Abstract
In adulthood, both alcohol (ethanol) and stress are known to suppress hippocampal neurogenesis in male rats. Similarly, most studies report that prenatal alcohol exposure (PAE) reduces cell proliferation and/or cell survival in the hippocampus of adult males. Furthermore, PAE is known to have marked effects on behavioral and hypothalamic–pituitary–adrenal (HPA) responsiveness to stressors. However, no studies have examined the modulation of adult hippocampal neurogenesis by stress in PAE animals. We hypothesized that, in accordance with previous data, PAE would suppress basal levels of adult hippocampal neurogenesis, and further that stress acting on a sensitized HPA axis would have greater adverse effects on adult hippocampal neurogenesis in PAE than in control rats.
Adult male offspring from PAE, pair-fed (PF) control, and ad libitum-fed control (C) groups were subjected to restraint stress (9 days, 1 h/day) or left undisturbed. Rats were then injected with bromodeoxyuridine (BrdU) on day 10, perfused 24 h (proliferation) or 3 weeks (survival) later, and brains processed for BrdU immunohistochemistry. We found that (1) under non-stressed conditions, PAE rats had a small but statistically significant suppressive effect on levels of hippocampal neurogenesis and (2) unexpectedly, repeated restraint stress significantly reduced neurogenesis in C and PF, but not PAE rats. We speculate that the failure of PAE males to mount an appropriate (i.e. suppressive) neurogenic response to stressors, implies reduced plasticity and adaptability or resilience, which could impact negatively on hippocampal structure and function.
Keywords: Neurogenesis, alcohol, prenatal ethanol, corticosterone, hypothalamic–pituitary–adrenal axis, stress
Introduction
Events early in life can dramatically affect developing systems, permanently changing their structure and function in a process referred to as fetal or early programing (Welberg and Seckl 2001). Such reorganization of key systems by early environmental events may link early life experiences to long-term health consequences (Matthews et al. 2002). Prenatal exposure to alcohol (ethanol) is an early life insult that can result in a wide range of adverse outcomes, collectively described under the umbrella term fetal alcohol spectrum disorder (FASD) (Sokol et al. 2003), with fetal alcohol syndrome at the most severe end of the spectrum (Jones and Smith 1973; Jones et al. 1973). Changes resulting from prenatal exposure to alcohol can include central nervous system dysfunction, growth deficiencies, behavioral, and cognitive alterations such as hyperactivity and hyperresponsivity to stressors, dysregulation of many physiological functions, and deficits in learning, response inhibition, and appropriate use of environmental cues (Riley and McGee 2005). Of particular relevance to the present study is the finding that the hypothalamic–pituitary–adrenal (HPA) axis, a key system that mediates physiological responses to stressors, is markedly altered following prenatal alcohol exposure (PAE). Children with FASD often show increased basal and/or stress levels of cortisol (Ramsay et al. 1996; Jacobson et al. 1999; Haley et al. 2006). Similarly, in animal models of FASD, long-term changes in central HPA regulation and increased stress responsiveness have been observed (Taylor et al. 1984; Nelson et al. 1986; Lee et al. 2000; Glavas et al. 2007; Weinberg et al. 2008), indicating fetal programing of the HPA axis.
The hippocampus is a primary target for corticosteroids (McEwen et al. 1968; De Kloet et al. 1998), containing a high concentration of glucocorticoid receptors and mediating negative feedback by glucocorticoids on HPA activity. In humans and in animal models, excessive levels of corticosteroids are associated with reduced hippocampus volume and impaired hippocampus-dependent performance (Lupien and McEwen 1997; Lupien et al. 1998; Starkman et al. 2001; McEwen 2004). Importantly, the hippocampus is also susceptible to the teratogenic effects of PAE. For example, the left hippocampus may be smaller in children with FASD compared to control children (Riikonen et al. 1999; Willoughby et al. 2008), and these children also show poorer spatial performance (Willoughby et al. 2008). Similarly, in animal models, PAE typically results in learning impairments in hippocampus-dependent tasks (Berman and Hannigan 2000), as well as impaired long-term potentiation, a cellular model for learning and memory (Sutherland et al. 1997; Richardson et al. 2002; Savage et al. 2002; Christie et al. 2005). Together, these findings demonstrate abnormal hippocampal development and function following PAE, which may underlie or exacerbate HPA dysregulation.
New neurons are continually produced and integrated into the dentate gyrus throughout life (Cameron and McKay 2001). Studies suggest that hippocampal neurogenesis plays a role in learning, memory (Gould et al. 1999; Shors et al. 2001), and depression (Duman et al. 2000; Malberg et al. 2000), as well as in the ability of the brain to process, adapt, and respond to stimuli (Duman et al. 2000). Exposure to acute or chronic stress in prenatal life (Lemaire et al. 2000, 2006; Mandyam et al. 2008) or in adulthood (Holmes and Galea 2002; Mirescu et al. 2004; Mirescu and Gould 2006) suppresses both cell proliferation and cell survival in the hippocampus of adult male rats. Similarly, PAE can reduce levels of hippocampal neurogenesis, but interestingly, these deficits were attenuated with exercise (Redila et al. 2006). Early postnatal ethanol exposure (postnatal 4–9 d, during the brain growth spurt) similarly reduced the number of neurons in the dentate gyrus of young (50 d) and mature (80 d) male rats (Klintsova et al. 2007). Furthermore, while a study on mice found no significant differences between PAE and control males in basal levels of adult hippocampal neurogenesis, PAE eliminated the enhancement in neurogenesis seen in controls after exposure to an enriched environment (Choi et al. 2005). Collectively, these findings indicate that PAE alters neurogenesis and/or the neurogenic response to a treatment. However, no studies have examined the modulation of adult hippocampal neurogenesis by stress in PAE animals. This issue is important in view of the altered hippocampal volume, impaired cognition, increased responsiveness to stressors, and higher rates of neuropsychiatric disorders, including depression and anxiety, shown in both human (Famy et al. 1998; Barr et al. 2006; O’Connor and Paley 2006) and animal (Hellemans et al. 2009) studies of FASD.
The present study investigated the effects of prenatal ethanol exposure and repeated stress in adulthood on both hippocampal cell proliferation and cell survival in adult male rats. We hypothesized that, consistent with previous studies from our laboratory and others, prenatal ethanol exposure would suppress basal levels of hippocampal neurogenesis in our model. Moreover, in view of the data suggesting that both stress and PAE can suppress neurogenesis, and that PAE sensitizes the HPA response to later life stressors, we hypothesized that there would be an additive effect of PAE and stress, i.e. the expected stress-induced suppression of neurogenesis would be significantly greater in PAE than in control rats.
Materials and methods
Breeding of animals
Adult virgin female (250–275 g; n = 36) and male (275–300 g; n = 18) Sprague–Dawley rats were obtained from Charles River Laboratories (St Constant, PQ, Canada). Rats were group housed by sex and maintained on a 12:12 h light/dark cycle (lights on 06:00 h). The ambient temperature in the colony room was controlled at 21–22°C. Rats were given ad libitum access to water and standard rat chow (Jamieson’s Pet Food Distributors Ltd, Delta, BC, Canada). One to two weeks following arrival, each female was paired with a male in a stainless steel suspended cage (25 × 18 × 18 cm), with a wire mesh front and floor. Wax paper was placed under the cages and checked daily for the presence of vaginal plugs indicating day 1 of gestation (GD1). Once a vaginal plug was found, the female was assigned to one of the three experimental groups (described below) and housed alone in a clear polycarbonate cage lined with bedding. All animal use and care procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (Council 1996), and were approved by the University of British Columbia Animal Care Committee. At the beginning of breeding, rats were weighed and each pair-fed (PF) control dam was yoked to a weight-matched ethanol-treated dam.
Prenatal treatments
Rats were housed in polycarbonate cages (24 × 16 × 46 cm) with pine shavings as bedding. On the first day of pregnancy (GD1), females were randomly assigned to one of the three prenatal treatment groups: (1) Ethanol (PAE), fed a liquid ethanol diet ad libitum (n = 9 dams); (2) PF, fed an isocaloric liquid control diet, in the same amount consumed by a PAE partner (g/kg/body weight per day of gestation), to control for nutritional effects of the reduced food intake that occurs with ethanol consumption (n = 8 dams); and (3) Control (C) group, fed lab chow and water, ad libitum (n = 10 dams). All dams had ad libitum access to water throughout gestation and lactation.
For the ethanol-containing diets, we used 95% ethanol (Commercial Alcohol Inc., Chatham, Ont., Canada). The liquid diets were formulated in our laboratory to provide an optimal nutrition, with 36% of the total calories derived from ethanol. Maltose–dextrin was isocalorically substituted for ethanol in the liquid control diet (PF group). Diets were supplied by Dyets Inc., Bethlehem, PA, USA. Fresh diets were presented daily at 16:00–17:00 h. This feeding schedule permits maintenance of relatively normal glucocorticoid rhythms in the PF dams, as the glucocorticoid rhythm in animals receiving a reduced ration, such as PF animals, re-entrains to the time of feeding (Krieger 1974; Gallo and Weinberg 1981). Every day, diet bottles from the previous day were removed and weighed to determine the amount of diet consumed. Experimental diets were provided to dams until GD 21, at which time they were replaced with standard laboratory chow ad libitum. We have previously shown that pregnancy outcome and lactation are more successful if the alcohol diet is removed prior to parturition (Weinberg 1989). Pregnant females remained undisturbed except for routine cage changing and weighing on GDs 1, 7, 14, and 21. On postnatal day 1 (PND1), pups were weighed and litters randomly culled to 10 (5 females and 5 males when possible), to control for any confounding effects of litter size or sex ratio. If necessary, pups were cross-fostered from a litter in the same prenatal treatment group on the same day to maintain litter size or sex ratio. Dams and pups were weighed on PND 1, 7, 14, and 21; pups were weaned on PND22, after which the offspring were pair-housed by litter and sex, with ad libitum access to standard rat chow and water. We utilized adult (90–120 days old) male PAE, PF, and C offspring in this study, as all data available to date, examining PAE and neurogenesis, come from males and they show a consistent decrease in hippocampal neurogenesis in response to stress. To control for litter effects, only 1 or 2 pups from any one litter were assigned to each adult treatment condition.
Blood ethanol concentration measurements
To determine the maximal (or near maximal) blood ethanol concentration (BEC) achieved by ethanol-treated dams, blood samples were taken from the tail on GD15, 2 h after the presentation of the ethanol diet, to coincide with the end of a major eating bout. Tails were briefly warmed in a water bath, dried, and 1–2 mm of the tip sliced off with a razor. The tail was gently stroked, and 300–400 μl blood was collected into a 1.5 ml Eppendorf tube. Blood samples were allowed to coagulate for 2 h at room temperature and then centrifuged at 1880g for 20 min at 4°C to separate serum. Serum was collected and stored at −20°C until the time of assay. BECs were measured using a Alcohol Reagent Set, Pointe Scientific Inc. (Lincoln Park, MI, USA; minimum detectable concentration of ethanol of 2 mg/dl).
Restraint stress, bromodeoxyuridine injections, perfusion–fixation of brains, and blood collection
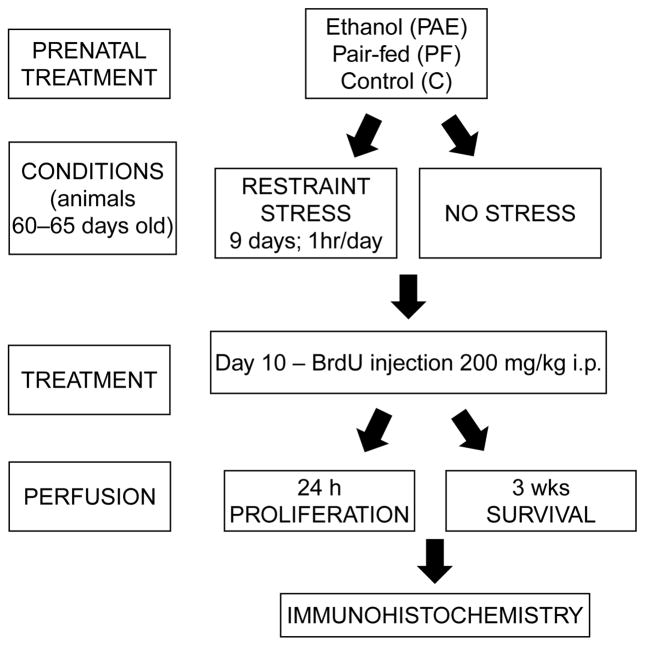
The experimental groups and time line of the procedures are presented in Figure 1. The effects of repeated restraint stress on the proliferation and survival of new cells were examined following 9 days of restraint stress. Rats in the stressed condition were confined in polyvinyl chloride restraint tubes for 1 h/day for nine consecutive days (12:00–13:00 h), during which time control rats were left undisturbed (non-stressed) in their home cages. Restraint tubes were 5.5 × 20 cm (inner diameter × length) for rats weighing less than 380 g and 7.5 × 20 cm (inner diameter × length) for rats weighing more than 380 g at testing. The front cap of the tube had four ventilation holes 1 cm apart and the end cap had a 1.5 cm diameter opening for the tail. Restraint is primarily a psychological stressor, and causes no pain or injury (Briski and Gillen 2001).
Figure 1.
Diagram presenting the experimental groups and time line of the procedures.
Twenty-four hours following the last restraint stress, all rats were injected i.p. with 200 mg/kg bromodeoxyuridine (BrdU; a DNA synthesis marker) and singly housed. This dose of BrdU is nontoxic and widely used in the field (Cameron and McKay 2001; Redila et al. 2006). BrdU is a thymidine analog that is incorporated into the DNA of dividing cells during the synthetic phase of the cell cycle. BrdU can be used to accurately assess cell proliferation if the animals are given one injection and perfused within 24 h, as one mitotic division occurs in approximately 24 h (Cameron and McKay 2001). BrdU can also be used to assess cell survival by perfusing animals weeks after injection to track survival of BrdU-immunoreactive (BrdU-IR) cells. Three weeks after BrdU injection, the majority of BrdU-IR cells will begin to express mature neuronal protein (NeuN) (Taupin 2007; Galea et al. 2008). Accordingly, rats were perfused either 24 h (proliferation group) or 3 weeks (survival group) after BrdU injection. Rats were anesthetized with a lethal dose of chloral hydrate (35% wt/vol; 1 ml per 100 g body weight; i.p. injection), the chest cavity was opened, blood was collected from the heart into EDTA-containing tubes for measurement of corticosterone (CORT) concentration, and rats were perfused transcardially with 0.9% saline for 10 min, followed by 4% formaldehyde in phosphate buffer (PFA) for 20 min, using perfusion pumps (Welch Pumps, VWR, Mississauga, Ont., Canada). Brains were removed and stored in PFA for 4 h after perfusion and then saturated in 20% sucrose in PFA at 4°C and kept in this buffer until sectioning. Coronal sections (40 μm) were obtained throughout the entire extent of the hippocampus using a microtome cryostat (HM 505E; MICROM International GmbH, Walldorf, Germany).
BrdU immunohistochemistry
Tissue was processed to reveal BrdU labeling by applying solutions to a series of every 10th section from the anterior–posterior extent of the hippocampus of each rat (10–14 sections per rat; 5–6 rats per group). All incubations were performed at room temperature unless stated otherwise. Brain sections were rinsed overnight at 4°C in TBS (0.1 M tris-PFA in 0.9% saline; pH 7.4). The next day, sections were rinsed in TBS (3×10 min), incubated in 0.6% H2O2 for 30 min and then rinsed again in TBS. Tissue was then incubated in 2 N HCl for 30 min at 37°C, incubated in 0.1 M borate buffer for 10 min, rinsed in TBS (3×10 min) and blocked with 3% normal horse serum (NHS) in 0.3% Triton X in TBS (TBS+) for 30 min. Tissue was then incubated overnight in mouse monoclonal antibody against BrdU (Roche Diagnostic Corp., Indianopolis, IN, USA) at a dilution of 1:200 in 3% NHS/TBS+ at 4°C. The following day, tissue was rinsed in TBS and incubated in mouse secondary antibody IgG (Vector Laboratories, Burlingame, CA, USA) at a dilution of 1:100 in 3% NHS/TBS+ for 4 h. Following another rinse in TBS, tissue was incubated in an avidin–biotin conjugate (Elite Kit, 1:50; Vector Laboratories) for 2 h and rinsed in TBS. Sections were reacted for 5 min in 0.02% diaminobenzidine (Sigma-Aldrich, St Louis, MO, USA) with H2O2. Finally, sections were rinsed in TBS and mounted on slides, counterstained with Cresyl Violet acetate (ACROS ORGANICS, Fair Lawn, NJ, USA), dehydrated in graded ethanol, cleared with CitriSolv and cover-slipped with Per-mount (Fisher Scientific, Fair Lawn, NJ, USA).
Fluorescent double-staining immunohistochemistry
Eight sections per rat (n = 4–5 per group) from a series of hippocampus slices, adjacent to those used for quantifying cell survival, were simultaneously processed for BrdU (DNA synthesis marker) and NeuN (neuronal marker) immunohistochemistry to assess the phenotype of BrdU-IR cells. Unless otherwise stated, all sections were rinsed several times with TBS between steps. Sections were blocked in 3% normal donkey serum (NDS) in TBS+ (Chemicon, Temecula, CA, USA) for 30 min followed by incubation with mouse anti-NeuN (Chemicon) at a dilution of 1:100 in 3% NDS/TBS+ for 48 h at 4°C. The next day, tissue was blocked in 3% NDS/TBS+ for 30 min, followed by incubation in donkey anti-mouse Alexa 488-conjugated antibody at a dilution of 1:200 (Invitrogen, Burlington, Ont., Canada) in 3% NDS/TBS+ for 4 h. Sections were then rinsed in TBS, fixed in 4% formaldehyde for 10 min, rinsed in 0.9% saline (3×10 min), incubated in 2 N HCl at 37°C for 30 min, and rinsed again in 0.9% saline. Next, tissue was blocked in 3% NDS/TBS+ for 30 min and incubated in rat anti-BrdU antibody (Oxford Biotechnology Ltd, Kidlington, Oxfordshire, UK) at a dilution of 1:250 in 3% NDS/TBS+ for 48 h at 4°C. The following day, tissue was blocked again in 3% NDS/TBS+ for 30 min and incubated overnight in donkey anti-rat Cy3-conjugated antibody (Jackson Immunoresearch, West Grove, PA, USA) at a dilution of 1:200 in 3% NDS/TBS+. Sections were mounted on microscope slides and coverslipped with 2.5% PVA-DABCO (Sigma, Oakville, Ont., Canada) and stored in the dark at 4°C.
Quantification of data
Slides were labeled with codes prior to analysis so that at the time of manual counting, the experimenters were blind to treatment condition. All BrdU-IR cells in the granule cell layer (GCL, including the subgranular zone (SGZ), defined as a 50 μm band between the inner edge of the GCL and the hilus) and hilus of the dentate gyrus were counted manually in every stained section throughout the rostral–caudal extent of the hippocampus. Thus, the counting frame was the entire GCL and SGZ. BrdU-IR cells were counted in the hilus and compared to the counts in the granule cell region for a number of reasons: (1) to determine whether any effects of treatment on cell counts in the GCL are due to generalized effects on blood–brain barrier permeability; (2) progeny from progenitor cells in the hilus give rise to a different population of cells that are mainly glial cells, compared to those from progenitor cells in the SGZ, which give rise to cells that are mainly neurons (Cameron et al. 1993), and (3) new neurons in the hilus are considered ectopic (McCloskey et al. 2006; Scharfman and Hen 2007). We considered as BrdU-IR, those cells in which there was visible 3,3′-diaminobenzidine brown product, either completely filling the nucleus or in a punctate pattern within the nucleus. To estimate total BrdU-IR cell numbers, BrdU-IR cells were counted in every 10th section in both hemispheres under a 100× objective using a E600 microscope (Nikon), and these counts were summed and the total number was multiplied by 10 to estimate the total number of BrdU-IR cells in the entire GCL, consistent with previous studies (Gould 1999; Cameron and McKay 2001; Mazzucco et al. 2006; Mirescu and Gould 2006; Redila et al. 2006; Spritzer and Galea 2007). As section thickness was 40 μm and we counted cells in every 10th section, the distance between two counted sections was 400 μm. We excluded cells that were touching the uppermost focal plane of each slide to avoid counting cells that are sectioned (caps). For the phenotyping of cells, 25 randomly selected BrdU-IR cells in each of eight sections per brain were analysed to determine whether they also expressed NeuN, a protein found in mature neurons (BrdU/NeuN-IR). A subset of BrdU/NeuN-IR cells found under epifluorescence was further examined under a confocal microscope (BioRad 200, Philadelphia, PA, USA) to confirm double labeling. The data are presented as percentage of double-labeled cells per all analysed BrdU-IR cells. Depending on availability of tissue, our sample size was 4–5 rats per group. For volume measurements, an Image J (NIH) software was used to digitize images of the dentate gyrus. Area measurements were then obtained and used to calculate the volume of GCL + SGZ and hilus using Cavalieri’s principle. Because there were no statistically significant differences in the GCL volume among groups, we report total number of BrdU-IR cells.
Radioimmunoassay for CORT
Blood samples were collected from the heart at the time of perfusion, as noted above, and centrifuged at 2140g for 10 min at 4°C to separate the plasma, which was transferred into 600 μl Eppendorf tubes and stored at −80°C until assayed using a commercial kit (MP Biomedicals, Orangeburg, NY, USA). The antiserum cross-reacts were 100% for CORT, 2.3% for deoxycorticosterone, 0.47% for testosterone, 0.17% for progesterone, and 0.05% for aldosterone. The minimum detectable CORT concentration was 0.25 μg/dl and the intra- and inter-assay coefficients of variation were 1.55 and 4.26%, respectively.
Statistical analysis
Body weight data were analysed using repeated-measures analysis of variance (ANOVA) for the factors of prenatal group (PAE, PF, and C), sex, and day, as appropriate, with day as a repeated measure. The number of BrdU-IR cells and the percentage of double-labeled cells were analysed using repeated-measures ANOVAs with prenatal group and treatment (non-stress and stress) as the between-subjects factors and region (GCL, hilus) as the within-subjects factor. The CORT data were analysed using ANOVA with prenatal group (PAE, PF, and C) and treatment (non-stress and stress) as the between-subjects factors. All statistical analyses were performed using a Statistica 6.0 software (StatSoft, Inc., Tulsa, OK, USA). Significant main or interaction effects were followed by Fisher’s LSD post-hoc tests. In addition, a priori tests were conducted to explore our hypotheses that basal levels of hippocampal neurogenesis would be suppressed in our model, and that stress would have greater adverse effects on adult neurogenesis in PAE than in control rats.
Results
Developmental data
Blood ethanol concentration
The mean BEC for ethanol-exposed dams was 144.4 ± 31.32 (SD) mg/dl, measured ~2 h after lights off, consistent with previous studies that have employed the same breeding and feeding protocols (Sliwowska et al. 2008; Lan et al. 2009). It has been shown that BECs of this level induce biological and behavioral deficits in the offspring (Sliwowska et al. 2006; Weinberg et al. 2008).
Dam body weights during gestation and lactation
Analysis of maternal body weights during gestation revealed significant main effects of group (F(2,24) = 17.40, p < 0.001) and time (F(3,72) = 512.76, p < 0.001), and a group × time interaction (F(6,72) = 17.08, p < 0.001). Fisher’s post-hoc analysis revealed that while there were no significant differences in body weights among groups on GD1 (all p > 0.74), both PAE and PF dams had lower body weights than C dams on GDs 7, 14, and 21 (all p < 0.001; Table I). Analysis of maternal body weights during lactation revealed a main effect of time (F(3,72) = 128.20, p < 0.001) and a group × time interaction (F(6,72) = 5.665, p < 0.001). Fisher’s post-hoc analyses revealed that on lactation day 1 (LD 1) both PAE and PF females had lower body weights than C females ( p < 0.001). On LD 7 and LD 14, only PF females had lower body weights than C females (p < 0.008 and p < 0.028, respectively), while on LD 21, there were no differences in maternal body weights among groups (all p > 0.59; Table I).
Table I.
Developmental data from PAE, PF, and C dams and offspring from birth to weaning.
| Pregnancy outcome variables | Treatment
|
||
|---|---|---|---|
| PAE | PF | Control | |
| Number of pregnant dams | 9 | 8 | 10 |
| Maternal death | 1 | 0 | 0 |
| Perinatal death | 4 | 0 | 0 |
| Length of gestation (days) ±SEM | 22.9 ± 0.3 | 22.9 ± 0.2 | 22.8 ± 0.2 |
| Dam weight (g) mean ± SEM | |||
| GD1 | 267.9 ± 2.3 | 269.9 ± 2.7 | 264.3 ± 2.3 |
| GD7 | 276.9 ± 2.9* | 268.8 ± 3.7* | 293.3 ± 3.8 |
| GD14 | 303.0 ± 6.4* | 292.3 ± 4.6* | 329.2 ± 5.2 |
| GD21 | 372.9 ± 10.9* | 363.0 ± 9.1* | 426.4 ± 6.7 |
| LD1 | 302.0 ± 6.4* | 299.7 ± 7.7* | 326.4 ± 5.4 |
| LD7 | 341.3 ± 5.8 | 337.4 ± 7.5* | 344.5 ± 3.7 |
| LD14 | 359.3 ± 7.5 | 355.8 ± 8.0* | 353.7 ± 5.2 |
| LD21 | 338.6 ± 5.3 | 341.6 ± 5.3 | 335.4 ± 4.4 |
| Litter size | 15.3 ± 0.7 | 16.1 ± 0.5 | 15.8 ± 0.3 |
| Offspring weight (g) mean ± SEM | |||
| Males | |||
| PND1 | 5.7 ± 0.2 | 6.1 ± 0.2 | 6.7 ± 0.1 |
| PND7 | 15.3 ± 0.6 | 16.8 ± 0.4 | 17.6 ± 0.4 |
| PND14 | 30.3 ± 0.8* | 33.1 ± 0.8 | 33.4 ± 0.8 |
| PND21 | 45.1 ± 1.4* | 51.5 ± 1.4* | 54.9 ± 2.1 |
For dam weight data: for GDs 7, 14 and 21: E = PF < C ( p < 0.001); for lactation day 1 (LD1): PAE = PF < C ( p < 0.001); for lactation day 7 (LD7): PF < C ( p < 0.001); for lactation day 14 (LD14): PF < C ( p < 0.028). For male offspring weight data: for postnatal day 14 (PND14): PAE < C ( p < 0.02); for postnatal day 21 (PND21): PAE = PF < C ( p < 0.001). GD, gestational day; LD, lactation day; PND, postnatal day; PAE, rats prenatally exposed to ethanol; PF, pair-fed rats; and C, control rats.
Indicates difference vs. control. Please note: one maternal death of a PAE female and four perinatal deaths in the PAE group (perinatal death refers to the number of dead pups).
Offspring body weights
Analysis of pup body weights in the pre-weaning period revealed significant main effects of group (F(2,24) = 9.892, p < 0.001) and time (F(3,72) = 888.7, p < 0.001), and a group × time interaction (F(6,72) = 5.086, p < 0.001). There were no significant group differences in body weights on PNDs 1–8 ( p > 0.06), but body weights were lower in PAE than in C pups on PND15, and lower in PAE than both PF and C pups on PND21 ( p < 0.001; Table I). Thus, PF but not PAE pups showed catch-up growth during the pre-weaning period.
Cell proliferation and cell survival in adulthood
Dentate gyrus volume did not differ among groups
As expected, the volume of the hilus was greater than that of the GCL (main effect of region, F(1,28) = 430.8, p < 0.001; Table II). There were no other significant main effects or interactions.
Table II.
Mean GCL volume (mm3) in prenatal ethanol exposure (PAE), PF, and C adult male rats at 3 weeks post BrdU injections (survival).
| Mean GCL volume ± SEM | Mean hilus volume ± SEM | |
|---|---|---|
| Basal | ||
| PAE | 1.464 ± 0.116 | 4.144 ± 0.490 |
| PF | 1.335 ± 0.113 | 3.827 ± 0.546 |
| C | 1.272 ± 0.086 | 4.733 ± 0.620 |
| Stress | ||
| PAE | 1.309 ± 0.092 | 4.180 ± 0.434 |
| PF | 1.187 ± 0.157 | 2.707 ± 0.163 |
| C | 1.141 ± 0.133 | 3.506 ± 0.417 |
Note: Each point is a mean ± SEM of 5–6 rats. Volume of hilus > volume of GCL ( p < 0.001) for all groups.
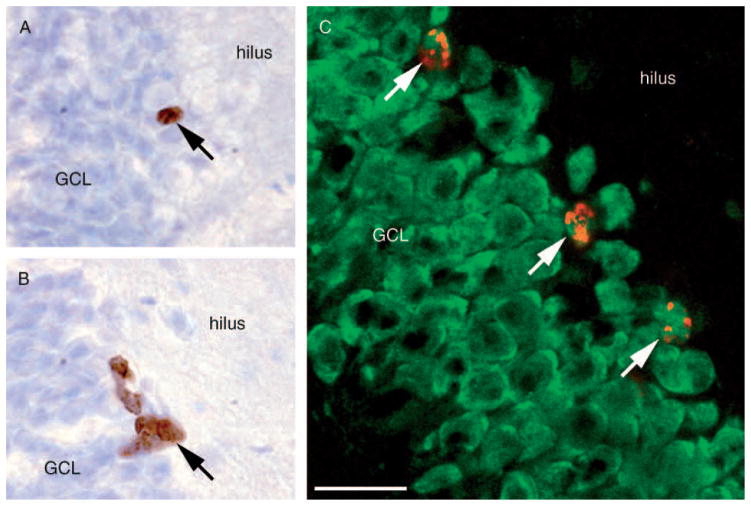
The majority of BrdU-labeled cells express NeuN
The majority (73–83%) of BrdU-IR cells expressed NeuN (Figure 2C, Table III). There was a main effect of region on the proportion of cells immunoreactive for both BrdU and NeuN (F(1,22) = 332.1 p < 0.001), with more double-labeled cells in the GCL + SGZ than in the hilus. There were no other main effects or interactions ( p > 0.18; Table III).
Figure 2.
BrdU-IR cells in the GCL of dentate gyrus at (A) 3 weeks (survival) and (B) 24 h (proliferation) after injection. (C) Confocal image of BrdU/NeuN-IR cells in the GCL of dentate gyrus of the hippocampus. BrdU-IR cells are indicated by arrows. The scale bar is 20 μm in (A)–(C).
Table III.
Mean percentage of double (BrdU/NeuN) labeled cells ± SEM across groups and treatment (n = 4–5 rats/group).
| Groups | Treatment
|
|
|---|---|---|
| Basal | Stress | |
| PAE | 76.0 ± 3.6 | 73.6 ± 3.0 |
| PF | 81.2 ± 3.9 | 76.5 ± 6.0 |
| C | 76.0 ± 4.4 | 83.0 ± 3.0 |
Note: PAE, Prenatal ethanol exposure; PF, pair-fed; and C, control.
No significant differences among prenatal groups.
Ethanol-treated rats had lower levels of cell survival than control rats, and stress reduced cell survival in control and PF but not in ethanol-treated rats
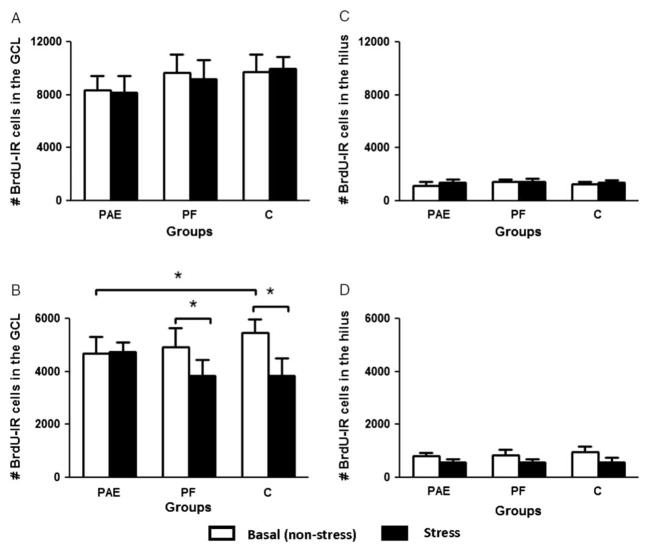
Rats in all groups had BrdU-IR cells in the GCL of the dentate gyrus at both 24 h and 3 weeks after BrdU injection (Figure 2). Prenatal ethanol exposure did not affect cell proliferation, determined by the number of BrdU-IR cells in rats perfused 24 h after BrdU injection, under either non-stressed or stressed conditions (all p > 0.39, Figure 3A). There was, however, an expected significant main effect of region, with more proliferating BrdU-IR cells in the GCL than the hilus (F(1,30) = 293.4, p < 0.0001).
Figure 3.
(A) Mean (±SEM) number of BrdU-IR cells in the dentate gyrus 24 h after BrdU injection (n = 5–6 rats/group). Prenatal ethanol exposure (PAE) did not significantly affect cell proliferation nor did stress influence cell proliferation in any group (all p > 0.39). (B) Mean (±SEM) number of BrdU-IR cells in the dentate gyrus 3 weeks after BrdU injection (n = 5–6 rats/group). Stress decreased cell survival in control (C; p < 0.001) and PF ( p < 0.02) rats but not in the PAE group ( p < 0.87) rats. Prenatal ethanol exposure reduced the survival of BrdU-IR cells under non-stressed conditions ( p < 0.04, one-tailed). Asterisks indicate significant differences between groups. (C), and (D) Mean (±SEM) number of BrdU-IR cells in the hilus 24 h (C) or 3 weeks (D) after BrdU injection (n = 5–6 rats/group). Prenatal ethanol exposure did not significantly affect cell proliferation or cell survival in the hilus. GCL, granule cell layer.
The ANOVA on cell survival revealed main effects of treatment (F(1,28) = 4.57, p < 0.05), and region (F(1,28) = 430.8, p < 0.001), a treatment × region interaction (F(1,28) = 4.27, p < 0.05), and a trend for a three-way interaction among group, treatment, and region ( p < 0.095; Figure 3B). Post-hoc analysis of the treatment × region interaction revealed that overall, stress reduced BrdU-IR cell number in the GCL + SGZ ( p < 0.03) but not in the hilus ( p > 0.52). We then performed a priori comparisons to test our hypotheses that PAE would reduce cell survival and that stress would have greater adverse effect on PAE than on control rats. We found that under non-stressed conditions, PAE rats had significantly lower numbers of BrdU-IR cells surviving in the GCL + SGZ than C ( p < 0.04, one-tailed) rats, while PF rats were intermediate and did not differ from either PAE ( p < 0.57) or C ( p < 0.22; Figure 3B). Importantly, exposure to stress reduced cell survival in the GCL + SGZ in C and PF but not PAE rats (C, p < 0.001; PF, p < 0.02 and E, p < 0.87, Figure 3B). There were no significant differences among groups in the hilus (Figures 3C,D).
Plasma CORT concentrations
CORT concentrations were measured in cardiac blood samples obtained under terminal anesthesia with the chest opened. As illustrated in Table IV, plasma CORT concentrations obtained under these conditions represent mild to moderate stress levels rather than basal levels, indicating that the collection procedure itself was not rapid enough to obtain a measure of true basal hormone concentrations. At 24 h after BrdU injection, CORT concentrations were higher in rats previously exposed to stress than in non-stressed rats ( p < 0.01), but there were no significant effects of prenatal treatment. At 3 weeks after the end of stress exposure and BrdU injection, CORT concentrations were affected by both prior stress treatment and prenatal ethanol exposure (Table IV). Post-hoc analysis of the group × treatment interaction (F(2,28) = 3.95, p < 0.03), indicated that CORT concentrations were higher in C males previously exposed to stress than in their non-stressed counterparts ( p < 0.02), whereas there were no effects of stress condition for PAE or PF males. In addition, for rats in the non-stressed condition, CORT concentrations in PAE males were higher than in PF males ( p < 0.02) and marginally higher than in C males ( p = 0.059).
Table IV.
Terminal plasma CORT concentrations, 24 h (proliferation) or 3 weeks (survival) after BrdU injection.
| Prenatal treatment group | CORT concentrations (μg/dl) (mean ± SEM) 24 h after BrdU injection
|
CORT concentrations (μg/dl) (mean ± SEM) 3 weeks after BrdU injection
|
||
|---|---|---|---|---|
| Non-stressed | Stressed* | Non-stressed | Stressed | |
| PAE | 12.89 ± 1.61 | 30.61 ± 4.02 | 16.58 ± 4.09†‡ | 11.52 ± 3.51 |
| PF | 10.19 ± 2.66 | 15.67 ± 4.35 | 6.52 ± 1.26 | 12.50 ± 2.36 |
| C | 15.49 ± 3.42 | 22.29 ± 6.57 | 8.07 ± 1.19 | 19.72 ± 3.97¶ |
At 24 h, plasma CORT concentration was higher in rats previously exposed to stress than in non-stressed rats (*p < 0.01). No significant differences were found among the prenatal groups.
At 3 weeks, for non-stressed;
PAE > PF ( p < 0.02);
PAE > C ( p = 0.059);
For C rats previously exposed to stress (stressed) > non-stressed, p < 0.02. PAE, prenatal ethanol exposure; PF, pair-fed (PF); C, control. n = 6 rats per group.
Discussion
The present data demonstrate that prior exposure to repeated restraint stress reduced neurogenesis in PF and C, but not PAE rats. Furthermore, male rats exposed to ethanol in utero had a small but statistically significant suppression in basal levels of hippocampal neurogenesis. However, there were no significant differences among prenatal groups in cell proliferation or dentate gyrus volume under either stressed or non-stressed conditions. Of note, approximately 80% of BrdU-IR cells were NeuN-IR, regardless of prenatal treatment or exposure to stressors in adulthood.
Volume of the dentate gyrus in PAE, PF, and C rats
In the present study, consistent with a previous report (Maier and West 2001), we found no statistically significant differences in dentate gyrus volume among prenatal treatment groups. In contrast, other studies have reported reduced dentate gyrus granule cell density and cell number in 10-day-old rat pups exposed to ethanol during early postnatal life (the brain growth spurt or third trimester equivalent) (Livy et al. 2003), and reductions in hippocampal volume in 3-day-old PAE pups (Zimmerberg and Reuter 1989). Interestingly, there are equivocal findings on hippocampus volume in human studies as well. For example, one study found that a small percentage of children (3 out of 17) exposed prenatally to alcohol, exhibited relatively small hippocampi (Autti-Ramo et al. 2002), whereas other studies found similar hippocampus sizes in the control and FASD subjects (Archibald et al. 2001; Geuze et al. 2005). Interestingly, in contrast to non-exposed children, alcohol-exposed children may also have asymmetrical hippocampi, such that the right hippocampus is significantly larger than the left, but not significantly different from those of control subjects (Riikonen et al. 1999; Willoughby et al. 2008). The timing of prenatal exposure, the age of the offspring, and BECs may all contribute to the different results obtained by different groups of investigators.
Basal rates of neurogenesis in the hippocampus are lower in PAE rats
The disruptive effects of exposure to ethanol in utero on the development of many brain structures are well known (West et al. 1981; Miller and Dow-Edwards 1988; Miller 1995; Maier and West 2001; Mattson et al. 2001; Bookstein et al. 2007), but only recently investigations have begun to explore the effects of PAE on adult hippocampal neurogenesis. Our finding of reduced hippocampal neurogenesis in PAE males under basal conditions is consistent with previous studies in rats, which reported reductions in both cell proliferation and cell survival following prenatal ethanol (Redila et al. 2006) or reduced numbers of mature neurons in the dentate gyrus following early postnatal ethanol exposure (Klintsova et al. 2007). In contrast, a study in mice found no significant differences between PAE and control males in basal levels of adult hippocampal neurogenesis (Choi et al. 2005). Methodological differences, including age, handling conditions, the species used, alcohol, and BrdU administration paradigms may all influence the observed effects of PAE on cell proliferation and/or survival and these factors need to be considered in comparing results among studies. For example, rats in the present study were adults (90–120 days) which remained pair-housed and undisturbed for several weeks prior to testing, whereas Redila and collaborators used 50-day-old males that were singly housed prior to BrdU injection. Males at 50 days of age are in the late stages of puberty (Lewis et al. 2002; Gomez et al. 2004; Romeo et al. 2006), when dramatic fluctuations in ACTH, CORT, and testosterone levels, and altered metabolic responsiveness to stressors occur, all of which could influence hippocampal neurogenesis (Akana et al. 1999; Gomez and Dallman 2001; Gomez et al. 2002; Viau et al. 2005). Similarly, species differences between mice and rats in physiological and metabolic function could also influence blood alcohol concentrations and/or impact of alcohol on the brain, and hence influence the results obtained. Nevertheless, although consistent with previous work in rats, we interpret our data cautiously, as the reduction in basal levels of neurogenesis in the present study were seen only using a one-tailed test based on an a priori hypothesis, and thus may not be a robust finding.
The possible role of the HPA hormones in mediating alterations in neurogenesis in PAE rats remains to be determined. The data do suggest that prior exposure to restraint stress differentially altered the response to the stress of blood collection at 24 h vs. 3 weeks. At 24 h following the end of restraint stress and BrdU injection, CORT concentrations were higher in all rats previously exposed to stress than in non-stressed rats, whereas at 3 weeks, CORT concentrations were affected by both prior stress and prenatal ethanol exposure. However, because our blood collection method in itself appears to have induced a mild stress response, and thus did not allow an assessment of true basal hormone levels, we cannot, at this time, draw conclusions about the possible role of the HPA hormones in mediating either the reduced basal levels of neurogenesis or the absence of the normal neurogenic response to stress seen in PAE rats.
Possible significance of the lack of stress-induced suppression in adult neurogenesis in PAE rats
In view of previous data suggesting that both stress and PAE can suppress neurogenesis, and that PAE sensitizes the HPA response to later life stressors, we hypothesized that there would be an additive effect of PAE and stress, i.e. that stress would have significantly greater adverse effects on hippocampal neurogenesis in PAE than in control rats. Unexpectedly, we found exactly the opposite: while PF and C males showed a significant stress-induced suppression of neurogenesis, PAE rats showed no change in neurogenesis, i.e. a blunting of the expected stress-induced suppression of cell survival. One possible interpretation of these data is that PAE had protective effects against stress-induced damage, as measured by cell survival. Interestingly, a number of studies examining effects of alcohol consumption in adulthood have shown that alcohol in moderation (not, more than 2 drinks/day) may have cardioprotective and neuroprotective effects (Collins et al. 2009). However, this is in marked contrast to the data on ethanol exposure of the fetus, where neurotoxicity is typically observed (Farber et al. 2004). Indeed, there are no known reports in the literature demonstrating protective effects of PAE. Therefore, we believe that a more likely interpretation of our data is that the absence of the normal neurogenic response to stress represents a maladaptive outcome. In healthy organisms, during stress, energy is redirected away from non-essential systems, including digestion, growth, and reproduction, so that all resources can be used to cope with the stressor. Thus, we suggest that the stress-induced suppression of neurogenesis seen in control male rats may be adaptive, redirecting energy to increase coping and thus increasing adaptability and resiliency in the face of challenge. For example, it has been shown that during exposure to 21 days of restraint stress, the glucocorticoid hormones may act together with excitatory amino acids to mediate a reversible stress-induced atrophy or remodeling of apical dendrites in the CA3 region of the hippocampus (Magarinos and McEwen 1995). While this type of plasticity does have some adverse effects on learning and short-term memory (McEwen 2004), it was suggested that in the long term it may be beneficial or adaptive, in that a retraction of dendrites may reduce cell death caused by overexposure to glutamate and glucocorticoids (Magarinos and McEwen 1995). Such structural plasticity is an example of resilience and adaptability, particularly evident in the young brain, which can withstand and adapt to challenge. The term allostasis, meaning “achieving stability through change”, refers to physiological mediators such as the HPA hormones, catecholamines, and cytokines, whose production varies according to environmental challenges and demands to allow the organism to respond and adapt, and hence cope with stressors (McEwen 2002). However, the cumulative cost to the body of adapting repeatedly to adverse psychosocial or physical situations can lead to allostatic load, and dysregulated activity of these mediators of allostasis—failure to shut off or habituate, or failure to turn on when needed (McEwen 2002). We propose that because rats prenatally exposed to ethanol have a sensitized or dysregulated HPA axis, they are more vulnerable to the adverse effects of stressors, and when exposed to stressors later in life thus show reduced plasticity and ability to adapt. In the present study, this is reflected in loss of the normal stress-induced decrease in neurogenesis, seen in control rats. Support for this possibility comes from data of Choi et al. (2005) who found that while control adult mice benefited from an enriched environment by doubling the number of new neurons in the dentate gyrus, PAE mice did not show this increased neurogenesis in response to enrichment. Similarly, it was shown that although voluntary exercise increased hippocampal neurogenesis in both PAE and control rats, suggesting adaptability in the adult brain, PAE rats never reached the levels of neurogenesis seen in their control counterparts (Redila et al. 2006). Moreover, postnatal handling, which typically modulates HPA responses and enhances learning and performance in normal animals (Levine 1967, 1969; Meaney et al. 1989), does not attenuate the HPA hyperresponsiveness and spatial navigation deficits in adult PAE animals (Gabriel et al. 2000, 2002). This further indicates reduced resilience and an inability to benefit from stimulation in PAE animals. In view of the discussion above, we speculate that the failure of PAE males to maintain an appropriate (i.e. suppressive) neurogenic response to stressors implies reduced adaptability, and possibly an inability to maintain homeostasis, which could have a negative impact on hippocampal structure and function. In turn, these effects on the hippocampus could adversely impact learning and memory, as well as appropriate neuroendocrine responses to stressors, and could play a role in increasing vulnerability to the development of affective disorders later in life.
Acknowledgments
We would like to thank Linda Ellis, Wayne Yu, Devon O’Rourke, Krysta Cochran, Stephanie Lieblich, Paxton Bach, Dr Xingqi Zhang and Dr Kim Hellemans, for expert help with this study. This study was funded by a BC Ministry of Children and Family Development Grant (via HELP) to LAMG and JW. The views presented in the paper are solely those of the authors and do not represent the policy of HELP or the Province of British Columbia.
Support
JHS is supported by IMPART (CIHR training grant); JMB, CKB, and NL were supported by NSERC PGS scholarships. This research was supported by a grant from the BC Ministry of Children and Family Development (through the UBC Human Early Learning Partnership) to LAMG and JW. Partial support also came from NIH/NIAAA AA007789 to JW and CIHR to LAMG. LAMG is a Michael Smith Senior Scholar.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akana SF, Strack AM, Hanson ES, Horsley CJ, Milligan ED, et al. Interactions among chronic cold, corticosterone and puberty on energy intake and deposition. Stress. 1999;3:131–146. doi: 10.3109/10253899909001118. [DOI] [PubMed] [Google Scholar]
- Archibald S, Fennema-Notestine C, Gamst A, Riley E, Mattson S, Jernigan T. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Autti-Ramo I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol. 2002;44:98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- Barr HM, Bookstein FL, O’Malley KD, Connor PD, Huggins JE, Streissguth AP. Binge drinking during pregnancy as a predictor of psychiatric disorders on the structured clinical interview for DSM-IV in young adult offspring. Am J Psychiatry. 2006;163:1061–1065. doi: 10.1176/ajp.2006.163.6.1061. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: Spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Connor PD, Huggins JE, Barr HM, Pimentel KD, Streissguth AP. Many infants prenatally exposed to high levels of alcohol show one particular anomaly of the corpus callosum. Alcohol Clin Exp Res. 2007;31:868–879. doi: 10.1111/j.1530-0277.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- Briski K, Gillen E. Differential distribution of Fos expression within the male rat preoptic area and hypothalamus in response to physical vs. psychological stress. Brain Res Bull. 2001;55:401–408. doi: 10.1016/s0361-9230(01)00532-9. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Choi IY, Allan AM, Cunningham LA. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcohol Clin Exp Res. 2005;29:2053–2062. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci. 2005;21:1719–1726. doi: 10.1111/j.1460-9568.2005.04004.x. [DOI] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Mukamal KJ, Gray MO, Parks DA, Das DK, Korthuis RJ. Alcohol in moderation, cardioprotection, and neuroprotection: Epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res. 2009;33:206–219. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am J Psychiatry. 1998;155:552–554. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- Farber NB, Heinkel C, Dribben WH, Nemmers B, Jiang X. In the adult CNS, ethanol prevents rather than produces NMDA antagonist-induced neurotoxicity. Brain Res. 2004;1028:66–74. doi: 10.1016/j.brainres.2004.08.065. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Yu W, Ellis L, Weinberg J. Postnatal handling does not attenuate hypothalamic–pituitary–adrenal hyperresponsiveness after prenatal ethanol exposure. Alcohol Clin Exp Res. 2000;24:1566–1574. [PubMed] [Google Scholar]
- Gabriel KI, Johnston S, Weinberg J. Prenatal ethanol exposure and spatial navigation: Effects of postnatal handling and aging. Dev Psychobiol. 2002;40:345–357. doi: 10.1002/dev.10023. [DOI] [PubMed] [Google Scholar]
- Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL. Endocrine regulation of cognition and neuroplasticity: Our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol. 2008;62:247–260. doi: 10.1037/a0014501. [DOI] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: Interactive effects of dietary restriction and schedule of feeding. J Nutr. 1981;111:208–218. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry. 2005;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on basal limbic-hypothalamic–pituitary–adrenal regulation: Role of corticosterone. Alcohol Clin Exp Res. 2007;31:1598–1610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Gomez F, Dallman MF. Manipulation of androgens causes different energetic responses to cold in 60- and 40-day-old male rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R262–R273. doi: 10.1152/ajpregu.2001.280.1.R262. [DOI] [PubMed] [Google Scholar]
- Gomez F, Houshyar H, Dallman MF. Marked regulatory shifts in gonadal, adrenal, and metabolic system responses to repeated restraint stress occur within a 3-week period in pubertal male rats. Endocrinology. 2002;143:2852–2862. doi: 10.1210/endo.143.8.8929. [DOI] [PubMed] [Google Scholar]
- Gomez F, Manalo S, Dallman MF. Androgen-sensitive changes in regulation of restraint-induced adrenocorticotropin secretion between early and late puberty in male rats. Endocrinology. 2004;145:59–70. doi: 10.1210/en.2003-0565. [DOI] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: A possible role in learning. Trends Cogn Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30:2055–2064. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: Fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2009 doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Galea LA. Defensive behavior and hippocampal cell proliferation: Differential modulation by naltrexone during stress. Behav Neurosci. 2002;116:160–168. [PubMed] [Google Scholar]
- Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev Psychopathol. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth AP. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res. 2007;31:2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Sliwowska JH, Viau V, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic–pituitary–adrenal function across the estrous cycle. Alcohol Clin Exp Res. 2009;33:1075–1088. doi: 10.1111/j.1530-0277.2009.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic–pituitary–adrenal axis of rats exposed to alcohol in utero: Role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000;16:515–528. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN. Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biol Psychiatry. 2006;59:786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Levine S. An endocrine theory of infantile stimulation. In: Ambrose A, editor. Stimulation in infancy. London: Academic Press; 1969. pp. 45–63. [Google Scholar]
- Lewis EM, Barnett JF, Jr, Freshwater L, Hoberman AM, Christian MS. Sexual maturation data for Crl Sprague–Dawley rats: Criteria and confounding factors. Drug Chem Toxicol. 2002;25:437–458. doi: 10.1081/dct-120014794. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: Effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23:49–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Crawford EF, Eisch AJ, Rivier CL, Richardson HN. Stress experienced in utero reduces sexual dichotomies in neurogenesis, microenvironment, and cell death in the adult rat hippocampus. Dev Neurobiol. 2008;68:575–589. doi: 10.1002/dneu.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SG, Owen D, Banjanin S, Andrews MH. Glucocorticoids, hypothalamo-pituitary–adrenal (HPA) development, and life after birth. Endocr Res. 2002;28:709–718. doi: 10.1081/erc-120016991. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- Mazzucco CA, Lieblich SE, Bingham BI, Williamson MA, Viau V, Galea LA. Both estrogen receptor alpha and estrogen receptor beta agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141:1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- McCloskey DP, Hintz TM, Pierce JP, Scharfman HE. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 2006;24:2203–2210. doi: 10.1111/j.1460-9568.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: Allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann NY Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- Miller MW. Generation of neurons in the rat dentate gyrus and hippocampus: Effects of prenatal and postnatal treatment with ethanol. Alcohol Clin Exp Res. 1995;19:1500–1509. doi: 10.1111/j.1530-0277.1995.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Dow-Edwards DL. Structural and metabolic alterations in rat cerebral cortex induced by prenatal exposure to ethanol. Brain Res. 1988;474:316–326. doi: 10.1016/0006-8993(88)90445-3. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Taylor AN, Lewis JW, Poland RE, Redei E, Branch BJ. Pituitary-adrenal responses to morphine and footshock stress are enhanced following prenatal alcohol exposure. Alcohol Clin Exp Res. 1986;10:397–402. doi: 10.1111/j.1530-0277.1986.tb05112.x. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Paley B. The relationship of prenatal alcohol exposure and the postnatal environment to child depressive symptoms. J Pediatr Psychol. 2006;31:50–64. doi: 10.1093/jpepsy/jsj021. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants’ adrenocortical reactivity to stress. J Pediatr Psychol. 1996;21:833–840. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16:305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Richardson DP, Byrnes ML, Brien JF, Reynolds JN, Dringenberg HC. Impaired acquisition in the water maze and hippocampal long-term potentiation after chronic prenatal ethanol exposure in the guinea-pig. Eur J Neurosci. 2002;16:1593–1598. doi: 10.1046/j.1460-9568.2002.02214.x. [DOI] [PubMed] [Google Scholar]
- Riikonen R, Salonen I, Partanen K, Verho S. Brain perfusion SPECT and MRI in foetal alcohol syndrome. Dev Med Child Neurol. 1999;41:652–659. doi: 10.1017/s0012162299001358. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: An overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Karatsoreos IN, McEwen BS. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm Behav. 2006;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Savage DD, Becher M, de la Torre AJ, Sutherland RJ. Dose-dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcohol Clin Exp Res. 2002;26:1752–1758. doi: 10.1097/01.ALC.0000038265.52107.20. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science. 2007;315:336–338. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Sliwowska J, Zhang X, Weinberg J. Prenatal ethanol exposure and fetal programming: Implications for endocrine and immune development and long-term health. In: Miller M, editor. Brain development. Normal processes and the effects of alcohol and nicotine. New York: Oxford University Press; 2006. pp. 153–181. [Google Scholar]
- Sliwowska JH, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J. Effects of prenatal ethanol exposure on regulation of basal hypothalamic–pituitary–adrenal activity and hippocampal 5-HT1A receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology. 2008;33:1111–1123. doi: 10.1016/j.psyneuen.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. Jama. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Berent S, Schork MA, Schteingart DE. Elevated cortisol levels in Cushing’s disease are associated with cognitive decrements. Psychosom Med. 2001;63:985–993. doi: 10.1097/00006842-200111000-00018. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Nelson LR, Branch BJ, Kokka N, Poland RE. Altered stress responsiveness in adult rats exposed to ethanol in utero: Neuroendocrine mechanisms. Ciba Found Symp. 1984;105:47–65. doi: 10.1002/9780470720868.ch4. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol exposure alters adrenocortical development of offspring. Alcohol Clin Exp Res. 1989;13:73–83. doi: 10.1111/j.1530-0277.1989.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: Foetal programming, the hypothalamic–pituitary–adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- West JR, Hodges CA, Black AC., Jr Prenatal exposure to ethanol alters the organization of hippocampal mossy fibers in rats. Science. 1981;211:957–959. doi: 10.1126/science.7466371. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc. 2008;14:1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Reuter JM. Sexually dimorphic behavioral and brain asymmetries in neonatal rats: Effects of prenatal alcohol exposure. Brain Res Dev Brain Res. 1989;46:281–290. doi: 10.1016/0165-3806(89)90291-5. [DOI] [PubMed] [Google Scholar]