancement of HPA activity than that in controls, with the greatest effects in proestrus. Together, these findings suggest that estradiol may have differential modulatory effects on the stress system of PAE compared with control females.
5-HT neurons of the DR project to all area of the forebrain and have a crucial role in mood disorders (see Bethea et al., 2009). Thus, loss of 5-HT neurons in OVX females in our PAE model could lead to behavioral problem. Hellemans and colleagues (2008, 2010) have shown that the combination of PAE and chronic mild stress (CMS) in adulthood increased depressive- and anxiety-like behaviors in a sexually dimorphic manner. PAE males exposed to CMS spent less time in the open arms of the elevated plus maze and had fewer open-arm entries compared with controls, whereas CMS reduced only the frequency of open-arm entries in PAE females. PAE females but not males showed greater levels of “behavioral despair” in the forced swim test, whereas PAE males and females both showed deficits in social behavior, but in different aspects of behavior and at different times of testing compared with their control counterparts (Hellemans et al., 2010). Studies by Hofmann and colleagues (2005) also found sexually dimorphic links between PAE, 5-HT function, and behavioral outcome. Anxiolytic effects of the 5-HT1A receptor agonist, 8-OH-DPAT, were found primarily in PAE females (Hofmann et al., 2005), who also showed a greater hypothermic response to 8-OH-DPAT and a greater rate of wet-dog shakes in response to the 5-HT2A/2C receptor agonist DOI, compared with controls (Hofmann et al., 2002). Thus, it appears that alterations in activity or function of the 5-HT system by PAE may have long-lasting effects that may present in a sexually dimorphic manner, supporting a modulatory role for the sex steroid hormones in these effects.
Deficits in the 5-HT system have been reported in patients with FASD (Riikonen et al., 2005), and these individuals are also at high risk for “secondary” disabilities, including mental health problems, such as depression and anxiety (O’Connor and Kasari, 2000; Pei et al., 2011; Streissguth et al., 2004). These findings together with our current data may provide insight into the development of novel treatment strategies. Clinical studies have shown that administration of ovarian steroids may be effective in alleviating some symptoms of depression and anxiety (e.g., Frey et al., 2008; Schmidt et al., 2000). Moreover, both basic science and clinical evidence support a neuroprotective role of E2 and P4 in decreasing vulnerability to neurodegenerative stimuli (Bethea and Reddy, 2010; Bethea et al., 2009; Stein, 2011). E2 and P4 are known to regulate gene and protein expression in 5-HT neurons in a manner that would increase 5-HT production and turnover as well as increase 5-HT neuronal firing and decrease 5-HT degradation. For example, ovarian steroids had a neuroprotective role in preventing DNA fragmentation and cell death in the DR in Rhesus macaques following OVX (Lima and Bethea, 2010). Moreover, ovarian steroids increased the expression of antiapoptotic genes and/or down-regulated proapoptotic genes in rodents and monkeys (Bethea and Reddy, 2010; Garcia-Segura et al., 1998). Mechanisms underlying neuroprotection by estradiol include prevention of cell death and promotion of cell survival by induction of axonal sprouting and promotion of synaptic transition (Garcia-Segura et al., 1998). P4 actions include reduction of proapoptotic and increase in antiapoptotic enzymes and modiffcation of the expression of brain derived neurotrophic growth factor (BDNF; Coughlan et al., 2009; Djebaili et al., 2004).
In this context, our finding that progesterone reversed the effects of estradiol on of 5-HT-ir neurons in estradiol-primed (OVX + E2 + P4) PAE females requires further investigation. While P4 may have protective effects in control animals, it is possible that PAE may alter the levels or function of this hormone during development or that PAE may change sensitivity of the 5-HT system to P4. Alternatively, P4 may have protective effects under certain conditions, such as the effects on apoptosis and BDNF noted above, but, in the context of its action in counteracting certain effects of estradiol, may have adverse effects under other conditions.
In summary, we present novel findings demonstrating that PAE has a long-term impact on the 5-HT system of female rats and that this impact is modulated by estradiol and P4. Keeping in mind the role of the 5-HT system in cognitive function and regulation of mood, alterations in this system could lead to cognitive and mental health problems such as anxiety and depression as seen with PAE, and conversely, enhanced survival of 5-HT neurons would support cognition and mental health. Our data have potential implications for understanding 5-HT dysfunction in depression and anxiety disorders and particularly, in females, as well as the higher rates of these mental health problems in individuals with FASD. Use of the female sex hormones as adjunctive treatments in depression/anxiety disorders might be a novel approach to treatment of mental health problems in this vulnerable population.
ACKNOWLEDGMENTS
The authors are grateful to Linda Ellis and Wayne Yu for expert assistance in all aspects of this research and would like to thank Dr. F. C. Zhou for advice on 5-HT-ir cell analysis. This work was supported by R37 AA007789 and RO1 AA022460 to JW and a fellowship from IMPART (Canadian Institutes for Health Research STIHR) to JHS.
REFERENCES
- Azmitia EC. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology. 1999;21:33S–45S. doi: 10.1016/S0893-133X(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Serotonin and brain: evolution, neuroplasticity, and homeostasis. Int Rev Neurobiol. 2007;77:31–56. doi: 10.1016/S0074-7742(06)77002-7. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Liao B, Chen YS. Increase of tryptophan hydroxylase enzyme protein by dexamethasone in adrenalectomized rat midbrain. J Neurosci. 1993;13:5041–5055. doi: 10.1523/JNEUROSCI.13-12-05041.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP. Effect of ovarian hormones on genes promoting dendritic spines in laser-captured serotonin neurons from macaques. Mol Psychiatry. 2010;15:1034–1044. doi: 10.1038/mp.2009.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Front Neuroendocrinol. 2009;30:212–238. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F. Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Brain Res Rev. 1993;18:1–32. doi: 10.1016/0165-0173(93)90005-k. [DOI] [PubMed] [Google Scholar]
- Cone RI, Davis GA, Goy RW. Effects of ovarian steroids on serotonin metabolism within grossly dissected and microdissected brain regions of the ovariectomized rat. Brain Res Bull. 1981;7:639–644. doi: 10.1016/0361-9230(81)90111-8. [DOI] [PubMed] [Google Scholar]
- Coughlan T, Gibson C, Murphy S. Progesterone, BDNF and neuroprotection in the injured CNS. Int J Neurosci. 2009;119:1718–1740. doi: 10.1080/00207450903116430. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Diagle M, Picard V, Barden N. Effect of acute and chronic 17 beta-estradiol treatment on serotonin and 5-hydroxyindole acetic acid content of discrete brain nuclei of ovariectomized rat. Exp Brain Res. 1983;51:73–76. doi: 10.1007/BF00236804. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Kuo A, Tajuddin N. Effects of in utero ethanol exposure on the developing serotonergic system. Alcohol Clin Exp Res. 1991;15:678–684. doi: 10.1111/j.1530-0277.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Felton TM, Auerbach SB. Changes in gamma-aminobutyric acid tone and extracellular serotonin in the dorsal raphe nucleus over the rat estrous cycle. Neuroendocrinology. 2004;80:152–157. doi: 10.1159/000082356. [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol. 1998;25:764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner BE, Rosie R, Grace O, Quinn JP. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16:325–344. doi: 10.1007/BF02088099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey BN, Lord C, Soares CN. Depression during menopausal transition: a review of treatment strategies and pathophysiological correlates. Menopause Int. 2008;14:123–128. doi: 10.1258/mi.2008.008019. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Cardona-Gomez P, Naftolin F, Chowen JA. Estradiol upregulates Bcl-2 expression in adult brain neurons. NeuroReport. 1998;9:593–597. doi: 10.1097/00001756-199803090-00006. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Piletz J, Halaris A. Influence of gonadal hormones on neurotransmitters, receptor, cognition and mood. Clin Neuropharmacol. 1992;15:590A–591A. doi: 10.1097/00002826-199201001-00306. [DOI] [PubMed] [Google Scholar]
- Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcohol Clin Exp Res. 2001;25:487–495. [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–175. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann CE, Patyk IA, Weinberg J. Prenatal ethanol exposure: sex differences in anxiety and anxiolytic response to a 5-HT1A agonist. Pharmacol Biochem Behav. 2005;82:549–558. doi: 10.1016/j.pbb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Hofmann CE, Simms W, Yu WK, Weinberg J. Prenatal ethanol exposure in rats alters serotonergic-mediated behavioral and physiological function. Psychopharmacology. 2002;161:379–386. doi: 10.1007/s00213-002-1048-8. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Lan N, Hellemans KG, Ellis L, Viau V, Weinberg J. Role of testosterone in mediating prenatal ethanol effects on hypothalamic-pituitary-adrenal activity in male rats. Psychoneuroendocrinology. 2009a;34:1314–1328. doi: 10.1016/j.psyneuen.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Sliwowska JH, Viau V, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic-pituitary-adrenal function across the estrous cycle. Alcohol Clin Exp Res. 2009b;33:1075–1088. doi: 10.1111/j.1530-0277.2009.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfumey L, Mannoury La Cour C, Froger N, Hamon M. 5-HT-HPA interactions in two models of transgenic mice relevant to major depression. Neurochem Res. 2000;25:1199–1206. doi: 10.1023/a:1007683810230. [DOI] [PubMed] [Google Scholar]
- Lima FB, Bethea CL. Ovarian steroids decrease DNA fragmentation in the serotonin neurons of non-injured rhesus macaques. Mol Psychiatry. 2010;15:657–668. doi: 10.1038/mp.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SE, Chen WJ, West JR. Prenatal binge-like alcohol exposure alters neurochemical profiles in fetal rat brain. Pharmacol Biochem Behav. 1996;55:521–529. doi: 10.1016/s0091-3057(96)00282-1. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Montes GS, Luque EH. Effects of ovarian steroids on vaginal smears in the rat. Acta Anat (Basel) 1988;133:192–199. doi: 10.1159/000146639. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Recent advances in the neurobiology of depression. Psychopharmacol Bull. 2002;36:6–23. [PubMed] [Google Scholar]
- Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: animal models of an adverse life event. Am J Psychiatry. 2002;159:1265–1283. doi: 10.1176/appi.ajp.159.8.1265. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Kasari C. Prenatal alcohol exposure and depressive features in children. Alcohol Clin Exp Res. 2000;24:1084–1092. [PubMed] [Google Scholar]
- Ohta K, Sakata-Haga H, Fukui Y. Alteration in anxiety-related behaviors and reduction of serotonergic neurons in raphe nuclei in adult rats prenatally exposed to ethanol. Congenit Anom (Kyoto) 2010;50:105–114. doi: 10.1111/j.1741-4520.2010.00269.x. [DOI] [PubMed] [Google Scholar]
- Pei J, Denys K, Hughes J, Rasmussen C. Mental health issues in fetal alcohol spectrum disorder. J Ment Health. 2011;20:438–448. doi: 10.3109/09638237.2011.577113. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun W, Druse MJ. Dopamine, serotonin, and acid metabolites in brain regions from the developing offspring of ethanol-treated rats. J Neurochem. 1985;44:57–62. doi: 10.1111/j.1471-4159.1985.tb07112.x. [DOI] [PubMed] [Google Scholar]
- Riikonen RS, Nokelainen P, Valkonen K, Kolehmainen AI, Kumpulainen KI, Kononen M, Vanninen RL, Kuikka JT. Deep serotonergic and dopaminergic structures in fetal alcoholic syndrome: a study with nor-beta-CIT-single-photon emission computed tomography and magnetic resonance imaging volumetry. Biol Psychiatry. 2005;57:1565–1572. doi: 10.1016/j.biopsych.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Sari Y, Powrozek T, Zhou FC. Alcohol deters the outgrowth of serotonergic neurons at midgestation. J Biomed Sci. 2001;8:119–125. doi: 10.1007/BF02255980. [DOI] [PubMed] [Google Scholar]
- Sari Y, Zhou FC. Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcohol Clin Exp Res. 2004;28:941–948. doi: 10.1097/01.alc.0000128228.08472.39. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, developmental ‘programming’ and the risk of affective dysfunction. Prog Brain Res. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- Sliwowska JH, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J. Effects of prenatal ethanol exposure on regulation of basal hypotha-lamic-pituitary-adrenal activity and hippocampal 5-HT1A receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology. 2008;33:1111–1123. doi: 10.1016/j.psyneuen.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG. Progesterone in the treatment of acute traumatic brain injury: a clinical perspective and update. Neuroscience. 2011;191:101–106. doi: 10.1016/j.neuroscience.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Sundstrom E, Kolare S, Souverbie F, Samuelsson EB, Pschera H, Lunell NO, Seiger A. Neurochemical differentiation of human bulbospinal monoaminergic neurons during the first trimester. Brain Res Dev Brain Res. 1993;75:1–12. doi: 10.1016/0165-3806(93)90059-j. [DOI] [PubMed] [Google Scholar]
- Tajuddin NF, Druse MJ. A persistent deficit of serotonin neurons in the offspring of ethanol-fed dams: protective effects of maternal ipsapirone treatment. Brain Res Dev Brain Res. 2001;129:181–188. doi: 10.1016/s0165-3806(01)00199-7. [DOI] [PubMed] [Google Scholar]
- Takeoka A, Kubasak MD, Zhong H, Roy RR, Phelps PE. Serotonergic innervation of the caudal spinal stump in rats after complete spinal transection: effect of olfactory ensheathing glia. J Comp Neurol. 2009;515:664–676. doi: 10.1002/cne.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama Y, Reddy AP, Bethea CL. Neuroprotective actions of ovarian hormones without insult in the raphe region of rhesus macaques. Neuroscience. 2008;154:720–731. doi: 10.1016/j.neuroscience.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Li TK, Goodlett C, Azmitia EC. Deviations in brain early serotonergic development as a result of fetal alcohol exposure. Neurotox Res. 2002;4:337–342. doi: 10.1080/10298420290030532. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek TA. Fetal alcohol exposure reduces serotonin innervation and compromises development of the forebrain along the serotonergic pathway. Alcohol Clin Exp Res. 2005;29:141–149. doi: 10.1097/01.alc.0000150636.19677.6f. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK, Goodlett CR, Li T. Prenatal alcohol exposure retards the migration and development of serotonin neurons in fetal C57BL mice. Brain Res Dev Brain Res. 2001;126:147–155. doi: 10.1016/s0165-3806(00)00144-9. [DOI] [PubMed] [Google Scholar]