Abstract
Background
Deficits in executive function have been associated with risk for relapse. Data from previous studies suggest that relapse may be triggered by stress and drug-paired cues and that there are significant sex differences in the magnitude of these responses. The aim of this study was to examine the impact of the pharmacological stressor and alpha-2 adrenergic receptor antagonist yohimbine and cocaine cues on executive function in cocaine-dependent men and women.
Methods
In a double-blind placebo controlled cross-over study, cocaine-dependent men (n=12), cocaine-dependent women (n=27), control men (n=31) and control women (n=25) received either yohimbine or placebo prior to two cocaine cue exposure sessions. Participants performed the Connors’ Continuous Performance Test II prior to medication/placebo administration and immediately after each cue exposure session.
Results
Healthy controls had a significant increase in commission errors under the yohimbine condition [RR (95% CI)=1.1 (1.0–1.3), X21=2.0, p=0.050]. Cocaine-dependent individuals exhibited a significant decrease in omission errors under the yohimbine condition [RR (95% CI)=0.6 (0.4–0.8), X21=8.6, p=0.003]. Cocaine-dependent women had more omission errors as compared to cocaine-dependent men regardless of treatment [RR (95% CI)=7.2 (3.6–14.7), X21=30.1, p<0.001]. Cocaine-dependent women exhibited a slower hit reaction time as compared to cocaine-dependent men [Female 354±13 vs. Male 415±14; t89=2.6, p=0.012].
Conclusions
These data add to a growing literature demonstrating significant sex differences in behaviors associated with relapse in cocaine-dependent individuals.
Keywords: cocaine, sex differences, yohimbine, executive control, stress, drug-cues
1. INTRODUCTION
Substance use disorders are characterized by compulsive drug-seeking behavior despite the deleterious consequences of repeated drug use. Relapse rates are particularly high among cocaine-dependent (CD) populations (McKay et al., 1995; McMahon, 2001; Sinha et al., 1999). Recent studies suggest that prior stress enhances the ability of drug-paired cues to elicit craving and relapse (Feltenstein et al., 2011; Feltenstein and See, 2006; Moran-Santa Maria et al., 2014). In addition, deficits in executive function appear to play a critical role in the maintenance of substance use disorders. For example, among cocaine-dependent individuals engaged in cognitive behavioral therapy, baseline performance on tests of impulsivity and attention predicted treatment retention, engagement and relapse (Carroll et al., 2011). Moreover, data from neuroimaging studies of cocaine-dependent individuals demonstrate hyperactivity in the anterior cingulate and orbitofrontal cortices during exposure to drug cues and hypoactivity during performance on tests of executive function, suggesting that chronic drug use alters executive control of impulsive behavior and underscores attentional bias to drug-paired cues (Goldstein and Volkow, 2002; Copersino et al., 2004; DiGirolamo et al., 2015; Hester and Garavan, 2004; Kaufman et al., 2003). Despite these data surprisingly little is known about the impact of stress and drug-paired cues on executive function in cocaine dependent men and women.
Impulsive action is an aspect of executive function that involves the inability to inhibit inappropriate behavior. Impulsive action can be measured in the laboratory using continuous performance tests that require the participants to inhibit the initiation of a response to a non-target. Failure to inhibit responding to a non-target is considered a commission error. In general, human laboratory tests suggest that cocaine-dependent individuals exhibit greater commission errors than healthy controls (Fillmore and Rush, 2002; Gooding et al., 2008; Kaufman et al., 2003). Impairments in impulsive action such as using more drug than intended, failure to reduce drug use, failure to inhibit drug taking despite the consequences are characteristic of substance use disorders (APA 2013 DSM-V). However, the extent to which exposure to triggers of relapse affects impulsive action has not been assessed in cocaine-dependent individuals.
Sustained attention is also a critical component of executive function and involves the ability to establish and maintain focus during a goal directed task. In the laboratory continuous performance tests assess sustained attention by the average number of times the participant fails to respond to a target (omission errors). Cocaine-dependent individuals demonstrate significantly more omission errors than healthy controls (Gooding et al., 2008; Moeller et al., 2005; Soar et al., 2012). With regard to substance use disorders deficits in sustained attention predict poorer responses to behavioral treatments that require active participation in therapy and learning of coping skills (Aharonovich et al., 2008; Verdejo-Garcia et al., 2012). In addition, a breakdown in attention control mechanisms has been hypothesized to contribute to impulsive action and relapse (Kenemans et al., 2005; Sutherland et al., 2012). Understanding the impact of stress and cues on sustained attention could provide significant insight into the relapse process.
A growing literature suggests that risk for cue-induced relapse may be potentiated by prior exposure to a stressor (Feltenstein et al., 2011; Moran-Santa Maria et al., 2014). For example, the alpha-2 adrenergic receptor antagonist yohimbine potentiates cue-induced reinstatement of both cocaine and heroin seeking behavior in rodents and in humans (Banna et al., 2010; Feltenstein et al., 2011; Moran-Santa Maria et al., 2014). In addition, footshock potentiates cue-induced reinstatement of cocaine and ethanol-seeking behavior in rodents (Buffalari and See, 2011; Liu and Weiss, 2002). In addition, there are significant sex differences in responses to stress and drug cues (Feltenstein et al., 2011; Moran-Santa Maria et al., 2014). Despite these findings, little is known about the relationship between relapse triggers and executive function in cocaine-dependent men and women.
Of note, studies of executive function in cocaine-dependent subjects have mostly been conducted in men. This is particularly noteworthy as studies of heavy drinkers have found that women appear to exhibit greater deficits in inhibitory control as compared to men (Nederkoorn et al., 2009; Townshend and Duka, 2005). In addition, women may be more vulnerable than men to stress and cue-related drug craving and relapse. For example, cocaine-dependent women reported greater anxiety and drug craving in response to yohimbine and drug-paired cues than cocaine-dependent men (Moran-Santa Maria et al., 2014). Compared with male rodents, female rodents exhibit greater stress potentiation of cue-induced cocaine-seeking behavior (Feltenstein et al., 2011). Moreover, cocaine-dependent women demonstrate significantly greater cue-related deactivation in brain regions involved in executive control as compared to cocaine-dependent men (Volkow et al., 2011). Studies examining the effects of stress and drug cues on executive function in cocaine-dependent men and women may contribute to our understanding of the relapse process.
This study was conducted as part of a larger study examining sex differences in stress and cue reactivity in cocaine-dependent men and women (Moran-Santa Maria et al., 2014). Specifically, the overarching goal of the larger project was to identify the potentiative effects of yohimbine on conditioned response to drug-paired cues in cocaine-dependent men and women and sex matched controls. As part of this study we examined the impact of the yohimbine and drug-paired cues on different aspects of executive function using a continuous performance test in cocaine-dependent men and women and sex-matched control groups. Given our previous findings, we hypothesized that together yohimbine and drug cues would increase impulsivity and inattention in comparison to drug cues alone (Moran-Santa Maria et al., 2014).
2. MATERIALS AND METHODS
2.1 Subjects
Male and female cocaine-dependent individuals and sex-matched healthy controls were recruited for the larger parent study. Study participants were recruited primarily via media advertisements over a 48-month period. Written informed consent was obtained from each participant before the study assessments were administered. Inclusion criterion for the cocaine-dependent groups included meeting Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for cocaine-dependence in the 90-days prior to the study. Exclusion criterion for the cocaine-dependent groups included (1) DSM-IV criteria for substance dependence except alcohol or marijuana within the past 60 days. Exclusion criteria for the control groups included (1) DSM-IV criteria for current or lifetime dependence on alcohol or any drugs of abuse and (2) DSM-IV criteria for current abuse of alcohol or any illicit drugs. General exclusion criteria included (1) pregnancy, nursing, or ineffective means of birth control; (2) premenstrual dysphoric disorder; (3) history of or current significant hematological, endocrine, cardiovascular, pulmonary, renal, gastrointestinal, or neurological diseases; (4) history of or current psychotic, panic, eating, or bipolar affective disorders; (5) current major depressive and PTSD; (6) history of or current medical conditions that might affect HPA axis activity; (7) synthetic glucocorticoid or exogenous steroid therapy within one month of testing; (8) psychotropic medications with the exception of selective serotonin reuptake inhibitors, opiates or opiate antagonists, benzodiazepines, antipsychotics, b-blockers and other medications that might interfere with HPA axis activity or physiologic measurements; (9) acute illness or fever; (10) body mass index > 35 and (11) unwillingness or inability to maintain abstinence from alcohol and other drugs of abuse (except nicotine) for three days prior to the cue-reactivity sessions.
2.2 Assessment
Participants meeting pre-screening criteria were evaluated for study eligibility using the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998). The substance use module of the Structured Clinical Interview for DSM-IV was used to assess current and lifetime substance use disorders (First et al., 1994). A medical history and physical examination were completed to assess for medical exclusions. Participants meeting inclusion criteria and no exclusion criteria were scheduled to complete the study procedures. A total of 232 individuals were consented but did not meet study criteria. Fifty eight individuals were consented, met study criteria were enrolled and did not complete study procedures.
2.3 Study Procedures
Participants completed two cue reactivity sessions conducted on consecutive days. On the first day of testing, participants arrived at the Medical University of South Carolina’s (MUSC’s) Research Nexus at 10:00 a.m. Urine pregnancy tests were administered to female participants prior to study participation. Smokers were provided with a nicotine patch. Abstinence was assessed using self-reports, urine drug screens (Roche Diagnostics, Indianapolis, Indiana), and breathalyzer tests (AlcoSensor III, Intoximeters, Inc., St. Louis, Missouri). If the pregnancy and drug tests were negative (with the exception of tetrahydrocannabinol; THC), the study procedures continued. Participants with THC positive UDS were allowed to participate.
The study utilized a double-blind placebo-controlled design. At 11:00 a.m. participants performed the Conners’ Continuous Performance Test II (CPT II Version 5 for Windows; described below). One hour later, they received either the alpha-2 adrenergic receptor antagonist yohimbine (Spectrum Laboratories, Inc., New Brunswick, New Jersey) 21.6 mg or a matching placebo capsule. Yohimbine has been shown to increase noradrenergic transmission, heart rate, blood pressure and subjective anxiety (Charney et al., 1983). The MUSC Investigational Drug Service (IDS) compounded the yohimbine and placebo capsules and was responsible for treatment randomization. Half of the participants were randomized to receive the placebo capsule on day 1 while the other half to receive the yohimbine capsule on day 1. Study participants were seated in a private room, given lunch and were allowed to read until the testing procedures began. The cue reactivity session began at 2:00 p.m. Briefly, the participant was asked to recall a time when they were using cocaine and recount, in as much detail as possible including their heart rate, breathing, and the “rush” of the first hit. The participant was then asked to view and handle cocaine cues for two minutes. For individuals who used crack cocaine the cues consisted of a small bag of fake crack cocaine, the subject’s preferred style of pipe, a lighter, and a $20 bill. For powder or intravenous users, cues consisted of a $20 bill and fake cocaine. Afterwards the participant watched a five-minute film of individuals using cocaine (Coffey et al., 2002). Immediately after the cue reactivity session the participant completed the CPT-II. At the end of the test session the participant spent the night in a private room in the Medical University Hospital. The next day, the participant returned to the Research Nexus and repeated the study procedures with the alternate pharmacologic treatment after which they spent a second night in the hospital. At 11:00 a.m. on the morning following the second test day, the participant was compensated and discharged from the Research Nexus.
Each participant completed the CPT-II on an IBM compatible laptop computer. The CPT-II has high test-retest reliability in measures of attention and impulsive action (Weafer et al., 2013). The participants were asked to click on the mouse when any letter except the letter “X” appeared on the screen. Letters appear on the screen for 250 milliseconds. The interval between letter presentations (interstimulus interval) varied between 1, 2 and 4 seconds. The CPT-II is divided into six blocks and 90% of the letters in each block were targets (letters other than “X”). Each block included three sub-blocks of different interstimulus interval, for a total of 18 blocks. The CPT-II takes approximately 14 minutes to complete. Participants were given a practice trial prior to the test session. Participants completed the CPT-II four times (prior to placebo administration, post placebo administration and cue exposure, prior to yohimbine administration and post yohimbine and cue exposure).
2.4 Study Analysis
A Wilcoxon Rank sum test was used to evaluate baseline continuous demographic and clinical measures across drug use groups while the Pearson Chi-Square test was used to assess the association among categorical and ordinal demographic and clinical variables at baseline. To assess the hypothesis that there is an association between treatment with yohimbine and response to cocaine cues, generalized linear mixed effects models were developed that included pre and post cue measurements under both the yohimbine and placebo conditions. Omission errors and reaction time were used to measure attention. Commission errors were used to measure impulsivity. Omission and commission errors were examined using count models (log linear); the data showed evidence of over-dispersion and thus a negative binomial distribution was used to model the data. Hit reaction time (RT) was assessed from the point at which any target letter appeared on the screen until the mouse was clicked. Hit reaction time data were analyzed assuming a Gaussian distribution with an identity link. Prior to analysis of RT data, assumptions of homoscedasticity and normality in the residual were checked and data were natural logarithm transformed as necessary to conform to model assumptions. For each outcome (Omission count, commission count, and mean RT), a single model was fit containing the main effects of cocaine use status, medication assignment, sex, time within each visit (pre-post cue) and all high level interactions between primary independent variables. Pair-wise comparisons between pre and post cue time points as well as between cocaine and healthy control subjects were assessed using model based estimates and associated standard errors derived from contrast statements built within each outcome model. Effects of drug administration (carryover effects) and cue-reactivity session (day 1 vs. day 2) were tested in all models. No effects of order were determined to significantly affect model estimates; however, responses were lower during the second presentation of the cue (day 2) and thus study day was controlled for in all analysis models. Sex and study day interactions with design variables (cocaine use status, medication assignment and time within visit) as well as the effect of positive THC UDS at baseline were investigated in all statistical models. There were no significant interactions between study design variables and study day noted in any model (all p>0.50). Study baseline rates were collected as the CPT-II scores completed prior to any drug administration on the first study day. Baseline error rates and response times were tabulated and compared between cocaine dependent and healthy control subjects as well as between males and females. Results from the analysis of error rates are presented as risk ratios (RR) and associated 95% confidence intervals (95% CI). Results from the analysis of RTs are presented as mean group differences and associated 95% confidence intervals. Due to relatively small within group sample sizes and sample size imbalance across groups, all model based significance testing was done using a generalized score test. The score test has shown to be robust to both small samples and imbalance as compared to the Wald test.
All statistical analyses were performed using SAS, version 9.3 (SAS Institute, Cary, N.C.). Significance for all planned pair-wise comparisons was set at a 2-sided alpha level of 0.05 and no correction for multiple testing was applied to reported values.
3. RESULTS
3.1 Demographics
Data were collected from groups of cocaine-dependent men and women as well as non-dependent healthy control men and women. Participants were part of a larger parent study designed to assess sex differences in responses to stress and drug cues (Moran-Santa Maria et al., 2014). Of the 119 participants in the parent study, 99 (83.2%) had CPT-II task scores available. Additionally, four participants had data removed prior to analysis due to high commission error rates (>80%) coupled with very low omission error rates, indicative of constant response. Baseline demographic and clinical characteristics between dependence groups are presented in Table 1. Cocaine-dependent subjects were older and more likely to be unemployed as compared to healthy control subjects. Age was significantly associated with omission errors, commission errors and RT and was included as a covariate in all models. Employment status was included in initial model development but was not a significant predictor of error rates or mean response times and was removed from the final analysis models.
Table 1.
| Cocaine-Dependent | Healthy Controls | |||||
|---|---|---|---|---|---|---|
| CD (n=39) | Male (n=12) | Female (n=27) | HC (n=56) | Male (n=31) | Female (n=25) | |
| Age (years, se) | 41 (1.7)* | 38 (3.1) | 42 (2.1) | 33 (1.7) | 32 (2.2) | 34 (2.6) |
| African American (%) | 62 | 75 | 55 | 48 | 52 | 44 |
| Married (%) | 26 | 8 | 14 | 23 | 22 | 24 |
| HS Graduate (%) | 82 | 83 | 81 | 91 | 84 | 81 |
| Employed (%) | 29** | 33 | 14 | 46 | 42 | 52 |
| Smoker (%) | 87 | 83 | 89 | 73 | 84 | 61 |
| Positive THC UDS (%) | 15 | 25 | 11 | 18 | 19 | 16 |
| Alcohol Dependent (%) | 20 | 33 | 14 | |||
| Total Years of Use | 13.4 (1.4) | 14.4 (2.6) | 13 (1.7) | NA | NA | NA |
| Days Since Last Use | 16.7 (2.8) | 20.5 (7.6) | 14.9 (2.2) | NA | NA | NA |
| Shipley Score (mean, se) | 90 (2.0) | 87.2 (5.5) | 91 (3.0) | 98 (2.6) | 98.4 (2.7) | 97.3 (3.4) |
| ADHD (%) | 2.3 | 8 | 0 | 0 | 0 | 0 |
Denotes significant difference from healthy control subjects Z=−3.315, p<0.01
Denotes significant difference from healthy control subjects X2 (1,N=95) = 7.19 p< 0.01
3.2 Baseline CPT-II Response
Prior to the administration of the study drug on day 1, CPT-II responses were measured across all participants. As anticipated, there were no difference between those randomized to receive yohimbine as compared to placebo (omission: X21=0.1, p=0.812; commission: X21=1.3, p=0.250; RT: F1,86=0.3, p=0.591). There was, however, a differential omission error rate between men and women in the cocaine dependent and healthy control study groups [Group × Sex interaction; X21=6.1, p=0.013]. Data show that there was a significantly greater rate of omission errors in cocaine-dependent women as compared to cocaine-dependent men [RR (95% CI)=4.4 (1.5–13.0), X21=4.3, p=0.038]. Conversely, in the healthy control participants, the women showed slightly lower omission error rates as compared to men [RR (95% CI)=0.4 (0.2–1.0), X21=3.1, p=0.078]. The baseline sex differential between groups seen in the omission error rates was not present in the analysis of baseline commission error rates [Group × Sex interaction; X21=0.5, p=0.484]. Baseline mean reaction times did not differ between cocaine dependent and healthy control subjects [387±14 vs. 368±10; p=0.333] or between men and women [364±13 vs. 391±10; p=0.080]. There was also no differential effect of sex across cocaine use status rates [Group × Sex interaction; p=0.207].
3.3 CPT-II Cue Response
Omission Errors
There was no significant difference in the overall frequency of omission errors between those randomized to receive yohimbine as compared to placebo [RR (95% CI)=1.3 (0.7–2.4), X21=0.5, p=0.467]. However, there was a significant decrease in omission errors following the cocaine cue specifically in cocaine-dependent participants under the yohimbine condition [RR (95% CI)=0.6 (0.4–0.8), X21=4.5, p=0.034] that was not present under the placebo condition [RR (95% CI)=0.9 (0.4–2.0), X21=0.0, p=0.849] (Figures 1A and 1B; see accompanying table in Supplementary Materials1). This effect under the yohimbine condition appears to be primarily evident in cocaine-dependent males [RR (95% CI)=0.5 (0.3–0.7), X21=3.9, p=0.048] rather than cocaine-dependent females [RR (95% CI)=0.7 (0.3–1.2), X21=0.9, p=0.334]. Omission error model results showed a significant group by sex interaction [X21=10.8, p=0.001] indicating that the difference in errors between cocaine dependent and healthy control participants varied by sex. Investigation of the interaction shows that cocaine-dependent women had significantly more omission errors as compared to cocaine-dependent men both prior to and following presentation of the cue [RR (95% CI)=7.2 (3.6–14.7), X21=10.5, p=0.001] (Figures 2A and 2B; see accompanying table in Supplementary Material2). However, there was no evidence of this effect in the healthy controls [RR (95% CI)=0.7 (0.3–1.5), X21=0.9, p=0.350].
Fig. 1.
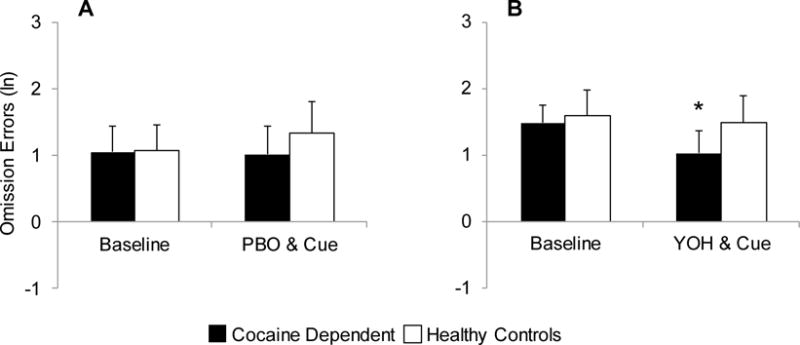
Comparison of omission errors between cocaine-dependent and healthy control subjects after placebo (PBO) and cue exposure (A) and yohimbine (YOH) and cue exposure (B). Data are presented as the mean + se. * Denotes a significant difference from baseline.
Fig. 2.
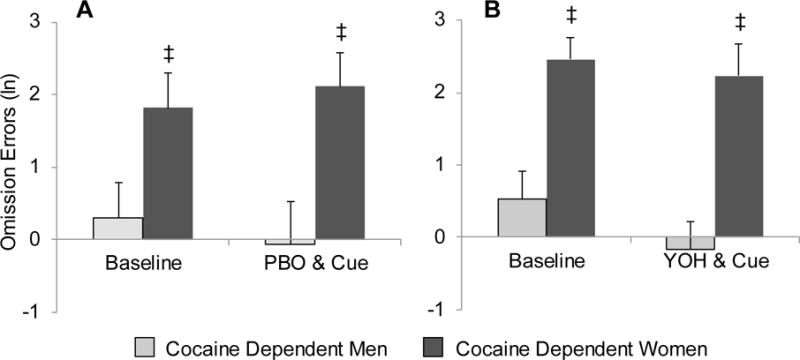
Comparison of omission errors between cocaine-dependent men and cocaine-dependent women after placebo (PBO) and cue exposure (A) and yohimbine (YOH) and cue exposure (B). Data are presented as the mean + se. ‡ Denotes a significant sex difference.
Commission Errors
There was no significant difference in the overall frequency of commission errors between those randomized to receive yohimbine as compared to placebo [RR (95% CI)=1.1 (0.9–1.4), X21=0.5, p=0.488]. However, healthy controls had a moderate increase in commission errors under the yohimbine condition [RR (95% CI)=1.1 (1.0–1.3), X21=3.1, p=0.070] that wasn’t present in the cocaine-dependent subjects [RR (95% CI)=0.9 (0.8–1.1), X21=0.6, p=0.428] (Figures 3A and 3B; see accompanying table in Supplementary Material3). The sex difference across cocaine-dependent status observed in omission errors was not present in commission errors [X21=1.7, p=0.200] (Figures 4A and 4B; see accompanying table in Supplementary Material4).
Fig. 3.
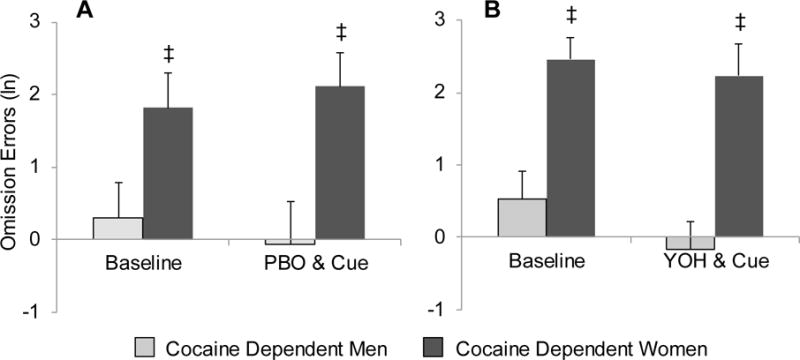
Comparison of commission errors and reaction time between cocaine-dependent and healthy control subjects after placebo (PBO) and cue exposure (A & C) and yohimbine (YOH) and cue exposure (B & D). Data are presented as the mean + se. * Denotes a significant difference from baseline.
Fig. 4.
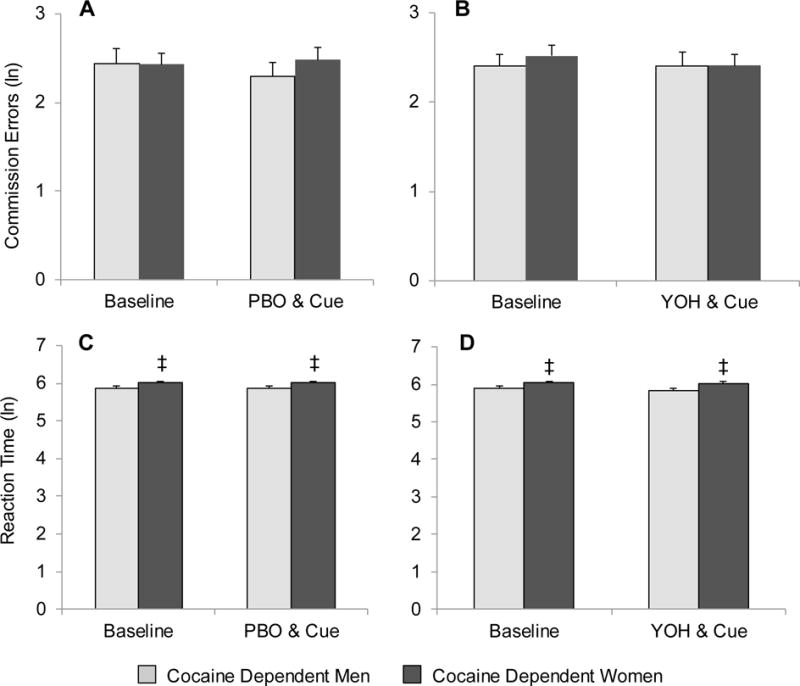
Comparison of commission errors and reaction time between cocaine-dependent men and cocaine-dependent women after placebo (PBO) and cue exposure (A & C) and yohimbine (YOH) and cue exposure (B & D). Data are presented as the mean + se. ‡ Denotes a significant sex difference.
Reaction Times
Hit reaction times did not differ across drug condition [F1,79=0.05, p=0.829] or cocaine use status [F1,89=0.58, p=0.449] (Figures 3C and 3D; see accompanying material in Supplementary Material5). Sex differentially impacted hit reaction times in cocaine-dependent and healthy control subjects [F1,83=4.9, p=0.029]. Cocaine-dependent female participants had significantly slower hit reaction times as compared to cocaine-dependent men [Female 354±13 vs. Male 415±14; t89=2.6, p=0.012] (Figures 4C and 4D) while healthy control women and men had similar hit reaction times [Female 368±13 vs. Male 374±14; t89=0.4, p=0.689].
4. DISCUSSION
In this study we examined the effects of the pharmacological stressor yohimbine and drug cues on laboratory measures of impulsivity and attention in cocaine-dependent individuals and sex-matched control groups. Yohimbine was selective in altering aspects of executive function in cocaine-dependent individuals. Of note, cocaine-dependent women exhibited more omission errors and had a longer reaction time than cocaine-dependent men regardless of treatment. To our knowledge, this is the first study to investigate executive function following exposure to a pharmacological stressor and drug-paired cues in cocaine-dependent individuals and to systematically investigate sex differences in these responses.
In the present study the drug cue and the placebo had no effect on omission errors, commission errors or reaction time. Performance on tests of executive function is best with an intermediate level of noradrenergic activity in the prefrontal cortex and worse with either too much or too little noradrenergic activity (Arnsten and Li, 2005; Berridge and Waterhouse, 2003). Drug cues increase noradrenergic activity in cocaine-dependent subjects (Sinha et al., 2003). Thus, we expected that cocaine-dependent subjects would exhibit deficits in executive function following exposure to the drug cue under the placebo condition. It is possible that in the laboratory cue-related increases in noradrenergic activity are below the threshold necessary to affect impulsive action and sustained attention.
In contrast to our hypothesis, yohimbine together with the drug cue reduced omission errors in cocaine-dependent subjects but not in healthy controls. The decrease in omission errors appeared to be driven by cocaine-dependent men as cocaine-dependent women exhibited significantly more omission errors and a longer mean response time than cocaine-dependent men under both the placebo and yohimbine conditions. Lower numbers of omission errors coupled without a change in reaction time suggests that these findings were specific to improvements in attention and not due to a change in the speed of the motor response and suggest that yohimbine may improve laboratory measures of attention in cocaine-dependent men. Post synaptic alpha-2 receptors play a pertinent role in attention. It is possible that the improvement in attention was facilitated by cocaine-induced increases in the sensitivity of alpha-2 receptors. Cocaine-dependent women exhibited both slower reaction times than cocaine-dependent men. Although slow reaction times can reflect a sluggish motor response, cocaine-dependent women also exhibited and higher omission errors suggesting that cocaine-dependent women exhibit deficits in laboratory measures of sustained attention. In support, there were no differences in the number of omission errors or the mean response time between healthy control women and healthy control men, suggesting that the sex differences found in the present study were specific to cocaine-using individuals. In agreement, data from previous studies of smokers and heavy drinkers, found that women exhibit greater deficits in executive function as compared to men (Fields et al., 2009; Nederkoorn et al., 2009; Townshend and Duka, 2005). Still others have found no sex differences in measures of impulsivity or attention in cocaine-dependent individuals (van der Plas et al., 2009). Differences in drug use history and the type of laboratory tasks across studies may explain these disparate findings. Previously we reported that yohimbine and drug cues increased craving and anxiety in cocaine-dependent women (Moran-Santa Maria et al., 2014), thus we had anticipated that yohimbine and the drug cue would cause significant impairments in executive function in cocaine-dependent women. The absence of deficits in cognitive function suggests that alpha-2 receptors play a greater role in drug craving than in mediating executive function in cocaine-dependent women.
There was no effect of yohimbine and drug cues on commission errors in cocaine-dependent subjects. Both clinical and preclinical studies have found that the alpha-2 agonist guanfacine, which decreases noradrenergic activity, decreased measures of impulsivity (Fox et al., 2015; Terry et al., 2014), thus we expected that yohimbine would increase commission errors in cocaine-dependent subjects. It is unlikely that the present findings were due to cocaine-induced ceiling effect as there were no differences in baseline commission errors between cocaine-dependent and healthy control subjects. Yohimbine increased commission errors in healthy controls. These findings are consistent with data from previous studies demonstrating that elevated noradrenergic activity stimulates post synaptic alpha-1 noradrenergic receptors and impairs executive function in healthy controls (Arnsten and Li, 2005; Arnsten et al., 1999; Swann et al., 2005). Thus, taken together with the present findings it appears that the sensitivity of alpha-1 adrenergic receptors may be altered in cocaine-dependent individuals.
There are a few limitations to the present findings. We did not collect any blood markers of noradrenergic activity; however, others have shown that similar doses of yohimbine increase plasma noradrenergic metabolites in healthy controls (Swann et al., 2013). In addition, in the previous study we demonstrated that both the cocaine-dependent and healthy controls exhibited significant increases in heart rate following yohimbine administration, suggesting that the dose of yohimbine was sufficient to produce an active physiologic response (Moran-Santa Maria et al., 2014). A second limitation is that we did not measure ovarian hormones levels in our subjects. A previous study found significant effects of ovarian hormones on laboratory tests of executive function (Colzato et al., 2010). Thus, it is possible that hormones played a significant role in the current findings. The relationship between noradrenergic activity and executive function has been suggested to operate according to an inverted “U” (Yerkes, 1908). Therefore, different doses of yohimbine may produce different results between the cocaine-dependent and healthy control groups. It is also important to note that yohimbine is a pharmacological stressor, thus it is unclear how the present findings would translate to psychosocial stressors. Finally we did not include a yohimbine neutral-cue group, which would have allowed us to delineate between the effects of the stressor alone and that of the cue presentations. Future studies assessing the effects of steroid hormones on executive function in cocaine-dependent individuals may be warranted.
Despite these limitations, this is the first study of stress and cue-exposure on measures of executive function in cocaine-dependent individuals. Moreover these findings demonstrate important sex differences in laboratory measures of impulsivity and attention in cocaine-dependent subjects. Importantly, these findings add to a growing literature demonstrating important sex differences in risk factors for relapse in cocaine-dependent individuals and support the development of specific treatment interventions for men and women with substance use disorders.
Supplementary Material
Highlights.
Yohimbine was selective in altering aspects of executive function in cocaine-dependent individuals.
Yohimbine reduced omission errors in cocaine-dependent men.
Cocaine-dependent women exhibited more omission errors and had a longer reaction time than cocaine-dependent men regardless of treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: …
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: …
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Conflict of Interest
Authors Moran-Santa Maria, Baker, and Prisciandaro declare no conflict of interest. Kathleen Brady lists: Consultant AstraZeneca Pharmaceuticals. Aimee McRae lists: Forest Pharmaceuticals medication provided for separate NIH grant.
Contributors
Drs. Brady and McRae-Clark designed the study and wrote the protocol. Drs. Moran-Santa Maria and McRae-Clark coordinated the study. Mr. Baker and Dr. Prisciandaro performed the statistical analyses and contributed to the results section. Dr. Moran-Santa Maria participated in data analysis and wrote the manuscript. All authors have contributed to and have approved the final manuscript.
References
- Aharonovich E, Brooks AC, Nunes EV, Hasin DS. Cognitive deficits in marijuana users: Effects on motivational enhancement therapy plus cognitive behavioral therapy treatment outcome. Drug Alcohol Depend. 2008;95:279–283. doi: 10.1016/j.drugalcdep.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208:144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 2011;213:19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, Babuscio TA, Brewer JA, Potenza MN, Ball SA, Martino S, Rounsaville BJ, Lejuez CW. Cognitive function and treatment response in a randomized clinical trial of computer-based training in cognitive-behavioral therapy. Subst Use Misuse. 2011;46:23–34. doi: 10.3109/10826084.2011.521069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Redmond DE., Jr Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Saladin ME, Drobes DJ, Brady KT, Dansky BS, Kilpatrick DG. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug Alcohol Depend. 2002;65:115–127. doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Hertsig G, van den Wildenberg WP, Hommel B. Estrogen modulates inhibitory control in healthy human females: evidence from the stop-signal paradigm. Neuroscience. 2010;167:709–715. doi: 10.1016/j.neuroscience.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Serper MR, Vadhan N, Goldberg BR, Richarme D, Chou JC, Stitzer M, Cancro R. Cocaine craving and attentional bias in cocaine-dependent schizophrenic patients. Psychiatry Res. 2004;128:209–218. doi: 10.1016/j.psychres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- DiGirolamo GJ, Smelson D, Guevremont N. Cue-induced craving in patients with cocaine use disorder predicts cognitive control deficits toward cocaine cues. Addict Behav. 2015;47:86–90. doi: 10.1016/j.addbeh.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S, Collins C, Leraas K, Reynolds B. Dimensions of impulsive behavior in adolescent smokers and nonsmokers. Exp Clin Psychopharmacol. 2009;17:302–311. doi: 10.1037/a0017185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- First MB, Frances AJ, Pincus HA, Vettorello N, Davis WW. DSM-IV in progress. Changes in substance-related, schizophrenic, and other primarily adult disorders. Hosp Community Psychiatry. 1994;45:18–20. doi: 10.1176/ps.45.1.18. [DOI] [PubMed] [Google Scholar]
- Fox H, Sofuoglu M, Sinha R. Guanfacine enhances inhibitory control and attentional shifting in early abstinent cocaine-dependent individuals. J Psychopharmacol. 2015;29:312–323. doi: 10.1177/0269881114562464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding DC, Burroughs S, Boutros NN. Attentional deficits in cocaine-dependent patients: converging behavioral and electrophysiological evidence. Psychiatry Res. 2008;160:145–154. doi: 10.1016/j.psychres.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenemans JL, Bekker EM, Lijffijt M, Overtoom CC, Jonkman LM, Verbaten MN. Attention deficit and impulsivity: selecting, shifting, and stopping. Int J Psychophysiol. 2005;58:59–70. doi: 10.1016/j.ijpsycho.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Alterman AI, Cacciola JS, Kaplan MR. An examination of the cocaine relapse process. Drug Alcohol Depend. 1995;38:35–43. doi: 10.1016/0376-8716(95)01098-j. [DOI] [PubMed] [Google Scholar]
- McMahon RC. Personality, stress, and social support in cocaine relapse prediction. J Subst Abuse Treat. 2001;21:77–87. doi: 10.1016/s0740-5472(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology (Berl) 2014;21:4157–4165. doi: 10.1007/s00213-014-3555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederkoorn C, Baltus M, Guerrieri R, Wiers RW. Heavy drinking is associated with deficient response inhibition in women but not in men. Pharmacol Biochem Behav. 2009;93:331–336. doi: 10.1016/j.pbb.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Soar K, Mason C, Potton A, Dawkins L. Neuropsychological effects associated with recreational cocaine use. Psychopharmacology (Berl) 2012;222:633–643. doi: 10.1007/s00213-012-2666-4. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychological And Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Swann AC, Birnbaum D, Jagar AA, Dougherty DM, Moeller FG. Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biol Psychiatry. 2005;57:1209–1211. doi: 10.1016/j.biopsych.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Cox B, Steinberg JL, Moeller FG. Norepinephrine and impulsivity: effects of acute yohimbine. Psychopharmacology (Berl) 2013;229:83–94. doi: 10.1007/s00213-013-3088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Callahan PM, Schade R, Kille NJ, Plagenhoef M. Alpha 2A adrenergic receptor agonist, guanfacine, attenuates cocaine-related impairments of inhibitory response control and working memory in animal models. Pharmacol Biochem Behav. 2014;126:63–72. doi: 10.1016/j.pbb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol Clin Exp Res. 2005;29:317–325. doi: 10.1097/01.alc.0000156453.05028.f5. [DOI] [PubMed] [Google Scholar]
- Van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2009;31:706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Betanzos-Espinosa P, Lozano OM, Vergara-Moragues E, Gonzalez-Saiz F, Fernandez-Calderon F, Bilbao-Acedos I, Perez-Garcia M. Self-regulation and treatment retention in cocaine dependent individuals: a longitudinal study. Drug Alcohol Depend. 2012;122:142–148. doi: 10.1016/j.drugalcdep.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Goldstein RZ, Alia-Klein N, Wong C. Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PLoS One. 2011;6:e16573. doi: 10.1371/journal.pone.0016573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Baggott MJ, de Wit H. Test-retest reliability of behavioral measures of impulsive choice, impulsive action, and inattention. Exp Clin Psychopharmacol. 2013;21:475–481. doi: 10.1037/a0033659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit formation. J Comp Neurol Psychol. 1908;18:459–482. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


