Abstract
Background
Chronic pain is common during childhood and adolescence and is associated with negative outcomes such as increased severity of pain, reduced function (e.g. missing school), and low mood (e.g. high levels of depression and anxiety). Psychological therapies, traditionally delivered face-to-face with a therapist, are efficacious at reducing pain intensity and disability. However, new and innovative technology is being used to deliver these psychological therapies remotely, meaning barriers to access to treatment such as distance and cost can be removed or reduced. Therapies delivered with technological devices, such as the Internet, computer-based programmes, smartphone applications, or via the telephone, can be used to deliver treatment to children and adolescents with chronic pain.
Objectives
To determine the efficacy of psychological therapies delivered remotely compared to waiting-list, treatment-as-usual, or active control treatments, for the management of chronic pain in children and adolescents.
Search methods
We searched four databases (CENTRAL, MEDLINE, EMBASE, and PsycINFO) from inception to June 2014 for randomised controlled trials of remotely delivered psychological interventions for children and adolescents (0 to 18 years of age) with chronic pain. We searched for chronic pain conditions including, but not exclusive to, headache, recurrent abdominal pain, musculoskeletal pain, and neuropathic pain. We also searched online trial registries for potential trials. A citation and reference search for all included studies was conducted.
Selection criteria
All included studies were randomised controlled trials that investigated the efficacy of a psychological therapy delivered remotely via the Internet, smartphone device, computer-based programme, audiotapes, or over the phone in comparison to an active, treatment-as-usual, or waiting-list control. We considered blended treatments, which used a combination of technology and face-to-face interaction. We excluded interventions solely delivered face-to-face between therapist and patient from this review. Children and adolescents (0 to 18 years of age) with a primary chronic pain condition were the target of the interventions. Each comparator arm, at each extraction point had to include 10 or more participants.
Data collection and analysis
For the analyses, we combined all psychological therapies. We split pain conditions into headache and mixed (non-headache) pain and analysed them separately. Pain, disability, depression, anxiety, and adverse events were extracted as primary outcomes. We also extracted satisfaction with treatment as a secondary outcome. We considered outcomes at two time points: first immediately following the end of treatment (known as ’post-treatment’), and second, any follow-up time point post-treatment between 3 and 12 months (known as ’follow-up’). We assessed all included studies for risk of bias.
Main results
Eight studies (N = 371) that delivered treatment remotely were identified from our search; five studies investigated children with headache conditions, one study was with children with juvenile idiopathic arthritis, and two studies included mixed samples of children with headache and mixed (i.e. recurrent abdominal pain, musculoskeletal pain) chronic pain conditions. The average age of children receiving treatment was 12.57 years.
For headache pain conditions, we found one beneficial effect of remotely delivered psychological therapy. Headache severity was reduced post-treatment (risk ratio (RR) = 2.65, 95% confidence interval (CI) 1.56 to 4.50, z = 3.62,p < 0.01, number needed to treat to benefit (NNTB) = 2.88). For mixed pain conditions, we found only one beneficial effect: psychological therapies reduced pain intensity post-treatment (standardised mean difference (SMD) = −0.61, 95% CI −0.96 to −0.25, z = 3.38, p < 0.01). No effects were found for reducing pain at follow-up in either analysis. For headache and mixed conditions, there were no beneficial effects of psychological therapies delivered remotely for disability post-treatment and a lack of data at follow-up meant no analyses could be run. Only one analysis could be conducted for depression outcomes. We found no beneficial effect of psychological therapies in reducing depression post-treatment for headache conditions. Only one study presented data in children with mixed pain conditions for depressive outcomes and no data were available for either condition at follow-up. Only one study presented anxiety data post-treatment and no studies reported follow-up data, therefore no analyses could be run. Further, there were no data available for adverse events, meaning that we are unsure whether psychological therapies are harmful to children who receive them. Satisfaction with treatment is described qualitatively.
‘Risk of bias’ assessments were low or unclear. We judged selection, detection, and reporting biases to be mostly low risk for included studies. However, judgements made on performance and attrition biases were mostly unclear.
Authors’ conclusions
Psychological therapies delivered remotely, primarily via the Internet, confer benefit in reducing the intensity or severity of pain after treatment across conditions. There is considerable uncertainty around these estimates of effect and only eight studies with 371 children contribute to the conclusions. Future studies are likely to change the conclusions reported here. All included trials used either behavioural or cognitive behavioural therapies for children with chronic pain, therefore we cannot generalise our findings to other therapies. However, satisfaction with these treatments was generally positive. Larger trials are needed to increase our confidence in all conclusions regarding the efficacy of remotely delivered psychological therapies. Implications for practice and research are discussed.
PLAIN LANGUAGE SUMMARY
Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents
Background
Children and adolescents with chronic pain often report their pain as hurting too much (intense) and happening too often (frequent). The pain can affect their ability to function physically and that can leave them feeling anxious or depressed. The most common types of chronic pain in children and adolescents are headaches and recurrent abdominal pain. A therapist, physically together with a patient or family (a method often called face-to-face) traditionally delivers psychological therapies, such as cognitive behavioural therapy or behavioural therapy. These therapies can include components such as relaxation techniques, coping strategies, and behavioural strategies, all of which have been found to benefit children by reducing pain and improving physical functioning. However, new technologies now allow therapy to be delivered without needing to be face-to-face with a therapist. Therapies delivered remotely promise to make treatments easier to access because they remove the need for travel. They may also be less expensive. By technology we mean the Internet, computer-based programmes, smartphone applications, and the telephone.
Review questions
Can psychological therapies, delivered remotely using technology, help children and adolescents with chronic pain to have less pain, to improve physical functioning, and to have fewer symptoms of depression and anxiety? Are any improvements greater than those reported by children who are waiting to be treated (waiting-list control), or being treated in other ways (active control)?
Study characteristics
We conducted the search through to June 2014. We found eight studies including 371 children and adolescents. Five studies treated children with headache, one study treated children with juvenile idiopathic arthritis, and two studies included mixed samples of children, some who had headache and some with other chronic pain conditions. The average age of children receiving the interventions was 12.6 years. Four trials delivered therapy via the internet, two trials used CD-ROMs, one trial delivered therapy via audiotapes, and one trial delivered therapy via the telephone. All therapies delivered were either cognitive behavioural therapy or behavioural therapy. We looked at six outcomes; pain, physical functioning, depression, anxiety, adverse events, and satisfaction with treatment.
Key results
We split the painful conditions into two groups and analysed them separately. The first group included children with headache pain. The second group included children with other painful conditions (e.g. recurrent abdominal pain, musculoskeletal pain), known as ‘mixed pain’. Psychological therapies delivered remotely (primarily via the Internet) were beneficial at reducing pain for children and adolescents with headache pain and mixed pain when assessed immediately following treatment. However, we found no effects of treatment on physical functioning post-treatment for headache and mixed pain conditions. There was also no effect on depression for headache conditions post-treatment. Satisfaction was described qualitatively in the trials and was generally positive. However, we could not assess this outcome using any numbers. For all other outcomes, no data were available for analysis. There was no description of adverse events reported in the included studies.
Currently, there are very few studies investigating this treatment. Caution should be taken when interpreting these results as they are based on a small number of studies with few children. However, this is a growing field and more trials using cognitive behavioural therapy and other psychological therapies are needed to determine the efficacy of remotely delivered therapies.
BACKGROUND
Description of the condition
Episodes of chronic pain are surprisingly common during childhood and adolescence (Perquin 2000). Epidemiological studies report that girls experience more pain than boys and that pain increases during early adolescence (King 2011). Further, risk of developing a pain condition is higher for children of a lower socioeconomic status (King 2011). The most commonly reported pain problems are headache, recurrent abdominal pain, musculoskeletal pain, and back pain (King 2011). Some children with chronic pain report high levels of pain as well as depression and anxiety (Gauntlett-Gilbert 2007; Kaczynski 2011). Children can also suffer impairments in their physical and social functioning, such as attending school less often (Cohen 2011). The detrimental effects of chronic pain can also impact parents, who report significant distress and anxiety (Jordan 2007; Maciver 2010).
Description of the intervention
Psychological therapies, delivered individually or in groups to children and families, significantly reduce pain and disability in children with chronic pain (Eccleston 2014). However, many young people do not receive psychological treatments for chronic pain due to barriers to access such as a shortage of providers, expense, and geographic distance from treatment centres (Palermo 2013; Peng 2007). This has led to consideration of innovative methods of delivery and calls to assess whether psychological interventions can be delivered effectively when remote from the patient using technology such as the Internet (Palermo 2009). The Internet is widely available to a large number of children and adolescents. For example, in the UK 83% of households had Internet access in 2013 (ONS 2013), in the US 72% (USDC 2013), and in Australia 79% (ABS 2012), meaning that access to health information and treatment is potentially available to many.
Different terms are used within this growing field, broadly described as e-health, telemedicine, telecare, minimal therapist contact, and distance treatment. Here, we adopt ’remotely delivered therapies’ to define psychological therapies delivered without, or with limited face-to-face contact with the therapist. Therapies are typically delivered via technology, principally the Internet, but could also be delivered via telephone, written materials, or stand-alone computer programmes. Therapies may also be combined or blended by including both face-to-face and remote components. These interventions can be delivered in the home or community (i.e. outside the clinic or hospital setting) without the physical presence of a therapist.
How the intervention might work
Psychological therapies (as discussed in Eccleston 2014) are used in paediatric pain practice to reduce pain symptoms, disability, and negative mood associated with pain conditions, and to modify social-environmental factors to enhance the child’s adaptive functioning. This field is currently dominated by cognitive behavioural therapies (CBT) and behavioural therapies (BT) that have components such as relaxation, biofeedback, imagery, parent operant strategies, and coping skills training.
Recognising the advantages of reaching more children in their homes with remotely delivered interventions, earlier studies relied on low levels of technology, including written self help manuals, portable biofeedback monitors, and relaxation audiotapes (e.g. Burke 1989; McGrath 1992). As technological advances became available, intervention delivery options expanded to personal computers via CD-ROM applications and then to programmes/applications via the Internet. The delivery of psychological therapies over the Internet is becoming more common (March 2008; Richardson 2010; Tait 2010). The potential benefits of a successful programme include improved access, improved scale of coverage, and lowered cost (Marks 2009; Palermo 2009). However, the change of a delivery mechanism from face-to-face delivery to remote delivery via technology arguably changes the content, intensity, and force of a treatment. The move away from face-to-face delivery is not simply a change in the route of administration. The transformation of a treatment to a reliance on communication technology (instead of face-to-face interaction with a therapist) may involve critical changes in aspects of the treatment thought crucial to its success. For example, treatment where a therapist is not present may influence treatment participation and impact treatment outcomes (Fry 2009).
There may also be different therapeutic opportunities available using interactive and communication technologies. As described in the behavioural change model for Internet interventions (Ritterband 2009), user characteristics interact with website characteristics to produce behaviour change. For example, Internet-delivered therapies may work by better matching and designing technology to maximise the therapeutic benefits (e.g. 24-hour access to skills training), or there may be a blend to these solutions that function differently dependent upon user characteristics. Typically, authors are not explicit about how the technology may have changed the intervention itself, but earlier remotely delivered therapies were informed by the question of equivalence: can a remotely delivered therapy perform as well as a face-to-face therapy? More recent trials treat the remotely delivered therapy as a package and ask: can a remotely delivered therapy achieve better outcomes than a comparison group or can remotely delivered therapy be efficacious in achieving positive change in meaningful treatment outcomes?
Why it is important to do this review
Psychological therapies delivered remotely (principally but not exclusively via the Internet) have now developed into stand-alone treatments, and are investigated as stand-alone treatments. A Cochrane review has previously summarised the evidence of psychological therapies for the management of chronic pain in children and adolescents (Eccleston 2014). This was first authored in 2003, and updated in 2009, 2012, and most recently in 2014. Earlier updates combined remote and face-to-face office-based treatment delivery. However, we believe it is important to separate them so that the evidence can be separately evaluated. This review should be considered a sister review to the Eccleston 2014 update, which now excludes treatments delivered remotely. A similar distinction has also been made in the Cochrane reviews on psychological therapies for the management of chronic pain in adults: face-to-face (Williams 2012) and Internet-delivered (Eccleston 2014b).
OBJECTIVES
To determine the efficacy of psychological therapies delivered remotely compared to waiting-list, treatment-as-usual, or active control treatments, for the management of chronic pain in children and adolescents.
METHODS
Criteria for considering studies for this review
Types of studies
We searched for randomised controlled trials (RCTs) that delivered psychological therapies remotely to children and adolescents with chronic pain.
Types of participants
We included children and adolescents under the age of 18 years. The intervention had to primarily target the child or adolescent with chronic or recurrent pain, defined as pain lasting for three months or longer. Pain conditions typically (but not exclusively) fall into the categories of headache, musculoskeletal pain, neuropathic pain, and recurrent abdominal pain. We excluded pain associated with life-limiting (e.g. cancer) or other primary conditions (e.g. diabetes). For the trial to be included, we required 10 or more participants to be in each arm of the trial at each extracted time point of post-treatment or follow-up.
Types of interventions
Included studies investigated treatments that were primarily psychological and included recognisable psychotherapeutic content, or were based on an existing psychological framework. Trials had to include at least one comparator arm. Therapies had to aim to improve pain outcomes and function; we excluded therapies that solely aimed to manage child or adolescent mood. Psychological therapies had to be delivered remotely, using technology such as the Internet, computer programme, smartphone application, audiotapes, or telephone. Therapies delivered face-to-face are included in Eccleston 2014, and are not included in this review. We also considered therapies that used blended treatments, combining both face-to-face contact and a remote component for inclusion in this review. However, the intention of included trials (stated or inferred) was to deliver the majority of the treatment remotely from the therapist. As a guide, we excluded studies that conducted over 30% of contact time (assessment or therapy) face-to-face. Interventions that had a primary aim to monitor symptoms or aid communication (such as with a treatment team) were excluded.
Types of outcome measures
Primary outcomes
We extracted five primary outcomes from each study; pain symptoms, disability, depression, anxiety, and adverse events.
Secondary outcomes
We extracted satisfaction with treatment as a secondary outcome.
Search methods for identification of studies
Electronic searches
We searched the following databases for studies from inception to the present day:
CENTRAL (CRSO) searched on 3rd June 2014;
MEDLINE (OVID) 1946 to 2nd June 2014;
EMBASE (OVID) 1974 to 2nd June 2014;
PsycINFO (OVID) 1806 to May week 4 2014.
A search strategy for MEDLINE was devised and adapted for the other databases listed (see Appendix 1 for all search strategies).
Searching other resources
We conducted a reference search and citation search of all included studies in order to identify additional studies not found in our database search. We also contacted authors for any further studies. Relevant reviews retrieved by the database searches were examined to identify any further trials. In addition, trial registries, including the metaRegister of controlled trials (mRCT) (www.controlledtrials.com/mrct/), ClinicalTrials.gov (clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/) were searched for trials. There were no limitations on publication date or language.
Data collection and analysis
Selection of studies
Two authors (EF, EL) independently selected and read potential studies for inclusion. A third author (CE) arbitrated any disagreements. We selected studies according to the following criteria:
Children and adolescents under the age of 18 years with a chronic pain condition.
N ≥ 10 in each arm of the trial at each extracted time point.
A primarily psychological therapy used in at least one arm of each included trial.
Therapies with a primary aim to change thoughts or behaviours of the child to assist with the management of, or coping with, chronic pain.
Therapies that were principally delivered remotely.
See PRISMA flow diagram for search results (Figure 1), as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Figure 1.
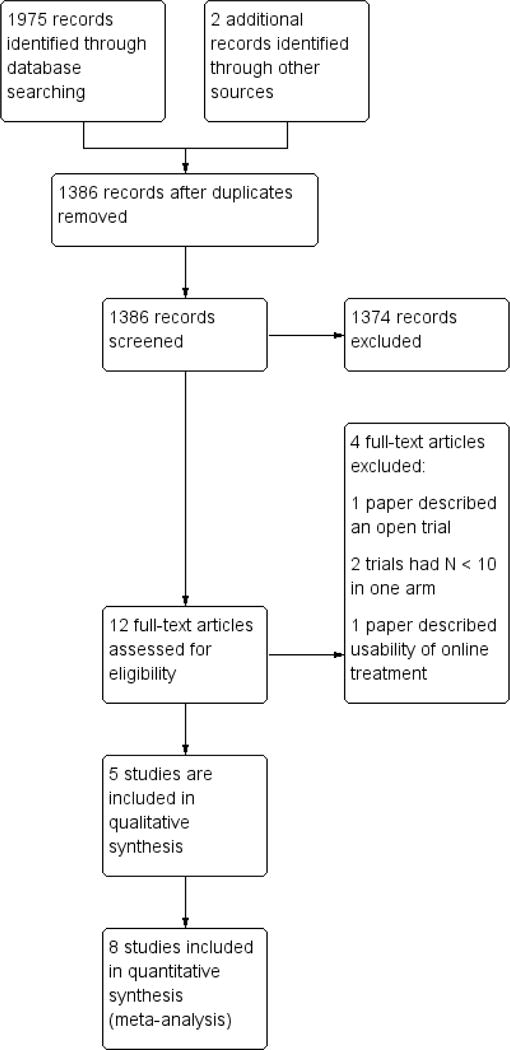
Study flow diagram.
Data extraction and management
Two authors (EF, EL) independently extracted data from the studies. Disagreements were first discussed between the two authors, and arbitrated by a third author (CE) if no agreement could be found. First, study characteristics were extracted from each of the studies. These included patient demographics and characteristics of the psychological therapies including delivery type, duration of treatment, when and where treatment was accessed, engagement in treatment, type of control condition, and follow-up periods. Second, data for each of the five primary outcomes and secondary outcome were extracted at post-treatment and follow-up. If studies reported incomplete data, study authors were contacted.
Assessment of risk of bias in included studies
We assessed risk of bias using The Cochrane Collaboration’s ’Risk of bias’ tool. This outlines four biases: selection bias, detection bias, attrition bias, and reporting bias. Selection bias was judged by random sequence generation and allocation bias. Detection bias was judged by blinding of personnel and participants, and blinding of outcome assessors. Attrition bias was judged by incomplete outcome reporting. Finally, reporting bias was used to judge selective reporting.
It was not possible to assess the quality of outcomes using the GRADE criteria due to the small number of studies. However, in future updates, when more data are available, we will assess the quality of outcomes post-treatment and at follow-up. Two ’Summary of findings’ tables will be produced; one for headache outcomes and one for mixed pain conditions. Only the seven most important outcomes can be included in each ’Summary of findings’ table, therefore, we will select the seven outcomes that include the highest number of participants. We will use a four-tiered rating system to rate outcomes a ’high’, ’moderate’, ’low’, or ’very low’. Outcomes will be assessed on risk of bias, inconsistency, indirectness, imprecision, and publication bias (Balshem 2011; Higgins 2011). An assessment of high quality is given when “we are very confident that the true effect lies close to that of the estimate of the effect”, moderate quality is judged when “we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different”, low quality is given when “our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect”, and very low quality is judged when “we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect” (p. 404, Balshem 2011).
Measures of treatment effect
We categorised chronic pain conditions into headache and mixed pain conditions. Mixed pain conditions refer to painful conditions such as musculoskeletal pain, neuropathic pain, and recurrent abdominal pain. Due to the small number of studies in this area, we combined these conditions in analyses to provide the overall effectiveness of psychological therapies delivered remotely. If a study reported both headache and mixed pain conditions, we entered data into both analyses where appropriate. We analysed pain symptoms, disability, depression, and anxiety at two time points (post-treatment and follow-up). Adverse events were extracted and described. Satisfaction with treatment was defined as any measure, based on self report (child or parent), that aimed to assess how useful the treatment was, satisfaction with the outcome of therapy, and likeability and preference for the treatment. When studies used more than one measure for a given outcome, we extracted the most reliable or commonly used. We defined post-treatment as the time point immediately following treatment. Follow-up was defined as the time point between 3 and 12 months following post-treatment. If more than one time point was available, the latter of the two was extracted. Due to this novel method of delivery of psychological interventions, there are currently only a small number of studies that can be included in analyses. Therefore, we did not categorise studies by therapy type or control type (i.e. active versus waiting-list) and results are directly comparable to Eccleston 2014. In total, there are 20 possible analyses, categorised by four headings:
Treatment versus control, post-treatment, headache conditions;
Treatment versus control, follow-up, headache conditions;
Treatment versus control, post-treatment, mixed pain conditions;
Treatment versus control, follow-up, mixed pain conditions.
Data synthesis
We pooled data using Review Manager 5.3 (RevMan 2014). Headache conditions are typically reported with dichotomous data for pain symptoms defined by a 50% reduction of pain symptoms. Mixed pain conditions (e.g. musculoskeletal pain, neuropathic pain, and recurrent abdominal pain) are typically reported with continuous data for pain symptoms. For dichotomous data, we calculated risk ratios (RRs), 95% confidence intervals (CIs) and number needed to treat to benefit (NNTB). For continuous data, we report standardised mean differences (SMDs) and 95% CIs. Mantel-Haenszel methods were used to analyse dichotomous data and random-effects models were used to analyse continuous data.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses to investigate the technology type and intensity used in the trials (e.g. Internet versus telephone; low intensity technology versus high intensity technology) were planned. Further, we also planned to determine the difference in effect between trials that included a human support component (blended therapy) versus those without human support that were exclusively delivered remotely, as additional support during trials delivered via the Internet has been found to influence outcomes of participants (Law 2012). However, due to the small number of trials we were unable to conduct these analyses.
RESULTS
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Characteristics of included studies [ordered by study ID]
| Connelly 2006 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment, 2 months, 3 months | |
| Participants | End of treatment: n = 36 Start of treatment: n = 37 Sex: 18 F, 19 M Mean age: 10.0 (range 7 to 12) Source: clinic Diagnosis: headache Mean years of pain: not given |
|
| Interventions | ”CD-ROM behavioural” “Wait-list neurology TAU” |
|
| Outcomes | Primary pain outcome: clinical reduction in headache frequency Primary disability outcome: Ped-MIDAS Primary depression outcome: none Primary anxiety outcome: none Primary satisfaction outcome: none Measures reported: Total pain (headache diary) Pediatric Migraine Disability Assessment (Ped-MIDAS) |
|
| Notes | Funding source: educational grant from AstraZeneca LP Declarations of interest: none stated |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomly assigned to one of two groups by a research assistant using a uniform random numbers table.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “Randomly assigned to one of two groups by a research assistant using a uniform random numbers table.” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes |
Low risk | “Study neurologists remained blind to randomisation condition throughout the study. Chances of unbinding were limited because follow-up appointments with the study neurologist were scheduled for 2 months following the initial assessment.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Measures completed at home and mailed back |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition is described, however significant descriptions between completers and non-completers were not reported |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Cottrell 2007 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment, and 8 months | |
| Participants | End of treatment: n = 30, follow-up n = 28 Start of treatment: n = 30 Sex: 15 F, 15 M Mean age: 14.1 (SD 1.91) Source: referral by neurologist and community advertisement Diagnosis: headache Duration (mean): unknown |
|
| Interventions | “STOP Migraines treatment” – behavioural treatment delivered via telephone Triptan treatment |
|
| Outcomes | Primary pain outcome: none Primary disability outcome: hours disabled by headache Primary depression outcome: none Primary anxiety outcome: none Primary satisfaction outcome: satisfaction from participant feedback Measures reported: Participant feedback including evaluation of the manual, relaxation tapes, home biofeedback equipment, telephone versus clinic treatment format, satisfaction, and quality of relationship Daily diary including headache duration, headache severity, number of hours participant was totally disabled Migraine Specific Quality of Life Questionnaire – Adolescent |
|
| Notes | Funding source: National Institutes of Health (NINDS #N32374) Declarations of interest: Dr. O’Donnell is an employee of OrthoNeuro Inc |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Thus, 34 adolescents were randomized to treatment (16 TT and 18 TAT).” Comment: probably done; description of randomisation not provided |
| Allocation concealment (selection bias) | Unclear risk | No description found in text Comment: probably not done |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | No description found in text Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition completely reported; significant differences between completers and non-completers were not reported |
| Selective reporting (reporting bias) | High risk | Outcomes incompletely reported |
| Hicks 2006 | ||
| Methods | RCT 2 arms. Assessed at pre-treatment, 1 month post-treatment, 3 months | |
| Participants | End of treatment: n = 37, 1-month follow-up n = 37, 3-month follow-up n = 32 Start of treatment: n = 47 Sex: 30 F, 17 M Mean age: 11.7 (range 9 to 16) Source: advertisements in media, physicians’ offices and school Diagnosis: headache and RAP Duration (mean): 3 years |
|
| Interventions | “Internet CBT (with Internet and phone)” “Standard Care (Wait List)” |
|
| Outcomes | Primary pain outcome: clinical reduction in headache frequency (headache analysis) and mean pain intensity (mixed pain conditions analysis) Primary disability outcome: none Primary depression outcome: none Primary anxiety outcome: none Primary satisfaction outcome: none Measures reported: Pain diary Numeric rating scale frequency Numeric rating scale intensity Pediatric Quality of Life Inventory Parental Quality of Life |
|
| Notes | Funding source: Peter Samuelson STARB RIGHT Foundation 2002 Dissertation Award in paediatric psychology and the Canadian Pain Society Small Grant for Local and Regional Initiatives. McGrath is supported by a Canada Research Chair Declarations of interest: none stated | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The 47 participants were stratified by age and pain severity and randomly assigned by blocks to either the treatment condition or the standard medical care wait-list condition.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “The 47 participants were stratified by age and pain severity and randomly assigned by blocks to either the treatment condition or the standard medical care wait-list condition.” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | No description found in text Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Measures completed at home and submitted online |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition completely reported; significant differences between completers and non-completers were not reported |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| McGrath 1992 | ||
| Methods | RCT 3 arms. Assessed at pre-treatment, post-treatment, 3 months and 1-year follow-up | |
| Participants | End of treatment: n = 74 Start of treatment: n = 87 Sex: 63 F, 24 M Mean age: not given(range 11 to 18 years) Source: paediatricians and family physicians Diagnosis: migraine Mean years of pain not given: minimum 3 months |
|
| Interventions | “Therapist administered cognitive behavioural/stress coping/relaxation training” “Self-administered cognitive behavioural/stress coping/relaxation training” “Information and support” |
|
| Outcomes | Primary pain outcome: headache diary Primary disability outcome: none Primary depression outcome: Poznanski Depression Scale Primary anxiety outcome: none 1. Headache index 2. Efficiency of treatment 3. Poznanski Depression Scale |
|
| Notes | Funding source: National Health and Welfare Research and Development Program of Canada Declaration of interest: Dr. McGrath was supported by a Career Scientist Award of the Ontario Ministry of Health |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomised to 1 of the 8-week treatments” Comment: probably done; no method described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text Comment: probably not done |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | No description found in text Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Measures completed at home |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition is described, however significant differences between completers and non-completers are not reported |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported |
| Palermo 2009 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment and post-treatment | |
| Participants | End of treatment: n = 44 Start of treatment: n = 48 Sex: 35 F, 13 M Mean age: 14.8 (SD 2.0) Source: medical centre in the Pacific Northwest USA Diagnosis: headache (25% of the sample), abdominal pain (50% of the sample), or musculoskeletal pain (25% of the sample) Mean years of pain: 30 months |
|
| Interventions | “Internet-delivered family cognitive-behavioural therapy” “Wait-list control group” |
|
| Outcomes | Primary pain outcome: clinical reduction in headache frequency (headache analysis) and mean pain intensity (mixed pain conditions analysis) Primary disability outcome: Child Activity and Limitations Interview Primary depression outcome: Revised Child Anxiety and Depression Scale Primary anxiety outcome: none Primary satisfaction outcome: treatment acceptability and satisfaction Measures reported: Daily pain intensity NRS (averaged over 7 days) Usual pain intensity over the past month NRS Child Activity Limitations Interview Revised Child Anxiety and Depression Scale Protect subscale from Adult Responses to Children’s Symptoms Treatment acceptability and satisfaction |
|
| Notes | Funding source: National Institutes of Health/National Institute of Child Health and Human Development (Grant HD050674; PI: Palermo) and by a grant from the Doernbecher Foundation Declarations of interest: authors have no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A fixed allocation randomisation scheme was used. Specifically, we used blocked randomisation with blocks of 10 to assign participants to the two treatment conditions during the course of randomisation. An online random number generator was used to produce the blocked randomisation. Group assignments were identified by ID number in sealed envelopes. Following completion of all pre-treatment assessments, a research coordinator opened the sealed envelope to reveal the group assignment.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “A fixed allocation randomisation scheme was used. Specifically, we used blocked randomisation with blocks of 10 to assign participants to the two treatment conditions during the course of randomisation. An online random number generator was used to produce the blocked randomisation. Group assignments were identified by ID number in sealed envelopes. Following completion of all pre-treatment assessments, a research coordinator opened the sealed envelope to reveal the group assignment.” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | No description found in text Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Measures completed at home and submitted online or mailed back |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition completely reported; significant differences between completers and non-completers were not reported |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Rapoff 2014 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment and post-treatment | |
| Participants | End of treatment: n = 22 Start of treatment: n = 35 Sex: 25 F, 10 M Mean age: 10.2 (SD 1.75) Source: paediatric headache clinics at 1 university and 2 children’s hospitals Diagnosis: headache Mean years of pain: unknown |
|
| Interventions | “Headstrong programme” “Education” |
|
| Outcomes | Primary pain outcome: none Primary disability outcome: Pediatric Migraine Disability Assessment Primary depression outcome: none Primary anxiety outcome: none Primary satisfaction outcome: none Measures reported: Headache diaries including frequency, intensity/severity, and duration Pediatric Migraine Disability Assessment Pediatric Quality of Life Inventory |
|
| Notes | Funding source: National Institutes of Health (National Institute of Neurological Disorders and Stroke), R01-NS046641 (PI: Michael Rapoff) Declarations of interest: authors have no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Participants were stratified by age (7–9 and 10–12) and randomly assigned following baseline to one of the two groups (education control or Headstrong).” Comment: probably done; description of randomisation not provided |
| Allocation concealment (selection bias) | Unclear risk | No description found in text Comment: probably not done |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | No description found in text Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Measures completed at home and mailed back |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition completely reported; significant differences between completers and non-completers were not reported |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Stinson 2010 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment and post-treatment | |
| Participants | End of treatment: n = 39 Start of treatment: n = 46 Sex: 31 F, 15 M Mean age: 14.6 (SD 1.5) Source: 4 paediatric tertiary care centres Diagnosis: juvenile idiopathic arthritis Mean years of pain: 6.4 (SD 4.6) |
|
| Interventions | “Internet treatment” “Attentional control group” |
|
| Outcomes | Primary pain outcome: Recall Pain Inventory Primary disability outcome: Juvenile Arthritis Quality of Life Questionnaire Primary depression outcome: none Primary anxiety outcome: Perceived Severity of Stress Questionnaire Primary satisfaction outcome: none Measures reported: Recall Pain Inventory Juvenile Arthritis Quality of Life Questionnaire Perceived Severity of Stress Questionnaire Medical Issues, Exercise, Pain and Social Support Questionnaire Children’s Arthritis Self-Efficacy scale JIA-specific Child Adherence Report Questionnaire Parent Adherence Report Questionnaire |
|
| Notes | Funding source: The Canadian Arthritis Network and The Arthritis Society Declarations of interest: Drs. Feldman and McGrath (co-authors) hold Canada Research Chairs |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A fixed allocation randomisation scheme was used. Specifically, blocked randomisation was employed. An online random number generator was used to produce the blocked randomisation. Group assignments were identified by ID number in sealed envelopes during the recruitment period.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “A fixed allocation randomisation scheme was used. Specifically, blocked randomisation was employed. An online random number generator was used to produce the blocked randomisation. Group assignments were identified by ID number in sealed envelopes during the recruitment period.” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | No description found in text Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Measures completed at home and submitted online |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition completely reported; significant differences between completers and non-completers were not reported |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Trautmann 2010 | ||
| Methods | RCT. 3 arms. Assessed at pre-treatment, post-treatment, 6 months | |
| Participants | End of treatment: n = 55, follow-up n = 40 Start of treatment: n = 68 Sex: 36 F, 30 M Mean age: 12.7 (SD 2.2) Source: newspaper adverts and websites Diagnosis: headache (migraine, tension type headache or combined headache) Mean years of pain: 2.8 (SD 3.0) |
|
| Interventions | “Cognitive behavioural therapy, self-help and management” “Applied relaxation group” “Education” |
|
| Outcomes | Primary pain outcome: clinical reduction in headache frequency Primary disability outcome: none Primary depression outcome: Children’s Depression Inventory Primary anxiety outcome: Pain Catastrophising Scale Primary satisfaction outcome: none Measures reported: Children’s Depression Inventory Pain diary Children’s Depression Inventory Pain Catastrophising Scale Health-related quality of life (KINDL-R) Strength and difficulties questionnaire |
|
| Notes | Funding source: German Research Foundation (Number: KR756/16-2) Declarations of interest: none stated | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “All participants were randomly assigned to one of the three conditions. The randomly ordered list of groups was used to assign sequentially enrolled participants to two intervention groups and the active control condition.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “The first author randomly selected participants according to a computer-generated randomisation list by using the ’select cases’ random selection option.” Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes |
Unclear risk | No description found in text Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Measures completed at home and mailed back |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition is described. “Furthermore, no significant differences were found between dropouts and completers” |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
CBT: cognitive behavioural therapy
F: female
M: male
NRS: numerical rating scale
Ped-MIDAS: Pediatric Migraine Disability Assessment
RAP: Recurrent abdominal pain
RCT: randomised controlled trial
SD: standard deviation
TAU: treatment as usual
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bonnert 2014 | Open trial, no control group |
| Long 2009 | Usability evaluation of online treatment |
| Merlijn 2005 | N < 10 in at least 1 arm of the trial |
| Trautmann 2008 | N < 10 in at least 1 arm of the trial |
Results of the search
We conducted a search of four databases that produced 1384 papers after duplicates were removed. A further two were identified from other sources (see Figure 1). From the 12 papers identified and read in full, eight were included and four were excluded.
Included studies
Eight studies met the inclusion criteria for this review. Five trials investigated psychological therapies delivered remotely for children with headache (Connelly 2006; Cottrell 2007; McGrath 1992; Rapoff 2014; Trautmann 2010), one assessed juvenile idiopathic arthritis (Stinson 2010), and two included headache and non-headache conditions (i.e. recurrent abdominal pain and musculoskeletal pain) meaning that we entered them in both headache and mixed pain analyses where appropriate (Hicks 2006; Palermo 2009). Children were recruited via hospitals or clinics (n = 5), adverts in the media or community (n = 1), or a combination of advertisements in clinics and the community (n = 2). All children recruited into trials were diagnosed with their primary condition by a medical professional. A total of 371 participants entered into treatment and 342 participants finished, giving a retention rate of 92%. Girls (58%) outnumbered boys (42%). The mean age of participants was 12.57 years (standard deviation (SD) 1.85, range 10 to 14 years).
Six studies included two arms, and two studies included three (McGrath 1992; Trautmann 2010). For McGrath 1992, we compared the remotely delivered cognitive behavioural treatment to the active control group. For Trautmann 2010, we combined two remotely delivered treatment conditions (cognitive behavioural therapy and applied relaxation) and compared this to the control condition (education). Most treatments were delivered via the Internet (Hicks 2006; Palermo 2009; Stinson 2010; Trautmann 2010), two studies delivered treatment via CD-ROM (Connelly 2006; Rapoff 2014), one study delivered treatment via audiotapes (McGrath 1992), and the remaining study delivered treatment primarily via the telephone (Cottrell 2007). Control conditions differed between studies. Three studies used a waiting-list control (Connelly 2006; Hicks 2006; Palermo 2009), and five studies used active controls. The active controls included studies that delivered education online (Rapoff 2014; Trautmann 2010), prescribed children triptan medication (Cottrell 2007), delivered a credible placebo therapy (e.g. discussed triggers, brainstorming stressful situations; McGrath 1992), and had research assistants discuss “own best efforts” over the telephone with children (Stinson 2010). All treatments were delivered at home and included phone calls, emails, or a combination of both on a weekly basis to deliver treatment, check engagement, or answer questions. See Table 1 for a summary of the characteristics of treatment and control conditions.
Table 1.
Description of remotely delivered treatments
| Study | Description of treatment | Description of control |
|---|---|---|
| Connelly 2006 |
Name of treatment programme: Headstrong Therapy type: cognitive behavioural therapy Mode of delivery: CD-ROMs, plus weekly telephone calls with a study therapist Content: children completed 3 modules: education, relaxation, and thought-changing. Parents completed 1 module on pain behaviour modification. Each module included assignments for home practice Support: weekly telephone calls with a study therapist were used to answer questions Programme features: all components of the CD-ROMs were fully narrated and included developmentally appropriate graphics, language and music Duration: 4 modules completed over 4 weeks plus weekly phone calls from a study therapist (unknown duration). Each module could be completed in 1 hour |
Control type: waiting-list control Mode of delivery: N/A Content: participants continued with the recommendations of their neurologist, and were contacted weekly by phone by study staff to encourage completion of assessments Duration: 2 months, after which participants were offered the Headstrong programme |
| Cottrell 2007 |
Name of treatment programme: STOP Migraines Therapy type: cognitive behavioural therapy Mode of delivery: STOP Migraines patient manual and 2 relaxation tapes, plus weekly telephone calls with study therapist. Adolescents received 8 weekly phone calls from a study therapist. Parents spoke with the study therapist during the first 2 phone calls. Phone calls were guided by a standardised treatment manual Content: adolescents completed 8 chapters: education, recognising and monitoring headache-related stress, introduction to relaxation training, relaxation methods for coping with headache, cue-controlled relaxation and thermal biofeedback training, stress management, activity pacing, relapse prevention. Parents completed 2 chapters: how to support the adolescent’s effective use of the treatment programme, and recognising and rewarding effective coping Support: adolescents received 8 weekly phone calls from study therapists focused on reviewing material, providing instruction in behavioural skills, and assigning homework. Parents received 2 phone calls from study therapists focused on clarifying the study protocol, increasing parents’ awareness of the adolescents’ responsibilities in the study, and explaining how to best support the adolescent’s efforts at migraine management Programme features: not described Duration: 8 chapters completed over 8 weeks plus weekly 30-minute phone calls with the study therapist. Duration of readings in the patient manual was not described |
Control type: active (triptan treatment) Mode of delivery: N/A Content: adolescents in the control group were prescribed either 5 mg rizatriptan (n = 13) or 2.5 mg zolmitriptan (n = 2), based on each participant’s past experience and the judgement of the neurologist. Adolescents were asked to take the medication within 30 minutes after the migraine headache became moderate to severe in pain intensity Duration: 4 clinic visits over 8 months with the study neurologist to evaluate triptan use and side effects (baseline, 1, 3 and 8 months) |
| Hicks 2006 |
Name of treatment programme: Help Yourself Online Therapy type: cognitive behavioural therapy Mode of delivery: Internet plus personalised relaxation tape and weekly email or telephone calls with a study therapist Content: children completed 7 online chapters covering pain education, relaxation techniques, cognitive strategies, activity pacing, lifestyle choices, and relapse prevention. Parents completed 2 online chapters focused on encouraging healthy behaviour. Each chapter ended with a knowledge quiz. Children were assigned skills to practice each week, which were then reviewed with the study therapist via alternating email or telephone contact Support: study staff contacted parents twice during the treatment period Duration: 1 chapter per week for 7 weeks plus email or telephone contact with the study therapist. Average duration of contact with the study therapist was 187 minutes per family Programme features: each chapter included a knowledge quiz All participants received a personalised relaxation tape |
Control type: waiting-list control Mode of delivery: N/A Content: participants were reminded by study staff to see their physician as needed while waiting to start the treatment programme Duration: 7 weeks, after which participants were offered the Help Yourself Online programme |
| McGrath 1992 |
Name of treatment programme: Help Yourself: A Treatment for Migraine Headaches Therapy type: cognitive behavioural therapy Mode of delivery: manual and audiotapes Content: the 8-week course included coping and relaxation strategies. Adolescents were contacted each week by the therapist to answer questions and check progress Support: weekly phone calls from a therapist Duration: 8-week programme, 1 chapter delivered each week Programme features: not described |
Control type: therapist administered or active control Mode of delivery: face-to-face or via the telephone Content: the therapist administered therapy was the same as the CBT for the treatment group. However, children were trained face-to-face by a therapist. For the active control, children were given a list of common headache triggers and brainstorming techniques for stressful situations during a session with a therapist. They were then called weekly by the therapist to monitor their progress Duration: 8 weeks |
| Palermo 2009 |
Name of treatment programme: Web-based Management of Adolescent Pain (Web-MAP) Therapy type: cognitive behavioural therapy Mode of delivery: Internet Content: Web-MAP includes separate websites for children and parents. Children completed 8 modules on pain education, recognising stress and negative emotions, relaxation methods, distraction methods, cognitive methods, sleep hygiene and lifestyle factors, staying active, and relapse prevention. Parents completed 8 modules on pain education, recognising stress and negative emotions, operant training, modelling, sleep hygiene and lifestyle, communication, and relapse prevention. Each module included a knowledge quiz and a behavioural assignment Support: online coaches provided personalised feedback on behavioural assignments via a message centre Duration: participants completed 8 modules over 8 to 10 weeks. Each module could be completed in 30 minutes Programme features: the website included interactive fields, which allowed for tailored and personalised assignments and instructions, interactive animations, audio files of relaxation exercises, and video files of peer models |
Control type: waiting-list control Mode of delivery: N/A Content: participants continued with standard care offered through the pain clinic, although were asked not to start psychotherapy for pain management during the 8-week period Duration: 8 to 10 weeks, after which participants were offered Web-MAP |
| Rapoff 2014 |
Name of treatment programme: Headstrong Therapy type: cognitive behavioural therapy Mode of delivery: CD-ROMs plus workbook and weekly phone calls with a study therapist Content: children completed 3 modules: education, re laxation, and problem-solving and stress management. Parents completed 1 module on pain behaviour modification. The workbook included supplementary materials Duration: each module was divided into six 10-minute lessons. Children completed one 10-minute lesson per day for 4 weeks. Parents completed one 10-minute lesson per day for one week. Each lesson included a knowledge quiz and homework assignment Support: weekly phone calls with study therapist were used to answer questions about the CD-ROMs and to remind participants about record keeping Programme features: graphics, audio narration, music, clickable progress controls, passwords to mark progress through the programme, and homework assignments. A workbook had supplementary material required to complete the treatment. Parents were given a manual containing instructions and technical assistance information |
Control type: active (education control) Mode of delivery: CD-ROM Content: children completed modules on education about primary headaches and health habits. Parents were given a manual on how to use the educational programme Duration: each module was divided into six 10-minute lessons. Children completed one 10-minute lesson per day for 4 weeks |
| Stinson 2010 |
Name of treatment programme: Teens Taking Charge: Managing Arthritis Online Therapy type: cognitive behavioural therapy Mode of delivery: Internet, plus weekly telephone calls from a study therapist Content: adolescents completed 12 modules, which included education about arthritis, managing symptoms (pain, stiffness, fatigue), managing stress and negative thoughts, relaxation, distraction, other types of care (exercise, nutrition, splints), self monitoring and supports, lifestyle issues, and issues related to transition to adulthood. Parents completed 2 modules focused on encouraging healthy behaviour. Each module includes a knowledge quiz and homework assignments. Parents were also able to view the materials on the teen website Support: weekly scripted telephone calls with a study coach were used to review homework assignments, quiz responses, module content, and problem-solve around skills implementation. The website also included a discussion board that was monitored by the study coach Duration: children completed 12 modules over 12 weeks. Each module took between 20 and 30 minutes to complete. Participants received an average of 1.6 telephone calls per week with the average duration of calls being 17.3 minutes (range 7 to 30 minutes) Programme features: the web programme is multi-layered and interactive, and includes a discussion board monitored by a study coach. Adolescents use a progress tracker in the web programme to monitor progress on personal goals |
Control type: active (attention control) Mode of delivery: telephone Content: adolescents received weekly phone calls from a research assistant to discuss their “own best efforts” at managing their arthritis Duration: participants received a mean of 1.4 phone calls per week. Average duration of calls was 3 minutes (range 2 to 6 minutes) |
| Trautmann 2010 |
Name of treatment programme: Therapy type: cognitive behavioural therapy Mode of delivery: Internet and a relaxation CD Content: there were 2 treatment groups in this trial; cognitive behavioural group (CBG) and applied relaxation group (APG) The CBG completed modules on headache education, stress management, progressive relaxation techniques, cognitive restructuring, self assurance, and problem-solving. Participants received a CD with relaxation instructions, and children could download relaxation instructions from a website The APG completed modules on headache education, progressive relaxation, cue-controlled relaxation, and an applied relaxation CD Support: both groups received weekly email support from study therapists. Emails were standardised and included a knowledge quiz to determine whether participants had read the assigned material and completed the assigned exercises. Participants also received 2 booster emails after the completion of treatment focused on reminders of skills learned and encouragement to continue daily practice Duration: participants completed 1 module per week for 6 weeks. Participants received weekly email support from study therapists during treatment and 2 booster emails after the completion of treatment Programme features: relaxation CD, email support from study therapists, option to download and print material from the website to review and practice |
Control type: active (education control) Mode of delivery: Internet Content: adolescents received access to the headache education module and had weekly email contact with study therapists. Emails focused on review of headache diary from the previous week Duration: weekly email contact with study therapists |
CBT: cognitive behavioural therapy
N/A: not applicable
Three trials were supported by grants from the National Institutes of Health. One trial was funded by a pharmaceutical and biologics company (Connelly 2006). The remaining trials were supported by research foundations, government-backed research councils, or awards. Three studies did not have a statement about conflict of interest, two studies declaredthat the authors did not have a conflict of interest, two studies stated that authors were members of research funding bodies, and the final trial reported at least one conflict of interest (see Characteristics of included studies for more detail).
Excluded studies
Four papers were excluded from this review. One studies was excluded as it was conducted as an open trial (Bonnert 2014). A second paper, Long 2009, evaluated the usability of an online study already included in the review (Palermo 2009). We excluded a further two studies as they included fewer than 10 participants in at least one arm of the trial at an extraction time point (Merlijn 2005; Trautmann 2008).
Risk of bias in included studies
We carried out ’Risk of bias’ assessments on all included studies (for a summary see Figure 2 and Figure 3).
Figure 2.
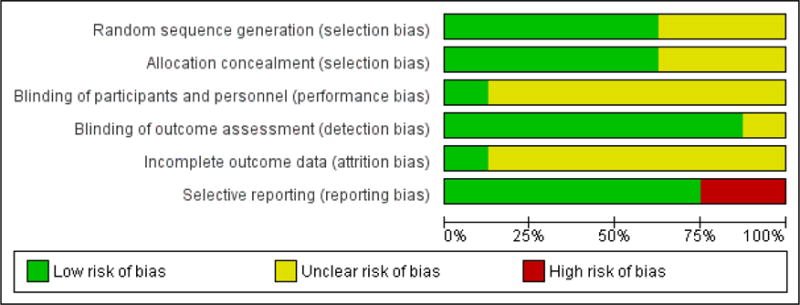
’Risk of bias’ graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.

’Risk of bias’ summary: review authors’ judgements about each risk of bias item for each included study.
For selection bias (randomisation sequence generation and allocation concealment bias), five studies gave a detailed description of randomisation and allocation concealment and were judged to have a low risk of bias. The remaining three studies did not give a clear description and so were judged unclear.
Only one study was found to have a low risk of bias for blinding of participants and personnel, the remaining studies did not give any description of attempts to blind participants and personnel so were judged unclear. Seven studies asked children to complete measures at home, and these were either submitted online or returned to the research team via mail and therefore were given low risk of bias. One study did not give a description of how measures were taken and therefore was marked unclear.
Only one study reported attrition fully (i.e. described attrition throughout the trial and differences between completers and non-completers), so was judged to have low risk of bias. The remaining seven trials reported attrition, but did not comment on whether completers and non-completers of treatment were significantly different, so they remain unclear.
Finally, for selective reporting bias, six of the studies reported outcomes in full in the published manuscript. Two studies did not report full outcomes so were judged to be high risk of bias. More detail on the ’Risk of bias’ judgements can be found in the Characteristics of included studies.
Effects of interventions
The pain outcomes extracted below differ between headache and mixed conditions (see Table 2 for a scorecard of results). For headache conditions we extracted dichotomous outcomes. For mixed pain conditions we extracted continuous pain outcomes. To provide further clarity, the extracted pain measures and justification are outlined here.
Table 2.
Scorecard of results
| Psychological therapies (remotely delivered) for the management of chronic pain in children | ||||
|---|---|---|---|---|
| Headache | Non-headache | |||
| Post-treatment | Follow-up | Post-treatment | Follow-up | |
| Pain | Effect (6) | No effect (3) | Effect (3) | No data (1) |
| Disability | No effect (3) | No data (1) | No effect (2) | No data (0) |
| Depression | No effect (2) | No data (0) | No data (1) | No data (0) |
| Anxiety | No data (1) | No data (0) | No data (1) | No data (0) |
Number indicated in brackets denotes number of studies entered into analyses.
The International Headache Society and American Headache Society provides guidance on how to measure headache pain in adults and children. Guidelines for trials of behavioural and pharmacological treatments for chronic and recurrent headache recommend reporting headache frequency as the primary outcome variable and pain intensity and duration as secondary outcome variables (Andrasik 2005; Penzien 2005; Tfelt-Hansen 2012). Therefore, we preferentially extracted data for children and adolescents who reported at least 50% reduction of headache frequency in both the treatment and control groups; this was possible in four studies (Connelly 2006; McGrath 1992; Rapoff 2014; Trautmann 2010). When headache frequency was not reported or available, we extracted data for children and adolescents who reported at least 50% reduction pain intensity in both the treatment and control groups (Hicks 2006; Palermo 2009). Headache pain outcomes are hereby known as ’headache severity’. For mixed conditions, we extracted mean pain intensity across all trials.
Treatment versus control for headache conditions
Primary outcomes
Pain symptoms
Six studies (N=247) were included in the analysis to investigate whether psychological therapies delivered remotely were beneficial for reducing pain in children with headache conditions post-treatment, and three studies (N=85) were included in the analysis at follow-up. Psychological therapies delivered remotely have a beneficial effect at achieving at least 50% reduction of headache severity post-treatment (risk ratio (RR) = 2.65, 95% confidence interval (CI) 1.56 to 4.50, z = 3.62, p < 0.01, number needed to treat to benefit (NNTB) = 2.88; Analysis 1.1; Figure 4). However, this effect was not maintained at follow-up (RR= 1.56, 95% CI 0.67 to 3.68, z = 1.03, p = 0.30; Analysis 2.1; Figure 5).
Figure 4.
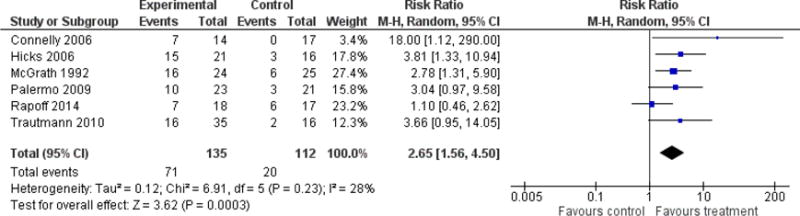
Forest plot of comparison: 1 Headache conditions treatment versus control (post-treatment), outcome: Achievement of at least 50% reduction in headache severity.
Figure 5.
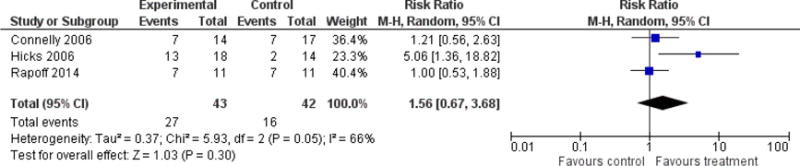
Forest plot of comparison: 2 Headache conditions treatment versus control (follow-up), outcome: Achievement of at least 50% reduction in headache severity.
Disability
Three studies (N=114) were included in the analysis to assess whether psychological therapies delivered remotely were beneficial at reducing disability post-treatment. The therapies had no effect (standardised mean difference (SMD) = −0.37, 95% CI −0.88 to 0.15, z = 1.40, p = 0.16; Analysis 1.2). Only one study was available at follow-up, therefore no analysis was run.
Depression
For depression, two studies (N = 103) had data available to determine whether psychological therapies were beneficial at reducing depressive symptoms. The analysis revealed no effect of therapies (SMD = 0.02, 95% CI −0.38 to 0.43, z = 0.12,p = 0.91; Analysis 1.3). There were no data available for analysis at follow-up.
Anxiety
Only one study was available to assess whether psychological therapies were beneficial for reducing anxiety symptoms post-treatment, and no data were available at follow-up, therefore no conclusions can be drawn.
Adverse events
None of the included studies reported adverse events. All trials had dropouts either post-treatment or at follow-up, or both. Connelly 2006, Cottrell 2007, and Trautmann 2010 gave full reasons for dropouts. However, Hicks 2006, McGrath 1992, Palermo 2009, and Rapoff 2014 did not give full reasons for dropouts.
Secondary outcome
Satisfaction with treatment
Satisfaction was measured in four studies (Cottrell 2007; Hicks 2006; Palermo 2009; Trautmann 2010). Due to the heterogeneity of measures used and the use of waiting-list controls (satisfaction ratings are inappropriate to measure in this group), we were unable to meta-analyse the data. Cottrell 2007 asked participants allocated to the treatment group to report their satisfaction with three components of treatment. For each component, more than half the participants indicated that they were satisfied. Thirteen of 15 participants indicated satisfaction with deep breathing, relaxation, thermal biofeedback, and mental imagery for management of headaches, 11 of 15 indicated satisfaction with pain coping and pain transformation imagery, and 9 of 15 reported satisfaction with the stress management skills component. No satisfaction scores were reported for the triptan control group. Hicks 2006 measured satisfaction in the treatment group using a visual analogue scale and reported that child and parent satisfaction were positively correlated. Palermo 2009 measured satisfaction for the treatment group using the Treatment Evaluation Inventory – Short Form (Kelley 1989) and reported global satisfaction of children and parents in the treatment group as moderate to high. The waiting-list controls for Hicks 2006 and Palermo 2009 were not asked to report satisfaction. Finally, Trautmann 2010 asked all participants and their parents to report their degree of satisfaction. The findings revealed that the applied relaxation (treatment) group were more satisfied compared to the education (control) group. However, there were no significant differences between the cognitive behavioural (treatment) group and the applied relaxation (treatment) group or the education (control) group. Connelly 2006 and McGrath 1992 did not report satisfaction outcomes.
Treatment versus control for mixed pain conditions
Primary outcomes
Pain symptoms
Three studies (N=131) were included in the analysis to investigate whether psychological therapies were beneficial for reducing pain intensity for children with mixed pain conditions at post-treatment. The analysis revealed a beneficial effect for reducing pain intensity (SMD = −0.61, 95% CI −0.96 to −0.25, z = 3.38, p < 0.01; Analysis 3.1). Only one study included follow-up data and therefore no conclusions can be drawn.
Disability
Two studies (N=94) were included in the analysis to determine if psychological therapies delivered remotely were beneficial for reducing disability in children with mixed pain conditions. The analysis did not reveal an effect of therapies (SMD = −0.50, 95% CI −1.02 to 0.02, z = 1.90, p = 0.06; Analysis 3.2). No data were available for analysis at follow-up.
Depression and anxiety
Only one study could be included in an analysis to determine how beneficial psychological therapies delivered remotely are for reducing depression and anxiety post-treatment in children with mixed pain conditions, and no data were available for either analysis at follow-up, therefore no conclusions can be drawn.
Adverse events
None of the studies reported adverse events. Stinson 2010 gave full reasons regarding participants who dropped out, however Hicks 2006 and Palermo 2009 did not give reasons regarding dropouts.
Secondary outcome
Satisfaction with treatment
Three studies reported results on satisfaction. Hicks 2006 and Palermo 2009 are described above. Stinson 2010 used a questionnaire developed by the investigators of the trial. The study reported that participants in the treatment group were satisfied with treatment. No information is provided regarding the satisfaction of the ’own best efforts’ control group. Similar to the headache group, satisfaction data could not be entered into a meta-analysis.
DISCUSSION
Summary of main results
This systematic review included eight trials (N = 371) that delivered psychological therapies remotely to children and adolescents with chronic pain complaints. Chronic pain conditions were split into headache conditions and mixed pain conditions (including juvenile idiopathic arthritis, musculoskeletal pain, and recurrent abdominal pain). Psychological therapies were beneficial at reducing headache severity for children with headache and pain intensity for children with mixed pain conditions post-treatment. No beneficial effect was found for psychological therapies in improving disability for children with headache and mixed pain conditions post-treatment. Two studies involving children with headache reported depression outcomes, but we found no beneficial effect. For the remaining analyses, data could not be meta-analysed due to lack of data and therefore, no conclusions can be drawn. None of the included studies reported adverse events so we do not know whether children entered into any treatment or comparison group experienced adverse events, and we have no data to inform a judgement about the safety of the treatments. Satisfaction with treatment between the treatment group and the control group was only appropriate in four trials that used active controls. From these four trials only one trial reported satisfaction scores for all conditions (Trautmann 2010), therefore satisfaction scores were qualitatively reported. Overall, trial authors reported that satisfaction of treatment groups was positive. We were unable to assess the quality of evidence using GRADE or conduct the subgroup analyses planned in the protocol due to the lack of studies.
Overall completeness and applicability of evidence
Similar to reviews investigating face-to-face therapies for children with chronic pain (Eccleston 2014; Fisher 2014), the studies included in this review were dominated by cognitive behavioural or behavioural treatments. Due to the emerging nature of this field, only a small number of studies could be included, meaning that we are not confident in making strong conclusions about remotely delivered interventions. Further trials are needed before we can be confident of the effects of psychological therapies delivered remotely for this population, and for which outcomes they are efficacious. Trials should include core outcomes as recommended by PedIMMPACT (McGrath 2008), including anxiety and depression outcomes. Most included studies had publication dates after this guidance was published, yet many omit key recommended clinical trial outcomes for chronic pain in children. We were unable to conduct meta-analyses for most depression and anxiety outcomes due to lack of data. Mood outcomes are very important when considering children with chronic pain and functional disability; they have been found to be significantly associated with disability outcomes (Simons 2012). Only two studies reported depression outcomes and one trial reported anxiety outcomes. Satisfaction should also be measured in both the treatment and active control groups to determine whether satisfaction with treatment delivered remotely is higher compared to an active control. Nevertheless, the trials provide promising evidence that treatments delivered remotely can be beneficial for children with chronic pain.
Going further, greater consensus is needed on how pain outcomes should be measured. Pain outcomes for headache and mixed pain conditions still vary. The International Headache Society and American Headache Society have published guidelines of preferred outcome measures in trials of behavioural and pharmacological interventions for individuals with recurrent and chronic headache (Andrasik 2005; Penzien 2005; Tfelt-Hansen 2012). Although these guidelines recommend reporting multiple measures of headache severity including pain intensity and duration, headache frequency is widely considered to be the primary outcome of interest in these trials. These guidelines also recommend reporting clinically significant reduction in pain using a criterion of 50% reduction in headache frequency. In the current review, few trials followed these reporting guidelines, with the majority of trials for youth with headache not reporting clinically significant change in headache frequency. In contrast, pain intensity is the most widely reported outcome in trials for youth with mixed chronic pain conditions. Similarly, clinically significant change is typically reported as the proportion of youth achieving 50% reduction in pain intensity.
Quality of the evidence
Due to the low number of studies included in this review, GRADE assessments of the quality of evidence were not conducted. Risk of bias assessments were conducted on all included studies, however, there were two noticeable ’Risk of bias’ categories where the majority of studies did not have a low risk of bias, reducing the quality of the evidence. First, only one study gave an adequate description of blinding of participants. Second, attrition bias was also incompletely reported in the included trials. Authors should analyse and report data between completers and non-completers of treatment to ensure that they are not retaining a particular type of patient. Achieving a low risk of bias judgement across all ’Risk of bias’ categories is attainable if authors are clear, transparent, and attentive when conducting and reporting trials.
Agreements and disagreements with other studies or reviews
This review is intended to be a sister review to Eccleston 2014, which assesses face-to-face psychological interventions for children with chronic pain. Face-to-face interventions have previously been the ’go-to’ delivery type in this field and therefore, unsurprisingly, Eccleston 2014 included over four times as many studies and seven times as many participants (N = 37 studies, N = 2111 participants). Similar to the current review, the face-to-face review split pain conditions by headache and mixed/non-headache pain conditions, revealing seven effects of psychological treatments. For headache conditions, psychological interventions have a beneficial effect on pain and disability post-treatment and at follow-up, and on anxiety at post-treatment. For non-headache pain conditions, two beneficial effects were found post-treatment for pain intensity and disability (Eccleston 2014). The average ages in both reviews were similar (mean age current review = 12.57 years; mean age Eccleston 2014 = 12.45 years). Girls outnumbered boys in both and recruitment methods were similar. Cognitive behavioural and behavioural therapies were the predominant treatment choice in both reviews. Some of the trials included in this review stated that they created online material using manuals from face-to-face treatments. A non-Cochrane review investigating the overall efficacy of psychological therapies delivered face-to-face and remotely has been conducted (Fisher 2014). Further, this review summarises the evidence by pain condition.
Other systematic reviews have investigated the efficacy of remotely delivered psychological therapies to both children and adults (e.g. Eccleston 2014b; Macea 2010; Stinson 2009). Eccleston 2014b summarised evidence from 15 studies that delivered therapy for adults with chronic pain via the Internet and found seven effects. First, therapies reduced pain and disability post-treatment for those adults with a headache condition. For adults with non-headache pain conditions, beneficial effects were found for pain, disability, depression, and anxiety post-treatment, and for disability at follow-up. Macea 2010 investigated web-based cognitive behavioural therapy (CBT) interventions for adults and children with chronic pain. Eleven studies were identified and a meta-analysis revealed small reductions in pain symptoms for the web-based CBT conditions. Other outcomes (e.g. disability, mood) were not investigated. Summaries of the literature have also been conducted exclusively for children. Stinson 2009 searched for interventions that were delivered via the Internet for subacute or chronic health conditions. Other forms of technology (e.g. CD ROM) were excluded. Nine studies met the inclusion criteria, of which one study included pain patients (Hicks 2006; also included in this review). Due to the heterogeneity of outcome measures and conditions, data could not be synthesised in a meta-analysis. Similarly, Andersson 2011 presented a qualitative review of Internet-delivered psychological therapies for children with diabetes, cancer, pain, and other health conditions.
Reviews have attempted to summarise the efficacy of Internet-delivered psychological interventions in other areas, such as depression and anxiety disorders (Arnberg 2014). However, similar to this review, the evidence base is small and of low quality. Only one study including children could be identified and therefore could not be entered into a meta-analysis (Arnberg 2014). A meta-analysis using a broader criterion of remotely delivered or e-therapies for anxiety and depression revealed 26 studies (National Collaborating Centre for Mental Health). The strongest evidence found that computerised cognitive behavioural therapies were beneficial for children and adolescents with depression and for decreasing anxiety in general populations. However, the evidence was judged to be low quality.
AUTHORS’ CONCLUSIONS
Implications for practice
There is promise that psychological therapies delivered remotely for children and adolescents with chronic pain can be beneficial, however, more high quality evidence is needed before we can be confident of how effective psychological therapies delivered remotely for this population are. There is evidence that remotely delivered psychological interventions are beneficial for reducing headache severity post-treatment, and have a moderate beneficial effect of reducing pain intensity for children with mixed pain conditions post-treatment. However, a maximum of six studies (n = 253) were included in these analyses meaning that further trials are very likely to change either the direction, strength, or both, of the effects found in this review. Analyses revealed no effects for disability and depression in headache, or for disability for mixed pain conditions. No other analyses could be conducted due to lack of data, meaning we are unsure whether remotely delivered interventions are beneficial for depression in children with mixed pain conditions or anxiety across all conditions, and whether psychological therapies can maintain effects at follow-up for most outcomes across all pain conditions. All delivered therapies were behavioural or cognitive behavioural in nature.
Implications for research
This field is still small but growing rapidly. Preliminary findings presented in this review are promising but future studies should build on the proposals outlined here. A recently published commentary provides guidance for authors embarking on designing and conducting a randomised controlled eHealth intervention with children (Wu 2014). This article highlights the challenges of developing, implementing, and disseminating eHealth interventions using the experience of the author group who have conducted a variety of eHealth trials.
The expense of developing and conducting an intervention is inevitable and can be particularly costly for an intervention delivered remotely, depending on the complexity (Wu 2014). Previous trials have found that fully automated, remotely delivered interventions are effective for children with encopresis (Ritterband 2008). Similar automated interventions should be trialled with children with chronic pain. Further, other types of therapies delivered remotely should be trialled to investigate whether they can produce equivalent or increased effects for children with chronic pain. To date, this field has been heavily dominated by cognitive behavioural therapies; alternative psychotherapeutic interventions are being attempted or developed. Remotely delivered therapies are likely, eventually, to be provided as the first choice of treatment for many and it would be helpful to investigate whether particular therapies are more relevant for particular patients (Morley 2013).
We encourage multi-centre randomised controlled trials (RCTs) of remotely delivered psychological interventions for children with chronic pain. We propose that future RCTs should include the following components:
At least two arms, including (at minimum) a treatment group and a placebo comparator. Placebo comparators that control for technology use (e.g. online education) will strengthen the study designs.
The number of participants at completion and follow-up of the trial to be in excess of 10 participants per arm, and should be closer to 100.
Trials should assess the outcome domains recommended by McGrath 2008 for inclusion in clinical trials of children and adolescents with chronic pain. At minimum, trials should measure and report pain intensity, disability, depression, and anxiety outcomes. Adverse events should also be measured and reported in published manuscripts.
Trials should report a 50% reduction in pain frequency, intensity, and duration for headache trials and intensity for mixed pain conditions between baseline and post-treatment/follow-up for intervention and control groups. For mixed conditions, a consensus should be met so that pain measures are standardised within pain conditions.
Trials should also report satisfaction with treatment in both treatment and control arms of trials, so that we are able to assess whether adolescents are more satisfied with psychological therapies compared to control arms.
Trialling of fully automated interventions (without any human support) would provide a more scalable option by lessening the burden on therapists and other healthcare professionals.
Including full descriptions of technology components (e.g. interactive elements, human support, etc.) to allow for better understanding of potentially effective features of remotely delivered interventions.
Trialling of other psychological therapies (beyond cognitive behavioural therapy) for children and adolescents with chronic pain.
Acknowledgments
We would like to thank Joanne Abbott for designing the search strategy; the referees; and the Pain, Palliative and Supportive Care (PaPaS) Group editorial team for their helpful comments in revising the review.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
SOURCES OF SUPPORT
Internal sources
No sources of support supplied
External sources
-
University Research Studentship Graduate School Award, University of Bath, UK.
Emma Fisher is a PhD student funded by the University Research Studentship Graduate School Award, University of Bath.
-
National Institutes of Health, USA.
Tonya Palermo was supported by grant number 2K24HD060068-07
-
The National Institute for Health Research (NIHR), UK.
Christopher Eccleston was supported by NIHR Cochrane Programme Grant: 13/89/29 – Addressing the unmet need of chronic pain: providing the evidence for treatments of pain.
APPENDICES
Appendix 1. Search strategies
CENTRAL (CRSO) search strategy
MeSH DESCRIPTOR Pain EXPLODE ALL TREES
MeSH DESCRIPTOR Headache Disorders EXPLODE ALL TREES
MeSH DESCRIPTOR Fibromyalgia
((pain* or headache* or migraine* or fibromyalgia* or neuralgia*)):TI,AB,KY
#1 OR #2 OR #3 OR #4
MeSH DESCRIPTOR Child EXPLODE ALL TREES
MeSH DESCRIPTOR adolescent
MeSH DESCRIPTOR Infant
((child* or infant* or baby or babies or preschooler* or pre-schooler* or toddler* or schoolchild* or girl* or boy* or adolescen* or teen*)):TI,AB,KY
#6 OR #7 OR #8 OR #9
MeSH DESCRIPTOR Internet EXPLODE ALL TREES
((internet or web or blog* or “social media” or online or www or email* or e-mail*)):TI,AB,KY
MeSH DESCRIPTOR Telecommunications EXPLODE ALL TREES
((telemedicine or tele-medicine)):TI,AB,KY
((telehealth or tele-health)):TI,AB,KY
((ehealth or e-health)):TI,AB,KY
((mobile health or mhealth or m-health)):TI,AB,KY
ICT:TI,AB,KY
(((inform* or communicat* or interact*) near6 (computer* or technolog* or software))):TI,AB,KY
(((health* or treat* or therap* or intervention* or assist* or selfmanag* or self-manag*) near6 (computer* or technolog* or software))):TI,AB,KY
(“world wide web”):TI,AB,KY
((telephone* or phone* or mobile* or cellphone* or apps or text* or SMS or smartphone*)):TI,AB,KY
((virtual reality or augmented reality or VR or AR)):TI,AB,KY
#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23
#5 AND #10 AND #24
MEDLINE (OVID) search strategy
exp Pain/
exp Headache Disorders/
Fibromyalgia/
(pain* or headache* or migraine* or fibromyalgia* or neuralgia*).tw.
or/1–4
exp Child/
Adolescent/
Infant/
(child* or infant* or baby or babies or preschooler* or pre-schooler* or toddler* or schoolchild* or girl* or boy* or adolescen* or teen*).tw.
or/6–9
exp Internet/
(Internet or web or blog* or “social media” or online or www or email* or e-mail*).tw.
exp Telecommunications/
(telemedicine or tele-medicine).tw.
(telehealth or tele-health).tw.
(ehealth or e-health).tw.
(mobile health or mhealth or m-health).tw.
ICT.tw.
((inform* or communicat* or interact*) adj6 (computer* or technolog* or software)).tw.
((health* or treat* or therap* or intervention* or assist* or selfmanag* or self-manag*) adj6 (computer* or technolog* or software)).tw.
“world wide web”.tw.
(telephone* or phone* or mobile* or cellphone* or apps or text* or SMS or smartphone*).tw.
(virtual reality or augmented reality or VR or AR).tw.
or/11–23
5 and 10 and 24
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
or/26–32
exp animals/not humans.sh.
33 not 34
25 and 35
EMBASE (OVID) search strategy
exp Pain/
exp Headache Disorders/
Fibromyalgia/
(pain* or headache* or migraine* or fibromyalgia* or neuralgia*).tw.
or/1–4
exp Child/
Adolescent/
Infant/
(child* or infant* or baby or babies or preschooler* or pre-schooler* or toddler* or schoolchild* or girl* or boy* or adolescen* or teen*).tw.
or/6–9
exp Internet/
(internet or web or blog* or “social media” or online or www or email* or e-mail*).tw.
exp Telecommunications/
(telemedicine or tele-medicine).tw.
(telehealth or tele-health).tw.
(ehealth or e-health).tw.
(mobile health or mhealth or m-health).tw.
ICT.tw.
((inform* or communicat* or interact*) adj6 (computer* or technolog* or software)).tw.
((health* or treat* or therap* or intervention* or assist* or selfmanag* or self-manag*) adj6 (computer* or technolog* or software)).tw.
“world wide web”.tw.
(telephone* or phone* or mobile* or cellphone* or apps or text* or SMS or smartphone*).tw.
(virtual reality or augmented reality or VR or AR).tw.
or/11–23
5 and 10 and 24
random$.tw.
factorial$.tw.
crossover$.tw.
cross over$.tw.
cross-over$.tw.
placebo$.tw.
(doubl$ adj blind$).tw.
(singl$ adj blind$).tw.
assign$.tw.
allocat$.tw.
volunteer$.tw.
Crossover Procedure/
double-blind procedure.tw.
Randomized Controlled Trial/
Single Blind Procedure/
or/26–40
(animal/or nonhuman/) not human/
41 not 42
25 and 43
PsycINFO (OVID) search strategy
exp Pain/
exp Headache/
Fibromyalgia/
(pain* or headache* or migraine* or fibromyalgia* or neuralgia*).tw.
or/1–4
(child* or infant* or baby or babies or preschooler* or pre-schooler* or toddler* or schoolchild* or girl* or boy* or adolescen* or teen*).tw.
exp Internet/
(internet or web or blog* or “social media” or online or www or email* or e-mail*).tw.
exp Telecommunications/
(telemedicine or tele-medicine).tw.
(telehealth or tele-health).tw.
(ehealth or e-health).tw.
(mobile health or mhealth or m-health).tw.
ICT.tw.
((inform* or communicat* or interact*) adj6 (computer* or technolog* or software)).tw.
((health* or treat* or therap* or intervention* or assist* or selfmanag* or self-manag*) adj6 (computer* or technolog* or software)).tw.
“world wide web”.tw.
(telephone* or phone* or mobile* or cellphone* or apps or text* or SMS or smartphone*).tw.
(virtual reality or augmented reality or VR or AR).tw.
or/7–19
5 and 6 and 20
clinical trials/
(randomis* or randomiz*).tw.
(random$ adj3 (allocat$ or assign$)).tw.
((clinic$ or control$) adj trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
(crossover$ or “cross over$”).tw.
random sampling/
Experiment Controls/
Placebo/
placebo$.tw.
exp program evaluation/
treatment effectiveness evaluation/
((effectiveness or evaluat$) adj3 (stud$ or research$)).tw.
or/22–34
21 and 35
DATA AND ANALYSES
Comparison 1. Headache conditions: treatment versus control (post-treatment)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Achievement of at least 50% reduction in headache severity | 6 | 247 | Risk Ratio (M-H, Random, 95% CI) | 2.65 [1.56, 4.50] |
| 2 Disability | 3 | 114 | Std. Mean Difference (IV, Random, 95% CI) | −0.37 [−0.88, 0.15] |
| 3 Depression | 2 | 103 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [−0.38, 0.43] |
Comparison 2. Headache conditions: treatment versus control (follow-up)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Achievement of at least 50% reduction in headache severity | 3 | 85 | Risk Ratio (M-H, Random, 95% CI) | 1.56 [0.67, 3.68] |
Comparison 3. Mixed conditions: treatment versus control (post-treatment)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 3 | 131 | Std. Mean Difference (IV, Random, 95% CI) | −0.61 [−0.96, −0.25] |
| 2 Disability | 2 | 94 | Std. Mean Difference (IV, Random, 95% CI) | −0.50 [−1.02, 0.02] |
Analysis 1.1.
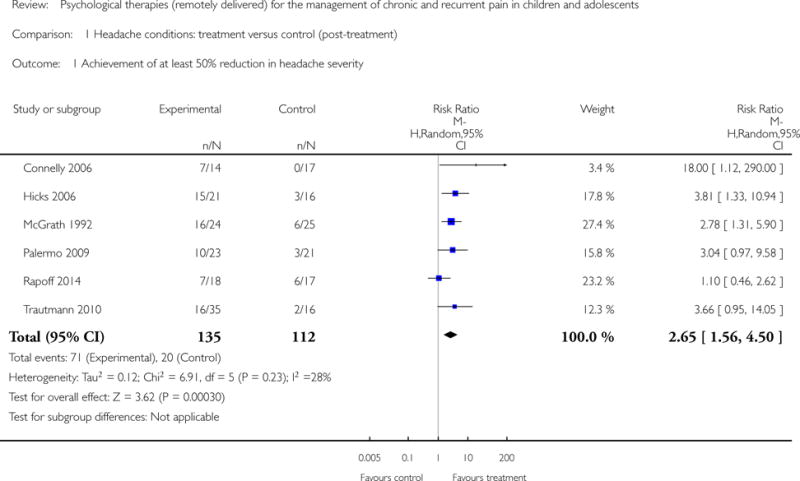
Comparison 1 Headache conditions: treatment versus control (post-treatment), Outcome 1 Achievement of at least 50% reduction in headache severity.
Analysis 1.2.
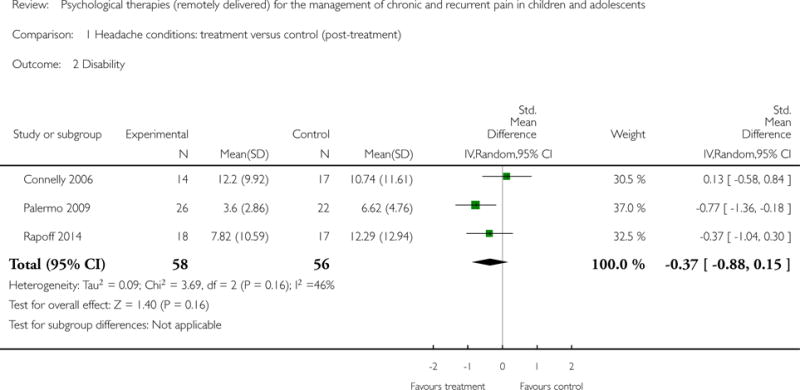
Comparison 1 Headache conditions: treatment versus control (post-treatment), Outcome 2 Disability.
Analysis 1.3.
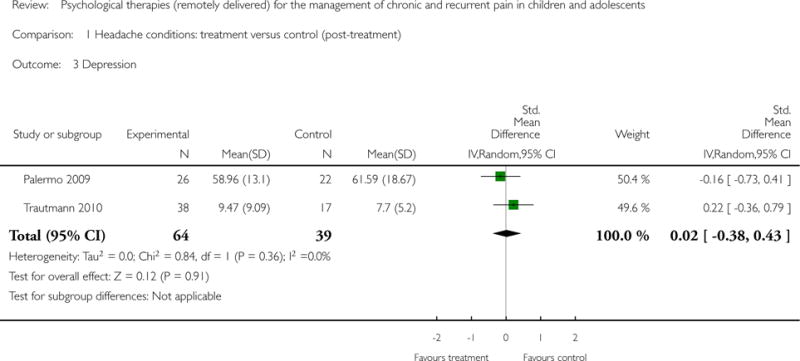
Comparison 1 Headache conditions: treatment versus control (post-treatment), Outcome 3 Depression.
Analysis 2.1.
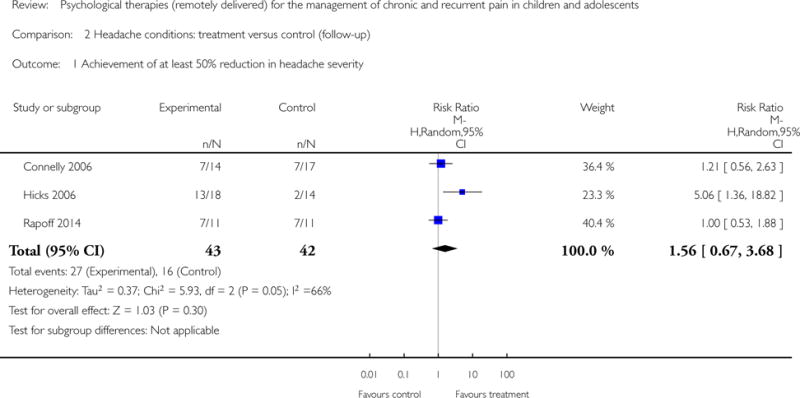
Comparison 2 Headache conditions: treatment versus control (follow-up), Outcome 1 Achievement of at least 50% reduction in headache severity.
Analysis 3.1.
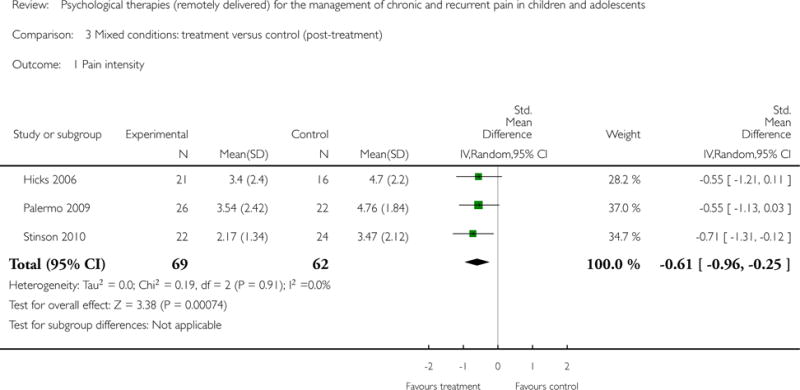
Comparison 3 Mixed conditions: treatment versus control (post-treatment), Outcome 1 Pain intensity.
Analysis 3.2.
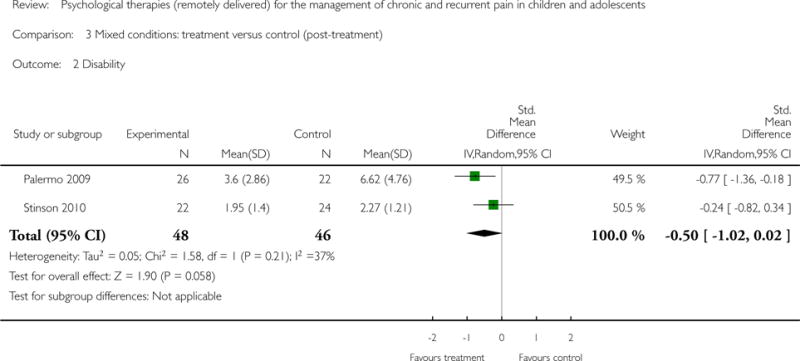
Comparison 3 Mixed conditions: treatment versus control (post-treatment), Outcome 2 Disability.
Footnotes
CONTRIBUTIONS OF AUTHORS
Emma Fisher (EF) oversaw the authoring of the manuscript, searched, obtained, and selected relevant studies, entered data into RevMan, carried out and interpreted the analysis, and will be responsible for updating the review.
Emily Law (EL) searched, obtained and selected relevant studies, carried out and interpreted the analysis, and drafted the final manuscript.
Tonya Palermo (TP) carried out and interpreted the analysis, and drafted the final manuscript.
Christopher Eccleston (CE) carried out and interpreted the analysis, arbitrated selection of studies, and drafted the final manuscript.
DECLARATIONS OF INTEREST
EF, EL, TP, and CE have no relevant declarations of interest.
For transparency we declare that we have received research support from charity, government, and industry sources at various times, but none relate to this review.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
In the protocol introduction, we stated that interventions delivered by audiotapes or written manuals were included in Eccleston 2014. After consideration, the review author team decided to include this mode of remotely delivered intervention, as some interventions included audiotapes or written material in combination with another form of intervention (e.g. telephone calls).
REFERENCES
* Indicates the major publication for the study
References to studies included in this review
- Connelly 2006 {published data only}.Connelly M, Rapoff MA, Thompson N, Connelly W. Headstrong: A pilot study of a CD-ROM intervention for recurrent pediatric headache. Journal of Pediatric Psychology. 2006;31:737–47. doi: 10.1093/jpepsy/jsj003. [DOI] [PubMed] [Google Scholar]
- Cottrell 2007 {published data only}.Cottrell C, Drew J, Gibson J, Holroyd K, O’Donnell F. Feasibility assessment of telephone-administered behavioral treatment for adolescent migraine. Headache. 2007;47:1293–302. doi: 10.1111/j.1526-4610.2007.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks 2006 {published data only}.Hicks CL, von Baeyer CL, McGrath PJ. Online psychological treatment for pediatric recurrent pain: a randomized evaluation. Journal of Pediatric Psychology. 2006;31:724–36. doi: 10.1093/jpepsy/jsj065. [DOI] [PubMed] [Google Scholar]
- McGrath 1992 {published data only}.McGrath PJ, Humphreys P, Keene D, Goodman JT, Lascelles MA, Cunningham J, et al. The efficacy and efficiency of a self-administered treatment for adolescent migraine. Pain. 1992;49:321–4. doi: 10.1016/0304-3959(92)90238-7. [DOI] [PubMed] [Google Scholar]
- Palermo 2009 {published data only}.Palermo TM, Wilson AC, Peters M, Lewandowski A, Somhegyi H. Randomized controlled trial of an internet delivered family cognitive behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146(1–2):205–13. doi: 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoff 2014 {published data only}.Rapoff MA, Connelly M, Bickel JL, Powers SW, Hershey AD, Allen JR, et al. Headstrong intervention for pediatric migraine headache: a randomized clinical trial. Journal of Headache and Pain. 2014;15(12):1–10. doi: 10.1186/1129-2377-15-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson 2010 {published data only}.Stinson JN, McGrath PJ, Hodnett ED, Feldman BM, Duffy CM, Huber AM, et al. An internet-based self-management program with telephone support for adolescents with arthritis: a pilot randomized controlled trial. Journal of Rheumatology. 2010;37(9):1944–52. doi: 10.3899/jrheum.091327. [DOI] [PubMed] [Google Scholar]
- Trautmann 2010 {published data only}.Trautmann E, Kroner-Herwig B. A randomized controlled trial of internet-based self-help training for recurrent headache in childhood and adolescence. Behaviour Research and Therapy. 2010;48:28–37. doi: 10.1016/j.brat.2009.09.004. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Bonnert 2014 {published data only}.Bonnert M, Ljótsson B, Hedman E, Andersson J, Arnell H, Benninga M, et al. Internet-delivered cognitive behavior therapy for adolescents with functional gastrointestinal disorders – an open trial. Internet Interventions. in press. [Google Scholar]
- Long 2009 {published data only}.Long AC, Palermo TM. Brief report: Web-based management of adolescent chronic pain: development and usability testing of an online family cognitive behavioral therapy program. Journal of Pediatric Psychology. 2009;35(5):511–6. doi: 10.1093/jpepsy/jsn082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlijn 2005 {published data only}.Merlijn VPBM, Hunfeld JAM, Van Der Wouden JC, Hazebroek-Kampschreur AAJM, Van Suijlekom-Smit LWA, Koes BW, et al. A cognitive-behavioural program for adolescents with chronic pain – a pilot study. Patient Education and Counseling. 2005;59(2):126–34. doi: 10.1016/j.pec.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Trautmann 2008 {published data only}.Trautmann E, Kroner-Herwig Internet-based self-help training for children and adolescents with recurrent headache: a pilot study. Behavioural and Cognitive Psychotherapy. 2008;36(2):241–5. [Google Scholar]
Additional references
- ABS 2012.Australian Bureau of Statistics. Use of Information Technology. www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/1301.0~2012~Main%20Features~Use%20of%20information%20technology~174 (accessed October 2013)
- Andersson 2011.Andersson G, Ljótsson B, Weise C. Internet-delivered treatment to promote health. Current Opinion in Psychiatry. 2011;24(2):168–72. doi: 10.1097/YCO.0b013e3283438028. [DOI] [PubMed] [Google Scholar]
- Andrasik 2005.Andrasik F, Powers SW, McGrath PJ. Methodological considerations in research with special populations: children and adolescents. Headache. 2005;45:520–5. doi: 10.1111/j.1526-4610.2005.05104.x. [DOI] [PubMed] [Google Scholar]
- Arnberg 2014.Arnberg FK, Linton SJ, Jultcrantz M, Heintz E, Jonsson U. Internet-delivered psychological treatments for mood and anxiety disorders: a systematic review of their efficacy, safety, and cost-effectiveness. PloS One. 2014;9(5):e98118. doi: 10.1371/journal.pone.0098118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshem 2011.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology. 2011;64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Burke 1989.Burke EJ, Andrasik F. Home- vs. clinic-based biofeedback treatment for pediatric migraine: results of treatment through one-year follow-up. Headache: The Journal of Head and Face Pain. 1989;29(7):434–40. doi: 10.1111/j.1526-4610.1989.hed2907434.x. [DOI] [PubMed] [Google Scholar]
- Cohen 2011.Cohen LL, Vowles KE, Eccleston C. The impact of adolescent chronic pain on functioning: disentangling the complex role of anxiety. Journal of Pain. 2010;11(11):1039–46. doi: 10.1016/j.jpain.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Eccleston 2014.Eccleston C, Palermo TM, Williams ACDC, Lewandowski Holley A, Morley S, Fisher E, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews. 2014;(5) doi: 10.1002/14651858.CD003968.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston 2014b.Eccleston C, Fisher E, Craig L, Duggan GB, Rosser BA, Keogh E. Psychological therapies (Internet-delivered) for the management of chronic pain in adults. Cochrane Database of Systematic Reviews. 2014;(2) doi: 10.1002/14651858.CD010152.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher 2014.Fisher E, Heathcote L, Palermo TM, Williams ACDC, Lau J, Eccleston C. Systematic review and meta-analysis: psychological therapies for children with chronic pain. Journal of Pediatric Psychology. 2014;39(8):763–82. doi: 10.1093/jpepsy/jsu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry 2009.Fry JP, Neff RA. Periodic prompts and reminders in health promotion and health behavior interventions: systematic review. Journal of Medical Internet Research. 2009;11(2):e16. doi: 10.2196/jmir.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntlett-Gilbert 2007.Gauntlett-Gilbert J, Eccleston C. Disability in adolescents with chronic pain: patterns and predictors across different domains of functioning. Pain. 2007;131(1–2):132–41. doi: 10.1016/j.pain.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Higgins 2011.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011 Available from www.cochrane-handbook.org.
- Jordan 2007.Jordan AL, Eccleston C, Osborn M. Being a parent of the adolescent with complex chronic pain: an interpretative phenomenological analysis. European Journal of Pain. 2007;11(1):49–56. doi: 10.1016/j.ejpain.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Kaczynski 2011.Kaczynski KJ, Simons LE, Lewis Claar R. Anxiety, coping, and disability: a test of mediation in a pediatric chronic pain sample. Journal of Pediatric Psychology. 2011;36(8):932–41. doi: 10.1093/jpepsy/jsr024. [DOI] [PubMed] [Google Scholar]
- Kelley 1989.Kelley ML, Heffer R, Gresham F, Elliot S. Development of a modified treatment evaluation inventory. Journal of Psychopathology and Behavioural Assessment. 1989;11:235–47. [Google Scholar]
- King 2011.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–38. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Law 2012.Law EF, Murphy LK, Palermo TM. Evaluating treatment participation in an internet-based behavioral intervention for pediatric chronic pain. Journal of Pediatric Psychology. 2012;37(8):893–903. doi: 10.1093/jpepsy/jss057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macea 2010.Macea DD, Gajos K, Calil YAD, Fregni F. The efficacy of web-based cognitive behavioral interventions for chronic pain: a systematic review and meta-analysis. Journal of Pain. 2010;11(10):917–29. doi: 10.1016/j.jpain.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Maciver 2010.Maciver D, Jones D, Nicol M. Parents’ experiences of caring for a child with chronic pain. Qualitative Health Research. 2010;20(9):1272–82. doi: 10.1177/1049732310367499. [DOI] [PubMed] [Google Scholar]
- March 2008.March S, Spence SH, Donovan CL. The efficacy of an internet-based cognitive-behavioral therapy intervention for child anxiety disorders. Journal of Pediatric Psychology. 2008;34(5):474–87. doi: 10.1093/jpepsy/jsn099. [DOI] [PubMed] [Google Scholar]
- Marks 2009.Marks I, Cavanagh K. Computer-aided psychological treatments: evolving issues. Annual Review of Clinical Psychology. 2009;5:121–41. doi: 10.1146/annurev.clinpsy.032408.153538. [DOI] [PubMed] [Google Scholar]
- McGrath 2008.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Journal of Pain. 2008;9(9):771–83. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Morley 2013.Morley S, Eccleston C, Williams ACDC. Examining the evidence of psychological treatments for chronic pain: time for a paradigm shift? Pain. 2013;154:1929–31. doi: 10.1016/j.pain.2013.05.049. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health.National Collaborating Centre for Mental Health. E-therapies systematic review for children and young people with mental health problems. 2014 https://www.minded.org.uk/course/view.php?id=122. (accessed August 2014)
- ONS 2013.Office for National Statistics. Internet access – households and individuals. 2013 www.ons.gov.uk/ons/dcp171778.322713.pdf (accessed October 2013)
- Palermo 2013.Palermo TM. Remote management of pediatric pain. In: Schmidt RF, Gebhart GF, editors. Encyclopedia of Pain. 2nd. New York: Springer; 2013. pp. 3389–93. [Google Scholar]
- Peng 2007.Peng P, Stinson JN, Chiniere M, Dion D, Intrater H, Lefort S, et al. STOPPAIN Investigators Group Dedicated multidisciplinary pain management centres for children in Canada: the current status. Canadian Journal of Anesthesia. 2007;54(12):985–91. doi: 10.1007/BF03016632. [DOI] [PubMed] [Google Scholar]
- Penzien 2005.Penzien DB, Andrasik F, Freidenberg BM, Houle TT, Lake AE, Lipchik GL, et al. Guidelines for trials of behavioral treatments for recurrent headache, first edition: American Headache Society behavioral clinical trials workgroup. Headache. 2005;45(Suppl 2):S110–32. doi: 10.1111/j.1526-4610.2005.4502004.x. [DOI] [PubMed] [Google Scholar]
- Perquin 2000.Perquin CW, Hazebroek-Kampscheur AAJM, Hunfeld JAM, Bohnene AM, van Suijlekom-Smit LWA, Passchier J, et al. Pain in children and adolescents: a common experience. Pain. 2000;87(1):51–8. doi: 10.1016/S0304-3959(00)00269-4. [DOI] [PubMed] [Google Scholar]
- RevMan 2014.The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- Richardson 2010.Richardson T, Stallard P, Velleman S. Computerised cognitive behavioural therapy for the prevention and treatment of depression and anxiety in children and adolescents: a systematic review. Clinical Child and Family Psychological Review. 2010;13(3):275–90. doi: 10.1007/s10567-010-0069-9. [DOI] [PubMed] [Google Scholar]
- Ritterband 2008.Ritterband LM, Ardalan K, Thorndike FP, Magee J, Saylor DK, Cox DJ, et al. Real world use of an internet intervention for pediatric encopresis. Journal of Medical Internet Research. 2008;10(2):e16. doi: 10.2196/jmir.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritterband 2009.Ritterband LM, Thorndike FP, Cox DJ, Kovatchev BP, Gonder-Frederick LA. A behavior change model for internet interventions. Annals of Behavioral Medicine. 2009;38(1):18–27. doi: 10.1007/s12160-009-9133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons 2012.Simons LE, Sieberg CB, Claar RL. Anxiety and functional disability in a large sample of children and adolescents with chronic pain. Pain Research & Management. 2012;17(2):93–7. doi: 10.1155/2012/420676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson 2009.Stinson J, Wilson R, Gill N, Yamada J, Holt J. A systematic review of internet-based self-management interventions for youth with health conditions. Journal of Pediatric Psychology. 2009;35(5):495–510. doi: 10.1093/jpepsy/jsn115. [DOI] [PubMed] [Google Scholar]
- Tait 2010.Tait RJ, Christensen H. Internet-based interventions for young people with problematic substance use: a systematic review. Medical Journal of Australia. 2010;192(11 Suppl):S15–21. doi: 10.5694/j.1326-5377.2010.tb03687.x. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen 2012.International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia. 2012;32(1):6–38. doi: 10.1177/0333102411417901. [DOI] [PubMed] [Google Scholar]
- USDC 2013.US Department of Commerce. Computer and Internet Use in the United States; population characteristics. www.census.gov/prod/2013pubs/p20-569.pdf (accessed October 2013)
- Williams 2012.Williams ACDC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews. 2012;(11) doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu 2014.Wu YP, Steele RG, Connelly MA, Palermo TM, Ritterband LM. Commentary: Pediatric eHealth Interventions: common challenges during development, implementation, and dissemination. Journal of Pediatric Psychology. 2014;39(6):312–23. doi: 10.1093/jpepsy/jsu022. [DOI] [PMC free article] [PubMed] [Google Scholar]


