Abstract
Attention-deficit hyperactivity disorder (ADHD) is a common childhood-onset psychiatric condition with a strong genetic component. Evidence from pharmacological, clinical and animal studies has suggested that the nicotinic system could be involved in the disorder. Previous studies have implicated the nicotinic acetylcholine receptor α4 subunit gene, CHRNA4, in ADHD. Particularly, a polymorphism in the exon 2–intron 2 junction of CHRNA4 has been associated with severe inattention defined by latent class analysis. In the current study, we used the transmission disequilibrium test (TDT) to investigate four polymorphisms encompassing this region of CHRNA4 for association with ADHD in a sample of 264 nuclear families from Toronto. No significant evidence of biased transmission was observed for any of the marker alleles for ADHD defined as a categorical trait (all subtypes included), although one haplotype showed marginal evidence of under-transmission. No association was found with the ADHD predominantly inattentive subtype or with symptom dimension scores of inattention. On the contrary, nominally significant evidence of association of individual markers was obtained for the ADHD combined subtype and with teacher-rated hyper-activity–impulsivity scores, with the same haplotype being under-transmitted. Based on our results and others, CHRNA4 may be involved in ADHD; however, its role in ADHD symptomatology remains to be clarified.
Keywords: Attention-deficit/hyperactivity disorder, CHRNA4, genetics, nicotinic receptor, transmission/disequilibrium test
Attention-deficit hyperactivity disorder (ADHD) is a common childhood-onset psychiatric condition affecting 4–12% of children worldwide (Faraone et al. 2003) with a tendency to persist into adolescence and adulthood (Clarke et al. 2005). Family, twin and adoption studies have shown that this disorder is highly heritable (Biederman & Faraone 2005; Thapar et al. 1999) and multiple susceptibility genes are likely to be involved.
As currently recognized by the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV), the behavioral symptoms of ADHD load into two separate dimensions, one reflecting inattentive behavior and the other a combination of hyperactive and impulsive behavior. Twin studies have shown that the symptoms of inattention and hyperactivity/impulsivity are primarily explained by shared genetic influences; however, each symptom dimension of ADHD was also shown to be under unique genetic influence (Levy et al. 2001; Rasmussen et al. 2004; Sherman et al. 1997).
Catecholamine system dysfunction, particularly in the dopaminergic system, has been suggested in ADHD by pharmacological, imaging, molecular genetic and animal studies (Davids et al. 2003; Durston 2003; Seeman & Madras 1998; Thapar et al. 2005; Viggiano et al. 2003). Accumulating evidence indicate a potential role for the nicotinic system in modulating dopamine neurotransmission. Nicotinic acetylcholine receptors (nAChRs) are expressed in regions densely innervated by dopaminergic neurons (Arroyo-Jimenez et al. 1999; Gotti et al. 2006; Klink et al. 2001) and activation of presynaptic nAChRs is known to facilitate dopamine release in the nucleus accumbens and in the striatum (Grady et al. 2002; Picciotto et al. 1998). In addition, nAChRs signaling was shown to regulate the dopamine transporter gene transcription and function (Li et al. 2004; Parish et al. 2005), potentially affecting dopamine uptake.
Attention-deficit hyperactivity disorder is associated with an increased risk of early initiation of cigarette smoking (Milberger et al. 1997) and, consequently, a high prevalence of cigarette smoking is observed in children with ADHD as they reach adolescence and adulthood (Biederman et al. 2006; Lambert & Hartsough 1998). Lower cessation (stop smoking) ratios were reported for boys with ADHD compared with the general population (Pomerleau et al. 1995). In addition, maternal smoking during pregnancy was shown to be a significant risk factor for development of ADHD and ADHD symptoms for the offspring (Barman et al. 2004; Batstra et al. 2003; Kotimaa et al. 2003; Thapar et al. 2003). Clinical and animal studies have shown that nicotine receptor stimulation plays a role, either directly or by interactions with other neurotransmitters, in several executive function processes such as response inhibition, attention and working memory (Newhouse et al. 2004; Rezvani & Levin 2001). These processes are thought to underlie the cognitive and behavioral difficulties experienced by children with ADHD (Arnsten & Li 2005; Lijffijt et al. 2005; Luman et al. 2005; Martinussen et al. 2005; Willcutt et al. 2005). Specifically, nicotine or nicotinic agonists have been shown to improve attention in adult smokers and nonsmokers without attention deficits and adults with ADHD (Levin et al. 1998; Mancuso et al. 1999; Wilens et al. 1999, 2006), making nicotinic system genes attractive susceptibility genes for ADHD.
Neuronal nAChRs are ligand-gated ion channels composed of five subunits. Molecular analyses have identified nine alpha (α2–α10) and three beta (β2–β4) subunits in the central nervous system (Dani & Bertrand 2007), with the majority of high-affinity binding sites provided by receptors consisting of α4 and β2 subunits. Nicotinic agonists shown to improve ADHD symptoms in adults bind selectively to α4–β2 high-affinity complexes.
The gene coding for the nAChR α4 subunit, CHRNA4, contains six exons spanning ~17 kb on chromosome 20q13.2–13.3 (Steinlein et al. 1994, 1996). Genetic polymorphisms in the CHRNA4 gene have been proposed to be associated with several psychiatric or behavioral disorders, including autosomal-dominant nocturnal frontal lobe epilepsy (Combi et al. 2004), febrile convulsions (Chou et al. 2003), Alzheimer’s disease (Kawamata & Shimohama 2002), alcohol dependence (Kim et al. 2004) and vulnerability to nicotine addiction (Feng et al. 2004; Li et al. 2005).
Because of the strong indication for the involvement of the nicotinic acetylcholine system in ADHD, the CHRNA4 gene has been tested as a candidate for ADHD in several genetic studies. Evidence of association was found with a dinucleotide repeat in intron 1 in an analysis of DSM-IV ADHD symptom scores among 326 individuals (271 cases with Tourette syndrome and 55 controls) (Comings et al. 2000), while Kent et al. (2001) found no significant evidence of association for a Cfo1 restriction site polymorphism in exon 5 in a study of 68 trios with DSM-IV-defined ADHD. Using families selected from a twin sample from Missouri (178 families), Todd et al. (2003) reported a relationship between a polymorphism in the exon 2–intron 2 junction, and severe inattention problems defined by a latent class analysis. Using the same markers, Bobb et al. (2005) did not find evidence for association with DSM-IV ADHD using a sample of 163 ADHD cases and 129 controls (also analyzed as families with 192 available parental DNAs). Finally, in a recent analysis of 51 genes including CHRNA4 in 674 families with a child meeting the DSM-IV ADHD combined subtype criteria, Brookes et al. (2006) reported nominal evidence of association for one marker in the 5′ flanking region of CHRNA4. Although the results from these studies are conflicting, some of the studies were based on small sample sizes, and further the studies used different phenotypes for analysis and the samples had different clinical characteristics. In three of the studies, the majority or all of the samples was composed of the DSM-IV combined ADHD subtype (Bobb et al. 2005; Brookes et al. 2006; Kent et al. 2001), whereas over half of Todd et al.’s sample was composed of children with severe inattention problems.
In this study, we investigated the association between CHRNA4 and ADHD in a sample of ADHD families collected in Toronto. Four markers in the CHRNA4 promoter-intron 2 region and their haplotypes were tested for evidence of biased transmission in relation to ADHD or DSM-IV ADHD subtypes using the transmission disequilibrium test (TDT). Relationship between these variants and the symptom dimensions of inattention and hyperactivity/impulsivity, and with cognitive measures of verbal short-term and working memory were also assessed using quantitative analyses.
Materials and methods
Diagnostic assessment and subjects
Probands and affected siblings between 7 and 16 years old were referred to the Child Development and Neuropsychiatry Clinics at the Hospital for Sick Children, Toronto, and met DSM-IV criteria for ADHD. Diagnosis was based on information from semi-structured interviews of parents [Parent Interview for Child Symptoms (PICS-IV)] (Ickowicz et al. 2006) and teachers [Teacher Telephone Interview (TTI-IV)] (Tannock et al. 2002). Clinical information was also obtained from the following standardized questionnaires and assessments: Conners’ Parent and Teacher Rating Scales – Revised (Conners 1997), Ontario Child Health Survey Scales – Revised (Boyle et al. 1993), Wide-Range Achievement Test – Revision 3 (Wilkinson 1993), Clinical Evaluation of Language Fundamentals, third edition (Semel et al. 1995), Children’s Depression Inventory (Kovacs 1995) and Children’s Manifest Anxiety Scale (Reynolds & Richmond 1985). Children who scored below 80 on both the Performance and Verbal Scales of the Weschler Intelligence Scale for Children, 3rd Edition (WISC-III) (Kaplan et al. 1999; Wechsler 1991) were excluded from the study, as were children who exhibited neurological or chronic medical illness, Tourette syndrome, chronic multipletics, bipolar affective disorder, psychotic symptoms or other anxiety, depressive or developmental disorders that might account for their behavior. All children were free of medication for 24 h before assessment. This protocol was approved by the Hospital for Sick Children’s Research Ethics Board and informed written consent or assent (children) was obtained for all participants.
The study sample comprised 264 nuclear families recruited in the Toronto area, for a total of 313 affected children (81% boys). The majority of the families reported their ethnic background to be of European Caucasian descent, while 10% of families were of other or mixed background, including Chinese, African, Indian and Native Canadians. Both parents were genotyped in 192 families. The distribution of the affected children among the three DSM-IV ADHD subtypes was: 14% of the predominantly hyperactive/impulsive subtype, 24% of the predominantly inattentive subtype and 62% of the combined subtype.
For the quantitative analysis, we used the symptom scores obtained for each dimension from the PICS-IV and TTI-IV semi-structured interviews, as described previously (Laurin et al. 2005). These are clinician ratings of the symptoms based on behavioral description elicited from parents or teachers. Verbal short-term and working memory was assessed using the digit span subtest of the WISC-III. This test provides two subscale scores (digits forward, digits backward), which index the ability to store and manipulate auditory–verbal information, respectively.
Isolation of DNA and marker typing
DNA was extracted from blood leukocytes using a high salt method (Miller et al. 1988). We examined four markers in CHRNA4: rs755203 in the promoter and rs2273505, rs6090384 and rs3787141 in intron 2. rs2273505 and rs6090384 were genotyped by restriction enzyme digest. They were both amplified on the same fragment using the following primers: 5′-CCTGCACCTGAGCCACTG-3′ and 5′-ACGCTCT-GAATCAACCCTTG-3′. Polymerase chain reaction (PCR) amplification (20 μl volume) was carried out with 60 ng of genomic DNA using the PCR Enhancer system (Invitrogen Tech-LineSM; Invitrogen, Carlsbald, CA, USA), supplemented with 1.5 mM MgCl2, for 35 cycles of 94°C, 40 s; 59°C, 40 s and 72°C, 40 s. PCR products were digested using the enzymes NlaIII (New England Biolabs, Berverly, MA, USA) for rs2273505 and HinP1I (New England Biolabs) for rs6090384. NlaIII restriction fragments for rs2273505 (allele G: 334 and 48 bp; allele A: 212, 170 and 48 bp) and HinP1I fragments for rs6090384 (allele G: 212 and 170 bp; allele A: 382 bp) were visualized by ethidium bromide staining on 2.5% agarose gels.
The markers rs755203 (C_8838223_10, Assay-on-Demand®; Applied Biosystems, Foster City, CA, USA) and rs3787141 (C__25800787_10, Assay-on-Demand®; Applied Biosystems) were genotyped with the ABI 7900-HT Sequence Detection Systems (Applied Biosystems) using the TaqMan 5′ nuclease assay for allelic discrimination. The PCR reactions (5 μl) contained 30 ng of genomic DNA, 2.5 μl of TaqMan Universal PCR Master Mix and 0.1 μl of allelic discrimination mix. The thermal cycling conditions were 95°C for 10 min and 50 cycles of 95°C, 15 s; and the annealing temperatures were 58 and 59°C, respectively, 1 min.
Statistical analysis
For categorically defined ADHD, we examined the allelic transmission of markers using the extended TDT program (Sham & Curtis 1995) and the haplotype transmission with TRANSMIT version 2.5, using the robust estimator of variance option (Clayton 1999). Quantitative trait TDT analyses, examining transmission of individual alleles or haplotypes in relation to dimensional symptom scores and short-term and working memory measures were carried out using the FBAT program version 1.5.5, with the additive model of inheritance (Horvath et al. 2001; Laird et al. 2000). We used population-based mean scores for the tests as an offset value to mean center the trait. P-values were not corrected for multiple tests. We did not observe significant departure from the Hardy–Weinberg equilibrium for the genotype frequencies.
Results
Based on a previous report showing evidence of association between severe inattention problems defined by latent class analyses, and markers at the exon 2–intron 2 junction of the CHRNA4 gene (Todd et al. 2003), we performed a family-based association study using four markers encompassing this same region of CHRNA4. Two markers, rs2273505 and rs6090384, were investigated in the previous association report. Although rs2273506 was also assessed in the report of Todd et al. (2003), it was not included in the present study because of evidence of complete linkage disequilibrium (LD) with rs2273505. Instead, we selected rs755203 in the promoter and rs3787141 located in CHRNA4 intron 2.
We first tested for biased transmission of marker alleles using the TDT statistic with categorically defined ADHD (all subtypes). As shown in Table 1, we did not observe significant evidence of biased transmission for any of the marker alleles. Similarly, no significant evidence for over-transmission was observed for any of the resulting four-marker haplotypes, although one low-frequency haplotype (7.2%) was marginally under-transmitted (P = 0.041) (Table 2).
Table 1.
Allele frequencies and TDT analysis of four SNPs in the CHRNA4 gene
| Allele | rs755203
|
rs2273505
|
rs6090384
|
rs3787141
|
||||
|---|---|---|---|---|---|---|---|---|
| G | A | G | A | G | A | T | C | |
| Frequency | 0.477 | 0.523 | 0.926 | 0.074 | 0.943 | 0.057 | 0.925 | 0.075 |
| DSM-IV ADHD (all subtypes) | ||||||||
| Transmitted | 108 | 105 | 40 | 26 | 25 | 18 | 37 | 26 |
| Not transmitted | 105 | 108 | 26 | 40 | 18 | 25 | 26 | 37 |
| χ2 (1 df) | 0.042 | 2.970 | 0.581 | 1.921 | ||||
| P-value | 0.837 | 0.085 | 0.446 | 0.166 | ||||
| DSM-IV predominantly inattentive subtype | ||||||||
| Transmitted | 30 | 28 | 8 | 9 | 7 | 6 | 6 | 9 |
| Not transmitted | 28 | 30 | 9 | 8 | 6 | 7 | 9 | 6 |
| χ2 (1 df) | 0.069 | 0.059 | 0.077 | 0.600 | ||||
| P-value | 0.793 | 0.808 | 0.782 | 0.439 | ||||
| DSM-IV combined subtype | ||||||||
| Transmitted | 55 | 61 | 25 | 12 | 9 | 10 | 24 | 12 |
| Not transmitted | 61 | 55 | 12 | 25 | 10 | 9 | 12 | 24 |
| χ2 (1 df) | 0.310 | 4.568 | 0.053 | 4.000 | ||||
| P-value | 0.578 | 0.033 | 0.819 | 0.046 | ||||
Table 2.
CHRNA4 haplotype frequencies and TRANSMIT analysis
| Haplotype* | Frequency | Transmission
|
Var (O-E) | χ2 (1 df) | P | |
|---|---|---|---|---|---|---|
| Observed† | Expected‡ | |||||
| DSM-IV ADHD (all subtypes)§ | ||||||
| AGGT | 0.492 | 290.92 | 288.51 | 66.61 | 0.087 | 0.768 |
| GGGT | 0.379 | 214.90 | 206.18 | 57.41 | 1.325 | 0.250 |
| GAGC | 0.072 | 34.97 | 43.58 | 17.82 | 4.161 | 0.041 |
| AGAT | 0.038 | 16.04 | 18.22 | 9.22 | — | — |
| GGAT | 0.021 | 11.12 | 11.86 | 4.09 | — | — |
| DSM-IV ADHD predominantly inattentive subtype¶ | ||||||
| AGGT | 0.492 | 69.98 | 70.21 | 20.97 | 0.003 | 0.960 |
| GGGT | 0.379 | 44.02 | 44.53 | 16.78 | 0.015 | 0.902 |
| GAGC | 0.072 | 11.98 | 10.91 | 4.67 | 0.244 | 0.622 |
| AGAT | 0.038 | 4.99 | 5.16 | 3.04 | — | — |
| GGAT | 0.021 | 3.01 | 3.14 | 1.02 | — | — |
| DSM-IV ADHD combined subtype** | ||||||
| AGGT | 0.492 | 163.44 | 158.85 | 35.29 | 0.597 | 0.440 |
| GGGT | 0.379 | 118.49 | 116.44 | 30.47 | 0.138 | 0.711 |
| GAGC | 0.072 | 15.99 | 23.77 | 7.23 | 8.375 | 0.004 |
| AGAT | 0.038 | 9.05 | 8.84 | 4.09 | — | — |
| GGAT | 0.021 | 6.01 | 5.02 | 1.24 | — | — |
Haplotypes with frequency >0.005 are listed.
Test statistic representing the observed number of transmissions.
Expected value of the test statistic under the null hypothesis of no association.
Global χ2 on 3 df for haplotypes with frequencies >5% = 5.688, P = 0.127.
Global χ2 on 3 df for haplotypes with frequencies >5% = 0.251, P = 0.969.
Global χ2 on 3 df for haplotypes with frequencies >5% = 8.430, P = 0.038.
In light of results previously reported for a severe inattention latent class and trends for association of the DSM-IV inattention subtype, we conducted TDT analyses for DSM-IV predominantly inattentive subtype. No evidence of association between CHRNA4 variants or haplotypes and this subtype was observed (Tables 1 and 2). However, the number of informative transmissions for this subgroup was small and is thus not conclusive. We next analyzed the marker alleles using a quantitative approach for ADHD inattentive symptom scores as reported by parents and teachers and found no evidence of relationship (Table 3).
Table 3.
FBAT analysis of CHRNA4 allele and haplotype transmission in relation to ADHD symptom scores
| Marker allele/haplotype | No. of families | Inattentive symptoms
|
Hyperactive/impulsive symptoms
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S* | E(S)† | Var(S) | Z | P | S* | E(S)† | Var(S) | Z | P | ||
| Parent Interview for Child Symptoms | |||||||||||
| rs755203 G | 128 | 654.0 | 640.1 | 1072.6 | 0.424 | 0.671 | 622.3 | 619.6 | 1106.8 | 0.081 | 0.935 |
| rs755203 A | 680.1 | 694.0 | −0.424 | 698.5 | 701.2 | −0.081 | |||||
| rs2273505 G | 49 | 378.2 | 350.1 | 322.6 | 1.562 | 0.118 | 353.0 | 323.0 | 307.9 | 1.712 | 0.087 |
| rs2273505 A | 98.8 | 126.8 | −1.562 | 85.6 | 115.7 | −1.712 | |||||
| rs6090384 G | 36 | 261.2 | 256.7 | 213.9 | 0.311 | 0.775 | 254.9 | 244.5 | 232.5 | 0.682 | 0.495 |
| rs6090384 A | 81.0 | 85.6 | −0.311 | 71.1 | 81.5 | −0.682 | |||||
| rs3787141 T | 44 | 319.7 | 304.7 | 294.9 | 0.871 | 0.384 | 313.0 | 290.3 | 294.3 | 1.326 | 0.185 |
| rs3787141 C | 101.8 | 116.7 | −0.871 | 86.6 | 109.4 | −1.326 | |||||
| AGGT | 113 | 702.8 | 713.7 | 1027.2 | −0.340 | 0.734 | 713.4 | 708.1 | 1031.5 | 0.167 | 0.868 |
| GGGT | 112 | 555.5 | 528.5 | 892.4 | 0.905 | 0.366 | 555.0 | 532.3 | 908.2 | 0.754 | 0.451 |
| GAGC | 42 | 100.7 | 120.7 | 279.3 | −1.193 | 0.233 | 86.5 | 115.5 | 272.4 | −1.756 | 0.079 |
| AGAT | 26 | 50.7 | 52.7 | 132.9 | −0.176 | 0.860 | 46.6 | 56.3 | 158.2 | −0.771 | 0.441 |
| GGAT | 10 | 33.9 | 32.6 | 74.1 | 0.148 | 0.882 | 36.3 | 33.1 | 73.9 | 0.372 | 0.710 |
| Teacher Telephone Interview | |||||||||||
| rs755203 G | 126 | 617.1 | 639.6 | 1207.5 | −0.646 | 0.518 | 499.8 | 509.9 | 970.4 | −0.325 | 0.745 |
| rs755203 A | 697.5 | 675.1 | 0.646 | 508.8 | 498.6 | 0.325 | |||||
| rs2273505 G | 48 | 353.9 | 328.1 | 302.2 | 1.482 | 0.138 | 340.8 | 301.0 | 317.4 | 2.231 | 0.026 |
| rs2273505 A | 92.5 | 118.3 | −1.482 | 66.3 | 106.0 | −2.231 | |||||
| rs6090384 G | 37 | 225.6 | 233.7 | 187.6 | −0.591 | 0.554 | 200.0 | 203.3 | 197.1 | −0.231 | 0.817 |
| rs6090384 A | 86.0 | 77.9 | 0.591 | 71.0 | 67.8 | 0.231 | |||||
| rs3787141 T | 43 | 313.9 | 296.4 | 280.2 | 1.044 | 0.297 | 320.3 | 284.5 | 316.2 | 2.010 | 0.044 |
| rs3787141 C | 93.5 | 111.0 | −1.044 | 68.3 | 104.0 | −2.010 | |||||
| AGGT | 110 | 695.1 | 692.5 | 1015.5 | 0.082 | 0.935 | 514.3 | 516.9 | 854.8 | −0.090 | 0.928 |
| GGGT | 108 | 512.6 | 510.2 | 922.0 | 0.080 | 0.936 | 488.0 | 456.2 | 824.0 | 1.110 | 0.267 |
| GAGC | 41 | 93.6 | 115.3 | 305.1 | −1.242 | 0.214 | 67.0 | 108.7 | 334.1 | −2.283 | 0.022 |
| AGAT | 26 | 57.6 | 49.3 | 116.9 | 0.765 | 0.444 | 44.5 | 36.9 | 103.1 | 0.751 | 0.453 |
| GGAT | 10 | 33.3 | 28.8 | 60.5 | 0.576 | 0.565 | 29.8 | 30.6 | 84.0 | −0.096 | 0.924 |
Test statistic.
Expected value of the test statistic under the null hypothesis of no association.
In contrast, we obtained nominally significant over-transmission of the rs2273505-G (P = 0.033) and rs3787141-T (P = 0.046) alleles for families with children meeting the DSM-IV criteria for the ADHD combined subtype (Table 1). One haplotype, including the alternate alleles of the two associated markers above (A and C, respectively), was significantly under-transmitted (GAGC: P = 0.004) (Table 2). This is the same haplotype that showed evidence of under-transmission with categorical ADHD. However, these findings should be interpreted cautiously because ADHD subtype analysis leads to a lower number of informative transmissions for each marker and the frequency of the under-transmitted haplotype is low (7.2%). For this reason, we did not analyze the predominantly hyperactive/impulsive subtype because the number of informative transmission would be too small to be conclusive.
Of note is that we observed over-transmission of the rs2273505-G for the combined subtype in our sample, while the opposite allele, rs2273505-A, showed a strong trend toward over-transmission for the DSM-IV-defined inattentive subtype in the report of Todd et al. (uncorrected P = 0.046, corrected P = 0.089). We also found marginally significant evidence for association of the individual alleles rs2273505-G and rs3787141-T with the teacher-rated hyperactivity–impulsivity scores (P = 0.026 and P = 0.044, respectively) (Table 3). Haplotype analysis showed a significant negative relationship between the same under-transmitted haplotype G–A–G–C, and teacher-rated hyperactivity–impulsivity scores (Z = −2.283, P = 0.022) (Table 3).
Finally, as nicotine has been suggested to modulate short-term and working memory processes, we also tested the relationship between CHRNA4 alleles and verbal short-term and working memory measures in this sample. No relationship between this gene and verbal short-term and working memory was observed for individual markers or any of the observed haplotypes (data not shown).
Discussion
In this study, we assessed the association between CHRNA4 and ADHD in a clinically ascertained sample. We limited our study to four polymorphisms in the gene region previously implicated in ADHD, i.e. 5′ flanking region to intron 2 (Brookes et al. 2006; Comings et al. 2000; Todd et al. 2003). No association was observed between these variants and DSM-IV categorically defined ADHD or with inattentive symptoms. We found, however, marginal evidence of association between CHRNA4 variants and the DSM-IV ADHD combined subtype and with hyperactive/impulsive symptom scores. We also observed the under-transmission of a low-frequency haplotype for DSM-IV categorical ADHD and the DSM-IV ADHD combined subtype.
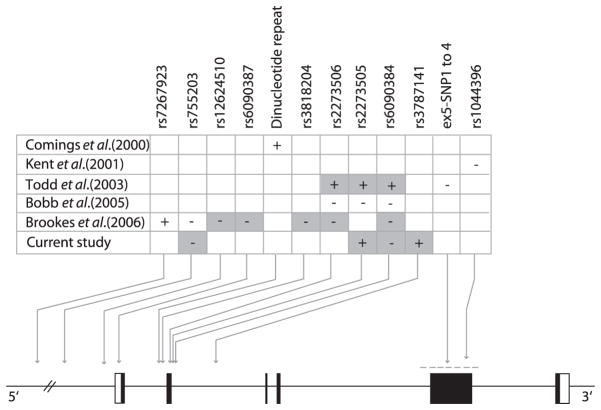
This report is the fourth showing evidence of association between ADHD and the CHRNA4 gene, despite different ADHD phenotypes or markers/alleles being associated (see Fig. 1). Differences in the markers and alleles associated may reflect differences in the linkage disequilibrium with the true and as yet unidentified risk variant(s) and because of ethnic/population differences, divergent linkage disequilibrium patterns lead to the association of different alleles.
Figure 1. Schematic representation of the CHRNA4 gene and association results reported for ADHD.
Lower panel shows the structure of the CHRNA4 gene. The six exons of the gene are represented by boxes, with black boxes for coding sequences and empty boxes for untranslated regions. Association findings are shown in the top panel with positive (+) and negative (−) results for the different studies. Of note, the Todd et al. findings presented here are not corrected for multiple tests and only rs6090384 remains significant after correction in that study. Markers involved in haplotypes showing evidence of association are shaded. Haplotypes from this study and Todd et al. were under-transmitted. The haplotype reported by Brookes et al. has a very low frequency (17 transmissions). Twelve other single nucleotide polymorphisms (SNPs), from intron 2 to 3′ untranslated region, have been tested by Brookes et al. (not shown here) and were all negative.
Differences in sample characteristics exist between the study samples. We used a clinically ascertained sample, while Todd et al. selected their sample from a birth record-based twin sample, which they first screened for the presence of three or more inattentive symptoms endorsed by a parent. The children then went through a clinical assessment and the ones that met DSM-IV criteria for ADHD were used to test for association with the DSM-IV-defined subtypes. The screening procedure based on inattention symptoms used by Todd and colleagues may have led to an over-representation of the children with inattentive problems, as illustrated by the high proportion of the primarily inattentive subtype for children meeting the DSM-IV criteria in Todd et al.’s sample (58% compared with 24% in our sample). This may have increased power for the analysis of the severe inattention latent class and the DSM-IV inattention subtype compared with other samples. Brookes et al.’s sample included families with only the ADHD combined subtype (93.5% male probands), while Kent et al.’s 68 trios (87% male probands) were also predominantly of the combined subtype (84%), with few of the inattentive subtype (7%, 5 probands) and Bobb et al.’s study of 163 probands (53% male probands) was composed of 94% of children with the combined subtype with 6% inattentive subtype. Finally, Comings et al.’s method of assessment was completely different based on ADHD symptom scores for individuals with Tourette syndrome.
In addition, the Missouri sample was highly enriched with female probands (45.6% vs. 19% in our sample). Important gender differences in symptomatology have been observed for ADHD. Attention-deficit hyperactivity disorder-affected girls exhibit greater intellectual impairment, lower levels of hyperactivity and lower rates of other externalizing behaviors compared with boys (Biederman et al. 2002; Gaub & Carlson 1997; Newcorn et al. 2001).
With regard to methodology, we did not perform analysis for latent-class-defined ADHD subtypes as reported by Todd et al. because examination of our sample showed that we would have a very small sample size for each group, especially for the severe inattentive class (less prevalent), owing to the predominance of the DSM-IV combined subtype in our clinical sample.
The severe inattention subtype as defined by latent class analysis is thought to represent a relatively pure primary inattention subset of those meeting DSM-IV criteria for predominantly inattentive subtype. The genetic factors involved in the severe inattention latent class might be different from the genetic factors involved in the inattention symptoms that are also present in children with hyperactivity/impulsivity symptoms. Thus, our analysis of inattention symptoms defined quantitatively and previous analysis of the latent-class-defined inattention subtype may not be comparable. As Todd et al.’s results are stronger for latent-class-defined inattention subtype than for DSM-IV-defined inattentive subtype, we cannot exclude the possibility that the same variants are associated with pure inattention problems that could not be detected with the current sample. However, the most significant single marker finding from that study was for the marker rs6090384 with the latent class severe inattention group (P = 0.007, corrected P = 0.015). This marker was also significant for the analysis of all children with ADHD (P = 0.028) and the inattentive DSM-IV subtype (P = 0.039); however, these two analyses did not stand up to correction for multiple testing (P = 0.055 and P = 0.076, respectively). Thus, we would have expected a similar trend in our entire sample and our inattentive subtype, but this was not seen. Instead, we obtained positive results for the DSM-IV combined subtype and hyperactive/impulsive symptom scores suggesting that CHRNA4 variants could be associated more with combined or hyperactive-impulsive problems in our sample.
Interestingly, although research on nicotine has focused primarily on attention processes and working memory, pre-natal exposure or acute administration of nicotine has been shown to stimulate locomotor activity levels in rodents (Benwell & Balfour 1992; Newman et al. 1999; Tizabi et al. 2000), and CHRNA4-deficient mice exhibit increases in several components of their ethogram, including locomotion, rearing and sniffing, over the course of habituation to a novel environment (Ross et al. 2000). Furthermore, maternal smoking has also been associated with symptoms of hyperactivity in children (Kotimaa et al. 2003).
In summary, using a family-based sample, we found nominal evidence of association between CHRNA4 and ADHD, particularly with the DSM-IV ADHD combined subtype and with hyperactive/impulsive symptoms. We were unable to show an association between CHRNA4 and inattentive symptoms albeit this may be the result of sample characteristics. Based on our results and the findings from previous studies, the involvement of CHRNA4 in ADHD still remains unclear, although the 5′ region-intron 2 of the gene has repeatedly shown association with the disorder. Further investigation of CHRNA4 5′ region, including regulatory regions is thus warranted.
Acknowledgments
This work was supported by Postdoctoral Fellowships from the Hospital for Sick Children Research Training Centre (N.L.) and the Canadian Institutes of Health Research (N.L.) and by grants from The Hospital for Sick Children Psychiatry Endowment Fund (C.L.B.), and the Canadian Institutes of Health Research MT14336 and MOP14336 (C.L.B.).
References
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arroyo-Jimenez MM, Bourgeois JP, Marubio LM, Le Sourd AM, Ottersen OP, Rinvik E, Fairen A, Changeux JP. Ultrastructural localization of the alpha4-subunit of the neuronal acetylcholine nicotinic receptor in the rat substantia nigra. J Neurosci. 1999;19:6475–6487. doi: 10.1523/JNEUROSCI.19-15-06475.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman SK, Pulkkinen L, Kaprio J, Rose RJ. Inattentiveness, parental smoking and adolescent smoking initiation. Addiction. 2004;99:1049–1061. doi: 10.1111/j.1360-0443.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early Hum Dev. 2003;75:21–33. doi: 10.1016/j.earlhumdev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV, Braaten E, Doyle A, Spencer T, Wilens TE, Frazier E, Johnson MA. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry. 2002;159:36–42. doi: 10.1176/appi.ajp.159.1.36. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, Snyder LE, Faraone SV. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psychol Med. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- Bobb AJ, Addington AM, Sidransky E, Gornick MC, Lerch JP, Greenstein DK, Clasen LS, Sharp WS, Inoff-Germain G, Wavrant-De Vrieze F, Arcos-Burgos M, Straub RE, Hardy JA, Castellanos FX, Rapoport JL. Support for association between ADHD and two candidate genes: NET1 and DRD1. Am J Med Genet B Neuropsychiatr Genet. 2005;134:67–72. doi: 10.1002/ajmg.b.30142. [DOI] [PubMed] [Google Scholar]
- Boyle MH, Offord DR, Racine Y, Fleming JE, Szatmari P, Sanford M. Evaluation of the revised Ontario Child Health Study scales. J Child Psychol Psychiatry. 1993;34:189–213. doi: 10.1111/j.1469-7610.1993.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Brookes K, Xu X, Chen W, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006;11:934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- Chou IC, Lee CC, Huang CC, Wu JY, Tsai JJ, Tsai CH, Tsai FJ. Association of the neuronal nicotinic acetylcholine receptor subunit alpha4 polymorphisms with febrile convulsions. Epilepsia. 2003;44:1089–1093. doi: 10.1046/j.1528-1157.2003.t01-1-44702.x. [DOI] [PubMed] [Google Scholar]
- Clarke S, Heussler H, Kohn MR. Attention deficit disorder: not just for children. Intern Med J. 2005;35:721–725. doi: 10.1111/j.1445-5994.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- Clayton D. A generalization of the transmission/disequilibrium test for uncertain-haplotype transmission. Am J Hum Genet. 1999;65:1170–1177. doi: 10.1086/302577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combi R, Dalpra L, Tenchini ML, Ferini-Strambi L. Autosomal dominant nocturnal frontal lobe epilepsy – a critical overview. J Neurol. 2004;251:923–934. doi: 10.1007/s00415-004-0541-x. [DOI] [PubMed] [Google Scholar]
- Comings DE, Gade-Andavolu R, Gonzalez N, Wu S, Muhleman D, Blake H, Chiu F, Wang E, Farwell K, Darakjy S, Baker R, Dietz G, Saucier G, MacMurray JP. Multivariate analysis of associations of 42 genes in ADHD, ODD and conduct disorder. Clin Genet. 2000;58:31–40. doi: 10.1034/j.1399-0004.2000.580106.x. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ Rating Scales – Revised. Multi-Health Systems Inc; Toronto, Canada: 1997. [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Res Brain Res Rev. 2003;42:1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- Durston S. A review of the biological bases of ADHD: what have we learned from imaging studies? Ment Retard Dev Disabil Res Rev. 2003;9:184–195. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Niu T, Xing H, Xu X, Chen C, Peng S, Wang L, Laird N. A common haplotype of the nicotine acetylcholine receptor alpha 4 subunit gene is associated with vulnerability to nicotine addiction in men. Am J Hum Genet. 2004;75:112–121. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997;36:1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [(3)H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype – phenotype associations. Eur J Hum Genet. 2001;9:301–306. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- Ickowicz A, Schachar R, Sugarman R, Chen S, Millette C, Cook L. The parent interview for child symptoms (PICS): a situation-specific clinical-research interview for attention deficit hyperactivity and related disorders. Can J Psychiatry. 2006;50:325–328. doi: 10.1177/070674370605100508. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Fein D, Kramer J, Delis D, Morris R. WISC-III PI Manual. The Psychological Corporation; San Antonio TX: 1999. [Google Scholar]
- Kawamata J, Shimohama S. Association of novel and established polymorphisms in neuronal nicotinic acetylcholine receptors with sporadic Alzheimer’s disease. J Alzheimers Dis. 2002;4:71–76. doi: 10.3233/jad-2002-4201. [DOI] [PubMed] [Google Scholar]
- Kent L, Middle F, Hawi Z, Fitzgerald M, Gill M, Feehan C, Craddock N. Nicotinic acetylcholine receptor alpha4 subunit gene polymorphism and attention deficit hyperactivity disorder. Psychiatr Genet. 2001;11:37–40. doi: 10.1097/00041444-200103000-00007. [DOI] [PubMed] [Google Scholar]
- Kim SA, Kim JW, Song JY, Park S, Lee HJ, Chung JH. Association of polymorphisms in nicotinic acetylcholine receptor alpha 4 subunit gene (CHRNA4), mu-opioid receptor gene (OPRM1), and ethanol-metabolizing enzyme genes with alcoholism in Korean patients. Alcohol. 2004;34:115–120. doi: 10.1016/j.alcohol.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotimaa AJ, Moilanen I, Taanila A, Ebeling H, Smalley SL, McGough JJ, Hartikainen AL, Jarvelin MR. Maternal smoking and hyperactivity in 8-year-old children. J Am Acad Child Adolesc Psychiatry. 2003;42:826–833. doi: 10.1097/01.CHI.0000046866.56865.A2. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Manual: The Children’s Depression Inventory. Multi-Health Systems; Toronto, Canada: 1995. [Google Scholar]
- Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genet Epidemiol. 2000;19(Suppl 1):S36–S42. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Laurin N, Misener VL, Crosbie J, Ickowicz A, Pathare T, Roberts W, Malone M, Tannock R, Schachar R, Kennedy JL, Barr CL. Association of the calcyon gene (DRD1IP) with attention deficit/hyperactivity disorder. Mol Psychiatry. 2005;10:1117–1125. doi: 10.1038/sj.mp.4001737. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology (Berl) 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levy F, McStephen M, Hay DA. The diagnostic genetics of ADHD symptoms and subtypes. In: Levy F, Hay D, editors. Attention-Genes and ADHD. Brunner-Routledge; Hove, UK: 2001. [Google Scholar]
- Li S, Kim KY, Kim JH, Park MS, Bahk JY, Kim MO. Chronic nicotine and smoking treatment increases dopamine transporter mRNA expression in the rat midbrain. Neurosci Lett. 2004;363:29–32. doi: 10.1016/j.neulet.2004.03.053. [DOI] [PubMed] [Google Scholar]
- Li MD, Beuten J, Ma JZ, Payne TJ, Lou XY, Garcia V, Duenes AS, Crews KM, Elston RC. Ethnic- and gender-specific association of the nicotinic acetylcholine receptor alpha4 subunit gene (CHRNA4) with nicotine dependence. Hum Mol Genet. 2005;14:1211–1219. doi: 10.1093/hmg/ddi132. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Mancuso G, Warburton DM, Melen M, Sherwood N, Tirelli E. Selective effects of nicotine on attentional processes. Psychopharmacology (Berl) 1999;146:199–204. doi: 10.1007/s002130051107. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcorn JH, Halperin JM, Jensen PS, et al. Symptom profiles in children with ADHD: effects of comorbidity and gender. J Am Acad Child Adolesc Psychiatry. 2001;40:137–146. doi: 10.1097/00004583-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Newman MB, Shytle RD, Sanberg PR. Locomotor behavioral effects of prenatal and postnatal nicotine exposure in rat offspring. Behav Pharmacol. 1999;10:699–706. doi: 10.1097/00008877-199911000-00017. [DOI] [PubMed] [Google Scholar]
- Parish CL, Nunan J, Finkelstein DI, McNamara FN, Wong JY, Waddington JL, Brown RM, Lawrence AJ, Horne MK, Drago J. Mice lacking the alpha4 nicotinic receptor subunit fail to modulate dopaminergic neuronal arbors and possess impaired dopamine transporter function. Mol Pharmacol. 2005;68:1376–1386. doi: 10.1124/mol.104.004820. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen ER, Neuman RJ, Heath AC, Levy F, Hay DA, Todd RD. Familial clustering of latent class and DSM-IV defined attention-deficit/hyperactivity disorder (ADHD) subtypes. J Child Psychol Psychiatry. 2004;45:589–598. doi: 10.1111/j.1469-7610.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. What I Think and Feel (RCMAS) Western Psychological Services; Los Angeles, CA: 1985. [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, Drago J. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Madras BK. Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry. 1998;3:386–396. doi: 10.1038/sj.mp.4000421. [DOI] [PubMed] [Google Scholar]
- Semel E, Wing E, Secord W. Clinical Evaluation of Language Fundamentals-Third Edition (CELF-3) The Psychological Corporation; San Antonio, TX: 1995. [Google Scholar]
- Sham PC, Curtis D. An extended transmission/disequilibrium test (TDT) for multi-allele marker loci. Ann Hum Genet. 1995;59:323–336. doi: 10.1111/j.1469-1809.1995.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Sherman DK, Iacono WG, McGue MK. Attention-deficit hyperactivity disorder dimensions: a twin study of inattention and impulsivity-hyperactivity. J Am Acad Child Adolesc Psychiatry. 1997;36:745–753. doi: 10.1097/00004583-199706000-00010. [DOI] [PubMed] [Google Scholar]
- Steinlein O, Smigrodzki R, Lindstrom J, Anand R, Kohler M, Tocharoentanaphol C, Vogel F. Refinement of the localization of the gene for neuronal nicotinic acetylcholine receptor alpha 4 subunit (CHRNA4) to human chromosome 20q13.2-q13.3. Genomics. 1994;22:493–495. doi: 10.1006/geno.1994.1420. [DOI] [PubMed] [Google Scholar]
- Steinlein O, Weiland S, Stoodt J, Propping P. Exon-intron structure of the human neuronal nicotinic acetylcholine receptor alpha 4 subunit (CHRNA4) Genomics. 1996;32:289–294. doi: 10.1006/geno.1996.0119. [DOI] [PubMed] [Google Scholar]
- Tannock R, Hum M, Masellis M, Humphries T, Schachar R. Teacher Telephone Interview for Children’s Academic Performance, Attention, Behavior and Learning: DSM-IV Version (TTI-IV) The Hospital for Sick Children; Toronto, Canada: 2002. Unpublished Document. [Google Scholar]
- Thapar A, Holmes J, Poulton K, Harrington R. Genetic basis of attention deficit and hyperactivity. Br J Psychiatry. 1999;174:105–111. doi: 10.1192/bjp.174.2.105. [DOI] [PubMed] [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, Harold G, Hay D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am J Psychiatry. 2003;160:1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- Thapar A, O’Donovan M, Owen MJ. The genetics of attention deficit hyperactivity disorder. Hum Mol Genet. 2005;14(Spec No 2):R275–R282. doi: 10.1093/hmg/ddi263. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Russell LT, Nespor SM, Perry DC, Grunberg NE. Prenatal nicotine exposure: effects on locomotor activity and central [125I]alpha-BT binding in rats. Pharmacol Biochem Behav. 2000;66:495–500. doi: 10.1016/s0091-3057(00)00171-4. [DOI] [PubMed] [Google Scholar]
- Todd RD, Lobos EA, Sun LW, Neuman RJ. Mutational analysis of the nicotinic acetylcholine receptor alpha 4 subunit gene in attention deficit/hyperactivity disorder: evidence for association of an intronic polymorphism with attention problems. Mol Psychiatry. 2003;8:103–108. doi: 10.1038/sj.mp.4001257. [DOI] [PubMed] [Google Scholar]
- Viggiano D, Ruocco LA, Sadile AG. Dopamine phenotype and behaviour in animal models: in relation to attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2003;27:623–637. doi: 10.1016/j.neubiorev.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. Harcourt Brace & Co; San Antonio, TX: 1991. [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, Soriano J, Fine C, Abrams A, Rater M, Polisner D. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Verlinden MH, Adler LA, Wozniak PJ, West SA. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit/hyperactivity disorder in adults: results of a pilot study. Biol Psychiatry. 2006;59:1065–1070. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test 3-Revision 3. Jastak Associates; Wilmington, DE: 1993. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]