Abstract
The color of turmeric (薑黃 jiāng huáng) is because of a substance called curcumin. It has different pharmacological effects, such as antioxidant and anti-inflammatory properties. Nicotine is a major pharmacologically active substance in cigarette smoke. It is mainly metabolized in the liver and causes devastating effects. This study was designed to evaluate the protective role of curcumin against nicotine on the liver in mice.
Forty-eight mice were equally divided into eight groups; control (normal saline), nicotine (2.5 mg/kg), curcumin (10, 30, and 60 mg/kg) and curcumin plus nicotine-treated groups. Curcumin, nicotine, and curcumin plus nicotine (once a day) were intraperitoneally injected for 4 weeks. The liver weight and histology, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and serum nitric oxide levels have been studied.
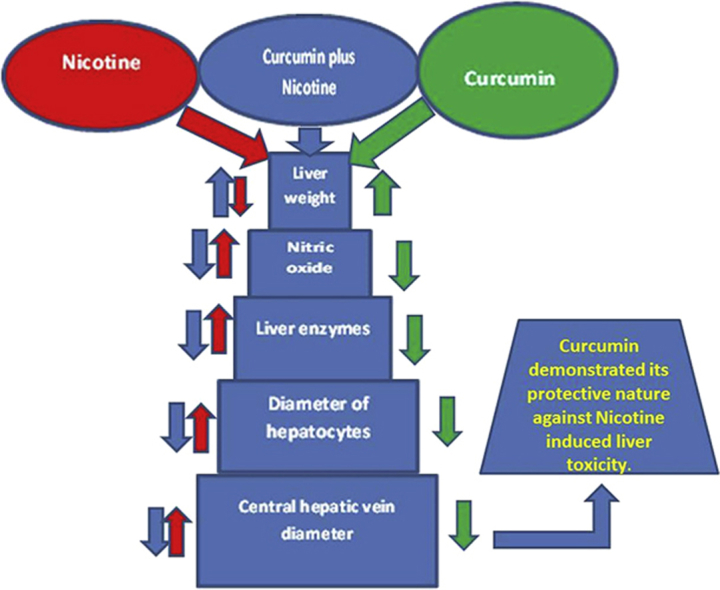
The results indicated that nicotine administration significantly decreased liver weight and increased the mean diameter of hepatocyte, central hepatic vein, liver enzymes level, and blood serum nitric oxide level compared with the saline group (p < 0.05). However, curcumin and curcumin plus nicotine administration substantially increased liver weight and decreased the mean diameter of hepatocyte, central hepatic vein, liver enzymes, and nitric oxide levels in all groups compared with the nicotine group (p < 0.05).
Curcumin demonstrated its protective effect against nicotine-induced liver toxicity.
Keywords: curcumin, damage, liver, mice, nicotine
Graphical abstract
1. Introduction
A large and increasing number of patients in the world use medicinal plants and herbs for health purposes.1 Curcuma longa (turmeric; 薑黃 jiāng huáng), one of the oldest plants, belongs to the Zingiberaceae family, which has long been used in traditional medicine for blood purification, digestion, arthritis treatment, liver protection, and as an anti-inflammatory agent.2 The color of turmeric is because of a chemical called curcumin, which comprises 3–4% of tumeric.3 Numerous studies have reported the antioxidant properties, anti-mutation and antitumor effects, and carcinogenic characteristics of curcumin.4 Curcumin affects the metabolism of arachidonic acid by inhibiting the phosphorylation of phospholipase A2 (PLA2), decreasing the expression of cyclooxygenase (COX)-2 gene and inhibiting the catabolic activity of COX-5. These effects induce the anti-inflammatory activity of curcumin.5 In addition, curcumin decreases the expression of different inflammatory cytokines such as interleukin (IL)-1, tumor necrosis factor (TNF)- α, IL-6, and chemokines.6 Curcumin shows efficacy in promoting wound healing as well as treating liver ailments, urinary tract diseases, and hepatitis.7 Pharmacologically, curcumin exhibits a wide range of effects including anti-inflammatory, hypo-cholesterolemic, and anti-infection activities and as well as anticarcinogenic effects.8, 9 Although approximately 4000 components are present in the cigarette, nicotine is a highly toxic organic compound containing nitrogen and alkaloid, which are mostly found in tobacco, and it is responsible for some of the deleterious effects of smoking.10 Nicotine affects a variety of cellular processes including altered gene expression.11 Exposure to nicotine produces oxidative tissue injuries in the mouse, often resulting in a depletion of glutathione content and a decrease in the activity of some oxygen free radical scavengers, such as catalase and superoxide dismutase.12 The liver is considered to be the major site of nicotine biotransformation, and nicotine exerts a number of adverse physiological effects on the liver.13 Nicotine is absorbed through the lungs during smoking and is rapidly metabolized in the liver, which induces three major adverse effects on the liver: toxic (direct or indirect), immunological, and oncogenic.14 Smoking causes liver cell injury and exerts genotoxic effects on rat liver.15 Current studies on curcumin effects have not reported on the protective effect of curcumin against nicotine; therefore, the current study was conducted to analyze the protective effect of curcumin to offset the damage induced by nicotine in the liver of male mice.
2. Materials and methods
2.1. Chemicals
Curcumin (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; C21H20O6) powder (Merck, Germany) was purchased, The powder was dissolved in absolute ethanol (C2H5OH) and diluted with normal saline (0.9%) to prepare different doses. Also, the nicotine solution (1-metyl-2-3-pyridel-pirolidin; C10H14N2, Merck, Germany) was also purchased and was diluted by normal saline (0.9%) for administration (Fig. 1).
Fig. 1.
Structure of nicotine and curcumin.
2.2. Experimental protocol
The mice were randomly divided into eight groups of six: control group (normal saline; 1 mL distilled water/daily); nicotine-treated group (2.5 mg/kg); 3) nicotine + curcumin 10 mg/kg treated group; nicotine + curcumin 30 mg/kg treated group; nicotine + curcumin 60 mg/kg treated group; curcumin 10 mg/kg treated group; curcumin 30 mg/kg treated group; and curcumin 60 mg/kg treated group. Nicotine was administered intraperitoneally once a day for 4 weeks. Curcumin and nicotine plus curcumin were administered intraperitoneally in animals.16, 17, 18
2.3. Animals
Forty-eight Balb/c male mice with weight range of 27–30 g were purchased from Tehran Razi Institute. Animals were kept at the temperature of 22 ± 2°C, under controlled environmental conditions, 12/12 hours light/dark cycle and free access to water and food. Maintenance and care of experimental animals complies with National Institutes of Health guidelines.19 Experiments were designed to conform with the International Guiding Principles for Biomedical Research Involving Animals (1985).
2.4. Liver weight and collection of blood serum
At the end of the experimental period, all animals were deeply anesthetized with ether. Blood was collected from the right ventricle, and serum separated and stored at −80°C for measurement of nitric oxide. They were then killed. Livers were removed and weighed on a microbalance sensitive to 0.001 mg (Precisa 125A, Switzerland) and recorded.20
2.5. Histological analysis
For the histological evaluation of the hepatic structures, the lower 1-cm-long part of the right lobe of the liver in transverse pieces was removed, washed in saline, and fixed in 10% formalin at room temperature for 72 hours. After tissue fixation, it was thoroughly washed under running water and dehydrated in ascending concentration ethanol, cleared in xylene, and then embedded in soft paraffin. Thin sections (5 μm) were cut using a microtome (Leica RM 2125, Leica Microsystems Nussloch GmbH, Germany) and stained with hematoxylin and eosin. The preparation was examined with an Olympus BX-51T-32E01 research microscope connected to a DP12 Camera with 3.34-million pixel resolution and Olysia Bio software (Olympus Optical Co. LTD, Tokyo, Japan).21
2.6. Morphometric measurements
For each hepatocyte, the total cellular area was measured. The outline of each hepatocyte was measured after taking an image with a 40 × objective lens. The longest and shortest axis were measured in the drawing of each hepatocyte in order to estimate the mean diameter (mean axis). At least 50 hepatocytes from each zone (total, 100) were measured in each liver. A separate measurement for central hepatic vein was performed, using the same methodology.22, 23
2.7. Griess assay
Nitric oxide was measured based on Griess colorimetric assay. Accordingly, N-1-naphthylenediamine (NEED), sulfonamide solutions, and nitrite standards were prepared. To measure nitrite concentration in serum, after defreezing the serum samples, 100 μL of the sample serum was deproteinized by zinc sulfate and transferred to the wells. One hundred microliters of chloride vanadium, 50 μL sulfonamide, and 50 μL NEED solutions were added afterward. The samples were incubated in the temperature of 30°C in darkness. The optical density of samples was measured using an enzyme-linked immunosorbent assay reader (Hyperion, Germany) at the wavelength of 540 nm.24
2.8. Biochemical analysis
The liver was minced and homogenized (10% w/v) in ice-cold 0.1M sodium phosphate buffer (pH 7.4). The homogenate was centrifuged twice at 10,000 rpm for 15 to 20 min at 4°C to obtain enzyme fraction. The resultant supernatant was used for various biochemical assays. Activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed using the method of Reitman and Frankel.25 Activities of alkaline phosphatase (ALP) were determined according to the protocol described in the laboratory practical manual.26
2.9. Statistical analysis
All the quantitative data were presented as mean ± standard deviation. One-way analysis of variance followed by least significant difference post hoc test were performed to determine the statistical significance between different groups using SPSS software package 16.0 (Statistical Package for the Social Sciences, version 16.0, SPSS Inc, Chicago, Illinois, USA). A p value <0.05 was considered significant.
3. Results
3.1. Liver weight
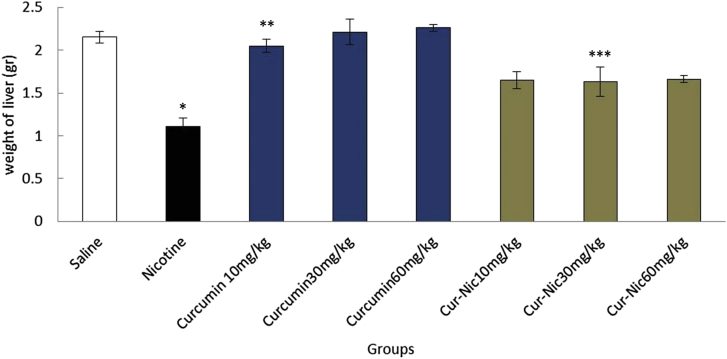
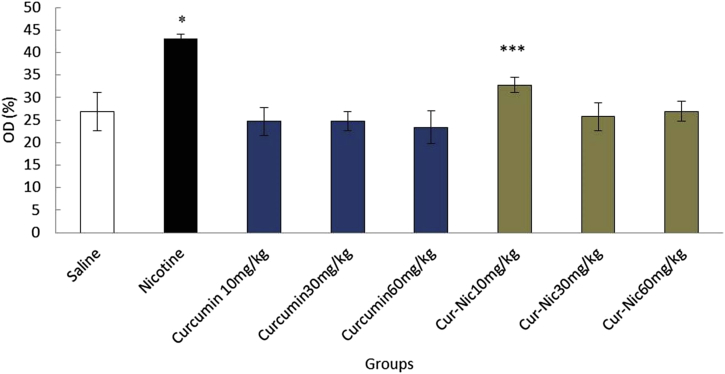
In the current study, the effective dose of nicotine (2.5 mg/kg) caused a significant decrease in the liver weight of the mice compared with the saline group (p < 0.05). Moreover, liver weight was significantly decreased in animals treated with curcumin and curcumin plus nicotine in all doses in comparison with the nicotine group (p < 0.05) (Fig. 2).
Fig. 2.
*Significant decrease of liver weight in the nicotine group compared with the saline group (p < 0.05). **Significant increase in all groups of curcumin compared with the nicotine group (p < 0.05). ***Significant increase all groups of curcumin-nicotine compared with the nicotine group (p < 0.05).
3.2. Morphometric measurements
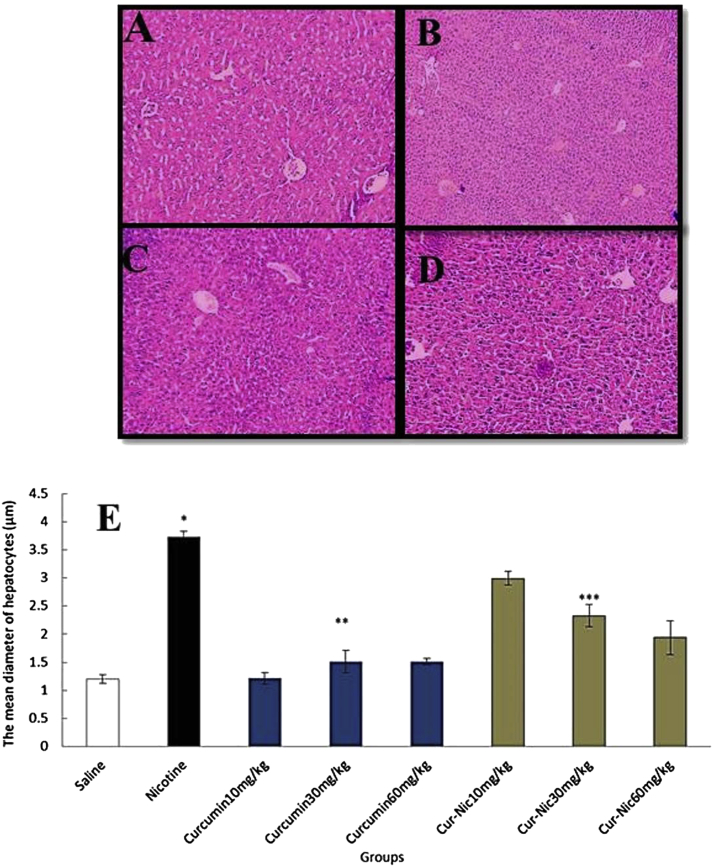
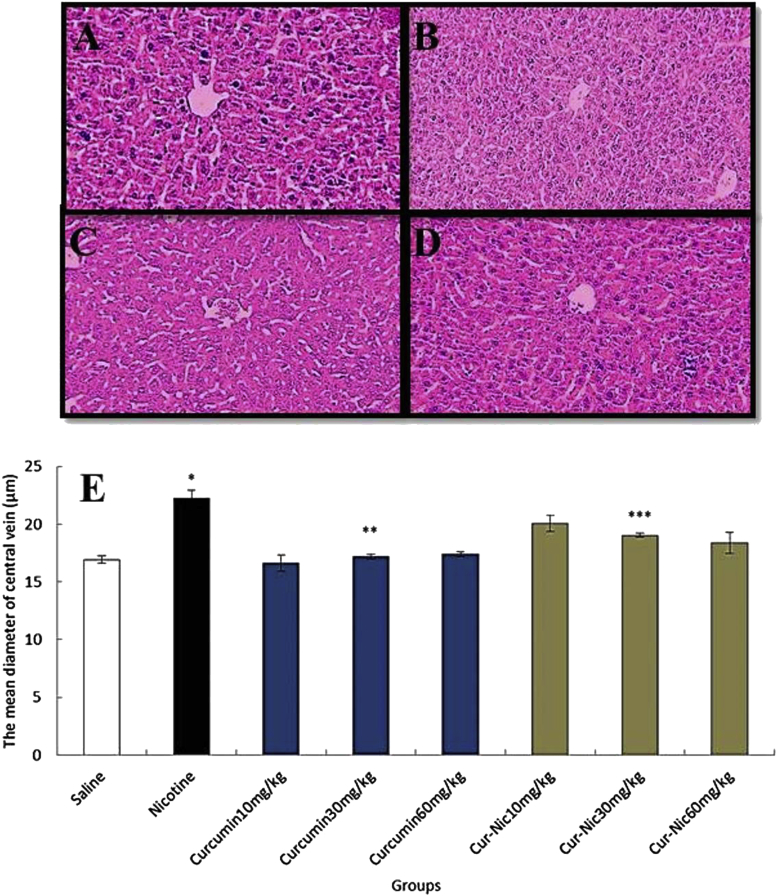
The mean diameter of hepatocytes and central hepatic vein was substantially increased in the nicotine administration group in comparison with the saline group (p < 0.05). Further, curcumin and curcumin plus nicotine caused a significant decrease in the mean diameter of hepatocytes and central hepatic vein in all treated groups in comparison with nicotine group administration (p < 0.05) (Fig. 3, Fig. 4).
Fig. 3.
(E) *Significant increase of the mean hepatocytes diameter in the nicotine group compared with the saline group (p < 0.05). **Significant decrease in all groups of curcumin administration compared with the nicotine group (p < 0.05). ***Significant decrease in all groups of curcumin-nicotine administration compared with the nicotine group (p < 0.05). (A) Saline group. (B) Nicotine group. (C) Curcumin 60 mg/kg. (D) Curcumin (60 mg/kg) plus nicotine (magnification 100×).
Fig. 4.
(E) *Significant increase of the mean central hepatic vein diameter in the nicotine group compared with the saline group (p < 0.05). **Significant decrease in all groups of curcumin administration compared with the nicotine group (p < 0.05). ***Significant decrease in all groups of curcumin-nicotine administration compared with the nicotine group (p < 0.05). (A) Saline group. (B) Nicotine group. (C) Curcumin 60 mg/kg. (D) Curcumin (60 mg/kg) plus nicotine (magnification 100×).
3.3. Nitric oxide
The mean nitric oxide in blood serum increased significantly (2.5 mL/kg) in the nicotine group in comparison with the saline group (p < 0.05). Also, the mean nitric oxide in blood serum decreased significantly in curcumin and curcumin plus nicotine in all groups in comparison with the nicotine group (Fig. 5).
Fig. 5.
*Significant increase of nitric oxide in the nicotine group compared with the saline group (p < 0.05). **Significant decrease in all groups of curcumin administration compared with the nicotine group (p < 0.05). ***Significant decrease in all groups of curcumin-nicotine administration compared with the nicotine group (p < 0.05).
3.4. Biochemical analysis
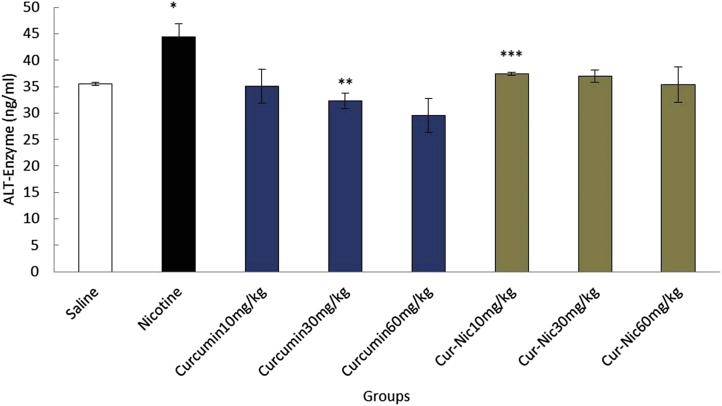
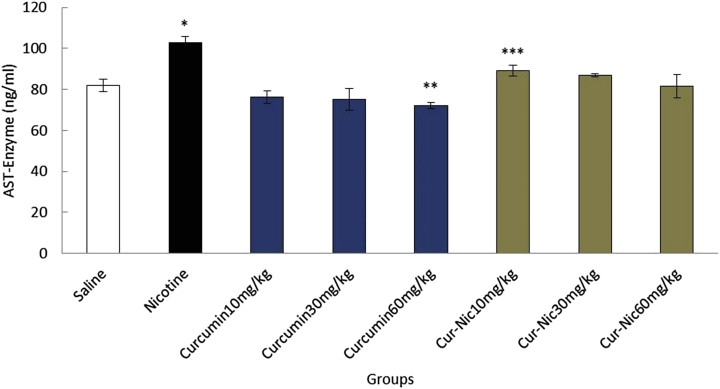
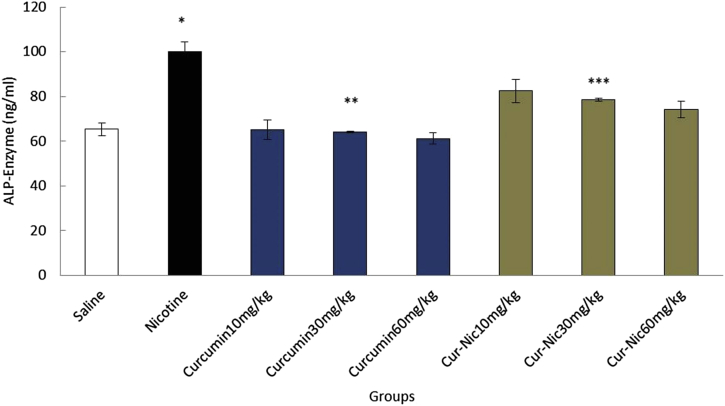
Nicotine (2.5 mL/kg) caused a significant increase in the mean of ALT, AST, and ALP enzymes compared with the saline group (p < 0.05). In addition, the mean of ALT, AST, and ALP enzymes decreased significantly in curcumin and curcumin plus nicotine in all groups in comparison with the nicotine group (p < 0.05) (Fig. 6, Fig. 7, Fig. 8).
Fig. 6.
*Significant increase of alanine aminotransferase (ALT) enzyme in the nicotine group compared with the saline group (p < 0.05). **Significant decrease in all groups of curcumin compared with nicotine group administration (p < 0.05). ***Significant decrease all groups of curcumin -nicotine compared with the nicotine group (p < 0.05).
Fig. 7.
*Significant increase of aspartate aminotransferase (AST) AST enzyme in the nicotine group compared with the saline group (p < 0.05). ** Significant decrease in all groups of curcumin compared with nicotine group administration (p < 0.05). *** Significant decrease in all groups of curcumin-nicotine compared with the nicotine group (p < 0.05).
Fig. 8.
*Significant increase of alkaline phosphatase (ALP) enzyme in the nicotine group compared with the saline group (p < 0.05). **Significant decrease in all groups of curcumin compared with nicotine group administration (p < 0.05). ***Significant decrease in all groups of curcumin-nicotine compared with the nicotine group (p < 0.05).
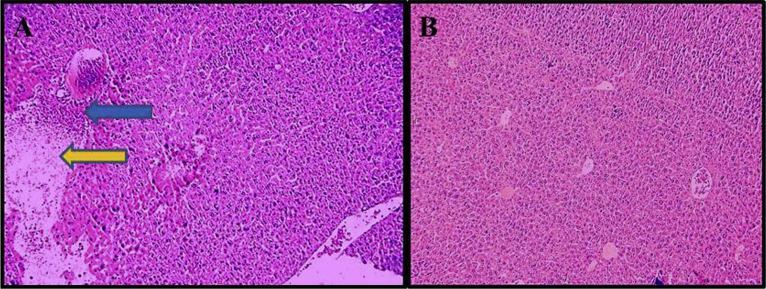
Liver damage induced by Nicotine administration included histological changes of liver confirmed by hematoxylin and eosin staining (Fig. 9).
Fig. 9.
Histological changes of liver hematoxylin-eosin staining. (A) Nicotine group. Arrows point to the histological changes of nicotine administration, Blue arrow pointing to lymphocytic infiltration and Yellow arrow pointing to enlargement of central hepatic vein (B) Saline group (control).
4. Discussion
The liver is an organ that plays a significant role in the oxidation of fatty acids and carbohydrates.13 Curcumin, with its high antioxidant power, exerts its protective effect on the liver.27 Moreover, curcumin can have protective effects on lipid peroxidation in rat liver in oxidative stress conditions, can collect free radicals, and can increase intracellular glutathione levels.27 The current study investigated the protective effects of curcumin against the disorders induced by nicotine administration, which affected liver weight, diameter of hepatocytes and central vein, serum level of liver enzymes (AST, ALT, and ALP), and nitric oxide secretion level. In the current study, nicotine administration significantly increased the serum level of AST, ALT, and ALK enzymes in comparison with the control group, which mostly approached the normal level with curcumin administration. Necrosis or cell membrane damage can cause the release of these enzymes into blood. However, the serum level of these enzymes is associated with liver performance.28 It seems that nicotine causes the destruction of hepatic cell membrane, thereby releasing cytozomal enzymes of hepatocytes and finally increasing their serum level.29 In the groups that received curcumin, the amount of these enzymes was reduced, which indicates the protective effects of curcumin against the toxicity induced by nicotine. The obtained results may be due to the antioxidant effects and reduction of oxidative stress in curcumin.30 Further, because TNF-α and IL-1 play a role in the induction of hepatic necrosis, curcumin can reduce the effects of toxicity by inhibiting the secretion of TNF-α and IL-1 by macrophages.31 The findings of the current study were in line with the results of Fu et al, which reported curcumin as a factor to reduce the increased hepatic enzyme due to Chemokine (C–C motif) ligand 4 (CCl4) administration.32 The results of liver weight analysis showed a significant decrease in liver weight between the nicotine and control (saline) groups; however, liver weight increased after curcumin administration in comparison with the nicotine group. The metabolic disorders in rats caused by nicotine administration might cause the weight loss, which was largely compensated by curcumin administration33 The results of the current study were also in agreement with the findings of Chuang et al, who reported curcumin as the factor to increase liver weight.34 Moreover, the results confirmed the findings of the study carried out by Willis et al, in which nicotine was reported to reduce the weight of different organs, including the liver.35 The histopathological results obtained in this study revealed the increase in diameter of mean hepatocytes and central veins due to nicotine administration. The change in the size of hepatocytes and central veins seems to be the result of an increase in the metabolic activity of cells to excrete toxins from the body during the toxification process.36 It seems that free radicals induced by nicotine metabolism trigger lipid peroxidation and reaction to DNA and membrane proteins, thereby causing cell damage.37 Antioxidant compounds such as curcumin can induce inhibitory effects on cytochrome P450, prevent nicotine metabolism, and consequently reduce the production of free radicals.38 Tetrahydro curcumin is one of the most important metabolites of curcumin and an active biological component of turmeric (薑黃 jiāng huáng) in the cytosol of hepatic cells. This substance seems to have extensive antioxidant activities in both in vitro and in vivo conditions.39 The findings of this study were also in line with the results of the study by Suñer et al, in which they reported nicotine administration resulted in increased size and vascularity of choroidal neovascularization.40 The results obtained from the measurement of nitric oxide in blood indicated a significant increase between the nicotine and control (saline) groups; however, nicotine administration reduced nitric oxide level. Nitric oxide is a free radical that is produced in mammalian cells and interferes with regulation of physiological processes, and its production increase is associated with induction of various diseases.41 The hydroxyl radicals produced by nitric oxide seem to interfere with the pathogenesis process and hepatic toxicity.42 Nicotine can induce the release of noradrenalin in paraventricular nucleus and amygdala through direct effect on the nuclei of solitary tract and N-methyl-D-aspartate receptors, which in turn stimulate the production of nitric oxide and noradrenergic activity of neurons.43 Nicotine absorption in the body seems to be followed by the increase of serum nitric oxide level and oxidative stress.43 Curcumin is structurally a polyphenol and exerts a protective effect on lipid oxidation in the liver in oxidative stress conditions, and acts as a collector of free radicals of oxygen and enhancer of intracellular glutathione.44 Thus, it can destroy the nitric oxide system (protein enzymes, substrates, and cofactors) and reduce nitric oxide production.42 The results of the study conducted by Zhao et al indicated that nicotine can decrease angiogenesis in gastric mucosa through inhibition of nitric oxide production and prevention of new cells' production process,45 which are in contrast with the findings obtained in the current study. However, the results of the current study confirm the findings of Muller and Schepers, showing the increase of peroxynitrite formation due to cigarette smoke, an element known to damage DNA.46
5. Conclusion
The current investigation demonstrates that nicotine has the potential to cause substantial damage in liver tissues and curcumin may improve some of this liver damage in mice. Therefore, curcumin could be useful to protect the liver from nicotine toxicity. The antioxidant effects of curcumin may be a major reason for its positive effect on liver parameters. However, further studies are required to define its exact mechanism of action.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
We sincerely and gratefully thank the Kermanshah University of Medical Sciences for financial support of this project [No. 93085].
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Jalili C., Salahshoor M.R., Moradi S., Pourmotabbed A., Motaghi M. The therapeutic effect of the aqueous extract of boswellia serrata on the learning deficit in kindled rats. Int J Prev Med. 2014;5:563–568. [PMC free article] [PubMed] [Google Scholar]
- 2.Ishita C., Kaushik B., Uday B., Banerjee R. Turmeric and curcumin biological actions and medicinal applications. Curr Sci. 2004;87:44–53. [Google Scholar]
- 3.Agarwal A., Nallella K., Allamaneni S., Said T. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2008;8:616–627. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 4.Daniel S., Limson J., Dairam A., Watkins G., Daya S. Through metal binding curcumin protects against lead-and cadmium–induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J Inorg Biochem. 2004;98:266–275. doi: 10.1016/j.jinorgbio.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Jungil H., Mousumi B., Jihyeuny J., Jae-Ha R., Xiaoxin C., Shengmin S. Modulation of arachidonic acid metabolism by curcumin and related B-diketone derivatives: effect on cytosolic phospholipase A, cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25:1671–1679. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- 6.Chigurupati S., Son T., Hyun D., Lathia J., Mughal M., Savell J. Lifelong running reduces oxidative stress and degenerative changes in the testes of mice. J Endocrinol. 2008;199:333–341. doi: 10.1677/JOE-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menon V., Sudheer A., Kalpana C. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 8.Kalpana C., Sudheer A., Rajasekharan K., Menon V. Comparative effects of curcumin and its synthetic analogue on tissue lipid peroxidation and antioxidant status during nicotine-induced toxicity. Med J. 2007;48:124–130. [PubMed] [Google Scholar]
- 9.Balcerek M., Matławska I. Preventive role of curcumin in lung cancer. Przegl Lek. 2005;62:1180–1181. [PubMed] [Google Scholar]
- 10.Jalili C., Salahshoor M.R., Naseri A. Protective effect of utrica diocia against nicotine-induced damage on reproductive parameters in male mice. Iran J Reprod Med. 2014;12:401–408. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S., Day I., Ye S. Nicotine induced changes in gene expression by human coronary artery endothelial cells. Atherosclerosis. 2001;154:277–283. doi: 10.1016/s0021-9150(00)00475-5. [DOI] [PubMed] [Google Scholar]
- 12.Li S., Lu D., Zhang Y., Zhang Y. Long-term treatment of hydrogen-rich saline abates testicular oxidative stress induced by nicotine in mice. J Assist Reprod Genet. 2014;31:109–114. doi: 10.1007/s10815-013-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Zayadi A. Heavy smoking and liver. World J Gastroenterol. 2006;12:6098–6101. doi: 10.3748/wjg.v12.i38.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hukkanen J., Jacob P., Benowitz N. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 15.Bandyopadhyaya G., Sinha S., Chattopadhyay B., Chakraborty A. Protective role of curcumin against nicotine-induced genotoxicity on rat liver under restricted dietary protein. Eur J Pharmacol. 2008;588:151–157. doi: 10.1016/j.ejphar.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Gawish A.M., Ramadan S., Hassan A.M., Issa A.M. Morphometrical, histopathological and cytogenetical ameliorating effects of green tea extract on nicotine toxicity of the testis of rats. J Cytol Histol. 2010;1:105–110. [Google Scholar]
- 17.Ghadami M.R., Pourmotabed A., Khademi N. The protective effect of curcumin on scopolamine induced spatial learning and memory deficits in rats. J Kermanshah Univ Med Sci. 2012;16:201–210. [Google Scholar]
- 18.Lari P., Abnous K., Imenshahidi M., Rashedinia M., Razavi M., Hosseinzadeh H. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol Ind Health. 2013;52:218. doi: 10.1177/0748233713475519. [DOI] [PubMed] [Google Scholar]
- 19.Jalili C., Salahshoor M.R., Pourmotabbed A. The effects of aqueous extract of Boswellia Serrata on hippocampal region CA1 and learning deficit in kindled rats. Res Pharm Sci. 2014;9:351–358. [PMC free article] [PubMed] [Google Scholar]
- 20.Basu S., Haldar N., Bhattacharya S., Biswas S., Biswas M. Hepatoprotective activity of Litchi chinensis leaf against paracetamol-induced liver damage in rats. Mid East J Sci Res. 2014;20:292–296. [Google Scholar]
- 21.Hematológico E. Action of trivalent chromium on rat liver structure. Histometric and haematological studies. Int J Morphol. 2006;24:197–203. [Google Scholar]
- 22.Zaitoun A., Apelqvist G., Al-Mardini H., Gray T., Bengtsson F., Record C. Quantitative studies of liver atrophy after portacaval shunt in the rat. J Surg Res. 2006;131:225–232. doi: 10.1016/j.jss.2005.11.587. [DOI] [PubMed] [Google Scholar]
- 23.Sala M., Komesu M., Lopes R., Maia G. Karyometric study of basal cell carcinoma. Braz Dental J. 1994;5:11–14. [PubMed] [Google Scholar]
- 24.Khazaei M., Zarei M., Sharifi M.R., Pourshanazari A.A. The effect of maintenance and reversal of DOCA-Salt hypertension on extravasation of macromolecules and serum nitric oxide concentration in male rats. Pathophysiology. 2011;18:201–206. doi: 10.1016/j.pathophys.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Reitman S., Frankel A. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am J Clin Pathol. 1957;28:53–56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 26.Sadashivam S., Manickam A. 2nd ed. New Age International Publisher; India: 1996. Biochemical Methods; pp. 121–124. [Google Scholar]
- 27.Wilken R. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:4–9. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berrahall A., Hajjaji N. Antioxidant enzymes activities and bilirubin level in a adult rats treated with load. C R Biol. 2007;330:581–588. doi: 10.1016/j.crvi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Shakhanbeh J. Teucrium polium inhibits nerve conduction and carrageenan induced inflammation in the rat skin. Turk J Med Sci. 2001;21:15–31. [Google Scholar]
- 30.El-Maraghy S.A., El-Sawalhi M.M. Hepatoprotective potential of crocin and curcumin against iron overload-induced biochemical alterations in rat. Afric J Biochem Res. 2009;3:215–221. [Google Scholar]
- 31.Nelson A., Sidney D. Molecular mechanisms of the hepatotoxity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- 32.Fu Y., Zheng S., Lin J., Ryerse J., Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 33.Ebrahimi S., Sadeghi H., Pourmahmoudi A., Askarian S., Askari S. Protective effect of zizphus vulgaris extract, on liver toxicity in laboratory rats. Armaghan Danesh. 2011;16:172–180. [Google Scholar]
- 34.Chuang S., Kuo M., Hs C. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis. 2000;21:331–335. doi: 10.1093/carcin/21.2.331. [DOI] [PubMed] [Google Scholar]
- 35.Willis D., Popovech M., Gany F., Hoffman C., Blum J., Zelikoff J. Toxicity of gutkha, a smokeless tobacco product gone global: is there more to the toxicity than nicotine. Int J Environ Res Public Health. 2014;11:919–933. doi: 10.3390/ijerph110100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakr A., Saber A. Ameliorative effect of ginger (zingiber officinale) on mancozeb fungicide induced liver injury in albino rats. Aust J Basic Appl Sci. 2007;1:650–656. [Google Scholar]
- 37.Cordova C., Netto C., Yunes R. Protective properties of butanolic extract of the Calendula officinalis L. (marigold) against lipid peroxidation of rat liver microsomes and action as free radical scavenger. Redox Rep. 2002;7:95–102. doi: 10.1179/135100002125000325. [DOI] [PubMed] [Google Scholar]
- 38.Chen B., Zhu Z., Chen M., Dong W., Li Z. Three-dimensional quantitative structure–activity relationship study on antioxidant capacity of curcumin analogues. J Mol Struct. 2014;1061:134–139. [Google Scholar]
- 39.Khorsandi L. The protective effect of turmeric (curcuma longa) extract on acetaminophen induced liver damage in mice. Daru. 2008;16.3:155–159. [Google Scholar]
- 40.Suñer I., Espinosa-Heidmann G., Marin-Castano E., Hernandez P., Pereira-Simon S., Cousins S. Nicotine increases size and severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2004;45:311–317. doi: 10.1167/iovs.03-0733. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi M. In-vitro antioxidant and in-vivo hepatoprotective activity of leucas ciliata leaves. Rec Nat Prod. 2010;2:124–130. [Google Scholar]
- 42.Hon W.M., Wei M., Kang Hoe L., Hoon Eng K. Nitric oxide in liver diseases. Ann NY Acad Sci. 2002;962:275–295. doi: 10.1111/j.1749-6632.2002.tb04074.x. [DOI] [PubMed] [Google Scholar]
- 43.Lallemand F., Dravolina O., De Witte P. Nicotine-induced changes of glutamate and arginine in naive and chronically alcoholised rats:an in vivo microdialysis study. Brain Res. 2006;1111:48–60. doi: 10.1016/j.brainres.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 44.Shen S.Q. Protective effect of curcumin against liver warm ischemia reperfusion injury in rat model is associated with regulation of heat shock protein and antioxidant enzymes. World J Gastroenterol. 2007;13:1953–1961. doi: 10.3748/wjg.v13.i13.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao R., Sharp B.M. Nicotine-induced norepinephrine release in hypothalamic paraventricular nucleus and amygdale is mediated by N-methyl-D-aspartate receptors and nitric oxide in the nucleus tractus solitarius. J Pharmacol Exp Ther. 2007;320:837–844. doi: 10.1124/jpet.106.112474. [DOI] [PubMed] [Google Scholar]
- 46.Muller T., Schepers G. Evidence for peroxynitrite as an oxidative stress-inducing compound of aqueous cigarette smoke fraction. Carcinogenesis. 1997;18:295–301. doi: 10.1093/carcin/18.2.295. [DOI] [PubMed] [Google Scholar]