Abstract
Angiosarcoma (AS) is a rare sarcoma subtype showing considerable clinicopathologic and genetic heterogeneity. Most radiation-induced AS show MYC gene amplifications, with a subset of cases harboring KDR, PTPRB and PLCG1 mutations. Despite recent advances, the genetic abnormalities of most primary AS remain undefined. Whole transcriptome sequencing was initiated in 2 index cases of primary soft tissue AS with epithelioid morphology occurring in young adults for novel gene discovery. The candidate abnormalities were validated and then screened by targeted sequencing and FISH in a large cohort of 120 well-characterized AS. Findings were subsequently correlated with the status of KDR, PLCG1, MYC and FLT4 gene abnormalities. The clinicopathologic relevance and prognostic significance of these genetic changes were analyzed by statistical methods. Concurrent CIC mutations and CIC rearrangements were identified in both index cases, with a CIC-LEUTX fusion detected in one case. Upon screening, an additional visceral AS in a young adult had a complex CIC rearrangement, while 6 others harbored only CIC mutations. All 3 CIC-rearranged AS lacked vasoformation and had a solid growth of round, epithelioid to rhabdoid cells, showing immunoreactivity for CD31 and ERG and sharing a transcriptional signature with other round cell sarcomas, including CIC-rearranged tumors. Overall, CIC abnormalities occurred in 9% (9/98) of cases, affecting younger patients with primary AS, with an inferior disease-free survival. In contrast, PLCG1 and KDR mutations occurred in both primary and secondary AS, accounting for 9.5% and 7%, respectively, with a predilection for breast and bone/viscera location, regardless of MYC status. MYC amplification was present in most secondary AS related to breast cancer (91%) compared to other causes (25%) or primary AS (7%). FLT4-amplified AS lacked PLCG1/KDR mutations, occurring predominantly in MYC-amplified population, and showed poor prognosis.
Keywords: angiosarcoma, CIC, KDR, PLCG1, MYC, FLT4
INTRODUCTION
Angiosarcomas (AS) are mesenchymal tumors recapitulating endothelial cell differentiation and displaying variable degree of vascular formation.1 Although AS account for only 2% of soft tissue sarcomas, they have a highly aggressive behavior and dismal overall survival.2 The age range at diagnosis varies significantly, with a peak incidence in the 7th decade. AS are extremely rare in children and in young adults, outside of the breast location. Furthermore, AS occur in a wide range of anatomic sites, including, skin (mainly head and neck and post-radiation), breast, soft tissue, bone, and viscera. Although most AS present de novo, a significant number of patients develop AS secondary to radiotherapy, and less commonly to chronic lymphedema or toxic carcinogens. Moreover, there is a broad spectrum of morphologic appearances, spanning from well-formed vasoformative spaces with minimal cytologic atypia to solid sheets of epithelioid or spindled cells lacking vasoformation.1
Cytogenetically, most AS are characterized by complex karyotypes, without recurrent chromosomal changes being described.3,4 By gene expression profiling, AS show distinctive up-regulation of genes related to angiogenesis and endothelial cell receptors compared to other sarcoma types.5,6 Recurrent somatic mutations involving angiogenic signaling pathways, such as KDR, PTPRB, and PLCG1, have been identified in about 40% of AS, while only rare mutations were reported in RAS, PIK3CA, TP53, FLT4, and TIE1.6–9 PTPRB mutations with or without co-existing PLCG1 mutations have been reported exclusively in secondary or MYC-amplified AS.8 In contrast, KDR mutations have been reported in both radiation-induced AS after breast cancer and primary AS (breast and heart).6,9 High level of MYC amplification is the hallmark of most post-radiation and chronic lymphedema-associated AS, with only a small subset of primary AS sharing this abnormality.10–12 Analysis of MYC amplification by fluorescence in situ hybridization (FISH) or MYC expression by immunohistochemistry has been confirmed by a number of studies as a reliable ancillary technique to distinguish radiation-related AS from other radiation-induced conditions, particularly atypical vascular lesions (AVL).11–14
Despite this recent progress accelerated by the next generation sequencing methodology, the underlying pathogenesis of most AS remains undefined, particularly of primary AS and AS occurring in younger patients. In this study we sought to investigate further novel genetic abnormalities by whole transcriptome sequencing of 2 index cases of primary soft tissue AS displaying an epithelioid phenotype. The candidate abnormalities were then screened in a large and diverse cohort of AS spanning different clinicopathologic features and compared to other known genetic events, such as PLCG1/KDR mutations and MYC/FLT4 amplification.
MATERIALS AND METHODS
Patient Collection
The Department of Pathology files at Memorial Sloan Kettering Cancer Center, New York were searched for the diagnosis of AS. A total of 120 cases had adequate material available for further molecular analysis, with 62 cases being included in our prior studies.6,7,11,15 The diagnosis was confirmed by the presence of vasoformative features microscopically and/or immunoreactivity for endothelial cell markers, i.e. CD31 and ERG. The relevant clinical information and follow-up was obtained from the electronic medical records. This study was approved by the Institutional Review Board 02-060.
RNA Sequencing and Data Mining using FusionSeq and Mutation Detection Algorithms
In 5 primary AS, including the 2 index cases (AS1, AS2) of soft tissue epithelioid AS, lacking vasoformative features and the remaining 3 cases with conventional histology (2 scalp, AS25, AS32, and 1 breast, AS110) for comparison, RNA was extracted from frozen tissue and subjected to transcriptome sequencing. Total RNA was prepared in accordance with the standard Illumina mRNA sample preparation instructions. Paired-end RNA-sequencing at read lengths of 50 or 51 bp was performed on the HiSeq 2000 platform. All reads were independently aligned with the STAR alignment software against the human genome sequence (hg19) and a splice junction library, simultaneously.16 The mapped reads were analyzed with FusionSeq method to identify potential fusion transcripts.17 Potential mutated genes were searched by MuTect (var 1.15)18 and VarScan (var 2.3.8)19 variant callers with the annotation added by Variant Effect Predictor tool provided by Ensembl. The candidate fusion genes and mutations were validated subsequently.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
An aliquot of the RNA extracted by Trizol Reagent (Invitrogen, Carlsbad, CA) was used to confirm the potential fusion transcripts. After cDNA synthesis by SuperScript® III First-Strand Synthesis Kit (Invitrogen), PCR was performed using the Clontech Advantage 2 PCR Enzyme System kit (Clontech, Mountain View, CA) with the following primers: CIC exon 19 forward primer, 5′-CTGGTCATGCAGCTCTTTCAGG–3′, and LEUTX exon 3 reverse primer, 5′–GTGTCATCTTCCCCTGGCAAGTC–3′. Amplified products were sent for sequencing confirmation.
Targeted Sequencing for Hot Spots Mutations
Genomic DNA was extracted from frozen or formalin-fixed paraffin-embedded tissue by the phenol/chloroform protocol or the QIAamp DNA FFPE Tissue Kit (Qiagen), respectively. Based on the RNA sequencing results and the reviewed literature, the hotspot targeted regions included CIC exons 5, 15, 18–2020, PLCG1 exons 11 and 188,21, and KDR exons 15, 16, and 246,9. The products were amplified with Clontech Advantage 2 PCR Enzyme System (see Supplemental Table S1 for PCR primers). All mutations were verified bi-directionally by Sanger sequencing.
Fluorescence in situ Hybridization (FISH)
FISH on interphase nuclei of tissue sections was performed by applying custom probes from bacterial artificial chromosomes (BACs) flanking or targeting CIC, MYC, FLT4 genes (Supplemental Table S2). BAC clones were chosen according to UCSC genome browser and obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (CHORI) (Oakland, CA). DNA from individual BACs was isolated and labeled with different fluorochromes in a nick translation reaction. The slides were pretreated, denatured, and hybridized by probes. After overnight incubation, the slides were serially washed and mounted with DAPI in an antifade solution.22 Two hundred successive nuclei were examined using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems, Newton, MA). A positive score for gene rearrangement was interpreted when at least 20% of the nuclei showed a break-apart signal. Nuclei with incomplete set of signals were omitted. An amplification was defined as the presence of >10 signals (ratio to control centromeric probe >10) or tight clustered signals characteristic of homogeneous staining regions (hsr), as previously described.10,15
Western Blotting for CIC protein expression
Frozen tissue from the 2 index AS cases and 10 control samples, including 5 CIC wild-type AS, 2 CIC-DUX4 positive small blue round cell tumors (SBRCTs), 3 gastrointestinal stromal tumors (GISTs), were homogenized with Tissue Protein Extraction Reagent (Thermo Scientific, Waltham, MA) supplemented with protease inhibitors. Electrophoresis and immunoblotting were performed with the polyclonal anti-CIC antibody (ABN446, 1:1,000, Millipore, Billerica, MA). A β-actin expression was used as reference with the monoclonal anti-actin antibody (8H10D10, 1:10,000, Cell Signaling, Danvers, MA). Following hybridization with the secondary antibodies, the blots were incubated with Immun-Star horseradish peroxidase luminal/enhancer (Bio-Rad, Hercules, CA) and exposed onto Kodak Biomax MR Film (Eastman Kodak, Rochester, NY).
Statistical Analysis
Statistical analysis was conducted using SPSS software (version 20; IBM, New York, NY). The associations between the clinicopathological factors and the genetic status were evaluated by Pearson’s χ2 test, Fisher exact test, or Mann-Whitney U test according the variable attribute. The disease free survival (DFS) was measured from the date of surgery to the date of recurrence; and overall survival (OS) from the date of surgery to the last follow-up or death. Kaplan-Meier estimate was performed to calculate the DFS and OS, and the statistical significance of different variables was examined by the log-rank test. The significant parameters in Kaplan-Meier analysis were subjected to multivariate Cox regression model to examine the statistic significance. Two-sided p values were calculated and, p <0.05 was considered to be significant for all statistical analyses.
RESULTS
The entire cohort consisted of 34 (28%) men and 86 (72%) women, with a mean age of 58 years at diagnosis (median 62 years; range 13–91). There were 73 (61%) primary and 47 (39%) secondary AS. The anatomic locations for primary AS included breast (22, 30%), head and neck (14, 19%), extremity (12, 16%), viscera (11, 15%), trunk (9, 12%), and body cavity (5, 7%). Aside from the mammary and visceral cases, the remaining primary AS were classified into cutaneous (14, 19%), soft tissue (18, 25%), and intra-osseous (8, 11%) subtypes. Secondary AS occurred following radiation for breast cancer (33) or other tumors (9), or in the setting of long-standing lymphedema (5). The average interval between radiotherapy and AS diagnosis was 11.3 years (median 8.5 years, range 4–70 years). Secondary AS occurred in the breast/chest wall (35, 76%; 33 s/p radiation for breast cancer, 1 s/p radiation for hemangiomatosis, 1 with chronic lymphedema due to thoracic surgery), extremity (6, 13%), head and neck (2), internal organs (2), and trunk (1).
CIC gene rearrangements identified in a subset of epithelioid AS in young adults, lacking vasoformative features
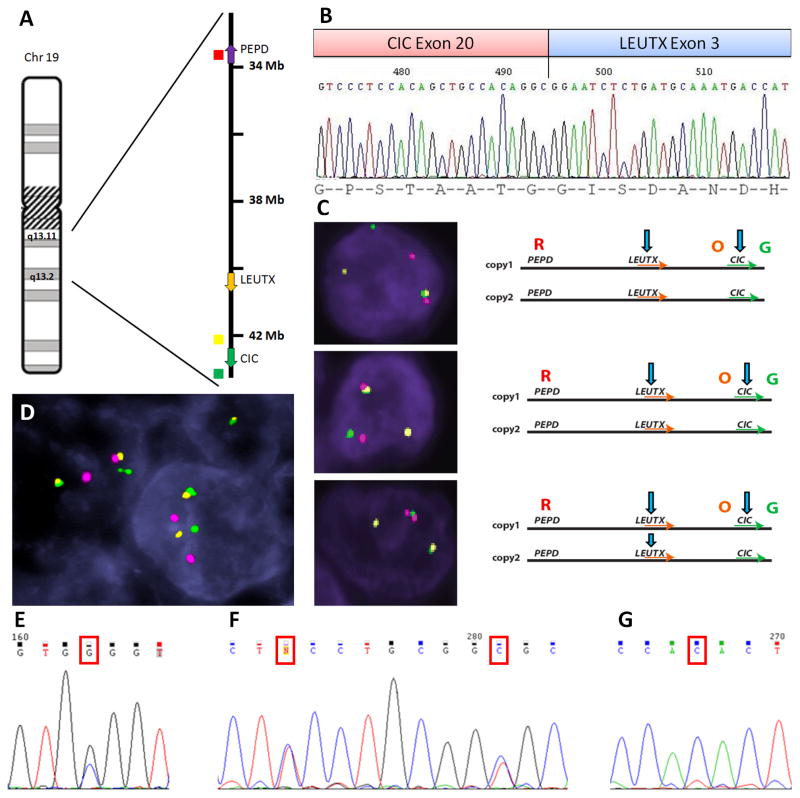
The fusion candidate identified by FusionSeq analysis in AS1 was a novel intra-chromosomal CIC-LEUTX fusion; the 2 genes being 2.5 Mb apart at 19q13.2 locus with similar directions of transcription (Fig. 1A). The chimeric transcript was confirmed by RT-PCR and composed of CIC exon 20 in-frame fused to LEUTX exon 3 (Fig. 1B). Due to the close vicinity of CIC and LEUTX genes, a 3-color FISH assay was employed with an additional PEPD gene probe, 6.2 Mb away from LEUTX. The result confirmed the CIC rearrangements, with a complex pattern (Fig. 1C).
Figure 1. CIC gene alterations in AS.
(A) The schematic diagram of chromosome 19q13 locus, encompassing CIC, LEUTX, and PEPD genes (arrows, direction of transcription; color dots indicate the BAC location used for 3-color FISH). (B) RT-PCR confirmed the chimeric CIC exon 20–LEUTX exon 3 transcript. (C) 3-color FISH confirmed the CIC and LEUTX break-apart with a complex and heterogeneous pattern in AS1: 3 different cells showing a variable pattern of rearrangement, with smaller and bigger gaps between the 3 BACs used (O, orange, CIC centromeric; G, green, CIC telomeric, R, red, PEPD; arrows indicate possible breaks) (D) CIC intra-chromosomal rearrangement in AS2 by FISH, with consistent gaps between red, orange and green BAC probes. Spectrum of CIC mutations in AS including heterozygous exon 15 mutations (p.G1190R, AS1)(E); synchronous exon 19 mutations (p.S1461F and p.R1465S, AS6)(F), and homozygous exon 19 mutations (p.Y1460H, AS7)(G).
In AS2, the Fusionseq algorithm did not reveal a robust fusion candidate, although a few chimeric CIC-DUX reads were identified. The FISH break-apart assay confirmed the presence of CIC rearrangements, with small and constant gaps, typical for an intra-chromosomal translocation (Fig. 1D) and similar to the complex pattern seen in AS1. The FISH fusion assay using CIC and DUX4 probes did not confirm a fused signal between these 2 genes (data not shown).
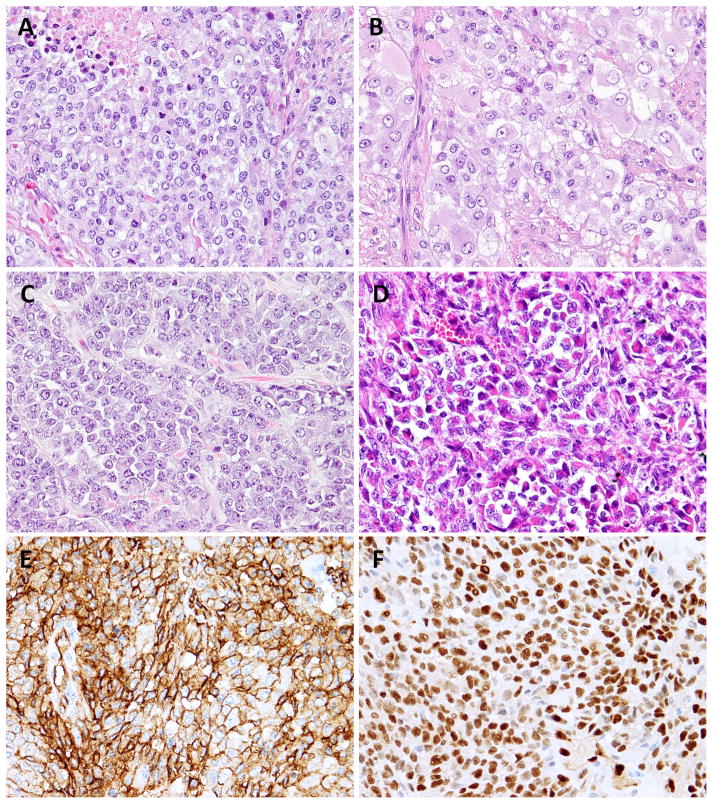
Forty-eight additional AS were screened by FISH for CIC gene abnormalities, with emphasis on young age, soft tissue location and epithelioid morphology. One additional CIC-rearrangement was found in a renal AS (AS3) from a 21 year-old female. In fact, 2 of the 3 CIC-rearranged AS occurred in patients younger than the age of 30 (Table 1). Morphologically, all 3 shared an epithelioid phenotype, composed of nests and solid sheets without identifiable vascular structures (Fig. 2A–D). The tumor cells ranged from a primitive small round to a polygonal appearance, with pale eosinophilic to amphophilic cytoplasm and vesicular nuclei with conspicuous nucleoli (Fig. 2A–C). AS3 showed a distinctive rhabdoid phenotype, with eccentric nuclei and paranuclear densely eosinophilic cytoplasm, arranged in a pseudo-alveolar pattern (Fig. 2D). Despite brisk mitotic activity (>10MF/10 HPFs) and geographic areas of necrosis, none showed significant nuclear pleomorphism. Immunohistochemically, all 3 cases demonstrated diffuse and strong CD31 and ERG expression and focal cytokeratin reactivity (Fig. 2E–F). The AS3 in addition showed CD34 positivity, while AS2 displayed focal membranous CD99 staining. S100 protein and desmin were negative in all cases. As AS3 showed loss of INI1 expression, raising the possibility of a proximal-type epithelioid sarcoma, further FISH analysis performed showed no SMARCB1 gene abnormalities (data not shown).23
Table 1.
Clinicopathologic features of CIC-mutated or rearranged angiosarcomas.
| Case |
CIC Mutation |
CIC Translocation |
Age/ Sex |
Location | Etiology | Cytomorphology | Vascular Formation |
Size (cm) |
Initial Stage |
Recur (month) |
Follow-up (month) |
Final Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | p.G1190R (exon 15) | CIC-LEUTX | 26/F | Thigh (st) | Primary | Round/epithelioid | UD | 7.5 | T2bN0M0 | 10 | 33 | DOD |
| 2* | p.S1422W (exon 18) | Positive | 47/F | Back (st) | Primary | Round | UD | 8.5 | T2bN1M0 | - | 13 | NED |
| 3 | Wild type | Positive | 21/F | Kidney | Primary | Round/rhabdoid | UD | 3.5 | T1bN1M0 | 21 | 23 | AWD |
| 4 | p.T1161S (exon 15) | Negative | 40/F | Breast | Primary | Flattened | WD | 9 | T2N0M0 | 17 | 28 | DOD |
| 5 | p.E1222G (exon 15) | Negative | 41/F | Spleen | Secondary | Pleomorphic | MD | 18 | T2bN0M0 | 5 | 10 | AWD |
| 6 | p.S1461F, p.R1465S (exon 19) | Negative | 21/M | Neck (st) | Primary | Spindled/round | PD | 2.5 | T1bN0M0 | 3 | 12 | AWD |
| 7 | p.Y1460H (exon 19) | Negative | 77/M | Scalp (skin) | Primary | Epithelioid/spindled | PD | 0.6 | T1aN0M0 | 12 | 55 | DOD |
| 8 | p.V1505I (exon 20) | Negative | 34/M | Pelvis (st) | Primary | Pleomorphic | PD | 9 | T2bN1M0 | 2 | 2 | AWD |
| 9 | p.P1549L (exon 20) | Negative | 60/M | Scalp (skin) | Primary | Epithelioid | MD | 1.6 | T1aN0M0 | - | 4 | NED |
Tested by RNA sequencing;
st: soft tissue, UD: undifferentiated, PD: poorly differentiated, MD: moderately differentiated, WD: well differentiated, DOD: dead of disease, NED: no evidence of disease, AWD: alive with disease
Figure 2. Pathologic features of CIC-rearranged AS.
Solid sheets of monotonous epithelioid cells, with moderate to abundant eosinophilic cytoplasm, and vesicular nuclei (A,B; AS1). Small epithelioid to round cells with scant, light eosinophilic cytoplasm and vesicular nuclei with inconspicuous nucleoli (C; AS2). Distinctive rhabdoid cells arranged in vague pseudoalveolar and nested pattern (D; AS3) All 3 cases expressed diffuse and strong CD31 (E) and ERG (F) immunoreactivity.
CIC missense mutations occur preferentially in primary AS of younger patients
The MuTect and VarScan algorithms applied on the RNAseq data identified co-existent missense CIC mutations in both index cases, but not in the remaining 3 AS with classic vasoformative morphology. AS1 harbored a CIC exon 15 mutation and AS2 a CIC exon 18 mutation, both being validated by Sanger sequencing in the tumor DNA, but not present in the germline DNA extracted from normal tissue (Fig. 1E). As additional hot spot mutations were reported in oligodendroglioma, including CIC exons 5, 19, and 2024, we screened an additional 95 AS for these 5 exons by direct sequencing. The results showed an additional 6 cases carrying 7 different missense mutations (Table 1). The mutations were distributed in CIC exons’ 15 and 18–20, but, in contrast to oligodendroglioma, no CIC mutations were identified in exon 5, the site of the highly conserved HMG (high-mobility group) box domain. AS6 had two synchronous CIC exon 19 mutations and AS7 harbored a homozygous exon 19 mutation (Fig. 1F, G). No CIC mutation was present in AS3.
In total we identified 9 (9%) AS harboring various CIC alterations, including gene rearrangements and/or missense mutations; their clinicopathologic features being summarized in Table 1. There were 4 males and 5 females, with a mean age of 41 years (versus 59 years in CIC-wild type cases, p = 0.02). Soft tissue AS was the most common type affected by this abnormality, followed by visceral, cutaneous, and breast locations. All except one were primary AS; only one case being possibly related to radiation (AS5, spleen), having a remote history of chemoradiation for ovarian germ cell tumor 35 years prior. Nevertheless, none of the AS with CIC-alterations demonstrated MYC amplification. All except one lacked other genetic abnormalities; only one primary breast AS (AS4) showed coexisting CIC and PLCG1 mutations.
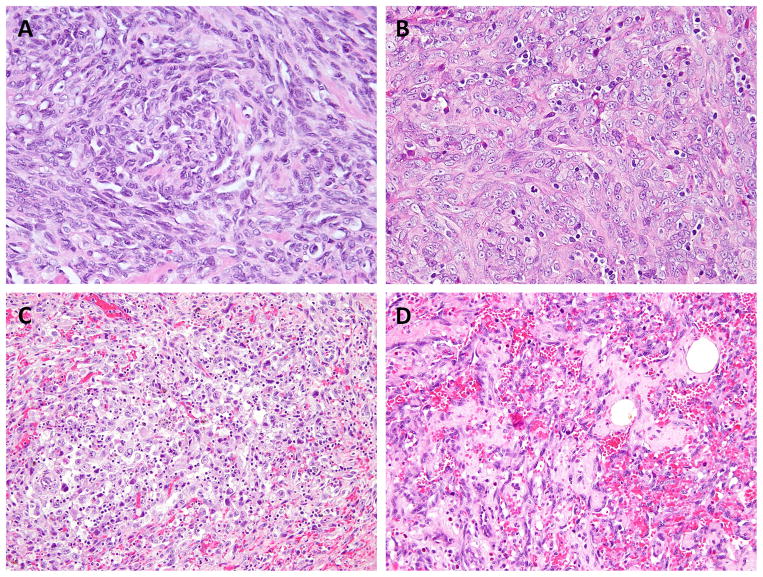
Aside from the index cases, there were 2 additional CIC-mutant AS displaying an epithelioid phenotype, with limited vasoformation: a deep-seated soft tissue AS from the neck in a 21 year-old man, harboring 2 distinct CIC mutations (AS6) and a cutaneous scalp AS bearing a homozygous CIC mutation (AS7) (Fig. 3A, B). The remaining 4 cases with single heterozygous CIC mutations exhibited a more classic morphology, with well to moderately formed vascular channels and pleomorphic cytology (Fig. 3C, D). The breast AS with concurrent PLCG1 and CIC mutations demonstrated well-differentiated anastomosing vascular channels (Fig. 3D). Thus, 3 of 6 (50%) AS cases presenting outside the breast location in adults younger than 30 years showed the presence of CIC gene abnormalities.
Figure 3. The morphologic spectrum of CIC-mutated AS.
A solid growth is noted in AS6 harboring double CIC mutations (A) and AS7 bearing CIC homozygous mutations (B); pleomorphic tumor cells and inconspicuous vascular formation in AS8 with single CIC mutation (C); well-formed anastomosing vascular channels in AS4 with concurrent CIC and PLCG1 mutations (D).
CIC-rearranged AS show up-regulation of PEA3 family of transcription factors and overexpression of CIC protein
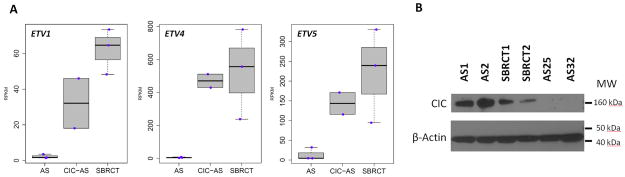
By unsupervised hierarchical clustering of RNA sequencing data of >100 cases of various sarcoma types, the 2 index AS cases with CIC-gene rearrangements showed an overlapping gene signature with a group of SBRCTs, including CIC-DUX4 positive cases (data not shown). Remarkably, there was a significant overexpression of PEA3 family of ETS genes, such as ETV1, ETV4, and ETV5, as well as WT1 and VGF, commonly seen in the CIC-DUX signature of SBRCT (Fig. 4A and Supplemental Table S3).25–27 Additionally, only a modest upregulation of angiogenesis and endothelial related genes, such as TIE1, FLT4, and PECAM1 (CD31), were noted.
Figure 4.
(A) High ETV1, ETV4, ETV5 overexpression in CIC-rearranged AS (CIC-AS) and SBRCTs, but not in classic AS (AS25, AS32, AS110) (RPKM, Reads Per Kilobase per Million mapped reads). (B) CIC overexpression (160 kDa) by western blotting in AS1-2 and CIC-DUX4-positive SBRCTs (SBRCT1-2), compared to CIC-negative AS (AS25, AS32) (control actin 43 kDa, lower part).
The Western blot revealed high CIC protein expression in the 2 index AS cases, at similar levels as the 2 CIC-DUX4-positive SBRCTs, whereas the control group of conventional AS and GISTs showed low, if any, protein levels (Fig. 4B).
PLCG1/KDR mutations are mutually exclusive but occur in similar AS subsets
A total of 11 (9.5%) AS harbored PLCG1 mutations of the 116 informative cases (Supplemental Table S4). All except one occurred in females, with a mean age of 53 years (range 23–82). The PLCG1-mutated AS were evenly distributed among primary (6 cases) and secondary AS (5 cases), the latter being all related to radiation for breast cancer. Among the primary AS there were 4 mammary and 2 visceral, one each in the heart and spleen. Most PLCG1 mutations (9, 82%) occurred in exon 18 (p.R707Q, 8; p.R707L), affecting the auto-inhibitory Src homology 2 (cSH2) domain, with only 2 in exon 11 (p.S345F), within the PLCx catalytic domain. Both PLCG1 p.S345F mutations occurred in primary AS, one each in breast and spleen. These mutations were mutually exclusive from KDR mutations, but all 5 PLCG1-mutated secondary AS showed co-existent MYC amplifications. Histologically, the PLCG1-mutated AS exhibited a wide morphologic spectrum, ranging from well formed vascular spaces to solid sheets of spindled to epithelioid endothelial cells, with low to high grade nuclear features.
KDR missense mutations were found in 8 (7%) of 113 informative cases (Supplemental Table S4), seen exclusively in female patients (mean age, 56 years; range 23–77). Five KDR mutations occurred in primary breast AS and 2 in secondary AS after radiotherapy for breast cancer, co-exhibiting MYC amplification. Only one KDR-mutant AS occurred outside the breast/chest wall area, in the lumbar spine. The most common hot spot mutation was KDR exon 16 (codon 771; transmembrane domain) seen in 5 cases. Additional less common spots included KDR exon 15 mutation (codon 717 and 731, extracellular domain Ig-like C2 type 7) in 2 cases and KDR exon 24 (codon 1065, kinase domain) in 1 case. In keeping with our previous study, KDR-mutated AS demonstrated a wide spectrum of low to high grade phenotype, with variable vasoformation and solid growth.6
As both KDR and PLCG1 genes are involved in the VEGFR2 (vascular endothelial growth factor receptor 2) signaling pathway, it is not surprising that activating KDR/PLCG1 mutations are present in similar AS subsets, such as primary or secondary breast AS (15/52, 29%) and bone/viscera (3/19, 16%) locations (p = 0.012).
MYC and FLT4 amplifications occur preferentially in AS related to breast cancer radiotherapy
The status of MYC gene copy number abnormalities was analyzed by FISH in 112 informative cases. MYC gene amplification was identified in 39 cases. It was detected in the overwhelming majority of AS secondary to radiation for breast cancer (28/31, 90%) and AS related to chronic lymphedema (4/4, 100%). The incidence was significantly lower in AS secondary to radiation for other tumor types (2/8, 25%), such as lymphoma, hemangiomatosis, salivary gland carcinoma, germ cell tumor, and prostatic cancer. The lowest incidence was seen in primary AS (5/69, 7%), occurring only in breast, bone, soft tissue, and skin. MYC amplification was significantly associated with female gender (p = 0.002) and older age (p < 0.001), most likely related to the high frequency of AS related to radiation for breast cancer and the lag time of post-radiation sarcoma. FLT4 copy number status was also assessed in 110 cases, showing gene amplifications in 6 AS (5.5%), all except one being co-amplified with MYC (5/39 versus 1/71, p = 0.022). All except one FLT4-amplified cases occurred in secondary AS either related to radiation therapy for breast cancer or lymphedema. Only one case occurred in a primary scalp cutaneous AS. None of them had PLCG1/KDR mutations. Histologically, FLT4-amplified AS exhibited mostly a solid, high-grade histology with variable epithelioid and spindled cell phenotype.
A summary of all molecular findings are depicted in Supplemental Fig. S1, providing an overview of the genetic landscape of AS subsets.
Survival Analysis
Among 108 cases with follow-up information, the median follow-up duration was 27.3 months (range, 0.3–234). Tumor recurrence occurred in 67 cases (70%), at a median disease-free duration of 16 months. Fifty patients (46%) died of disease and the median overall survival duration was 55 months (95% CI, 37.1–72.3), with a 5-year survival rate of 44.5%. Among the different anatomic locations, the intra-osseous/visceral AS showed the shortest OS (p < 0.001, a median 22 mo, for intra-osseous AS; 10 mo for visceral AS)(Supplemental Table S5). Secondary AS and the presence of CIC alterations conferred an inferior DFS and remained statistically significant by multivariate Cox regression model (p = 0.007, HR 2.691, 95% CI 1.314–5.512; p = 0.006, HR 3.462, 95% CI 1.419–8.443, respectively). The presence of MYC amplification had only a trend towards worse DFS (p=0.083) and OS (p = 0.062), but the presence of FLT4 amplification was associated with a short OS (p = 0.037).
DISCUSSION
Despite significant progress being made on the discovery of molecular abnormalities driving AS pathogenesis, the pace of further understanding is hindered by the broad clinical and pathologic heterogeneity of this disease. Even at the molecular level, AS emerges as a multifaceted disease, with mostly non-overlapping genetic signatures across clinical subsets; the majority of the alterations described to date, such as MYC/FLT4 amplifications and PLCG1/PTPRB mutations, affecting secondary AS. Although at the gene expression level, AS subtypes share a distinct transcriptional profile with up-regulation of angiogenic factors and endothelial receptors, compared to other tumor types1,2,5,6, this feature is most likely reflective of its line of differentiation, rather than overlapping oncogenic driver events. A particular limited knowledge surrounds primary AS, such as cutaneous or soft tissue locations, where no recurrent mutations have been identified to date. Furthermore, no insight has been gained in AS occurring in younger patients, which are often highly aggressive and exhibit a high grade epithelioid or spindled phenotype with scarce vasoformative features.28 Thus, our study focused on these orphan subsets, taking advantage of two index cases of primary soft tissue AS occurring in young patients with available material for paired-end RNA sequencing. The results are remarkable with identification of similar CIC gene rearrangements co-existing with CIC mutations in both cases. The extended screening further identifies a renal AS in a young patient with CIC gene rearrangements by FISH, as well as additional 6 cases with missense mutations. In total, CIC alterations were detected in 9% of AS cases showing a predilection for young patients and lacking MYC/FLT4 amplifications. CIC, a human homolog of the Drosophila gene capicua, belongs to the HMG-box family.29 CIC binding to target promoters and enhancers suppresses transcription in the absence of signaling. The activation of CIC protein through phosphorylation results in either attenuation of CIC DNA binding activity or prevents its nuclear import, relieving the default inhibitory ability of CIC.30,31,32 CIC gene abnormalities have been described in a variety of cancers, such as oligodendroglioma, medulloblastoma, breast cancer, and small blue round cell tumors (SBRCT).20,25,33–35 CIC-related fusions account for two-thirds of EWSR1-negative SBRCTs, with the CIC-DUX4 fusion exhibiting high chimeric CIC protein expression and an enhanced transcriptional activity of CIC downstream targets, including the PEA3 family genes, such as ETV1, ETV4, and ETV5.25,35 Remarkably, the 2 index AS harboring CIC rearrangements, shared the gene signature of CIC-fusion positive SBRCTs, with up-regulation of ETV1, ETV4, ETV5 genes. Moreover, the CIC fusion partners, LEUTX and DUX4, belong to the same class of paired (PRD) homeobox genes36, and KAT6A-LEUTX fusion has been reported in a therapy-related acute myeloid leukemia.37
A high frequency (69–78%) of homozygous CIC mutations has been reported in oligodendrogliomas with 1p/19q deletion.20,38,39 The biallelic CIC inactivation caused by mutation and deletion also results in up-regulation of the CIC-targeted genes (ETV1, ETV4, ETV5, CCND1).27 Most CIC mutations reported in oligodendroglioma occur in the HMG-domain or are protein-truncating mutations. In contrast, CIC mutations identified in AS clustered in exons 18–20 (single, double or homozygous mutations), targeting the nuclear localization and repressor activity of the CIC protein.29
These findings bring forward an underappreciated disconnect between tumor differentiation and pathogenesis. On one hand the 2 index AS cases expressed all markers of endothelial lineage (CD31, ERG), while on the other hand displayed the gene signature characteristic of its driving genetic abnormality, the CIC-rearrangement, with up-regulation of PEA3 transcription factors. A somewhat similar example in a different vascular tumor type is the TFE3-fusion positive epithelioid hemangioendothelioma, where the endothelial lineage markers are expressed despite the oncogenic activation of TFE3 gene signature40. As these concepts evolve, it remains to be determined if the therapy of these CIC-rearranged AS should target the line of differentiation, i.e. endothelial receptors using cytotoxic therapy or VEGFR-inhibitors typically used for conventional AS, or target the PEA3-transcriptional signature, thus being managed with Ewing sarcoma type protocols. The latter approach seems to be more in keeping with today’s ‘basket trials’ strategy, enrolling patients based on their specific genetic alterations rather than the histologic type.
In a recent study, Behjati et al8 identified PTPRB and PLCG1 in 26% of AS, being restricted to radiation-associated/MYC-amplified AS. The 3 PLCG1 mutations (p.R707Q) co-existed with PTPRB mutations and occurred in 9% of AS tested. The same PLCG1 hot spot mutation was subsequently identified in 3 out of 10 cardiac AS tested.9 A different PLCG1 missense mutation (p.S345F) was also more recently reported in cutaneous (19%) and nodal (13%) peripheral T-cell lymphoma.21,41 Our study screened a large cohort of AS cases for both hot spots and found 11 (9.5%) PLCG1-mutated cases, confirming that p.R707Q mutation occurs in both primary (breast, heart) and secondary AS. Our results also show PLCG1 p.S345F in 2 primary AS (breast, spleen), a previously unreported finding. PLCG1 encodes phospholipase Cγ1 (PLCγ1), a member of the phosphatidylinositol-specific phospholipase-C (PI-PLC) family, which generates inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), triggering Ca2+-dependent signaling and protein kinase C (PKC) activation, respectively. The p.S345F mutation alters the PLCx protein catalytic domain, thus activating the downstream calmodulin/calcineurin signaling through facilitating the interaction with lipids and its phosphodiesterase activity.21 In contrast, the codon 707 mutation maps at the c-terminal Src homology 2 (cSH2) domain8,9; mutations at this site preventing the intramolecular interaction of phosphorylated PLCγ1 and triggering constitutive activation of DAG and IP-dependent signaling.
In our previous study, we reported activating KDR mutations in 10% of AS, being only identified in primary breast or secondary AS after breast cancer.6 Kunze et al reported two cases of cardiac AS harboring KDR mutation each in the extracellular immunogloulin domain (R720W) and transmembrane domain (T771K).9 The present investigation identifies 4 new cases (7% in total), including one case arising in the vertebral bone. The most common hot spot was KDR codon 771 in exon 16 (transmembrane domain, 5 cases), followed by KDR codons 717 and 731 in exon 15 (extracellular domain Ig-like C2 type 7, 2 cases) and KDR exon 24 codon 1065 (kinase domain, 1 case). Our results also show that KDR and PLCG1 mutations, accounting for 17% of AS cases, are mutually exclusive and evenly distributed among primary and secondary AS, mainly affecting breast and only occasionally present in bone or viscera, with no impact on morphology or outcome. Both KDR and PLCG1 mutations share a common VEGFR2/PLCγ1 signaling pathway. KDR, also known as VEGFR2 (vascular endothelial growth factor receptor 2), is a member of human VEGFR family of receptor tyrosine kinases (VEGFR1-3), with critical regulatory functions in vasculogenesis and angiogenesis upon binding to various VEGFs.42,43 Although activating KDR mutations are relatively rare in AS, KDR is universally overexpressed in AS6 through alternative yet undefined mechanisms. Therapy with anti-VEGFR agents, such as sorafenib, which targets VEGFR2 and Ras kinase43, have shown limited antitumor activity.44 An alternative mechanism of VEGFR activation in secondary AS is the presence of FLT4 (a.k.a. VEGFR3) amplification, which mostly occurs in association with MYC amplification.11,14 FLT4 amplification was identified in 13% (5/39) of MYC-amplified AS, mutually exclusive from KDR/PLCG1 mutations, and associated with a poor outcome. All except one FLT4-amplified secondary AS occurred in the breast/chest wall area, being co-amplified with MYC. Only one case of a primary scalp AS had FLT4 amplification in the absence of MYC abnormalities by FISH. A recent study suggested that diffuse and strong cytoplasmic FLT4 immunostaining is a potential surrogate ancillary test for FLT4 gene amplification by FISH.14
MYC deregulation is a well-known oncogenic event involving a wide array of human cancers, lying at the crossroads of diverse signal transduction pathways, including cell growth, metabolism, differentiation, transformation, cell cycle, and angiogenesis.45 High level MYC amplification at the 8q24.21 locus was first shown by Manner and colleagues in 55% of secondary AS using array-comparative genomic hybridization10. Subsequently, larger studies showed that MYC amplification is the hallmark of radiation-induced AS, occurring in the majority of secondary AS, but not in other radiation-related sarcomas or AVLs.11,13 However, more recent studies identified MYC amplification in rare cases of primary AS, including breast, bone, visceral and skin.4,12,15,46 Compared to FISH assay, the immunohistochemical analysis detects a higher number of MYC overexpression in primary AS.12,46 Our findings support prior results of MYC amplification in a small subset (7%) of primary AS, encompassing breast and somatic soft tissue. In contrast MYC amplification occurs in 91% of secondary AS related to breast cancer radiation or lymphedema.
In conclusion, this is the largest molecular investigation to date of a diverse cohort of 120 AS patients. We describe recurrent, novel, CIC gene rearrangements in a subset of young adults with primary soft tissue and visceral AS, with or without concurrent CIC mutations, associated with up-regulation of CIC-target genes, such as ETV1, ETV4, and ETV5. An additional subset of AS harbored only missense CIC mutations, occurring with predilection in younger patients with primary AS, and being associated with a highly aggressive clinical course. The mutually exclusive KDR/PLCG1 mutations were present in both primary and secondary AS, but restricted to breast, bone and viscera, with no histologic or prognostic correlates. Our results show that in total, the CIC, PLCG1, KDR, MYC and FLT4 genetic abnormalities account for around 50% of our study cohort. Albeit our study did dot examine abnormalities in PTPRB, TP53, RAS, PIK3CA, etc, described in other studies 7–9. However, the molecular alterations of a significant number of cases remain to be elucidated. The divergent genetic abnormalities emerging in different AS subsets promise both challenges and opportunities in designing future clinical trials of targeted therapy in this disease.
Supplementary Material
The AS molecular classification includes emerging genetic groups such as: the KDR/PLCG1 mutant-AS involving both primary and secondary AS; AS with CIC-alterations affecting mainly primary lesions in younger patients, with poor outcome; secondary AS with co-amplification of MYC and FLT4. NA, not available; RT, radiotherapy; WT, wild type; NED, no evidence of disease; DOD, dead of disease; DOO, dead of other causes; AWD, alive with disease.
Footnotes
Conflict of interest: none
References
- 1.Antonescu C. Malignant vascular tumors--an update. Mod Pathol. 2014;27(Suppl 1):S30–38. doi: 10.1038/modpathol.2013.176. [DOI] [PubMed] [Google Scholar]
- 2.Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. The Lancet. Oncology. 2010;11:983–991. doi: 10.1016/S1470-2045(10)70023-1. [DOI] [PubMed] [Google Scholar]
- 3.Guillou L, Aurias A. Soft tissue sarcomas with complex genomic profiles. Virchows Arch. 2010;456:201–217. doi: 10.1007/s00428-009-0853-4. [DOI] [PubMed] [Google Scholar]
- 4.Verbeke SL, de Jong D, Bertoni F, et al. Array CGH analysis identifies two distinct subgroups of primary angiosarcoma of bone. Genes Chromosomes Cancer. 2015;54:72–81. doi: 10.1002/gcc.22219. [DOI] [PubMed] [Google Scholar]
- 5.Itakura E, Yamamoto H, Oda Y, et al. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. Journal of surgical oncology. 2008;97:74–81. doi: 10.1002/jso.20766. [DOI] [PubMed] [Google Scholar]
- 6.Antonescu CR, Yoshida A, Guo T, et al. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res. 2009;69:7175–7179. doi: 10.1158/0008-5472.CAN-09-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Italiano A, Chen CL, Thomas R, et al. Alterations of the p53 and PIK3CA/AKT/mTOR pathways in angiosarcomas: a pattern distinct from other sarcomas with complex genomics. Cancer. 2012;118:5878–5887. doi: 10.1002/cncr.27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behjati S, Tarpey PS, Sheldon H, et al. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet. 2014;46:376–379. doi: 10.1038/ng.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunze K, Spieker T, Gamerdinger U, et al. A recurrent activating PLCG1 mutation in cardiac angiosarcomas increases apoptosis resistance and invasiveness of endothelial cells. Cancer Res. 2014;74:6173–6183. doi: 10.1158/0008-5472.CAN-14-1162. [DOI] [PubMed] [Google Scholar]
- 10.Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34–39. doi: 10.2353/ajpath.2010.090637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo T, Zhang L, Chang NE, et al. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer. 2011;50:25–33. doi: 10.1002/gcc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginter PS, Mosquera JM, MacDonald TY, et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014;45:709–716. doi: 10.1016/j.humpath.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75–85. doi: 10.1038/modpathol.2011.134. [DOI] [PubMed] [Google Scholar]
- 14.Cornejo KM, Deng A, Wu H, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. 2015;46:868–875. doi: 10.1016/j.humpath.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Italiano A, Thomas R, Breen M, et al. The miR-17-92 cluster and its target THBS1 are differentially expressed in angiosarcomas dependent on MYC amplification. Genes Chromosomes Cancer. 2012;51:569–578. doi: 10.1002/gcc.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sboner A, Habegger L, Pflueger D, et al. FusionSeq: a modular framework for finding gene fusions by analyzing paired-end RNA-sequencing data. Genome biology. 2010;11:R104. doi: 10.1186/gb-2010-11-10-r104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nature biotechnology. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koboldt DC, Zhang Q, Larson DE, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome research. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333:1453–1455. doi: 10.1126/science.1210557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaque JP, Gomez-Lopez G, Monsalvez V, et al. PLCG1 mutations in cutaneous T-cell lymphomas. Blood. 2014;123:2034–2043. doi: 10.1182/blood-2013-05-504308. [DOI] [PubMed] [Google Scholar]
- 22.Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114–1124. doi: 10.1002/gcc.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Loarer F, Zhang L, Fletcher CD, et al. Consistent SMARCB1 homozygous deletions in epithelioid sarcoma and in a subset of myoepithelial carcinomas can be reliably detected by FISH in archival material. Genes Chromosomes Cancer. 2014;53:475–486. doi: 10.1002/gcc.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahm F, Koelsche C, Meyer J, et al. CIC and FUBP1 mutations in oligodendrogliomas, oligoastrocytomas and astrocytomas. Acta neuropathologica. 2012;123:853–860. doi: 10.1007/s00401-012-0993-5. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura-Saito M, Yamazaki Y, Kaneko K, et al. Fusion between CIC and DUX4 up-regulates PEA3 family genes in Ewing-like sarcomas with t(4;19)(q35;q13) translocation. Hum Mol Genet. 2006;15:2125–2137. doi: 10.1093/hmg/ddl136. [DOI] [PubMed] [Google Scholar]
- 26.Specht K, Sung YS, Zhang L, et al. Distinct transcriptional signature and immunoprofile of CIC-DUX4 fusion-positive round cell tumors compared to EWSR1-rearranged Ewing sarcomas: further evidence toward distinct pathologic entities. Genes Chromosomes Cancer. 2014;53:622–633. doi: 10.1002/gcc.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleize V, Alentorn A, Connen de Kerillis L, et al. CIC inactivating mutations identify aggressive subset of 1p19q codeleted gliomas. Annals of neurology. 2015;78:355–374. doi: 10.1002/ana.24443. [DOI] [PubMed] [Google Scholar]
- 28.Deyrup AT, Miettinen M, North PE, et al. Angiosarcomas arising in the viscera and soft tissue of children and young adults: a clinicopathologic study of 15 cases. Am J Surg Pathol. 2009;33:264–269. doi: 10.1097/PAS.0b013e3181875a5f. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez G, Shvartsman SY, Paroush Z. The Capicua repressor--a general sensor of RTK signaling in development and disease. Journal of cell science. 2012;125:1383–1391. doi: 10.1242/jcs.092965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jimenez G, Guichet A, Ephrussi A, et al. Relief of gene repression by torso RTK signaling: role of capicua in Drosophila terminal and dorsoventral patterning. Genes & development. 2000;14:224–231. [PMC free article] [PubMed] [Google Scholar]
- 31.Astigarraga S, Grossman R, Diaz-Delfin J, et al. A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling. EMBO J. 2007;26:668–677. doi: 10.1038/sj.emboj.7601532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dissanayake K, Toth R, Blakey J, et al. ERK/p90(RSK)/14-3-3 signalling has an impact on expression of PEA3 Ets transcription factors via the transcriptional repressor capicua. The Biochemical journal. 2011;433:515–525. doi: 10.1042/BJ20101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CJ, Chan WI, Scotting PJ. CIC, a gene involved in cerebellar development and ErbB signaling, is significantly expressed in medulloblastomas. J Neurooncol. 2005;73:101–108. doi: 10.1007/s11060-004-4598-2. [DOI] [PubMed] [Google Scholar]
- 34.Sjoblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 35.Italiano A, Sung YS, Zhang L, et al. High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes Chromosomes Cancer. 2012;51:207–218. doi: 10.1002/gcc.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland PW, Booth HA, Bruford EA. Classification and nomenclature of all human homeobox genes. BMC biology. 2007;5:47. doi: 10.1186/1741-7007-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chinen Y, Taki T, Tsutsumi Y, et al. The leucine twenty homeobox (LEUTX) gene, which lacks a histone acetyltransferase domain, is fused to KAT6A in therapy-related acute myeloid leukemia with t(8;19)(p11;q13) Genes Chromosomes Cancer. 2014;53:299–308. doi: 10.1002/gcc.22140. [DOI] [PubMed] [Google Scholar]
- 38.Yip S, Butterfield YS, Morozova O, et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012;226:7–16. doi: 10.1002/path.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eisenreich S, Abou-El-Ardat K, Szafranski K, et al. Novel CIC point mutations and an exon-spanning, homozygous deletion identified in oligodendroglial tumors by a comprehensive genomic approach including transcriptome sequencing. PloS one. 2013;8:e76623. doi: 10.1371/journal.pone.0076623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antonescu CR, Le Loarer F, Mosquera JM, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer. 2013;52:775–784. doi: 10.1002/gcc.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manso R, Rodriguez-Pinilla SM, Gonzalez-Rincon J, et al. Recurrent presence of the PLCG1 S345F mutation in nodal peripheral T-cell lymphomas. Haematologica. 2015;100:e25–27. doi: 10.3324/haematol.2014.113696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signalling - in control of vascular function. Nature reviews. Molecular cell biology. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 43.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes & cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for patients with advanced angiosarcoma: a phase II Trial from the French Sarcoma Group (GSF/GETO) The oncologist. 2012;17:260–266. doi: 10.1634/theoncologist.2011-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shon W, Sukov WR, Jenkins SM, et al. MYC amplification and overexpression in primary cutaneous angiosarcoma: a fluorescence in-situ hybridization and immunohistochemical study. Mod Pathol. 2014;27:509–515. doi: 10.1038/modpathol.2013.163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The AS molecular classification includes emerging genetic groups such as: the KDR/PLCG1 mutant-AS involving both primary and secondary AS; AS with CIC-alterations affecting mainly primary lesions in younger patients, with poor outcome; secondary AS with co-amplification of MYC and FLT4. NA, not available; RT, radiotherapy; WT, wild type; NED, no evidence of disease; DOD, dead of disease; DOO, dead of other causes; AWD, alive with disease.