Abstract
In Brief Patients with severe insulin resistance require >2 units/kg of body weight or 200 units/day of insulin. Yet, many patients do not achieve glycemic targets despite using very high doses of insulin. Insulin can cause weight gain, which further contributes to worsening insulin resistance. This article describes the pharmacological options for managing patients with severe insulin resistance, including the use of U-500 insulin and newer agents in combination with insulin.
An increasing number of patients have severe insulin resistance and require large doses of insulin. Managing patients with severe insulin resistance is challenging because it is difficult to achieve good glycemic control using conventional treatment approaches (1). Moreover, weight gain, hypoglycemia, regimen complexity, and cost are frequent concerns as insulin doses escalate.
Insulin resistance is characterized by an impaired response to either endogenous or exogenous insulin (2). Although insulin resistance is a common feature of type 2 diabetes, cases of severe insulin resistance remain relatively uncommon but are likely increasing as the prevalence of diabetes and obesity surges. The degree of insulin resistance can be measured using the euglyemic insulin clamp technique, but this is not a clinically useful method of determining whether a patient has severe insulin resistance in practice (3). The most widely reported and clinically useful definitions of severe insulin resistance are based on exogenous insulin requirements using either the number of units per kilogram of body weight per day or the total daily dose (1). Patients who require >1 unit/kg/day are considered to have insulin resistance, and those requiring >2 units/kg/day have severe resistance (3). Alternatively, a total daily insulin dose of >200 units is commonly considered to be evidence of severe insulin resistance. Large total daily dose requirements create practical problems with regard to insulin delivery because 1) a large volume of standard U-100 insulin can be painful to administer and 2) the onset and duration of insulin activity can be altered with high-volume doses (4).
Evaluating Patients
There are several known causes of severe insulin resistance, including several rare disorders and genetic conditions (Table 1) (3). Several medications are known to contribute to insulin resistance, including glucocorticoids, protease inhibitors, atypical antipsychotics, and calcineurin inhibitors. In patients with severe insulin resistance, an effort should be made to discontinue such agents or switch to alternative medications if possible (5).
TABLE 1.
Causes of Severe Insulin Resistance (3)
Syndromes of severe insulin resistance
|
Medications
|
Endocrine disorders
|
| Anti-insulin antibodies |
| HIV-associated lipodystrophy |
Physiological causes
|
Pseudoresistance
|
Poor medication-taking behaviors or “pseudoresistance” should be ruled out before modifying or intensifying therapy. Pseudoresistance may be the result of nonadherence, poor injection technique, improper insulin storage, or malingering for secondary gain. Pseudoresistance can be ruled out by conducting a modified insulin tolerance test (3). During such a test, patients are administered a witnessed dose of short-acting insulin in the clinic, and their blood glucose is monitored every 30 minutes for a period of 4–8 hours. Patients should be fasting for the test and should have a blood glucose level >150 mg/dL. A witnessed insulin dose approximately equal to what an average person with diabetes might require (insulin dose [units] = blood glucose [mg/dL] – 100 / (1,500 / weight [kg] × 1.0)] should be given. If there is not an appropriate drop in blood glucose within 4 hours, a second dose should be given. If normoglycemia or hypoglycemia is not achieved after either dose, the test confirms that a patient likely has severe insulin resistance.
Pharmacological Treatment Options
There are currently no guidelines or consensus statements describing how best to treat patients with severe insulin resistance. Until recently, insulin was the only therapy available to treat those with severe insulin resistance. Despite the use of high doses of insulin, however, many patients do not reach their glycemic targets and are hampered by undesirable adverse effects such as hypoglycemia and weight gain. To mitigate some of these challenges, several new therapies have emerged and can be used in combination with insulin.
Concentrated Insulin Products
Concentrated insulin products can help improve the delivery of insulin when very large doses are needed. U-500 insulin is five times more concentrated than standard U-100 insulin and is considered the cornerstone of therapy for most patients with severe insulin resistance (6). When daily insulin doses exceed 200 units/day, the volume of U-100 insulin needed makes insulin delivery challenging. Available insulin syringes can deliver a maximum of 100 units, and insulin pen devices can deliver only 60–80 units per injection. In addition, the administration of doses >1 mL in volume can be painful and may alter insulin absorption (7).
Two new concentrated insulin pen products are now available in the United States—U-200 insulin lispro and U-300 insulin glargine. There is no reported experience using these new concentrated insulin products in patients with severe insulin resistance, and clinical trials comparing these products to U-500 insulin have not been conducted. Although they are two to three times more concentrated than U-100 insulin, U-300 insulin glargine and U-200 insulin lispro can only deliver a maximum of 80 and 60 units per injection, respectively. They do offer the theoretical advantage of providing a dose of insulin in a smaller injection volume than would be required with U-100 (8,9).
Similar to U-100 regular insulin, the onset of activity for U-500 regular insulin is ∼30–45 minutes. However, the time to peak activity (4–6 hours) and duration of action (12–14 hours) for U-500 is most similar to NPH insulin (6).
In several crossover studies in which patients with severe insulin resistance were switched from U-100 insulin products to U-500 regular insulin, significant improvements in glycemic control have been observed (10–18). An analysis of nine studies (n = 310 patients) found that U-500 reduced mean A1C by 1.59% (95% CI 1.26–1.92) in patients who previously used a multiple daily injection (MDI) regimen with various U-100 insulin products. At baseline, these patients had an A1C between 9.1 and 11.3% and a total daily insulin requirement of 219–391 units. They were followed for 6–36 months. Weight gain was a substantial problem, with a mean increase of 4.4 kg (95% CI 2.4–6.4) in body weight. The mean total daily insulin dose increased by 52 units (95% CI 20–84) (19). U-500 insulin delivered by continuous subcutaneous insulin infusion (CSII) is a potential option. In one study, U-500 delivered via CSII reduced mean A1C by 1.1% (P = 0.026) in a cohort of patients with severe insulin resistance who were switched from a variety of insulin regimens, including U-500 insulin via MDI (20).
The risk of severe hypoglycemia does not appear to increase when patients are switched from U-100 to U-500 insulin (6,19). However, some studies have reported an increase in mild hypoglycemic events, defined as symptomatic episodes that did not require assistance (13,15). One retrospective study reported an increase in the frequency of mild hypoglycemic episodes only during the first 3 months after transitioning to U-500 insulin (13).
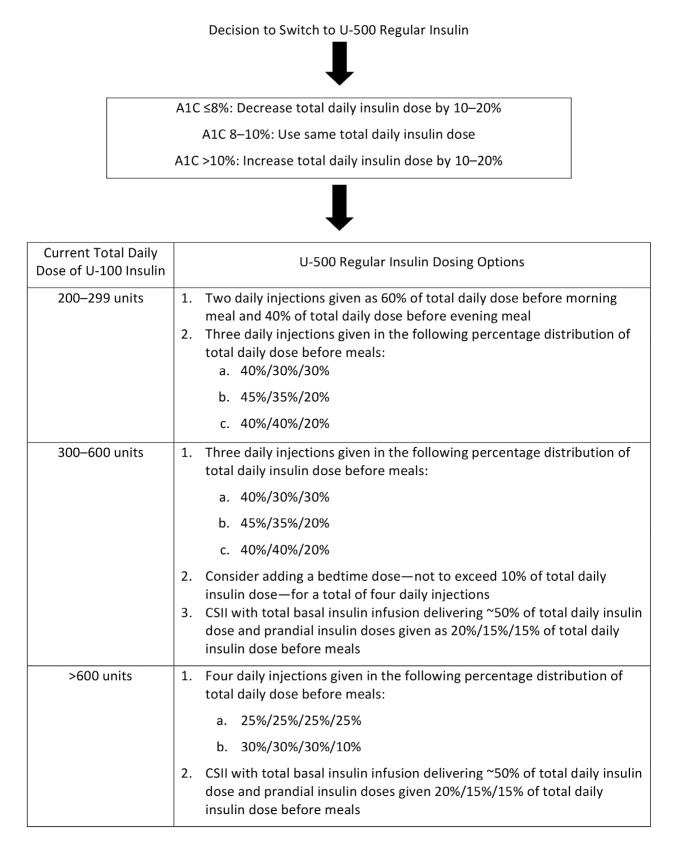
When transitioning a patient from U-100 to U-500 insulin, the dose and dosing frequency should be determined based on the patient’s current A1C and total daily insulin dose. Dosing algorithms have not yet been prospectively evaluated (Figure 1); nonetheless, they have been widely used. In general, U-500 should be given at least 30 minutes before a meal. One of the biggest drawbacks to using U-500 insulin is the potential for dosing errors that can lead to severe hypoglycemia, coma, or death. Clear communications between the prescriber, pharmacist, and patient are crucial. When prescribing and dispensing U-500 regular insulin, the dose should be expressed in both units and volume (mL) to be administered. To minimize the risk of errors, a 0.5–1 mL tuberculin syringe with a 29-gauge or higher needle should be used to administer each dose—not a U-100 insulin syringe (6,21).
FIGURE 1.
U-500 regular insulin initial dosing recommendations (6,55).
Metformin
The American Diabetes Association recommends metformin as the initial pharmacological option for most people with type 2 diabetes. It has a strong record of safety and efficacy, as well as a favorable effect on weight (22). Although it is common practice to combine metformin with insulin, metformin use has not been specifically evaluated in the setting of severe insulin resistance.
In most studies of U-500 regular insulin, patients have been permitted to continue metformin use (6,23,24). In patients who do not have severe insulin resistance, metformin use reduces insulin requirements and has a positive impact on glycemic control and weight. A meta-analysis of 26 randomized, controlled trials assessed the effects of metformin plus insulin versus insulin alone. Metformin combined with insulin resulted in a significant reduction in A1C (mean difference –0.60%, P <0.001) and lower insulin requirements (mean difference –18.9 units/day, P <0.001) when compared to insulin therapy alone. Moreover, weight gain was mitigated with combination therapy (mean difference –1.68 kg, P <0.001). The largest study conducted to date combining metformin with insulin therapy is the HOME (Hyperinsulinemia: the Outcome of its Metabolic Effects) study (25). The HOME study randomized 390 patients with type 2 diabetes currently using basal-bolus insulin regimens to either metformin titrated to 850 mg three times daily or placebo. At baseline, patients’ mean total daily insulin dose was ∼70 units, mean A1C was 7.8%, and mean body weight was 86 kg. At the end of the 16-week treatment period, those in the metformin group had a significant reduction in A1C of 0.9% compared to 0.3% in the placebo group (P <0.0001). Total mean daily insulin requirements were reduced by ∼10% (7.2 units) from baseline in the metformin group (P <0.0001), along with a small but statistically significant reduction in weight of 0.4 kg (P <0.0001).
Metformin is known to cause gastrointestinal (GI) side effects, which can lead to discontinuation of therapy (26). This typically manifests as diarrhea and less commonly as nausea or abdominal cramping (22,26). One option to mitigate these effects is to use the extended-release formulation of metformin, which has been shown to reduce the frequency of any GI side effects, including diarrhea (27).
Use of metformin in the setting of mild to moderate renal impairment is controversial. The potential risk of lactic acidosis appears to be negligible in the absence of other risk factors. Current U.S. Food and Drug Administration (FDA)-approved labeling for metformin still recommends against using metformin in men with a serum creatinine ≥1.5 mg/dL and in women with a serum creatinine ≥1.4 mg/dL (28). However, a Cochrane review including 347 studies concluded that the incidence of lactic acidosis was 4.3 cases per 100,000 patient-years in those treated with metformin compared to 5.4 cases per 100,000 patients for those not treated with metformin (29). Several organizations, including the American Geriatric Society, and the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines now advocate for continued metformin use as long as a patient’s estimated glomerular filtration rate is >30 mL/min (30,31).
Glucagon-Like Peptide 1 Receptor Agonists
Glucagon-like peptide 1 (GLP-1) receptor agonists stimulate the GLP-1 receptors in the pancreas and thereby increase insulin release and inhibit glucagon secretion, but only in the presence of elevated blood glucose (32). A recent meta-analysis of 15 studies showed that a GLP-1 receptor agonist combined with basal insulin was superior to basal-bolus insulin combinations in patients with type 2 diabetes (33). The GLP-1 receptor agonist–basal insulin combination led to significantly improved glycemic control and reduced body weight without increasing the risk of hypoglycemia when compared to basal-bolus insulin alone. These features have sparked interest in using GLP-1 receptor agonists in patients with severe insulin resistance (23,24).
In one recent, prospective, open-label study, 37 obese patients using basal-bolus insulin with total daily insulin requirements >100 units/day and a baseline A1C >6.5% were randomized to receive either liraglutide titrated to 1.8 mg/day or continued uptitration of their insulin doses (24). Seventeen patients were using U-500 insulin at baseline. Mean baseline A1C (7.8%) was similar in the two groups, but the mean total daily insulin dose was greater in the liraglutide group (199 vs. 171 units/day) and baseline weight was greater in the insulin treatment group (112 vs. 131 kg). At 6 months, both groups had statistically significant improvements in A1C compared to baseline. However, the A1C reduction was significantly greater in the liraglutide treatment group than in the insulin treatment group (mean A1C at 6 months 7.14 vs. 7.40%, P = 0.047). Moreover, time spent in the euglycemic range (blood glucose 70–180 mg/dL) based on continuous glucose monitoring increased from 57% of the time at baseline to 75% of the time at 6 months in the liraglutide group. The liraglutide group experienced significant weight loss (–5.3 kg, P <0.001) compared to a nonsignificant weight gain in the insulin-only treatment group (0.4 kg, P = 0.595). The mean total daily insulin dose in the liraglutide group dropped by 34% from 199 to 132 units (P <0.0001), whereas insulin requirements in the insulin titration group remained relatively unchanged, with a nonsignificant 4% increase from 171 to 178 units (P = 0.453). Hypoglycemia (blood glucose <70 mg/dL) based on continuous glucose monitoring was similar in both groups (<5% of the time) at baseline and 6-month follow-up. There were no episodes of severe hypoglycemia requiring assistance in any subjects during the study.
In another small, open-label, prospective study, 30 obese patients with type 2 diabetes treated with U-100 insulin and requiring >200 units/day of insulin were randomized to U-500 insulin or U-500 plus exenatide titrated to 10 μg twice daily (34). All patients in both treatment groups also received metformin in doses of 1,700–2,550 mg/day. Baseline A1C (9.2 vs. 8.7%), weight (118 vs. 119 kg), and total daily insulin dose (237 vs. 253 units) were reasonably similar in the two groups. After 6 months of treatment, there were significant improvements in glycemic control in both groups compared to baseline, but there was no difference in the between-group comparison (P = 0.80). There was a slight but nonsignificant mean weight loss in the exenatide group (–0.4 kg) and a slight but nonsignificant mean weight gain (3.6 kg) in the U-500 insulin-only group compared to baseline, with a between-group difference (4.0 kg) that was not statistically different at 6 months (P = 0.07). Similarly, total daily insulin doses were slightly lower in the U-500 insulin (–7 units/day) and exenatide (–27 units) treatment groups at 6 months compared to baseline, but the within- and between-group differences were not statistically significant. None of the patients experienced severe hypoglycemia requiring assistance, and mild symptomatic episodes were more frequent in the exenatide treatment group (50 vs. 11 episodes, P <0.001) over the 6-month treatment period.
A small, retrospective, observational study analyzed the effects of adding liraglutide to U-500 insulin in 15 obese patients with severe insulin resistance (23). The cohort’s mean baseline A1C was 8.5%, total daily insulin dose was 192 units, and weight was 137 kg. After 12 weeks of liraglutide treatment at a dose of 1.2 or 1.8 mg/day, there was a significant mean reduction in A1C (1.4%, P = 0.0001), total daily insulin dose (44 units, P = 0.0001), and weight (5.1 kg, P = 0.0001). Hypoglycemia, defined as blood glucose <70 mg/dL, occurred in eight patients, but there were no severe episodes requiring assistance.
Nausea and vomiting are the most common adverse effects associated with GLP-1 receptor agonist use, but they are often transient. It is a dose-dependent phenomenon, and the incidence varies among the FDA-approved products in this class (35).
Although combination therapy with GLP-1 receptor agonists and basal-bolus insulin is not approved by the FDA, there are several potential benefits when adding these agents to U-100 insulin, including improvement in glycemic control, weight loss, and reduced insulin requirements (24). Some patients may be able to discontinue prandial insulin use. However, it remains unclear whether combining U-500 insulin with a GLP-1 receptor agonist is an effective or cost-effective strategy. More studies are needed comparing GLP-1 receptor agonists to U-500 regular insulin to define which patients with severe insulin resistance would benefit most from these therapies.
Sodium-Glucose Cotransporter 2 Inhibitors
Sodium-glucose cotransporter 2 (SGLT2) inhibitors increase the excretion of urinary glucose, thereby reducing plasma glucose concentrations independent of the presence of insulin (36). These medications have been shown to reduce body weight, and this is one of the features that has prompted studies in obese patients treated with insulin.
A 52-week, double-blind, placebo-controlled trial of 563 obese patients with uncontrolled type 2 diabetes on a total daily insulin dose >60 units were randomized to receive once-daily empagliflozin 10 mg, empagliflozin 25 mg, or placebo (37). During weeks 19–40 of this study, insulin doses were titrated to achieve specified glucose targets. The mean A1C of study subjects at baseline was 8.3%, and patients had an average daily insulin dose of 92 units. At 18 weeks, A1C was reduced by 0.4% in the empagliflozin 10 mg group and by 0.5% in the 25 mg group compared to placebo (P <0.001), and these improvements in glycemic control were largely sustained at 52 weeks in both treatment groups. When compared to insulin requirements in the placebo group, the mean total daily insulin dose in the empagliflozin 10 mg group was 8.8 units lower (P = 0.004) and in the 25 mg group was 11.2 units lower (P <0.001). Patients who received empagliflozin lost ∼2 kg of weight compared to a 0.4-kg weight gain in the placebo group (P <0.001). Objectively confirmed hypoglycemia during the 52-week study occurred in a similar percentage of patients in the empagliflozin 10 mg (51.1%), empagliflozin 25 mg (57.7%), and placebo (58.0%) groups. Three patients each in the placebo and empagliflozin 10 mg groups and 1 patient in the empagliflozin 25 mg group had severe hypoglycemia requiring assistance.
SGLT2 inhibitors are known to increase the risk of urinary tract and genital mycotic infections, particularly in women with a history of these infections (38). Some patients experience orthostatic hypotension and changes in renal function secondary to the osmotic diuresis. Thus, in patients with severe insulin resistance who have very poor glycemic control, SGLT2 inhibitors are not the best choice because these patients are already at higher risk for dehydration and developing genitourinary tract infections.
Available data suggest that SGLT2 inhibitors, when combined with insulin therapy, may lead to modest improvements in glycemic control and modest weight loss without increasing the risk of hypoglycemia. However, given the lack of data regarding the benefits of SGLT2 inhibitors in patients with severe insulin resistance, it is premature to recommend their use.
Dipeptidyl Peptidase-4 Inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors prolong the activity of endogenous GLP-1 and glucose insulinotropic polypeptide by preventing their breakdown and potentiating their actions. On the surface, DPP-4 inhibitors, which are taken orally, would appear to be an attractive alternative to GLP-1 receptor agonists for the management of patients with severe insulin resistance. Indeed, all FDA-approved DPP-4 inhibitors have been studied in combination with insulin. However, none have been studied in patients with severe insulin resistance (39). When combined with insulin, DPP-4 inhibitors produce roughly similar reductions in mean A1C of 0.4–0.6% over a period of 24–52 weeks (40–43). However, unlike treatment with GLP-1 receptor agonists, DPP-4 inhibitors, when combined with insulin, do not promote weight loss or reduce total daily insulin requirements. Although DPP-4 inhibitors are well tolerated and typically do not increase the risk of hypoglycemia, their role in the management of patients with severe insulin resistance is questionable (22,39).
Pramlintide
Pramlintide is a synthetic analog of amylin, a neuroendocrine hormone that is cosecreted with insulin from pancreatic β-cells. It is effective as an adjunct to insulin therapy in patients with type 1 or type 2 diabetes by reducing postprandial glucose excursions (44). None of the studies conducted to date have specifically examined the benefits of pramlintide in patients with severe insulin resistance, but two studies in patients with poorly controlled type 2 diabetes provide some insights. Patients in these placebo-controlled studies received pramlintide in doses ranging from 90 to 300 µg/day (45,46). The mean baseline A1C was ∼9% in both studies, and the total daily insulin dose was 60–70 units. After 52 weeks, there was a statistically significant reduction in A1C of 0.6–0.7% and in weight of 1.4–1.5 kg. In one study, insulin total daily dose requirements increased in all treatment groups but to a lesser degree in the pramlintide groups (46).
Severe hypoglycemia requiring assistance may occur during the first 4 weeks of treatment with pramlintide, but beyond the initial treatment period, the risk appears to be similar to placebo (45). To mitigate the potential risk of severe hypoglycemia, the manufacturer recommends reducing prandial insulin doses when initiating pramlintide, and this practice also would be prudent in patients with severe insulin resistance (44). Nausea is a common adverse effect associated with pramlintide use (45,46), but it does not appear to be dose dependent and is typically transient, subsiding after 4–8 weeks of therapy.
Pramlintide produces modest improvements in glycemic control and weight without significantly increasing the risk of hypoglycemia. Its major drawback is frequent subcutaneous administration; it must be taken two or three times daily before meals. In the setting of severe insulin resistance, pramlintide use might result in reduced insulin requirements and significantly greater weight loss, but data are lacking. GLP-1 receptor agonists appear to be a better option to achieve these goals.
Thiazolidinediones
Thiazolidinediones (TZDs) improve insulin sensitivity by increasing insulin-dependent glucose disposal and decreasing hepatic glucose output. Given their mechanism of action, TZDs commonly have been combined with insulin in practice. TZDs have not been evaluated specifically in the setting of severe insulin resistance, but a few studies have examined their use in combination with insulin (47). A double-blind trial of 222 patients with uncontrolled type 2 diabetes (mean A1C of 8.5%) requiring insulin (mean total daily dose of 56 units/day) were randomized to pioglitazone (titrated to 45 mg/day) or placebo after titrating basal insulin doses to achieve a fasting blood glucose <140 mg/dL. After 20 weeks, both groups experienced a reduction in A1C of 1.4–1.6%, but the difference between the groups was not statistically significant. The mean daily insulin dose was lower in the pioglitazone group (mean difference 12 units, P <0.001), but more patients in the pioglitazone group experienced hypoglycemia (46 vs. 31%, P <0.005). Weight gain was greater in the pioglitazone group than the placebo group (4.4 vs. 2.2 kg, statistical comparison not reported). The magnitude of weight gain appears to be comparable to what patients might experience when switching to U-500 insulin. However, the lack of improvement in glycemic control, coupled with potential serious adverse effects (e.g., heart failure and fractures), limit the usefulness of this class of agents in patients with severe insulin resistance (6,22).
Other Therapies
Several other oral antidiabetic therapies are available, but there is a lack of evidence and experience using these agents in patients with severe insulin resistance. Given the very high insulin requirements of patients with severe insulin resistance, sulfonylureas or meglitinides are not likely to have any clinical utility. α-Glucosidase inhibitors (AGIs) reduce prandial hyperglycemia by retarding hydrolysis of complex carbohydrates. The AGI acarbose was studied in patients with type 2 diabetes with a mean total daily dose of insulin of 62 units and baseline A1C of 8.7%. After 26 weeks of therapy, patients who received acarbose had a significant reduction in A1C of 0.7%. However, these findings have not been replicated in patients with severe insulin resistance, and dose-dependent GI effects (i.e., flatulence, diarrhea, and abdominal pain) often limit use of these agents (48). Colesevelam is a bile acid sequesterant approved to treat hyperlipidemia and type 2 diabetes. The mechanism for its glucose-lowering effect is unknown (49). It has been shown to produce a modest reduction in A1C of 0.5% in patients treated with ∼75 units of insulin/day in one 16-week study (50). Bromocriptine is a dopamine receptor agonist indicated as adjunct therapy for type 2 diabetes, but its efficacy has not been evaluated in patients taking insulin (51).
Conclusion
Several medications are available to treat patients with type 2 diabetes, but few have been studied in the setting of severe insulin resistance (Table 2). The majority of patients with severe insulin resistance will likely have taken metformin. For patients who are currently taking metformin with insulin therapy, the metformin should be continued based on its track record of safety and efficacy, low cost, and potential to reduce the long-term complications related to diabetes (52). In the absence of a true contraindication, metformin should be added to high-dose U-100 insulin therapy if patients are able to tolerate it. However, switching patients to U-500 regular insulin or adding a GLP-1 receptor agonist will produce greater reductions in blood glucose and be more likely to achieve glycemic goals. U-500 regular insulin has the most data and greatest clinical experience to support its use in patients with severe insulin resistance, but weight gain is a problem that can escalate insulin resistance and dose requirements over time. GLP-1 receptor agonists, although not as potent as U-500 regular insulin in terms of A1C reduction, are an attractive alternative for obese patients with severe insulin resistance (53). SGLT2 inhibitors and pramlintide have a favorable impact on weight and can be considered. Other treatment options offer limited benefits but may be useful in specific patient circumstances. There is a paucity of evidence regarding the optimal treatment of patients with severe insulin resistance, and many questions remain unanswered.
TABLE 2.
Pharmacological Treatment Options in the Setting of Severe Insulin Resistance
| Medication Class | A1C Lowering* | Hypoglycemia Risk | Weight Effect | Ease of Use | Tolerability Issues | Relative Cost† |
| Treatment options that have been evaluated in patients with severe insulin resistance | ||||||
| U-500 regular insulin | ↓↓↓ | ↑ | ↑↑ | Subcutaneous; two to four times daily | $$$$ | |
| GLP-1 receptor agonists | ↓↓ | ←→ | ↓↓ | Subcutaneous; once daily or once weekly | Nausea, vomiting | $$$ |
| Metformin | ↓ | ←→ | ←→,↓ | One to four tablets once or twice daily | Diarrhea, loose stools | $ |
| Treatment options that have not been evaluated in patients with severe insulin resistance | ||||||
| SGLT-2 inhibitors | ↓ | ←→ | ↓ | Oral; once daily | Urogenital infections | $$$ |
| DPP-4 inhibitors | ↓ | ←→ | ←→ | Oral; once daily | Well tolerated | $$$ |
| TZDs | ↓ | ←→ | ↑↑ | Oral; once daily | Lower extremity edema, new-onset heart failure | $$$ |
| Pramlintide | ↓ | ↑, ←→ | ↓ | Subcutaneous; two to three times daily | Nausea, vomiting | $$$$ |
| Sulfonylureas | ↓ | ↑ | ↑ | Oral; once or twice daily | $ | |
| Meglitinides | ↓ | ↑ | ↑, ←→ | Oral; two or three times daily | $$ | |
| α-Glucosidase inhibitors | ↓ | ←→ | ←→ | Oral; three times daily | Flatulence, GI distress | $ |
| Colesevelam | ↓ | ←→ | ←→ | Oral; one packet or six tablets once daily | Constipation | $$$ |
| Bromocriptine | ↓ | ←→ | ←→ | Oral; four to six tablets once daily | Nausea, vomiting, somnolence, rhinitis, dizziness | $$$ |
Additional A1C lowering in previously treated patients; ↓ = 0.5–1%, ↓↓ = 1–1.5%, ↓↓↓ =1.5–2%.
Relative cost per 30-day supply; $ = <$100, $$ = $100–299, $$$ = $300–750, $$$$ = >$750 based on average wholesale price (54).
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
References
- 1.Crasto W, Hackett JE, Nayyar V, et al. Insulin U-500 in severe insulin resistance in type 2 diabetes mellitus. Postgrad Med J 2009;85:219–222 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Consensus development conference on insulin resistance. Diabetes Care 1997;21:310–314 [DOI] [PubMed] [Google Scholar]
- 3.Ovalle F. Clinical approach to the patient with diabetes mellitus and very high insulin requirements. Diabetes Res Clin Pract 2010;90:231–242 [DOI] [PubMed] [Google Scholar]
- 4.Galloway JA, Root MA, Bergstrom R, et al. Clinical pharmacologic studies with human insulin (recombinant DNA). Diabetes Care 1982;5(Suppl. 2):12–22 [DOI] [PubMed] [Google Scholar]
- 5.Rehman A, Setter SM, Vue MH. Drug-induced glucose alterations, part 2: drug-induced hyperglycemia. Diabetes Spectrum 2011;24:234–238 [Google Scholar]
- 6.Lane WS, Cochran EK, Jackson JA, et al. High-dose insulin therapy: is it time for U-500 insulin? Endocr Pract 2009;15:71–79 [DOI] [PubMed] [Google Scholar]
- 7.Binder C. Absorption of injected insulin: a clinical-pharmacological study. Acta Pharmacol Toxicol 1969;27(Suppl. 2):1–84 [DOI] [PubMed] [Google Scholar]
- 8.Humalog Kwikpen (insulin lispro) injection [package insert]. Indianapolis, IN, Lilly USA, 2015
- 9.Toujeo (insulin glargine) U-300 injection [package insert]. Bridgewater, NJ, Sanofi-Aventis US, 2015
- 10.Davidson MB, Navar MD, Echeverry D, et al. U-500 regular insulin: clinical experience and pharmacokinetics in obese, severely insulin resistant type 2 diabetic patients. Diabetes Care 2010;33:281–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wafa WS, Khan MI. Use of U-500 regular insulin in type 2 diabetes. Diabetes Care 2006;29:2175–2176 [DOI] [PubMed] [Google Scholar]
- 12.Nayyar V, Lawrence I, Kong MF, et al. Long-term follow-up of patients on U-500 insulin: a case-series. Pract Diabetes Int 2010;27:194–197 [Google Scholar]
- 13.Dailey AM, Williams S, Taneja D, et al. Clinical efficacy and patient satisfaction with U-500 insulin use. Diabetes Res Clin Pract 2010;88:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg R, Lawrence IG, Akinsola MO, et al. Improved glycemic control in severely insulin resistant, insulin treated diabetic patients with U-500 Human Actrapid over two year follow-up. (Abstract). Diabetologia 2004;47(Suppl. 1):A58 [Google Scholar]
- 15.Boldo A, Comi RJ. Clinical experience with U500 insulin: risks and benefits. Endocr Pract 2012;18:56–61 [DOI] [PubMed] [Google Scholar]
- 16.Quinn SL, Lansang MC, Mina D. Safety and effectiveness of U-500 insulin therapy in patients with insulin-resistant type 2 diabetes mellitus. Pharmacotherapy 2011;31:695–702 [DOI] [PubMed] [Google Scholar]
- 17.Ziesmer AE, Kelly KC, Guerra PA, et al. U-500 regular insuline use in insulin-resistant type 2 diabetic verteran patients. Endocr Pract 2012;18:34–38 [DOI] [PubMed] [Google Scholar]
- 18.Granata JA, Nawarskas AD, Resch ND, et al. Evalutating the effect of U-500 insulin therapy on glycemic control in verterans with type 2 diabetes. Clinical Diabetes 2015;33:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reutrakul S, Weoblewski K, Brown RL. Clinical use of U-500 regular insulin: review and meta-analysis. J Diabetes Sci Technol 2012;6:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane WS. Use of U-500 regular insulin by continuous subcutaneous insulin infusion in patients with type 2 diabetes and severe insulin resistance. Endocr Pract 2006;12:251–256 [DOI] [PubMed] [Google Scholar]
- 21.Humulin R. U-500 concentrated insulin [package insert]. Indianapolis, IN, Eli Lilly and Co., 1996 [Google Scholar]
- 22.American Diabetes Association Standards of medical care in diabetes—2015. Diabetes Care 2015;38(Suppl. 1):S1–S94 [PubMed] [Google Scholar]
- 23.Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther 2011;13:592–595 [DOI] [PubMed] [Google Scholar]
- 24.Lane W, Weinrib S, Rappaport J, et al. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab 2014;16:827–832 [DOI] [PubMed] [Google Scholar]
- 25.Wulffele M, Kooy A, Lehert P, et al. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care 2002;25:2133–2140 [DOI] [PubMed] [Google Scholar]
- 26.Ponssen HH, Elte JWF, Lehert P, et al. Combined metformin and insulin therapy for patients with type 2 diabetes mellitus. Clin Ther 2000;22:709–718 [DOI] [PubMed] [Google Scholar]
- 27.Blonde L, Dailey GE, Jabbour SA, et al. Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: results of a retrospective cohort study. Curr Med Res Opin 2004;20:565–572 [DOI] [PubMed] [Google Scholar]
- 28.Lipska KJ. Bailer CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care 2011;34:1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salpeter S, Greyber E, Pasternak G, et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2010;14:CD002967. [DOI] [PubMed] [Google Scholar]
- 30.KDIGO KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150 [DOI] [PubMed] [Google Scholar]
- 31.America Geriatrics Society Expert Panel Guidelines abstracted from the American Geriatrics Society guidelines for improving the care of older adults with diabetes mellitus: 2013 update. J Am Geritr Soc 2013;61:2020–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care 2011;34(Suppl. 2):279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 2014;384:2228–2234 [DOI] [PubMed] [Google Scholar]
- 34.Distiller LA, Nortje H, Wellmann H, et al. A 24-week prospective, randomized, open label, treat-to-target pilot study of obese type 2 diabetes patients with severe insulin resistance plus the addition of exenatide on the efficacy of U-500 regular insulin plus metformin. Endocr Pract 2014;20:1143–1150 [DOI] [PubMed] [Google Scholar]
- 35.Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015;6:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valentine V. The role of the kidney and sodium-glucose cotransporter 2 inhibition in diabetes management. Clinical Diabetes 2012;30:151–155 [Google Scholar]
- 37.Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 2014;37:1815–1823 [DOI] [PubMed] [Google Scholar]
- 38.Lajara R. The potential role of sodium glucose co-transporter 2 inhibitors in combination therapy for type 2 diabetes mellitus. Expert Opin Pharmacother 2014;15:2565–2685 [DOI] [PubMed] [Google Scholar]
- 39.Charbonnel B, Schhweizer A, Dejager S. Combination therapy with DPP-4 inhibitors and insulin in patients with type 2 diabetes mellitus: what is the evidence? Hosp Pract 1995;41:93–107 [DOI] [PubMed] [Google Scholar]
- 40.Rosenstock J, Rendell MS, Gross JL, et al. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1c) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab 2009;11:1145–1152 [DOI] [PubMed] [Google Scholar]
- 41.Vilsboll T, Rosenstock J, Yki-Jarvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2010;12:167–177 [DOI] [PubMed] [Google Scholar]
- 42.McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care 2013;36:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnett AH, Charbonnel B, Li J, et al. Saxagliptin add-on therapy to insulin with or without metformin for type 2 diabetes mellitus: 52-week safety and efficacy. Clin Drug Investig 2015;35:179–185 [DOI] [PubMed] [Google Scholar]
- 44.Symlin (pramlintide) injection [package insert]. Wilmington, DE, AstraZeneca, 2014
- 45.Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care 2003;26:784–790 [DOI] [PubMed] [Google Scholar]
- 46.Ratner RE, Want LL, Fineman MS, et al. Adjunctive therapy with the amylin analogue pramlintide leads to a combined improvement in glycemic and weight control in insulin-treated subjects with type 2 diabetes. Diabetes Technol Ther 2002;4:51–61 [DOI] [PubMed] [Google Scholar]
- 47.Berhanu P, Perez A, Yu S. Effect of pioglitazone in combination with insulin therapy on glycaemic control, insulin dose requirement and lipid profile in patients with type 2 diabetes previously poorly controlled withbes combination therapy. Diabetes Obes Metab 2007;9:512–520 [DOI] [PubMed] [Google Scholar]
- 48.Kelley DE, Bidot P, Freedman Z, et al. Efficacy and safety of acarbose in insulin-treated patients with type 2 diabetes. Diabetes Care 1998;21:2056–2061 [DOI] [PubMed] [Google Scholar]
- 49.Handelsman Y. Role of bile acid sequestrants in the treatment of type 2 diabetes. Diabetes Care 2011;34(Suppl. 2):S244–S250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldberg RB, Fonesca VA, Truitt KE, et al. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med 2008;168:1531–1540 [DOI] [PubMed] [Google Scholar]
- 51.Cycloset (bromocriptine) oral [package insert]. San Diego, CA, Santarus, 2010 [Google Scholar]
- 52.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Eng J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 53.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342–362 [DOI] [PubMed] [Google Scholar]
- 54.RedBook Online. Ann Arbor MI, Truven Health Analytics, 2015. Available by subscription only from http://www.redbook.com/redbook/online. Accessed 13 March 2015
- 55.Cochran E, Musso C, Gorden P. The use of U-500 in patients with extreme insulin resistance. Diabetes Care 2005;28:1240–1244 [DOI] [PubMed] [Google Scholar]