Abstract
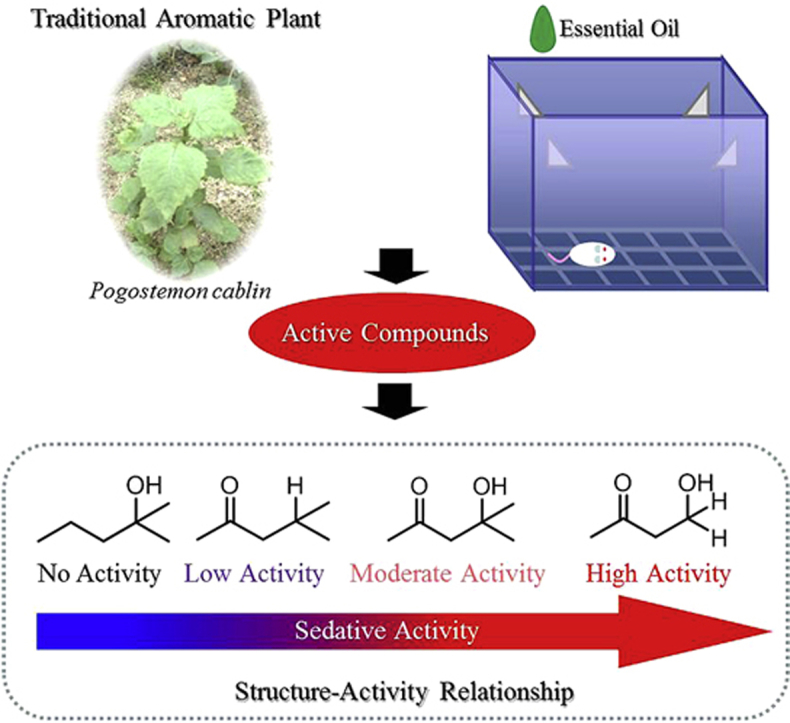
Plants rich in essential oils, such as Pogostemon cablin (P. cablin; 廣藿香 guǎng huò xiāng), have been used for aromas and as herbal medicines since ancient times because of their sedative effects. We investigated the sedative effects of hexane extract from P. cablin using locomotor activity in mice. Inhalation of P. cablin hexane extract exhibited significant sedative activity in a dose-dependent manner. In order to isolate the active constituents, the extract was fractionated and diacetone alcohol was identified as an active compound. Inhalation of diacetone alcohol significantly reduced murine locomotor activity in a dose-dependent manner, and this effect was not observed in olfaction-impaired mice. We examined the structure–activity relationship of diacetone alcohol and similar compounds. The ketone group at the two-position and number of carbons may play important roles in the sedative activity of diacetone alcohol.
Keywords: behavioral pharmacology, diacetone alcohol, inhalation, Labiatae, Pogostemon cablin, sedative effect, structure–activity relationship
Graphical abstract
1. Introduction
In Japan, there is a traditional custom of burning incense or enjoying scents from scent bags, which are made of Japanese paper or cloth containing aromatic herbal medicines and cause the scent to be emitted for a long time without the use of a flame. Typically, mixtures of minced herbal medicines have been used, such as dried herbs of Pogostemon cablin (P. cablin; 廣藿香 guǎng huò xiāng), bark of Cinnamomum cassia (肉桂 ròu guì), heartwood of Santalum album (檀香 tán xiāng), and roots of Hedychium spicatum (草果藥 cǎo guǒ yào), Illicium verum (八角 bā jiǎo), Syzygium aromaticum (丁香 dīng xiāng), Nardostachys chinensis (甘松 gān sōng), and Dryobalanops aromatica (冰片 bīng piàn). The extracts of these herbal medicines have been reported to have insecticidal and bactericidal potentials.1, 2 However, the effects of the scents, that is, inhaled volatile components, have not been sufficiently examined. Recently, aromatherapy, in which unhealthy people inhale essential oil components may prevent or treat physical and mental ailments, has received considerable attention. We conducted simple experiments using mice to examine the aromatic components of P. cablin and their sedative effects. P. cablin is also blended in the formula of traditional Chinese medicine (中醫 zhōng yī; TCM) and frequently used for its aroma. In previous studies, the scavenging effects of reactive oxygen species,3 antitrypanosomal activities4 of the extract, and decreases of human relative sympathetic activities due to inhaled oil have been reported.5 In this study, we evaluated the effects of inhalation of the extract of P. cablin and its active components on mouse locomotor activity. Additionally, we examined the structure–activity relationships of the active component, diacetone alcohol.
2. Materials and methods
2.1. Materials
Dried leaves of P. cablin (廣藿香 guǎng huò xiāng) were purchased from Mitsuboshi Pharmaceutical Co. Ltd. (Nara, Japan). Lavender oil used as a positive control for the inhalation studies was purchased from Nacalai Tesque, Inc. (Kyoto, Japan) and its batch number was the same as that in our previously reported studies.6, 7, 8 Gas chromatography–mass spectrometry (GC-MS) analysis showed that this lavender oil contained 41.3% linalool and 48.2% linalyl acetate.8 Triethyl citrate (Merck, Darmstadt, Germany), an odorless solvent, was used to dissolve the scent components. Chlorpromazine hydrochloride and pentobarbital were obtained from Mitsubishi Tanabe Pharma Corporation (Osaka, Japan). Diacetone alcohol, 1,1-dimethyl-1-butanol, isobutyl methyl ketone, 4-hydroxy-2-butanone, zinc sulfate, and corn oil were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
2.2. Animals
Animal experiments were designed following recommendations from the Animal Research Committee of Kyoto University, Kyoto, Japan. Experimental procedures involving the animals and their care were conducted in conformity with the institutional guidelines that are in compliance with Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science, and Technology, Japan (2006). Male 4-week-old ddY mice (25–30 g) were purchased from Japan SLC (Shizuoka, Japan). The mice were housed in colony glass cages at a temperature of 25 °C and a relative humidity of 50–60% with a light–dark cycle of 12 hours prior to being used for the experiments. They were fed standard pellet chow and water ad libitum. All behavioral observations were conducted between 10:00 and 17:00. The sedative activity of the scent components was evaluated in mice assessing their spontaneous motor activity in an open-field test as described in our previous report.6 Briefly, fragrance components were dissolved in triethyl citrate (400 μL total), and four filter paper disks were permeated with the solution. The disks were placed on the wall of a glass cage (W 60 cm × L 30 cm × H 34 cm) using Scotch tape, and the vapor of the solution pervaded the glass cage by natural diffusion. Sixty minutes after charging the solution, a mouse was placed into the center of the glass cage and was monitored by a video camera for another 60 minutes. In a further experiment, diacetone alcohol was dissolved in corn oil and intraperitoneally injected into mice. The frequency of the mouse crossing lines drawn on the bottom of the glass cage at 10 cm intervals was determined every 5 minutes for 60 minutes. The area under the curve representing total locomotor activity for 60 minutes was calculated by the trapezoidal rule. Another experiment using pentobarbital was conducted in accordance with our previous paper.8
2.3. Olfaction impairment of mice by zinc sulfate treatment
Zinc sulfate treatment was conducted as previously reported.9 Nasal drops (0.1 mL) of 1% zinc sulfate solution were carefully administered through a guide cannula only to the left nasal cavity of 4-week-old male ddY mice in a dorsal position under pentobarbital anesthesia. To achieve sufficient effects on the olfactory epithelium, mice received the nasal drops of zinc sulfate solution and were kept in a dorsal position for 10 minutes. Two days after the treatment, mice with sufficiently impaired olfaction were used in the experiment. The impaired olfaction was confirmed by an olfaction test using acetic acid. The test was conducted as previously reported.10 Cotton wool with 500 μL of 100% acetic acid dropped onto it was placed at one end of a glass cage. Cotton wool with 500 μL of distilled water dropped onto it was placed at the other end of the glass cage. The glass cage bottom was separated into halves by drawing a line. The behaviors of the mice in this glass cage were observed for 15 minutes. Then, the time spent in the acetic acid or distilled water area was recorded.
2.4. GC-MS analysis
Qualitative analysis of volatile components was conducted as follows: GC-MS analysis of the compounds in vapor was performed on a G7000-M9000/3DQMS (Hitachi, Tokyo, Japan) under the following operating conditions: column: fused silica capillary column TC-WAX (Hewlett Packard, Palo Alto, CA, USA), 60 m × 0.25 mm, 0.25 μm film thickness; column temperature: 40–120 °C increasing at 16 °C/min, 5 minutes at 120 °C, 120–130 °C increasing at 1 °C/min, 15 minutes at 130 °C, 130–200 °C increasing at 20 °C/min, 20 minutes at 200 °C; carrier gas: He, 147.1 kPa; and ionization energy: 15 eV. The methods used for the adsorption and desorption of the volatile components using solid-phase microextraction were described in a previous report.11 GC-MS analysis of the compounds present in the examined liquid was performed on a Hewlett Packard 5890 (Hewlett Packard) and AUTOMASS (JEOL, Tokyo, Japan) under the following operating conditions: column: fused silica capillary column TC-WAX (Hewlett Packard), 60 m × 0.25 mm, 0.25-μm film thickness; column temperature: 40–130 °C increasing at 2 °C/min, 25 minutes at 130 °C, 130–140 °C increasing at 2 °C/min, 15 minutes at 140 °C, 140–200 °C increasing at 15 °C/min, 30 minutes at 200 °C; injector: 180 °C, carrier gas: helium, 45 cm/min; column head pressure: 100 kPa; injection volume: 1 μL; and ionization energy: 70 eV. The chemical components were identified by comparison with the retention times, mass and ion spectra from an MS data library (NIST 02), of authentic standard compounds.
2.5. Lavender oil
The lavender oil used in this study was from Nacalai Tesque, Inc (Kyoto, Japan), and was analyzed by GC and GC-MS to identify the main components. GC-MS analysis was performed on a Hewlett Packard 5890 (Hewlett Packard) connected with AUTOMASS (JEOL) with the above-mentioned operating conditions. Chemical components were identified by comparing their retention time and mass spectra with those in the MS data library (NIST 02), and authentic standards.
2.6. Measurement of evaporated compound in a glass cage
A compound dissolved in triethyl citrate was dropped onto filter paper. Then, the filter paper was weighed and placed in a closed glass cage. The filter paper was removed at 1 hour and weighed again. Weight difference from that immediately after dropping was regarded as the amount of compound that evaporated per hour in the glass cage.
2.7. Extraction of P. cablin leaves and fractionation of their oil
Dried leaves of P. cablin (5.0 kg) were extracted with 2 L of hexane by simply standing for 2 days and the solvent was evaporated in reduced pressure to yield 198 g of essential oil. The oil (13.7 g) was fractionated by silica gel column chromatography (7 cm inner diameter × 25 cm) eluting with a solvent mixture of hexane-AcOEt (4:1) to yield Fraction 1 (4.3 g), Fraction 2 (5.3 g), and Fraction 3 (3.4 g).
2.8. Evaluation of lipophilicity
The lipophilicity of compounds was examined using Marvin Sketch (ChemAxon, Budapest, Hungary) on the basis of Log P values, a common logarithm of n-octanol/water partition coefficient.12
2.9. Statistical analysis
All values are expressed as the mean ± standard error of the mean. Statistical analyses were carried out with Dunnett test and Student t test13 using GraphPad Instat (GraphPad Software, San Diego, CA, USA). A probability level of p < 0.05 was taken to be statistically significant in the analyses.
3. Results and discussion
3.1. Lavender oil
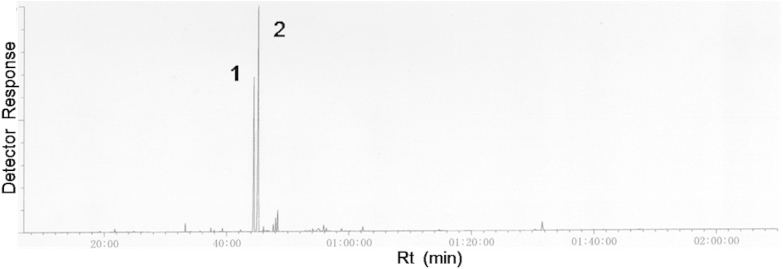
Qualitative characterization of the lavender oil showed that linalool and linalyl acetate were the main components (44.52 minutes and 45.27 minutes in Fig. 1, respectively). This oil was used as a positive control in inhalation experiments.
Fig. 1.
Total ion chromatograms of gas chromatography–mass spectrometry analysis of lavender oil. Numbered peaks are as follows: 1. linalool; 2. linalyl acetate. Rt = retention time.
3.2. Inhibitory effects of hexane extracts of P. cablin (廣藿香 guǎng huò xiāng) on locomotor activity and its active ingredients
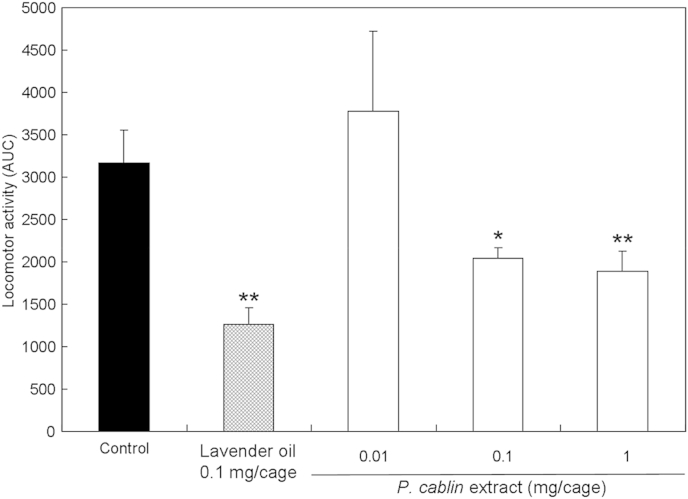
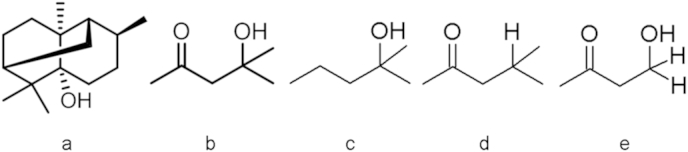
Mice were treated with vapor of the hexane extract of P. cablin (0.01 mg/cage, 0.1 mg/cage, or 1 mg/cage) and their locomotor activities were measured. The extract showed significantly reduced locomotor activity in a dose-dependent manner (Fig. 2). The hexane extract (13.7 g) of P. cablin was fractionated by silica gel column chromatography to yield Fraction 1 (4.3 g), Fraction 2 (5.3 g), and Fraction 3 (3.4 g). Inhalation of Fraction 1 at a dose corresponding to 1 mg/cage showed no effect on locomotor activities. However, inhalation of Fractions 2 and 3 showed significant sedative effects. The values of area under the curve for 60 minutes, which is an index of total locomotor activities, were significantly reduced to about 47% and 34% compared with that of the control group (p < 0.05) by the inhalation of Fraction 2 (0.25 mg/cage) and Fraction 3 (0.25 mg/cage), respectively. According to solid-phase microextraction-GC-MS analysis, Fraction 2 contained patchouli alcohol and Fraction 3 contained diacetone alcohol as major constituents (Fig. 3). Thus, patchouli alcohol and diacetone alcohol may be compounds responsible for the sedative activity of the hexane extract of P. cablin.
Fig. 2.
Total spontaneous motor activity of mice treated with Pogostemon cablin extract. The extract was put in the glass cage, and the locomotor activity of the mice that inhaled the vapor of the extract was evaluated. Data are shown as area under the curve (AUC) of their activity for 60 minutes, and expressed as the mean values ± standard error of the mean of six mice. Statistical differences were calculated using analysis of variance, followed by Dunnett test. *p < 0.05, **p < 0.01 compared with control group (treated with vehicle).
Fig. 3.
Chemical structure of compounds from Pogostemon cablin extract and related compounds. (A) Patchouli alcohol; (B) diacetone alcohol; (C) 1,1-dimethyl-1-butanol; (D) isobutyl methyl ketone; and (E) 4-hydroxy-2-butanone.
3.3. Activities of diacetone alcohol
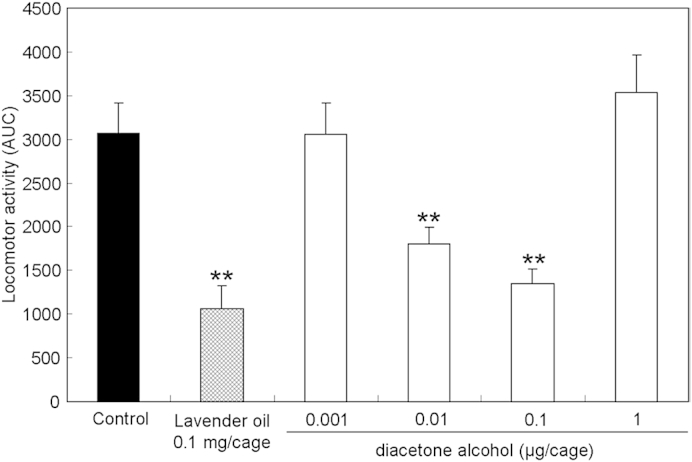
Inhalation of single diacetone alcohol at the dosages of 0.01 μg/cage and 0.1 μg/cage significantly reduced locomotor activities over the entire observation period. The dosage of 0.01 μg/cage was comparable to that with 1 mg/cage of P. cablin extract (Fig. 4).
Fig. 4.
Total spontaneous motor activity of mice treated with diacetone alcohol. Diacetone alcohol was put in the glass cage, and the locomotor activity of the mice that inhaled the vapor of the compound was evaluated. Data are shown as the mean of area under the curve ± standard error of the mean of six mice. Statistical differences were calculated using analysis of variance, followed by Dunnett test. ** p < 0.01 compared with control group (treated with vehicle).
The amount of diacetone alcohol actually evaporated in a cage was measured. At first, it was confirmed that the amount of volatilization in glass cages was correlated with the quantity of the samples dropped onto filter papers. When 10 mg of diacetone alcohol was dropped in a cage, 4.21 mg of the compound volatilized in the glass cage in 1 hour. When mice were placed inside the glass cage, 25% of the compound dropped onto filter paper was already volatilized in the cage.
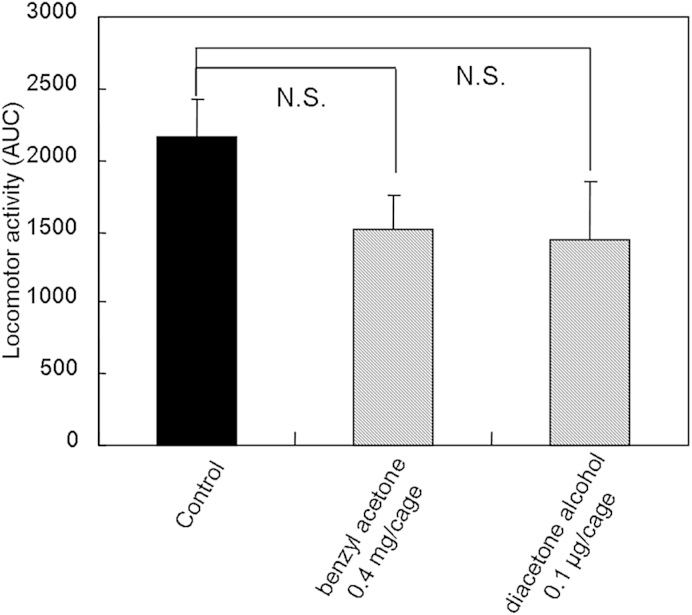
The compounds, inhaled through the nasal cavity, enter the body through the olfactory epithelium via olfactory receptors.14 Some molecules may be absorbed via the lungs into the body.15 To elucidate the pathway behind the effect of diacetone alcohol on locomotor activity, olfactory epithelium in mice was impaired using zinc sulfate.6, 9 Two days after the treatment with zinc sulfate, mice showed lower locomotor activity than untreated mice, but exhibited no abnormal behaviors. The severity of smell disorders was examined by an acetic acid test. Unlike the untreated mice, the zinc sulfate-treated mice showed no avoidance behavior for acetic acid (data not shown). These results suggest that zinc sulfate treatment severely damaged mouse olfaction. The inhalation of diacetone alcohol (0.1 μg/cage) in the mice with impaired olfactory epithelium did not reduce locomotor activity (Fig. 5), suggesting that a pathway through the olfactory epithelium played an important role in the sedative activities of inhaled diacetone alcohol.
Fig. 5.
Total spontaneous motor activity of six mice with dysfunction of olfactory epithelium treated with benzyl acetone or diacetone alcohol. Mice were treated with zinc sulfate to impair their olfactory epithelium. Data are shown as the mean values ± standard error of the mean of five mice. Statistical differences versus the control group were calculated using analysis of variance, followed by Dunnett test. N.S. = not significant.
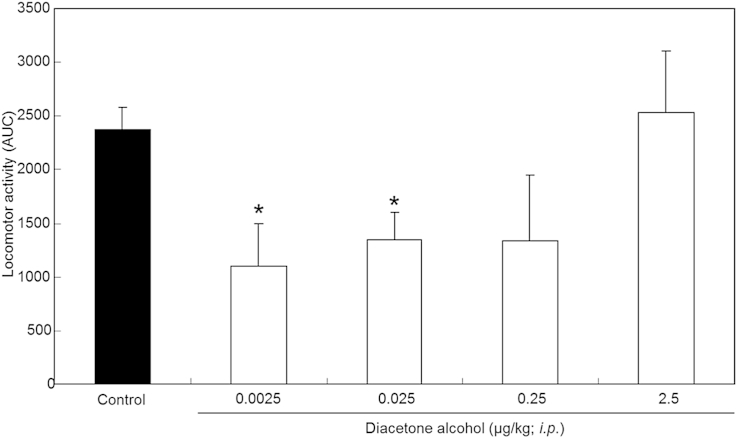
To examine the sedative activities of diacetone alcohol directly administered into the body, diacetone alcohol dissolved in corn oil was intraperitoneally administered. Intraperitoneal injection of 0.0025 μg/kg and 0.025 μg/kg diacetone alcohol significantly reduced locomotor activity (Fig. 6). These dosages were equivalent to about 1/100,000 and 1/10,000 of the dose via inhalation at 0.1 μg/cage. Intraperitoneal administration of diacetone alcohol exhibited sedative activity at a low dosage, suggesting that this compound might not exhibit its sedative effects via olfaction. It is predicted that the absorption of diacetone alcohol from olfactory epithelium into the circulation is important for the exhibition of sedative activity. Mice, treated by 2.5 μg/kg diacetone alcohol, showed not sedation but excitement. This result may indicate the effective concentration range of diacetone alcohol.
Fig. 6.
Total spontaneous motor activity of mice intraperitoneally treated with diacetone alcohol. Diacetone alcohol was intraperitoneally injected into the mice, and the locomotor activity of the mice was evaluated. Data are shown as the mean values of area under the curve ± standard error of the mean of six mice. Statistical differences were calculated using analysis of variance, followed by Dunnett test. * p < 0.05 compared with control group (treated with vehicle).
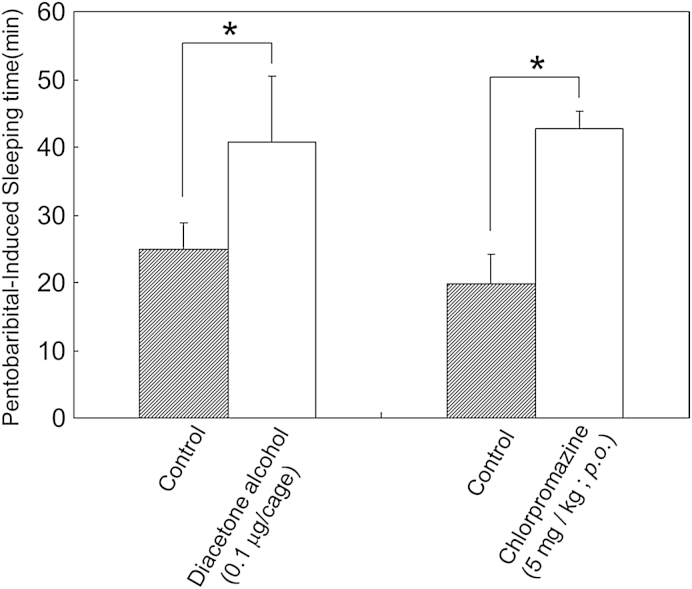
The effects of diacetone alcohol on pentobarbital-induced sleep onset latency and sleep duration in mice were investigated. Mice intraperitoneally injected with pentobarbital (25 mg/kg) fell asleep about 6 minutes after the administration and slept for around 20 minutes. Inhalation of 0.1 μg/cage of diacetone alcohol significantly prolonged the sleep time compared with that of the control group (p < 0.05) and the effect was similar to that for the set level of chlorpromazine (5 mg/kg, per os; Fig. 7). Pentobarbital induces sleep by decreasing neural excitability via direct and/or indirect activation of GABAA receptors.16 Chlorpromazine is known to prolong pentobarbital-induced sleep time through an antagonistic action in dopaminergic, noradrenergic, and serotonin neurons.17 The prolongation of pentobarbital-induced sleeping time by aromatic compounds, such as terpinyl acetate and phenethyl alcohol, or essential oils, such as lemon and jasmine oil, has also been reported.18 GABAA receptors have been implicated in the prolongation of pentobarbital-induced sleeping time by the inhalation of essential oil.19 Diacetone alcohol exhibited effects comparable to those of chlorpromazine, a potent depressant for patients coping with sleep disturbance.
Fig. 7.
Effects of the inhalation of diacetone alcohol (0.1 μg) or oral treatment of chlorpromazine in mice on pentobarbital-induced sleeping time. Diacetone alcohol was put into the glass cage. Data are shown as the mean ± standard error of the mean of six mice. Statistical differences vs. the control group were calculated by Student t test. * p < 0.05 versus the control group.
3.4. Structure–activity relationship of diacetone alcohol
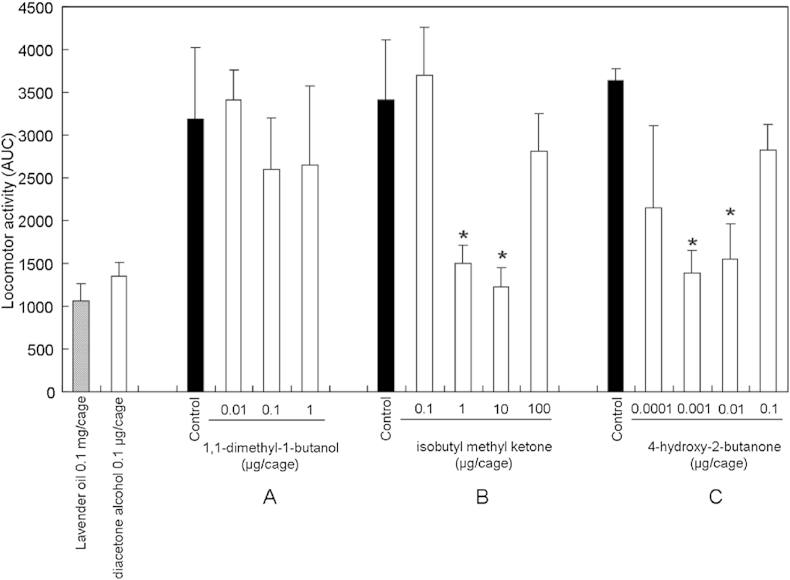
Scent molecules with similar molecular structures have been systematically investigated to demonstrate that scent receptors showed different responses depending on the carbon numbers and functional groups of the scent molecules.20, 21 In an experiment using rabbits, the ketone group on a linear compound was shown to play an important role in the neural activation of the dorsal olfactory bulb.22 An electrophysiological experiment on the structure–activity relationship using HEK293 cells with forced expression of olfactory receptor suggested that the hydroxyl and aldehyde groups on a linear compound played important roles in olfactory receptor activation.23 An experiment using dogs suggested that linear compounds with the same functional groups had more potent stimulatory effects on olfaction as their main chains became shorter, and that compounds with three or four carbons had the most potent stimulatory effects on olfaction, although the results differed depending on functional groups.24 Diacetone alcohol has a main chain consisting of five linear carbons with both ketone and hydroxyl groups. With respect to the above three features, which may influence diacetone alcohol activity, the sedative activities of 1,1-dimethyl-1-butanol (Fig. 3C) with the ketone group at the 2-position replaced by hydrogen, isobutyl ketone (Fig. 3D) with the hydroxy group at the 4-position replaced by hydrogen, and 4-hydroxy-2-butanone (Fig. 3D) with the two methyl groups at the 4-position replaced by hydrogen were examined. Locomotor activity was not significantly inhibited by the inhalation of 1,1-dimethyl-1-butanol at the dosages of 0.01μg/cage, 0.1μg/cage, and 1 μg/cage (Fig. 8A), suggesting that the ketone group at the 2-position in diacetone alcohol plays an important role in the sedative activity. The inhalation of isobutyl methyl ketone alone at the dosages of 1 μg/cage and 10 μg/cage significantly reduced locomotor activity (Fig. 8B). Thus, the hydroxyl group at the 4-position did not play an important role. The inhalation of 4-hydroxy-2-butanone also significantly reduced the locomotor activity at the dosages of 0.001 μg/cage and 0.01 μg/cage (Fig. 8C), suggesting that a linear compound with a smaller carbon number exhibited markedly higher activity. The saturated vapor pressures of 4-hydroxy-2-butanone and diacetone alcohol at 25 °C are 0.857 mmHg and 1.71 mmHg, respectively.14, 15 For samples on filter papers soaked under the same conditions, the amount of 4-hydroxy-2-butanone in the air may be smaller than that of diacetone alcohol. Additionally, 4 mg of each diacetone alcohol, 1,1-dimethyl-1-butanol, isobutyl methyl ketone, or 4-hydroxy-2-butanone, was dropped onto filter paper to examine the amount of evaporation in a glass cage in 1 hour. In terms of the amount of evaporation, isobutyl methyl ketone was largest (2.22 mg) and 4-hydroxy-2-butanone was smallest (1.24 mg). These results suggest that there is no positive correlation between sedative activities and the amount of evaporation. Additionally, the degrees of lipophilicity of diacetone alcohol, 1,1-dimethyl-1-butanol, isobutyl methyl ketone, and 4-hydroxy-2-butanone, expressed as the partition coefficients (Log P) of n-octanol/water, were 0.22, 1.50, 1.54, and −0.47, respectively. Specifically, both diacetone alcohol and 4-hydroxy-2-butanone showed higher activities at low concentrations and much lower lipophilicity than the other two compounds. The intraperitoneal injection of valproic acid and its analogs, linear compounds with a main chain consisting of seven carbons, resulted in potent anticonvulsant effects when Log p was 1.84–2.64.25 Compounds that exert sedative effects when directly administered into the body are highly lipophilic, while the compounds that exert sedative effects when inhaled are highly soluble in water. Scent molecules inhaled in the nasal cavity are dissolved into the mucus covering the olfactory epithelium, and bind to the scent receptors on the villi of olfactory cells. Thus, the absorption of the compounds in the olfactory epithelium is important for olfactory response.26 An investigation on the compounds containing oxygen as a functional group demonstrated that the compounds with higher water solubility induced a stronger response in the olfactory bulb glomeruli.27 In this experiment, the related compounds of diacetone alcohol showed markedly higher activity as their water solubility increased. The olfactory epithelium surface in the nasal cavity is wetted with mucus and rich in water. The inhaled compounds are dissolved in this mucus to enter the tissues. Thus, as water solubility increases, more compounds are absorbed through the olfactory epithelium into the body, resulting in increased olfactory response and higher sedative activity.
Fig. 8.
Total spontaneous motor activity of mice treated with 1,1-dimethyl-1-butanol, isobutyl methyl ketone, or 4-hydroxy-2-butanone. Data are shown as the mean values ± standard error of the mean of six mice. Statistical differences were calculated using ANOVA, followed by Dunnett test. * p < 0.05 compared with control group (treated with vehicle).
4. Conclusion
The inhalation of hexane extract of P. cablin (廣藿香 guǎng huò xiāng) showed sedative activity in mice, and its main active compound was diacetone alcohol. The ketone group in the structure and the carbon number of the main chain should play important roles in the exhibition of sedative activity. P. cablin plays a vital role in the effect of traditional Japanese scent bags; however, further studies on this issue are required.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Bläske V.U., Hertel H., Forschler B.T. Repellent effects of isoborneol on subterranean termites (Isoptera: Rhinotermitidae) in soils of different composition. J Econ Entomol. 2003;96:1267–1274. [PubMed] [Google Scholar]
- 2.Cava R., Nowak E., Taboada A., Marin-Iniesta F. Antimicrobial activity of clove and cinnamon essential oils against Listeria monocytogenes in pasteurized milk. J Food Prot. 2007;70:2757–2763. doi: 10.4315/0362-028x-70.12.2757. [DOI] [PubMed] [Google Scholar]
- 3.Kim H.W., Cho S.J., Kim B.Y., Cho S.I., Kim Y.K. Pogostemon cablin as ROS scavenger in oxidant-induced cell death of human neuroglioma cells. eCAM. 2010;7:239–247. doi: 10.1093/ecam/nem176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiuchi F., Matsuo K., Itano Y. Screening of natural medicines used in Vietnam for trypanocidal activity against epimastigotes of Trypanosoma cruzi. Nat Med. 2002;56:64–68. [Google Scholar]
- 5.Haze S., Sakai K., Gozu Y. Effects of fragrance inhalation on sympathetic activity in normal adults. Jpn J Pharm. 2002;90:247–253. doi: 10.1254/jjp.90.247. [DOI] [PubMed] [Google Scholar]
- 6.Takemoto H., Ito M., Shiraki T., Yagura T., Honda G. Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J Nat Med. 2008;62:41–46. doi: 10.1007/s11418-007-0177-0. [DOI] [PubMed] [Google Scholar]
- 7.Takemoto H., Yagura T., Ito M. Evaluation of volatile components from spikenard: valerena-4,7(11)-diene is a highly active sedative compound. J Nat Med. 2009;63:380–385. doi: 10.1007/s11418-009-0340-x. [DOI] [PubMed] [Google Scholar]
- 8.Ito K., Ito M. Sedative effects of vapor inhalation of the essential oil of Microtoena patchouli and its related compounds. J Nat Med. 2011;65:336–343. doi: 10.1007/s11418-010-0502-x. [DOI] [PubMed] [Google Scholar]
- 9.Kimura Y., Furukawa M., Kamide M., Sakumoto M., Miwa T., Umeda R. Experimental study on the effect of the topical application of steroids on olfactory disturbance in mice. Nippon Jibiinkoka Gakkai Kaiho. 1989;92:1869–1875. doi: 10.3950/jibiinkoka.92.1869. [DOI] [PubMed] [Google Scholar]
- 10.Chuah M.I., Tennent R., Jacobs I. Response of olfactory Schwann cells to intranasal zinc sulfate irrigation. J Neurosci Res. 1995;42:470–478. doi: 10.1002/jnr.490420405. [DOI] [PubMed] [Google Scholar]
- 11.Bais H.P., Dattatreya B.S., Ravishankar G.A. Production of volatile compounds by hairy root cultures of Cichorium intybus L. under the influence of fungal elicitors and their analysis using solid-phase micro extraction gas chromatography–mass spectrometry. J Sci Food Agric. 2003;83:769–774. [Google Scholar]
- 12.Sugano K. Fraction of a dose absorbed estimation for structurally diverse low solubility compounds. Int J Pharm. 2011;405:79–89. doi: 10.1016/j.ijpharm.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 13.Wallenstein S., Zucker C.L., Fleiss J.L. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Soudry Y., Lemogne C., Malinvaud D., Consoli S.M., Bonfils P. Olfactory system and emotion: common substrates. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:18–23. doi: 10.1016/j.anorl.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Struve M.F., Wong V.A., Marshall M.W., Kimbell J.S., Schroeter J.D., Dorman D.C. Nasal uptake of inhaled acrolein in rats. Inhal Toxicol. 2008;20:217–225. doi: 10.1080/08958370701864219. [DOI] [PubMed] [Google Scholar]
- 16.Chweh A.Y., Swinyard E.A., Wolf H.H. Hypnotic action of pentobarbital in mice: a possible mechanism. Exp Neurol. 1987;97:70–76. doi: 10.1016/0014-4886(87)90282-2. [DOI] [PubMed] [Google Scholar]
- 17.Sadre N.L., Tiwari N.M. Prolongation of chloral hydrate and pentobarbitone sleeping time by chlorpromazine and histamine in mice. Arch Int Pharmacodyn Ther. 1966;163:6–10. [PubMed] [Google Scholar]
- 18.Tsuchiya T., Tanida M., Uenoyama S., Nakayama Y., Ozawa T. Effects of olfactory stimulation on the sleep time induced by pentobarbital administration in mice. Brain Res Bull. 1991;26:397–401. doi: 10.1016/0361-9230(91)90013-a. [DOI] [PubMed] [Google Scholar]
- 19.Koo B.S., Park K.S., Ha J.H., Park J.H., Lim J.C., Lee D.U. Inhibitory effects of the fragrance inhalation of essential oil from Acorus gramineus on central nervous system. Biol Pharm Bull. 2003;26:978–982. doi: 10.1248/bpb.26.978. [DOI] [PubMed] [Google Scholar]
- 20.Guerrieri F., Schubert M., Sandoz J.C., Giurfa M. Perceptual and neural olfactory similarity in honeybees. PLoS Biol. 2005;3:60. doi: 10.1371/journal.pbio.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaluza J.F., Breer H. Responsiveness of olfactory neurons to distinct aliphatic aldehydes. J Exp Biol. 2000;203:927–933. doi: 10.1242/jeb.203.5.927. [DOI] [PubMed] [Google Scholar]
- 22.Imamura K., Mataga N., Mori K. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. I. Aliphatic compounds. J Neurophysiol. 1992;68:1986–2002. doi: 10.1152/jn.1992.68.6.1986. [DOI] [PubMed] [Google Scholar]
- 23.Araneda R.C., Kini A.D., Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- 24.Tonosaki K., Tucker D. Olfactory receptor cell responses of dog and box turtle to aliphatic n-acetates and aliphatic n-fatty acids. Behav Neural Biol. 1982;35:187–199. doi: 10.1016/s0163-1047(82)91193-1. [DOI] [PubMed] [Google Scholar]
- 25.Elmazar M.M.A., Hauck R.S., Nau H. Anticonvulsant and neurotoxic activities of twelve analogues of valpronic acid. J Pharm Sci. 1992;82:1255–1258. doi: 10.1002/jps.2600821214. [DOI] [PubMed] [Google Scholar]
- 26.Yang G.C., Scherer P.W., Zhao K., Mozell M.M. Numerical modeling of odorant uptake in the rat nasal cavity. Chem Senses. 2007;32:273–284. doi: 10.1093/chemse/bjl056. [DOI] [PubMed] [Google Scholar]
- 27.Johnson B.A., Arguello S., Leon M. Odorants with multiple oxygen-containing functional groups and other odorants with high water solubility preferentially activate posterior olfactory bulb glomeruli. J Comp Neurol. 2007;502:468–482. doi: 10.1002/cne.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]