Abstract
Background
Innate immune dysfunction after major burn injuries increases the susceptibility to organ failure. Lipid mediators of inflammation resolution, e.g. Resolvin D2 (RvD2), have been shown recently to restore neutrophil functionality and reduce mortality rate in a rat model of major burn injury. However, the physiological mechanisms responsible for the benefic activity of RvD2 are not well understood.
Design
Prospective randomized animal investigation.
Setting
Academic research setting.
Subjects
Wistar male rats.
Interventions
Animals were subjected to a full thickness skin burn of 30% total body surface area. Two hours after burn, 25 ng/kg RvD2 was administered i.v. and repeated every day, for 8 days. At day 10 post burn (pb), 2 mg/kg of lipopolysaccharide (LPS) was administered i.v., and the presence of renal and hepatic injuries was evaluated at day 11 pb by immunohistochemistry and relevant blood chemistry.
Measurements and Main Results
In untreated animals, we found significant tissue damage in the kidney and liver, consistent with acute tubular necrosis and multifocal necrosis, and changes in blood chemistry, reflecting the deterioration of renal and hepatic functions. In RvD2 treated rats, we detected less tissue damage and significantly lower values of blood urea nitrogen (BUN) (26.4±2.1 vs. 36.0±9.3 mg/dl, p≤0.001), alanine aminotransferase (ALT) (266.5±295.2 vs. 861.8±813.7 U/l, p≤0.01), and total bilirubin (0.13±0.05 vs. 0.30±0.14 mg/dl, p≤0.01), compared to untreated animals. The mean blood pressure of all animals was above 65 mmHg, indicating adequate tissue perfusion throughout the experiments. We measured significantly larger amounts of chromatin in the circulation of untreated compared to RvD2 treated rats (575.1±331.0 vs. 264.1±122.4 ng/ml, p≤0.05) and identified neutrophil extracellular traps (NETs) in kidney and liver tissues from untreated rats, consistent with the tissue damage.
Conclusions
Pathological changes in kidney and liver tissues in a rat model of major burn and endotoxin insults are ameliorated by RvD2.
Keywords: Burn, Sepsis, Rats, Acute Kidney Injury, Liver Injury, Resolvin D2, NETs (Neutrophil Extracellular Traps), Megalin
Introduction
Patients with major burn injuries often develop organ failure complications that increase mortality. One-third of patients with major burn injury have been reported to develop acute kidney injury (AKI) (1, 2), a condition associated with a high mortality rate (3, 4) and often require renal replacement therapy (5). Changes in liver function and size are also common, and could alter hepatic protein synthesis, acute-phase responses, and be followed by liver failure (6–8). Thus, the prevention of AKI and liver dysfunction are critical treatment goals after major burns (1). However, current treatment options are limited, because multiple conditions contribute to organ failure and the mechanisms by which major burns could lead to organ failure are not well understood. Recent genomic data suggests a role for the dysfunctional innate immune reactions in multi-organ failure after major burns and trauma (9). In particular, a central role for neutrophils, which represent the largest population of innate immune cells in the circulation, is emerging. The role of neutrophils is supported by experimental results showing that removing neutrophils from the circulation before burn injury provides protection against organ damage in animal models. Several mechanisms by which excessive activation of neutrophils after burns is detrimental have been proposed (10), more recently including the release of neutrophil extracellular traps (NETs) (11, 12). In one recent study, we measured the neutrophil function with high precision over time, in a rat model of thermal injury and subsequent septic insult (13). We observed that neutrophil motility was defective in the days after burn injury and found that these defects could be restored by lipid mediators of inflammation resolution. In particular, Resolvin D2 (RvD2) (14–16), when administered repeatedly after the injury resulted in restored neutrophil speed and directionality and was correlated with improved survival in that model of burn and septic injury (13). However, the mechanisms by which lipid mediators improve survival after burn injuries remain poorly understood.
To better understand how the lipid mediators contribute to improved survival during complications after major burns, we examined the pathology of kidney, liver, and other vital organs in a rat model of burn and endotoxin insult. We also investigated changes in hemodynamics, blood biochemistry, and tissue histology in response to RvD2 treatment. We identified neutrophil extracellular traps (NETs) in tissues as a potential trigger for the secondary injury that occurs after major burns.
Materials and Methods
Rat model of burn injury
Wistar male rats, weighting 300–400 g (Charles River Laboratories, Wilmington, MA) were used in the experiments, after a 4 to 7 day acclimatization period. Rats were assigned randomly to 8 study groups, which were subject of sham burn or burn procedures, with or without LPS, with or without RvD2 treatment. At least 7 rats were included in each of the burn groups, and at least 3 in each of the sham burn groups. The procedure for thermal injury was performed as previously described (13), with changes in the anesthesia protocol to reduce mortality after LPS injection. Specifically, before the experiments, rats were anesthetized with 50 mg/kg Ketamine and 5 mg/kg Xylazine intraperitoneally. Isoflurane 1–3 % inhalant was added as needed. The fur of the dorsal surface was shaved and rats were placed in a mold exposing 30 % of total body surface area to precisely control the area of burn injury. The exposed area was immersed in boiling water for 12 seconds, producing a full thickness burn. Rats in the sham burn groups followed the same procedure but were exposed to water at room temperature. Immediately after burn or sham-burn injury, all rats received fluid resuscitation with 40 ml/kg of normal saline i.p.. All rats were kept on a thermostat water blanket until recovery from anesthesia, when they were moved into individual cages. Buprenorphine at a dose of 0.05 mg/kg was injected s.c. before the anesthesia and continued every 12 hours in the first 48 hours after this procedure. All procedures were conducted using aseptic techniques. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Massachusetts General Hospital.
Secondary endotoxin insult and study end-point
Rats were injected with 2 mg/kg of the lipopolysaccharide (LPS) derived from Escherichia coli 0111:B4 (Sigma-Aldrich, St. Louis, MO) via tail vein at day 10 post burn (day 10 pb) or sham-burn. Vehicle solution (0.9% saline) was injected to control rats. After the LPS injection, all rats were monitored closely for 24 hours, after which they were euthanized.
Resolvin D2 treatment
RvD2 (Cayman Chemical, Ann Arbor, MI, USA) was administered starting at 2 hours after the burn procedure, every day, for 8 days (day 0 pb to day 7 pb), as previously described in detail (13). 25 ng/kg of RvD2 in 0.3 % ethanol was administrated via tail vein injection. Vehicle (0.3 % ethanol) was administered to control rats. After restraining the rats with appropriate size devices, the tail was placed in warm (30–35 °C) water for 3 minutes to dilate the veins. A 25 G needle attached to a 1 ml syringe filled with 500 μL/kg of RvD2 or vehicle was inserted to the tail vein and the solution injected slowly. The rats were released from the restraint as soon as the bleeding stopped.
Blood collection and measurement
To measure blood chemistry, the blood was drawn from the tail vein at three time points: before the burn procedure (day 0 pb), before the LPS injection (day 10 pb), and 24 hours after injecting LPS (day 11 pb). Blood chemistry was analyzed at the Center for Comparative Medicine (CCM) veterinary clinical pathology laboratory of Massachusetts General Hospital. To measure plasma genomic DNA levels, additional blood samples were collected at the end of the study using sodium citrate as anticoagulant. The blood samples were immediately processed by centrifugation (1,500 g × 10 min, 4°C) to isolate plasma, which was quickly stored at −80°C. Plasma genomic DNA was measured using a commercially available diagnostic kit, Quant-iT PicoGreen ds DNA assay kit (Invitrogen, Eugene, OR). After 24 hours of injecting LPS, all rats were euthanized with 200 mg/kg of sodium pentobarbital i.v. and tissue samples were collected for analysis (day 11 pb).
Non-invasive hemodynamic measurement
To monitor the hemodynamic state of the rats after burn injury, we measured their blood pressure and pulse rate using a non-invasive tail cuff method (CODA2, Kent Scientific Co., Torrington, CT). Measurements were performed at day 0 (before the injury), at day 10 pb, before the LPS injection, and then at 12 hours (day 10.5 pb) and 24 hours (day 11 pb) after the LPS injection. During the procedure, which was usually completed in less than 10 minutes, rats were restrained and kept on a thermostat water blanket. To reduce the potential for artifacts due to stress during animal restraint, rats were briefly anesthetized using 1–2 % isoflurane. To reduce the potential for cross-infections, the restraining device was cleaned thoroughly after each measurement.
Tissue histology
To examine the histology of kidney and liver after injury, tissues were harvested at day 11 pb and fixed in 10 % phosphate buffered formalin. Paraffin embedded tissues were sliced into 4 μm sections and stained with hematoxylin and eosin (H&E). To quantify kidney injury, we used a semi-quantitative score for renal tubular injury as described (17), which is commonly used for assessing kidney injury in murine septic models (18, 19). Briefly, two trained observers, blinded to the experimental conditions, analyzed each slide and evaluated the extent of tubular pathology, including the presence of tubular casts, tubular dilatation/flattening, and tubular degeneration/vacuolization. The observers assigned a score graded from 0 (Normal, less than 5 % tubules altered, in three random microscopy fields), 1 (Mild, 5–33 %), 2 (Moderate, 34–66 %), to 3 (Severe, more than 66 %). We used a similar scale for evaluating liver tissue sections, for which we quantified the extent of degeneration or edematous changes of hepatocytes, infiltration of inflammatory cells in the portal area, and spotty necrosis, as previously described (20, 21).
Immunohistology for megalin expression in the proximal tubules
Kidney sections were de-paraffinized by two changes of xylene (5 min each) and washed with descending concentrations of ethanol (100 % x 2, 90 %, 70 %, 50 %, 30 %, 5 min each wash) before transferring the sections to PBS. Sections were washed in PBS for 15 min, treated with 1 % SDS (4 min) for antigen retrieval (22), washed with PBS (3 × 5 min), and blocked with 1 % BSA for 30 min. Primary antibody against megalin (rabbit-polyclonal; a generous gift from Dr. Daniel Biemesderfer, Yale University School of Medicine) was diluted in DAKO antibody diluent (DAKO, Carpinteria, CA) and applied to the tissue overnight at 4 °C. Following incubation, tissues were washed with PBS (3 × 5 min). Secondary antibody raised in goat, conjugated with alexa fluor 488 (Jackson Immuno Research Laboratories, West Grove, PA), was diluted in DAKO (1:200) before applying for 45 min at room temperature. Slides were cover-slipped using Vectashield (Vector Laboratories, Burlingame, CA) and analyzed using a Nikon A1R confocal microscope. For quantification, the mean intensity of each tubular region was measured using NIS Elements software (Nikon). For this, 5 proximal tubules per image were outlined manually, in 3 images per animal (i.e., ~15 tubules per animal), and the mean fluorescence intensity of the outlined area was determined for each tubule. An average intensity was calculated for all images from the same animal, and then for all the animals in the same group.
Immunohistochemistry for Neutrophil extracellular traps (NETs)
To study the neutrophil extracellular traps (NETs) in tissues, we relied on immunohistochemistry. After de-waxing, the tissues were washed with Tris-Buffered Saline (TBS, Abcam, Cambridge, MA) (3 × 5 min) and blocked with 1 % BSA for 1 hour. Each tissue was stained with PL2-6 antibodies (specific for histone and DNA (23, 24)). To improve the performance of the antibodies and signal to background ratios, we employed both ultra sensitive avidin-biotin-peroxidase complex (ABC) mouse IgG staining kit (Thermo Scientific, Rockford, IL) and the tyramide signal amplification, using the TSA plus cyanine 3 kit (PerkinElmer, Waltham, MA). TSA amplification reagent was diluted 1:50 in TSA diluent. To further verify the neutrophil origin of the detected extracellular chromatin, we performed double staining, using Anti-Neutrophil Elastase Antibody (Abcam) and Goat Anti-Rabbit IgG H&L Alexa Flour® 488 antibody (Abcam). After staining, we mounted the slides using Vectashield HardSet mounting medium with DAPI (Vector Laboratories) and checked for the presence of NETs in the tissue.
Statistical analysis
Experimental data was expressed as mean and standard deviation of the mean (SD). The number of animals in each experiment and number of experimental repeats is presented in figure captions. Statistical tests performed include One-way ANOVA, Two–way ANOVA with repeated measure, and Multiple Comparison tests. Differences were determined to be significant for p values less than or equal to 0.05 (in the figures *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001).
Results
Resolvin D2 treatment improves survival at 24 hours, after burn and endotoxin
We monitored and treated the animals in the burn and sham-burn groups for up to 11 days (Fig. 1A). Two animals in the burn-untreated group (20 %) died within 24 hours after the second septic insult (N=10 rats, N=7 experiments). All animals in the burn and RvD2 treatment groups (N=7 rats, N=4 experiments), as well as sham burn treated (N=3 rats, N=2 experiments) and untreated groups (N=3 rats, N=3 experiments) survived over 24 hours after LPS injection. The survival rate in the current experiments was higher compared to our previous experiments (13), likely due to optimization of the general anesthesia protocol in this study. However, the rapid deterioration of health and subsequent death in less than 24 hours, prompted us to consider the possibility that one or more vital organs was being affected in this double insult model.
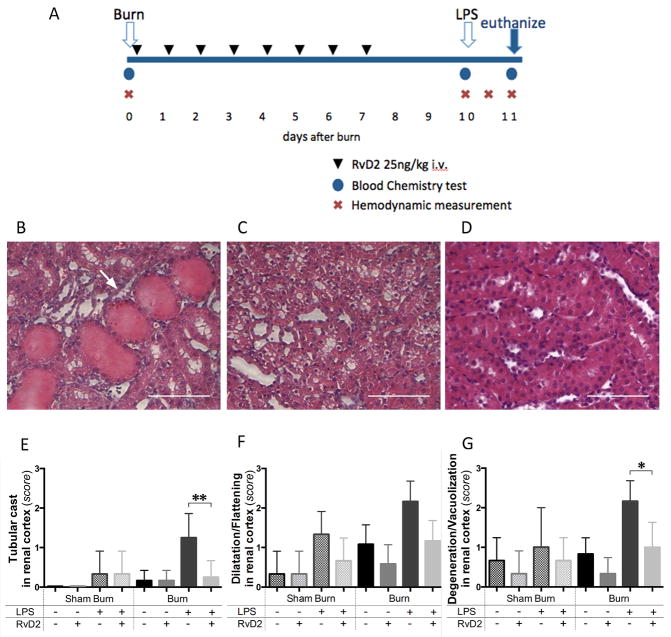
Figure 1. Timeline of the burn injuries, treatment, and renal cortex injury.
A. Blood samples were collected before the burn procedure (day 0), before injecting LPS (day 10 pb), and 24 hours after LPS (day 11 pb). Hemodynamic measurements were also performed at the same time points. One additional measurement was performed at 12 hours after LPS injection. For animals receiving treatment, 25 ng/kg of RvD2 were administered first at 2 hours after burn injury (day 0) and continued daily until day 7 after burn. B, C. Representative section of renal cortex at day 11 pb, in burn and LPS, untreated group. Tubular injuries were observed, including tubular cast formation (arrows), and ballooning degeneration/vacuolization of renal proximal tubular cells (H&E, ×400, scale bar 50 μm). D. Light microscopic image at day 11 pb in burn and LPS, RvD2 treated group, indicated that tubular injury was reduced by RvD2 treatment in renal cortex (H&E, ×400, scale bar 50 μm). E – G. Quantitative analysis of pathological changes in the renal cortex (N=3 rats total, N=2–3 experiments for each sham burn group; N=6 rats total, N=3–4 experiments for each burn group). We employed semi-quantitative scores (from 0 (normal) to 3 (severe)), as previously described (17)). We assessed the presence of tubular casts, tubular dilatation/flattening, and tubular degeneration/vacuolization in the renal cortex. In all comparisons, RvD2 treatment reduced the pathological changes of tubular cells in renal cortex (Burn/LPS vs. Burn/LPS/RvD2; **p≤0.01, ns, and *p≤0.05, respectively).
Resolvin D2 treatment reduces secondary organ injury after burn and endotoxin
We identified pathological changes in the kidneys of rats with burn injury. These changes included the presence of casts, tubular dilatation, and ballooning degeneration of proximal tubules in the renal cortex and were more significant in animals receiving LPS compared to the other burn groups (Fig. 1B, C). Kidneys from rats treated with RvD2 after burn and LPS injection displayed significantly fewer pathological changes compared with the non-treated rats (Fig. 1D). We measured significant reductions in proximal tubules cast formation, tubular dilatation, and ballooning degeneration (p≤0.01, ns, and p≤0.05, respectively, N=6 rats, N=3–4 experiments), indicating that RvD2 treatment attenuates tubular injuries (Fig. 1E–G). The pathological changes in the kidney, in the burn only group and sham burn with LPS injection group were also ameliorated by RvD2 treatment. We did not find signs of tissue necrosis in any of the groups. The glomeruli and distal tubules showed no changes after burn and LPS insults.
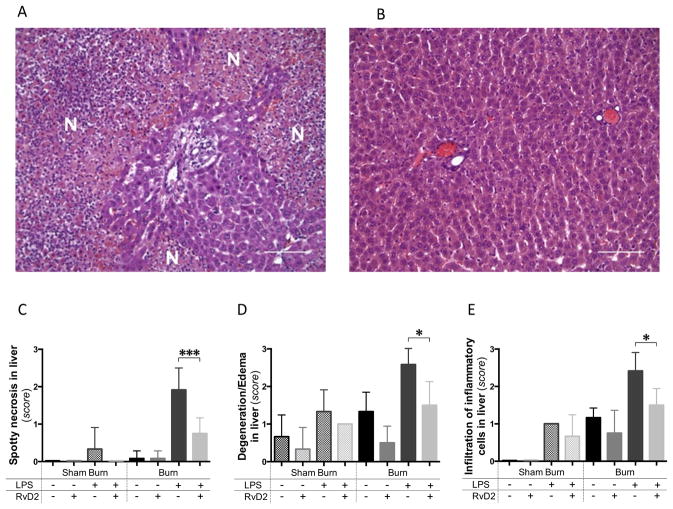
Main pathological changes in the liver were in the form of hepatic tissue necrosis and infiltration of inflammatory cells in the peri-portal area. These changes were observed with the highest frequency in the burn and LPS groups (Fig. 2A). The diffuse necrosis occurred in almost 50 % of the hepatocytes in the burn and LPS groups. The injury was significantly diminished in the RvD2 treated group (p≤0.001, N=6 rats, N=3–4 experiments) (Fig. 2B, C). Ballooning degeneration or edematous changes occurred predominantly in rats with burns and LPS, and were suppressed in the RvD2 treated group (p≤0.05, N=6 rats, N=3–4 experiments) (Fig. 2D). Neither the burn injury alone, nor the LPS alone resulted in significant necrotic damage of the liver tissue. Pathological change scores for the kidney and hepatic tissue damages were similar in burn-only group and the LPS injection in sham burn group. We did not observe any significant pathological changes in lung tissues from rats with burns and LPS compared to healthy controls (data not shown).
Figure 2. Pathology of the liver after burn injury and endotoxin injection with and without Resolvin D2 treatment.
A. Spotty necrosis of the liver at day 11 pb, after burn and LPS, untreated group (24 hours after LPS injection). Several necrotic foci (N) were identified in the liver tissue sections, in the burn and LPS group. Large numbers of inflammatory cells, including neutrophils, macrophages, and lymphocytes infiltrated the portal area (H&E, ×200, scale bar 100 μm). B. Representative section of liver at day 11 pb, in the burn and LPS with RvD2 treatment groups. Spotty necrotic changes were significantly diminished in the RvD2 treatment group (H&E, ×200, scale bar 100 μm). C – E. Quantitative analysis of pathological changes in the liver (N=3 rats total, N=2–3 experiments for each sham burn group; N=6 rats total, N=3–4 experiments for each burn group). We quantified the spotty necrosis, degeneration or edematous changes of hepatocytes, and infiltration of inflammatory cells in the portal area. RvD2 treatment suppressed these impaired pathological changes in liver (Burn/LPS vs. Burn/LPS/RvD2; *p≤0.05). In particular, RvD2 treatment reduced spotty necrotic changes in hepatocytes (Burn/LPS vs. Burn/LPS/RvD2; ***p≤0.001).
Hemodynamic changes in burn groups
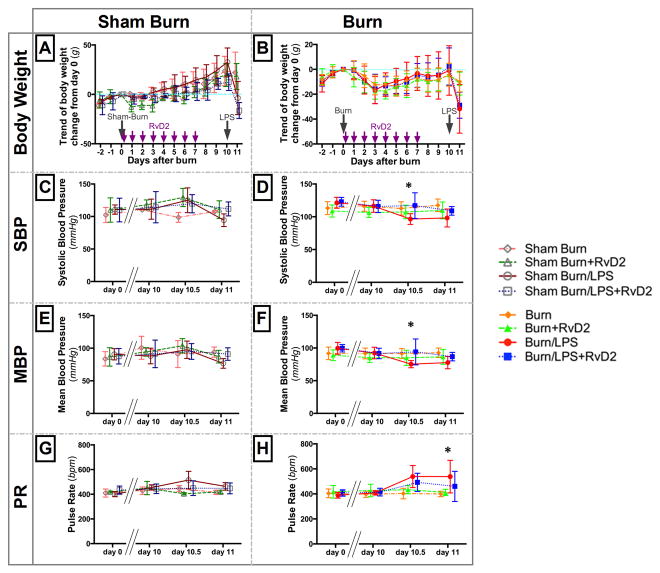
Recent studies suggested that AKI could develop despite adequate resuscitation after burn injury (2). To verify that the kidney and liver pathologies seen after burn and LPS are not secondary of hemodynamic changes, we measured blood pressure and pulse rate before and after the two injuries. The systolic blood pressure decreased on average by 20 % in untreated animals after injecting LPS (day 10.5 pb, day 11 pb) compared to their blood pressure before burns (day 0) and before endotoxin (day 10 pb) (Fig. 3D). We found significant differences between untreated and treated groups after injecting LPS, at day 10.5 pb (SBP: 96.5±8.1 vs. 117.0±19.5 mmHg, p≤0.05; MBP: 75.3±5.5 vs. 94.0±19.9 mmHg, p≤0.05, N=6 rats, N=3–4 experiments). However, the Mean Blood Pressure (MBP) was over 65 mmHg throughout the study in all groups, suggesting that organ perfusion was adequate throughout the experiment (Fig. 3E, F). Moreover, the pulse rate (PR) in the burn and LPS group was significantly higher after the injection of LPS (538±130 bpm, N=6 rats, N=4 experiments) compared to the RvD2 treated group (460±120 bpm, p≤0.05, N=6 rats, N=3 experiments) (Fig. 3H). In the sham burn group, the measurements did not show any significant hemodynamic changes throughout this study (Fig. 3C, E, G).
Figure 3. Hemodynamic changes in rats after burns and endotoxin, in treated and untreated groups.
A, B. Body weight change in sham burn (A) and burn groups (B). Rats in sham burn groups lost few % of total body weight in the first 1 or 2 days due to general anesthesia, and recovered quickly by day 4 pb. Burned rats (all) lost about 5 % of total body weight in the 3 or 4 days post burn injury, and recovered at 10 days after burn. The body weight loss was comparable in treated and untreated groups (orange and green lines). After LPS injection, both RvD2 treated (blue) and untreated (red) groups showed comparable body weight loss. C–F. Systolic and mean blood pressure in sham burn (C, E) and burn groups (D, F). We monitored hemodynamic changes using non-invasive tail cuff measurement at four points, day 0 (pre burn), day 10 pb (before LPS), and then at 12 (day10.5 pb) and 24 (day 11 pb) hours after the LPS injection. After LPS the systolic blood pressure (SBP) and the mean blood pressure (MBP) decreased slightly in untreated groups. Differences between untreated and treated rats were significant at 12 hours after LPS (Burn/LPS vs. Burn/LPS/RvD2; *p≤0.05). It is important to note that MBP was maintained above 65 mmHg for all animals throughout the study. G, H. Pulse rate (PR) increased after LPS, more in untreated than in RvD2 treated group (H). The difference between untreated and treated animals increased at 24 hours after endotoxin in burn and LPS groups (Burn/LPS vs. Burn/LPS/RvD2; *p≤0.05). Hemodynamic condition, including SBP, MBP, and PR, did not change significantly in all sham burn groups (A–H: N=3 rats total, N=2–3 experiments for each sham burn group; N=6 rats total, N=3–4 experiments for each burn group).
Body weight changes
Burn group rats lost 5 % of body weight in the first 3 days after the 30 % TBSA burn injury, and recovered by day 10 pb (Fig. 3B). Rats in sham burn groups also lost their weight, likely due to general anesthesia at day 0, and recovered by day 4 pb (Fig. 3A). There were no significant differences between the weight in untreated and RvD2 treated groups throughout our experiments. The weight loss was much larger in groups receiving LPS; up to 10 % of total body weight, regardless of burn or treatment.
Blood chemistry changes after burn and endotoxin are ameliorated by Resolvin D2 treatment
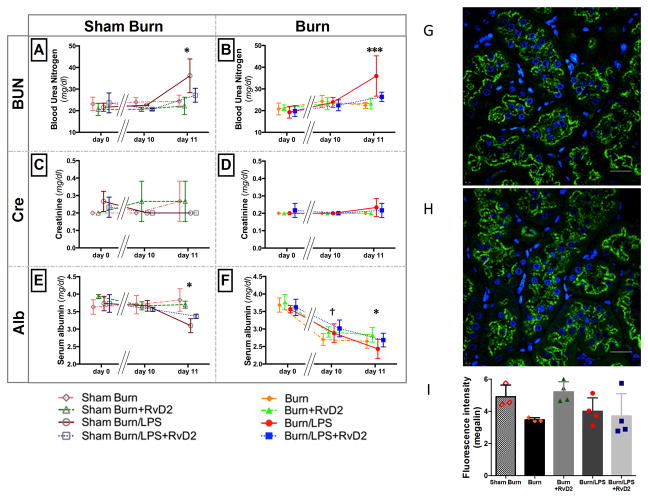
To probe the systemic effect of the pathological changes in the kidney, we compared blood urea nitrogen (BUN) and serum creatinine at three points, day 0 (before burn), day 10 pb (pre injecting LPS), and day 11 pb (24 hours after injecting LPS). Blood urea nitrogen (BUN) in rats with burns increased after LPS injection (Fig. 4B), and the increase was significantly more prominent in the non-treatment group (36.0±9.3 mg/dl, N=6 rats, N=4 experiments) compared with in RvD2 treated group (26.4±2.1 mg/dl, p≤0.001, N=6 rats, N=3 experiments). Interestingly, BUN in sham burn rats increased after LPS injection (Fig. 4A). However, in all groups, serum creatinine values did not change significantly in the 24 hours after the injection of LPS (Fig. 4C, D). Serum albumin values were significantly lower in both severe burn and second septic insult untreated groups, compared to the RvD2 treated rats (†: Burn vs. Burn/RvD2, 2.7±0.2 vs. 2.9±0.1 g/dl, p≤0.05; *: Burn/LPS vs. Burn/LPS/RvD2, 2.4±0.3 vs. 2.7±0.2 g/dl, p≤0.05, N=6 rats, N=3–4 experiments) (Fig. 4F). In sham burn groups, levels of serum albumin were preserved during the treatment period, and decreased sharply after LPS injection (Fig. 4E). These changes are consistent with dysfunction of kidney (filtration or reabsorption) and liver (synthesis). They are unlikely to represent losses at the wound site, which was maintained dry throughout the experiments. Overall, the RvD2 treatment prevented the deterioration of blood chemistry relevant to renal and hepatic functions after major burns and LPS injection.
Figure 4. Blood chemistry changes for renal function and Megalin staining of renal proximal tubules.
Blood chemistry tests were performed at day 0 (pre burn), day 10 pb (before LPS), and day 11 pb (24 hours after LPS) (A–F: N=3 rats total, N=2–3 experiments for each sham burn group; N=6 rats total, N=3–4 experiments for each burn group). A, B. The renal function marker, blood urea nitrogen (BUN), increased significantly after LPS injection, and restored by RvD2 pre treatment (A: Sham Burn/LPS vs. Sham Burn/LPS/RvD2; *p≤0.05). In burned rats, BUN increased more significantly after the second septic insult (Burn/LPS vs. Burn/LPS/RvD2; ***p≤0.001). C, D. Serum creatinine values did not change in any of the groups. E, F. In sham burn groups, serum albumin values were preserved during treatment period, and decreased after LPS, in both treated and untreated groups. The decrease in serum albumin after LPS was ameliorated by RvD2 treatment (E: Sham Burn/LPS vs. Sham Burn/LPS/RvD2; *p≤0.05). On the other hand, levels of serum albumin decreased after burn and even further after LPS, in both treated and untreated groups. The decrease in serum albumin after burn and burn and LPS was also ameliorated by RvD2 treatment (F: Burn vs. Burn/RvD2; †p≤0.05, Burn/LPS vs. Burn/LPS/RvD2; *p≤0.05). G, H. Representative images of renal proximal tubules at day 11 pb in burn only group (G) and burn + RvD2 treatment group (H). Green fluorescence shows megalin, and the blue fluorescence shows the nuclei. Megalin is localized in the apical brush border region of the proximal tubule. Staining intensity after burn injury is higher in the RvD2 treated group. Scale bars, 50 μm. I. Quantitative analysis of fluorescence intensity of megalin staining. Megalin intensity decreased after burn and LPS groups, and was restored in the burn and RvD2 treatment group, but not in the burn and LPS and RvD2 group (Sham Burn and Burn groups N=3 rats total; all other groups N=4 rats total).
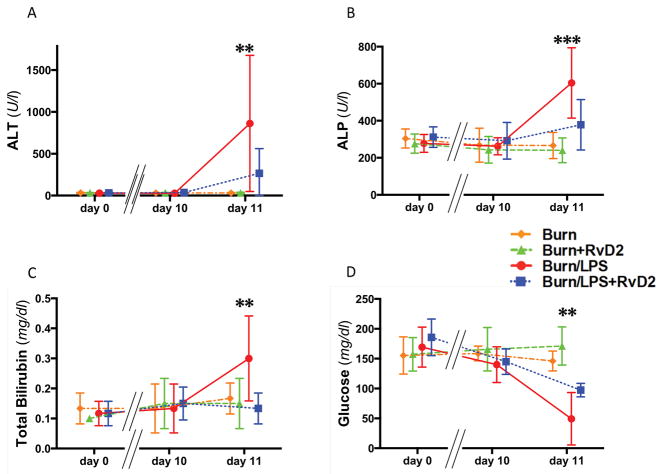
To probe the systemic effect of the pathological changes in the liver, blood biochemistry relevant to liver function was also monitored along the course of experiment. We compared alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin, and glucose levels at three points, day 0 (before burn), day 10 pb (pre injecting LPS), and day 11 pb (24 hours after injecting LPS). Serum ALT and ALP values increased after injecting LPS in the burn without treatment group compared with the treated group (ALT: 861.8±813.7 vs. 266.5±295.2 U/l, p≤0.01; ALP: 604.2±190.0 vs. 378.2±135.9 U/l, p≤0.001, N=6 rats, N=3–4 experiments) (Fig. 5A, B). Serum total bilirubin values, which are used for evaluating multiple organ failure in SOFA score (25) and MODS score (26), increased significantly after injecting LPS in the non-treated group (0.30±0.14 mg/dl, N=6 rats, N=4 experiments) compared with the RvD2 treated group (0.13±0.05 mg/dl, p≤0.01, N=6 rats, N=3 experiments) (Fig. 5C). Interestingly, we also found that in the untreated group, blood glucose values decreased significantly after injecting LPS (49.3±43.9 mg/dl, N=6 rats, N=4 experiments), but remained close to normal in the corresponding RvD2 treated group (97.3±11.2 mg/dl, p≤0.01, N=6 rats, N=3 experiments) (Fig. 5D).
Figure 5. Blood chemistry changes for hepatic function in rats after burns and endotoxin, in treated and untreated groups.
A – C. After LPS, as second septic insult post burn, we measured significant increases in serum ALT, ALP, and total bilirubin at day 11 pb (Burn/LPS vs. Burn/LPS/RvD2; **p≤0.01, ***p≤0.001, and **p≤0.01, respectively). Changes were significantly reduced in the RvD2 treated group. D. Levels of blood glucose were significantly depressed after LPS injection in the untreated vs. treated group (Burn/LPS vs. Burn/LPS/RvD2; **p≤0.01) (A–D: N=6 rats total, N=3–4 experiments for each burn group).
Megalin expression levels in the proximal tubules
We stained proximal tubules for megalin - a 600-kDa transmembrane receptor protein that is involved in the proximal tubular reabsorption of proteins, hormones and other components of the glomerular ultrafiltrate (27). We quantified megalin expression levels and found reduced expression after burn injury compared to sham burn rats. Levels of megalin expression after burn injury retuned to control values in the RvD2 treated group (Fig. 4G, H). These results are consistent with a reduction of reabsorbing function in proximal tubules after severe burn injury. However, we did not detect any differences in the burn and endotoxin groups, regardless of RvD2 treatment (Fig. 4I). This is consistent with previous reports that LPS can act directly on the rat proximal tubular cells and directly alter the expression of megalin (28).
Plasma genomic DNA values after burn and endotoxin are lower in the Resolvin D2 treated rats
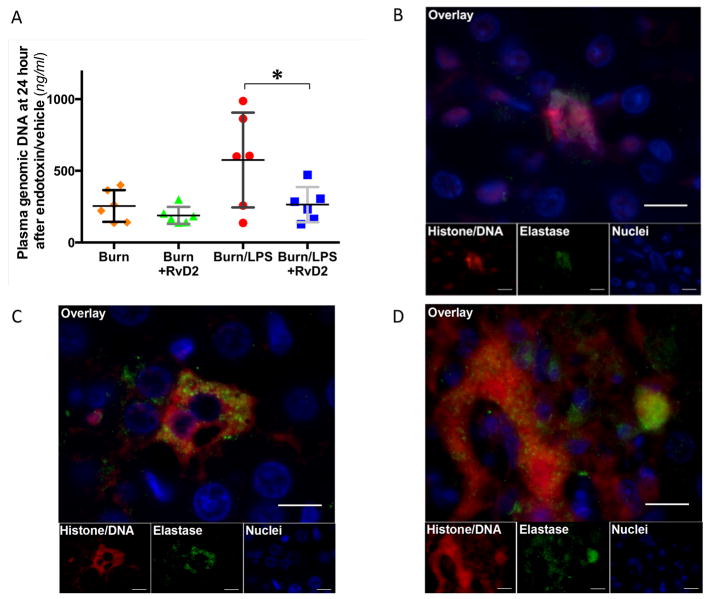
To investigate the possible mechanisms responsible for the observed pathology, we focused on the release of neutrophil extracellular traps from overly-activated neutrophils. We measured the changes in the plasma levels of genomic DNA and found lower values at day 0 before burn procedure (average 83.9±31.5 ng/ml) compared to 6 hours post burn injury (565.5±213.5 ng/ml, N=2 rats). Plasma DNA levels decreased to 254.6±110.9 ng/ml (N=6 rats) at day 11 pb in the burn-untreated group (N=19 rats, Fig. 6A). RvD2 treatment suppressed the early increase in the burn only group (188.7±59.6 ng/ml, ns, N=6 rats, N=4 experiments). Plasma genomic DNA values increased to significantly higher levels in the burn and LPS without treatment group (575.1±331.0 ng/ml, N=6 rats, N=4 experiments), and were less affected by the RvD2 treatment (264.1±122.4 ng/ml, p≤0.05, N=6 rats, N=3 experiments).
Figure 6. Neutrophil Extracellular Traps and Plasma genomic DNA values 24 hours after endotoxin.
A. Plasma genomic DNA values at day 11 pb (24 hours after injecting 2 mg/kg LPS) in burn groups increase after the second septic insult (N=6 rats total, N=3–4 experiments for each burn group). Administering RvD2 suppresses the increase in plasma genomic DNA (Burn/LPS vs. Burn/LPS/RvD2; *p≤0.05). B. Fluorescence microscopic detection of NETs in renal cortex at day 11 pb in burn and LPS group. NETs are identified by fluorescence from histone/DNA staining (red) and neutrophil elastase (green). Nuclei of intact parenchymal cells are identified by cell-permeant DAPI staining (blue). NETs appear to be located outside of proximal tubular cells in the renal cortex. Scale bar, 10 μm. C. Immunohistochemistry section of hepatic tissues at day 11 pb in burn and LPS groups. High fluorescence intensity, corresponding to the presence of NETs can be observed outside of the hepatocyte in burn and LPS without treatment groups. Scale bar, 10 μm. D. Representative section of hepatic focal necrosis at day 11 pb in burn and LPS groups (immunohistochemistry). High fluorescence intensity of histone/DNA and elastase, corresponding to large amounts of NETs can be observed at area of necrosis. Scale bar, 10 μm.
The amount of neutrophil extracellular traps (NETs) present in the kidney and liver after burn and endotoxin is reduced in Resolvin D2 treated animals
In the burn and LPS without treatment groups, we identified extracellular histone/DNA complexes (by the PL2-6 antibody) in the renal cortex, outside of the proximal tubular cells (Fig. 6B). These are likely to be derived from neutrophils, based on the co-localization of neutrophil elastase and chromatin. The area of tissue occupied by the NETs in the cortex was significantly greater in untreated compared to RvD2 treated animals with burn and LPS insults. In liver tissue, we found high fluorescence intensity corresponding to the presence of NETs between hepatocytes (Fig. 6C). Moreover, we could find the large amounts of neutrophil elastase colocalized in the necrosis area (Fig. 6D). The accumulation of NETs in the liver was significantly reduced in the RvD2 treatment group compared with the non-treatment group and correlated with lower amount of liver tissue damage.
Discussion
In this study, we show that resolvin D2 can ameliorate kidney and liver injury in a rat model that combines major burn and LPS insults. Using this model, we document the presence of histological changes in the renal cortex and liver, and measure the deterioration of blood chemistry reflecting the acute dysfunction of these organs. We found that the burn injury and LPS together have a synergistic effect on distant tissue damage, which appears to be larger than either one insult alone. For example, we observed significant damage to the liver in the double hit model, whereas neither the burn injury nor the LPS alone result significant necrotic damage in liver. We demonstrate that these changes could be prevented by the administration of RvD2 starting at 2 hours after burn injury, once a day for more than one week. The RvD2 treatment is most potent in the double hit model, when RvD2 administered in the time between the thermal injury and LPS insult appears to be disrupting the synergy between the two insults.
Lipopolysaccharide (LPS) injection is a common model for the study of innate immune response during infections, and serves as a surrogate for an infection with gram negative bacteria (29). The features of this model are relevant to the common clinical situation when an infection develops in patients with major burn injury. The LPS injection model is useful for stimulating systemic innate immune responses (30), and avoids the local complications and variability of using live microbes. Moreover, in one recent study study, LPS injection and Cecum Ligation procedures, yielded comparable survival trends and cytokine levels after burn injury (13). Consequently, in this study, we used LPS for the second septic insult after major burn.
Several studies have demonstrated the restorative ability of resolvins in various other conditions besides burns (13, 31), which include sepsis (32), inflammatory pain (33), colitis (34), ischemic kidney injury (14), and vascular injury (35). A common denominator for these actions was the restoration of neutrophil functionality (15, 16) and innate immune homeostasis (14–16, 31–38). Consistent with previous studies, the dose of RvD2 administration in this study was based on previous optimizations of the amount, duration, and types of resolvin that restore neutrophil functionality (13). It is important to emphasize that one of the implications of restored neutrophil activity is better protection against infections and that RvD2 was reported to enhance the anti-bacterial function in neutrophils (13, 32). Moreover, long-term studies involving RvD2 administration have been reported in some disease models (33, 35), suggesting that the beneficial effect on neutrophils could be maintained over time. Interestingly, the amount of RvD2 involved in this study is in the nanogram range, suggesting that the compound may be acting on specific targets, yet to be identified (39–41). Together, the results of current and previous studies, converge towards RvD2 being an ideal candidate for the restoration of immune system responses in the context of the systemic inflammatory responses after major burns (42–44).
Our histology findings suggest that chromatin derived from neutrophils as NETs may contribute to the complications of vital organ dysfunction after major burn. Overly active neutrophils have been proposed as potential link between the systemic inflammation and secondary organ injury after major burns, supported by genomic (45) and functional measurements (46). Over-activated neutrophils can release their chromatin as NETs (12, 47–51), and results in significant tissue damage (52) (e.g. in cystic fibrosis (53), lupus and associated nephritis (54), or acute respiratory distress syndrome (55)). Higher concentrations of chromatin in the circulation has been previously documented in burn and other critically ill patients (56–59), supporting the relevance of our model for human disease. Previous studies have suggested that the presence of MPO and elastase in tissues may be responsible for organ and tissue damages (60). Our finding that RvD2 treatment reduces the amount of NETs in vital organs provides new support for a mechanism involving the over-activation of neutrophils. This possibility is further substantiated by the beneficial effects of the normalization of neutrophil functionality by RvD2 after burn and endotoxin insults, measured previously using microfluidic devices (13). Future studies to understand the mechanisms by which RvD2 reduces the release of NETs may help develop effective therapies to reduce kidney and liver dysfunction in burn patients.
One more unexpected result from our study is the finding that RvD2 treatment could prevent the lower glycemia levels which would otherwise occur after LPS injection. Hyper metabolism is a prominent feature of major burn injury, with two fold increases in resting energy expenditure and a more than 50 % increase in gluconeogenesis (61). Part of the beneficial effect of RvD2 treatment could be explained by the reduction in damage to liver tissue, but other mechanisms may be involved as well (62). One example is the reductions of the stress and pain in the treated group, a possibility that will require further investigation.
The relevance of our findings from the animal models to patients is worth discussing in light of recent analysis in humans which has documented the presence of significant levels of endogenously produced RvD2 in healthy individuals (63). Moreover, recent studies in trauma patients suggest that higher levels of lipid mediators in human patients correlate with less complications after trauma (64). Together, the previous studies and current findings converge on the possibility that neutrophils participate in the distal tissues damage. They also raise the possibility that new therapies involving the lipid mediators of inflammation resolution, which showed benefit in relevant animal models (13, 32, 65, 66), could eventually be effective in patients after major burn or trauma injuries.
In conclusion, our study shows that the beneficial effect of RvD2 after burns and endotoxin insults could be explained by the prevention of acute kidney and liver injuries, potentially mediated by the reduction in the accumulation of NETs in kidney and liver.
Acknowledgments
Financial Support: This work was supported in part by funds from Shriners Hospital for Children and U.S. National Institutes of Health grants GM-092804 (DI), DK042956 (DB), DK082782 and DK097443 (AV). Additional support for the Program in Membrane Biology Microscopy Core comes from the Boston Area Diabetes and Endocrinology Research Center (DK57521) and the MGH Center for the Study of Inflammatory Bowel Disease (DK43351).
We would like to thank Drs. Takeshi Yamagiwa and Hassan Albadawi, and Ms. Florence Lin for help with the blood pressure measurements in rats.
Footnotes
Work was performed at Massachusetts General Hospital and Shriners Hospital for Children, Boston, MA, USA
Copyright form disclosures: Dr. Irimia received support for article research from the National Institutes of Health (NIH) and Shriners Hospitals. His institution received funding from the NIH. Dr. Inoue received support for article research from the NIH, the Boston Area Diabetes and Endocrinology Research Center, and the MGH Center for the Study of Inflammatory Bowel Disease. He disclosed off-label product use (resolvin d2). Drs. Yu, Zhao, Nair, and Tompkins received support for article research from the NIH. Dr. Brown received support for article research from the NIH and received funding from Exosome Diagnostics. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Mustonen KM, Vuola J. Acute renal failure in intensive care burn patients (arf in burn patients) Journal of burn care & research: official publication of the American Burn Association. 2008;29:227–237. doi: 10.1097/BCR.0b013e31815f3196. [DOI] [PubMed] [Google Scholar]
- 2.Mosier MJ, Pham TN, Klein MB, et al. Early acute kidney injury predicts progressive renal dysfunction and higher mortality in severely burned adults. Journal of burn care & research: official publication of the American Burn Association. 2010;31:83–92. doi: 10.1097/BCR.0b013e3181cb8c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmieri T, Lavrentieva A, Greenhalgh DG. Acute kidney injury in critically ill burn patients. Risk factors, progression and impact on mortality. Burns: journal of the International Society for Burn Injuries. 2010;36:205–211. doi: 10.1016/j.burns.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Kallinen O, Maisniemi K, Bohling T, et al. Multiple organ failure as a cause of death in patients with severe burns. Journal of burn care & research: official publication of the American Burn Association. 2012;33:206–211. doi: 10.1097/BCR.0b013e3182331e73. [DOI] [PubMed] [Google Scholar]
- 5.Chung KK, Juncos LA, Wolf SE, et al. Continuous renal replacement therapy improves survival in severely burned military casualties with acute kidney injury. The Journal of trauma. 2008;64:S179–185. doi: 10.1097/TA.0b013e3181608676. discussion S185–177. [DOI] [PubMed] [Google Scholar]
- 6.Jeschke MG, Micak RP, Finnerty CC, et al. Changes in liver function and size after a severe thermal injury. Shock. 2007;28:172–177. doi: 10.1097/shk.0b013e318047b9e2. [DOI] [PubMed] [Google Scholar]
- 7.Diao L, Marshall AH, Dai X, et al. Burn plus lipopolysaccharide augments endoplasmic reticulum stress and nlrp3 inflammasome activation and reduces pgc-1alpha in liver. Shock. 2014;41:138–144. doi: 10.1097/SHK.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tadros T, Traber DL, Herndon DN. Hepatic blood flow and oxygen consumption after burn and sepsis. The Journal of trauma. 2000;49:101–108. doi: 10.1097/00005373-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. The Journal of experimental medicine. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazeldine J, Hampson P, Lord JM. The impact of trauma on neutrophil function. Injury. 2014;45:1824–1833. doi: 10.1016/j.injury.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Clark SR, Ma AC, Tavener SA, et al. Platelet tlr4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nature medicine. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 12.Meng W, Paunel-Gorgulu A, Flohe S, et al. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit Care. 2012;16:R137. doi: 10.1186/cc11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurihara T, Jones CN, Yu YM, et al. Resolvin d2 restores neutrophil directionality and improves survival after burns. Faseb J. 2013;27:2270–2281. doi: 10.1096/fj.12-219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffield JS, Hong S, Vaidya VS, et al. Resolvin d series and protectin d1 mitigate acute kidney injury. Journal of immunology. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 15.Kasuga K, Yang R, Porter TF, et al. Rapid appearance of resolvin precursors in inflammatory exudates: Novel mechanisms in resolution. Journal of immunology. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mas E, Croft KD, Zahra P, et al. Resolvins d1, d2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clinical chemistry. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 17.Nomura A, Nishikawa K, Yuzawa Y, et al. Tubulointerstitial injury induced in rats by a monoclonal antibody that inhibits function of a membrane inhibitor of complement. The Journal of clinical investigation. 1995;96:2348–2356. doi: 10.1172/JCI118291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham PN, Dyanov HM, Park P, et al. Acute renal failure in endotoxemia is caused by tnf acting directly on tnf receptor-1 in kidney. The Journal of Immunology. 2002;168:5817–5823. doi: 10.4049/jimmunol.168.11.5817. [DOI] [PubMed] [Google Scholar]
- 19.Guo R, Wang Y, Minto AW, et al. Acute renal failure in endotoxemia is dependent on caspase activation. Journal of the American Society of Nephrology: JASN. 2004;15:3093–3102. doi: 10.1097/01.ASN.0000145530.73247.F5. [DOI] [PubMed] [Google Scholar]
- 20.Oz HS, Chen TS, Neuman M. Nutrition intervention: A strategy against systemic inflammatory syndrome. JPEN Journal of parenteral and enteral nutrition. 2009;33:380–389. doi: 10.1177/0148607108327194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oz HS, McClain CJ, Nagasawa HT, et al. Diverse antioxidants protect against acetaminophen hepatotoxicity. Journal of biochemical and molecular toxicology. 2004;18:361–368. doi: 10.1002/jbt.20042. [DOI] [PubMed] [Google Scholar]
- 22.Brown D, Lydon J, McLaughlin M, et al. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (sds) Histochemistry and cell biology. 1996;105:261–267. doi: 10.1007/BF01463929. [DOI] [PubMed] [Google Scholar]
- 23.Losman MJ, Fasy TM, Novick KE, et al. Monoclonal autoantibodies to subnucleosomes from a mrl/mp(−)+/+ mouse. Oligoclonality of the antibody response and recognition of a determinant composed of histones h2a, h2b, and DNA. Journal of immunology. 1992;148:1561–1569. [PubMed] [Google Scholar]
- 24.Kramers K, Stemmer C, Monestier M, et al. Specificity of monoclonal anti-nucleosome auto-antibodies derived from lupus mice. Journal of autoimmunity. 1996;9:723–729. doi: 10.1006/jaut.1996.0094. [DOI] [PubMed] [Google Scholar]
- 25.Vincent JL, Moreno R, Takala J, et al. The sofa (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive care medicine. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 26.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Critical care medicine. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Mahadevappa R, Nielsen R, Christensen EI, et al. Megalin in acute kidney injury: Foe and friend. American journal of physiology Renal physiology. 2014;306:F147–154. doi: 10.1152/ajprenal.00378.2013. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber A, Theilig F, Schweda F, et al. Acute endotoxemia in mice induces downregulation of megalin and cubilin in the kidney. Kidney international. 2012;82:53–59. doi: 10.1038/ki.2012.62. [DOI] [PubMed] [Google Scholar]
- 29.Wessels BC, Wells MT, Gaffin SL, et al. Plasma endotoxin concentration in healthy primates and during e. Coli-induced shock. Critical care medicine. 1988;16:601–605. doi: 10.1097/00003246-198806000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: Endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohr S, Patel SJ, Sarin D, et al. Resolvin d2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spite M, Norling LV, Summers L, et al. Resolvin d2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park CK, Xu ZZ, Liu T, et al. Resolvin d2 is a potent endogenous inhibitor for transient receptor potential subtype v1/a1, inflammatory pain, and spinal cord synaptic plasticity in mice: Distinct roles of resolvin d1, d2, and e1. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bento AF, Claudino RF, Dutra RC, et al. Omega-3 fatty acid-derived mediators 17(r)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin d1 and resolvin d2 prevent experimental colitis in mice. Journal of immunology. 2011;187:1957–1969. doi: 10.4049/jimmunol.1101305. [DOI] [PubMed] [Google Scholar]
- 35.Miyahara T, Runge S, Chatterjee A, et al. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2013;27:2220–2232. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serhan CN, Hong S, Gronert K, et al. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. Journal of Experimental Medicine. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuhofer A, Zeyda M, Mascher D, et al. Impaired local production of proresolving lipid mediators in obesity and 17-hdha as a potential treatment for obesity-associated inflammation. Diabetes. 2013;62:1945–1956. doi: 10.2337/db12-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claria J, Dalli J, Yacoubian S, et al. Resolvin d1 and resolvin d2 govern local inflammatory tone in obese fat. Journal of immunology. 2012;189:2597–2605. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnamoorthy S, Recchiuti A, Chiang N, et al. Resolvin d1 receptor stereoselectivity and regulation of inflammation and proresolving micrornas. Am J Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bang S, Yoo S, Yang TJ, et al. 17(r)-resolvin d1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. British journal of pharmacology. 2012;165:683–692. doi: 10.1111/j.1476-5381.2011.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norling LV, Dalli J, Flower RJ, et al. Resolvin d1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: Receptor-dependent actions. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher CJ, Jr, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The soluble tnf receptor sepsis study group. The New England journal of medicine. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 43.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA: the journal of the American Medical Association. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lainée P, Efron P, Tschoeke SK, et al. Delayed neutralization of interferon-γ Prevents lethality in primate gram-negative bacteremic shock. Critical care medicine. 2005;33:797–805. doi: 10.1097/01.ccm.0000159090.80228.57. [DOI] [PubMed] [Google Scholar]
- 45.Kotz KT, Xiao W, Miller-Graziano C, et al. Clinical microfluidics for neutrophil genomics and proteomics. Nature medicine. 2010;16:1042–1047. doi: 10.1038/nm.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones CN, Moore M, Dimisko L, et al. Spontaneous neutrophil migration patterns during sepsis after major burns. PLoS One. 2014;9:e114509. doi: 10.1371/journal.pone.0114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinkmann V, Zychlinsky A. Beneficial suicide: Why neutrophils die to make nets. Nature reviews Microbiology. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 48.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 49.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. The Journal of cell biology. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng W, Paunel-Gorgulu A, Flohe S, et al. Deoxyribonuclease is a potential counter regulator of aberrant neutrophil extracellular traps formation after major trauma. Mediators of inflammation. 2012;2012:149560. doi: 10.1155/2012/149560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altrichter J, Zedler S, Kraft R, et al. Neutrophil-derived circulating free DNA (cf-DNA/nets), a potential prognostic marker for mortality in patients with severe burn injury. European Journal of Trauma and Emergency Surgery. 2010;36:551–557. doi: 10.1007/s00068-010-0013-1. [DOI] [PubMed] [Google Scholar]
- 52.Kessenbrock K, Krumbholz M, Schonermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamutcu R, Rowland JM, Horn MV, et al. Clinical findings and lung pathology in children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165:1172–1175. doi: 10.1164/ajrccm.165.8.2104090. [DOI] [PubMed] [Google Scholar]
- 54.Hakkim A, Furnrohr BG, Amann K, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abrams ST, Zhang N, Manson J, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2012;187:160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keshari RS, Jyoti A, Dubey M, et al. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PloS one. 2012;7:e48111. doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamaguchi S, Hirose T, Akeda Y, et al. Identification of neutrophil extracellular traps in the blood of patients with systemic inflammatory response syndrome. The Journal of international medical research. 2013;41:162–168. doi: 10.1177/0300060513475958. [DOI] [PubMed] [Google Scholar]
- 58.Hirose T, Hamaguchi S, Matsumoto N, et al. Presence of neutrophil extracellular traps and citrullinated histone h3 in the bloodstream of critically ill patients. PloS one. 2014;9:e111755. doi: 10.1371/journal.pone.0111755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McIlroy DJ, Jarnicki AG, Au GG, et al. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. Journal of critical care. 2014;29:1133, e1131–1135. doi: 10.1016/j.jcrc.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 60.Fujimura N, Obara H, Suda K, et al. Neutrophil elastase inhibitor improves survival rate after ischemia reperfusion injury caused by supravisceral aortic clamping in rats. The Journal of surgical research. 2013;180:e31–36. doi: 10.1016/j.jss.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 61.Yu Y, Tompkins R, Ryan C, et al. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. Journal of Parenteral and Enteral Nutrition. 1999;23:160–168. doi: 10.1177/0148607199023003160. [DOI] [PubMed] [Google Scholar]
- 62.Hellmann J, Tang Y, Kosuri M, et al. Resolvin d1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colas RA, Shinohara M, Dalli J, et al. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. American journal of physiology Cell physiology. 2014 doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orr SK, Butler KL, Hayden DL, et al. Gene expression of pro-resolving lipid mediator pathways is associated with clinical outcomes in trauma patients. Critical Care Medicine. 2015 doi: 10.1097/CCM.0000000000001312. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bohr S, Patel SJ, Sarin D, et al. Resolvin d2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013;21 doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu B, Walker J, Spur B, et al. Effects of lipoxin a4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins, leukotrienes, and essential fatty acids. 2015;94:55–64. doi: 10.1016/j.plefa.2014.11.005. [DOI] [PubMed] [Google Scholar]