Abstract
Background
Rats prenatally exposed to ethanol (E) typically show increased hypothalamic-pituitary-adrenal (HPA) responses to stressors in adulthood. Importantly, prenatal ethanol may differentially alter stress responsiveness in male and female offspring, suggesting a role for the gonadal hormones in mediating the effects of ethanol on HPA activity. We investigated the role of ethanol-induced changes in hypothalamic-pituitary-gonadal (HPG) activity in the differential HPA regulation observed in E compared to control females across the estrous cycle.
Methods
Peripheral hormones and changes in central neuropeptide mRNA levels were measured across the estrous cycle in adult female offspring from E, pair-fed (PF) and ad libitum-fed control (C) dams.
Results
Ethanol females showed normal estrous cyclicity (vaginal smears) but delayed sexual maturation (vaginal opening). Both HPG and HPA activity were differentially altered in E (and in some cases, PF) compared to control females as a function of estrous cycle stage. In relation to HPG activity, E and PF females had higher basal and stress estradiol (E2) levels in proestrus compared to other phases of the cycle, and decreased GnRH mRNA levels compared to C females in diestrus. Further, E females had greater variation in LH than PF and C females across the cycle, and in proestrus, only E females showed a significant LH increase following stress. In relation to HPA activity, both basal and stress CORT levels and overall ACTH levels were greater in E than in C females in proestrus. Furthermore, AVP mRNA levels were increased overall in E compared to PF and C females.
Conclusions
These data demonstrate ethanol-induced changes in both HPG and HPA activity that are estrous phase-specific, and support the possibility that changes in HPA activity in E females may reflect differential sensitivity to ovarian steroids. E females appear to have an increased HPA sensitivity to E2, and a possible shift toward AVP regulation of HPA activity. That PF were similar to E females on some measures suggests that nutritional effects of diet or food restriction played a role in mediating at least some of the changes observed.
Keywords: Prenatal Ethanol, Stress, Pituitary-Adrenal, Pituitary-Gonadal, Estrous Cycle
Evidence from both human and animal studies indicates that alcohol is a teratogen that can cause malformations, intrauterine death, growth retardation, central nervous system abnormalities, and behavioral and physiological deficits (Abel and Berman, 1994; Abel and Dintcheff, 1978; Jones and Smith, 1973; Jones et al., 1973; Sokol and Clarren, 1989; Stratton et al., 1996; Weinberg, 1993; Zajac and Abel, 1992). Among the physiological abnormalities, maternal alcohol intake is known to alter many aspects of offspring endocrine function, including activity and regulation of the hypothalamic-pituitary-adrenal (HPA) and -gonadal (HPG) axes.
Studies have shown, for example, that alcohol-exposed infants exhibit higher basal cortisol levels (Jacobson et al., 1999; Ramsay et al., 1996) and increased responses to a heel-stick blood draw (Jacobson et al., 1999) and to a psychologically stressful “still-face” procedure (Haley et al., 2006) compared to nonexposed infants. Animal studies support and extend these findings. While basal corticosterone (CORT) levels are typically normal, ethanol-exposed (E) offspring are generally hyperresponsive to stressors. Enhanced and/or prolonged CORT, adrenocorticotropin (ACTH) and β-endorphin responses to stressors including footshock, ether, restraint, cold and immune challenges, and to drugs such as alcohol and morphine, have been reported in E compared to control rats (Angelogianni and Gianoulakis, 1989; Kim et al., 1999; Nelson et al., 1986; Weinberg et al., 1996), and both behavioral and HPA hyperresponsiveness to maternal separation stress have been observed in nonhuman primates (Schneider et al., 2004). Importantly, data suggest that central regulation of HPA activity is altered under both basal and stress conditions, and that both increased HPA drive and deficits in feedback regulation probably underlie the HPA hyperresponsiveness that is observed (Gabriel et al., 2005; Glavas et al., 2006, 2007; Lee et al., 1990, 2000; Osborn et al., 1996).
Interestingly, while HPA hyperresponsiveness is a robust pheonomenon, occurring in both male and female offspring, patterns of responsiveness may differ depending on the stressor, hormone, and time course measured (Lee and Rivier, 1996; Weinberg, 1988, 1992; Weinberg et al., 1996). This raises the possibility that the gonadal hormones may play a role in mediating prenatal ethanol effects on HPA activity. Indeed, a large body of evidence shows that the adrenal and gonadal axes develop in parallel and interact bidirectionally. The HPA hormones modulate HPG activity, and the effects of gonadal hormones on HPA function have been demonstrated at all levels of the axis. In general, estradiol (E2) activates, and androgens inhibit HPA function, mainly by changing the production and secretion of cortocotropin-releasing hormone (CRH) and/or arginine vasopressin (AVP) in the paraventricular nucleus (PVN) of the hypothalamus (Almeida et al., 1992; Bingaman et al., 1994; Bohler et al., 1990; Lund et al., 2004; Patchev et al., 1995; Roy et al., 1999; Viau et al., 2003, 2005). Furthermore, sex differences are seen at all levels of the HPA axis. For example, females have higher basal and stress levels of ACTH and CORT than males (Handa et al., 1994; Le Mevel et al., 1978, 1979; Young, 1995), as well as higher levels of CRH protein and CRH mRNA in the PVN (Watts and Swanson, 1989).
Importantly, prenatal ethanol exposure is known to alter HPG development and maturation in both male and female offspring. Of relevance to the present study, sexual maturation (vaginal opening) (Boggan et al., 1979; Creighton-Taylor and Rudeen, 1991; Esquifino et al., 1986; McGivern and Yellon, 1992), onset of follicle stimulating hormone (FSH) secretion (Wilson and Handa, 1997), and onset of puberty (McGivern and Yellon, 1992) may be delayed in E females. Altered developmental patterns of prolactin and luteinizing hormone (LH) secretion have also been reported in E compared to control females (Esquifino et al., 1986), and juvenile E females show increased sensitivity to exogenous gonadotropins compared to controls (Rudeen and Hagaman, 1988). Alterations in LH and FSH activity and responsiveness following ovariectomy and hormone replacement have also been observed, suggesting ethanol-induced changes in both pituitary and central regulation (Handa et al., 1985; Wilson et al., 1995). In addition, E females exhibit a decreased pituitary response in vitro to hormone challenge (Creighton-Taylor and Rudeen, 1991) as well as an earlier incidence of acyclicity (premature reproductive aging) than controls (McGivern et al., 1995).
In most studies to date examining HPA regulation in E females, including our own, females have been tested randomly across the phases of the estrous cycle. This has allowed us to demonstrate differences in prenatal ethanol effects in females versus males, but has resulted in increased variability among females, and has prevented us from correlating phase of the cycle with HPA activity and thus understanding possible alterations in HPG-HPA interactions induced by ethanol. In the present study, we utilized estrous cycle effects to examine the possible role of the ovarian steroids in mediating the differential changes in HPA activity in E compared to control females. Our experimental questions were: 1) Do E females show normal sexual maturation (vaginal opening) and estrous cyclicity? 2) If E females are cycling, then do they differ from controls in adrenal and gonadal hormone levels at different phases of the estrous cycle? 3) Do differences between E and control females across the estrous cycle occur under both basal and stress conditions? and 4) Do central measures of HPA and HPG activity across the estrous cycle differ in E compared to control females? We reasoned that ethanol-induced changes in HPG activity could contribute to differential regulation of HPA activity by the sex steroids in E compared to control females. We hypothesized that alterations in HPA responsiveness observed in E females compared to their control counterparts may be mediated, at least partly, by ethanol-induced changes in HPG regulation and/or HPG-HPA interactions.
MATERIALS AND METHODS
Animals and Breeding
Male (275 to 300 g, n = 10) and female (230 to 275 g, n = 31) Sprague–Dawley rats were obtained from Charles River Laboratories (St Constant, PQ, Canada). Rats were group-housed by sex and maintained on a 12:12 hours light/dark cycle (lights on at 06:00 hours), with controlled temperature (21 to 22°C), and ad libitum access to standard lab chow (Jamieson’s Pet Food Distributors Ltd., Delta, BC, Canada) and water. One to two weeks following arrival, males were placed singly in stainless steel suspended cages (25 × 18 × 18 cm), with mesh front and floor, together with a female. Wax paper under the cages was checked daily, and the presence of a vaginal plug indicated day 1 of gestation (G 1). All animal use and care procedures approved by the University of British Columbia Animal Care Committee.
Diets and Feeding
On G 1, females were singly housed in polycarbonate cages (24 × 16 × 46 cm) with pine shavings bedding and randomly assigned to 1 of 3 treatment groups: 1) Ethanol, liquid ethanol diet (36% ethanol-derived calories) and water, ad libitum (n = 9); 2) pair-fed (PF), liquid control diet with maltose-dextrin isocalorically substituted for ethanol, and intake matched to the amount consumed by an E partner (g/kg body weight/gestation d), and water ad libitum (n = 12); 3) control (C): standard lab chow and water, ad libitum (n = 10). The liquid diets (Dyets, Inc., Bethlehem, PA) were formulated in our lab to provide adequate nutrition to pregnant rats regardless of ethanol intake. All animals were provided with fresh diet daily within 1.5 hours prior to lights off to prevent a shift of CORT circadian rhythms, which may occur in PF animals who are on a restricted feeding schedule (Gallo and Weinberg, 1981). Experimental diets were continued through G 21, and beginning on G 22 animals were provided ad libitum access to standard lab chow and water, which they received throughout lactation. Pregnant dams were handled only on G 1, G 7, G 14, and G 21 for cage changing and weighing. On postnatal day 1 (PN 1), pups were weighed and litters were randomly culled to 10 (5 males and 5 females when possible). If necessary, cross fostering of pups occurred with pups from the same prenatal treatment group born on the same day, to maintain the litter size. Dams and pups were weighed on PN 1, PN 8, PN 15, and PN 22. On PN 22, pups were weaned and group-housed by litter and sex. Female pups were pair-housed starting around PN 40.
Blood Ethanol Levels
To determine the maximal or near maximal blood ethanol level (BEL) achieved by E dams, tail blood samples from 3 randomly chosen ethanol-treated dams were taken on G 15, 2 hours after lights off, which typically follows a major eating bout. Blood samples were allowed to coagulate for 2 hours at room temperature and then spun down at 2,200 × g for 20 minutes at 4°C. Serum was collected and stored at −20°C until the time of assay. BELs were measured using Pointe Scientific Inc. Alcohol Reagent Set (Lincoln Park, MI). The minimum detectable concentration of ethanol is 2 mg/dl.
Vaginal Openings
Vaginal opening is an index of sexual maturation in rodents, since sexually immature rats have a closed orifice. Beginning at 28 days of age, females were examined daily at 10:00 hours for evidence of vaginal opening. Age at vaginal opening was recorded.
Vaginal Smears
The 4 to 5 days estrous cycle in adult female rats consists of metestrus (diestrus I), diestrus (diestrus II), proestrus, and estrus. For simplicity, we combined the 2 diestrus phases because the ovarian hormone profiles are similar.
To verify if all females have normal estrous cycles, we tracked the estrous cycle by vaginal smears for 10 consecutive days (approximately 2 cycles), at 10:00 to 11:00 hours each day, 2 weeks prior to testing. Postmortem vaginal smears were done to verify the stage of the cycle at termination. Smears were done by vaginal lavage using a blunt tipped disposable pipette and ~ 0.3 ml physiological saline. The material collected was then applied to a slide and air-dried. Specimens were then stained with 1% toluene blue and cytology examined under a light microscope. Criteria for stage of cycle were as follows: diestrus – presence of abundant leucocytes and a few nucleated epithelial cells and cornified cells (irregular cells without nuclei); proestrus – predominance of nucleated epithelial cells with a few cornified cells; estrus – predominant presence of large cornified cells (Marcondes et al., 2002; Montes and Luque, 1988).
Stress Procedure
Female offspring, 90 to 120 days, (n = 151 in total: 43 E, 59 PF, 49 C) were used in this study. Rats were decapitated prior to (0 minutes, basal condition) or 30 minutes after (stress condition) the onset of a 30 minutes restraint stress. The stress procedure consisted of placement into polyvinyl chloride restraint tubes (5.5 × 20 cm, inner diameter × length) for a 30-minute period. The front cap of the tube had 4 holes 1 cm apart to allow for ventilation and the end cap had a 1.5 cm opening for the tail. Restraint is primarily a psychological stressor, and causes no pain or injury. All testing was done within the circadian trough at 09:30 to 12:00 hours. Animals were decapitated within 30 seconds of touching the cage (0 minute) or the restraint tube (30 minutes). Trunk blood was collected into ice-chilled polystyrene tubes containing 200 μl 0.5 M EDTA (to prevent coagulation) and 4 μg/ml aprotinin (to protect ACTH from denaturation). Due to the short time frame (30 minutes) of restraint stress in the present study, we measured only basal mRNA levels of gonadotropin-releasing hormone (GnRH), CRH and arginine vasopression (AVP), as changes in mRNA expression normally peak at about 3 to 4 hours poststress (Ma et al., 1997). Thus, brains from animals in the 0 minute (basal) condition were rapidly removed and immediately frozen on dry ice, then stored at −80°C until sectioning for measurement of mRNA levels.
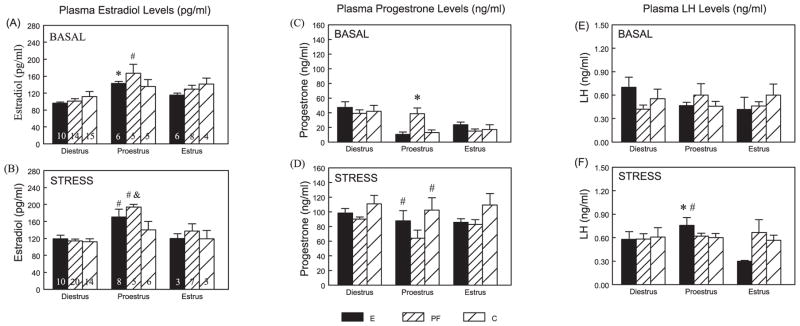
Because animals were terminated in the morning, in order to obtain basal CORT levels at the circadian trough, and because animals are typically in full proestrus in the late afternoon or early evening (within a few hours of lights off) (Park et al., 1990; Porkka-Heiskanen et al., 1994), we found fewer animals in proestrus (n’s = 5 to 8) and estrus (n’s = 3 to 8) than in diestrus (n’s = 10 to 20). The n per prenatal treatment, time and estrous phase is shown in Fig. 1A, B.
Fig. 1.
Plasma E2 (pg/ml) (A, B), progesterone (ng/ml) (C, D), and LH (ng/ml) (E, F) levels across the estrous cycle at basal and stress conditions in E, PF, and C females (mean ± SEM). The n’s in each prenatal treatment, time and estrous phase is shown in (A) and (B). For E2 levels, E: proestrus > diestrus under basal conditions (*p < 0.01), and proestrus > diestrus = estrus under stress conditions (#p’s < 0.05). PF: Proestrus > Diestrus = Estrus under both basal and stress conditions (#p’s < 0.05). In proestrus: PF > C (&p < 0.05) following stress. For progesterone levels, in proestrus, PF > E = C under basal conditions (*p < 0.001), and stress > basal in E and C females only (#p < 0.001). For LH levels, E: in proestrus, stress > basal (*p < 0.01), and proestrus > estrus under stress conditions (#p < 0.05).
To control for litter effects, only 1 female per litter from each of E, PF, and C conditions at each phase of the estrus cycle, was taken for analysis of brain neuropeptide mRNA levels and hormone measurements. The only exception was for hormone measurements in diestrus females, where a second female from some litters was included. This was due to the fact that in order to prevent any disturbance prior to termination, stage of cycle was verified by postmortem vaginal smears.
Radioimmunoassays
Blood samples were centrifuged at 2,200 × g for 10 minutes at 0°C. Plasma was transferred into 600 μl Eppendorf tubes and stored at −80°C until assayed.
Estradiol
Plasma E2 levels were measured using an adaptation of a 17β-estradiol radioimmunoassays (RIA) kit (ICN Biomedicals, Inc., Costa Mesa, CA) with [125I] 17β-estradiol as tracer and all reagent volumes halved. The 17β-estradiol antibody cross-reacts 100% with 17β-estradiol, 20% with estrone, 1.51% with estriol, 0.68% with 17α-estradiol, but not with progesterone, testosterone, cortisol, aldosterone, or cholesterol (<0.01%). The minimum detectable 17β-estradiol concentration was 10 pg/ml, and the intra- and inter-assay coeffeicients of variation were 4.7 and 9.1%respectively.
Progesterone
Plasma progesterone levels were measured using an adaptation of progesterone RIA kit (ICN Biomedicals) with [125I] progesterone as tracer and all reagent volumes halved. The progesterone antibody cross-reacts 100% with progesterone, 5.41% with 20α-dihydroprogesterone, 3.8% with desoxycorticosterone, 0.7% with CORT, 0.16% with testosterone, but not with 17α-estradiol, 17β-estradiol, aldosterone, or cortisol (<0.01%). The minimum detectable progesterone concentration was 0.2 ng/ml, and the intra- and inter-assay coeffeicients of variation were 3.6 and 6.7% respectively.
Luteinizing Hormone
Plasma LH levels were measured by RIA in the laboratory of Dr. A.F. Parlow, NIDDK, National Hormone and Peptide Programme (Harbor-UCLA Medical Centre, Torrance, CA). The cross-reactivity with other pituitary hormones was negligible. The minimum detectable LH concentration of the assay was 0.1 ng/ml. The intra- and inter-assay coefficients of variation were less than 10% (Attademo et al., 2004).
Corticosterone
Total CORT (bound plus free) levels were measured by RIA in plasma extracted in absolute ethanol (Kaneko et al., 1981; Weinberg and Bezio, 1987). Antiserum was obtained from MP Biomedicals (Orangeburg, NY), tritiated CORT tracer from Mandel Scientific (Guelph, ON, Canada) and CORT for standards from Sigma Chemical Co (St Louis, MO). Dextran-treated charcoal (Fisher Scientific Ltd., Nepean, ON, Canada) was used to absorb free CORT after incubation. The antiserum cross-reacts 100% for CORT, 2.3% for desoxycorticosterone, 0.47% for testosterone, 0.17% for progesterone and 0.05% for aldosterone. The minimum detectable CORT concentration was 0.25 μg/dl and the intra- and inter-assay coefficients of variation were 1.55 and 4.26%respectively.
Adrenocorticotrophin
Plasma ACTH levels were measured using an adaptation of an ACTH RIA kit (Diasorin Inc., Stillwater, MS) with [125I] ACTH as tracer and all reagent volumes halved. The ACTH antibody cross-reacts 100% with porcine ACTH1–39 and human ACTH1–24, but not with α-melanocyte-stimulating hormone (MSH), β-endophin, β-lipotropin (<0.1%). The minimum detectable ACTH concentration was 20 pg/ml, and the intra- and inter-assay coeffeicients of variation were 3.9 and 6.5% respectively.
In Situ Hybridization
Brain Preparation
Brains from animals under basal conditions were sectioned coronally into 20 μm sections through the rostral part of the medial preoptic area (MPOA) (Bregma −0.12 mm), and 14 μm sections through the extent of the PVN (Bregma −1.80 mm) (Paxinos and Watson, 2005). Frozen sections were thaw-mounted onto gelatin-coated slides and stored at −80°C.
Oligonucleotide Probes and Labeling
Oligonucleotide probes were used to measure GnRH mRNA in the MPOA, and CRH and AVP mRNA in the PVN. Probes were synthesized at the Oligonucleotide Synthesis Laboratory, University of British Columbia as follows: antisense GnRH (5′-TTCAGTATTTCTCTTCCCCCCAGGGCGCAACCCATAGGACCAGTGCTG-3′) (Zoeller et al., 1988), antisense CRH (5′-CAGTTTCCTGTTGCTGTGAGCTTGCTGAGCTAACTGCTCTGCCCTGGC-3′) (Jingami et al., 1985; Young et al., 1986a), and antisense AVP (5′-GTAGACCCGGGGCTTGGCAGAATCCACGGACTCTTGTGTCCCAGCCAG-3′) (Ivell and Richter, 1984; Young et al., 1986b). Sense strand oligos for GnRH, CRH, and AVP mRNA were used as negative controls, and yielded no positive hybridization signal. Probes were 3′ tail labeled with 35S-dATP (Amersham Biosciences, Piscataway, NJ) using terminal deoxytransferase (New England Biolabs Inc., Pickering, ON, Canada) as per supplier protocol. Probes were purified using G-25 Sephadex Columns (Roche Scientific, Indianapolis, IN). 1 M dithiotreitol (DTT) was added to prevent oxidation.
Hybridization With Oligonucleotides
The procedure for in situ hybridization is described in greater detail elsewhere (Lan et al., 2006). Briefly, sections were thawed and prehybridized as follows: fixed in formalin for 30 minutes, acetylated for 10 minutes in 0.1 M triethanolamine-hydrochloride −0.9% NaCl (pH 8.0 + 0.25% acetic anhydride), dehydrated through a graded series of ascending concentrations of ethanol, and air-dried. Hybridization buffer (50% formamide, 3× SSC, 1× Denhardt’s solution, 100 μg/ml yeast tRNA, 25 mM sodium phosphate buffer (pH 7.4), 10% dextran sulphate, 55 mM DTT, 30% deionized water), was applied (probe activity for AVP: 2 × 105 cpm/section; CRH: 2.72 × 105 cpm/section; GnRH: 4.5 × 105 cpm/section), coverslipped and incubated overnight at 37°C in 50% formamide humidified containers. Coverslips were removed and slides were washed in 2× SSC (20 minutes) twice, 2× SSC/0.01 M DTT (45°C, 20 minutes), 1× SSC (45°C, 15 minutes), 1× SSC/50% formamide (45°C, 30 minutes), 1× SSC (10 minutes), 0.5× SSC (10 minutes). Sections were dipped briefly in NF water 5 times then plunged into 70% ethanol (5 minutes), then air dried overnight. Sections hybridized for CRH and AVP mRNA detection were exposed to Kodak BioMax MR film (Eastman Kodak Co., Rochester, NY) for 6 days and 1 hour, respectively. All slides were dipped in Kodak NTB2 autoradiography emulsion (Eastman Kodak Co.) diluted 1:1 (deionized H2O) dilution and exposed for 24 days for GnRH mRNA, 19 days for CRH and overnight for AVP in dessicated, light tight boxes at 4°C. Slides were developed with Kodak D-19 developer at 14°C and fixed with Kodak Polymax T fixer at 14°C, then counterstained with Cresyl Violet for anatomical reference.
Densitometric Analysis
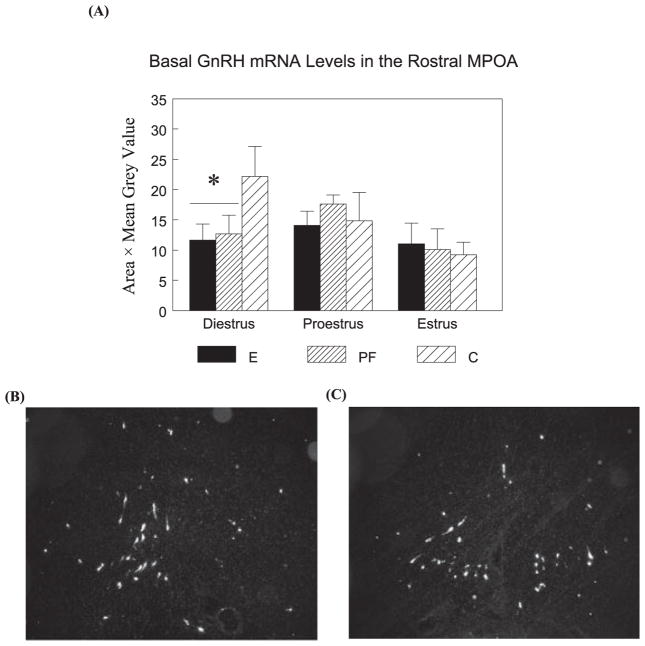
The densitometric analysis methods are the same as described previously (Lan et al., 2006). GnRH, CRH, and AVP mRNA positive cells were visualized with a Q-imaging monochrome 12-bit camera attached to a Zeiss Axioskop 2 microscope. Images were captured using Northern Elite 6.0v (Empix Imaging Inc., Mississauga, ON, Canada) and semiquantitative densitometric analyses were performed using Image J 1.33v software (National Institutes of Health, Bethesda, MD). For GnRH mRNA, 2 anatomically matched sections were evaluated from each brain, corresponding to the region of the rostral MPOA that surrounds the rostral tip of the third ventricle (see Fig. 2B, C). Signal was measured by outlining clusters of labeled neurons and measuring the pixel area and the mean OD (grain density measurements over individual clusters of neurons). Total GnRH mRNA levels for each section were calculated by pixel area × mean OD for each cluster and then summing the values of all the clusters. Values from the 2 sections were averaged to represent the total GnRH mRNA levels of each animal.
Fig. 2.
Basal GnRH mRNA levels (mean ± SEM, n = 4 to 6 per group) in the rostral MPOA (A) in E, PF, and C females and representative dark-field photomicrographs of nuclear emulsion-dipped sections demonstrating GnRH mRNA pattern in the rostral MPOA (B, Bregma 0.00 mm level; C, Bregma 0.12 mm level). E = PF < C in diestrus (*p’s < 0.05).
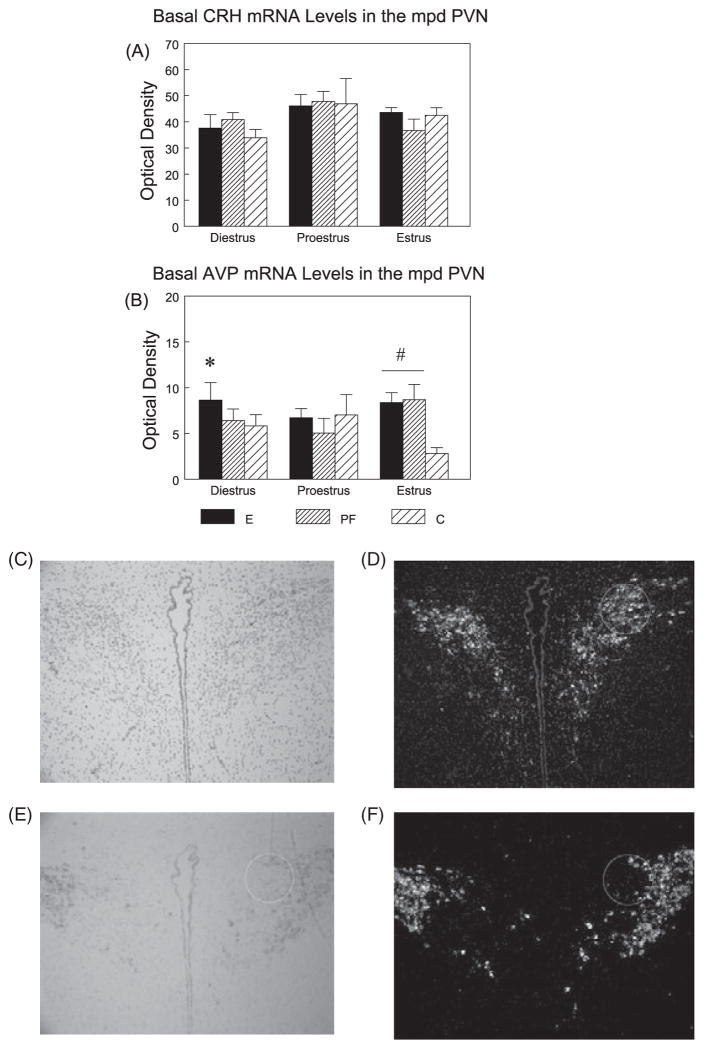
The mean optical density (OD) of hybridization signal was measured under dark-field illumination for CRH and AVP mRNA signals (Fig. 6). Densitometric analysis was conducted over the medial parvocellular dorsal division (mpd) of the PVN for both CRH and AVP (Swanson and Sawchenko, 1983). The mpd PVN was traced by outlining a fixed circle (0.75 in diametre; scale 300 pixels/inch) under bright field. Illumination was then switched to dark-field, the tracing was restored over the image, and OD measurements were taken. AVP sections were matched for rostrocaudal level based on the signal pattern, and CRH measurement was carried out on the section adjacent to the AVP section. CRH OD was measured concurrently with AVP, such that the area of measurement used for CRH was then superimposed on the corresponding AVP image. Background signal was measured over a region immediately adjacent to the PVN. The corrected grey levels were averaged from 4 measurements through the extent of the mpd region of the PVN.
Fig. 6.
Basal CRH (A) and AVP (B) mRNA levels (mean ± SEM, n = 4 to 6 per group) in the medial parvocellular dorsal (mpd) PVN (outlined in circle) across the estrous cycle in E, PF, and C females, and representative bright-field and dark-field photomicrographs of nuclear emulsion-dipped sections demonstrating CRH (C, D) and AVP (E, F) mRNA patterns in the PVN. For CRH mRNA levels, proestrus > diestrus in C females only (p = 0.05). For AVP levels, overall (collapse phase) E > C (p < 0.05). In diestrus, E > PF = C(*p’s < 0.05); In estrus, E = PF > C (#p’s < 0.05).
Statistical Analyses
Developmental and body weight data were analyzed using repeated measures ANOVAs for the factors of prenatal group (E, PF, C) and day. Hormone data were first analyzed using three-way ANOVAs for the factors of prenatal group (E, PF, C), estrous phase (diestrus, proestrus, estrus), and time (0, 30 minutes), followed by further two-way (group X time) ANOVAs within each estrous phase, with the error term adjusted to reflect the error term from the three-way ANOVA, when appropriate. Neuropeptide mRNA levels were assessed under basal conditions, and analyzed using two-way (group × phase) ANOVAs. Newman–Keuls and Fisher least significant differences (LSD) post hoc tests were used to detect significant main and interaction effects, respectively. Further analyses utilized planned comparisons to test the a priori hypothesis that E females have differential HPA responsiveness across the estrous cycle compared to their control counterparts. Statistical significance was set at p < 0.05; p < 0.10 was considered a statistical trend. Note that p values shown in Table and Figure legends represent post hoc tests.
As noted above, we had n’s of 3 for some conditions (2 cases of 18). Based on a similar estrus cycle study which also had n’s of 3 for some cells (Figueiredo et al., 2002). We ran a power analysis to calculate their effect size, and then used that number to do an a priori test to calculate the sample size necessary for detecting significant 2-way interactions (group X phase) in the present study. We found that our sample size is well above the level needed for detection of significant effects in 2-way or 3-way interactions.
RESULTS
Pregnancy Outcome and Pup Development
Ethanol intake of pregnant females was consistently high throughout gestation, averaging 10.88 ± 0.47, 11.38 ± 0.40, 11.34 ± 0.27 g/kg BW/d for weeks 1, 2, and 3 of gestation, respectively, and resulting in BELs of 104.3 ± 9.2 mg/dl measured 2 hours after lights off on G 15.
Analysis of maternal body weights during gestation indicated significant main effects of group (F(2,26) = 9.04; p < 0.005) and day (F(3,78) = 1089.50; p < 0.001), and a group × day interaction (F(6,785) = 10.98; p < 0.001) (Table 1). E, PF, and C dams were similar in body weight on G 1 but by G 21, as expected, E and PF weighed significantly less than C dams (p < 0.001). Similarly, we found main effects of group (F(2,26) = 3.60; p < 0.05) and day (F(3,78) = 197.4; p < 0.001), and a group × day interaction (F(6,78) = 3.07; p < 0.01) for body weights during lactation (L). E dams weighed less than PF and C dams on L 1 (p < 0.001), whereas there were no differences in body weights among groups on L 22 and no significant differences among groups for gestational length, litter size or number of live born or stillborn pups.
Table 1.
Maternal Body Weights (g) During Gestation (Days 1 and 21) and Body Weights (g) of Female Offspring at Birth (Postnatal Day 1, PND1) and Weaning (PND22)
| Dams
|
Female pups
|
|||
|---|---|---|---|---|
| G1 | G21 | PND1 | PND22 | |
| E | 262.5 ± 2.0 | 364.9 ± 6.2* | 5.16 ± 0.08*# | 43.57 ± 1.24* |
| PF | 271.2 ± 2.9 | 376.7 ± 8.0* | 5.66 ± 0.09* | 44.95 ± 0.77* |
| C | 268.6 ± 1.8 | 411.9 ± 4.1 | 6.16 ± 0.10 | 50.96 ± 0.98 |
Values represent the mean ± SEM of average body weights per group in dams or litters (n = 10 to 11 per group).
p’s < 0.001 compared to C;
E < PF, p’s < 0.01.
Analysis of female pup body weights indicated significant main effects of group (F(2,26) = 18.693; p < 0.001) and day (F(3,78) = 3635.54; p < 0.001), and a group × day interaction (F(6,78) = 7.922; p < 0.001) (Table 1). E and PF pups weighed less than their C counterparts at birth (p < 0.001) and on PN 22 (p < 0.05), indicating that E and PF did not show catch up growth at weaning.
Vaginal opening was assessed as a marker of puberty/sexual development. Data shown in Fig. 3 indicate a delay in vaginal opening in E and PF females. Fifty percent of C females showed vaginal opening on PN 33, whereas vaginal opening did not occur in 50% of E and PF females until PN 34. Furthermore, the percentage of rats showing vaginal opening was lower in E compared to PF (χ2=8.28, p < 0.01) and C (χ2=2.85, p < 0.09) females on PN 32; and lower in E compared to C on PN 33 (χ2=3.87, p<0.05), PN 35 (χ2=3.71, p = 0.05), and PN 36 (χ2=4.13, p < 0.05). However, we found that females from all prenatal groups displayed normal estrous cycles in adulthood as assessed by vaginal smears taken over 10 days.
Fig. 3.
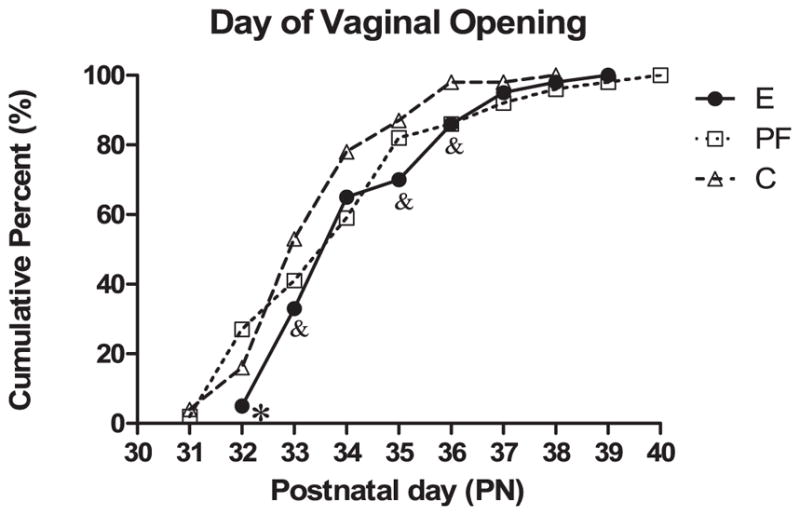
Percentages of rats showing vaginal opening. Values represent the cumulative percent of rats with vaginal opening observed on each postnatal day (n = 43 to 49 per group). *E < PF, p < 0.01, E < C, p < 0.09 on PN 32; &E < C on PN 33 and PN 36, p’s < 0.05, p = 0.05 on PN 35.
HPG Activity Under Basal and Stress Conditions
Plasma E2 Levels
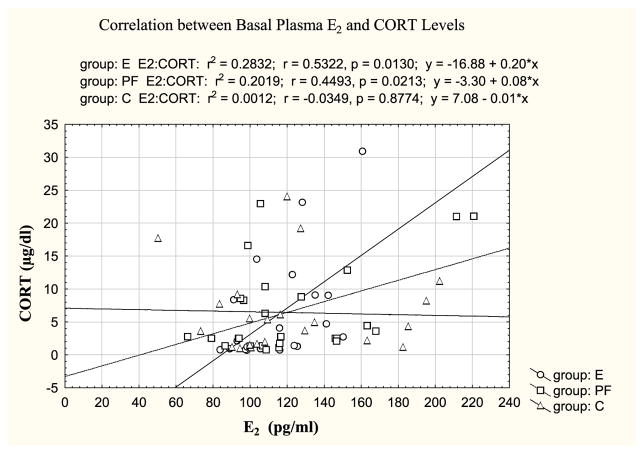
The three-way ANOVA indicated a main effect of estrous phase (F(2,130) = 27.83; p < 0.001) (Fig. 1A, B) and a trend for a group × phase interaction (F(4,130) = 2.22; p < 0.07). As expected, E2 levels overall were higher in proestrus than estrus (p < 0.001), and higher in estrus than diestrus (p < 0.01). Importantly, post hoc tests indicated that these differences were due primarily to greater changes in hormone levels across the cycle in E and PF compared to C females. Two-way ANOVAs within basal and stress conditions revealed that C females showed similar basal E2 levels across the cycle, and a trend for higher E2 levels in proestrus compared to diestrus (p < 0.08). In contrast, E females had significantly higher basal E2 levels in proestrus compared to diestrus (p < 0.01) and higher stress E2 levels in proestrus compared to both diestrus (p < 0.01) and estrus (p < 0.05); similarly, PF females had higher E2 levels in proestrus compared to both diestrus and estrus under both basal (p’s < 0.05) and stress (p’s < 0.01) conditions. In addition, stress E2 levels were higher in PF than C females in proestrus (p < 0.05), with a similar trend in E compared to C females that failed to reach significance (E > C, p < 0.09). Importantly, we found a positive correlation between basal E2 and CORT in E and PF but not C females (Fig. 4).
Fig. 4.
Positive correlations between basal E2 and CORT levels were found in E and PF (p’s < 0.05) but not C females.
The E2 levels detected in this study were higher than levels reported in some previous studies (Nequin et al., 1979) but similar to those in a study that used the same RIA kit (ICN Biomedicals) (Figueiredo et al., 2002). Although this RIA kit is specific for 17β-estradiol detection, 20% cross-reactivity occurs with estrone (manufacturer’s data), which could potentially have increased our values.
Plasma Progesterone Levels
Significant main effects of time (F(1,129) = 197.143; p < 0.001) and phase (F(2,129) = 7.69; p < 0.001), and a group × time interaction (F(2,129) = 5.557; p < 0.005) were found (Fig. 1C, D). Overall, progesterone levels were higher in diestrus than in proestrus and estrus, and showed an increase following 30-minute stress (p’s < 0.005). Inspection of Fig. 1C, D indicates that the overall group × time interaction was due primarily to the response of the PF group in proestrus. That is, a within phase group × time interaction (F(2,29) = 3.88; p < 0.05), indicated that during proestrus, PF had higher basal progesterone levels than E and C females, and only E and C females showed significant progesterone elevations following stress (p < 0.001).
Plasma LH Levels
Two-way ANOVAs (phase × time; group × time) revealed differential changes in prenatal treatment groups across the cycle and time. A trend for a main effect of phase in E females (F(2,36) = 2.39; p = 0.10) indicated that E females had significantly higher stress LH levels in proestrus compared to estrus (p < 0.05). Furthermore, in proestrus, a significant main effect of time (F(1,26) = 5.11; p < 0.05) indicated that E but not PF or C females showed a significant LH response to stress (30 > 0 minute, p < 0.01) (Fig. 1E, F). In contrast, PF and C females showed no differences in basal or stress levels of LH across the estrous cycle.
Basal GnRH mRNA in the Rostral MPOA
A two-way ANOVA followed by planned comparisons indicated that during diestrus, E and PF had lower basal GnRH mRNA levels in the rostral MPOA compared to C females (p’s < 0.05). There were no significant effects of prenatal treatment on GnRH mRNA levels across the estrous cycle (Fig. 2A).
HPA ACTIVITY UNDER BASAL AND STRESS CONDITIONS
Plasma Levels of CORT and ACTH
Corticosterone
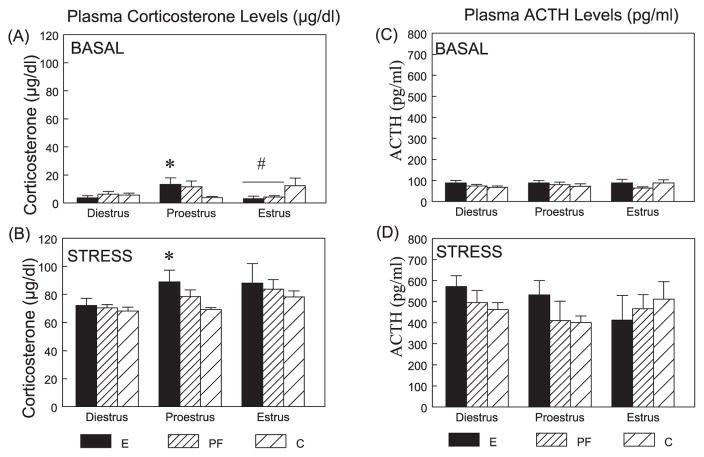
The three-way ANOVA indicated main effects of time (F(1,125) = 1165.66 p < 0.001) and phase (F(2,125) = 6.53; p < 0.005), and trends for group × time (F(2,125) = 2.55; p < 0.08) and time × phase (F(2,125) = 2.93; p < 0.06) interactions (Fig. 5A, B). Basal (0 minute) CORT levels were significantly higher in E than in C females in proestrus (p < 0.05), but were significantly lower in E and PF than in C females (p’s < 0.05) in estrus. As expected, CORT levels at 30-minute poststress were significantly higher than basal levels in all groups (p < 0.001), and overall, the CORT stress response was greater in proestrus (p < 0.05) and estrus (p < 0.005) than in diestrus. Importantly, in proestrus, E females had higher stress CORT levels than C females (p < 0.05).
Fig. 5.
Plasma CORT (μg/dl) (A, B) and ACTH (pg/ml) (C, D) levels across the estrous cycle at basal and stress conditions in E, PF, and C females. For CORT levels, in proestrus, E > C under both basal and stress conditions (*p’s < 0.05). In estrus, E = PF < C under basal conditions (#p’s < 0.05). For ACTH levels, overall (collapse time and phase), E > C (p < 0.05), and E > C (collapse time) in diestrus (p < 0.05).
Adrenocorticotropin
The three-way ANOVA indicated the expected main effect of time (F(1,121) = 246.39; p < 0.001) (Fig. 5C, D). Overall, ACTH levels at 30 minutes were elevated over basal levels in all groups (p’s < 0.001). Planned comparisons indicated that, in parallel with the CORT data, ACTH levels overall were higher in E compared to C females in proestrus (p < 0.05). E females also had higher ACTH levels overall than C females in diestrus (p < 0.05). Inspection of Fig. 5 indicates that the overall differences in ACTH can be attributed primarily to ACTH levels following stress.
Basal CRH and AVP mRNA in the mpd PVN
Cortocotropin-Releasing Hormone
A main effect of phase (F(2,48) = 3.82; p < 0.05) (Fig. 6A), indicated that overall, basal CRH mRNA levels in proestrus were significantly higher (p < 0.05) than in diestrus and marginally higher (p < 0.08) than in estrus. Interestingly, planned comparisons revealed that the significant phase effect is due mainly to C females. That is, for C females, CRH mRNA levels were higher in proestrus than in diestrus (p = 0.05), whereas for E (p > 0.16) and PF (p > 0.20) females, there were no significant differences in CRH mRNA levels across the cycle.
Arginine Vasopressin
The two-way ANOVA indicated a trend for an effect of group (F(2,37) = 2.88; p < 0.07) (Fig. 6B). Overall, basal AVP mRNA levels were significantly higher in E than in C females (p < 0.05); the difference between E and PF females failed to reach significance (p < 0.08). Furthermore, planned comparison revealed that these differences were due primarily to cycle phase. Thus, basal AVP mRNA levels were significantly higher in E compared to PF and C (p’s < 0.05) females in diestrus, and in E and PF compared to C females (p’s < 0.05) in estrus.
DISCUSSION
A number of novel findings demonstrate effects of prenatal ethanol exposure on both peripheral and central measures of HPG and HPA function, with the most marked effects observed during proestrus, suggesting that ethanol-induced changes in HPA activity may reflect, at least partly, differential regulation by ovarian steroids in E compared to control females.
Ethanol Effect on Pregnancy Outcome and Pup Development
Prenatal ethanol exposure had significant adverse effects on pregnancy outcome, as indicated by reduced birth weights in E male and female compared to control pups, and both E and PF pups had significantly lower weights than C pups at weaning. This indicates that catch-up growth did not occur, and that some developmental outcomes may be mediated, at least in part, by nutritional effects of diet. In addition, consistent with previous studies in both mice (Boggan et al., 1979) and rats (Creighton-Taylor and Rudeen, 1991; Esquifino et al., 1986; McGivern and Yellon, 1992), we found that prenatal ethanol exposure delayed sexual maturation, as measured by the time of vaginal opening. Fifty percent of C females showed vaginal opening on PN 33, whereas vaginal opening did not occur in 50% of E and PF females until PN 34. Furthermore, a significantly lower percentage of E compared to PF and/or C females showed vaginal opening on postnatal days 32, 33, 35, and 36.
Effects of Prenatal Ethanol on HPG Activity Across the Estrous Cycle
In the present study, animals were tested in the morning, at the trough of the circadian rhythm, in order to avoid the afternoon increase in basal CORT which, in itself, could alter HPG activity. While this timing did enable us to obtain low basal CORT and ACTH levels (see discussion below), it probably impacted our ability to detect optimal proestrus effects. That is, animals are typically in full proestrus in the late afternoon or early evening (within a few hours of lights off) (Park et al., 1990; Porkka-Heiskanen et al., 1994), and both E2 and progesterone peak at that time (Nequin et al., 1979). Thus, our animals were likely in very early proestrus at the time of termination. That progesterone levels were lower overall in proestrus than diestrus supports this suggestion. Nevertheless, we found, as expected, that estradiol levels were higher overall in proestrus than in estrus, and in estrus than in diestrus. These data are consistent with those of Isgor and colleagues (2002), who also measured hormone levels at the circadian trough. They reported that both E2 and progesterone levels were naturally lower than the potential peak levels that would occur in the afternoon of proestrus, and that there was a small but nonsignificant increase in E2 and decrease in progesterone levels in proestrus compared to the other phases of the cycle.
While E females appeared to show normal estrous cyclicity, our data demonstrate effects of prenatal treatment on HPG activity and regulation across the estrous cycle. Several of these effects appeared to be mediated, at least in part, by nutritional effects of diet and/or of food restriction. Under both basal and stress conditions, E and PF females had higher E2 levels in proestrus compared to other phases of the cycle, whereas C females showed similar E2 levels across the cycle, suggesting greater fluctuations in hormone levels in E and PF compared to C females. In addition, E and PF females showed decreased GnRH mRNA levels compared to C females in diestrus, and there were no differences among prenatal groups in GnRH mRNA levels across the estrous cycle. These data are consistent with those of Zoeller and Young, who reported high GnRH mRNA levels in the morning of diestrus day 1 and a decrease from diestrus day 2 to the morning of proestrus (Zoeller and Young, 1988). Similarly, Gore and Roberts, using quantitative ribonuclease protection assays, found increased GnRH mRNA levels in diestrus. However, since this was not paralleled by an increase in GnRH primary transcript, the authors proposed that this may be due to an increase in GnRH mRNA stability (Gore and Roberts, 1995). Another possibility is that the higher GnRH mRNA levels in C females in diestrus in the present study may reflect an increase in GnRH biosynthesis, which could restore the releasing pool of GnRH before the proestrus surge of GnRH release; if so, it would appear that E and PF females are deficient in this aspect of HPG regulation.
We found specific effects of ethanol on LH regulation, whereas changes in E2, progesterone and GnRH levels, appear to be mediated, at least partly, by the nutritional effects of diet. Consistent with previous data showing that LH levels in E animals typically normalize after 40 days of age (Esquifino et al., 1986), we found no significant differences in LH levels among prenatal treatment groups at any phase of the cycle. However, only E females showed a significant LH response to restraint stress during proestrus, and had higher LH levels in proestrus than in estrus, suggesting increased HPG activity despite down-regulation of GnRH.
The fact that testing occurred at the circadian trough may have influenced our results. For example, Wilson and colleagues found that following estrogen treatment, fetal ethanol-exposed animals had significantly greater LH levels in the evening than in the morning, indicating the presence of a preovulatory-like surge of LH. They also found that the afternoon surge of LH in response to estrogen treatment was significantly reduced in E females compared to controls (Wilson et al., 1995).
Regardless of the mechanism, however, our data clearly demonstrate long-term effects of prenatal treatment on HPG regulation, with some aspects of HPG activity enhanced and other aspects are blunted in E compared to control animals, and some effects mediated by the nutritional effects of diet or diet restriction.
Interestingly, we also found specific effect of pair-feeding on plasma ovarian hormone levels. During proestrus, stress E2 levels were higher in PF than C females, and basal progesterone levels were higher in PF compared to both E and C females. Further, PF females did not show the significant progesterone elevations following stress shown by E and C females. This pair-feeding effect may be due to the fact that pair-feeding is a treatment as well as a control.
The nutritional aspects of prenatal ethanol exposure are of fundamental importance in determining the toxic effect of ethanol. Ethanol administration may affect nutrient intake directly, or cause secondary malnutrition due to its deleterious effects at almost every level of the gastrointestinal tract, as well as its direct toxic effects on the digestive glands, liver, and pancreas (Lieber, 2005; Weinberg, 1984, 1985). In contrast, PF dams are fed a reduced ration, equivalent to that consumed by their E partners, such that both calorie and protein intake are significantly reduced in E and PF compared to control dams (Weinberg, 1985). Thus, in addition to controlling for the reduced food intake of ethanol-consuming dams, pair-feeding is also a treatment in itself (Weinberg, 1984). The relatively mild undernutrition of E dams could further exacerbate the effects of ethanol on the developing fetus. However, PF dams experience mild stress due to the hunger that accompanies consuming less than they would eat ad libitum. Thus, it is possible that the mechanisms underlying basal HPA dysregulation, while resulting in a similar HPA phenotype, may differ between E and PF animals rather than occurring along a continuum of effects on the same pathway. That is, changes in HPA regulation in E animals may result from direct effects of ethanol on the developing HPA axis, whereas changes in PF pups may be due to mild prenatal stress (Weinberg et al., 2008). Importantly, prenatal stress is known to be associated with an increased risk of cardiovascular, metabolic, and neuroendocrine disorders in adult life (Matthews, 2002; Seckl, 2004). Moreover, like prenatal ethanol exposure, both prenatal stress and prenatal undernutrition reprogram the HPA axis, altering HPA tone and stress responsiveness as well as temporal patterns of secretion (Koehl et al., 1999; Lucas, 2005; Walker, 2005). It is possible that prenatal exposure to mild stress in PF offspring may shift circadian rhythms such that at the time of sampling PF females were in a later phase of proestrus than E and C females, thus accounting for the differential hormone levels observed. That PF females were similar to E females on some measures suggests that nutritional effects of diet or diet restriction played at least some role in mediating the changes observed. Further studies are needed to investigate these possibilities.
Do HPG Changes Mediate HPA Alterations in E Compared to PF and C Females?
Previous studies have shown that in general, plasma CORT and ACTH levels are elevated during the proestrus phase of the estrous cycle, when E2 levels are at their peak (Atkinson and Waddell, 1997; Bohler et al., 1990; Buckingham et al., 1978; Carey et al., 1995; Raps et al., 1971; Viau and Meaney, 1991). Similarly, cyclic variations in HPA function are reflected by changes in CRH/AVP expression and synthesis, which are highest during the proestrus phase (Bohler et al., 1990; Greer et al., 1986; Hiroshige and Wada-Okada, 1973; Viau and Meaney, 2004).
Ovarian steroids, E2 and progesterone, play an important modulatory role in HPA responsiveness (Atkinson and Waddell, 1997; Burgess and Handa, 1992; Carey et al., 1995; Dayas et al., 2000; Figueiredo et al., 2007; Lund et al., 2004; Roy et al., 1999; Viau and Meaney, 1991, 2004; Young, 1995; Young et al., 2001). Both ACTH and CORT responses are enhanced by E2 treatment of ovariectomized (OVX) female rats (Carey et al., 1995; Viau and Meaney, 1991), and E2 can increase PVN CRH mRNA levels in OVX female rats (Patchev et al., 1995) and monkeys (Roy et al., 1999). Similarly, higher basal AVP mRNA levels were found in the PVN of estrogen-treated OVX females compared to intact males (Ferrini et al., 1997). In contrast, progesterone has been shown to inhibit the facilitatory effect of E2 on ACTH responses following stress (Viau and Meaney, 1991) and on CRH mRNA expression in the PVN (Roy et al., 1999).
In the present study, examination of HPA changes across the estrous cycle unmasked possible influences of the HPG hormones on HPA activity that could not be seen previously with observations collapsed across cycle stage. We found specific effects of ethanol on ACTH and AVP mRNA regulation, whereas changes in CORT and CRH mRNA levels appear to be partly mediated by nutritional effects of diet. Overall, across prenatal groups, the CORT stress response was greater in proestrus and estrus than in diestrus. Importantly, however, prenatal ethanol exposure induced even greater enhancement of HPA activity, with the greatest effects in proestrus. Both basal and stress CORT levels and overall ACTH levels were greater in E than in C females in proestrus. Moreover, stress-induced elevations in CORT occurred in parallel with plasma E2 levels in E and PF but not C females. In view of our finding that changes in ovarian steroid hormone levels were similar in E and PF females, these data support the possibility that changes in HPA activity may reflect differential sensitivity to or regulation by ovarian steroids in E and/or PF compared to control females. Consistent with this argument, we have previously shown that estrogen receptor β (ER-β) mRNA levels are upregulated in the PVN in E compared to PF and C females (Yamashita et al., unpublished data). ER-β modulates HPA responses to stress and is regulated by circulating CORT (Isgor et al., 2003). Moreover, estradiol may modulate behaviors and functions mediated by the amygdala and hypothalamus via differentially regulated ER subtypes (Osterlund et al., 1998). It remains to be determined whether HPA hyperresponsiveness in E females during proestrous is mediated, at least partly, by elevated levels of ER-β, or whether ER-β levels are elevated as a result of HPA hyperactivity. Moreover, we have recently shown that basal levels of hippocampal mineralocorticoid receptor (MR) mRNA are decreased in E compared to PF and C females, with the greatest effects in proestrus, whereas E (but not PF or C) females have higher basal hippocampal glucocorticoid receptor (GR) mRNA levels in proestrus than in estrous and diestrus (Sliwowska et al., 2008). These findings support the suggestions of increased basal HPA tone in E females, and a role for E2 in mediating these effects. Interestingly, the finding that basal progesterone levels were elevated in PF females during proestrus, but that E females showed higher CORT and ACTH levels than C females supports the suggestion that progesterone has an inhibitory effects on the facilitatory actions of E2 on HPA activity.
Importantly, we found a specific ethanol effect on AVP mRNA expression. E females had significantly higher basal AVP mRNA levels than PF and C in diestrus, and both E and PF had significantly higher basal AVP mRNA levels than C females in estrus. AVP is a weak ACTH secretagogue on its own, but acts synergistically with CRH and plays an important role in sustaining pituitary responsiveness during chronic stress (Aguilera, 1994; Gillies et al., 1982) or to a novel heterotypic stressor following repeated stress (Ma et al., 1999). Moreover, in a previous study comparing intact with gonadectomized males under basal and acute stress conditions, we saw no significant differences in CRH mRNA levels across time in any group, but lower AVP mRNA levels in intact E and PF compared to C males at 90-minute poststress onset (Lan et al., 2006). Together, these findings suggest that AVP may play a larger role than CRH in HPA regulation in E compared to control animals and that differences in stress responsiveness may not be entirely intrinsic to the HPA axis, but reflective of a differential modulatory role of the sex steroids.
In summary, findings from this study demonstrate that, despite normal estrous cyclicity, adrenal and gonadal hormones in E females respond differentially at different phases of the estrous cycle under both basal and stress conditions compared to responses of PF and/or C females. The data suggest that the ovarian steroids play an important role in mediating prenatal effects of ethanol on HPA function, with particularly marked differences among groups during proestrus. Effects on ACTH and LH levels, as well as on AVP mRNA levels, suggest specific effects of ethanol on HPA and HPG regulation, whereas changes in CORT and E2 activity and in CRH mRNA levels, appear to be partly mediated by the nutritional effects of diet or by the mild prenatal stress induced by pair-feeding. Nevertheless, taken together, these data suggest that E females have altered HPG-HPA interactions across the estrous cycle, and in particular, differential sensitivity to ovarian steroids. Our data support the hypothesis that altered HPG regulation and/or HPG-HPA interactions could contribute to the altered HPA responsiveness and regulation typically observed in E females.
Acknowledgments
A portion of these data has been presented as a poster at the Society for Neuroscience 2006 Annual Meeting, supported by SfN Chapters Graduate Student Travel Award to NL. We thank Linda Ellis and Wayne K. Yu for helping to organize and guide all aspects of the research, and Paxton Bach and Jessica Lam for their technical assistance on the experiments.
SOURCES OF FUNDING
This research was supported by NIH/NIAAA AA07789 to JW and VV, grants from the Canadian Institute for Advanced Research and the UBC Human Early Learning Partnership to JW, Fellowship from IMPART (CIHR Strategic Training Initiative in Health Research) to JHS, an NSERC Postgraduate Scholarship to AH, and an NSERC Canada Graduate Scholarship to NL.
References
- Abel EL, Berman RF. Long-term behavioral effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol. 1994;16:467–470. doi: 10.1016/0892-0362(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Abel EL, Dintcheff BA. Effects of prenatal alcohol exposure on growth and development in rats. J Pharmacol Exp Ther. 1978;207:916–921. [PubMed] [Google Scholar]
- Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15:321–350. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- Almeida OF, Hassan AH, Harbuz MS, Linton EA, Lightman SL. Hypothalamic corticotropin-releasing hormone and opioid peptide neurons: functional changes after adrenalectomy and/or castration. Brain Res. 1992;571:189–198. doi: 10.1016/0006-8993(92)90654-r. [DOI] [PubMed] [Google Scholar]
- Angelogianni P, Gianoulakis C. Prenatal exposure to ethanol alters the ontogeny of the beta-endorphin response to stress. Alcohol Clin Exp Res. 1989;13:564–571. doi: 10.1111/j.1530-0277.1989.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- Attademo AM, Sanchez-Borzone M, Lasaga M, Celis ME. Intracerebroventricular injection of neuropeptide EI increases serum LH in male and female rats. Peptides. 2004;25:1995–1999. doi: 10.1016/j.peptides.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology. 1994;59:228–234. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- Boggan WO, Randall CL, Dodds HM. Delayed sexual maturation in female C57BL/6J mice prenatally exposed to alcohol. Res Commun Chem Pathol Pharmacol. 1979;23:117–125. [PubMed] [Google Scholar]
- Bohler HC, Jr, Zoeller RT, King JC, Rubin BS, Weber R, Merriam GR. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Brain Res Mol Brain Res. 1990;8:259–262. doi: 10.1016/0169-328x(90)90025-9. [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Dohler KD, Wilson CA. Activity of the pituitary-adrenocortical system and thyroid gland during the oestrous cycle of the rat. J Endocrinol. 1978;78:359–366. doi: 10.1677/joe.0.0780359. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Creighton-Taylor JA, Rudeen PK. Fetal alcohol exposure and effects of LHRH and PMA on LH beta-mRNA expression in the female rat. Alcohol Clin Exp Res. 1991;15:1031–1035. doi: 10.1111/j.1530-0277.1991.tb05206.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Xu Y, Buller KM, Day TA. Effects of chronic oestrogen replacement on stress-induced activation of hypothalamic-pituitary-adrenal axis control pathways. J Neuroendocrinol. 2000;12:784–794. doi: 10.1046/j.1365-2826.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- Esquifino AI, Sanchis R, Guerri C. Effect of prenatal alcohol exposure on sexual maturation of female rat offspring. Neuroendocrinology. 1986;44:483–487. doi: 10.1159/000124690. [DOI] [PubMed] [Google Scholar]
- Ferrini MG, Grillo CA, Piroli G, de Kloet ER, De Nicola AF. Sex difference in glucocorticoid regulation of vasopressin mRNA in the paraventricular hypothalamic nucleus. Cell Mol Neurobiol. 1997;17:671–686. doi: 10.1023/A:1022538120627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology. 2002;143:2534–2540. doi: 10.1210/endo.143.7.8888. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP. Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab. 2007;292:E1173–E1182. doi: 10.1152/ajpendo.00102.2006. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Glavas MM, Ellis L, Weinberg J. Postnatal handling does not normalize hypothalamic corticotropin-releasing factor mRNA levels in animals prenatally exposed to ethanol. Brain Res Dev Brain Res. 2005;157:74–82. doi: 10.1016/j.devbrainres.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: interactive effects of dietary restriction and schedule of feeding. J Nutr. 1981;111:208–218. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: role of corticosterone. Alcohol Clin Exp Res. 2007;31:1598–1610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Yu WK, Weinberg J. Effects of mineralocorticoid and glucocorticoid receptor blockade on hypothalamic-pituitary-adrenal function in female rats prenatally exposed to ethanol. Alcohol Clin Exp Res. 2006;30:1916–1924. doi: 10.1111/j.1530-0277.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- Gore AC, Roberts JL. Regulation of gonadotropin-releasing hormone gene expression in the rat during the luteinizing hormone surge. Endocrinology. 1995;136:889–896. doi: 10.1210/endo.136.3.7867597. [DOI] [PubMed] [Google Scholar]
- Greer ER, Caldwell JD, Johnson MF, Prange AJ, Jr, Pedersen CA. Variations in concentration of oxytocin and vasopressin in the paraventricular nucleus of the hypothalamus during the estrous cycle in rats. Life Sci. 1986;38:2311–2318. doi: 10.1016/0024-3205(86)90638-7. [DOI] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30:2055–2064. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, McGivern RF, Noble ES, Gorski RA. Exposure to alcohol in utero alters the adult patterns of luteinizing hormone secretion in male and female rats. Life Sci. 1985;37:1683–1690. doi: 10.1016/0024-3205(85)90295-4. [DOI] [PubMed] [Google Scholar]
- Hiroshige T, Wada-Okada S. Diurnal changes of hypothalamic content of corticotropin-releasing activity in female rats at various stages of the estrous cycle. Neuroendocrinology. 1973;12:316–319. doi: 10.1159/000122179. [DOI] [PubMed] [Google Scholar]
- Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–845. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- Isgor C, Huang GC, Akil H, Watson SJ. Correlation of estrogen beta-receptor messenger RNA with endogenous levels of plasma estradiol and progesterone in the female rat hypothalamus, the bed nucleus of stria terminalis and the medial amygdala. Brain Res Mol Brain Res. 2002;106:30–41. doi: 10.1016/s0169-328x(02)00407-2. [DOI] [PubMed] [Google Scholar]
- Ivell R, Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci USA. 1984;81:2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev Psychopathol. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- Jingami H, Mizuno N, Takahashi H, Shibahara S, Furutani Y, Imura H, Numa S. Cloning and sequence analysis of cDNA for rat corticotropin- releasing factor precursor. FEBS Lett. 1985;191:63–66. doi: 10.1016/0014-5793(85)80994-7. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Kaneko K, Shinsako J, Dallman MF. Adrenal sensitivity to adrenocorticotropin varies diurnally. Endocrinology. 1981;109:70–75. doi: 10.1210/endo-109-1-70. [DOI] [PubMed] [Google Scholar]
- Kim CK, Giberson PK, Yu W, Zoeller RT, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic-pituitary-adrenal responses to chronic cold stress in rats. Alcohol Clin Exp Res. 1999;23:301–310. [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic- pituitary-adrenal activity in male rats. J Neuroendocrinol. 2006;18:672–684. doi: 10.1111/j.1365-2826.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Le Mevel JC, Abitbol S, Beraud G, Maniey J. Dynamic changes in plasma adrenocorticotrophin after neurotropic stress in male and female rats. J Endocrinol. 1978;76:359–360. doi: 10.1677/joe.0.0760359. [DOI] [PubMed] [Google Scholar]
- Le Mevel JC, Abitbol S, Beraud G, Maniey J. Temporal changes in plasma adrenocorticotropin concentration after repeated neurotropic stress in male and female rats. Endocrinology. 1979;105:812–817. doi: 10.1210/endo-105-3-812. [DOI] [PubMed] [Google Scholar]
- Lee S, Imaki T, Vale W, Rivier C. Effect of prenatal exposure to ethanol on the activity of the hypothalamic-pituitary-adrenal axis of the offspring: importance of the time of exposure to ethanol and possible modulating mechanisms. Mol Cell Neurosci. 1990;1:168–177. doi: 10.1016/1044-7431(90)90022-v. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C. Gender differences in the effect of prenatal alcohol exposure on the hypothalamic-pituitary-adrenal axis response to immune signals. Psychoneuroendocrinology. 1996;21:145–155. doi: 10.1016/0306-4530(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic-pituitary-adrenal axis of rats exposed to alcohol in utero: role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000;16:515–528. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Metabolism of alcohol. Clin Liver Dis. 2005;9:1–35. doi: 10.1016/j.cld.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Lucas A. Long-term programming effects of early nutrition – implications for the preterm infant. J Perinatol. 2005;25(Suppl 2):S2–S6. doi: 10.1038/sj.jp.7211308. [DOI] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004;16:272–278. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- Ma XM, Levy A, Lightman SL. Rapid changes in heteronuclear RNA for corticotrophin-releasing hormone and arginine vasopressin in response to acute stress. J Endocrinol. 1997;152:81–89. doi: 10.1677/joe.0.1520081. [DOI] [PubMed] [Google Scholar]
- Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- McGivern RF, McGeary J, Robeck S, Cohen S, Handa RJ. Loss of reproductive competence at an earlier age in female rats exposed prenatally to ethanol. Alcohol Clin Exp Res. 1995;19:427–433. doi: 10.1111/j.1530-0277.1995.tb01526.x. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Yellon SM. Delayed onset of puberty and subtle alterations in GnRH neuronal morphology in female rats exposed prenatally to ethanol. Alcohol. 1992;9:335–340. doi: 10.1016/0741-8329(92)90077-n. [DOI] [PubMed] [Google Scholar]
- Montes GS, Luque EH. Effects of ovarian steroids on vaginal smears in the rat. Acta Anat (Basel) 1988;133:192–199. doi: 10.1159/000146639. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Taylor AN, Lewis JW, Poland RE, Redei E, Branch BJ. Pituitary-adrenal responses to morphine and footshock stress are enhanced following prenatal alcohol exposure. Alcohol Clin Exp Res. 1986;10:397–402. doi: 10.1111/j.1530-0277.1986.tb05112.x. [DOI] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J, Schwartz NB. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod. 1979;20:659–670. doi: 10.1095/biolreprod20.3.659. [DOI] [PubMed] [Google Scholar]
- Osborn JA, Kim CK, Yu W, Herbert L, Weinberg J. Fetal ethanol exposure alters pituitary-adrenal sensitivity to dexamethasone suppression. Psychoneuroendocrinology. 1996;21:127–143. doi: 10.1016/0306-4530(95)00037-2. [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Park OK, Gugneja S, Mayo KE. Gonadotropin-releasing hormone gene expression during the rat estrous cycle: effects of pentobarbital and ovarian steroids. Endocrinology. 1990;127:365–372. doi: 10.1210/endo-127-1-365. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hayashi S, Orikasa C, Almeida OF. Implications of estrogen-dependent brain organization for gender differences in hypothalamo-pituitary-adrenal regulation. FASEB J. 1995;9:419–423. doi: 10.1096/fasebj.9.5.7896013. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier; Burlington, MA: 2005. [Google Scholar]
- Porkka-Heiskanen T, Urban JH, Turek FW, Levine JE. Gene expression in a subpopulation of luteinizing hormone-releasing hormone (LHRH) neurons prior to the preovulatory gonadotropin surge. J Neurosci. 1994;14:5548–5558. doi: 10.1523/JNEUROSCI.14-09-05548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants’ adrenocortical reactivity to stress. J Pediatr Psychol. 1996;21:833–840. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raps D, Barthe PL, Desaulles PA. Plasma and adrenal corticosterone levels during the different phases of the sexual cycle in normal female rats. Experientia. 1971;27:339–340. doi: 10.1007/BF02138184. [DOI] [PubMed] [Google Scholar]
- Roy BN, Reid RL, Van Vugt DA. The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology. 1999;140:2191–2198. doi: 10.1210/endo.140.5.6684. [DOI] [PubMed] [Google Scholar]
- Rudeen PK, Hagaman J. Ovarian stimulation by exogenous gonadotrophins in fetal ethanol-exposed immature rats. Experientia. 1988;44:714–715. doi: 10.1007/BF01941040. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW. Moderate level alcohol during pregnancy, prenatal stress, or both and limbic-hypothalamic-pituitary- adrenocortical axis response to stress in rhesus monkeys. Child Dev. 2004;75:96–109. doi: 10.1111/j.1467-8624.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151(Suppl 3):U49–U62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- Sliwowska JH, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J. Effects of prenatal ethanol exposure on regulation of basal hypothalamic-pituitary-adrenal activity and hippocampal 5-HT(1A) receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology. 2008;33:1111–1123. doi: 10.1016/j.psyneuen.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol RJ, Clarren SK. Guidelines for use of terminology describing the impact of prenatal alcohol on the offspring. Alcohol Clin Exp Res. 1989;13:597–598. doi: 10.1111/j.1530-0277.1989.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome—Diagnosis, Epidemiology, Prevention, and Treatment. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Viau V, Lee P, Sampson J, Wu J. A testicular influence on restraint-induced activation of medial parvocellular neurons in the paraventricular nucleus in the male rat. Endocrinology. 2003;144:3067–3075. doi: 10.1210/en.2003-0064. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Alpha1 adrenoreceptors mediate the stimulatory effects of oestrogen on stress-related hypothalamic-pituitary-adrenal activity in the female rat. J Neuroendocrinol. 2004;16:72–78. doi: 10.1111/j.1365-2826.2004.01122.x. [DOI] [PubMed] [Google Scholar]
- Walker CD. Nutritional aspects modulating brain development and the responses to stress in early neonatal life. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1249–1263. doi: 10.1016/j.pnpbp.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW. Diurnal variations in the content of prepro-corticotropin-releasing hormone messenger ribonucleic acids in the hypothalamic paraventricular nucleus of rats of both sexes as measured by in situ hybridization. Endocrinology. 1989;125:1734–1738. doi: 10.1210/endo-125-3-1734. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Nutritional issues in perinatal alcohol exposure. Neurobehav Toxicol Teratol. 1984;6:261–269. [PubMed] [Google Scholar]
- Weinberg J. Effects of ethanol and maternal nutritional status on fetal development. Alcohol Clin Exp Res. 1985;9:49–55. doi: 10.1111/j.1530-0277.1985.tb05049.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Hyperresponsiveness to stress: differential effects of prenatal ethanol on males and females. Alcohol Clin Exp Res. 1988;12:647–652. doi: 10.1111/j.1530-0277.1988.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol effects: sex differences in offspring stress responsiveness. Alcohol. 1992;9:219–223. doi: 10.1016/0741-8329(92)90057-h. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Prenatal alcohol exposure: endocrine function of offspring. In: Zakhari S, editor. Alcohol and the Endocrine System. National Institutes of Health; Bethesda, MD: 1993. pp. 363–382. [Google Scholar]
- Weinberg J, Bezio S. Alcohol-induced changes in pituitary-adrenal activity during pregnancy. Alcohol Clin Exp Res. 1987;11:274–280. doi: 10.1111/j.1530-0277.1987.tb01307.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Taylor AN, Gianoulakis C. Fetal ethanol exposure: hypothalamic- pituitary-adrenal and beta-endorphin responses to repeated stress. Alcohol Clin Exp Res. 1996;20:122–131. doi: 10.1111/j.1530-0277.1996.tb01054.x. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Handa RJ. Gonadotropin secretion in infantile rats exposed to ethanol in utero. Alcohol. 1997;14:497–501. doi: 10.1016/s0741-8329(97)00037-2. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Marshall MT, Bollnow MR, McGivern RF, Handa RJ. Gonadotropin-releasing hormone mRNA and gonadotropin beta-subunit mRNA expression in the adult female rat exposed to ethanol in utero. Alcohol Clin Exp Res. 1995;19:1211–1218. doi: 10.1111/j.1530-0277.1995.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Young EA. The role of gonadal steroids in hypothalamic-pituitary-adrenal axis regulation. Crit Rev Neurobiol. 1995;9:371–381. [PubMed] [Google Scholar]
- Young EA, Altemus M, Parkison V, Shastry S. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 2001;25:881–891. doi: 10.1016/S0893-133X(01)00301-3. [DOI] [PubMed] [Google Scholar]
- Young WS, III, Mezey E, Siegel RE. Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett. 1986a;70:198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]
- Young WS, III, Mezey E, Siegel RE. Vasopressin and oxytocin mRNAs in adrenalectomized and Brattleboro rats: analysis by quantitative in situ hybridization histochemistry. Brain Res. 1986b;387:231–241. doi: 10.1016/0169-328x(86)90029-x. [DOI] [PubMed] [Google Scholar]
- Zajac CS, Abel EL. Animal models of prenatal alcohol exposure. Int J Epidemiol. 1992;21(Suppl 1):S24–S32. doi: 10.1093/ije/21.supplement_1.s24. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Seeburg PH, Young WS., III In situ hybridization histochemistry for messenger ribonucleic acid (mRNA) encoding gonadotropin-releasing hormone (GnRH): effect of estrogen on cellular levels of GnRH mRNA in female rat brain. Endocrinology. 1988;122:2570–2577. doi: 10.1210/endo-122-6-2570. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Young WS., III Changes in cellular levels of messenger ribonucleic acid encoding gonadotropin-releasing hormone in the anterior hypothalamus of female rats during the estrous cycle. Endocrinology. 1988;123:1688–1689. doi: 10.1210/endo-123-3-1688. [DOI] [PubMed] [Google Scholar]