Abstract
Heart failure is associated with generalized insulin resistance. Moreover, insulin resistant states such as type 2 diabetes and obesity increases the risk of heart failure even after adjusting for traditional risk factors. Insulin resistance or type 2 diabetes alters the systemic and neurohumoral milieu leading to changes in metabolism and signaling pathways in the heart that may contribute to myocardial dysfunction. In addition, changes in insulin signaling within cardiomyocytes develop in the failing heart. The changes range from activation of proximal insulin signaling pathways that may contribute to adverse left ventricular remodeling and mitochondrial dysfunction to repression of distal elements of insulin signaling pathways such as forkhead (FOXO) transcriptional signaling or glucose transport which may also impair cardiac metabolism, structure and function. This article will review the complexities of insulin signaling within the myocardium and ways in which these pathways are altered in heart failure or in conditions associated with generalized insulin resistance. The implications of these changes for therapeutic approaches to treating or preventing heart failure will be discussed.
Subject Terms: Heart Failure, Hypertrophy, Remodeling, Metabolic Syndrome, Insulin action, insulin resistance, insulin receptor, cardiac hypertrophy
Cardiovascular disease (CVD) remains the leading cause of death globally in developed and developing economies 1. The morbidity from CVD is broad-based and includes consequences of atherosclerosis, hypertension leading to ischemic heart disease, stroke and heart failure. Heart failure is a major cause of death in industrialized nations and exceeds the mortality of most types of cancers. It is estimated that about 5.1 million patients are affected by heart failure in the United States. The annual incidence is approximately 670,000 cases, with about half of these patients dying within 5 years after the initial diagnosis 2. The associated annual costs are about $34.4 billion, resulting in an enormous economic burden 3. Thus, heart failure once it develops has a dismal prognosis that has not significantly improved despite advances in pharmacological therapies. Much work has been done to examine diverse signaling pathways that contribute to heart failure, pathological cardiac hypertrophy and adverse left ventricular remodeling and have been reviewed extensively 4, 5. A relatively understudied signaling pathway that could represent an important contributor to the pathophysiology of heart failure is insulin signaling, which will be the subject of this review.
The connection between abnormal insulin signaling and heart failure arises in part from the established epidemiological association between obesity, type 2 diabetes, insulin resistance and heart failure. Diabetes increases the risk for ischemic heart disease as evidenced by a two- to four-fold increase in myocardial infarction in diabetic patients compared to non-diabetics and is associated with a poorer prognosis 6. In addition, coexistence of ischemic cardiomyopathy and diabetes accelerate the progression to heart failure 7. Numerous epidemiologic studies identified insulin resistance and diabetes as risk factors for the development of heart failure independent of myocardial ischemia. The Framingham Heart Study revealed that diabetes independently increases the risk of heart failure by about 2-fold in men and about 5-fold in women compared with age-matched control subjects, independent of blood pressure and serum cholesterol levels 8, 9. Furthermore, a longitudinal study in the same cohort identified obesity as a risk factor for the future incidence of heart failure in both men and women 10. These data are supported by the United Kingdom Prospective Diabetes Study (UKPDS), which reported that the risk for developing heart failure increases by 16% for every 1% increase in serum HbA1c concentrations 11. Similar results reporting an association between diabetes and the incidence of heart failure have been provided by the Cardiovascular Health Study 12, the New Haven cohort of the Established Populations for Epidemiological Studies in the Elderly 13, post hoc analyses of clinical trials14 and have been reviewed comprehensively15.
In contrast, a number of studies reported a significant increase in survival in obese subjects in the context of heart failure 16, 17 and following myocardial infarction after adjusting for age, severity of illness, and gender 18. This resulted in the term “obesity paradox” which has been previously reviewed 19, 20. Although obesity and type 2 diabetes are well-described and commonly accepted risk factors for the development of cardiovascular disease, these studies suggest a protective role of obesity when cardiac decompensation occurs. Given that cachexia is independently associated with a worse prognosis in heart failure, the possibility exists that the obesity paradox could in part reflect the adverse consequences of cachexia, which would lower body weight. These lower body weight subjects with heart failure were reported for example to have higher circulating concentrations of tumor necrosis factor α (TNFα) 21, implying an adverse consequence of systemic inflammation in these subjects. In another cohort, the potential benefit of the obesity paradox in heart failure was seen in the elderly and those with acute heart failure and was not present in subject in diabetes 21. Thus many of the interactions between insulin resistance obesity and heart failure might differ between acute and chronic heart failure. The neurohumoral changes in obesity that might confer a survival benefit in acute heart failure are currently poorly understood. Moreover, the absence of a benefit in subjects with type 2 diabetes suggests that long-standing insulin resistance, hyperinsulinemia or hyperglycemia might offset any survival advantages that can be attributed to the “obesity paradox”. Thus, the pathophysiology of the increased risk of heart failure in insulin resistant states is complex and multifactorial, and the potential impact of generalized insulin resistance and hyperinsulinemia per se remains incompletely understood.
Moreover, studies in humans and animal models have revealed that heart failure is associated with generalized insulin resistance 22. Metabolic abnormalities observed in patients with advanced heart failure resulting in catabolism, cardiac cachexia and insulin resistance might correlate with decreased survival23. Although it is has not been definitively shown that insulin resistance in the context of heart failure directly contributes to a worse prognosis, a recent report suggested that ventricular unloading leads to improvement in whole body insulin sensitivity 24. This review will therefore explore the current state of knowledge linking generalized insulin resistance with heart failure with a focus on the physiological and pathophysiological roles of insulin signaling within the heart, the cardiac adaptations to generalized insulin resistance and the interaction between systemic insulin resistance and changes in myocardial insulin signaling, which may inform the pathophysiology of heart failure.
Insulin Signaling Pathways
Insulin mediates its signaling upon ligand binding to the insulin receptor, which is a member of the tyrosine kinase family of receptors and is closely related to and partially homologous with the receptor for insulin-like growth factor −1 (IGF-1). Upon ligand binding, insulin receptors are autophosphorylated, which in turn increases its tyrosine kinase activity for other substrates such as insulin receptor substrates (IRS) proteins (Figure 1). The activated insulin receptor interacts with IRS proteins and other binding partners to activate a network of intracellular signaling pathways including PI3K/Akt and MAP Kinases such as ERK 25, 26. As summarized in Figure 1, an essential initial step in IR/IGF-1R receptor signaling is activation of IRS proteins. IRS1 and IRS2 are the two most abundant IRS isoforms in the heart and are required for insulin-mediated activation of PI3K. PI3K consists of a p110 catalytic subunit and a p85 regulatory subunit, which catalyzes the generation of the lipid product phosphatidylinositol (3,4,5)-triphosphate (PIP3). This results in phosphoinositide dependent kinase 1 (PDPK1) mediated activation of the kinase Akt (protein kinase B). Three members of the Akt family have been identified. Akt1 and Akt2 represent the most abundant Akt isoforms in the heart. Akt mediates a variety of cellular processes by phosphorylating proteins involved in autophagy and members of the forkhead transcription factor (FOXO) family which inhibits nuclear translocation and transcriptional activity27 of promoters that encode pro-apoptotic signaling molecules, such as members of the BCL-2 family. The isoform-specific contribution of Akt on the regulation of these processes is incompletely understood. However, Akt1 but not Akt2 signaling has been identified to mediate somatic growth28, 29. Furthermore, it has been shown that Akt2 is required for insulin-stimulated glucose uptake and metabolism in isolated cardiomyocytes and this effect is independent of Akt130. Together, this suggests a predominant role for Akt1 in the regulation of somatic growth and for Akt2 in the regulation of metabolism. Inhibition of glycogen synthase kinase 3β (GSK-3β) by Akt-mediated phosphorylation promotes cardiac hypertrophy 31 and glycogen synthesis. In addition, Akt mediates phosphorylation of mechanistic target of Rapamycin (mTOR), a protein complex regulating somatic growth and protein synthesis. mTOR consists of two distinct complexes, mTORC1 and mTORC232. Among other components, mTOR Complex 1 (mTORC1) is composed of Raptor (regulatory-associated protein of mTOR)33. Rictor (Rapamycin-insensitive companion of mTOR) is part of mTOR Complex 2 (mTORC2)34. mTOR mediated phosphorylation of 70 kDa ribosomal protein S6 kinase (p70S6K) and 4E-binding protein 1 (4E-BP1)35 results in its dissociation from eukaryotic translation initiation factor 4E (eIF4E) and activation of cap-dependent translation36, which is attenuated by the mTOR inhibitor Rapamycin.
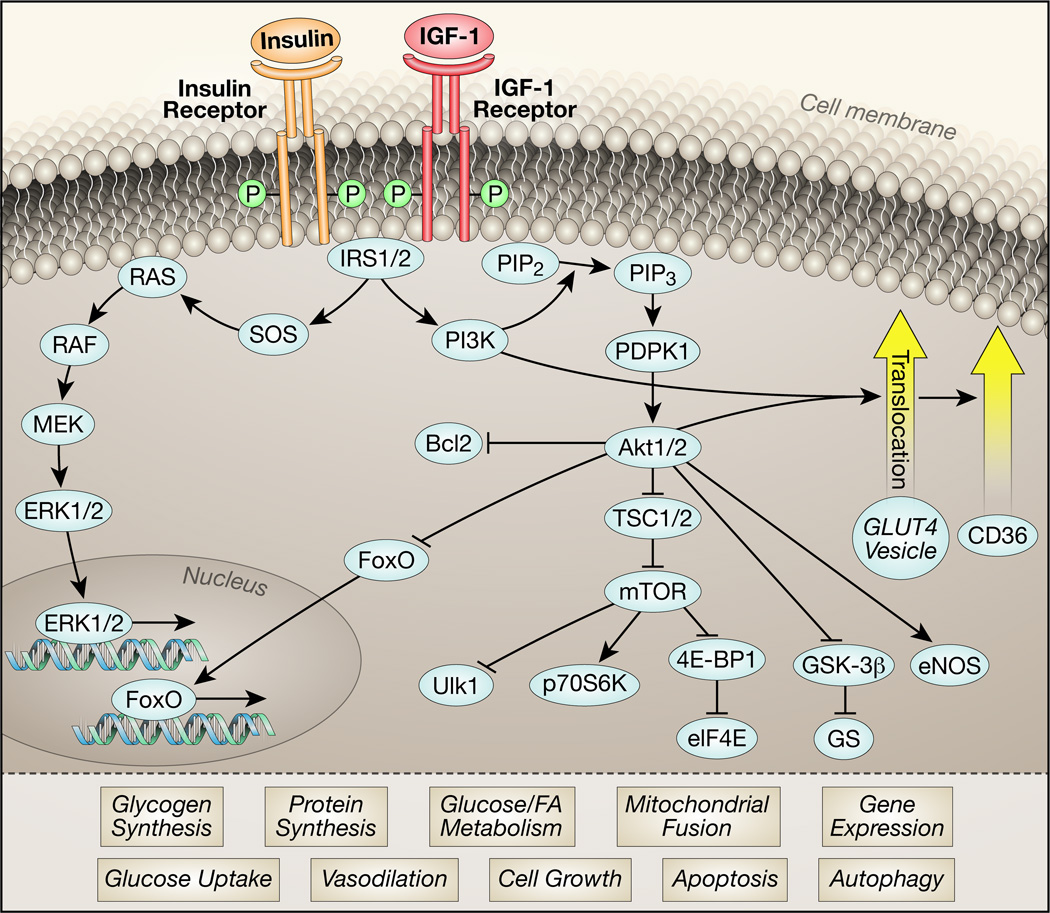
Figure 1. Schematic of insulin signaling pathway.
Simplified summary of key elements involved in insulin signal transduction that ultimately regulate multiple cellular processes. Upon binding to its ligand, insulin and IGF-1 receptors undergo autophosphorylation, which increases their tyrosine kinase activities. Tyrosine phosphorylation and activation of the docking proteins insulin receptor substrates 1 and 2 (IRS1/2), engages regulatory subunits of the phosphatidylinositol-3-kinase (PI3K) that generates phosphatidylinositol 3,4,5 tris phosphate (PIP3) from phosphatidylinositol 3,4, bis phosphate (PIP2). The serine threonine kinases phosphoinositide dependent protein kinase 1 (PDPK1) and Akt1 or Akt2 bind to PIP3 by their PH domains. PDPK1 phosphorylates Akt on Thr 308 and mTORC2 phosphorylates Akt1/2 on Ser 473. Once activated, Akt in turn phosphorylates multiple targets of which a subset is shown. Phosphorylation of these targets induces pleiotropic cellular responses: Bcl2 phosphorylation inhibits apoptosis, FOXO protein phosphorylation promotes nuclear exclusion, thereby repressing the expression of FOXO-regulated transcripts that mediate autophagy and apoptosis. TSC1/2 phosphorylation promotes mTOR activation, which increases mRNA translation, promotes protein synthesis, cell growth, mitochondrial fusion and also inhibits autophagy. Phosphorylation of glycogen synthase kinase (GSK) removes the repression of glycogen synthase, which in concert with increased availability of glucose promotes glycogen synthesis. Phosphorylation of endothelial nitric oxide synthase (NOSIII) or eNOS by Akt increases the generation of nitric oxide (NO) to promote vasodilation. Akt phosphorylation mediates the translocation of vesicles containing the GLUT4 glucose transporter in part by phosphorylating downstream targets such as AS160 (not shown), leading to increased glucose transport following insertion into the plasma membrane. Akt also mediates in part, translocation of the fatty acid translocase CD36. Insulin signaling also promotes activation of the mitogen activated protein kinases (ERK1/2) to increase the expression of various genes.
In addition to activating the Akt / mTOR signaling cascade and mediating GLUT4 translocation, insulin activates the mitogen-activated protein kinase (MAPK) / extracellular regulated kinase (ERK) pathway. This pathway involves activation of a cascade including Raf, MEK and ERK kinases, resulting in nuclear translocation of ERK and phosphorylation of transcription factors such as p62TCF, initiating a transcriptional program that mediates cellular differentiation and proliferation37. A variety of genetically altered mouse models with perturbed insulin / IGF-1 signaling has been utilized to investigate insulin signaling in the heart. Table 1 summarizes the characteristics of previously described transgenic models under basal conditions and superimposed pathological stressors.
Table 1.
Rodent models for perturbed insulin / IGF-1 signaling
| Basal phenotype | Phenotype following superimposed pathological stressor |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signaling molecule |
Genetic Manipul ation / Treatme nt |
Cardiac size |
Contractile Function |
Notes | Ref | Stressor | Cardiac size |
Contractile Function |
Notes | Ref |
| IR/IGF-1R | ||||||||||
| IR | CKO | ↓ | = / ↓ |
age-dependent mitochondrial dysfunction |
42, 73, 78, 137 | ISO | = | ↓ | catecholamine- mediated injury ↑ |
138 |
| AC | = | ↓ | susceptibility to heart failure ↑ |
137 | ||||||
| MI | = | ↓ | left ventricular dysfunction post MI ↑ |
139 | ||||||
| STZ | ↓ | = | mitochondrial dysfunction ↑ / cardiac efficiency ↓ |
162 | ||||||
| IPC | efficacy of IPC to reduce infarct size ↑ |
163 | ||||||||
| CKO het | = | = | 114 | AC | ↓ | ↑ | 114 | |||
| IGF-1R | CKO | = | = | 78 | ||||||
| MKO | ↑ | = | 164 | |||||||
| IR/IGF- 1R DKO |
MKO | ↓ | ↓↓ | 164 | ||||||
| IRS | ||||||||||
| IRS1 | CKO | ↓ | = | 76 | ||||||
| IRS2 | CKO | = | = | 76 | ||||||
| IRS1/2 DKO |
CKO | = / ↓ | ↓↓ | 43, 150 | ||||||
| MKO | = | ↓↓ | 165 | |||||||
| GKO | ND | ND | embryonic lethal? | 166 | ||||||
| CKOi | ↓ | ↓↓ | 151 | |||||||
| IRS3 | GKO | = | 167 | |||||||
| IRS4 | GKO | NR | 168 | |||||||
| PI3K | ||||||||||
| p85 | p85α MKO p85β GKO |
↓ | = | 169 | ||||||
| p110α | dn | ↓ | = | 170, 171 | AC | = | ↓ | 170, 172 | ||
| MI | = | ↓ | 173 | |||||||
| DCM | ↓ | survival following cross ↓ | 172 | |||||||
| ca | ↑ | = | 171 | AC | = | ↑ | 172 | |||
| MI | = | ↑ | 173 | |||||||
| DCM | ↑ | survival following cross ↑ | 172 | |||||||
| p110γ | GKO | = | ↑ | 174 | AC | ↓ | 175 | |||
| ISO | ↑ | 174 | ||||||||
| PDK1 | ||||||||||
| PDK1 | CKO | ↓↓ | β1-adrenergic receptor ↓ |
176, 177 | ||||||
| MKO | ↓ | ↓↓ | Dilated cardiomyopathy |
178 | ||||||
| Akt | ||||||||||
| Akt1 | GKO | = | = | 79, 136 | AC | ↑ | ↓ | 136 | ||
| GKO het | = | 114 | AC | ↑ | 114 | |||||
| Akt2 | GKO | = / ↑ | = / ↓ |
age-dependent hypertrophy and contractile dysfunction |
30, 79, 179 | AC | = | = | 30 | |
| ISO | ↑ | = | 179 | |||||||
| MI | ↓ | infarct size ↑ | 180 | |||||||
| Akt3 | COE | ↑ | ↓ | age-dependent contractile dysfunction |
181 | |||||
| GKO | = | 182 | ||||||||
| kdAkt | kd | = | = | 183 | ||||||
| caAkt | ca | ↑ | ↓ | age-dependent contractile dysfunction |
183 | |||||
| myrAkt | ↑ | = | 184 | MI | infarct size ↓ | 184 | ||||
| GSK-3β | ||||||||||
| GSK-3β | ca | = | 185 | AC | ↓ | 185 | ||||
| dn | ↑ | = | 186 | AC | = | ↑ | 186 | |||
| mTOR | ||||||||||
| mTOR | Rapamyc in |
= | = | 187, 188 | AC | ↓ | = / ↑ | 187, 188 | ||
| MI | ↓ | ↑ | LV remodelling and cell death ↓ |
189 | ||||||
| CKO (mTOR) |
↓ | ↓ ↓ | embryonic lethal | 190 | ||||||
| CKOi (mTOR) |
↓ ↓ | 191 | AC | ↓ | ↓↓ | rapid development of heart failure post AC |
191 | |||
| CKOi (raptor) |
↓ | autophagy ↑, Glucose oxidation ↑, Palmitate oxidation ↓ |
192 | AC | ↓ | ↓↓ | rapid development of heart failure post AC |
192 | ||
| CKOi (mTOR) |
= | ↓ | Glucose oxidation ↑, Palmitate oxidation ↓ |
193 | ||||||
| ca | = | = | 194 | |||||||
| kd | = | ↓ | 194 | ISO | = | = | hypertrophic response similar to WT |
194 | ||
| p70S6K | ||||||||||
| p70S6K1 | TG | ↑ | = | mutant with higher basal activity |
195 | AC | = | 195 | ||
| kd | = | = | 195 | |||||||
| GKO | = | 195 | AC | = | 195 | |||||
| p70S6K2 | kd | = | = | 195 | ||||||
| GKO | = | 195 | AC | = | 195 | |||||
| p70S6K1 /2 |
GKO | = | 195 | AC | = | 195 | ||||
| GLUT4 | ||||||||||
| GLUT4 | CKO | ↑ | = | 123, 196 | ||||||
| MKO (α-actin- Cre) |
↑ | ↓ | age-dependent contractile dysfunction |
197, 198 | ||||||
Changes are expressed compared to WT controls same treatment; ↑ increased; ↓ decreased; = no difference observed.
AC, aortic constriction; ca, constitutively active mutant; CKO het, cardiac-specific heterozygous knockout (αMHC-Cre); CKO, cardiac-specific knockout (αMHC-Cre); CKOi, inducible cardiac-specific knockout; COE, cardiac-specific overexpression; DCM, cross to transgenic mouse model of dilated cardiomyopathy; dn, dominant-negative mutant; GKO het, global heterozygous knockout; GKO, global knockout; IPC, ischemic preconditioning; ISO, Isoproterenol treatment; kd, kinase-deficient mutant; MI, Myocardial infarction; MKO, muscle-specific knockout (MCK-Cre, unless otherwise indicated); myrAkt, myristoylated Akt; STZ, streptozotocin treatment; TG, transgenic model; ND, not determined.
Insulin receptors are ubiquitously expressed and are highly abundant in tissues of the cardiovascular system such as the heart and endothelial cells 38. Given its identification as a hormone that is intimately involved in fuel metabolic homeostasis, much of the work on insulin signaling has focused on its impact on tissues such as adipose, liver, skeletal muscle and brain and the mechanisms by which insulin regulates glucose uptake, lipogenesis and hepatic glucose utilization for example 39–41 . Subsequent studies have revealed that insulin signaling by activating cell survival pathways may play an important role in the regulation of apoptosis and autophagy and cellular growth 42, 43. Thus a brief review of the physiological role of insulin signaling in the heart and vasculature is warranted to set the stage for a deeper discussion of the pathophysiological consequences of insulin signaling in heart failure.
Insulin Signaling in the Vasculature
The major cell types in the vasculature in which insulin signaling has been evaluated are endothelial and vascular smooth muscle cells. In endothelial cells, insulin signaling via PI3K and Akt leads to activation of endothelial nitric oxide synthase. Nitric oxide release not only promotes vasorelaxation, but may also activate anti-inflammatory and anti-atherosclerotic pathways. As such, impaired endothelial insulin signaling has been suggested to contribute to obesity and diabetes-related vascular dysfunction that may contribute to hypertension and accelerated atherosclerosis 44, 45. It is important to note that whereas there is broad consensus that impaired nitric oxide availability will contribute to vascular pathology in insulin resistant states, impairment of eNOS activity may occur independently of defects in insulin signaling per-se 46–48. Another important consideration in understanding impaired endothelial insulin signaling in the context of insulin resistant states is the concept of imbalanced insulin signaling, whereby peripheral hyperinsulinemia, leads to activation of certain branches of the insulin signaling pathway, while others remain inhibited. For example, in certain circumstances, whereas insulin signaling to PI3K-Akt and eNOS might be impaired in endothelial cells, activation of MAPK signaling pathways leading to induction of endothelin1 expression might remain active and indeed be activated by ambient hyperinsulinemia, thereby leading to increased vasoconstriction 49–51. Similarly, in vascular smooth muscle cells, the hyperinsulinemia that accompanies insulin resistant states has been suggested to be a contributor to vascular smooth muscle cell hyperplasia, which may promote intimal injury and vascular stenosis 52. The role of impaired insulin signaling in the coronary vasculature and the pathophysiology of heart failure in insulin resistant states is incompletely understood. However, subjects with insulin resistance and type 2 diabetes were shown to have significantly impaired endothelial-dependent coronary vasodilation 53. Thus the possibility exists that impaired coronary vasodilation in insulin resistant states could contribute to impaired myocardial contractile reserve in heart failure, particularly in subjects with insulin resistance.
Insulin Signaling and the Regulation of Substrate Metabolism in Cardiomyocytes
Like skeletal muscle, activation of insulin signaling pathways in cardiomyocytes leads to an increase in glucose uptake by promoting the translocation of GLUT4 containing vesicles to the plasma membrane 54. These vesicles ultimately fuse with the plasma membrane and expose GLUT4 proteins on the cellular surface. This process involves the modulation of filamentous actin networks, SNARE proteins, and small GTPases of the Rab family55. Insulin signaling mediates GLUT4 translocation at multiple checkpoints. Recently identified mechanisms include Akt-mediated phosphorylation of Akt substrate of 160 kDa (AS160), a GTPase-activating protein for the Rab family of small G proteins. Phosphorylation of AS160 in response to insulin regulates its interaction with 14-3-3 and inhibits AS160 GTPase activity. This results in increased Rab activity and translocation of GLUT4 vesicles to the plasma membrane 56, 57. It has also been shown that selective activation of Akt might be sufficient to stimulate GLUT4 translocation to an extent which is similar to insulin58. However, Akt-independent mechanisms have also been identified 57. In perfused hearts or in vivo, the increase in glucose utilization (uptake and oxidation) that is mediated by insulin signaling is associated with reduced fatty acid oxidation on the basis of the Randle phenomenon 42, 59.
Transmembrane uptake of fatty acids (FA) in cardiomyocytes is mediated in part by fatty acyl translocases of the FA transporter family and CD36 60. FAs are then converted to FA-CoA by the enzyme Acyl-CoA synthase (ACS) and may be stored in the form of triglycerides. However, excess lipids may also be shunted into non-oxidative pathways which results in the generation of toxic lipid intermediates. This eventually results in mitochondrial dysfunction61, perturbed cellular signaling62, incomplete FA oxidation and increased apoptosis63, a phenomenon called lipotoxicity (reviewed in 64). Lipotoxicity has been extensively studied in animal models of obesity and type 2 diabetes, including ob/ob, db/db mice (models for obesity and insulin resistance based on leptin deficiency or resistance, respectively) and Zucker fatty rats65. Importantly though, insulin also promotes the translocation of the fatty acid translocase CD36, which may increase FA uptake that may be partitioned towards lipid synthesis and storage 60. This insulin-mediated increase and translocation of CD36 is transduced by PI3K/Akt signaling pathways66 and may contribute to increased FA oxidation and increased myocardial oxygen consumption (mVO2) in hyperinsulinemic states that is often associated with increased availability of triglycerides and FA to the heart. Increased FA utilization is associated with decreased cardiac efficiency (cardiac work / mVO2). Studies in ob/ob and db/db mice suggest that mitochondrial uncoupling and increased reactive oxygen species (ROS) parallel the increase in FA oxidation67, 68. Mitochondria-derived ROS can activate uncoupling proteins (UCPs)69 allowing protons to bypass the ATP synthase of the electron transport chain and decrease coupling of mitochondrial ATP production to O2 consumption. As a consequence, fatty acid oxidation further increases, which subsequently decreases cardiac efficiency. Similar to these studies performed in animal models, a recent study using human myocardial samples from type 2 diabetics showed mitochondrial dysfunction and increased oxidative stress70. Furthermore, increased mVO2 along with increased FA oxidation was reported for young women with obesity, insulin resistance and increased body mass index71. Increased ROS production and mitochondrial uncoupling was not observed in rodent models of type 1 diabetes72. This suggests that ROS mediated mitochondrial uncoupling might not be attributable to hyperglycemia per se, but is more likely a consequence of generalized insulin resistance and type 2 diabetes. Interestingly, hearts from cardiomyocyte-specific insulin receptor knockout mice show increased ROS generation and mitochondrial uncoupling, in the absence of hyperglycemia73.
Insulin may also directly regulate mitochondrial metabolism by promoting mitochondrial fusion via a mechanism that involves the induction of OPA1 74. Short-term Insulin-mediated Akt activation may also activate pro-survival signaling pathways related to Akt inhibition of apoptotic signaling 75. Studies in gene targeted mice with cardiomyocyte-restricted loss of insulin receptors or IRS1 proteins have also suggested that insulin signaling may play an important trophic role within the heart 42. Thus, loss of insulin receptors or IRS-1 is associated with a reduction in cardiomyocyte size with little hemodynamic consequences in non-stressed animals 42, 76. In addition insulin/IGF-1 or IRS1 or IRS2 signaling pathways appear to be required for physiological cardiac hypertrophy in response to exercise 76–78. Insulin signaling also regulates mitochondrial oxidative capacity. Thus mice with genetic deletion of the insulin receptor, or IRS proteins develop reduced mitochondrial oxidative capacity via partially understood mechanisms that may involve repressed expression of nuclear-encoded mitochondrial genes 43, 73, 76. We have also shown that activation of PI3K signaling is responsible for increasing mitochondrial oxidative capacity in response to physiological cardiac hypertrophy 76, 79, 80. However, this effect of PI3K appears to be independent of changes in Akt signaling and as will be discussed later, constitutive activation of Akt appears to repress mitochondrial oxidative capacity.
Insulin Resistance
Insulin resistance has been classically defined as the inability of insulin to promote its metabolic actions in organs such as adipose tissue, skeletal muscle and liver (Figure 2). Insulin resistance is a hallmark of obesity and type 2 diabetes and also is a characteristic of heart failure. Thus in insulin resistant states there is impaired insulin-mediated glucose uptake in muscle and adipocytes, impaired suppression of hepatic glucose production and impaired suppression of lipolysis 81. Paradoxically, triglyceride synthesis and hepatic secretion of VLDL is increased on the basis of increased insulin mediated activation of lipogenesis 82. This underscores the important concept of selective insulin resistance, indicating that whereas certain targets of insulin signaling might be repressed in insulin resistant states other targets may retain their “sensitivity” 83. The metabolic milieu in insulin resistant individuals is therefore characterized by increased circulating concentrations of FFA, and triglycerides, and variable increases in glucose concentrations. Moreover, beta cells adapt to the insulin resistant environment by increasing insulin release, leading to hyperinsulinemia. In addition to these systemic metabolic abnormalities, insulin resistance is associated with additional changes to the systemic metabolic milieu including low-grade inflammation, increasing circulating concentrations of various cytokines and altered secretion of adipokines such as leptin, resistin and adiponectin 84, 85. Mechanisms for insulin resistance include ectopic accumulation of lipid intermediates in skeletal muscle and liver. In the liver, a proposed mechanism for steatosis is hyperinsulinemia-driven de novo lipogenesis in the face of excess caloric intake. The molecular mechanisms for hepatic insulin resistance are incompletely understood; however, increased endoplasmic reticulum stress, ROS generation and mitochondrial dysfunction have been suggested to contribute to the development of insulin resistance86. Ectopic lipid accumulation and oxidative stress have been proposed to contribute to skeletal muscle insulin resistance 87. Additional mechanisms contributing to insulin resistance include adipose tissue dysfunction that impairs the release of “insulin sensitizing” adipokines such as adiponectin, mitochondrial dysfunction, incomplete FA oxidation, and activation of inflammatory responses leading to increased promulgation of inflammatory cytokines such as TNFα and interleukins. Mechanistically, TNFα, IL-6 and free fatty acids (FFAs) activate intracellular kinases that induce serine phosphorylation of IRS proteins, thereby attenuating insulin signaling and inducing insulin resistance88. This complex pathophysiology of insulin resistance has been classically associated with obesity and a sedentary lifestyle. However, in heart failure generalized insulin resistance develops in patients with cardiac cachexia. Most studies seeking to explore the mechanisms for insulin resistance have been performed in subjects with obesity or type 2 diabetes. Fewer studies have been conducted in patients with heart failure.
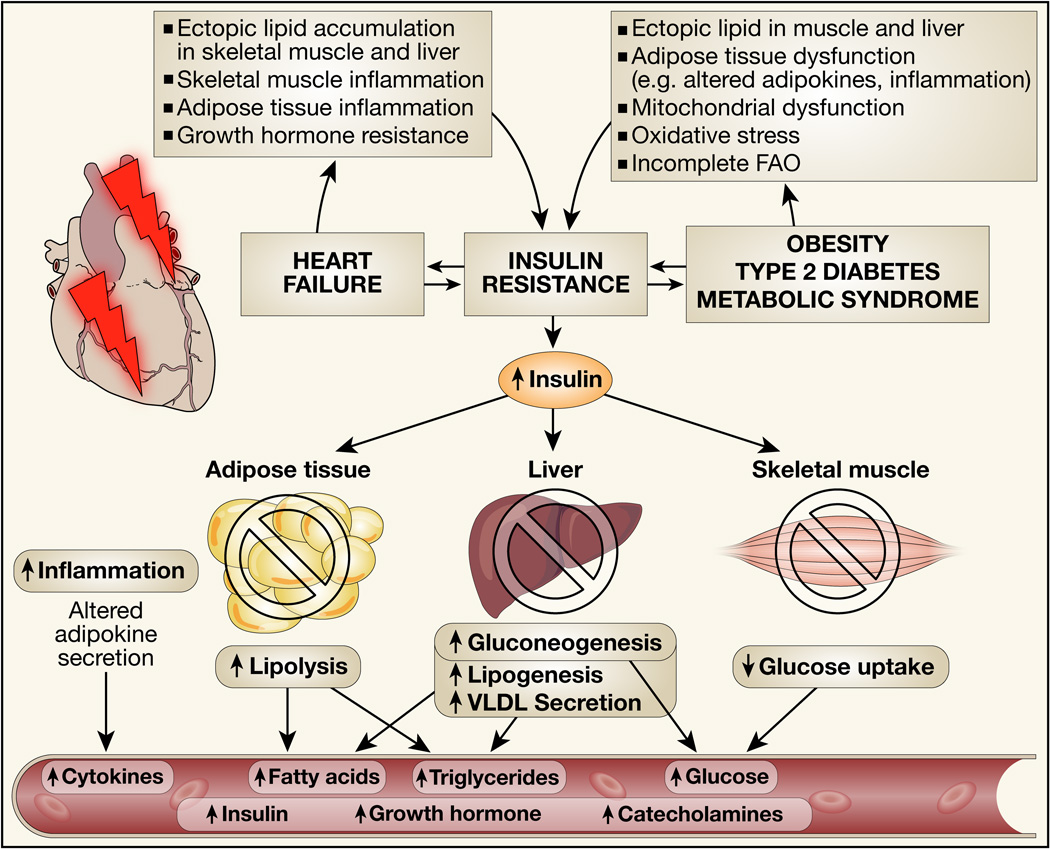
Figure 2. Summary of mechanisms that lead to insulin resistance in heart failure or in the metabolic syndrome.
Signaling events in skeletal muscle, liver, adipose tissue and brain (not shown) impair insulin signaling in each respective organ leading to metabolic perturbations as illustrated, which alter the systemic milieu in ways that may adversely impact cardiac structure and function.
Insulin resistance in heart failure also correlates with increased serum concentrations of proinflammatory cytokines, catecholamines, catabolic steroids and growth hormone89–93. In addition, decreased physical activity, loss of skeletal muscle mass and sympathetic over-activity, increases lipolysis thereby increasing the circulating concentrations of free fatty acids, which may further exacerbate insulin resistance in skeletal muscle and liver by promoting the accumulation of bioactive lipids. FFAs may further increase the activity of the sympathetic nervous system94, which may independently attenuate peripheral insulin signaling and reduce glucose utilization in skeletal muscle95. Furthermore, increased catecholamine concentrations stimulate hepatic glycogenolysis and gluconeogenesis leading to increased circulating glucose. Heart failure is also associated with hyperactivation of the renin-angiotensin-aldosterone system (RAAS), system, which has been implicated in the pathophysiology of insulin resistance 96. In addition to increasing circulating concentrations of angiotensinogen (originating from adipose tissue)97, chronic hyperinsulinemia may also increase the expression of angiotensin II receptors, which may further exacerbate adverse left ventricular remodeling98. Recent studies have also indicated that heart failure patients with insulin resistance also exhibit evidence of ectopic accumulation of bioactive lipids such as ceramide in their skeletal muscles, adipose tissue inflammation and hypo-adiponectinemia, all of which may contribute to generalized insulin resistance 99, 100. Moreover, skeletal muscle hypo-perfusion impairs skeletal muscle oxidative capacity. This induces growth hormone resistance and a state of low-grade inflammation which might also contribute to insulin resistance 101–103. Growth hormone resistance described in patients with heart failure results in decreased hepatic IGF-1 production in response to growth hormone, but circulating growth hormone concentrations increase 92. Increased growth hormone receptor signaling has been shown to induce insulin resistance in multiple tissues including the heart104–106, thereby exacerbating peripheral insulin resistance which might further impair cardiac function (Figure 2).
The changes in the metabolic milieu summarized above may contribute to the myocardial mal-adaptations that have been described in insulin resistant states, often referred to as diabetic cardiomyopathy and which have been extensively reviewed 107, 108. In brief and as outlined above, multiple mechanisms have been proposed to contribute to the increased vulnerability of the heart in insulin resistant states such as increased FA utilization, impaired mitochondrial oxidative capacity, mitochondrial dysfunction, decreased cardiac efficiency, oxidative stress, accumulation of bio-active lipids, inflammation, increased apoptosis, altered calcium metabolism and signaling, increased apoptosis and myocardial fibrosis. In addition, expansion of local adipose tissue depots might compromise cardiac function under conditions of obesity and type 2 diabetes. Epicardial adipose tissue (EAT) is a visceral-like adipose depot that is located on the surface of the ventricles, along the coronary arteries, and at the apex of the heart. Perivascular adipose tissue (PVAT) surrounds the blood vessels. EAT mass and diameter are increased in obesity 109, 110, and the volume of PVAT is associated with visceral obesity 111. Neither, EAT nor PVAT, are separated by a fascial layer from the surrounding tissue. Therefore, it has been suggested that adipokines and possibly fatty acids secreted from EAT and PVAT might directly contribute to the development of cardiovascular dysfunction in type 2 diabetes112. The induction of generalized insulin resistance and hyperinsulinemia in the context of pressure overload accelerates LV remodeling 113, 114. Thus it is plausible that insulin resistance that accompanies heart failure could further exacerbate myocardial dysfunction by similar mechanisms. There is a paucity of studies that have directly addressed the possible beneficial effects of therapeutic manipulations that might increase insulin sensitivity in patients with heart failure to determine if such changes will meaningfully impact LV function or alter long-term prognosis. In a cohort of patients with advanced heart failure the Schulze group recently reported that mechanical unloading resulted in a significant reduction in indices of systemic insulin resistance that occurred in concert with improvement in hemodynamic indices 24. Their study design did not address the question of whether or not the improvement in insulin sensitivity directly contributed to improved LV function. However, the authors reported that improvement in insulin sensitivity did correlate with reversal of certain metabolic and signaling abnormalities in the heart. An important question that these observations raise is: What are the contributions of altered insulin signaling and insulin resistance per se in cardiomyocytes and the potential contribution of these changes to heart failure and LV remodeling?
Cardiac Muscle Insulin Signaling in Insulin Resistant States or in Heart Failure
Prior to summarizing important studies in the field, it is important to discuss the various ways in which myocardial insulin resistance has been defined in the literature. In most instances, “insulin resistance” has been defined as a reduction in insulin-mediated myocardial glucose uptake. This definition is advantageous in the sense that it enables the inclusion of non-invasive studies that evaluate myocardial glucose utilization. The limitation however, is that this definition of myocardial insulin resistance does not account for changes in the activation of signaling intermediates that are downstream of the insulin receptor. For example, a reduction in GLUT4 protein could account for decreased insulin mediated glucose uptake in the face of an otherwise intact insulin signaling pathway, whereas impaired intracellular signaling could also reduce insulin-mediated glucose uptake even when GLUT4 protein levels are normal. As will be discussed, intact versus impaired intracellular signaling might influence LV remodeling in distinct ways.
Nearly all studies that have examined insulin-stimulated glucose uptake in humans or in animals with generalized insulin resistance, usually induced by diet-induced obesity, have demonstrated impaired insulin-mediated glucose uptake 115. Studies of myocardial glucose uptake in subjects with heart failure need to be interpreted in light of whether or not measurements of glucose uptake were performed under basal conditions or in response to physiological or pharmacological hyperinsulinemia as would occur during a euglycemic hyperinsulinemic glucose clamp. In many animal studies in which heart failure is induced by aortic banding (thoracic or abdominal) or by coronary artery ligation, basal glucose uptake has been reported to be increased, and this has been attributable to an increase in the GLUT1 transporter 116–119. However, when the augmentation of glucose uptake in response to insulin was evaluated, the fold increase in insulin-mediated glucose uptake was impaired relative to non-failing control groups 120. Importantly, in contrast to GLUT1, GLUT4 protein levels in the hearts of experimental models of heart failure or pathological cardiac hypertrophy are variable 117, 118, 121, 122 and the impact of GLUT4 expression and translocation in the context of heart failure is incompletely understood. Studies in transgenic mouse lines revealed that GLUT4 expression is dispensable for contractile function under basal conditions123, while these mice exhibited increased fibrosis124 and a poor response to ischemic injury125. In contrast, deletion of GLUT1 in the hearts of mice did not exacerbate heart failure progression following TAC 117. Thus it is likely that GLUT1 and GLUT4 mediated glucose transport may play divergent as well as overlapping roles in the modulation of myocardial glucose uptake in heart failure. It is important to note that whereas myocardial glucose utilization might be increased in compensated stages of LV hypertrophy and early in the course of heart failure, glucose utilization may be reduced in the hearts of patients and animals with advanced heart failure 126.
Insulin stimulation of the heart activates PI3K and Akt, which are requisite upstream signaling steps that promote GLUT4 translocation and increased glucose uptake. Studies of insulin-mediated activation of Akt in the heart in rodent models of generalized insulin resistance have yielded variable and potentially divergent results, which might be explicable by differences in the models used and differences in experimental conditions, such as dietary lipid composition. Studies based on 45% fat diets of varying durations have suggested that the ability of insulin to stimulate Akt might be augmented or preserved at a time when insulin-mediated glucose uptake is impaired 127. In contrast, studies in more severe models of insulin resistance such as ob/ob mice or following a more aggressive high-fat protocol with 60% fat leads to impaired insulin-mediated activation of Akt and with FOXO1 dephosphorylation and nuclear localization 128. It is important to put in context that the usual rodent diet is significantly lower in fat than human diets. Thus caution is warranted in extrapolating findings from rodent students in which a large increase in dietary fat is imposed for variable periods of time, given that species-specific differences may exist in the myocardial adaptations to a pathological lipid load, relative to those that might already be partially adapted to diets that are higher in fat content. In studies of failing hearts in experimental models, usually induced by transverse aortic constriction (on normal chow), Akt phosphorylation is increased in concert with increased IRS-1 phosphorylation 114, 129. In one study, genetically reducing the expression of insulin receptors, Akt1 or inducing systemic hypoinsulinemia reduced Akt phosphorylation and interestingly attenuated cardiac hypertrophy, and prevented adverse LV remodeling and heart failure 114.
Limited availability of human heart tissue has hampered the ability of investigators to evaluate changes in insulin signaling pathways in heart failure or insulin resistant states. Early studies suggested dynamic regulation of uncoupling protein and GLUT4 content in human heart atrial appendages in response to increasing circulating FA concentrations indicating that increased myocardial lipid delivery could repress myocardial GLUT4 130. Subsequently in an elegant study combining LV biopsy with metabolic analyses, Cook and colleagues reported that a significant reduction in plasma membrane GLUT4 accounted for the reduction in myocardial glucose utilization in humans with heart failure (average EF 27%) or in subjects with type 2 diabetes without heart failure despite minimal changes in overall GLUT4 content 131. The depletion of sarcolemmal GLUT4 was corroborated by animal studies that revealed that only two weeks of high fat feeding significantly reduced myocardial GLUT4 content and sarcolemmal translocation even prior to any weight gain and at a time when myocardial insulin signaling was preserved 127. Importantly, the human studies by Cook’s group also showed that PI3K and Akt signaling was substantially increased in the hearts of subjects with type 2 diabetes or heart failure and positively correlated with circulating insulin concentrations and with indices of insulin resistance. These studies suggest a paradigm whereby insulin-mediated glucose uptake in the heart in insulin resistant states could be dissociated from activation of insulin signaling to PI3K and Akt. The mechanisms contributing to reduced GLUT4 expression and translocation in the heart in obesity are incompletely understood, but could be due in part to altered regulation of SNARE proteins 131.
In contrast, in an analysis of left ventricular samples obtained from subjects with advanced heart failure at the time of left ventricular assist device (LVAD) implantation, Schulze and colleagues reported a significant repression in myocardial Akt phosphorylation that occurred in concert with evidence of accumulated bio-active lipids such as diacyl glycerol and ceramides leading to activation of protein kinase C isoforms that have been implicated as mediators of impaired insulin signaling 24. In materials obtained after mechanical unloading and following evidence of recovery of LV function, these signaling changes were reversed, leading to the conclusion that in end-stage heart failure there is significant repression of insulin signaling pathways and that recovery correlated with improvement in insulin action. In a study from a different group, mechanical unloading was associated with a decrease in the phosphorylation of the Akt target FOXO3 in human hearts 132. This study did not measure Akt phosphorylation, but in light of other studies that have reported activation of Akt signaling in human failing hearts, the implications of this observation is that in some cases ventricular unloading could potentially reduce myocardial Akt signaling.
The complexity of the Akt-FOXO interactions is further underscored by studies showing that 6-months of treatment with a 60% high fat diet leads to heart failure, which is associated with reduced Akt phosphorylation and dephosphorylation and nuclear localization of FOXO1 128. Genetic deletion of FOXO1 prevented the development of heart failure and the associated metabolic abnormalities that was induced by high fat feeding. Interestingly genetic deletion of FOXO1 also restored insulin sensitivity by reducing serine phosphorylation of IRS1 proteins and restoring Akt phosphorylation in cardiomyocytes in response to insulin stimulation. Thus FOXO activation could represent an important mechanism leading to impaired insulin signaling in heart failure that is induced by lipotoxic cardiomyopathy. It is noteworthy that additional post-translational modifications of FOXO proteins could potentially drive nuclear localization 133; therefore it will be of interest to determine if nuclear FOXO localization precedes impaired insulin-IRS1-Akt signaling in metabolic cardiomyopathy or occurs as a consequence of it.
Taken together, these studies underscore the complex responses of insulin signaling pathways in the myocardium to the changes in the systemic metabolic milieu that characterizes insulin resistant states or that accompany left ventricular remodeling in the failing heart. Current experimental data and studies in human samples could suggest a model whereby an early adaptation of the heart to systemic insulin resistance or to heart failure might be the repression of GLUT4 expression and GLUT4-mediated glucose transport. This occurs at a time when proximal insulin signaling to PI3K and Akt might remain intact and could potentially accelerate mal-adaptive ventricular hypertrophy. Over time, in response to persistent nutrient overload or to ongoing ventricular remodeling, FOXO activation (i.e. increased nuclear localization) might accelerate the desensitization of proximal insulin signaling leading to loss of Akt signaling and reduction in pro-survival signaling pathways that may further exacerbate cardiomyocyte loss. It has been proposed that myocardial insulin resistance might represent a mechanism by which the heart attempts to protect itself against nutrient overload in insulin resistant states134. Early on, attempts to over-ride myocardial insulin resistance could exacerbate the early phases of adverse ventricular remodeling. However, it is possible that over time, a transition may take place in which there is subsequent loss of proximal insulin signaling to Akt, which may exacerbate LV dysfunction via additional mechanisms related to further loss of trophic effects of insulin signaling on myocyte mass and mitochondrial capacity and loss of its anti-apoptotic actions, all of which could accelerate or exacerbate LV dysfunction (Figure 3). We will next explore potential mechanisms linking changes in myocardial insulin signaling to left ventricular dysfunction and adverse LV remodeling. Given the potential bi-phasic changes in insulin signaling that occur in the evolution of heart failure or systemic insulin resistance namely an activation of Akt, followed later by loss of insulin-mediated Akt signaling, pathways by which Akt activation may promote heart failure versus mechanisms linking impaired insulin signaling with heart failure will be discussed separately.
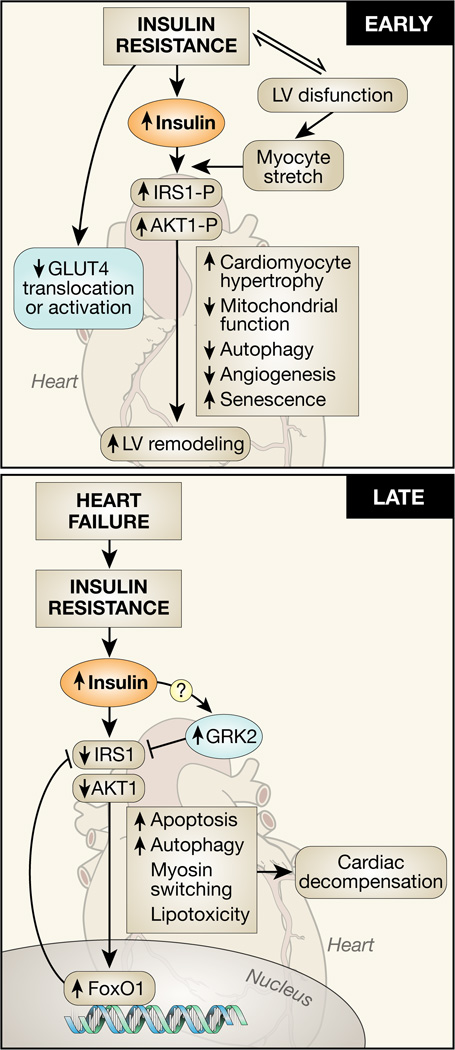
Figure 3. Molecular mechanisms linking increased or decreased insulin signaling with adverse left ventricular (LV) remodeling and heart failure progression.
In early stages of heart failure mediated by pressure overload, or in the early stages of the metabolic syndrome, hyperinsulinemia leads to activation of insulin receptor substrate 1(IRS1) and Akt1 to promote pathological hypertrophy, mitochondrial dysfunction and decreased autophagy, which contribute to accelerated LV remodeling. As heart failure progresses, despite systemic hyperinsulinemia insulin signaling pathways may become desensitized leading to loss of cytoprotective consequences of Akt signaling and persistent nuclear localization of forkhead (FOXO) proteins that will accelerate heart failure by various mechanisms such as increased apoptosis and exacerbation of lipotoxicity.
Excessive Insulin Signaling and Heart Failure
As summarized above, there is clear evidence that hyperactivation of proximal insulin signaling pathways such as increased IRS-1 phosphorylation and Akt hyperactivation may occur in the context of pathological cardiac hypertrophy and subsequent heart failure, and that reduction of insulin or Akt signaling in this context might reverse LV remodeling and preserve cardiac function 114. How might persistent activation of Akt lead to pathological hypertrophy? Interesting insights have been obtained from various lines of mutant mice with constitutive activation of various downstream components of the insulin signaling pathway. These models mimic the effects of hyperinsulinemia in type 2 diabetes and obesity on myocardial insulin signaling. Although constitutive activation of PI3K leads to compensated cardiac hypertrophy, our group recently showed that this leads to desensitization of insulin mediated glucose uptake followed by impaired ability of insulin to activate Akt 54. Of note this impairment in glucose uptake is due in part to a defect in activation of glucose transport following insertion GLUT4 in the plasma membrane.
In mice with inducible transgenic overexpression of Akt, cardiac hypertrophy is reversible and LV function preserved after short-term activation. However, if Akt expression is induced long-term, heart failure ensues, in part to a failure of angiogenesis. Importantly if Akt signaling is subsequently reduced in these failing hearts, the rate of progression to mortality is accelerated 135. It is tempting to speculate that this temporal relationship between an adverse consequence of Akt activation leading to pathological remodeling that is then exacerbated by reduction of Akt expression might parallel the temporal relationship between an initial increase in Akt signaling in earlier stages of heart failure that subsequently transitions to reduced Akt signaling in end-stage cardiomyopathy. We recently reported an additional mechanism by which persistent Akt signaling impairs mitochondrial energy metabolism that likely contributes to the Akt-mediated LV dysfunction. The mechanism for mitochondrial dysfunction is due to Akt-dependent repression of the expression nuclear-encoded mitochondrial genes via FOXO dependent and independent signaling pathways. We also showed that short-term activation of Akt promotes a metabolic switch characterized by increased glycolysis while impairing mitochondrial FA oxidation and we speculated that this short term adaptation might represent a compensatory response to protect mitochondrial integrity and capacity. Importantly the mitochondrial repression occurs early and is independent of the Akt-induced cardiac hypertrophy 129. However, in the long-term, a mismatch between impaired mitochondrial capacity and the increase in energetic requirements of Akt-mediated cardiac hypertrophy accelerates the onset of heart failure. Partial reduction of insulin or Akt signaling in murine models of TAC-induced cardiac hypertrophy prevents heart failure, which supports a model in which Akt-driven mechanisms might contribute to adverse LV remodeling 114. It is important to emphasize though, that in these studies Akt signaling was not abolished, but rather maintained at basal levels. In contrast, when Akt1 or insulin receptor signaling is completely abolished, then in response to stressors such as pressure overload, catecholamine toxicity or myocardial ischemia, all of these models exhibit a more rapid progression to heart failure 136–139. Taken together, these observations suggest that whereas residual insulin-PI3K-Akt signaling is required to maintain cardiomyocyte viability, excessive signaling by these pathways drives adverse LV remodeling and ventricular dysfunction via multiple mechanisms.
We recently described that hyperinsulinemia may impair myocardial contractility via a mechanism by which the G-protein receptor kinase (GRK2) leads to Gi biased β2AR signaling that inhibits adenylate cyclase, cAMP generation and cardiac contractility 140. The inhibition of βAR signaling via GRK2 by hyperinsulinemia requires insulin receptor and IRS-1 mediated signaling pathways (Figure 3). Ob/ob mice or mice that have been treated for 12-weeks with a 45% HFD, exhibit increased myocardial GRK2 content 141. GRK2 overexpression has been shown to reduce myocardial insulin signaling, and in germline GRK2 heterozygous KO mice, GLUT4 content is increased, insulin signaling was enhanced in aging mice or when these animals were placed on a high-fat diet 141, 142. Moreover, reduced GRK2 signaling promoted a “physiological cardiac hypertrophy” profile as animals aged. Thus GRK2 activation might also contribute to impairing myocardial insulin signaling with aging and in response to nutrient overload. GRK2 overactivation may also increase mitochondrial-mediated apoptosis by increasing mitochondrial calcium sensitivity, MPTP opening and cytochrome C release 142. Taken together, GRK2 activation could represent another important mechanism linking hyperinsulinemia with adverse LV remodeling. Although increased sympathetic activity is an established mechanism by which GRK2 is induced in the heart, whether or not hyperinsulinemia induces GRK2 by this mechanism remains to be definitively elucidated.
Suppression of PI3K signaling in the heart was reported to reduce signs of cardiac aging, in part by sustaining physiological levels of autophagy 143. Kim and colleagues also recently reported that aging was associated with increasing expression of IGF-1 receptors in cardiomyocytes and in mice with deficiency of myocardial IGF1R signaling, cardiac senescence and age-dependent cardiac fibrosis was reduced 144. Finally, hyperactivation of insulin signaling pathways will lead to autophagy suppression 145. Autophagy plays a critical role in organellar quality control, thus as has been recently reviewed hyperinsulinemic activation of Akt/mTOR signaling could suppress autophagy thereby contributing to LV dysfunction 27. In addition to Akt/mTOR-mediated suppression of autophagy, we also reported a novel mechanism that may occur in insulin resistant states and the metabolic syndrome by which bioactive lipids that accumulate in the heart will activate NOX2 via a PKC-dependent mechanism that ultimately inhibits lysosomal function and autophagic flux 146. In this model autophagic flux was impaired despite reduced phosphorylation of mTOR. Recent reports of the time course of autophagy following TAC suggests that although autophagy might be transiently induced, a repression of autophagy coincides with the transition from compensated hypertrophy to heart failure 147. Consistent with this model are many observations that mouse models with impaired expression of autophagy mediators develop LV dysfunction either at baseline or more rapid decompensation following pressure overload 148, 149. Thus constitutive repression of myocardial autophagy could represent a plausible mechanism by which hyperinsulinemia and activation of Akt signaling could impair left ventricular function and accelerate adverse LV remodeling. Impaired autophagy might also impair mitochondrial quality control. Whether or not insulin signaling specifically regulates mitophagy remains incompletely understood, and whether or not they are linked in heart failure is currently unknown.
Decreased Insulin Signaling and LV Remodeling
Mechanisms that are distinct from those associated with hyperactivation of insulin signaling pathways might also contribute to promoting LV dysfunction when insulin-PI3K and Akt signaling ultimately become impaired. In this regard, valuable insights have been obtained from genetic knockouts of components of the insulin signaling pathway (Table 1). It is important to emphasize that there is significant redundancy in insulin and IGF-1 signal transduction pathways 77. Thus, there is clear crosstalk between insulin and IGF-1R signaling pathways in the heart, which might reflect the relatively benign phenotypes of single knockouts of the IGF-1R or the IR respectively 42, 78. This is also true for IRS isoforms in that loss of IRS1 or IRS2 individually do not precipitate LV dysfunction in unstressed hearts and indeed we observed no differences in the ability of insulin to activate Akt or promote glucose uptake 76. However, combined deletion of IR and IGF-1receptors or IRS1 and IRS2 leads to catastrophic heart failure due in part to unrestrained autophagy, apoptosis and profound mitochondrial dysfunction 43, 150. Similar phenotypes have been observed in animals with targeted loss of the phosphoinositide dependent protein kinase (pdpk1), which is required for IR or IGF-1R dependent activation of Akt 80.
The second mechanism by which reduced insulin signaling might contribute to LV dysfunction is by persistent nuclear localization of FOXO1. Guo and colleagues in mice with cardiomyocyte-restricted deletion of IRS1 and IRS2 showed that they could ameliorate the heart failure in part by genetically deleting FOXO1 expression 150, 151. Similarly heart failure associated with long-term high-fat feeding could also be prevented when FOXO1 was deleted 128. It is likely that the mechanism by which persistent nuclear localization of FOXO proteins might lead to LV dysfunction is multifactorial. Mechanisms by which FOXO may mediate LV remodeling include myosin isoform switching, FOXO mediated activation of autophagic and atrophic pathways and FOXO-mediated activation of lipid metabolism pathways that would promote lipotoxicity 128, 132, 151. The potential bi-phasic response in insulin signaling is also of particular importance for the design of future studies. Therefore, it is of great interest to perform time course studies, especially in the context heart failure and insulin resistance.
Implications for Therapy and Future Research
Taken together, this review of the field identifies an important need for uniformity in determining changes in the specific components of the insulin signaling cascade at varying stages in the evolution from compensated cardiac hypertrophy to decompensated cardiac hypertrophy and ultimately to heart failure. Furthermore, a careful analysis of the relationship between altered myocardial insulin signaling in models of generalized insulin resistance in concert with cardiac adaptations to hemodynamic stress will advance our understanding of the complex interactions between generalized insulin resistance, myocardial insulin signaling and heart failure. To more closely mimic human disease, studies in which heart failure is induced in animals that have developed generalized insulin resistance would be informative. Generalized insulin resistance is usually evident in high-fat fed rodent models after high-fat diets of >10-week duration. We would also suggest that measuring glucose uptake in response to insulin gives only a partial picture of changes in insulin signaling in the heart and as such it is important to evaluate the phosphorylation states of IRS1/2, Akt1/2 and FOXO proteins under conditions of ambient insulinemia and in response to an insulin stimulus. These analyses would be informative if determined in conjunction with assays of signaling defects in skeletal muscle, adipose tissue and liver, as they will not only identify differences in the adaptations of these tissues to insulin resistance but also the potential for crosstalk between these classical insulin signaling targets and the heart in models of heart failure. Moreover, they will test the hypothesis that changes in insulin signaling, either an increase in the early stages followed by impaired signaling in late-stage heart failure, may independently contribute to the pathophysiology of heart failure.
Modulation of myocardial insulin signaling is not currently a consideration in the management of heart failure. As discussed above, there are profound changes in myocardial insulin signaling that develop in the failing heart and are also present in subjects with generalized insulin resistance that may sensitize the heart to LV dysfunction. This raises important questions regarding whether or not modulation of generalized insulin resistance, normalization of hyperinsulinemia and other disturbances in the metabolic milieu will alter the prognosis of heart failure. Second, it remains to be determined if strategies that will directly correct the changes in myocardial insulin signaling described above will reverse heart failure. Oxidative stress is a widely accepted mechanism for cardiac dysfunction in insulin resistant states and it was recently reported that targeted anti-oxidant therapy may partially restore abnormal insulin signaling and improve cardiac structure and function 152. Combined treatment with polyphenols resveratrol and S17834 also significantly restored cardiac function in diet-induced heart failure in rodent models via multiple mechanisms including reducing oxidative stress, reversing oxidative modifications of proteins, reducing hyperinsulinemia and increasing circulating adiponectin concentrations 153. As alluded to above, GRK2 could represent an attractive target that could ameliorate pathological LV remodeling via mechanisms that could involve direct modulation of insulin signaling pathways within cardiomyocytes as well as by effects on systemic metabolic homeostasis 141, 142, 154. Whereas ventricular unloading improved myocardial insulin signaling and lipotoxicity, it is not clear if the myocardial changes were primary or secondary to improvement in peripheral insulin resistance 24. It is probable that ventricular unloading by reversing some of the mechanisms that promote generalized insulin resistance in subjects with heart failure might have contributed to a feed-forward mechanism to increase myocardial recovery.
An important conundrum that has been increasingly recognized is the unique challenges in managing heart failure in the patient with type 2 diabetes and insulin resistance. Although it is clear that diabetic patients with heart failure will respond to conventional heart failure therapies, many recent clinical trials have revealed a disturbing association between multiple diabetes therapies and worsening of heart failure or increased heart failure hospitalization. This topic has been the subject of recent reviews 155, 156. However, it is clear that many diabetes therapies including thiazolidinediones and even insulin might be associated with volume expansion, which may exacerbate heart failure. Similar effects have also been observed with certain DDPIV inhibitors. Specifically, treatment with the DDPIV inhibitor saxagliptin resulted in increased heart failure hospitalization157. DPPIV degrades GLP-1 and other vasoactive peptides158 including brain natriuretic peptide (BNP), which though increased in heart failure could represent an adaptive response159. Moreover, the increase in circulating concentrations of insulin that occur as a result of incretin therapy could lead to ligand-dependent activation of insulin signaling pathways. Thus, if hyperactivation of insulin signaling indeed contributes to adverse ventricular remodeling in heart failure then a plausible mechanism for the relationship between these therapies and heart failure exacerbation could be the increase in myocardial insulin signaling and Akt hyperactivation. In contrast, metformin, which acts as an insulin sensitizer and reduces hyperinsulinemia might afford some degree of protection in terms of heart failure 160. Even though not directly proven, decreased circulating insulin levels following metformin treatment might attenuate cardiac insulin signaling and provide a potential explanation for the cardioprotective effects observed. A recent trial using a novel class of diabetes therapeutic, an inhibitor of the renal sodium glucose co-transporter (SGLT2) had a dramatic impact on subsequent risk of hospitalization for heart failure 161. It is not known if this beneficial effect was secondary to the mild volume depletion or anti-hypertensive effects of this agent or to effects on altering the metabolic milieu such as reducing circulating levels of insulin. Thus additional studies are required to determine if therapeutic strategies that directly impact the abnormal systemic milieu that characterizes the insulin resistant state will impact LV structure and function in heart failure and impact prognosis and survival.
Concluding Remarks
The heart is an insulin responsive organ and insulin signaling plays an important role in cardiovascular homeostasis. Cardiac hypertrophy and heart failure are associated with profound changes in myocardial insulin signaling. Moreover, heart failure is an insulin resistant state that leads to an altered metabolic milieu that will influence myocardial structure and function via mechanisms that are dependent or independent of alterations in myocardial insulin signaling. It is possible that strategies that might correct the systemic metabolic disturbances that are associated with insulin resistance could have an impact on the outcome and prognosis of subjects with heart failure. An important imperative for the future will be to design trials that can rigorously test this hypothesis. Second, an important opportunity for the future will be to identify and evaluate therapeutic strategies that might directly influence changes in myocardial insulin signaling that accompany heart failure. Given the dynamic nature of these changes, it will be important that subjects are carefully stratified or phenotyped to determine which insulin signaling pathways are perturbed and the pathophysiological mechanisms that will most likely benefit from targeted therapies.
Acknowledgments
Work in the Abel Laboratory has been supported by the following grants from the National Institutes of health R01HL10837, RO1 HL12357, R01 HL127764, R01 HL112413, R01DK092065, U01 HL087947, RO1 HL73167, R21DK073590 and by grants to trainees from the American Heart Association and the JDRF. EDA is an established investigator of the AHA. CR was supported by a postdoctoral grant from the German Research Foundation (DFG).
Nonstandard Abbreviations and Acronyms
- FA
Fatty Acids
- EAT
Epicardial adipose tissue
Footnotes
Disclosures: None
References
- 1.Norheim OF, Jha P, Admasu K, Godal T, Hum RJ, Kruk ME, Gomez-Dantes O, Mathers CD, Pan H, Sepulveda J, Suraweera W, Verguet S, Woldemariam AT, Yamey G, Jamison DT, Peto R. Avoiding 40% of the premature deaths in each country, 2010–30: Review of national mortality trends to help quantify the un sustainable development goal for health. Lancet. 2015;385:239–252. doi: 10.1016/S0140-6736(14)61591-9. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ American Heart Association Advocacy Coordinating C, Stroke C Council on Cardiovascular R, Intervention, Council on Clinical C , Council on E, Prevention, Council on A, Thrombosis, Vascular B Council on C Critical C Perioperative Resuscitation Council on Cardiovascular N, Council on the Kidney in Cardiovascular D, Council on Cardiovascular S, Anesthesia, Interdisciplinary Council on Quality of C, Outcomes R. Forecasting the future of cardiovascular disease in the united states: A policy statement from the american heart association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz K, Stathopoulou K, Schmid E, Eder P, Cuello F. Heart failure-specific changes in protein kinase signalling. Pflugers Archiv : European journal of physiology. 2014;466:1151–1162. doi: 10.1007/s00424-014-1462-x. [DOI] [PubMed] [Google Scholar]
- 5.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. The Journal of clinical investigation. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miettinen H, Lehto S, Salomaa V, Mahonen M, Niemela M, Haffner SM, Pyorala K, Tuomilehto J. Impact of diabetes on mortality after the first myocardial infarctionThe finmonica myocardial infarction register study group. Diabetes care. 1998;21:69–75. doi: 10.2337/diacare.21.1.69. [DOI] [PubMed] [Google Scholar]
- 7.Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. Journal of the American College of Cardiology. 2001;38:421–428. doi: 10.1016/s0735-1097(01)01408-5. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: The framingham study. The American journal of cardiology. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB, McGee DL. Diabetes cardiovascular disease The framingham study. JAMA : the journal of the American Medical Association. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 10.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. The New England journal of medicine. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 11.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (ukpds 35): Prospective observational study. Bmj. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: The cardiovascular health study. Journal of the American College of Cardiology. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 13.Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: A prospective community-based study. The American journal of medicine. 1999;106:605–612. doi: 10.1016/s0002-9343(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 14.Davis BR, Piller LB, Cutler JA, Furberg C, Dunn K, Franklin S, Goff D, Leenen F, Mohiuddin S, Papademetriou V, Proschan M, Ellsworth A, Golden J, Colon P, Crow R Antihypertensive, Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research G. Role of diuretics in the prevention of heart failure: The antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2006;113:2201–2210. doi: 10.1161/CIRCULATIONAHA.105.544031. [DOI] [PubMed] [Google Scholar]
- 15.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: An update. Diabetes care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 16.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: The obesity paradox. The American journal of cardiology. 2003;91:891–894. doi: 10.1016/s0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 17.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The obesity paradox: Body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 18.Hall JA, French TK, Rasmusson KD, Vesty JC, Roberts CA, Rimmasch HL, Kfoury AG, Renlund DG. The paradox of obesity in patients with heart failure. J Am Acad Nurse Pract. 2005;17:542–546. doi: 10.1111/j.1745-7599.2005.00084.x. [DOI] [PubMed] [Google Scholar]
- 19.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiological reviews. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. Journal of the American College of Cardiology. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 21.Takiguchi M, Yoshihisa A, Miura S, Shimizu T, Nakamura Y, Yamauchi H, Iwaya S, Owada T, Miyata M, Abe S, Sato T, Suzuki S, Suzuki H, Saitoh S, Takeishi Y. Impact of body mass index on mortality in heart failure patients. Eur J Clin Invest. 2014;44:1197–1205. doi: 10.1111/eci.12354. [DOI] [PubMed] [Google Scholar]
- 22.Velez M, Kohli S, Sabbah HN. Animal models of insulin resistance and heart failure. Heart failure reviews. 2014;19:1–13. doi: 10.1007/s10741-013-9387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 24.Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, Kato T, Khan R, Takayama H, Knoll R, Milting H, Chung CS, Jorde U, Naka Y, Mancini DM, Goldberg IJ, Schulze PC. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadowaki T, Kubota N, Ueki K, Yamauchi T. Snapshot: Physiology of insulin signaling. Cell. 2012;148:834–834. doi: 10.1016/j.cell.2012.02.004. e831. [DOI] [PubMed] [Google Scholar]
- 26.Kadowaki T, Ueki K, Yamauchi T, Kubota N. Snapshot: Insulin signaling pathways. Cell. 2012;148:624. doi: 10.1016/j.cell.2012.01.034. 624 e621. [DOI] [PubMed] [Google Scholar]
- 27.Riehle C, Abel ED. Insulin regulation of myocardial autophagy. Circulation journal : official journal of the Japanese Circulation Society. 2014;78:2569–2576. doi: 10.1253/circj.cj-14-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/pkbalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. The Journal of biological chemistry. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 29.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase akt2 (pkb beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 30.DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. Akt2 regulates cardiac metabolism and cardiomyocyte survival. The Journal of biological chemistry. 2006;281:32841–32851. doi: 10.1074/jbc.M513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: A novel regulator of cardiac hypertrophy and development. Circulation research. 2002;90:1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 32.Wullschleger S, Loewith R, Hall MN. Tor signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Mtor interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 34.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mtor, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 35.Schmelzle T, Hall MN. Tor, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 36.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4e-bp1 phosphorylation: A novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. Erks: A family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and ngf. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 38.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocrine reviews. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 39.Heni M, Kullmann S, Preissl H, Fritsche A, Haring HU. Impaired insulin action in the human brain: Causes, metabolic consequences. Nature reviews. Endocrinology. 2015;11:701–711. doi: 10.1038/nrendo.2015.173. [DOI] [PubMed] [Google Scholar]
- 40.Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U. Insulin resistance and impaired adipogenesis. Trends in endocrinology and metabolism: TEM. 2015;26:193–200. doi: 10.1016/j.tem.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Otero YF, Stafford JM, McGuinness OP. Pathway-selective insulin resistance and metabolic disease: The importance of nutrient flux. The Journal of biological chemistry. 2014;289:20462–20469. doi: 10.1074/jbc.R114.576355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, Abel ED. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. The Journal of clinical investigation. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riehle C, Wende AR, Sena S, Pires KM, Pereira RO, Zhu Y, Bugger H, Frank D, Bevins J, Chen D, Perry CN, Dong XC, Valdez S, Rech M, Sheng X, Weimer BC, Gottlieb RA, White MF, Abel ED. Insulin receptor substrate signaling suppresses neonatal autophagy in the heart. The Journal of clinical investigation. 2013 doi: 10.1172/JCI71171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rask-Madsen C, King GL. Mechanisms of disease: Endothelial dysfunction in insulin resistance, diabetes. Nature clinical practice. Endocrinology & metabolism. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 45.Symons JD, Abel ED. Lipotoxicity contributes to endothelial dysfunction: A focus on the contribution from ceramide. Reviews in endocrine & metabolic disorders. 2013;14:59–68. doi: 10.1007/s11154-012-9235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bharath LP, Ruan T, Li Y, Ravindran A, Wan X, Nhan JK, Walker ML, Deeter L, Goodrich R, Johnson E, Munday D, Mueller R, Kunz D, Jones D, Reese V, Summers SA, Babu PV, Holland WL, Zhang QJ, Abel ED, Symons JD. Ceramide-initiated protein phosphatase 2a activation contributes to arterial dysfunction in vivo. Diabetes. 2015;64:3914–3926. doi: 10.2337/db15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circulation research. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, Pettey D, Losee J, Duncan B, Gale D, Kowalski CA, Deeter N, Nichols A, Deesing M, Arrant C, Ruan T, Boehme C, McCamey DR, Rou J, Ambal K, Narra KK, Summers SA, Abel ED, Symons JD. Ceramide mediates vascular dysfunction in diet-induced obesity by pp2a-mediated dephosphorylation of the enos-akt complex. Diabetes. 2012;61:1848–1859. doi: 10.2337/db11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese zucker (fa/fa) rats. The Journal of clinical investigation. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, Park K, Li C, Rask-Madsen C, Mima A, Qi W, Mizutani K, Huang P, King GL. Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase c-beta isoform in the endothelium. Circulation research. 2013;113:418–427. doi: 10.1161/CIRCRESAHA.113.301074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park K, Li Q, Rask-Madsen C, Mima A, Mizutani K, Winnay J, Maeda Y, D’Aquino K, White MF, Feener EP, King GL. Serine phosphorylation sites on irs2 activated by angiotensin ii and protein kinase c to induce selective insulin resistance in endothelial cells. Molecular and cellular biology. 2013;33:3227–3241. doi: 10.1128/MCB.00506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isenovic ER, Kedees MH, Tepavcevic S, Milosavljevic T, Koricanac G, Trpkovic A, Marche P. Role of pi3k/akt, cpla2 and erk1/2 signaling pathways in insulin regulation of vascular smooth muscle cells proliferation. Cardiovascular & hematological disorders drug targets. 2009;9:172–180. doi: 10.2174/187152909789007034. [DOI] [PubMed] [Google Scholar]
- 53.Prior JO, Quinones MJ, Hernandez-Pampaloni M, Facta AD, Schindler TH, Sayre JW, Hsueh WA, Schelbert HR. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation. 2005;111:2291–2298. doi: 10.1161/01.CIR.0000164232.62768.51. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Y, Pereira RO, O’Neill BT, Riehle C, Ilkun O, Wende AR, Rawlings TA, Zhang YC, Zhang Q, Klip A, Shiojima I, Walsh K, Abel ED. Cardiac pi3k-akt impairs insulin-stimulated glucose uptake independent of mtorc1 and glut4 translocation. Molecular endocrinology (Baltimore, Md. 2013;27:172–184. doi: 10.1210/me.2012-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoffman NJ, Elmendorf JS. Signaling, cytoskeletal and membrane mechanisms regulating glut4 exocytosis. Trends Endocrinol Metab. 2011;22:110–116. doi: 10.1016/j.tem.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishikura S, Koshkina A, Klip A. Small g proteins in insulin action: Rab and rho families at the crossroads of signal transduction and glut4 vesicle traffic. Acta Physiol (Oxf) 2008;192:61–74. doi: 10.1111/j.1748-1716.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 57.Stockli J, Fazakerley DJ, James DE. Glut4 exocytosis. J Cell Sci. 2011;124:4147–4159. doi: 10.1242/jcs.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ng Y, Ramm G, Lopez JA, James DE. Rapid activation of akt2 is sufficient to stimulate glut4 translocation in 3t3-l1 adipocytes. Cell metabolism. 2008;7:348–356. doi: 10.1016/j.cmet.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 60.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiological reviews. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 61.Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond) 2008;114:195–210. doi: 10.1042/CS20070166. [DOI] [PubMed] [Google Scholar]
- 62.Yang R, Barouch LA. Leptin signaling and obesity: Cardiovascular consequences. Circulation research. 2007;101:545–559. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 63.Unger RH, Orci L. Lipoapoptosis: Its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 64.Wende AR, Abel ED. Lipotoxicity in the heart. Biochim Biophys Acta. 2010;1801:311–319. doi: 10.1016/j.bbalip.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, Hess JF. Leptin receptor missense mutation in the fatty zucker rat. Nature genetics. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 66.Chabowski A, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Bonen A. Insulin stimulates fatty acid transport by regulating expression of fat/cd36 but not fabppm. American journal of physiology. Endocrinology and metabolism. 2004;287:E781–E789. doi: 10.1152/ajpendo.00573.2003. [DOI] [PubMed] [Google Scholar]
- 67.Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 68.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: Direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 69.Echtay KS, Murphy MP, Smith RA, Talbot DA, Brand MD. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. The Journal of biological chemistry. 2002;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- 70.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. Journal of the American College of Cardiology. 2009;54:1891–1898. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 72.Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, Abel ED. Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes. 2008 doi: 10.2337/db08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boudina S, Bugger H, Sena S, O’Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H, Khalimonchuk O, Wayment B, Sheng X, Rodnick KJ, Centini R, Chen D, Litwin SE, Weimer BE, Abel ED. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–1283. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parra V, Verdejo HE, Iglewski M, Del Campo A, Troncoso R, Jones D, Zhu Y, Kuzmicic J, Pennanen C, Lopez-Crisosto C, Jana F, Ferreira J, Noguera E, Chiong M, Bernlohr DA, Klip A, Hill JA, Rothermel BA, Abel ED, Zorzano A, Lavandero S. Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the akt-mtor-nfkappab-opa-1 signaling pathway. Diabetes. 2014;63:75–88. doi: 10.2337/db13-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Si R, Tao L, Zhang HF, Yu QJ, Zhang R, Lv AL, Zhou N, Cao F, Guo WY, Ren J, Wang HC, Gao F. Survivin: A novel player in insulin cardioprotection against myocardial ischemia/reperfusion injury. Journal of molecular and cellular cardiology. 2011;50:16–24. doi: 10.1016/j.yjmcc.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 76.Riehle C, Wende AR, Zhu Y, Oliveira KJ, Pereira RO, Jaishy BP, Bevins J, Valdez S, Noh J, Kim BJ, Moreira AB, Weatherford ET, Manivel R, Rawlings TA, Rech M, White MF, Abel ED. Insulin receptor substrates are essential for the bioenergetic and hypertrophic response of the heart to exercise training. Molecular and cellular biology. 2014;34:3450–3460. doi: 10.1128/MCB.00426-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ikeda H, Shiojima I, Ozasa Y, Yoshida M, Holzenberger M, Kahn CR, Walsh K, Igarashi T, Abel ED, Komuro I. Interaction of myocardial insulin receptor and igf receptor signaling in exercise-induced cardiac hypertrophy. Journal of molecular and cellular cardiology. 2009 doi: 10.1016/j.yjmcc.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim J, Wende AR, Sena S, Theobald HA, Soto J, Sloan C, Wayment BE, Litwin SE, Holzenberger M, LeRoith D, Abel ED. Insulin-like growth factor i receptor signaling is required for exercise-induced cardiac hypertrophy. Molecular endocrinology (Baltimore, Md. 2008;22:2531–2543. doi: 10.1210/me.2008-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Neill BT, Kim J, Wende AR, Theobald HA, Tuinei J, Buchanan J, Guo A, Zaha VG, Davis DK, Schell JC, Boudina S, Wayment B, Litwin SE, Shioi T, Izumo S, Birnbaum MJ, Abel ED. A conserved role for phosphatidylinositol 3-kinase but not akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell metabolism. 2007;6:294–306. doi: 10.1016/j.cmet.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Noh J, Wende AR, Olsen CD, Kim B, Bevins J, Zhu Y, Zhang QJ, Riehle C, Abel ED. Phosphoinositide dependent protein kinase 1 is required for exercise-induced cardiac hypertrophy but not the associated mitochondrial adaptations. Journal of molecular and cellular cardiology. 2015;89:297–305. doi: 10.1016/j.yjmcc.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harbor perspectives in biology. 2014;6 doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Critical reviews in biochemistry and molecular biology. 2011;46:200–215. doi: 10.3109/10409238.2011.562481. [DOI] [PubMed] [Google Scholar]
- 83.Konner AC, Bruning JC. Selective insulin and leptin resistance in metabolic disorders. Cell metabolism. 2012;16:144–152. doi: 10.1016/j.cmet.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 84.Keane KN, Cruzat VF, Carlessi R, de Bittencourt PI, Jr, Newsholme P. Molecular events linking oxidative stress and inflammation to insulin resistance and beta-cell dysfunction. Oxidative medicine and cellular longevity. 2015;2015:181643. doi: 10.1155/2015/181643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bluher M. Adipokines - removing road blocks to obesity and diabetes therapy. Molecular metabolism. 2014;3:230–240. doi: 10.1016/j.molmet.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taube A, Eckardt K, Eckel J. Role of lipid-derived mediators in skeletal muscle insulin resistance. American journal of physiology. Endocrinology and metabolism. 2009;297:E1004–E1012. doi: 10.1152/ajpendo.00241.2009. [DOI] [PubMed] [Google Scholar]
- 88.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European heart journal. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 89.Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJ. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–534. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 90.Schulze PC, Kratzsch J, Linke A, Schoene N, Adams V, Gielen S, Erbs S, Moebius-Winkler S, Schuler G. Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. European journal of heart failure. 2003;5:33–40. doi: 10.1016/s1388-9842(02)00177-0. [DOI] [PubMed] [Google Scholar]
- 91.Hambrecht R, Schulze PC, Gielen S, Linke A, Mobius-Winkler S, Yu J, Kratzsch JJ, Baldauf G, Busse MW, Schubert A, Adams V, Schuler G. Reduction of insulin-like growth factor-i expression in the skeletal muscle of noncachectic patients with chronic heart failure. Journal of the American College of Cardiology. 2002;39:1175–1181. doi: 10.1016/s0735-1097(02)01736-9. [DOI] [PubMed] [Google Scholar]
- 92.Anker SD, Volterrani M, Pflaum CD, Strasburger CJ, Osterziel KJ, Doehner W, Ranke MB, Poole-Wilson PA, Giustina A, Dietz R, Coats AJ. Acquired growth hormone resistance in patients with chronic heart failure: Implications for therapy with growth hormone. Journal of the American College of Cardiology. 2001;38:443–452. doi: 10.1016/s0735-1097(01)01385-7. [DOI] [PubMed] [Google Scholar]
- 93.Doehner W, Rauchhaus M, Godsland IF, Egerer K, Niebauer J, Sharma R, Cicoira M, Florea VG, Coats AJ, Anker SD. Insulin resistance in moderate chronic heart failure is related to hyperleptinaemia, but not to norepinephrine or tnf-alpha. International journal of cardiology. 2002;83:73–81. doi: 10.1016/s0167-5273(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 94.Paolisso G, Manzella D, Rizzo MR, Ragno E, Barbieri M, Varricchio G, Varricchio M. Elevated plasma fatty acid concentrations stimulate the cardiac autonomic nervous system in healthy subjects. The American journal of clinical nutrition. 2000;72:723–730. doi: 10.1093/ajcn/72.3.723. [DOI] [PubMed] [Google Scholar]
- 95.Roden M. How free fatty acids inhibit glucose utilization in human skeletal muscle. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2004;19:92–96. doi: 10.1152/nips.01459.2003. [DOI] [PubMed] [Google Scholar]
- 96.Favre GA, Esnault VL, Van Obberghen E. Modulation of glucose metabolism by the renin-angiotensin-aldosterone system. American journal of physiology. Endocrinology and metabolism. 2015;308:E435–E449. doi: 10.1152/ajpendo.00391.2014. [DOI] [PubMed] [Google Scholar]
- 97.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386–393. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- 98.Samuelsson AM, Bollano E, Mobini R, Larsson BM, Omerovic E, Fu M, Waagstein F, Holmang A. Hyperinsulinemia: Effect on cardiac mass/function, angiotensin ii receptor expression, and insulin signaling pathways. Am J Physiol Heart Circ Physiol. 2006;291:H787–H796. doi: 10.1152/ajpheart.00974.2005. [DOI] [PubMed] [Google Scholar]
- 99.Empinado HM, Deevska GM, Nikolova-Karakashian M, Yoo JK, Christou DD, Ferreira LF. Diaphragm dysfunction in heart failure is accompanied by increases in neutral sphingomyelinase activity and ceramide content. European journal of heart failure. 2014;16:519–525. doi: 10.1002/ejhf.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan RS, Kato TS, Chokshi A, Chew M, Yu S, Wu C, Singh P, Cheema FH, Takayama H, Harris C, Reyes-Soffer G, Knoll R, Milting H, Naka Y, Mancini D, Schulze PC. Adipose tissue inflammation and adiponectin resistance in patients with advanced heart failure: Correction after ventricular assist device implantation Circulation. Heart failure. 2012;5:340–348. doi: 10.1161/CIRCHEARTFAILURE.111.964031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khawaja T, Chokshi A, Ji R, Kato TS, Xu K, Zizola C, Wu C, Forman DE, Ota T, Kennel P, Takayama H, Naka Y, George I, Mancini D, Schulze CP. Ventricular assist device implantation improves skeletal muscle function, oxidative capacity, and growth hormone/insulin-like growth factor-1 axis signaling in patients with advanced heart failure. Journal of cachexia, sarcopenia and muscle. 2014;5:297–305. doi: 10.1007/s13539-014-0155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Larsen AI, Lindal S, Myreng K, Ogne C, Kvaloy JT, Munk PS, Aukrust P, Yndestad A, Dickstein K, Nilsen DW. Cardiac resynchronization therapy improves minute ventilation/carbon dioxide production slope and skeletal muscle capillary density without reversal of skeletal muscle pathology or inflammation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2013;15:857–864. doi: 10.1093/europace/eus428. [DOI] [PubMed] [Google Scholar]
- 103.Toth MJ, Ades PA, Tischler MD, Tracy RP, LeWinter MM. Immune activation is associated with reduced skeletal muscle mass and physical function in chronic heart failure. International journal of cardiology. 2006;109:179–187. doi: 10.1016/j.ijcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 104.Miquet JG, Giani JF, Martinez CS, Munoz MC, Gonzalez L, Sotelo AI, Boparai RK, Masternak MM, Bartke A, Dominici FP, Turyn D. Prolonged exposure to gh impairs insulin signaling in the heart. Journal of molecular endocrinology. 2011;47:167–177. doi: 10.1530/JME-11-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krusenstjerna-Hafstrom T, Clasen BF, Moller N, Jessen N, Pedersen SB, Christiansen JS, Jorgensen JO. Growth hormone (gh)-induced insulin resistance is rapidly reversible: An experimental study in gh-deficient adults. The Journal of clinical endocrinology and metabolism. 2011;96:2548–2557. doi: 10.1210/jc.2011-0273. [DOI] [PubMed] [Google Scholar]
- 106.Xu J, Messina JL. Crosstalk between growth hormone and insulin signaling. Vitam Horm. 2009;80:125–153. doi: 10.1016/S0083-6729(08)00606-7. [DOI] [PubMed] [Google Scholar]
- 107.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 108.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cikim AS, Topal E, Harputluoglu M, Keskin L, Zengin Z, Cikim K, Ozdemir R, Aladag M, Yologlu S. Epicardial adipose tissue, hepatic steatosis and obesity. Journal of endocrinological investigation. 2007;30:459–464. doi: 10.1007/BF03346328. [DOI] [PubMed] [Google Scholar]
- 110.Kankaanpaa M, Lehto HR, Parkka JP, Komu M, Viljanen A, Ferrannini E, Knuuti J, Nuutila P, Parkkola R, Iozzo P. Myocardial triglyceride content and epicardial fat mass in human obesity: Relationship to left ventricular function and serum free fatty acid levels. The Journal of clinical endocrinology and metabolism. 2006;91:4689–4695. doi: 10.1210/jc.2006-0584. [DOI] [PubMed] [Google Scholar]
- 111.Schlett CL, Massaro JM, Lehman SJ, Bamberg F, O’Donnell CJ, Fox CS, Hoffmann U. Novel measurements of periaortic adipose tissue in comparison to anthropometric measures of obesity, and abdominal adipose tissue. International journal of obesity. 2009;33:226–232. doi: 10.1038/ijo.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. Journal of cellular and molecular medicine. 2010;14:2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raher MJ, Thibault HB, Buys ES, Kuruppu D, Shimizu N, Brownell AL, Blake SL, Rieusset J, Kaneki M, Derumeaux G, Picard MH, Bloch KD, Scherrer-Crosbie M. A short duration of high-fat diet induces insulin resistance and predisposes to adverse left ventricular remodeling after pressure overload. Am J Physiol Heart Circ Physiol. 2008;295:H2495–H2502. doi: 10.1152/ajpheart.00139.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, Tateno K, Moriya J, Yokoyama M, Nojima A, Koh GY, Akazawa H, Shiojima I, Kahn CR, Abel ED, Komuro I. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. The Journal of clinical investigation. 2010;120:1506–1514. doi: 10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abel ED, O’Shea KM, Ramasamy R. Insulin resistance: Metabolic mechanisms and consequences in the heart. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2068–2076. doi: 10.1161/ATVBAHA.111.241984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pereira RO, Wende AR, Crum A, Hunter D, Olsen CD, Rawlings T, Riehle C, Ward WF, Abel ED. Maintaining pgc-1alpha expression following pressure overload-induced cardiac hypertrophy preserves angiogenesis but not contractile or mitochondrial function. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:3691–3702. doi: 10.1096/fj.14-253823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Riehle C, Abel ED. Glut1 deficiency in cardiomyocytes does not accelerate the transition from compensated hypertrophy to heart failure. Journal of molecular and cellular cardiology. 2014;72:95–103. doi: 10.1016/j.yjmcc.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Anderson SM, Abel ED. Inducible overexpression of glut1 prevents mitochondrial dysfunction and attenuates structural remodeling in pressure overload but does not prevent left ventricular dysfunction. Journal of the American Heart Association. 2013;2:e000301. doi: 10.1161/JAHA.113.000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kolwicz SC, Jr, Tian R. Glucose metabolism and cardiac hypertrophy. Cardiovascular research. 2011;90:194–201. doi: 10.1093/cvr/cvr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD. Cardiac insulin-resistance decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circulation. Heart failure. 2013;6:1039–1048. doi: 10.1161/CIRCHEARTFAILURE.112.000228. [DOI] [PubMed] [Google Scholar]
- 121.Abel ED. Glucose transport in the heart. Frontiers in bioscience : a journal and virtual library. 2004;9:201–215. doi: 10.2741/1216. [DOI] [PubMed] [Google Scholar]
- 122.Riehle C, Wende AR, Zaha VG, Pires KM, Wayment B, Olsen C, Bugger H, Buchanan J, Wang X, Moreira AB, Doenst T, Medina-Gomez G, Litwin SE, Lelliott CJ, Vidal-Puig A, Abel ED. Pgc-1beta deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circulation research. 2011;109:783–793. doi: 10.1161/CIRCRESAHA.111.243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB. Cardiac hypertrophy with preserved contractile function after selective deletion of glut4 from the heart. The Journal of clinical investigation. 1999;104:1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Domenighetti AA, Danes VR, Curl CL, Favaloro JM, Proietto J, Delbridge LM. Targeted glut-4 deficiency in the heart induces cardiomyocyte hypertrophy and impaired contractility linked with ca(2+) and proton flux dysregulation. Journal of molecular and cellular cardiology. 2010;48:663–672. doi: 10.1016/j.yjmcc.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 125.Tian R, Abel ED. Responses of glut4-deficient hearts to ischemia underscore the importance of glycolysis. Circulation. 2001;103:2961–2966. doi: 10.1161/01.cir.103.24.2961. [DOI] [PubMed] [Google Scholar]
- 126.Sankaralingam S, Lopaschuk GD. Cardiac energy metabolic alterations in pressure overload-induced left and right heart failure (2013 grover conference series) Pulmonary Circulation. 2015;5:15–28. doi: 10.1086/679608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovascular research. 2009;82:351–360. doi: 10.1093/cvr/cvp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of foxo1 triggers diabetic cardiomyopathy in mice. The Journal of clinical investigation. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wende AR, O’Neill BT, Bugger H, Riehle C, Tuinei J, Buchanan J, Tsushima K, Wang L, Caro P, Guo A, Sloan C, Kim BJ, Wang X, Pereira RO, McCrory MA, Nye BG, Benavides GA, Darley-Usmar VM, Shioi T, Weimer BC, Abel ED. Enhanced cardiac akt/protein kinase b signaling contributes to pathological cardiac hypertrophy in part by impairing mitochondrial function via transcriptional repression of mitochondrion-targeted nuclear genes. Molecular and cellular biology. 2015;35:831–846. doi: 10.1128/MCB.01109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K. Uncoupling proteins in human heart. Lancet. 2004;364:1786–1788. doi: 10.1016/S0140-6736(04)17402-3. [DOI] [PubMed] [Google Scholar]
- 131.Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, Strickland N, Matsui T, Das S, Rosenzweig A, Punjabi P, Camici PG. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. European heart journal. 2010;31:100–111. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cao DJ, Jiang N, Blagg A, Johnstone JL, Gondalia R, Oh M, Luo X, Yang KC, Shelton JM, Rothermel BA, Gillette TG, Dorn GW, Hill JA. Mechanical unloading activates foxo3 to trigger bnip3-dependent cardiomyocyte atrophy. Journal of the American Heart Association. 2013;2:e000016. doi: 10.1161/JAHA.113.000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eijkelenboom A, Burgering BM. Foxos: Signalling integrators for homeostasis maintenance. Nature reviews. Molecular cell biology. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 134.Christopher BA, Huang HM, Berthiaume JM, McElfresh TA, Chen X, Croniger CM, Muzic RF, Jr, Chandler MP. Myocardial insulin resistance induced by high fat feeding in heart failure is associated with preserved contractile function. Am J Physiol Heart Circ Physiol. 2010;299:H1917–H1927. doi: 10.1152/ajpheart.00687.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. The Journal of clinical investigation. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 137.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: Effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 138.McQueen AP, Zhang D, Hu P, Swenson L, Yang Y, Zaha VG, Hoffman JL, Yun UJ, Chakrabarti G, Wang Z, Albertine KH, Abel ED, Litwin SE. Contractile dysfunction in hypertrophied hearts with deficient insulin receptor signaling: Possible role of reduced capillary density. Journal of molecular and cellular cardiology. 2005;39:882–892. doi: 10.1016/j.yjmcc.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 139.Sena S, Hu P, Zhang D, Wang X, Wayment B, Olsen C, Avelar E, Abel ED, Litwin SE. Impaired insulin signaling accelerates cardiac mitochondrial dysfunction after myocardial infarction. Journal of molecular and cellular cardiology. 2009;46:910–918. doi: 10.1016/j.yjmcc.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fu Q, Xu B, Liu Y, Parikh D, Li J, Li Y, Zhang Y, Riehle C, Zhu Y, Rawlings T, Shi Q, Clark RB, Chen X, Abel ED, Xiang YK. Insulin inhibits cardiac contractility by inducing a gi-biased beta2-adrenergic signaling in hearts. Diabetes. 2014;63:2676–2689. doi: 10.2337/db13-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lucas E, Jurado-Pueyo M, Fortuno MA, Fernandez-Veledo S, Vila-Bedmar R, Jimenez-Borreguero LJ, Lazcano JJ, Gao E, Gomez-Ambrosi J, Fruhbeck G, Koch WJ, Diez J, Mayor F, Jr, Murga C. Downregulation of g protein-coupled receptor kinase 2 levels enhances cardiac insulin sensitivity and switches on cardioprotective gene expression patterns. Biochimica et biophysica acta. 2014;1842:2448–2456. doi: 10.1016/j.bbadis.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 142.Woodall MC, Ciccarelli M, Woodall BP, Koch WJ. G protein-coupled receptor kinase 2: A link between myocardial contractile function and cardiac metabolism. Circulation research. 2014;114:1661–1670. doi: 10.1161/CIRCRESAHA.114.300513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Inuzuka Y, Okuda J, Kawashima T, Kato T, Niizuma S, Tamaki Y, Iwanaga Y, Yoshida Y, Kosugi R, Watanabe-Maeda K, Machida Y, Tsuji S, Aburatani H, Izumi T, Kita T, Shioi T. Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation. 2009;120:1695–1703. doi: 10.1161/CIRCULATIONAHA.109.871137. [DOI] [PubMed] [Google Scholar]
- 144.Ock S, Lee WS, Ahn J, Kim HM, Kang H, Kim HS, Jo D, Abel ED, Lee TJ, Kim J. Deletion of igf-1 receptors in cardiomyocytes attenuates cardiac aging in male mice. Endocrinology. 2016;157:336–345. doi: 10.1210/en.2015-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: Role of autophagy. Basic research in cardiology. 2011;106:1173–1191. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 146.Jaishy B, Zhang Q, Chung HS, Riehle C, Soto J, Jenkins S, Abel P, Cowart LA, Van Eyk JE, Abel ED. Lipid-induced nox2 activation inhibits autophagic flux by impairing lysosomal enzyme activity. Journal of lipid research. 2015;56:546–561. doi: 10.1194/jlr.M055152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shirakabe A, Ikeda Y, Zai P, Saito T, Sadoshima J. Autophagy protects the heart against pressure overload induced heart failure. Circulation. 2015;132:A17583. [Google Scholar]
- 148.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nature medicine. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 149.Takemura G, Kanamori H, Goto K, Maruyama R, Tsujimoto A, Fujiwara H, Seishima M, Minatoguchi S. Autophagy maintains cardiac function in the starved adult. Autophagy. 2009;5:1034–1036. doi: 10.4161/auto.5.7.9297. [DOI] [PubMed] [Google Scholar]
- 150.Qi Y, Xu Z, Zhu Q, Thomas C, Kumar R, Feng H, Dostal DE, White MF, Baker KM, Guo S. Myocardial loss of irs1 and irs2 causes heart failure and is controlled by p38alpha mapk during insulin resistance. Diabetes. 2013;62:3887–3900. doi: 10.2337/db13-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qi Y, Zhu Q, Zhang K, Thomas C, Wu Y, Kumar R, Baker KM, Xu Z, Chen S, Guo S. Activation of foxo1 by insulin resistance promotes cardiac dysfunction and beta-myosin heavy chain gene expression. Circulation. Heart failure. 2015;8:198–208. doi: 10.1161/CIRCHEARTFAILURE.114.001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ilkun O, Wilde N, Tuinei J, Pires KM, Zhu Y, Bugger H, Soto J, Wayment B, Olsen C, Litwin SE, Abel ED. Antioxidant treatment normalizes mitochondrial energetics and myocardial insulin sensitivity independently of changes in systemic metabolic homeostasis in a mouse model of the metabolic syndrome. Journal of molecular and cellular cardiology. 2015;85:104–116. doi: 10.1016/j.yjmcc.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Qin F, Siwik DA, Luptak I, Hou X, Wang L, Higuchi A, Weisbrod RM, Ouchi N, Tu VH, Calamaras TD, Miller EJ, Verbeuren TJ, Walsh K, Cohen RA, Colucci WS. The polyphenols resveratrol and s17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation. 2012;125:1757–1764. doi: 10.1161/CIRCULATIONAHA.111.067801. S1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vila-Bedmar R, Cruces-Sande M, Lucas E, Willemen HL, Heijnen CJ, Kavelaars A, Mayor F, Jr, Murga C. Reversal of diet-induced obesity and insulin resistance by inducible genetic ablation of grk2. Science signaling. 2015;8:ra73. doi: 10.1126/scisignal.aaa4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gilbert RE, Krum H. Heart failure in diabetes: Effects of anti-hyperglycaemic drug therapy. Lancet. 2015;385:2107–2117. doi: 10.1016/S0140-6736(14)61402-1. [DOI] [PubMed] [Google Scholar]
- 156.Udell JA, Cavender MA, Bhatt DL, Chatterjee S, Farkouh ME, Scirica BM. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: A meta-analysis of randomised controlled trials. The lancet. Diabetes & endocrinology. 2015;3:356–366. doi: 10.1016/S2213-8587(15)00044-3. [DOI] [PubMed] [Google Scholar]
- 157.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I, Committee S-TS. Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. The New England journal of medicine. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 158.Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: A comparative review. Diabetes, obesity & metabolism. 2011;13:7–18. doi: 10.1111/j.1463-1326.2010.01306.x. [DOI] [PubMed] [Google Scholar]
- 159.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, Vanderloo SE, McAlister FA. Comparative safety effectiveness of metformin in patients with diabetes mellitus and heart failure: Systematic review of observational studies involving 34,000 patients. Circulation. Heart failure. 2013;6:395–402. doi: 10.1161/CIRCHEARTFAILURE.112.000162. [DOI] [PubMed] [Google Scholar]
- 161.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. The New England journal of medicine. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 162.Bugger H, Riehle C, Jaishy B, Wende AR, Tuinei J, Chen D, Soto J, Pires KM, Boudina S, Theobald HA, Luptak I, Wayment B, Wang X, Litwin SE, Weimer BC, Abel ED. Genetic loss of insulin receptors worsens cardiac efficiency in diabetes. Journal of molecular and cellular cardiology. 2012 doi: 10.1016/j.yjmcc.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fullmer TM, Pei S, Zhu Y, Sloan C, Manzanares R, Henrie B, Pires KM, Cox JE, Abel ED, Boudina S. Insulin suppresses ischemic preconditioning-mediated cardioprotection through akt-dependent mechanisms. Journal of molecular and cellular cardiology. 2013;64:20–29. doi: 10.1016/j.yjmcc.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Laustsen PG, Russell SJ, Cui L, Entingh-Pearsall A, Holzenberger M, Liao R, Kahn CR. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Molecular and cellular biology. 2007;27:1649–1664. doi: 10.1128/MCB.01110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Long YC, Cheng Z, Copps KD, White MF. Insulin receptor substrates irs1 and irs2 coordinate skeletal muscle growth and metabolism via the akt and ampk pathways. Molecular and cellular biology. 2011;31:430–441. doi: 10.1128/MCB.00983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nature genetics. 1999;23:32–40. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- 167.Liu SC, Wang Q, Lienhard GE, Keller SR. Insulin receptor substrate 3 is not essential for growth or glucose homeostasis. The Journal of biological chemistry. 1999;274:18093–18099. doi: 10.1074/jbc.274.25.18093. [DOI] [PubMed] [Google Scholar]
- 168.Fantin VR, Wang Q, Lienhard GE, Keller SR. Mice lacking insulin receptor substrate 4 exhibit mild defects in growth, reproduction, glucose homeostasis American journal of physiology. Endocrinology and metabolism. 2000;278:E127–E133. doi: 10.1152/ajpendo.2000.278.1.E127. [DOI] [PubMed] [Google Scholar]
- 169.Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumo S, Cantley LC. Class ia phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Molecular and cellular biology. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. The EMBO journal. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, Buerger A, Izumo S, Jay PY, Jennings GL. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:612–617. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lin RC, Weeks KL, Gao XM, Williams RB, Bernardo BC, Kiriazis H, Matthews VB, Woodcock EA, Bouwman RD, Mollica JP, Speirs HJ, Dawes IW, Daly RJ, Shioi T, Izumo S, Febbraio MA, Du XJ, McMullen JR. Pi3k(p110 alpha) protects against myocardial infarction-induced heart failure: Identification of pi3k-regulated mirna and mrna. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:724–732. doi: 10.1161/ATVBAHA.109.201988. [DOI] [PubMed] [Google Scholar]
- 174.Oudit GY, Crackower MA, Eriksson U, Sarao R, Kozieradzki I, Sasaki T, Irie-Sasaki J, Gidrewicz D, Rybin VO, Wada T, Steinberg SF, Backx PH, Penninger JM. Phosphoinositide 3-kinase gamma-deficient mice are protected from isoproterenol-induced heart failure. Circulation. 2003;108:2147–2152. doi: 10.1161/01.CIR.0000091403.62293.2B. [DOI] [PubMed] [Google Scholar]
- 175.Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E. Pi3kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 176.Ito K, Akazawa H, Tamagawa M, Furukawa K, Ogawa W, Yasuda N, Kudo Y, Liao CH, Yamamoto R, Sato T, Molkentin JD, Kasuga M, Noda T, Nakaya H, Komuro I. Pdk1 coordinates survival pathways and beta-adrenergic response in the heart. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8689–8694. doi: 10.1073/pnas.0900064106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Di RM, Feng QT, Chang Z, Luan Q, Zhang YY, Huang J, Li XL, Yang ZZ. Pdk1 plays a critical role in regulating cardiac function in mice and human. Chinese medical journal. 2010;123:2358–2363. [PubMed] [Google Scholar]
- 178.Mora A, Davies AM, Bertrand L, Sharif I, Budas GR, Jovanovic S, Mouton V, Kahn CR, Lucocq JM, Gray GA, Jovanovic A, Alessi DR. Deficiency of pdk1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. The EMBO journal. 2003;22:4666–4676. doi: 10.1093/emboj/cdg469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Etzion S, Etzion Y, DeBosch B, Crawford PA, Muslin AJ. Akt2 deficiency promotes cardiac induction of rab4a and myocardial beta-adrenergic hypersensitivity. Journal of molecular and cellular cardiology. 2010;49:931–940. doi: 10.1016/j.yjmcc.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li X, Mikhalkova D, Gao E, Zhang J, Myers V, Zincarelli C, Lei Y, Song J, Koch WJ, Peppel K, Cheung JY, Feldman AM, Chan TO. Myocardial injury after ischemia-reperfusion in mice deficient in akt2 is associated with increased cardiac macrophage density. Am J Physiol Heart Circ Physiol. 2011;301:H1932–H1940. doi: 10.1152/ajpheart.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Taniyama Y, Ito M, Sato K, Kuester C, Veit K, Tremp G, Liao R, Colucci WS, Ivashchenko Y, Walsh K, Shiojima I. Akt3 overexpression in the heart results in progression from adaptive to maladaptive hypertrophy. Journal of molecular and cellular cardiology. 2005;38:375–385. doi: 10.1016/j.yjmcc.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 182.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for akt3/protein kinase bgamma in attainment of normal brain size. Molecular and cellular biology. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase b promotes organ growth in transgenic mice. Molecular and cellular biology. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated akt in the heart. The Journal of biological chemistry. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 185.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hirotani S, Zhai P, Tomita H, Galeotti J, Marquez JP, Gao S, Hong C, Yatani A, Avila J, Sadoshima J. Inhibition of glycogen synthase kinase 3beta during heart failure is protective. Circulation research. 2007;101:1164–1174. doi: 10.1161/CIRCRESAHA.107.160614. [DOI] [PubMed] [Google Scholar]
- 187.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 188.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mtor signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 189.Di R, Wu X, Chang Z, Zhao X, Feng Q, Lu S, Luan Q, Hemmings BA, Li X, Yang Z. S6k inhibition renders cardiac protection against myocardial infarction through pdk1 phosphorylation of akt. The Biochemical journal. 2012;441:199–207. doi: 10.1042/BJ20110033. [DOI] [PubMed] [Google Scholar]
- 190.Zhu Y, Pires KM, Whitehead KJ, Olsen CD, Wayment B, Zhang YC, Bugger H, Ilkun O, Litwin SE, Thomas G, Kozma SC, Abel ED. Mechanistic target of rapamycin (mtor) is essential for murine embryonic heart development and growth. PloS one. 2013;8:e54221. doi: 10.1371/journal.pone.0054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. Mtorc1 regulates cardiac function and myocyte survival through 4e-bp1 inhibition in mice. The Journal of clinical investigation. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, Krishnan J, Lerch R, Hall MN, Ruegg MA, Pedrazzini T, Brink M. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- 193.Zhu Y, Soto J, Anderson B, Riehle C, Zhang YC, Wende AR, Jones D, McClain DA, Abel ED. Regulation of fatty acid metabolism by mtor in adult murine hearts occurs independently of changes in pgc-1alpha. Am J Physiol Heart Circ Physiol. 2013;305:H41–H51. doi: 10.1152/ajpheart.00877.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shen WH, Chen Z, Shi S, Chen H, Zhu W, Penner A, Bu G, Li W, Boyle DW, Rubart M, Field LJ, Abraham R, Liechty EA, Shou W. Cardiac restricted overexpression of kinase-dead mammalian target of rapamycin (mtor) mutant impairs the mtor-mediated signaling and cardiac function. The Journal of biological chemistry. 2008;283:13842–13849. doi: 10.1074/jbc.M801510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Dorfman AL, Longnus S, Pende M, Martin KA, Blenis J, Thomas G, Izumo S. Deletion of ribosomal s6 kinases does not attenuate pathological, physiological, or insulin-like growth factor 1 receptor-phosphoinositide 3-kinase-induced cardiac hypertrophy. Molecular and cellular biology. 2004;24:6231–6240. doi: 10.1128/MCB.24.14.6231-6240.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li Y, Wende AR, Nunthakungwan O, Huang Y, Hu E, Jin H, Boudina S, Abel ED, Jalili T. Cytosolic, but not mitochondrial, oxidative stress is a likely contributor to cardiac hypertrophy resulting from cardiac specific glut4 deletion in mice. The FEBS journal. 2012;279:599–611. doi: 10.1111/j.1742-4658.2011.08450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kaczmarczyk SJ, Andrikopoulos S, Favaloro J, Domenighetti AA, Dunn A, Ernst M, Grail D, Fodero-Tavoletti M, Huggins CE, Delbridge LM, Zajac JD, Proietto J. Threshold effects of glucose transporter-4 (glut4) deficiency on cardiac glucose uptake and development of hypertrophy. Journal of molecular endocrinology. 2003;31:449–459. doi: 10.1677/jme.0.0310449. [DOI] [PubMed] [Google Scholar]
- 198.Huggins CE, Domenighetti AA, Ritchie ME, Khalil N, Favaloro JM, Proietto J, Smyth GK, Pepe S, Delbridge LM. Functional and metabolic remodelling in glut4-deficient hearts confers hyper-responsiveness to substrate intervention. Journal of molecular and cellular cardiology. 2008;44:270–280. doi: 10.1016/j.yjmcc.2007.11.020. [DOI] [PubMed] [Google Scholar]