Abstract
The positive roles of antioxidants on brain development and learning and memory have been suggested. Nigella sativa (NS) has been suggested to have antioxidant and neuroprotective effects. This study was done to investigate the effects of feeding by the hydro-alcoholic extract of NS during neonatal and juvenile growth on learning and memory of rats. The pregnant rats were kept in separate cages. After delivery, they were randomly divided into four Groups including: (1) control; (2) NS 100 mg/kg (NS 100); (3) NS 200 mg/kg (NS 200); and (4) NS 400 mg/kg (NS 400). Rats in the control group (Group 1) received normal drinking water, whereas Groups 2, 3, and 4 received the same drinking water supplemented with the hydro-alcoholic extract of NS (100 mg/kg, 200 mg/kg, and 400 mg/kg, respectively) from the 1st day after birth through the first 8 weeks of life. After 8 weeks, 10 male offspring from each group were randomly selected and tested in the Morris water maze (MWM) and passive avoidance (PA) test. Finally, the brains were removed and total thiol groups and malondialdehyde (MDA) concentrations were determined. In the MWM, treatment by 400 mg/kg extract reduced both the time latency and the distance traveled to reach the platform compared to the control group (p < 0.05–p < 0.01). Both 200 mg/kg and 400 mg/kg of the extract increased the time spent in the target quadrant (p < 0.05–p < 0.01). In the PA test, the treatment of the animals by 200 mg/kg and 400 mg/kg of NS extract significantly increased the time latency for entering the dark compartment (p < 0.05–p < 0.001). Pretreatment of the animals with 400 mg/kg of NS extract decreased the MDA concentration in hippocampal tissues whereas it increased the thiol content compared to the control group (p < 0.001). These results allow us to propose that feeding of the rats by the hydro-alcoholic extract of NS during neonatal and juvenile growth has positive effects on learning and memory. The effects might be due to the antioxidant effects.
Keywords: Juvenile, Learning, Memory, Neonatal, Nigella sativa, Oxidative stress
Graphical abstract

1. Introduction
The development of the brain is a highly complex and precisely timed process, which starts at gestation and continues throughout the juvenile stages to adolescence.1 The human brain grows fastest during the second and third trimesters of pregnancy and the first 2 years of postnatal life, reaching 83% of adult values by the end of the 2nd year.1 During these critical periods of growth and development, the number and/or size of the cells is influenced by the state of nutrition.2 It is suggested that changes in nutritional conditions affect neuronal structure, function, or connectivity and eventually may lead to long-lasting, even permanent, effects, which may contribute to brain disorders later in life.3 Neurodevelopmental alterations in the frontal/prefrontal cortex, striatum, and hippocampus, which are heavily involved in cognition, memory, emotion, and learning, are likely involved in the etiology of neuropsychiatric disorders like autism, substance use disorders, schizophrenia, Parkinson's disease, and Alzheimer's disease.4 Various dietary factors such as n-3 fatty acids, antioxidants, vitamins, minerals, curcumins, and flavonoids among others have been identified to have beneficial effects on brain functions including cognition.5 During the developmental period, the intake of n-3 and n-6 long-chain polyunsaturated fatty acids (LCPUFAs), particularly docosahexanoic acid (DHA), eicosapentaenoic acid (EPA), and arachidonic acid (AA), has been shown to be beneficial for the development of sensory, cognitive, and neuromotor systems in humans and animals.6
Antioxidants are well known for their beneficial effects on neurological actions.7 Basic animal studies conclude that antioxidants, in adequate amounts, improve cognitive performance. Blueberry extract, for example, not only improves memory tasks, but it also inhibits acetylcholinesterase (AChE), a synaptic enzyme that is inhibited in therapies for Alzheimer's disease, such as tacrine, donepezil, and rivastigmine.8 A study using a rodent model of Alzheimer's disease suggested that tau phosphorylation occurs as a compensatory response to oxidative stress.9 Epidemiological studies have suggested a link between antioxidant consumption and cognitive protection. Also, several clinical trials have confirmed the memory improving effects of antioxidants in patients with normal aging or Alzheimer's disease.10
Antioxidant compounds have also been shown to have an important role in brain development. An optimal supply of antioxidants such as vitamin E is thought to be beneficial to cognitive development in infants.11 Indeed, optimal supplementation of a specific nutrient during early life could influence or program long-term cognitive development, as well as development of major diseases well into adulthood.12 In recent decades, there has been a significant shift in thinking about nutrition from a preoccupation with meeting nutrient needs to a concern about its effect on health, including adult degenerative diseases, cancer, and cognitive function.13 It has been hypothesized that antioxidants could be prophylactic against central nervous system (CNS) diseases. Brain protein, lipid, and nucleic acid oxidation products increase at an accelerating pace with age.14
In traditional medicine, Nigella sativa (NS) was identified to have healing power. It has been used in the Middle East and Far East for treating diseases such as asthma, headache, dysentery, infections, obesity, back pain, hypertension, and gastrointestinal problems. There is a common Islamic opinion that NS is useful for all diseases except death.15, 16, 17, 18, 19, 20, 21 The main active ingredients isolated from NS seeds are thymoquinone (TQ), alkaloids (nigellidine, nigellimine, and nigellicine), vitamins such as thiamine, riboflavin, pyridoxine, niacin, and folic acid, minerals, and proteins.22 NS oil has been shown to drastically improve neuronal cell viability compared to untreated cerebellar neuron cell culture and to protect against beta-amyloid protein intoxication.23An in vitro study revealed that the methanolic extract of NS modulates the neuronal release of amino acid neurotransmitters including γ-aminobutyric acid (GABA), glycine, aspartate, and glutamate on cultured cortical neurons.24 NS oil also has antioxidant effects during cerebral ischemia–reperfusion injury in the rat hippocampus.25 Some studies showed that NS protects against hippocampal neurodegeneration.26 Recent studies revealed the positive modulating impact of NS on memory, attention, and cognition.27
The present study aimed to elucidate the effects of feeding NS during neonatal and juvenile growth on learning and memory of rats.
2. Materials and methods
2.1. Animals and treatments
Fourteen pregnant female Wistarrats (12 weeks old and weighing 220–250 g) were purchased from animal center of Mashhad University of Medical Sciences, Mashhad, Iran and kept in separate cages at 22 ± 2°C in a room with a 12-hour light/dark cycle (lights on at 7:00 am). They were randomly divided into four Groups including: (1) control; (2) NS 100 mg/kg (NS 100); (3) NS 200 mg/kg (NS 200); and (4) NS400 mg/kg (NS 400). The animals were treated according to the experimental protocol from the 1st day after delivery through the first 2 months of life.
Rats in the control group (Group 1) received normal drinking water whereas Groups 2, 3, and 4 received the same drinking water supplemented with the hydro-alcoholic extract of NS 100 mg/kg, 200 mg/kg, and 400 mg/kg, respectively.28 After 60 days, 10 male offspring from each group were randomly selected and examined in the Morris water maze (MWM) and passive avoidance (PA) tests. Animal handling and all related procedures were carried out in accordance with the rules set by Mashhad University of Medical Sciences Ethical Committee. PTU was purchased from Sigma (Sigma Aldrich Chemical Co.St. Louis, MO). Other chemicals which were used for biochemical assessments were purchased from Merck Company (Darmstadt, Germany).
2.2. MWM apparatus and procedures
A circular black pool (136 cm diameter, 60 cm high, and 30 cm deep) was filled with water (24–26°C). A circular platform (10 cm diameter, 28 cm high) was placed within the pool and was submerged approximately 2 cm below the surface of the water in the center of the southwest quadrant. Outside the maze, fixed visual cues (i.e., a computer, hardware, and posters) were present at various locations around the room. Before the experiment, each rat was handled daily for 3 days and habituated to the water maze for 30 seconds without a platform. The animals performed four trials on each of the 5 days, and each trial began with the rat being placed in the pool and released facing the side wall at one of four positions (the boundaries of the four quadrants, labeled north (N), east (E), south (S), and west (W). The release positions were randomly predetermined. For each trial, the rats were allowed to swim until they found and remained on the platform for 15 seconds. If 60 seconds had passed and the animals had not found the platform, they were guided to the platform by the experimenter and allowed to stay on the platform for 15 seconds. The rats were then removed from the pool, dried, and placed in a holding bin for 5 minutes. The time latency to reach the platform and the length of the swimming path were recorded using a video tracking system. On the 6th day, the platform was removed, and the animals were allowed to swim for 60 seconds. The time spent in the target quadrant was recorded for comparisons between groups.29, 30 All measurements were performed during the second half of the light cycle.
2.3. PA apparatus and procedures
A PA learning test based on negative reinforcement was used to examine memory. The apparatus consisted of a light and a dark compartment with a grid floor adjoining them through a small gate. The animals were accustomed to the behavioral apparatus during 2 consecutive days (5 minutes on each day) before the training session. On the 3rd day, the animals were placed in the light compartment, and the time latency for entering the dark compartment was recorded. In the training phase, the rats were placed in the light compartment facing away from the dark compartment. When the animals were entered completely into the dark compartment, they received an electric shock (2 mA, 2 seconds duration). The mice were then returned to their home cages. One, 24 hours, and 72 hours later (the retention phase or test phase), the animals were placed in the light compartment, and the time latency for entering the dark compartment as well as the time spent by the animals in the dark and light compartments were recorded and defined as the retention trial.31
2.4. Biochemical assessment
Finally, the animals were sacrificed and the cortical and hippocampal tissues were removed, weighed, and submitted to determination of total thiol (SH) contents and malondialdehyde (MDA) concentrations. Total SH groups were measured using DTNB (2,2′-dinitro-5,5′-dithiodibenzoic acid) as the reagent. This reagent reacts with the SH groups to produce a yellow-colored complex that has a peak absorbance at 412 nm.32 Briefly, 1 mL Tris–EDTA buffer (pH = 8.6) was added to 50 μL of brain homogenate in 1-mL cuvettes and the sample absorbance was read at 412 nm against Tris–EDTA buffer alone (A1). Then, 20 μL of DTNB reagent (10 mM in methanol) were added to the mixture and after 15 minutes (stored at laboratory temperature) the sample absorbance was read again (A2). The absorbance of the DTNB reagent was also read as a blank (B). The total thiol concentration (mM) was calculated from the following equation31, 33, 34, 35: Total thiol concentration (mM) = (A2 − A1 − B) × 1.07/0.05 × 13.6.
MDA levels, as an index of lipid peroxidation, were measured. MDA reacts with thiobarbituric acid (TBA) as a thiobarbituric acid reactive substance (TBARS) to produce a red-colored complex that has a peak absorbance at 535 nm. Two milliliters of a TBA/trichloroacetic acid (TCA)/hydrochloric acid (HCL) reagent was added to 1 mL of homogenate and the solution was heated in a water bath for 40 minutes. After cooling, the whole solution was centrifuged at 1000 × g for 10 minutes. The absorbance was measured at 535 nm.31, 34, 35 The MDA concentration was calculated as follows:
| C(m) = absorbance/(1.56 × 105) |
2.5. Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM). The data for time and distance during 5 days of the MWM were compared using repeated measures analysis of variance (ANOVA) followed by a post-hoc comparisons test. The data from the PA test as well as MDA and total thiol concentrations were compared using one-way ANOVA followed by a post-hoc comparisons test. Differences were considered statistically significant when p < 0.05.
3. Results
3.1. MWM test
Treatment with 400 mg/kg of the NS extract reduced the time latency to reach the platform compared to the control group (Fig. 1A and B; p < 0.01). In the group treated with 400 mg/kg of the extract, the distance traveled to reach the platform was lower than that of the control (Fig. 2A and B; p < 0.05). The administration of both 200 mg/kg and 400 mg/kg of the NS extract increased the time spent in the target quadrant (Fig. 3; p < 0.05 and p < 0.01).
Fig. 1.
Comparison of time latency between the groups. Data are presented as mean ± SEM. (A) Comparison of time latency each day between the groups; (B) comparison of group total time latencies over 5 days (n = 10 in each group). Rats in the control group received tap drinking water; NS 100, NS 200, and NS 400 groups received 100 mg/kg, 200 mg/kg, and 400 mg/kg of the NS extract, respectively, in drinking water. *p < 0.05 in comparison with the control group. NS = Nigella sativa; SEM = standard error of the mean.
Fig. 2.
Comparison of the traveled distance between the groups. Data are presented as mean ± SEM. (A) Comparison of the distance traveled each day between the groups; (B) comparison of the group total traveled distance over 5 days (n = 10 in each group). Rats in the control group received tap drinking water; NS 100, NS 200, and NS 400 groups received 100 mg/kg, 200 mg/kg, and 400 mg/kg of NS extract, respectively, in drinking water. **p < 0.01 in comparison with the control group. NS = Nigella sativa; SEM = standard error of the mean.
Fig. 3.
The results of the time spent in the target quadrant (Q1) and nontarget quadrants (Q2–Q4) during the probe trial on Day 6. Data are presented as mean ± SEM (n = 10 in each group). Rats in the control group received tap drinking water; NS 100, NS 200, and NS 400 groups received 100 mg/kg, 200 mg/kg, and 400 mg/kg of NS extract, respectively, in drinking water. The platform was removed, and the time spent in the target quadrant (Q1) and nontarget quadrants (Q2–Q4) was compared between the groups. *p < 0.05, **p < 0.01, ***p < 0.001 in comparison with the control group. NS = Nigella sativa; SEM = standard error of the mean.
3.2. PA test
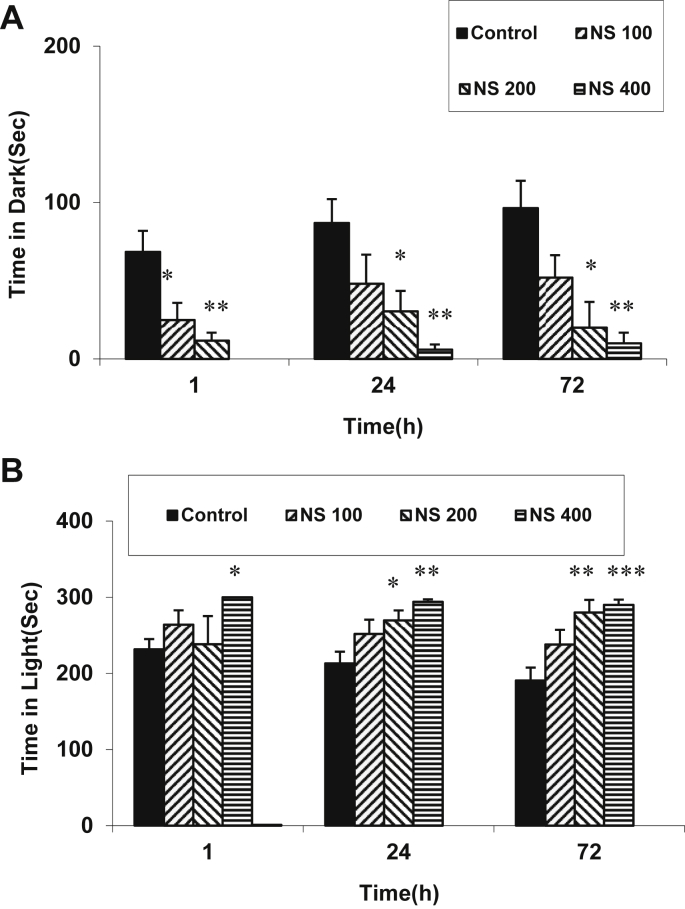
The treatment of the animals with 400 mg/kg of the NS extract significantly increased the time latency for entering the dark compartment at 1 hour after receiving a shock (Fig. 4A; p < 0.01). Treatment of the animals with 200 mg/kg and 400 mg/kg of the extract also increased the time latency at 24 hours after receiving a shock (Fig. 4A; p < 0.05 and p < 0.01). The results also showed that both 200 mg/kg and 400 mg/kg of the extract increased the time latency at 72 hours after receiving the shock (Fig. 4A; p < 0.01 and p < 0.001). Fig. 4B shows the number of entries to the dark compartment after the shock. Treatment with 400 mg/kg of the extract reduced the number of entries to the dark compartment at 1 hour, 24 hours, and 72 hours after the shock (p < 0.01–p < 0.001). Treatment of the animals with 200 mg/kg of the extract also decreased the number of entries to the dark compartment at 72 hours after receiving a shock (Fig. 4B; p < 0.05). The results also showed that treatment with 200 mg/kg and 400 mg/kg of the NS extract reduced the time spent in the dark compartment at 1 hour, 24 hours, and 72 hours after the shock (Fig. 5A; p < 0.05–p < 0.01). There was no significant difference between the animals treated with 100 mg/kg of the extract and the control group (Fig. 5A). There was no significant difference between the animals of the NS 100 and control groups when the time spent in the light compartment was compared. The time spent in light at both 24 hours and 72 hours after the shock (Fig. 5B; p < 0.05–p < 0.01) increased on treatment with 200 mg/kg of the extract. Treatment with 400 mg/kg of the extract increased the time spent in light at 1 hour, 24 hours, and 72 hours (Fig. 5B; p < 0.05–p < 0.001).
Fig. 4.
Comparison of (A) time latency for entering the dark compartment and (B) the number of entries to the dark compartment at 1 hour, 24 hours, and 72 hours after receiving the shock in the experimental groups. Data are presented as mean ± SEM (n = 10 in each group). Rats in the control group received tap drinking water; NS 100, NS 200, and NS 400 groups received 100 mg/kg, 200 mg/kg, and 400 mg/kg of NS extract, respectively, in drinking water. *p < 0.05, **p < 0.01 and ***p < 0.001 in comparison with the control group. NS = Nigella sativa; SEM = standard error of the mean.
Fig. 5.
Comparison of the total time spent in (A) the dark and (B) the light compartments at 1 hour, 24 hours, and 72 hours after receiving the shock in the experimental groups. Data are presented as mean ± SEM (n = 10 in each group). Rats in the control group received tap drinking water; NS 100, NS 200, and NS 400 groups received 100 mg/kg, 200 mg/kg, and 400 mg/kg of NS extract, respectively, in drinking water. *p < 0.05, **p < 0.01 and ***p < 0.001 in comparison with control group. NS = Nigella sativa; SEM = standard error of the mean.
3.3. Biochemical assessment results
Pretreatment of the animals with 400 mg/kg of the NS extract decreased the MDA concentration in hippocampal tissues compared to the control group (Fig. 6A; p < 0.001). In the NS 400 group, the hippocampal total thiol concentration was significantly higher than that of the control group (Fig. 6B; p < 0.001). Treatment of the animals with both 200 mg/kg and 400 mg/kg of the extract reduced the MDA concentrations in cortical tissues (Fig. 7A; p < 0.05 and p < 0.001). Administration of 100–400 mg/kg of the extract did not change the thiol content of cortical tissues compared to the control group (Fig. 7B).
Fig. 6.
(A) MDA concentrations and (B) total thiol concentrations in hippocampal tissues of four groups (n = 10 in each group). Rats in the control group received tap drinking water; NS 100, NS 200, and NS 400 groups received 100 mg/kg, 200 mg/kg, and 400 mg/kg of NS extract, respectively, in drinking water. ***p < 0.001 in comparison with the control group. MDA = malondialdehyde; NS = Nigella sativa; SEM = standard error of the mean.
Fig. 7.
(A) MDA concentrations and (B) total thiol concentrations in the cortical tissues of four groups (n = 10 in each group). Rats in the control group received tap drinking water; NS 100, NS 200, and NS 400 groups received 100 mg/kg, 200 mg/kg, and 400 mg/kg of NS extract, respectively, in drinking water. *p < 0.05 and ***p < 0.001 in comparison with the control group. MDA = malondialdehyde; NS = Nigella sativa; SEM = standard error of the mean.
4. Discussion
The results of the present study showed that dietary supplementation with NS during lactation and in the neonatal and juvenile periods enhanced learning and memory when the animals were examined in both the MWM and PA tests. In the present study, the NS extract also improved oxidative stress criteria in hippocampal and cortical tissues. To the best of our knowledge, the effect of NS on the CNS during the developmental period has not been investigated. However, it was previously reported that NS oil had ameliorative effects on rat performance in the T maze in the short-term memory deficit induced by scopolamine in adult rats.36 Other researchers showed that long-term administration of NS improved learning and memory in rats.27 The fixed oil of NS seeds has also been demonstrated to be effective against spatial cognitive impairments induced by chronic cerebral hypo-perfusion when the animals were examined in the MWM test.37
It has also been reported that NS has positive modulation effects on aged rats with memory impairments.27 NS was also protective against learning and memory deficiency in rats with diabetes.38 Another study showed a preventive effect of NS oil against hippocampal pyramidal cell loss.26 Pharmacological studies demonstrated that NS could be involved in AChE activity inhibition, thus retaining the effects of acetylcholine (ACh) in the encoding of new memories.39 It was previously shown that the hydro-alcoholic extract of NS prevented scopolamine-induced spatial memory deficits in rats; this was accompanied by inhibition of AChE activity as well as protection against brain tissue oxidative damage.40 Hippocampal neurodegeneration after chronic toluene exposure in rats was also prevented by NS oil and TQ.26
Besides antioxidant and neuroprotective effects, NS was reported to have many other therapeutic effects, such as antitumor, immunopotentiation, anti-inflammatory, and antimicrobial effects.22, 26 It has also been demonstrated that aqueous and methanolic extracts of NS seeds exerted a potent sedative and depressive effect on the CNS and also an analgesic property.23 In the present study using the MWM and PA tests, we showed for the first time that consumption of NS during development improves learning and memory. However, future studies need to be done to elucidate the exact mechanism(s) of the effects of the plant in developing brains. In this regard, the effects of the plant on neurogenesis are suggested. Clinical studies are also suggested to show the beneficial effects of the plant in humans. A systematic review also needs to examine the neuroprotective effects of the plant.
The beneficial effects of NS on memory are most likely due to the ability of one or more of its constituents to protect against the cellular damage caused by oxidative stress through its free radical scavenging properties.41 Such similar effects have also been reported by other well-known antioxidant compounds. It has been shown that the antioxidant vitamin E protected the neonatal granule cells against ethanol toxicity in the culture medium, and that this protection was paralleled by increases in the antiapoptotic proteins Bcl-2, Bcl-xl, and pAkt and decreased expression of proapoptotic Bcl-xs.42 It was also reported that in fetal rat hippocampal cells, vitamin E protected against ethanol-induced cell death, even when ethanol treatment was combined with ischemic conditions.43 Also, in cerebellar granule cells grown in vitro, this antioxidant compound inhibited ethanol-mediated activation of caspase-3, and diminished cellular membrane disruption.44 In vivo, this vitamin reduced cerebellar Purkinje cell death at a vulnerable neonatal age42 and restored the balance of the glutathione system following destructive ethanol-induced alterations and decreased lipid peroxidation in the rat CNS.45 Vitamin E also prevented ethanol-mediated reductions in brain mass and neuronal density in the embryonic chick CNS.46 Vitamin E has also protected against haloperidol-associated neurotoxicity and locomotor impairment in rats,47 prevented 1-methyl-4-phenylpyridinium (MPP+)-induced apoptosis of cerebellar granule cells,48 and reduced 6-hydroxydopamineneurotoxicity in adult rats.49 This antioxidant has also been explored as a possible therapeutic agent for both Alzheimer's disease and Parkinson's disease.50 It has also been shown that maternal administration of the vitamin C plus E regimen throughout gestation has limited efficacy and potential adverse effects as a therapeutic intervention for ethanol neurobehavioral teratogenicity.11
Researchers have shown that ethanol exposure during nervous system development produces a range of abnormalities, and in humans may lead to fetal alcohol syndrome, which has been attributed to oxidative stress processes or the insufficiency of protective antioxidants.51 Vitamin E has been suggested as a possible therapy for preventing or ameliorating the CNS damage seen in fetal alcohol syndrome.42
Ascorbic acid, the other important antioxidant vitamin, was also reported to be important for brain development.6 It has been speculated that ascorbic acid and its oxidized form are regulators of cell division.52 It was also reported that vitamin C was neuroprotective against ethanol and nicotine via the modulation of protein kinase A expression in prenatal and postnatal rat brain.53 Deficiency of vitamin A during pregnancy has also been shown to cause malformation of the fetal brain.54 It was reported that maternal vitamin A restriction caused altered brain development in offspring in terms of tissue weight, DNA, RNA, and protein levels, and biosynthesis of DNA and proteins.14
NS and its components are also well known for their antioxidant properties. The antioxidant effects of NS and TQ in carbon tetrachloride (CCl4)-induced oxidative injury in rat liver,55 isolated rat hepatocytes,56 hypercholesterolemic rats,57 and gentamicin-58 and cyclosporine-induced kidney injury have also been reported.58 NS oil has been reported to have a protective effect on the lipid peroxidation process during ischemia–reperfusion injury in the rat hippocampus.25
Consuming antioxidant nutrients, such as NS, could be one of the promising health strategies to help prevent oxidative damage to cells, particularly in the brain regions that are related to memory functions.41 In the present study, we showed that besides its effects on learning and memory, the extract lowered brain tissue MDA concentrations and increased thiol content. It seems that the beneficial effects of the plant on learning and memory are at least in part due to its antioxidative effects. However, more precise tests need to be carried out in the future.
The neuroprotective effects of NS have been shown previously. NS and TQ, the bioactive constituent of NS, have beneficial effects against the neurotoxic effects of lead in rats.59 Another study showed that TQ prevented neurotoxicity and amyloid beta (Aβ)-induced apoptosis, thereby confirming its potential to reduce the risk of developing Alzheimer's disease.24 TQ also has a protective role against ethanol-induced neuronal apoptosis in primary rat cortical neurons.60 Regarding these facts and the results of the present study, the antioxidative and neuroprotective effects of NS extract for brain tissues as possible mechanisms for enhancing effects on learning and memory in neonatal and juvenile growth are suggested.
5. Conclusion
In conclusion, the findings of this study imply that feeding NS during the neonatal and juvenile growth period improves learning and memory in rats.
Conflicts of interest
The authors do not have any direct financial relationship with the commercial identities mentioned in this article. Therefore, the authors have no conflicts of interest.
Acknowledgments
The results described in this paper were from a M.Sc. student thesis proposal. The authors would like to thank the Vice Presidency of Research of Mashhad University of Medical Sciences (Grant number: 921386) for financial support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Knickmeyer R.C., Gouttard S., Kang C. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winick M., Noble A. Cellular response in rats during malnutrition at various ages. J Nutr. 1966;9:300–306. doi: 10.1093/jn/89.3.300. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ari Y. Neuropaediatric and neuroarchaeology: understanding development to correct brain disorders. Acta Paed. 2013;102:331–334. doi: 10.1111/apa.12161. [DOI] [PubMed] [Google Scholar]
- 4.Langen M., Leemans A., Johnston P. Fronto-striatal circuitry and inhibitory control in autism: findings from diffusion tensor imaging tractography. Cortex. 2012;48:183–193. doi: 10.1016/j.cortex.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas A. Role of nutritional programming in determining adult morbidity. Arch Dis Child. 1994;71:288–290. doi: 10.1136/adc.71.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin A., Prior R., Shukitt-Hale B., Cao G., Joseph J.A. Effect of fruits, vegetables, or vitamin E-rich diet on vitamins E and C distribution in peripheral and brain tissues: implications for brain function. J Gerontol A Biol Sci Med Sci. 2000;55:B144–B151. doi: 10.1093/gerona/55.3.b144. [DOI] [PubMed] [Google Scholar]
- 8.Kamat C.D., Gadal S., Mhatre M., Williamson K.S., Pye Q.N., Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koudinov A., Kezlya E., Koudinova N., Berezov T. Amyloid-beta, tau protein, and oxidative changes as a physiological compensatory mechanism to maintain CNS plasticity under Alzheimer's disease and other neurodegenerative conditions. J Alzheimers Dis. 2009;18:381–400. doi: 10.3233/JAD-2009-1202. [DOI] [PubMed] [Google Scholar]
- 10.Zandi P.P., Anthony J.C., Khachaturian A.S. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 11.Nash C.M., Ibram F., Dringenberg H.C., Reynolds J.N., Brien J.F. Effects of maternal administration of vitamins C and E on ethanol neurobehavioral teratogenicity in the guinea pig. Alcohol. 2007;41:577–586. doi: 10.1016/j.alcohol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Lucas A. Programming by early nutrition: an experimental approach. J Nutr. 1998;128:401S–406S. doi: 10.1093/jn/128.2.401S. [DOI] [PubMed] [Google Scholar]
- 13.Barker D.J.P., Godfrey K.M., Gluckman P.D., Harding J.E., Owens J.A., Robinson J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 14.Smith C.D., Carney J.M., Tatsumo T., Stadtman E.R., Floyd R.A., Markesbery W.R. Protein oxidation in aging brain. Ann N Y Acad Sci. 1992;663:110–119. doi: 10.1111/j.1749-6632.1992.tb38654.x. [DOI] [PubMed] [Google Scholar]
- 15.Ali B.H., Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 16.Boskabady M.H., Farhadi J. The possible prophylactic effect of Nigella sativa seed aqueous extract on respiratory symptoms and pulmonary function tests on chemical war victims: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2008;14:1137–1144. doi: 10.1089/acm.2008.0049. [DOI] [PubMed] [Google Scholar]
- 17.Boskabady M.H., Javan H., Sajady M., Rakhshandeh H. The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam Clin Pharmacol. 2007;21:559–566. doi: 10.1111/j.1472-8206.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 18.Boskabady M.H., Keyhanmanesh R., Saadatloo M.A. Relaxant effects of different fractions from Nigella sativa L. on guinea pig tracheal chains and its possible mechanism(s) Indian J Exp Biol. 2008;46:805–810. [PubMed] [Google Scholar]
- 19.Boskabady M.H., Mohsenpoor N., Takaloo L. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 2010;17:707–713. doi: 10.1016/j.phymed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Boskabady M.H., Shafei M.N., Parsaee H. Effects of aqueous and macerated extracts from Nigella sativa on guinea pig isolated heart activity. Pharmazie. 2005;60:943–948. [PubMed] [Google Scholar]
- 21.Boskabady M.H., Shirmohammadi B., Jandaghi P., Kiani S. Possible mechanism(s) for relaxant effect of aqueous and macerated extracts from Nigella sativa on tracheal chains of guinea pig. BMC Pharmacol. 2004;4:3. doi: 10.1186/1471-2210-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salem M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 23.El-Naggar T., Gomez-Serranillos M.P., Palomino O.M., Arce C., Carretero M.E. Nigella sativa L. seed extract modulates the neurotransmitter amino acids release in cultured neurons in vitro. J Biomed Biotechnol. 2010;2010:398312. doi: 10.1155/2010/398312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ismail N., Ismail M., Mazlan M. Thymoquinone prevents beta-amyloid neurotoxicity in primary cultured cerebellar granule neurons. Cell Mol Neurobiol. 2013;33:1159–1169. doi: 10.1007/s10571-013-9982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosseinzadeh H., Parvardeh S., Asl M.N., Sadeghnia H.R., Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14:621–627. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Kanter M. Nigella sativa and derived thymoquinone prevents hippocampal neurodegeneration after chronic toluene exposure in rats. Neurochem Res. 2008;33:579–588. doi: 10.1007/s11064-007-9481-z. [DOI] [PubMed] [Google Scholar]
- 27.Bin Sayeed M.S., Asaduzzaman M., Morshed H., Hossain M.M., Kadir M.F., Rahman M.R. The effect of Nigella sativa Linn. seed on memory, attention and cognition in healthy human volunteers. J Ethnopharmacol. 2013;148:780–786. doi: 10.1016/j.jep.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Javanbakht J., Hobbenaghi R., Hosseini E. Histopathological investigation of neuroprotective effects of Nigella sativa on motor neurons anterior horn spinal cord after sciatic nerve crush in rats. Pathol Biol. 2013;61:250–253. doi: 10.1016/j.patbio.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Hosseini M., Hadjzadeh M.A., Derakhshan M. The beneficial effects of olibanum on memory deficit induced by hypothyroidism in adult rats tested in Morris water maze. Arch Pharm Res. 2010;33:463–468. doi: 10.1007/s12272-010-0317-z. [DOI] [PubMed] [Google Scholar]
- 30.Saffarzadeh F., Eslamizade M.J., Nemati Karimooy H.A., Hadjzadeh M.A., Khazaei M., Hosseini M. The effect of L-arginine on Morris water maze tasks of ovariectomized rats. Acta Physiol Hung. 2010;97:216–223. doi: 10.1556/APhysiol.97.2010.2.8. [DOI] [PubMed] [Google Scholar]
- 31.Pourganji M., Hosseini M., Soukhtanloo M., Zabihi H., Hadjzadeh M.A. Protective role of endogenous ovarian hormones against learning and memory impairments and brain tissues oxidative damage induced by lipopolysaccharide. Iran Red Crescent Med J. 2014;16:e13954. doi: 10.5812/ircmj.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 33.Hosseini M., Pourganji M., Khodabandehloo F., Soukhtanloo M., Zabihi H. Protective effect of L-arginine against oxidative damage as a possible mechanism of its beneficial properties on spatial learning in ovariectomized rats. Basic Clin Neurosci. 2012;3:36–44. [Google Scholar]
- 34.Khodabandehloo F., Hosseini M., Rajaei Z., Soukhtanloo M., Farrokhi E., Rezaeipour M. Brain tissue oxidative damage as a possible mechanism for the deleterious effect of a chronic high dose of estradiol on learning and memory in ovariectomized rats. Arq Neuropsiquiatr. 2013;71:313–319. doi: 10.1590/0004-282x20130027. [DOI] [PubMed] [Google Scholar]
- 35.Hosseini M., Harandizadeh F., Niazamand S., Soukhtanloo M., Mahmoudabady M. Antioxidant effect of Achillea wilhelmsii extract on pentylenetetrazole (seizure model)-induced oxidative brain damage in Wistar rats. Indian J Physiol Pharmacol. 2013;57:418–424. [PubMed] [Google Scholar]
- 36.El-Marasy S.A., El-Shenawy S.M., El-Khatib A.S., El-Shabrawy O.A., Kenawy S.A. Effect of Nigella sativa and wheat germ oils on scopolamine-induced memory impairment in rats. Bull FacPharmacy, Cairo Univ. 2010;50:81–88. [Google Scholar]
- 37.Azzubaidi M.S., Saxena A.K., Talib N.A., Ahmed Q.U., Dogarai B.B. Protective effect of treatment with black cumin oil on spatial cognitive functions of rats that suffered global cerebrovascular hypoperfusion. Acta Neurobiol Exp. 2012;72:154–165. doi: 10.55782/ane-2012-1888. [DOI] [PubMed] [Google Scholar]
- 38.Jalali M.R., Roghani M. The effect of Nigella sativa on learning and memory in male diabetic rats. Basic Clin Neurosci. 2009;1:32–34. [Google Scholar]
- 39.Hasselmo M.E. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosseini M., Mohammadpour T., Karami R., Rajaei Z., Sadeghnia H.R., Soukhtanloo M. Effects of the hydro-alcoholic extract of Nigella sativa on scopolamine-induced spatial memory impairment in rats and its possible mechanism. Chin J Integr Med. 2014 doi: 10.1007/s11655-014-1742-5. [DOI] [PubMed] [Google Scholar]
- 41.Sandhu K.S., Rana A.C. Evaluation of anti-Parkinson's activity of Nigella sativa (kalonji) seeds in chlorpromazine induced experimental animal model. Int J Pharm Pharm Sci. 2013;5 [Google Scholar]
- 42.Heaton M.B., Mitchell J.J., Paiva M. Amelioration of ethanol-induced neurotoxicity in the neonatal rat central nervous system by antioxidant therapy. Alcohol Clin Exp Res. 2000;24:512–518. Epub 2000/05/08. [PubMed] [Google Scholar]
- 43.Mitchell J.J., Paiva M., Heaton M.B. Vitamin E and beta-carotene protect against ethanol combined with ischemia in an embryonic rat hippocampal culture model of fetal alcohol syndrome. Neurosci Lett. 1999;263:189–192. doi: 10.1016/s0304-3940(99)00144-5. [DOI] [PubMed] [Google Scholar]
- 44.Siler-Marsiglio K.I., Shaw G., Heaton M.B. Pycnogenol and vitamin E inhibit ethanol-induced apoptosis in rat cerebellar granule cells. J Neurobiol. 2004;59:261–271. doi: 10.1002/neu.10311. [DOI] [PubMed] [Google Scholar]
- 45.Agar E., Bosnak M., Amanvermez R., Demir S., Ayyildiz M., Celik C. The effect of ethanol on lipid peroxidation and glutathione level in the brain stem of rat. Neuroreport. 1999;10:1799–1801. doi: 10.1097/00001756-199906030-00032. [DOI] [PubMed] [Google Scholar]
- 46.Miller R.R., Jr., Olson B.M., Rorick N., Wittingen A.L., Bullock M. Embryonic exposure to exogenous alpha- and gamma-tocopherol partially attenuates ethanol-induced changes in brain morphology and brain membrane fatty acid composition. Nutr Neurosci. 2003;6:201–212. doi: 10.1080/1028415031000119329. [DOI] [PubMed] [Google Scholar]
- 47.Post A., Rucker M., Ohl F. Mechanisms underlying the protective potential of alpha-tocopherol (vitamin E) against haloperidol-associated neurotoxicity. Neuropsychopharmacology. 2002;26:397–407. doi: 10.1016/S0893-133X(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Polo R.A., Soler G., Alvarez A., Fabregat I., Fuentes J.M. Vitamin E blocks early events induced by 1-methyl-4-phenylpyridinium (MPP+) in cerebellar granule cells. J Neurochem. 2003;84:305–315. doi: 10.1046/j.1471-4159.2003.01520.x. [DOI] [PubMed] [Google Scholar]
- 49.Perumal A.S., Gopal V.B., Tordzro W.K., Cooper T.B., Cadet J.L. Vitamin E attenuates the toxic effects of 6-hydroxydopamine on free radical scavenging systems in rat brain. Brain Res Bull. 1992;29:699–701. doi: 10.1016/0361-9230(92)90142-k. [DOI] [PubMed] [Google Scholar]
- 50.Fariss M.W., Zhang J.G. Vitamin E therapy in Parkinson's disease. Toxicology. 2003;189:129–146. doi: 10.1016/s0300-483x(03)00158-6. [DOI] [PubMed] [Google Scholar]
- 51.Guerri C., Montoliu C., Renau-Piqueras J. Involvement of free radical mechanism in the toxic effects of alcohol: implications for fetal alcohol syndrome. Free Rad Diagn Med. 1994:291–305. doi: 10.1007/978-1-4615-1833-4_20. [DOI] [PubMed] [Google Scholar]
- 52.Edgar J.A. Dehydroascorbic acid and cell division. Nature. 1970;227:24–26. doi: 10.1038/227024a0. [DOI] [PubMed] [Google Scholar]
- 53.Naseer M.I., Lee H.Y., Kim M.O. Neuroprotective effect of vitamin C against the ethanol and nicotine modulation of GABA(B) receptor and PKA-alpha expression in prenatal rat brain. Synapse. 2010;64:467–477. doi: 10.1002/syn.20752. [DOI] [PubMed] [Google Scholar]
- 54.Millen J.W., Woollam D.H., Lamming G.E. Congenital hydrocephalus due to experimental hypovitaminosis A. Lancet. 1954;267:679–683. doi: 10.1016/s0140-6736(54)90453-5. [DOI] [PubMed] [Google Scholar]
- 55.Kanter M., Coskun O., Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch Toxicol. 2006;80:217–224. doi: 10.1007/s00204-005-0037-1. [DOI] [PubMed] [Google Scholar]
- 56.Mansour M.A., Ginawi O.T., El-Hadiyah T., El-Khatib A.S., Al-Shabanah O.A., Al-Sawaf H.A. Effects of volatile oil constituents of Nigella sativa on carbon tetrachloride-induced hepatotoxicity in mice: evidence for antioxidant effects of thymoquinone. Res Commun Mol Pathol Pharmacol. 2001;110:239–251. [PubMed] [Google Scholar]
- 57.Ismail M., Al-Naqeep G., Chan K.W. Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic Biol Med. 2010;48:664–672. doi: 10.1016/j.freeradbiomed.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Yaman I., Balikci E. Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2010;62:183–190. doi: 10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Radad K., Hassanein K., Al-Shraim M., Moldzio R., Rausch W.D. Thymoquinone ameliorates lead-induced brain damage in Sprague Dawley rats. Exp Toxicol Pathol. 2014;66:13–17. doi: 10.1016/j.etp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Ullah I., Ullah N., Naseer M.I., Lee H.Y., Kim M.O. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci. 2012;13:11. doi: 10.1186/1471-2202-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]