Abstract
Background
Fetal Alcohol Spectrum Disorder (FASD) is associated with numerous neuro behavioral alterations, as well as disabilities in a number of domains, including a high incidence of depression and anxiety disorders. Prenatal alcohol exposure (PAE) also alters hypothalamic-pituitary-adrenal (HPA) function, resulting in increased responsiveness to stressors and HPA dysregulation in adulthood. Interestingly, data suggest that pre-existing HPA abnormalities may be a major contributory factor to some forms of depression, particularly when an individual is exposed to stressors later in life. We tested the hypothesis that exposure to stressors in adulthood may unmask an increased vulnerability to depressive- and anxiety-like behaviors in PAE animals.
Methods
Male and female offspring from prenatal alcohol (PAE), pair-fed (PF), and ad libitumfed control (C) treatment groups were tested in adulthood. Animals were exposed to 10 consecutive days of chronic mild stress (CMS), and assessed in a battery of well-validated tasks sensitive to differences in depressive- and / or anxiety-like behaviors.
Results
We report here that the combination of PAE and CMS in adulthood increases depressive- and anxiety-like behaviors in a sexually dimorphic manner. PAE males showed impaired hedonic responsivity (sucrose contrast test), locomotor hyperactivity (open field), and alterations in affiliative and nonaffiliative social behaviors (social interaction test) compared to control males. By contrast, PAE and, to a lesser extent, PF, females showed greater levels of “behavioral despair” in the forced swim test, and PAE females showed altered behavior in the final 5 minutes of the social interaction test compared to control females.
Conclusions
These data support the possibility that stress may be a mediating or contributing factor in the psychopathologies reported in FASD populations.
Keywords: Chronic Mild Stress, HPA Axis, FASD, Depression, Anxiety, Sex Differences
Alcohol is a teratogenic substance that can cause a spectrum of deleterious effects on the fetus if consumed during pregnancy (Mattson et al., 2001). At the extreme end of the spectrum is Fetal Alcohol Syndrome (FAS) (Jones et al., 1973), characterized by pre- and / or postnatal growth deficiency, the presence of specific craniofacial anomalies, and central nervous system dysfunction, including mental retardation, hyperactivity, and deficits in language, motor learning, and visuospatial functioning (Fryer et al., 2007; Mattson and Riley, 1998; Mattson et al., 1997; Roebuck et al., 1998). In cases where individuals exposed to alcohol prenatally do not display the full syndrome, they may be diagnosed with partial FAS or alcohol-related neurodevelopmental disorder (ARND). The umbrella term Fetal Alcohol Spectrum Disorder (FASD) is used to describe the broad range of outcomes associated with all levels of prenatal alcohol exposure (Stratton et al., 1996). In the United States approximately 1 in 100 live births is diagnosed with an FASD (Sampson et al., 1997).
Beyond the major deficits in behavioral, cognitive, and adaptive function that occur in individuals with FASD, one of the most commonly described disabilities in both children and adults is an elevated rate of mental health disorders, including depressive and anxiety disorders (Barr et al., 2006; Cryan et al., 2002; Famy et al., 1998; Streissguth et al., 1996). Currently, the biological mechanisms underlying this elevated rate are not known. However, studies have demonstrated that one of the consequences of prenatal exposure to alcohol is hyperactivity and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis. For example, heavy drinking at conception and during pregnancy are associated with higher basal cortisol levels (Jacobson et al., 1999; Ramsay et al., 1996) and increased responsiveness to stressors (Haley et al., 2006; Jacobson et al., 1999) in infants ranging from 2 to 13 months of age. Studies using animal models support and extend these findings. PAE rats typically show normal basal corticosterone (CORT) and adrenocorticotropin (ACTH) levels, but are hyperresponsive to a variety of stressors and to challenges with drugs, such as ethanol or morphine (Kim et al., 1996; Stein et al., 2009; Weinberg, 1988, 1992a, 1993; Weinberg et al., 1996). Central markers of HPA function similarly reflect altered regulation, including increased immediate early gene and CRH mRNA levels (Gabriel et al., 2005; Kim et al., 1999; Lee et al., 2000; Rivier et al., 1990), and deficits in feedback regulation of HPA activity (Glavas et al., 2006, 2007). Interestingly, sex differences in response are often observed, with PAE males and females showing different patterns of response depending on the nature and intensity of the stressor, time course of testing, and hormonal endpoint measured (Kim et al., 1996; Schneider et al., 2002; Taylor et al., 1982, 1983, 1988; Weinberg, 1985, 1992a,b; Weinberg and Gallo, 1982; Weinberg et al., 1986).
These data are intriguing in light of evidence that one of the most consistently observed biological alterations in depression and anxiety disorders is HPA dysregulation. For example, about half of severely depressed patients exhibit increased and / or sustained HPA activity following exposure to mild stressors (Murphy, 1991; Parker et al., 2003) or following HPA challenge tests, which appear to be particularly sensitive in revealing aspects of HPA dysfunction (Heuser et al., 1994; Holsboer et al., 1987). Thus, HPA hyperactivity is often manifest in nonsuppression in the dexamethasone (DEX) suppression test, and blunted ACTH responses to CRH but increased ACTH responses to DEX + CRH (Heuser et al., 1994; Holsboer et al., 1987). Moreover, successful antidepressant treatment typically normalizes HPA responses to challenge (Holsboer, 2001), and in patients where a neuroendocrine abnormality persists, the risk of relapse or resistance to treatment is much higher (Holsboer, 2000; de Kloet et al., 2005). Whether HPA abnormalities are a primary cause of depression, represent an illness marker, or are secondary to another initiating cause is not known. Nevertheless, it is possible that reprogramming of the HPA axis by PAE may, at least in part, underlie the development of depressive and anxiety-related pathologies in children and adults with FASD.
Relatively few studies have utilized animal models to explore whether PAE leads to increased depressive / anxiety-like behaviors. PAE does increase immobility in the forced swim test (Wilcoxon et al., 2005), which is attenuated by adrenalectomy (Wilcoxon and Redei, 2007), suggesting adrenal activity may, at least partly, underlie the expression of this behavior. PAE also appears to alter other aspects of the depression syndrome, such as locomotor activity and anxiety. However, data are often discrepant among studies (Carneiro et al., 2005; Dursun et al., 2006; Osborn et al., 1998a,b). A number of methodological factors may explain these discrepancies, among the most important of which are the animal model and test conditions employed. First, alterations in behavior and HPA regulation among PAE animals may become evident only if animals are tested following exposure to stress rather than in a basal state. We have recently shown, for example, that the combination of PAE and exposure to a 10-day chronic mild stress (CMS) paradigm (modified from the more prolonged and severe CMS paradigms often used in depression-related research, e.g., Willner, 1997, 2005) in adulthood increases anxiety-like behaviors on the elevated plus maze in a sexually dimorphic manner, with concomitant alterations in HPA and HPG reactivity (Hellemans et al., 2008). Importantly, it appears that exposure to CMS was critical in unmasking HPA dysregulation in PAE offspring, as there were fewer differences among prenatal groups not exposed to CMS. Second, depression is described as a multidimensional syndrome, and studies often neglect to consider this aspect, limiting their studies to 1 or 2 behavioral measures. And third, studies often make conclusions based on data from only male rats, despite significant evidence that depression in humans is 2 to 3 times more common in women than in men (Kessler, 2003), and that the neurobehavioral consequences of PAE are highly sexually dimorphic (Halasz et al., 1993; Kim et al., 1999; Taylor et al., 1982; Weinberg, 1988, 1992a,b).
The current study was undertaken to explore whether the combination of PAE and CMS alters depressive- and anxiety-like behaviors. Animals were tested in our multidimensional test battery that is sensitive to multiple aspects of depressive- and / or anxiety-like behaviors in rodents: sucrose contrast (anhedonia); Porsolt forced swim (behavioral despair); open field (locomotor hyperactivity); and social interaction (anxiety; social behaviors). Using our well-established animal model of prenatal alcohol exposure (Weinberg, 1988; Weinberg et al., 2008), we exposed adult male and female PAE, PF, and C offspring to 10 days of CMS. Immediately following CMS, separate cohorts of animals were tested in one of the 4 behavioral tests. Our results indicate that the combination of PAE and CMS in adulthood increases depressive- and anxiety-like behaviors in a sexually dimorphic manner.
MATERIALS AND METHODS
Breeding and Animals
Female Sprague-Dawley rats (223 to 300 g, n = 45) were obtained from Charles River Laboratories (St Constant, PQ, Canada) and male Sprague-Dawley rats (275 to 350 g, n = 18) were obtained from UBC Animal Care Centre, South Campus. Rats were group-housed by sex and maintained on a 12:12 hours light / dark cycle (lights on 06:00 AM), with controlled temperature (21 to 22°C), and ad libitum access to standard lab chow (Jamieson’s Pet Food Distributors Ltd., Delta, BC, Canada) and water. One to two weeks following arrival, male and female pairs were placed together in stainless steel suspended cages (25 × 18 × 18 cm), with mesh front and floor. Day 1 of gestation (G1) was indicated by the presence of a vaginal plug on wax paper beneath the breeding cages, which was checked daily. Animal use and care procedures were in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and the Canadian Council on Animal Care (National Research Council, 1996) guidelines and were approved by the University of British Columbia Animal Care Committee, protocol #A06-0017.
Diets and Feeding
On G1, females were singly housed in polycarbonate cages (24 × 16 × 46 cm) with pine shaving bedding and randomly assigned to 1 of 3 treatment groups. The ethanol (PAE) treatment group (n = 10) was offered ad libitum liquid ethanol diet (36% ethanol-derived calories) prepared by Dyets Inc. (Bethlehem, PA) and water. This diet is formulated to provide adequate nutrition to pregnant rats regardless of ethanol intake (Weinberg, 1985). The pairfed (PF) group (n = 10) was offered a liquid control diet with maltosedextrin isocalorically substituted for ethanol and water. Intake was matched to the amount consumed by an ethanol-treated partner (g / kg body weight / gestation day). The control (C) group (n = 10) was offered standard lab chow (Jamieson’s Pet Food Distributors Ltd.) and water ad libitum. All animals were provided with fresh diet daily within 1.5 hours prior to lights off to preventa shift of corticosterone (CORT) circadian rhythms, which occurs in animals such as those in the PF group who are on a restricted feeding schedule (Gallo and Weinberg, 1981). Experimental diets were continued through G21. Beginning on G22 all animals were provided with ad libitum access to standard laboratory chow and water, which they received through-out lactation. Pregnant dams were left undisturbed except for cage changing and weighing which occurred on G1, G7, G14, and G21. On postnatal day 1 (PN1), pups were weighed and litters randomly culled to 10 (5 males and 5 females when possible). All developmental data were previously reported in (Hellemans et al., 2008). Dams and pups were weighed on PN1, PN8, PN15, and PN22 (data not reported). On PN22, pups were ear notched for identification and group-housed by litter and sex. Animals were not cross-fostered in the present study as previous data have shown that ethanol-induced alterations in mother-pup interactions appear to result primarily from direct effects of ethanol on the pup rather than through alterations in maternal behavior. For example, pups prenatally exposed to ethanol show selective changes in isolation-induced ultrasonic vocalizations that may result in impaired maternal-infant communication (Barron and Gilbertson, 2005; Kehoe and Shoemaker, 1991; Marino et al., 2002; Vorhees, 1989), and a decreased ability to elicit retrieval behavior from both control and ethanol-exposed dams (Ness and Franchina, 1990). On PN40, 1 male and1 female pup from each litter were randomly assigned to a behavioral task and were pair-housed with another animal of the same sex, prenatal treatment, and behavioral task assignment.
Chronic Mild Stress Protocol
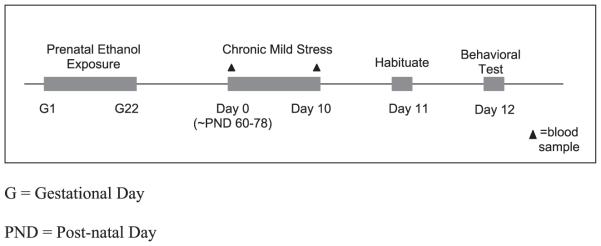
Prior to behavioral testing, animals were subjected to a randomized protocol of stressors (see Fig. 1). Because animals were born over a 10-day period, and entered the experiment in a staggered fashion, the age at which the chronic mild stress (CMS) procedure commenced ranged from 60 to 78 days of age. To explain our procedure in detail, there were 240 animals total tested in this study. Animals entered the study in cohorts, with each cohort randomized across litters. Each cohort was assigned to a specific behavioral test (e.g., cohort 1 were males tested in the FST; cohort 2 were females tested in the FST, cohort 3 were males tested in the OF, etc.). Thus, the greatest age difference within each cohort, i.e., within each behavioral test, was 10 days, and the age difference of animals between behavioral tests was a maximum of 18 days.
Fig. 1.
Experimental design.
On day 1 of CMS, within 2 hours of lights on (i.e., between 07:00 AM and 08:00 AM), basal blood samples for CORT were obtained via tail nick 1 mm from the tip of the tail, immediately upon removal from the home cage. Animals were not anesthetized prior to this procedure. Although tail nicking can be considered a mild stressor, the amount of behavioral and endocrine activation that might result from anesthesia would likely be greater than that resulting from this rapid procedure. Animals were also weighed, handled, and moved to a new facility in a neighboring building. These were considered the first 2 stressors of the 10-day CMS regimen. On days 2 to 9, all CMS animals were taken to a separate room and exposed to 2 different stressors: one between 08:00 AM and 12:00 PM, and the second between 01:00 PM and 04:00 PM, with a minimum of 2 hours between stressors. Each of the stressors is described in detail below (an example of the CMS schedule for 1 cohort is provided in Table 1). The order and type of stressor were randomized, but all animals received the same number of each stressor over the 10-day CMS exposure period. The stressors were: (i) 5-minute exposure to a 20 × 20 × 90 cm transparent Plexiglas platform mounted on a wooden post 90 cm high; (ii) 2-hour white noise (40 dB; Lafayette Instruments, Lafayette, IN, model #15800); (iii) overnight social isolation in hanging cages with wire mesh front and floor (20 × 23 × 18 cm); (iv) 30-minute restraint in PVC tubes (19 × 7 cm for male, 15 × 6 cm for female); (v) 1-hour soiled cage (cage-mates placed together in soiled cage of another pair of animals of the same sex); (vi) 2-hour cage tilt at a 30° angle; and (vii) 1-hour exposure to a novel cage (cage-mates re-housed in small, opaque cages (18 × 25 × 15 cm) without bedding, food, or water). On Day 10, a second basal blood sample was obtained via tail nick in CMS-exposed animals, as above; all animals were exposed to second stressor in the afternoon of the tenth day. Animals were also weighed on Days 4, 7, and 10 of the CMS exposure period (see Hellemans et al., 2008).
Table 1.
Example of Chronic Mild Stress Regimen
| Day 1 | Stressor 1 = Tail nick, handle, move, weigh |
| Day 2 | Stressor 2 = Cage tilt, novel cage |
| Day 3 | Stressor 3 = Platform, white noise |
| Day 4 | Stressor 4 = Social isolation and water deprivation, weigh |
| Day 5 | Stressor 5 = Restraint, soiled cage |
| Day 6 | Stressor 2 = Cage tilt, novel cage |
| Day 7 | Stressor 3 = Platform, white noise, weigh |
| Day 8 | Stressor 4 = Social isolation, water deprivation |
| Day 9 | Stressor 5 = Restraint, soiled cage |
| Day 10 | Stressor 1 = Tail nick, handle, weigh |
Behavioral Testing
Behavioral testing was conducted on Day 11, one day following the last CMS exposure. One cohort (n = 10) of PAE, PF, and C male and female rats was randomly assigned to each of the 4 behavioral tests: sucrose contrast (SC); forced swim (FST); open field (OF); or social interaction (SI). The testing room was adjacent to the colony room and was separate from the room where animals were exposed to stressors. Behaviors in the FST, OF, and SI were recorded using a digital camera (Panasonic, Mississauga, ON, Canada, CCTV Camera, Model No. WV-BP 334), and analyzed using Noldus Etho-vision, Wageningen, The Netherlands, v.3.1 software at a sampling rate of 4.994 samples / s for the OF and FST, and Noldus Observer v. 20 for the SI test. In addition, white noise at 30 dB was played in background to dampen random noise (“The Maker,” Model AM1100, Soundolier Inc., St Louis, MO). Behavioral testing took place under dim lighting conditions and all animals were tested during the light phase of their cycle (06:00 AM to 06:00 PM). Due to the large number of animals tested in this study, females were tested at random stages of their estrous cycle.
Sucrose Positive Contrast Test
Anhedonia was measured using the sucrose positive contrast test. On Day 11, animals were singly housed in polycarbonate cages (24 × 16 × 46 cm) and offered a 2.1% sucrose solution over 24 hours, for 4 consecutive days. The number of days of habituation at this concentration was based on pilot studies. Sucrose consumption for each 24-hour period was measured by recording the initial and final bottle weights for each 24-hour period, at 02:00 pm, or 6 hours after lights on. Fresh sucrose solution was offered daily. On the fifth day, the 2.1% solution was replaced with a 15% sucrose solution, and consumption was again measured for a 24-hour period. Animals were also offered ad libitum access to lab chow and water throughout the habituation and testing phase. To eliminate place preference effects, the positions of the water and sucrose bottles were alternated daily.
Porsolt Forced Swim Test
The apparatus consisted of transparent Plexiglas cylinders (males: 20 cm diameter, 60.5 cm height; females: 20 cm diameter, 43 cm height). The cylinders were filled with tap water maintained at 25 ± 1°C at a height of 30 cm (females) or 40 cm (males). The height was determined to prevent animals’ tails from touching the bottom of the tank (Detke et al., 1995). Activity was recorded on 2 consecutive days by a video camera placed directly facing both cylinders. This provided an unobstructed view of both animals and allowed for scoring of both swimming and immobility behaviors by the observer. On Day 1, animals were placed in the cylinders for 15 minutes. This pretest session allowed animals to experience the fact that escape is impossible (Borsini and Meli, 1988). On Day 2, animals were re-exposed to the cylinders for a 5-minute test. After each exposure, animals were partially dried with a towel and returned to their home cages. A rat was judged to be immobile when it remained floating, making only the necessary movements to keep its head above water (Borsini et al., 1986). Duration of immobility (seconds) was measured on both Day 1 and Day 2.
Open Field
The open field consisted of a square arena (80 × 80 cm) divided evenly into 4 smaller squares (average of 40 × 40 cm) enclosed by walls (30 cm) made of white plastic sheets. Behaviors were recorded by a video camera positioned directly above the apparatus to capture the image of all 4 squares simultaneously. The animals were habituated to the field for 5 minutes the day prior to the first test day. Animals were then placed into the field for 5 min / d over 3 consecutive days. Total distance traveled (cm) was recorded and analyzed. The apparatus was wiped clean with 5% acetic acid between animals.
Social Interaction
The social interaction test is a well-validated test of anxiety and social behavior (File and Hyde, 1978). The present protocol was adapted from Overstreet and colleagues (2002) and File and Hyde (1978). The social interaction test was run in the same arena as the open field, with similar lighting conditions. Animals were habituated to the field alone for 15 minutes the day before testing. On the following day, each experimental rat was placed in 1 quadrant of the field and a control animal of the same weight range (within 20 g) and sex was introduced into the arena. Animals were allowed to interact for a total of 15 minutes, and the entire session was digitally recorded. The first and last 5 minutes of each session were manually scored according to an all-occurrence sampling method, by an observer blind to the prenatal treatment, to measure both affiliative (allogrooming, mutual circling / follow, social rest) and non-affiliative (rough- and-tumble play, wrestling, pinning) social behavior in the experimental animals.
Corticosterone Radioimmunoassay
Total CORT (bound plus free) levels were measured using a commercially available kit (MP Biomedicals, Orangeburg, NY, cat. #07-120103). The antiserum cross-reacts 100% for corticosterone. The minimum detectable corticosterone concentration was 7.7 ng / ml and the intra- and inter-assay coefficients of variation were 7.1 and 7.2%, respectively.
Statistical Analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) v15.0 software. In each of the data sets, between-subjects factors were Sex (male or female) and Prenatal group (PAE, PF, or C). In addition, the within-subjects factor of Day was included to explore the change in CORT levels prior to (Day 1) and following (Day 10) CMS exposure. Data from the sucrose positive contrast test were corrected for body weight and analyzed using a mixed ANOVA, with Day (Precontrast vs. Postcontrast) as a repeated measures factor. The duration of immobility (seconds) in the forced swim test was analyzed using a mixed ANOVA, with Time as the repeated measures factor [Bin 1 (minutes 0 to 5); Bin 2 (minutes 5 to 10); Bin 3 (minutes 10 to 15) for Day 1; and minutes 1 to 5 for Day 2]. Data from open field test were also analyzed using a mixed ANOVA, with both Day (Day 1, 2, or 3) and Minute (1, 2, 3, 4, or 5) as repeated measures factors. In the social interaction test, data were analyzed within behavioral categories (Affiliative vs. Nonaffiliative) and analyzed across intervals (First 5 minutes vs. Last 5 minutes). Significant main effects or interactions were further explored for simple main effects; a Fisher’s LSD post hoc was used for comparisons of 3 groups or less, and a Sidak correction for >3 groups. In analyses that included a within-subjects factor, an estimated marginal means procedure with a Sidak correction was employed. Because the Sidak correction accurately controls for family wise α, an overall F test is not required to be significant in order to explore a priori differences between factors, i.e., planned comparisons (Cardinal and Aitken, 2005). For repeated measures analyses, the degrees of freedom (df) were corrected to more conservative values using the Huynh-Feldt epsilon ε (Huynh and Feldt, 1970) to correct for any violations of the sphericity assumption (Cardinal and Aitken, 2005). Corrected df are reported to 1 decimal place. Alpha was set at 0.05 for all analyses. All results are shown as means ± SEM.
RESULTS
Plasma Corticosterone Levels Pre- and Post-CMS
Mixed factors ANOVA on CORT levels revealed significant main effects of Day [F(1,44) = 4.04, p < 0.05], Sex [F(1,44) = 47.60, p < 0.05], and Prenatal Group [F(2,44) = 4.98, p < 0.05], as well asa Sex × Prenatal Group interaction [F(2,44) = 6.24, p < 0.05] (Fig. 2). As expected, basal CORT levels were higher in females than males. As well, and in line with our previous results, CORT levels overall were lower on Day 10 than on Day 1. Simple main effects analysis among females revealed that while all females showed a CORT decrease from Day 1 to Day 10, PF females had significantly higher basal CORT (although still well within the normal basal range) levels than both PAE and C females (p’s < 0.05). In contrast, PAE males had significantly higher basal CORT levels on Day 1 compared to PF and C males (p’s < 0.05) but did not differ from PF and C on Day 10. Further, PAE (p < 0.05), but not PF and C males showed a significant overall drop in basal CORT levels from Day 1 to Day 10. Once again, CORT levels in all groups were well within the normal basal range.
Fig. 2.
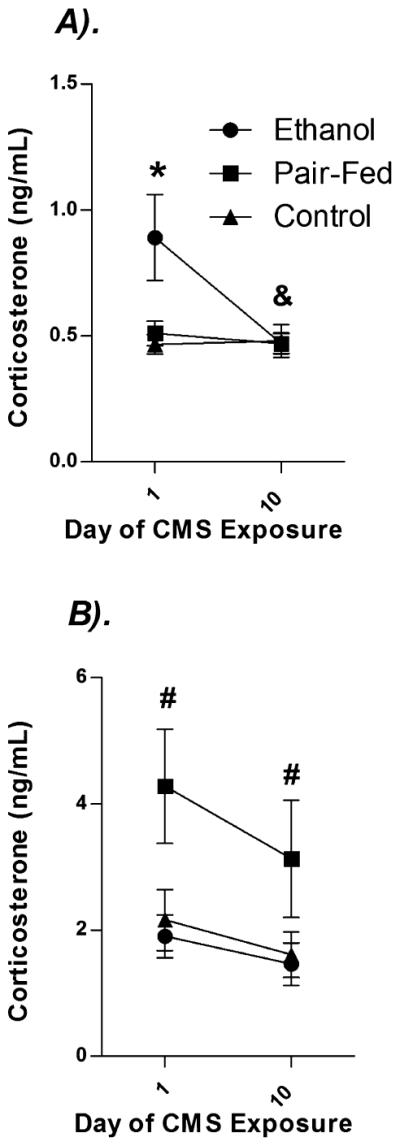
Effects of prenatal group and sex on plasma corticosterone (ng / ml). Data represent mean basal corticosterone (ng / ml) before (Day 1) and on the last day (Day 10) of CMS exposure in (A) males and (B) females. * denotes significantly different from PF and C animals. # denotes significantly different from PAE and C rats. & denotes significantly different between Days among PAE rats. n’s = 8 to 10 for each condition; p’s < 0.05.
Positive Sucrose Contrast Test
Figure 3 illustrates data from the positive sucrose contrast test. A main effect of Sex [F(1,49) = 23.59, p < 0.05] indicated that, as expected, males consumed less sucrose overall than females. Analysis of simple main effects in males revealed significant main effects of Day [F(1,24) = 14.12, p < 0.05] and Prenatal group [F(2,24) = 4.85, p < 0.05], as well as a Day × Prenatal group interaction [F(2,24) = 2.77, p < 0.05]. PAE males consumed more sucrose precontrast than PF and C males (p < 0.05). In addition, whereas PF and C males increased their consumption at the higher concentration (p’s < 0.05), PAE males showed no change in consumption over days (p > 0.05). By contrast, females in all Prenatal groups increased consumption of sucrose at the higher concentration [significant main effect of Day; F(1,25) = 54.42, p < 0.05].
Fig. 3.
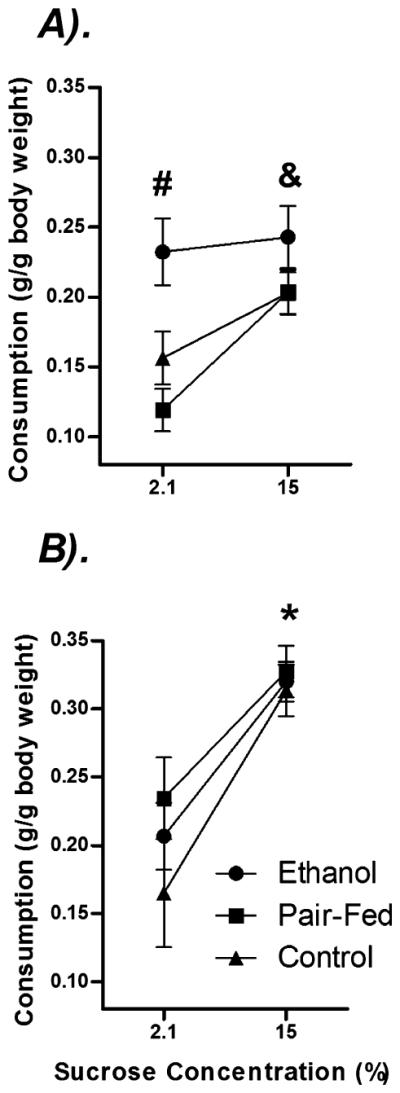
Effects of prenatal group and sex on behavior in the sucrose contrast test. Data represent the amount of sucrose consumed by body weight on Days 4 (“Pre”; 2.1% sucrose concentration) and 5 (“Post”; 15% sucrose concentration) in (A) males and (B) females exposed to chronic mild stress.* denotes a significant difference in consumption across days for all prenatal groups. & denotes significant difference in consumption across days for PF and C. # indicates that precontrast, PAE males consumed significantly more sucrose than PF and C males. n’s = 8 to 10 for each condition; p’s < 0.05.
Forced Swim Test
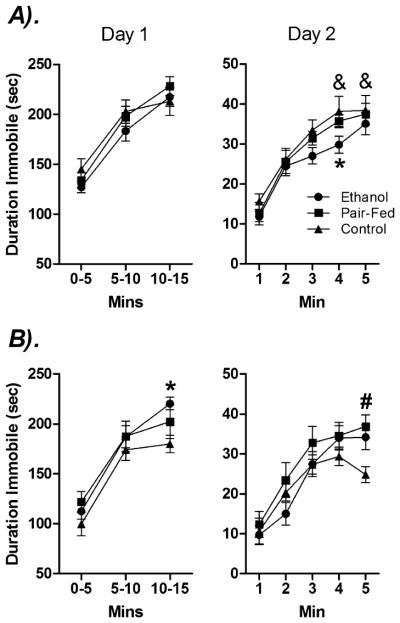
Day 1
Mixed factors ANOVA across the three 5-minute time bins revealed significant main effects of Time [F(2,108) = 287.14, p < 0.05] and Sex [F(1,54) = 5.651, p< 0.05], and a Prenatal Group × Time interaction [F(4,108) = 2.52, p < 0.05] for duration (seconds) of immobility (see Fig. 4). Overall, immobility increased over time, and males were more immobile than females. However, planned comparisons indicated that only PF and C males showed increased immobility during the first and last 5 minutes of testing (p’s < 0.05). In addition, planned comparisons between sexes for the significant Time × Prenatal Group interaction revealed that PAE females (p < 0.05) but not males spent more time immobile compared to their control counterparts.
Fig. 4.
Effects of prenatal group and sex on duration of immobility in the forced swim test. Data represent the time (s) spent immobile in (A) males and (B) females on Day 1 and Day 2. * denotes PAE significantly different from C animals. # denotes PAE and PF significantly different from C animals. & denotes that C males significantly more immobile than C females. n’s = 8 to 10 for each condition; p’s < 0.05.
Day 2
Mixed factors ANOVA on duration of immobility indicated a significant main effect of Time [F(4.00,201.28) = 113.78, p < 0.05], Sex [F(1,53) = 3.27, p = 0.07], and a Time × Sex × Prenatal Group [F(7.60,201.28) = 1.75, p = 0.09] trend (see Fig. 4). Planned comparisons revealed that PAE males were less immobile than C males in the fourth minute of testing, p < 0.05, whereas PAE and PF females were more immobile than C females in the last minute of testing, p < 0.05. Finally, C males were more immobile than C females in the final 2 minutes of the test period, p’s < 0.05.
Open Field
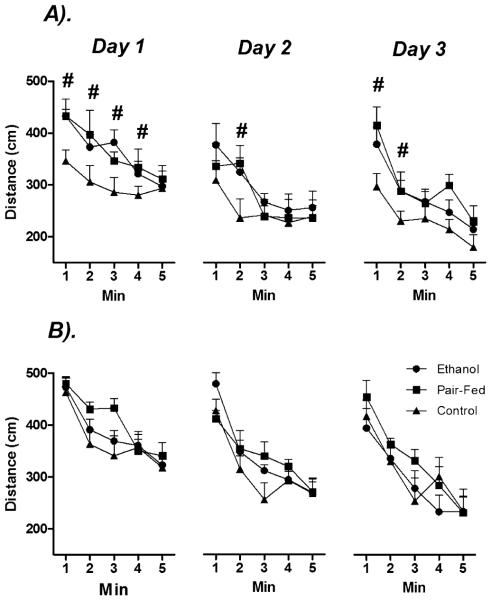
Figure 5 illustrates the significant main effects of Time (minutes) [F(3.58,135.88) = 41.27, p < 0.05] and Day [F(2,76) = 3.88, p < 0.05] and the significant Time × Day interaction [F(7.48,284.32) = 14.34, p < 0.05] on distance travelled in the open field. Overall activity decreased across Days and within each 5-minute testing period. There were also significant main effects of Sex [F(1,38) = 9.26, p < 0.05] and Prenatal Group [F(2,38) = 5.74, p < 0.05]: overall, females were more active than males. Moreover, planned comparisons revealed that the effects of Prenatal Group were due primarily to greater activity in PAE and PF compared to C males overall (p’s < 0.05), whereas there were no significant effects of Prenatal Group among females across Days or within each 5-minute test session (p’s > 0.05).
Fig. 5.
Effects of prenatal group and sex on locomotor activity in the open field. Data represent the total distance traveled (cm) in (A) males and (B) females during a 5-minute testing period across 3 consecutive days of testing. # denotes significantly different from C animals. n’s = 8 to 10 for each condition; p’s < 0.05.
Social Interaction Test
Duration
ANOVA performed on the duration of affiliative and nonaffiliative behaviors in the first and last 5-minute interval of testing yielded significant main effects of Behavior [F(1,50) = 8.36, p < 0.05] and Prenatal Group [F(2,50) = 3.52, p < 0.05], as illustrated in Fig. 6. Overall, animals spent more time in nonaffiliative behaviors and PAE reduced the frequency of both types of behavior relative to PF and C rats (p’s < 0.05). However, planned comparisons revealed that this effect was selective for male PAE rats in the first 5 minutes of testing (p < 0.05), and there were no significant effects of Prenatal Group or Time among females (p’s > 0.05).
Fig. 6.
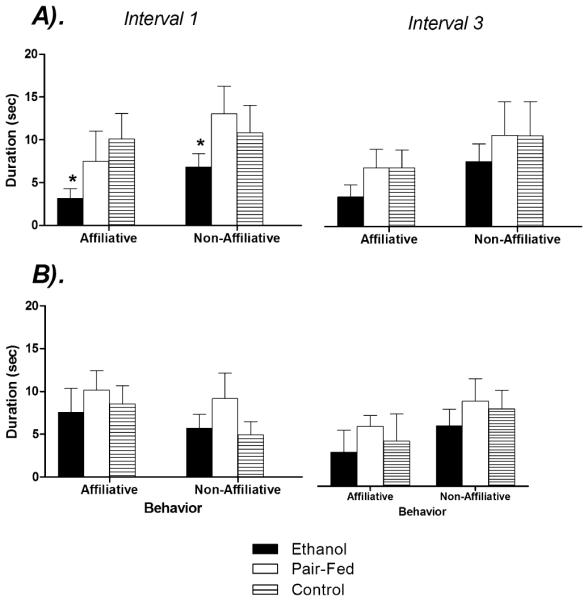
Effects of prenatal group and sex on the duration of behaviors in the social interaction test. Data represent the duration(s) of affiliative and nonaffiliative behaviors among (A) males and (B) females in the first (0 to 5 minutes) and third (11 to 15 minutes) intervals of a 15-minute test. * denotes significantly different from PF and C animals. n’s = 8 to 10 for each condition; p’s < 0.05.
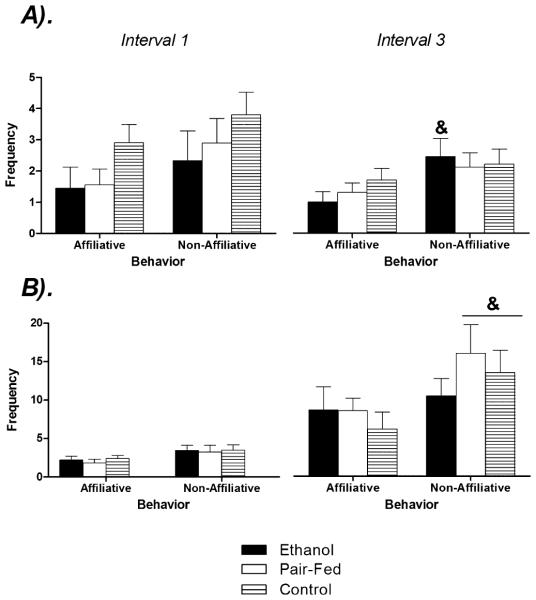
Frequency
Figure 7 illustrates the significant main effects of Behavior [F(1,52) = 21.59, p < 0.05] and Sex [F(2,52) = 3.52, p < 0.05] on the frequency of affiliative and nonaffiliative behaviors in the first and last interval of testing. Analysis of simple main effects by sex revealed that PAE (p < 0.05) but not PF and C (p’s > 0.05) males engaged in significantly higher numbers of nonaffiliative compared to affiliative behaviors in the final 5 minutes of testing. Among females, the opposite pattern emerged: whereas both PF and C females engaged in more nonaffiliative than affiliative behaviors in the final 5 minutes of testing (p’s < 0.05), PAE females did not differ between behavioral categories (p > 0.05).
Fig. 7.
Effects of prenatal group and sex on the frequency of behaviors in the social interaction test. Data represent the frequency of affiliative and nonaffiliative behaviors among (A) males and (B) females in the first (0 to 5 minutes) and third (11 to 15 minutes) intervals of a 15-minute test. & denotes significantly different than affiliative behaviors in the third interval. n’s = 8 to 10 for each condition; p’s < 0.05.
DISCUSSION
The results of the current study reveal that the combination of PAE and CMS in adulthood increases depressive- and anxiety-like behaviors in a sexually dimorphic manner. PAE males showed impaired hedonic responsivity (sucrose contrast test), locomotor hyperactivity (open field), and alterations in affiliative and nonaffiliative behaviors (social interaction) compared to control males. By contrast, PAE and, to a lesser extent, PF, females showed greater levels of behavioral despair in the forced swim test, and PAE females showed altered behavior in the final 5 minutes of the social interaction test compared to control females. In conjunction with our previous work showing that CMS leads to significant increases in CORT release among PAE animals following acute stress (Hellemans et al., 2008), these results support the possibility that fetal programming by PAE has long-term effects on sensitivity to stressors, resulting in increased behavioral and physiological responsiveness to stressors encountered later in life. In this respect, PAE may act as a predisposing factor for the increased prevalence of mood and anxiety disorders in FASD populations.
Anhedonia, or the inability to experience pleasure from rewarding stimuli, is one of the core symptoms of depression and can be easily studied in animal models. Although sucrose preference or intake tests are commonly employed, some have argued that a change in sucrose consumption may be mediated by a significant loss of body mass following CMS. As a possible solution to this confound, we opted to utilize the sucrose contrast test (Matthews et al., 1996) to study hedonic behavior. In the present study, the combination of PAE and CMS resulted in sexually dimorphic responses in the sucrose contrast test. Overall, females consumed more sucrose than males, even with a correction for body weight, supporting previous findings that control (i.e., nonfetal alcohol-exposed) females consume higher volumes of sweetfluids than males (Valenstein et al., 1967). Furthermore, all females and both PF and C males showed increased sucrose intake with increased concentration (i.e., increased reward value), whereas PAE males showed no increase in consumption with the shift in sucrose concentration. This lack of response could reflect insensitivity to the change in reward value of the sucrose, and may be interpreted as anhedonia. However, in view of our findings that PAE animals are less able to respond appropriately to environmental cues (Weinberg, 1988, 1992b), an alternate explanation is that PAE males could not discriminate between the low and high concentrations. Regardless of interpretation, our data indicate an adverse effect of prenatal alcohol exposure, and not prenatal stress or nutritional deprivation, as our PF males did demonstrate a significant contrast effect. Further, severe maternal dietary restriction does not appear to influence sucrose preference (water vs. 1% sucrose solution) in either male or female adult offspring (Jezova et al., 2002).
It may be argued that no significant alterations in sucrose consumption were evident in PAE males because they initially consumed higher amounts of sucrose than PF and C males prior to the contrast. Barron and colleagues (1995) also report elevated sucrose consumption in PAE males in their model of neonatal alcohol exposure. One explanation for the elevated intake among PAE males is that sucrose consumption may represent a compensatory response to modulate greater behavioral or HPA arousal (Dallman et al., 2003; Laugero et al., 2001; Pecoraro et al., 2004). These findings run counter to the Willner model, which purports that chronic stress results in reduced drive on appetitive activity and thus decreased consumption of sweet fluids in rodents (Willner, 1995; Willner et al., 1992). Of note, however, is that our adapted CMS protocol is much milder than the typical Willner CMS protocols. Nevertheless, due to the dearth of literature investigating PAE-induced alterations in hedonic behaviors, further studies are needed in order to understand fully the current findings.
In the forced swim test, PAE led to a significant increase in the duration of immobility among females, but not males. Importantly, the emergence of depressive-like behavior in females was first observed during the pretest session: PAE females were significantly more immobile than C females in the final 5 minutes of the 15-minute session on Day 1, and continued to show greater immobility on Day 2. Interestingly, on Day 2, PF females were also more immobile than C females in the last minute of the 5-minute test on Day 2. These studies support previous evidence that PAE leads to increased immobility in the FST in both males and females in adulthood (Slone and Redei, 2002; Wilcoxon et al., 2005), and that in general, females exhibit greater behavioral despair than males (Brotto et al., 2000; Drossopoulou et al., 2004; Mogil et al., 1993; Papaioannou et al., 2002), although conflicting data suggest higher immobility among males (Alonso et al., 1991; Barros and Ferigolo, 1998; Contreras et al., 1995).
According to Porsolt, prolonged floating time in the forced swim test reflects a state of “behavioral despair” (i.e., animals “give up” trying to escape), one of the characteristics of depression (Porsolt et al., 1977, 1978a), and therefore immobility is often interpreted as depressive-like behavior (Alonso et al., 1991, 2000; Carneiro et al., 2005; Porsolt et al., 1978b). This interpretation is further based on the finding that anti-depressants generally reverse the immobility in this task (e.g., (Armario et al., 1988; Detke et al., 1995; Morley-Fletcher et al., 2003; Overstreet and Griebel, 2004; Overstreet et al., 2004; Porsolt et al., 1978a,b; Sikiric et al., 2000; Tonissaar et al., 2008; Walf et al., 2004). However, the interpretation of increased immobility as a measure of behavioral despair is controversial, as some argue that immobility instead reflects a relatively successful coping strategy that employs energy-con-serving behavior (Borsini and Meli, 1988; Nishimura et al., 1988; Schechter and Chance, 1979; West, 1990). According to this view, immobility can be interpreted as an adaptive response that increases the chance of survival (Binik and Sullivan, 1983; Bruner and Vargas, 1994; Calil and Marcondes, 2006). Interestingly, our results showed that PAE males were less immobile than PF and C males. If immobility indeed reflects an adaptive strategy rather than behavioral despair, it would suggest that PAE males failed to cope with the stress, which could be interpreted as a failure in consolidation of aversive learning. However, this interpretation is speculative and future studies are required to explore fully the implications of these findings.
Our finding that females show somewhat greater immobility than males is consistent with clinical literature demonstrating that women are more susceptible to depression than men (Altemus, 2006; Weiss et al., 1999). It is likely that this sex difference is mediated, at least in part, by the interaction between the hypothalamic-pituitary-gonadal (HPG) and HPA axes, as immobility levels in the FST (Frye and Walf, 2002; Frye and Wawrzycki, 2003; Schneider and Popik, 2007) and HPA responses to stress (Dalla et al., 2005; Drossopoulou et al., 2004; Patchev and Almeida, 1998) differ across the estrus cycle. PAE also results in different patterns of HPA and HPG response and regulation following stressors compared to those in controls (Lan et al., 2006; Lee et al., 2003). Thus, an altered HPA-HPG interaction may be a significant factor underlying the robust sexual dimorphism observed in the epidemiology of mood disorders both in the general population, and in FASD populations (Streissguth et al., 1996). Although we did not explicitly measure stage of estrus cycle in the present study, we would expect significant increases in depressive-like behavior in proestrous, as previous studies from our laboratory revealed that PAE and PF animals show increased basal and stress estradiol levels and increased HPA responses to restraint stress compared to control females during proestrous, when estradiol levels are high (Lan et al., 2009). Moreover, hippocampal mineralocorticoid receptor (MR) mRNA levels are significantly decreased, and glucocorticoid receptor (GR) mRNA increased, during proestrus in PAE compared to PF and C females (Sliwowska et al., 2008).
On the open field, females overall had higher levels of locomotor activity than males, supporting previous evidence that females are hyperactive following CMS (Dalla et al., 2005; Westenbroek et al., 2003). In addition, PAE and PF males were significantly more active than C males across test days, consistent with previous data demonstrating hyperactivity on the OF following PAE (Riley, 1990; Randall et al., 1986; Rockman et al., 1989). Of relevance, increased activity on the OF has been linked to anxiety and fear (Carola et al., 2002; Crawley, 1985; Ennaceur et al., 2006; Prut and Belzung, 2003). Moreover, these findings have important clinical implications, as alterations in locomotor activity are one of the diagnostic criteria for major depression (American Psychiatric Association, 2000) and hyperactivity is one of the most commonly reported behavioral symptoms of individuals with FASD (Mattson and Riley, 1998; Mattson et al., 2001). It is important to note, however, that animals in the present study were all exposed to CMS in adulthood prior to testing. As CMS increases locomotor activity on the OF (Gronli et al., 2005), baseline activity levels among CMS-exposed C animals in this study are likely already elevated, which may have resulted in ceiling effects, particularly among females.
Finally, results from this study indicate sexually dimorphic alterations in social interactions among PAE animals. PAE males spent significantly less time in both affiliative and non-affiliative behaviors in the first 5 minutes of testing, and engaged in significantly more nonaffiliative compared to affiliative behaviors in the final 5 minutes of testing, which was not observed among PF and C males. By contrast, PAE females differed in frequency rather than duration of social behavior: PF and C females showed a greater frequency of nonaffiliative compared to affiliative behaviors by the last 5-minute of testing, whereas PAE females showed no change in the type of social behavior engaged in over time. Together, these data suggest that PAE leads to altered social interactions, which supports evidence from the clinical literature indicating that children, and to a lesser extent, adults, with FASD also show dysfunctional social behaviors (Grant et al., 2004; Rasmussen et al., 2008; Wiedemann and Holsboer, 1987). Moreover, prenatal stress (Laviola et al., 2004; Lee et al., 2007), olfactory bulbectomy (a novel model of depression, Wang et al., 2007), and CMS (Tonissaar et al., 2008) all reduce social activity in the social interaction test, particularly with respect to affiliative behaviors. This is not surprising, given that the social interaction test is a sensitive measure of anxiety (Louis et al., 2008) and PAE, prenatal stress, and CMS all generally increase anxiety behaviors in adulthood (Austin et al., 2005; D’Aquila et al., 1994; Estanislau and Morato, 2005; Gabriel et al., 2006; Hofmann et al., 2005; Lee et al., 2007; Mineur et al., 2006; Osborn et al., 1998a,b; Richardson et al., 2006). Although the current study did not have a non-CMS cohort, we can hypothesize that CMS induced high levels of anxiety (see Hellemans et al., 2008), and therefore lower levels of social interaction in controls. That our study was able to detect significant differences among PAE, PF, and C animals despite low baseline levels of behavior in C rats provides evidence for the power of the CMS model in unmasking the adverse effects of prenatal alcohol exposure. Again, these findings have significant clinical implications, as reductions in social behavior or altered social interactions constitute another of the diagnostic criteria of major depression (American Psychiatric Association, 2000). Thus, these data support the hypothesis that PAE increases depressive- and anxiety-like behaviors. Future studies are required to investigate whether and how CMS modulates these findings.
Consistent with our previous finding that CMS does not increase basal CORT levels (Hellemans et al., 2008), the present data demonstrate that CMS exposure actually decreases basal CORT levels across the CMS period for all females and for PAE males. Moreover, PAE males showed significantly higher basal CORT levels compared to controls prior to CMS exposure; whereas PF females had elevated basal CORT levels both prior to and following CMS exposure compared to PAE and C females. It is not surprising that we were able to detect these subtle prenatal effects in the present study, as we had significantly more animals in this (n = 120) than in the previous study (n = 60). Nonetheless, it is important to highlight that CORT levels are all well within the basal range, and cannot be considered stress values. Furthermore, other than a pair-feeding effect in females, prenatal groups did not differ by the final day of CMS exposure, which supports previous findings that PAE does not lead to differential levels of the HPA hormones at rest. By contrast, our previous results, using littermates of animals tested in the present study, demonstrate significant HPA hyperactivity following exposure to the acute stress of testing on the elevated plus maze (Hellemans et al., 2008). Specifically, post-test CORT levels were significantly higher in CMS-exposed compared to non-CMS-exposed males and females across all prenatal groups. As exposure to CMS in the current study resulted in anhedonia, locomotor hyperactivity, “behavioral despair,” and altered social interactions in PAE compared to PF and C animals, these findings support our hypothesis that PAE increases sensitivity to stressors, leading to increased responsivity to stressors in adulthood and consequently, increased expression of depressive- and anxiety-like behaviors.
Taken together, the results of the current study support and extend our previous findings suggesting that the combination of PAE and CMS in adulthood increases neurobehavioral measures of anxiety and depression (Hellemans et al., 2008). While our previous data demonstrated that the anxiogenic effects of PAE are unmasked by adult exposure to CMS, we now show that this extends to other behavioral measures of anxiety / depression, such as anhedonia, behavioral despair, locomotor activity, and social interaction, and demonstrate that these neurobehavioral alterations occur in a sexually dimorphic manner. These findings emphasize the importance of examining sex differences in outcome in studies using animal models of depression, as this is critical in elucidating mechanisms underlying the significant disparity in the epidemiology of depression between men and women. More-over, these data highlight the value of employing a multi-dimensional test battery rather than single behavioral tests in order to explore and characterize fully the range of deficits that may occur following PAE. Currently, we are exploring whether the behavioral deficits induced by PAE and CMS are accompanied by central changes in key brain regions that underlie both stress and depression. Future studies will also explore whether these neurobehavioral alterations can be attenuated or reversed by antidepressant therapy, and again, whether these therapeutic interventions may differ by sex. Studies of this nature have the potential to identify novel therapeutic targets that may ultimately improve the quality of life for those who are affected by FASD and affective disorders.
ACKNOWLEDGMENTS
The authors would like to thank Peter Chen (Noldus) for his assistance with the behavioral software, as well as Dr. Matthew N. Hill for his assistance with developing the chronic mild stress paradigm. As well, we’d like to acknowledge Linda Ellis, Elisha Yoon, James Song, Alison Walzak, and Jessica Lam for their expert assistance in this study. This research was supported by NIH / NIAAA grant AA007789, and grants from the BC Ministry of Children and Family Development (through the UBC Human Early Learning Partnership) and the Canadian Institute for Advanced Research to JW, and awards from the Michael Smith Foundation for Health Research and IMPART (CIHR) to KGCH.
REFERENCES
- Alonso SJ, Castellano MA, Afonso D, Rodriguez M. Sex differences in behavioral despair: relationships between behavioral despair and open field activity. Physiol Behav. 1991;49:69–72. doi: 10.1016/0031-9384(91)90232-d. [DOI] [PubMed] [Google Scholar]
- Alonso SJ, Damas C, Navarro E. Behavioral despair in mice after pre-natal stress. J Physiol Biochem. 2000;56:77–82. doi: 10.1007/BF03179902. [DOI] [PubMed] [Google Scholar]
- Altemus M. Sex differences in depression and anxiety disorders: poten-tial biological determinants. Horm Behav. 2006;50:534–538. doi: 10.1016/j.yhbeh.2006.06.031. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, American Psychiatric Association. Task Force on DSM-IV . Diagnostic and Statistical Manual of Mental Dis-orders: DSM-IV-TR. 4th American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Armario A, Gavalda A, Marti O. Forced swimming test in rats: effect of desipramine administration and the period of exposure to the test on struggling behavior, swimming, immobility and defecation rate. Eur J Phar-macol. 1988;158:207–212. doi: 10.1016/0014-2999(88)90068-4. [DOI] [PubMed] [Google Scholar]
- Austin MP, Leader LR, Reilly N. Prenatal stress, the hypothalamic-pituitary-adrenal axis, and fetal and infant neurobehaviour. Early Hum Dev. 2005;81:917–926. doi: 10.1016/j.earlhumdev.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Barr HM, Bookstein FL, O’Malley KD, Connor PD, Huggins JE, Streissguth AP. Binge drinking during pregnancy asa predictor of psychiatric dis-orders on the Structured Clinical Interview for DSM-IV in young adult off-spring. Am J Psychiatry. 2006;163:1061–1065. doi: 10.1176/ajp.2006.163.6.1061. [DOI] [PubMed] [Google Scholar]
- Barron S, Gilbertson R. Neonatal ethanol exposure but not neonatal cocaine selectively reduces specific isolation-induced vocalization waveforms in rats. Behav Genet. 2005;35:93–102. doi: 10.1007/s10519-004-0859-2. [DOI] [PubMed] [Google Scholar]
- Barron S, Razani LJ, Gallegos RA, Riley EP. Effects of neonatal etha-nol exposure on saccharin consumption. Alcohol Clin Exp Res. 1995;19:257–261. doi: 10.1111/j.1530-0277.1995.tb01500.x. [DOI] [PubMed] [Google Scholar]
- Barros HMT, Ferigolo M. Ethopharmacology of imipramine in the forced-swimming test: gender differences. Neurosci Biobehav Rev. 1998;23:279–286. doi: 10.1016/s0149-7634(98)00029-3. [DOI] [PubMed] [Google Scholar]
- Binik Y, Sullivan M. Sudden swimming deaths: a psychomotor reinter-pretation. Psychophysiology. 1983;20:670–681. doi: 10.1111/j.1469-8986.1983.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for evealing antidepressant activity? Psychopharmacology. 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Borsini F, Volterra G, Meli A. Does the behavioral “despair” test mea-sure “despair”? Physiol Behav. 1986;38:385–386. doi: 10.1016/0031-9384(86)90110-1. [DOI] [PubMed] [Google Scholar]
- Brotto L, Alasdair M, Barr B, Gorzalka BB. Sex differences in forced-swim and open-field test behaviors after chronic adrminstration of melato-nin. Eur J Pharmacol. 2000;402:87–93. doi: 10.1016/s0014-2999(00)00491-x. [DOI] [PubMed] [Google Scholar]
- Bruner CA, Vargas I. The activity of rats in a swimming situation as a function of water temperature. Physiol Behav. 1994;55:21–28. doi: 10.1016/0031-9384(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Calil CM, Marcondes FK. The comparison of immobility time in exper-imental rat swimming models. Life Sci. 2006;79:1712–1719. doi: 10.1016/j.lfs.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Aitken MRF. ANOVA for the Behavioural Sciences. Lawrence Erlbaum Associates; Mahwah, NJ: 2005. [Google Scholar]
- Carneiro LM, Diogenes JP, Vasconcelos SM, Aragao GF, Noronha EC, Gomes PB, Viana GS. Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol. Neurotoxicol Teratol. 2005;27:585–592. doi: 10.1016/j.ntt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- Contreras CM, Lara-Morales H, Molina-Hernaández M, Saavedra M, Arrellín-Rosas G. An early lesion of the lateral septal nuclei produces changes in the forced swim test depending on gender. Prog Neuropsycho-pharmacol Biol Psychiatry. 1995;19:1277–1284. doi: 10.1016/0278-5846(95)00266-9. [DOI] [PubMed] [Google Scholar]
- Crawley J. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food.”. Proc Natl Acad Sci U S A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aquila PS, Brain P, Willner P. Effects of chronic mild stress on per-formance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–867. doi: 10.1016/0031-9384(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swim-ming test differentially produced by serotonergic and noradrenergic antide-pressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, Papadopoulou-Daifoti Z. Sex differences in behavioral, neuro-chemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience. 2004;126:849–857. doi: 10.1016/j.neuroscience.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Dursun I, Jakubowska-Dogru E, Uzbay T. Effects of prenatal exposure to alcohol on activity, anxiety, motor coordination, and memory in young adult Wistar rats. Pharmacol Biochem Behav. 2006;85:345–355. doi: 10.1016/j.pbb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Chazot PL. Models of anxiety: responses of rats to novelty in an open space and an enclosed space. Behav Brain Res. 2006;171:26–49. doi: 10.1016/j.bbr.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Estanislau C, Morato S. Prenatal stress produces more behavioral alterations than maternal separation in the elevated plus-maze and in the elevated T-maze. Behav Brain Res. 2005;163:70–77. doi: 10.1016/j.bbr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am J Psychiatry. 1998;155:552–554. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippo-campus can modulate open field and forced swim test behavior of proe-strous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–326. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol expo-sure. Pediatrics. 2007;119:e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Glavas MM, Ellis L, Weinberg J. Postnatal handling does not normalize hypothalamic corticotropin-releasing factor mRNA levels in animals prenatally exposed to ethanol. Brain Res Dev Brain Res. 2005;157:74–82. doi: 10.1016/j.devbrainres.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Yu CL, Osborn JA, Weinberg J. Prenatal ethanol exposure alters sensitivity to the effects of corticotropin-releasing factor (CRF) on behavior in the elevated plus-maze. Psychoneuroendocrinology. 2006;31:1046–1056. doi: 10.1016/j.psyneuen.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: interactive effects of dietary restriction and schedule of feeding. J Nutr. 1981;111:208–218. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: role of corticosterone. Alcohol Clin Exp Res. 2007;31:1598–1610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Yu WK, Weinberg J. Effects of mineralocorticoid and glu-cocorticoid receptor blockade on hypothalamic-pituitary-adrenal function in female rats prenatally exposed to ethanol. Alcohol Clin Exp Res. 2006;30:1916–1924. doi: 10.1111/j.1530-0277.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- Grant T, Huggins J, Connor P, Pedersen JY, Whitney N, Streissguth A. A pilot community intervention for young women with fetal alcohol spec-trum disorders. Community Ment Health J. 2004;40:499–511. doi: 10.1007/s10597-004-6124-6. [DOI] [PubMed] [Google Scholar]
- Gronli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM, Ursin R. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav. 2005;84:571–577. doi: 10.1016/j.physbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Halasz I, Aird F, Li L, Prystowsky MB, Redei E. Sexually dimorphic effects of alcohol exposure in utero on neuroendocrine and immune functions in chronic alcohol-exposed adult rats. Mol Cell Neurosci. 1993;4:343–353. doi: 10.1006/mcne.1993.1044. [DOI] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prena-tal alcohol exposure. Alcohol Clin Exp Res. 2006;30:2055–2064. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Hellemans KGC, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alco-hol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–175. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone ⁄ CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hofmann CE, Patyk IA, Weinberg J. Prenatal ethanol exposure: sex dif-ferences in anxiety and anxiolytic response to a 5-HT1A agonist. Pharmacol Biochem Behav. 2005;82:549–558. doi: 10.1016/j.pbb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neu-ropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Holsboer F, von Bardeleben U, Wiedemann K, Muller OA, Stalla GK. Serial assessment of corticotropin-releasing hormone response after dexa-methasone in depression. Implications for pathophysiology of DST nonsup-pression. Biol Psychiatry. 1987;22:228–234. doi: 10.1016/0006-3223(87)90237-x. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Conditions under which mean square ratios in repeated measurements designs have exact F-distributions. J Am Stat Assoc. 1970;65:1582–1589. [Google Scholar]
- Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev Psychopathol. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- Jezova D, Skultetyova I, Makatsori A, Moncek F, Duncko R. Hypothalamo-pituitary-adrenocortical axis function and hedonic behavior in adult male and female rats prenatally stressed by maternal food restric-tion. Stress. 2002;5:177–183. doi: 10.1080/1025389021000010512. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ullenland CN, Streissguth AP. Patterns of mal-formation in offspring of chronic alcoholic mothers. Lancent. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Shoemaker W. Opioid-dependent behaviors in infant rats: effects of prenatal exposure to ethanol. Pharmacol Biochem Behav. 1991;39:389–394. doi: 10.1016/0091-3057(91)90197-a. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kim CK, Giberson PK, Yu W, Zoeller RT, Weinberg J. Effects of pre-natal ethanol exposure on hypothalamic-pituitary-adrenal responses to chronic cold stress in rats. Alcohol Clin Exp Res. 1999;23:301–310. [PubMed] [Google Scholar]
- Kim CK, Osborn JA, Weinberg J. In: Stress reactivity in fetal alcohol syn-drome, in Fetal Alcohol Syndrome: From Mechanism to Prevention. Abel EL, editor. Vol. 336. CRC Press; Boca Raton, FL: 1996. [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adapta-tion to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J. Prenatal ethanol exposure alters the effects of gonadectomy on hypo-thalamic-pituitary-adrenal activity in male rats. J Neuroendocrinol. 2006;18:672–684. doi: 10.1111/j.1365-2826.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Sliwowska JS, Viau V, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic-pituitary-adrenal function across the estrous cycle. Alcohol Clin Exp Res. 2009;33:1075–1088. doi: 10.1111/j.1530-0277.2009.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugero KD, Bell ME, Bhatnagar S, Soriano L, Dallman MF. Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenalectomized rats: a glucocorticoid-metabolic-brain axis? Endocrinology. 2001;142:2796–2804. doi: 10.1210/endo.142.7.8250. [DOI] [PubMed] [Google Scholar]
- Laviola G, Rea M, Morley-Fletcher S, Di Carlo S, Bacosi A, De Simone R, Bertini M, Pacifici R. Beneficial effects of enriched environment on adolescent rats from stressed pregnancies. Eur J Neurosci. 2004;20:1655–1664. doi: 10.1111/j.1460-9568.2004.03597.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Blanton CA, Rivier C. Prenatal ethanol exposure alters the responsiveness of the rat hypothalamic-pituitary-adrenal axis to nitric oxide. Alcohol Clin Exp Res. 2003;27:962–969. doi: 10.1097/01.ALC.0000076120.67014.ED. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypotha-lamic-pituitary-adrenal axis of rats exposed to alcohol in utero: role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000;16:515–528. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- Louis C, Stemmelin J, Boulay D, Bergis O, Cohen C, Griebel G. Addi-tional evidence for anxiolytic-and antidepressant-like activities of saredu-tant (SR48968), an antagonist at the neurokinin-2 receptor in various rodent-models. Pharmacol Biochem Behav. 2008;89:36–45. doi: 10.1016/j.pbb.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Marino MD, Cronise K, Lugo JN, Jr, Kelly SJ. Ultrasonic vocalizations and maternal-infant interactions in a rat model of fetal alcohol syndrome. Dev Psychobiol. 2002;41:341–351. doi: 10.1002/dev.10077. [DOI] [PubMed] [Google Scholar]
- Matthews K, Wilkinson LS, Robbins TW. Repeated maternal separa-tion of preweanling rats attenuates behavioral responses to primary and conditioned incentives in adulthood. Physiol Behav. 1996;59:99–107. doi: 10.1016/0031-9384(95)02069-1. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in chil-dren with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson S, Riley E, Gramling L, Delis D, Jones K. Heavy prenatal alcohjol exposure with or withour physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Mattson S, Schoenfeld A, Riley E. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Sternberg WF, Kest B, Marek P, Liebeskind JC. Sex differ-ences in the antagonism of swim stress-induced analgesia: effects of gonad-ectomy and estrogen replacement. Pain. 1993;53:17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Darnaudery M, Koehl M, Casolini P, Van Reeth O, Maccari S. Prenatal stress in rats predicts immobility behavior in the forced swim test: effects of a chronic treatment with tianeptine. Brain Res. 2003;989:246–251. doi: 10.1016/s0006-8993(03)03293-1. [DOI] [PubMed] [Google Scholar]
- Murphy BE. Steroids and depression. J Steroid Biochem Mol Biol. 1991;38:537–559. doi: 10.1016/0960-0760(91)90312-s. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Ness JW, Franchina JJ. Effects of prenatal alcohol exposure on rat pups’ ability to elicit retrieval behavior from dams. Dev Psychobiol. 1990;23:85–99. doi: 10.1002/dev.420230109. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Tsuda A, Oguchi M, Ida Y, Tanaka M. Is immobility of rats in the forced swim test “behavioral despair?”. Physiol Behav. 1988;42:93–95. doi: 10.1016/0031-9384(88)90266-1. [DOI] [PubMed] [Google Scholar]
- Osborn JA, Kim CK, Steiger J, Weinberg J. Prenatal ethanol exposure differentially alters behavior in males and females on the elevated plus maze. Alcohol Clin Exp Res. 1998a;22:685–696. [PubMed] [Google Scholar]
- Osborn JA, Yu C, Gabriel K, Weinberg J. Fetal ethanol effects on ben-zodiazepine sensitivity measured by behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1998b;60:625–633. doi: 10.1016/s0091-3057(98)00039-2. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Griebel G. Antidepressant-like effects of CRF1 receptor antagonist SSR125543 in an animal model of depression. Eur J Pharmacol. 2004;497:49–53. doi: 10.1016/j.ejphar.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Keeney A, Hogg S. Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. Eur J Pharmacol. 2004;492:195–201. doi: 10.1016/j.ejphar.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou A, Gerozissis K, Prokopiou A, Bolaris S, Stylianopoulou F. Sex differences in the effects of neonatal handling on the animal’s response to stress and the vulnerability for depressive behaviour. Behav Brain Res. 2002;129:131–139. doi: 10.1016/s0166-4328(01)00334-5. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Patchev V, Almeida O. Gender specificity in the neural regulation of the response to stress: new leads from classical paradigms. Mol Neurobiol. 1998;16:63–77. doi: 10.1007/BF02740603. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978a;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol. 1978b;51:291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- Porsolt R, Le Pichon M, Jalfre M. Depression: a new animal model sen-sitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The Open field as a paradigm to measure the effects of drugs on anxiety-like behavuirs: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two-and six-month-old infants’ adrenocortical reac-tivity to stress. J Pediatr Psychol. 1996;21:833–840. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall C, Becker H, Middaugh L. Effect of prenatal ethanol exposure on activity and shuttle avoidance behavior in adult C57 mice. Alcohol Drug Res. 1986;6:351–360. [PubMed] [Google Scholar]
- Rasmussen C, Talwar V, Loomes C, Andrew G. Brief report: lie-telling in children with fetal alcohol spectrum disorder. J Pediatr Psychol. 2008;33:220–225. doi: 10.1093/jpepsy/jsm069. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinol-ogy. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Riley EP. The long-term behavioral effects of prenatal alcohol exposure in rats. Alcohol Clin Exp Res. 1990;14:670–673. doi: 10.1111/j.1530-0277.1990.tb01225.x. [DOI] [PubMed] [Google Scholar]
- Rivier C, Imaki T, Vale W. Prolonged exposure to alcohol: effect on CRF mRNA levels, and CRF-and stress-induced ACTH secretion in the rat. Brain Res. 1990;520:1–5. doi: 10.1016/0006-8993(90)91685-a. [DOI] [PubMed] [Google Scholar]
- Rockman GE, Markert LE, Delrizzo M. Effects of prenatal ethanol exposure on ethanol-induced locomotor activity in rats. Alcohol. 1989/0;6:353–356. doi: 10.1016/0741-8329(89)90003-7. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:339–344. doi: 10.1111/j.1530-0277.1998.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Schechter M, Chance W. Non-specificity of “behavioral despair” as an animal model of depression. Eur J Pharmacol. 1979;60:139–142. doi: 10.1016/0014-2999(79)90212-7. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on develop-ment: perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- Schneider T, Popik P. Increased depressive-like traits in an animal model of premenstrual irritability. Horm Behav. 2007;51:142–148. doi: 10.1016/j.yhbeh.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Sikiric P, Separovic J, Buljat G, Anic T, Stancic-Rokotov D, Mikus D, Marovic A, Prkacin I, Duplancic B, Zoricic I, Aralica G, Lovric-Bencic M, Ziger T, Perovic D, Rotkvic I, Mise S, Hanzevacki M, Hahn V, Seiwerth S, Turkovic B, Grabarevic Z, Petek M, Rucman R. The antidepressant effect of an antiulcer pentadecapeptide BPC 157 in Porsolt’s test and chronic unpredictable stress in rats. A comparison with antidepressants. J Physiol Paris. 2000;94:99–104. doi: 10.1016/s0928-4257(00)00148-0. [DOI] [PubMed] [Google Scholar]
- Sliwowska JS, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J. Effects of prenatal ethanol exposure on basal hypothalamic-pituitary-adre-nal activity and hippocampal 5HT1A receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology. 2008;33:1111–1123. doi: 10.1016/j.psyneuen.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slone JL, Redei EE. Maternal alcohol and adrenalectomy: asynchrony of stress response and forced swim behavior. Neurotoxicol Teratol. 2002;24:173–178. doi: 10.1016/s0892-0362(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Stein EJ, da Silveira Filho NG, Machado DC, Hipolide DC, Barlow K, Nobrega JN. Chronic mild stress induces widespread decreases in thyroid hormone alpha1 receptor mRNA levels in brain–reversal by imipra-mine. Psychoneuroendocrinology. 2009;34:281–286. doi: 10.1016/j.psyneuen.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL. Final report to the Centers for Disease Control and Prevention on Grant No. RO4/CCR008515 (Tech. Report No. 96-06) University of Washington, Fetal Alcohol and Drug Unit; Seattle, WA: 1996. Understanding the occurence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE) [Google Scholar]
- Taylor AN, Branch BJ, Kokka N, Poland RE. Neonatal and long-term neuroendocrine effects of fetal alcohol exposure. Monogr Neural Sci. 1983;9:140–152. doi: 10.1159/000406886. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Liu SH, Kokka N. Long-term effects of fetal ethanol exposure on pituitary-adrenal response to stress. Pharmacol Bio-chem Behav. 1982;16:585–589. doi: 10.1016/0091-3057(82)90420-8. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Van Zuylen JE, Redei E. Maternal alcohol con-sumption and stress responsiveness in offspring. Adv Exp Med Biol. 1988;245:311–317. doi: 10.1007/978-1-4899-2064-5_25. [DOI] [PubMed] [Google Scholar]
- Tonissaar M, Mallo T, Eller M, Haidkind R, Koiv K, Harro J. Rat behavior after chronic variable stress and partial lesioning of 5-HT-ergic neurotransmission: effects of citalopram. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:164–177. doi: 10.1016/j.pnpbp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste prefer-ence for glucose and saccharin solutions. Science. 1967;156:942–943. doi: 10.1126/science.156.3777.942. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. A fostering ⁄ crossfostering analysis of the effects of prena-tal ethanol exposure in a liquid diet on offspring development and behavior in rats. Neurotoxicol Teratol. 1989;11:115–120. doi: 10.1016/0892-0362(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, Nabeshima T. Behavioural and neurochemical features of olfactory bulbectomized rats resembling depression with comorbid anxiety. Behav Brain Res. 2007;178:262–273. doi: 10.1016/j.bbr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Effects of ethanol and maternal nutritional status on fetal development. Alcohol Clin Exp Res. 1985;9:49–55. doi: 10.1111/j.1530-0277.1985.tb05049.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Hyperresponsiveness to stress: differential effects of prena-tal ethanol on males and females. Alcohol Clin Exp Res. 1988;12:647–652. doi: 10.1111/j.1530-0277.1988.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol effects: sex differences in offspring stress responsiveness. Alcohol. 1992a;9:219–223. doi: 10.1016/0741-8329(92)90057-h. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol exposure alters adrenocortical response to predictable and unpredictable stressors. Alcohol. 1992b;9:427–432. doi: 10.1016/0741-8329(92)90043-a. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Neuroendocrine effects of prenatal alcohol exposure. Ann N Y Acad Sci. 1993;697:86–96. doi: 10.1111/j.1749-6632.1993.tb49925.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Gallo PV. Prenatal ethanol exposure: pituitary-adrenal activity in pregnant dams and offspring. Neurobehav Toxicol Teratol. 1982;4:515–520. [PubMed] [Google Scholar]
- Weinberg J, Nelson LR, Taylor AN. In: Hormonal effects of fetal alcohol exposure, in Alcohol and Brain Development. West JR, editor. Oxford University Press; New York: 1986. pp. 310–342. [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Taylor AN, Gianoulakis C. Fetal ethanol exposure: hypo-thalamic-pituitary-adrenal and beta-endorphin responses to repeated stress. Alcohol Clin Exp Res. 1996;20:122–131. doi: 10.1111/j.1530-0277.1996.tb01054.x. [DOI] [PubMed] [Google Scholar]
- Weiss EL, Longhurst JG, Mazure CM. Childhood sexual abuse as a risk factor for depression in women: psychosocial and neurobiological correlates. Am J Psychiatry. 1999;156:816–828. doi: 10.1176/ajp.156.6.816. [DOI] [PubMed] [Google Scholar]
- West AP. Neurobehavioral studies of forced swimming: the role of learning and memory in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:863–877. doi: 10.1016/0278-5846(90)90073-p. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Ter Horst GJ, Roos MH, Kuipers SD, Trentani A, den Boer JA. Gender-specific effects of social housing in rats after chronic mild stress exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:21–30. doi: 10.1016/s0278-5846(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Wiedemann K, Holsboer F. Plasma dexamethasone levels in depression: oral vs. i.v. administration. Psychiatry Res. 1987;20:83–85. doi: 10.1016/0165-1781(87)90126-0. [DOI] [PubMed] [Google Scholar]
- Wilcoxon J, Kuo A, Disterhoft J, Redei E. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Mol Psychiatry. 2005;10:961–971. doi: 10.1038/sj.mp.4001694. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Redei EE. Maternal glucocorticoid deficit affects hypotha-lamic-pituitary-adrenal function and behavior of rat offspring. Horm Behav. 2007;51:321–327. doi: 10.1016/j.yhbeh.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Animal models of depression: validity and applications. Adv Biochem Psychopharmacol. 1995;49:19–41. [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsy-chobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedo-nia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]