Abstract
Aims
To determine whether a single course of antenatal dexamethasone alters resting cortisol at 3, 8 and 18 months corrected age in preterm infants.
Methods
Preterm infants born ≤32 weeks gestational age were recruited during 2001–2004 from a single neonatal intensive care unit. Resting salivary cortisol was collected at least once at 3, 8 and 18 months corrected age in a longitudinal cohort. A mixed-effects repeated measures analysis was used to accommodate cases with less than complete follow-up.
Results
One hundred and thirty three infants were included in the present study, contributing 266 cortisol samples. Of these, 107 infants had been exposed to a single course of antenatal dexamethasone and 26 not exposed to antenatal steroids. There was no significant main effect of antenatal steroids on resting cortisol at any age. This result was not altered after adjusting for gestational age at birth, neonatal cumulative pain, morphine exposure, mechanical ventilation days and post-natal steroid exposure.
Conclusions
No effect of a single course of dexamethasone on resting salivary cortisol, an indicator of hypothalamic-pituitary-adrenal axis function, was found in infancy up to 18 months corrected age in infants born very preterm.
Keywords: Antenatal glucocorticoids, Cortisol, Hypothalamic-pituitary-adrenal axis, Premature infants, Preterm
INTRODUCTION
Antenatal steroid therapy is a fundamental component in the management of preterm labour, resulting in improved survival and decreased neonatal morbidity for infants born at very low gestational age (GA) (1). Synthetic glucocorticoids accelerate foetal lung maturation, thus decreasing pulmonary complications of prematurity (1,2). However, antenatal synthetic glucocorticoids have been shown to penetrate the foetal brain and bind to glucocorticoid receptors (2). It is well established that the hypothalamic-pituitary-adrenal (HPA) axis is sensitive to programming by early life events (2). Even a brief exposure to glucocorticoids during vulnerable windows of brain development can induce permanent changes to HPA axis function, under resting and stress reactivity conditions, in the offspring of several mammalian species (2). In human preterm infants, much of the research examining the effects of antenatal steroid exposure on HPA axis regulation has involved multiple courses (3), and focused on the immediate post-natal period (4). Later, while still in the neonatal intensive care unit (NICU), one study reported decreased resting cortisol and a blunted cortisol response to heel prick at 4–6 weeks of age (5). However, another study showed no effect of antenatal steroids on urinary cortisol excretion at age 60 days (6).
To our knowledge, there are only two published studies beyond NICU discharge (7,8). One study showed altered resting cortisol and cortisol response to immunization in antenatal steroid-exposed preterms at 4 but not at 12 months chronological age. However, there was a wide range of antenatal steroid doses, with apparently only four of the infants non-exposed (7). A large randomized controlled trial of a single course of antenatal steroid versus placebo, with follow-up to adulthood, reported no change in morning cortisol in the exposed group; however, the study primarily included late preterms born at 34–35 weeks GA (8).
Importantly, we have previously reported that preterm infants born at extremely low gestational age (24–28 weeks) exhibited high resting cortisol levels at 8 and 18 months corrected age (CA) compared to controls (9,10), suggesting altered HPA function owing to programming of the HPA axis. However, the role of antenatal steroids was not evaluated. Given the dearth of human data beyond NICU discharge, the aim of the present study was to examine whether a single course of antenatal steroids altered resting cortisol levels (a marker of HPA axis regulation) from 3 to 18 months CA in infants born very preterm. To our knowledge, this is the first study to examine the effects of antenatal steroids on cortisol levels beyond the first year of life in preterm infants born at very low gestational age.
METHODS
Participants
Preterm infants participating in a study of long-term effects of neonatal pain-related stress in infants born ≤32 weeks GA (10), recruited between 2001 and 2004 from the main tertiary NICU in British Columbia, Canada. The present study is a secondary analysis of this cohort. Inclusion criteria were no major congenital anomalies or maternal heroin or cocaine use during pregnancy, and no major impairment. Infants exposed to a partial course or more than a single course of antenatal steroids were excluded, as well as one infant on hydrocortisone post-discharge.
Procedures and measures
Written consent was obtained in the NICU and at each visit, following approval by the Clinical Research Ethics Board of the University of British Columbia and Children's and Women's Hospitals. The infants were seen prospectively at least once at 3, 8 or 18 months CA. The visit was carried out at home at 3 months and in our research unit at 8 and 18 months CA. A saliva sample was collected under resting conditions when the infant was awake, alert and quietly settled on the mother's lap, shortly after arrival. All infants were healthy on the test day by parent report.
Cortisol
Cortisol was assayed from saliva and collected without stimulants, using a small cotton dental roll, then placed into a needleless syringe, and saliva expressed into a vial. Vials were stored at −20 °C until assayed using the Salimetrics High Sensitivity Salivary Cortisol Enzyme Immunoassay kit (Salimetrics LLC, Philadelphia, PA, USA). Intra-assay and inter-assay coefficients of variation were 2.92% and 3.41%, respectively.
Chart review
Medical and nursing chart review from birth to term equivalent was carried out by a single research nurse and included but was not limited to: antenatal steroid exposure and doses, gestational age at birth, Score for Neonatal Acute Physiology II (SNAP II) on day 1, number of days of mechanical ventilation, intraventricular haemorrhage (IVH, graded from neonatal head ultrasound), bronchopulmonary dysplasia (BPD, need for O2 at 36 weeks post-conceptional age), number of skin-breaking procedures, days on post-natal steroids, morphine and midazolam exposure. The regimen used in our centre during the study years was two doses of dexamethasone (12 mg), 12–24 h apart. Exposure to morphine and midazolam (IV and PO combined after appropriate conversion) from birth to term was calculated as the average dose (mg/kg) per day adjusted for daily weight multiplied by the number of days on the drug.
Data analysis
A mixed-effects repeated measures analysis was performed to accommodate cases with less than full follow-up (11,12). We examined whether the resting cortisol trajectory across age was altered because of the exposure to antenatal steroids by testing the interaction of antenatal steroid exposure on cortisol levels (log-transformed) across age, adjusting for neonatal clinical confounders.
RESULTS
Of the 248 parents approached, 156 consented to participate at the 3-month visit, 180 at 8 months and 182 at 18 months. Recruitment, exclusions and losses to follow-up are presented in Table 1. After exclusions for insufficient saliva sampling or feeding within 20 min prior to sampling, the final study sample comprised 133 infants contributing 266 cortisol samples. Of those, 40 infants had a valid cortisol sample at every visit (three samples), 53 infants had two samples and 40 infants had one sample.
Table 1.
Number of infants recruited, excluded and lost to follow-up
| 3 months | 8 months | 18 months | |
|---|---|---|---|
| Parents consented to the visit | 156 | 180 | 182 |
| Withdrew | 12 | 8 | 6 |
| Consented but not seen at visit | |||
| Died after consent obtained | 4 | 1 | – |
| Excluded for major sensory or motor impairment | 5 | 4 | 2 |
| Unable to reach the family to book the appointment | – | 3 | 1 |
| Did not attend booked visit (e.g. infant ill that day, family reasons) | 8 | 28 | 38 |
| Seen at visit but excluded | |||
| Multiple courses or a partial course of antenatal steroids, one infant exposed to hydrocortisone post-discharge | 26 | 31 | 20 |
| Invalid cortisol sample (insufficient saliva or fed <20 min prior) | 2 | 16 | 23 |
| Final N for the present study* | 99 | 89 | 78 |
The final N at each age is the number of eligible infants seen at that visit who had a valid resting cortisol sample (thus equals the number of samples, total = 266). Of the N = 133 infants in the present study, 40 infants had a valid resting cortisol sample at all three visits, 53 infants at two visits and 40 at one visit.
Saliva was collected at consistent times of day (mean ± SD): 11:30 am ± 1.5 h at the 3-month visit, 10:16 am ± 1.6 h at the 8-month visit and 9:51 am ± 0.8 h at the 18-month visit. Time from last awakening was (mean ± SD): 64 ± 54 min at the 3-month visit, 95 ± 98 min at the 8-month visit and 126 ± 58 min at the 18-month visit.
Antenatal steroid exposure
One hundred and seven infants were exposed to a single course of two doses of dexamethasone and 26 were non-exposed. Neonatal characteristics for each group are presented in Table 2.
Table 2.
Neonatal characteristics and morbidities by study group
| Non-exposed N = 26 | Exposed N = 107 | |
|---|---|---|
| GA (weeks) | 29.5 ± 2.9 | 29.1 ± 2.5 |
| Birth weight (g) | 1455 ± 597 | 1233 ± 423 |
| Male (%) | 61 | 47 |
| Out born (%)* | 27 | 6 |
| APGAR at 1 min | 6 ± 2 | 6 ± 2 |
| APGAR at 5 min | 8 ± 2 | 8 ± 1 |
| SNAP II score day 1 | 12.1 ± 14 | 13.2 ± 12.8 |
| Ventilation (days) | 15.9 ± 25 | 14.5 ± 24.1 |
| BPD (%) | 4 | 17 |
| IVH grade III-IV (%) | 4 | 1 |
| Cortisol (μg/dL) at 3 m† | 0.27 (0.13–0.54) | 0.21 (0.15–0.35) |
| Cortisol (μg/dL) at 8 m† | 0.17 (0.12–0.2) | 0.13 (0.11–0.24) |
| Cortisol (μg/dL) at 18 m† | 0.11 (0.06–0.15) | 0.13 (0.07–0.24) |
Mean ± SD.
p < 0.05.
Median (Interquartile range).
Post-natal steroid exposure
In the group exposed to antenatal steroids, 18 infants also received post-natal steroids: seven received dexamethasone alone, six received hydrocortisone alone and five received both of the drugs. In the group not exposed prenatally, four infants received post-natal steroids: one received dexamethasone alone, one received hydrocortisone alone and two received both.
Neonatal predictors of resting cortisol
Time of day was not significantly correlated with cortisol level and therefore was not considered further. Significant interactions with age at cortisol collection were found for gestational age at birth (p = 0.005), days of post-natal dexamethasone therapy (p = 0.008), days on mechanical ventilation (p = 0.023), number of skin-breaking procedures (p = 0.008) andcumulativemorphine(p = 0.028). Each of those neonatal factors was associated with lower cortisol level at 3 months, no relationship at 8 months and higher cortisol at 18 months.
Antenatal steroids in relation to resting cortisol at 3, 8 and 18 months
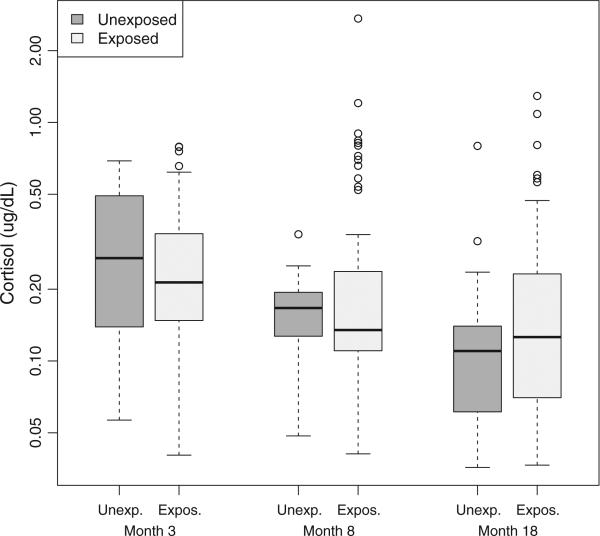
There was no significant main effect of antenatal steroids on resting cortisol across age (p = 0.80; see Fig. 1). The antenatal steroid by age interaction remained non-significant (p = 0.72) after adjusting for a significant interaction between gestational age at birth and corrected age at cortisol collection (p = 0.009). Results remained the same after further adjustment for neonatal cumulative pain, morphine, post-natal steroid exposure and ventilation. The analysis was re-run excluding the 22 infants exposed to post-natal steroids, and the results were unchanged. In a model with only main effects for antenatal steroid exposure, the estimated effect of antenatal steroid exposure on cortisol levels relative to non-exposure was 1.05 (95% confidence interval 0.83–1.33). Our finding provides 95% confidence that a reduction in cortisol was no greater than 17%, and a rise in cortisol was no greater than 33%, indicating no major effect of antenatal steroid exposure on later cortisol.
Figure 1.
Box plots for resting salivary cortisol at 3, 8 and 18 months CA by study group. Box plots: Cortisol values for exposed and non-exposed infants at each visit (median, interquartile range). Minimum and maximum limits shown with outliers identified as circles if the value exceeded the top or bottom quartile by more than 1.5 times the interquartile range.
DISCUSSION
The present study, to our knowledge, is the first to examine the impact of antenatal steroids on cortisol to 18 months CA with an appropriate sample of non-exposed infants for comparison. We found that infants exposed to a single course of antenatal dexamethasone had similar resting cortisol values compared to infants whose mothers had no antenatal steroid administered. We demonstrated that a difference of more than 33% in cortisol levels between the groups is unlikely, unlike reports in animals demonstrating a 50–60% change or more in resting cortisol or corticosterone levels after antenatal steroid exposure (13–15). Our results are consistent with the findings of Shulman et al. (6), which showed no effect of antenatal steroids on urinary cortisol levels to 60 days of age in preterm infants born at 26–30 weeks of GA. In contrast, Glover et al. (7) found antenatal steroid exposure to be associated with higher resting cortisol levels at 4 months chronological age in preterm infants born <32 weeks GA. However, there was a wide range of antenatal exposure, with as many as 16 doses at the upper end, and the study appeared to include only four non-exposed infants. In that study, the infants were tested at 4 months of age from birth, thus were far younger than the infants in the present study. Importantly, at 12 months from birth, resting cortisol was not altered, which is in line with our findings. Interestingly, Davis et al. (5) reported lower resting cortisol in a group of antenatal steroid-exposed pre-term infants born at 28–30 weeks of gestation, and tested at 34 weeks post-menstrual age, compared to a group of unexposed older preterms born at 34 weeks GA tested during the first days of life. Although the post-menstrual age at testing was comparable in both groups, the steroid-exposed infants were born more premature and exposed to more NICU experiences. Furthermore, neuroendocrine developmental shifts in the first weeks of life (10,16) and the association between greater previous exposure to procedural pain and suppressed cortisol response later in the NICU (17) make these results difficult to evaluate. The inconsistency of studies carried out early in life, reporting increase (7), decrease (5) or no change in resting cortisol (6), underlines the importance of following infants to later ages as was carried out in the current study. One large-scale study examined the effects of antenatal steroid exposure on former preterms into adulthood (8). After adjustment for demographic factors, no difference in early morning cortisol concentrations was found between antenatal steroid-exposed and non-exposed adults at 30 years of age. This cohort was born 1969 and 1974, had a median GA at delivery of 34–35 weeks and a mean birth weight 2290 g and above. These factors may limit the applicability of those results to the current very low gestational age preterm population.
While our finding of no effect of antenatal steroid exposure on resting cortisol at 3, 8 or 18 months is consistent with the only other study with an adequate sample size in infancy (6) and with the only study in adults (8), it is divergent from the vast animal data that support a major effect of antenatal steroids on later functioning of the HPA axis. Long-term alterations of the HPA axis following steroid exposure were found in offspring of several species, primarily related to glucocorticoid receptor modification in the brain and pituitary (18,19). Because hippocampal glucocorticoid receptors in humans are already expressed at 24 weeks of gestation (20), the time when antenatal steroids are administered in the management of preterm labour, this is concerning. Several important differences exist between human and animal studies, which may account for the different findings. First, there are known variations in neuroendocrine regulation of the maternal-placental-foetal unit across mammals (21). Second, the effects of antenatal steroid exposure on HPA activity in animal studies varied depending on the species, timing and duration of exposure and dose (14,18,19,22). A low dose of dexamethasone (0.5 mg/kg, close to the dose used in the human regimen) given prenatally in monkeys did not cause the hippocampal structural changes that were seen with higher doses (13). Last, animal studies are typically designed to expose the foetus to antenatal steroids during a normal gestation progressing to an undisturbed normal term delivery. In contrast, preterm infants are exposed to antenatal steroids during threatened preterm labour, a time of heightened maternal and foetal stress with high levels of endogenous maternal and foetal glucocorticoids, peaking during preterm delivery (23,24). Exogenous steroids given on top of already high levels of endogenous glucocorticoids may not produce additional long-term effects on the HPA axis. Importantly, in human studies that are more analogous to animal models, in which infants received antenatal steroids for preterm labour but delivered at term, alterations in HPA axis response were found (25,26), albeit inconsistently.
In the present study, our findings remained the same after adjusting for medical confounders such as post-natal steroid exposure, mechanical ventilation, gestational age, as well as cumulative procedural pain-related stress, previously shown to be correlated with cortisol levels (10). In our previous work, we found elevated levels of resting cortisol from 8 to 18 months CA in ELGA preterm infants compared to term controls (9,10). The results of our current study indicate that antenatal steroids did not contribute to these elevated levels.
While sex-dependent effects of antenatal steroids have been described in animal studies (19), we did not find sex differences, consistent with the only other study in humans that examined this factor (25). Because HPA axis regulation has been shown to be influenced by hypothalamic-pituitary-gonadal hormones (19), differences may only manifest later in life after reaching sexual maturity.
One of the strengths of our study is that we included only infants exposed to a single course of antenatal steroids, in order to reflect the standard and most common practice today. Furthermore, our non-exposed control group comprised preterm infants with a comparable degree of prematurity and exposure to early preterm labour and NICU experiences. The lack of an effect at any of the ages tested suggests that our findings are robust.
There are several limitations to our study. First, the timing of antenatal dexamethasone administration was unknown. However, while programming of the HPA axis is time-dependant in animals (27), no effect of gestational age at antenatal steroid administration on HPA axis regulation was found in human infants delivered at term (25). Second, because of the ethical concerns of inducing stress for research purposes in young children, we only studied resting cortisol. In the animal literature, altered HPA programming is found under both resting and reactivity conditions (13,14) following antenatal steroids. In humans, no association was reported between antenatal steroid exposure and response to immunization stress at 12 months of life (7), consistent with our results with resting cortisol. Finally, betamethasone is more frequently used (in the USA) for threatened preterm delivery than dexamethasone.
CONCLUSION
We found no effect of a single course of antenatal steroids on resting salivary cortisol in infancy from 3 to 18 months CA, suggesting that antenatal steroids are not likely to have a major impact on the HPA axis long term. Our findings are reassuring, given that for the foreseeable future, there will not be a change in practice using prenatal steroids.
Key notes.
In preterm infants, a single course of antenatal dexamethasone did not alter resting salivary cortisol, the end product of the hypothalamic-pituitary-adrenal (HPA) axis, in infancy up to 18 months corrected age.
This finding in humans is reassuring and differs from the vast animal literature suggesting altered programming of the HPA axis following administration of antenatal steroids.
ACKNOWLEDGEMENT
We thank the families who participated and Adi Keidar, Gisela Gosse and Ivan Cepeda for project management.
FUNDING
This study was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development grant R01 HD39783 to REG. REG holds a Senior Scientist award from the Child and Family Research Institute; SB holds a post-doctoral fellowship from the Louise and Allan Edwards Foundation; SPM holds a Tier II Canada Research Chair and a Scholar award from the Michael Smith Foundation for Health Research. JW is also supported by NIH/NIAAA R37 AA007789.
Abbreviations
- HPA
hypothalamic-pituitary-adrenal
- GA
gestational age
- CA
corrected age
- NICU
neonatal intensive care unit
- ELGA
extremely low gestational age
- BPD
bronchopulmonary dysplasia
- IVH
intraventricular haemorrhage
- SNAP II
Score for Neonatal Acute Physiology II
References
- 1.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Matthews SG, Kapoor A. Antenatal glucocorticoids and programming of neuroendocrine function and behavior. In: Lagercrantz H, Hanson MA, Ment LR, Peebles DM, editors. The newborn brain: neuroscience and clinical applications. 2nd ed. Cambridge University Press; Cambridge: 2010. pp. 366–7. [Google Scholar]
- 3.Ng PC, Wong GW, Lam CW, Lee CH, Fok TF, Wong MY, et al. Effect of multiple courses of antenatal corticosteroids on pituitary-adrenal function in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1999;80:F213–6. doi: 10.1136/fn.80.3.f213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis EP, Townsend EL, Gunnar MR, Georgieff MK, Guiang SF, Ciffuentes RF, et al. Effects of prenatal betamethasone exposure on regulation of stress physiology in healthy premature infants. Psychoneuroendocrinology. 2004;29:1028–36. doi: 10.1016/j.psyneuen.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Davis EP, Townsend EL, Gunnar MR, Guiang SF, Lussky RC, Cifuentes RF, et al. Antenatal betamethasone treatment has a persisting influence on infant HPA axis regulation. J Perinatol. 2006;26:147–53. doi: 10.1038/sj.jp.7211447. [DOI] [PubMed] [Google Scholar]
- 6.Shulman RJ, Heitkemper M, O'Brian Smith E, Lau C, Schanler RJ. Effects of age, feeding regimen, and glucocorticoids on catecholamine and cortisol excretion in preterm infants. JPEN J Parenter Enteral Nutr. 2001;25:254–9. doi: 10.1177/0148607101025005254. [DOI] [PubMed] [Google Scholar]
- 7.Glover V, Miles R, Matta S, Modi N, Stevenson J. Glucocorticoid exposure in preterm babies predicts saliva cortisol response to immunization at 4 months. Pediatr Res. 2005;58:1233–7. doi: 10.1203/01.pdr.0000185132.38209.73. [DOI] [PubMed] [Google Scholar]
- 8.Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365:1856–62. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 9.Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114:e77–84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thi-essen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150:151–6. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinheiro J, Bates D, DebRoy S, Sarkar D. R Core team. NLME: linear and nonlinear mixed effects models. R package version 3. 2009:1–96. [Google Scholar]
- 12.R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. Available at: http://www.R-project.org (version 2.11.0) [Google Scholar]
- 13.Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, et al. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–48. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 14.Sloboda DM, Moss TJ, Gurrin LC, Newnham JP, Challis JR. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol. 2002;172:71–81. doi: 10.1677/joe.0.1720071. [DOI] [PubMed] [Google Scholar]
- 15.Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–8. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- 16.Kidd S, Midgley P, Nicol M, Smith J, McIntosh N. Lack of adult-type salivary cortisol circadian rhythm in hospitalized preterm infants. Horm Res. 2005;64:20–7. doi: 10.1159/000087324. [DOI] [PubMed] [Google Scholar]
- 17.Grunau RE, Holsti L, Haley DW, Oberlander T, Weinberg J, Solimano A, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–9. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280:E729–39. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- 20.Noorlander CW, De Graan PN, Middeldorp J, Van Beers JJ, Visser GH. Ontogeny of hippocampal corticosteroid receptors: effects of antenatal glucocorticoids in human and mouse. J Comp Neurol. 2006;499:924–32. doi: 10.1002/cne.21162. [DOI] [PubMed] [Google Scholar]
- 21.Smith R, Nicholson RC. Corticotrophin releasing hormone and the timing of birth. Front Biosci. 2007;12:912–8. doi: 10.2741/2113. [DOI] [PubMed] [Google Scholar]
- 22.de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, Louw J, et al. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest. 2007;117:1058–67. doi: 10.1172/JCI30982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tropper PJ, Warren WB, Jozak SM, Conwell IM, Stark RI, Goland RS. Corticotropin releasing hormone concentrations in umbilical cord blood of preterm fetuses. J Dev Physiol. 1992;18:81–5. [PubMed] [Google Scholar]
- 24.Phocas I, Sarandakou A, Rizos D. Maternal serum total cortisol levels in normal and pathologic pregnancies. Int J Gynaecol Obstet. 1990;31:3–8. doi: 10.1016/0020-7292(90)90173-i. [DOI] [PubMed] [Google Scholar]
- 25.Schaffer L, Luzi F, Burkhardt T, Rauh M, Beinder E. Antenatal betamethasone administration alters stress physiology in healthy neonates. Obstet Gynecol. 2009;113:1082–8. doi: 10.1097/AOG.0b013e3181a1f0e6. [DOI] [PubMed] [Google Scholar]
- 26.Davis EP, Waffarn F, Sandman CA. Prenatal treatment with glucocorticoids sensitizes the hpa axis response to stress among full-term infants. Dev Psychobiol. 2011;53:175–83. doi: 10.1002/dev.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapoor A, Leen J, Matthews SG. Molecular regulation of the hypothalamic-pituitary-adrenal axis in adult male guinea pigs after prenatal stress at different stages of gestation. J Physiol. 2008;586:4317–26. doi: 10.1113/jphysiol.2008.153684. [DOI] [PMC free article] [PubMed] [Google Scholar]