Abstract
Objectives
TGF-β regulates immune and fibrotic responses of chronic pancreatitis (CP). The bone morphogenetic protein-2 (BMP-2) antagonist gremlin is regulated by TGF-β. Parathyroid hormone-related hormone (PTHrP) levels are elevated in CP. Here we investigated the crosstalk between TGF-β/BMP-2/gremlin and PTHrP signaling.
Methods
Reverse transcription/real-time PCR, ChIP, Western blotting, and transient transfection were used to investigate PTHrP regulation by TGF-β and BMP-2, and gremlin regulation by PTHrP. The PTHrP antagonist PTHrP (7-34) and acinar cells with conditional Pthrp gene deletion (PTHrPΔacinar) were used to assess PTHrP’s role in the pro-inflammatory and pro-fibrotic effects of TGF-β and gremlin.
Results
TGF-β increased PTHrP levels in acinar cells and pancreatic stellate cells (PSCs) through a Smad3-dependent pathway. TGF-β’s effects on levels of IL-6 and ICAM-1(acinar cells) and procollagen I and fibronectin (PSCs) were inhibited by PTHrP (7-34). PTHrPΔacinar suppressed TGF-β’s effects on IL-6 and ICAM-1. PTHrP increased gremlin in acinar cells, and inhibiting gremlin action suppressed TGF-β’s and PTHrP’s effects on IL-6 and ICAM-1. TGF-β-mediated gremlin upregulation was suppressed in PTHrPΔacinar cells. BMP-2 suppressed PTHrP levels in PSCs.
Conclusions
PTHrP functions as a novel mediator of the pro-inflammatory and pro-fibrotic effects of TGF-β. TGF-β and BMP-2 regulate PTHrP expression and PTHrP regulates gremlin levels.
Keywords: PTHrP, TGF-β, BMP-2, gremlin, acinar cells, stellate cells
INTRODUCTION
Chronic pancreatitis (CP) is a progressive inflammatory disease in which pancreatic acini are destroyed and replaced by fibrous tissue. In patients with CP, the disease course is the same regardless of etiology. A growing body of epidemiological, etiological, and experimental data indicates that CP results from the accumulated damage incurred during repeated bouts of acute pancreatitis (recurrent AP or RAP) (1). Acinar cell injury plays a major role in the pathophysiology of pancreatitis (2). Damaged acinar cells then release factors that lead to the generation of multiple inflammatory mediators and activation of pancreatic stellate cells (PSCs). Activated PSCs in turn produce pro-inflammatory molecules and autocrine factors to perpetuate the activated phenotype (3,4). TGF-β is a regulatory molecule with pleiotropic effects on cell proliferation, differentiation, migration, and survival and affects multiple biological processes, including development, carcinogenesis, fibrosis, wound healing, and immune responses (5). TGF-β secreted by acinar cells and PSCs in response to injury plays a key role in both the inflammatory and fibrotic responses observed in pancreatitis (6–9). Inhibition of TGF-β action protects the pancreas against chronic injury by preventing acinar cell apoptosis (10). The molecule plays a key role in PSC activation and increases extracellular matrix (ECM) deposition, leading to fibrosis (11–13). In humans, TGF-β levels are upregulated in both AP and CP (14–16).
Parathyroid hormone-related protein (PTHrP) exerts multiple effects in both normal and disease states, where it modulates critical cellular functions such as proliferation, apoptosis, and differentiation (17). The effects of PTHrP are mediated in part through paracrine and/or autocrine activation of the PTH/PTHrP receptor (PTH1R), a G protein-coupled receptor (GPCR) (18). In the normal pancreas, PTHrP is expressed by islet cells and regulates cell proliferation, apoptosis, and insulin release (19–21). PTHrP expression is very low in the normal exocrine pancreas, and is elevated after induction of pancreatitis by repeated hyperstimulation with the decapeptide cerulein and after pancreatic duct ligation (PDL) (22,23). Deletion of the Pthrp gene in acinar cells exerts a protective effect against both cerulein- and PDL-induced pancreatitis; this protective effect is accompanied by a decrease in pro-inflammatory cytokine release and reduced PSC activation and ECM deposition (23). PTHrP also exerts pro-inflammatory effects in the injured kidney, and in atherosclerosis and rheumatoid arthritis (24–28).
A positive feedback loop linking TGF-β and PTHrP was first described in breast cancer, where PTHrP was identified as an effector of TGF-β in bone metastases (29). Later studies have shown that TGF-β regulates PTHrP expression in multiple cell types, including chondrocytes, and hepatocellular carcinoma and hepatoma cells (30,31). Using the PTHrP signaling inhibitor PTHrP (7-34) and acinar cells with Pthrp gene deletion, here we investigated the role of PTHrP in the inflammatory and fibrotic effects conferred by activation of the TGF-β signaling axis in the pancreas.
Bone morphogenetic proteins (BMPs) are members of TGF-β superfamily with anti-fibrogenic functions in multiple organs (32–35). The BMP antagonist gremlin is regulated by TGF-β and exerts pro-fibrotic effects in multiple organs, including pancreas (36–39). Gremlin plays a pivotal role in maintaining balanced TGF-β/BMP signaling (40). Gremlin also exerts pro-inflammatory effects by activating the vascular endothelial growth factor receptor-2 (VEGFR2) signaling pathway (41). Here we investigated the cross-talk between PTHrP and TGF-β/BMP-2/gremlin signaling in acinar cells and PSCs, and asked whether the observed pro-inflammatory and pro-fibrotic effects of PTHrP involve modulation of this signaling loop.
MATERIALS AND METHODS
Materials
Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Norcross, GA). Tissue culture supplies were purchased from Gibco (Carlsbad, CA). Tamoxifen was obtained from Cayman Chemicals (San Antonio, TX). PTHrP (1-36) and PTHrP (7-34) were purchased from Polypeptide Laboratories (Torrance, CA) and Bachem (Torrance, CA), respectively. TGF-β, BMP-2, and gremlin were obtained from R&D Systems (Minneapolis, MN), and SIS3 was from Sigma (St. Louis, MO). The antibodies against PTHrP and gremlin used for Western blotting were from Santa Cruz Biotechnologies (Santa Cruz, CA), and Abcam (Cambridge, MA), respectively. The antibodies for Western blotting for phospho(p)Smad3 and Smad3 were from Cell Signaling Technology, Inc. (Billerica, MA). The anti-gremlin neutralizing antibody was from R&D Systems. For chromatin immunoprecipitation (ChIP), the ChIP assay kit and the anti-acetyl histone H3 antibody were from Millipore (Temecula CA). The Smad2/3 antibody was from Santa Cruz Biotechnologies.
Generation of mice with conditional knockout of the Pthrp gene in pancreatic acinar cells
All mice were housed in the animal facility at the University of Texas Medical Branch (UTMB) and were handled according to National Institutes of Health and UTMB guidelines. All procedures were approved by the Institutional Animal Care and Use Committee at the UTMB. Pthrp+/flox mice, generously provided by Dr. A. Karaplis, McGill University (42–44), were crossed with CD-1 mice. The heterozygous offspring was crossed with inducible-Cre transgenic mice [STOCK Tg(Ela1-Cre/ESR1)1Stof/J, Jackson Lab Stock Number 008861]. These mice have a tamoxifen-inducible Cre-mediated recombination system which is driven by the rat elastase 1 pancreatic promoter (45). The double heterozygous offspring were intercrossed to obtain the Pthrpflox/flox;Cre+ (homozygous) and Pthrpflox/flox;Cre− (control) mice used in this study. These mice were generated in collaboration with the Transgenic Mouse Facility at UTMB (directed by Dr. M. Wakamiya). The characterization of these mice has been described (23).
Induction of Cre recombinase activity
Cre recombinase activity was induced in Pthrpflox/flox;Cre+ mice by intraperitoneal (i.p.) injection of tamoxifen (20mg/ml, 100μl/mouse), once daily for 5 days (46). Corn oil was used as vehicle control. Previous studies established that similar responses were obtained from wild-type CD-1 mice and Pthrpflox/flox;Cre− mice injected with tamoxifen or corn oil and from Pthrpflox/flox;Cre+ injected with corn oil (23). Therefore, the latter mice were used as controls in this study.
Isolation, culture, and quality control of mouse acinar and stellate cells
Pancreata removed from 3–4 mice sacrificed under anesthesia were washed quickly with 3 ml of warm isolation buffer [PBS containing Ca2+ and Mg2+, soybean trypsin inhibitor (0.01%, Sigma) and 0.1% BSA] and digested for 15 min in warm isolation buffer containing collagenase type IV (0.3 mg/ml, Invitrogen). The digestion was facilitated mechanically with continuous pipetting. Collagenase was inactivated by addition of 6 ml cold isolation buffer. The cell suspension was washed 3x with cold isolation buffer and filtered to remove large debris. Acini were collected by centrifugation, resuspended in DMEM containing 10% FBS and 0.025% trypsin inhibitor, and seeded into multi-well plates coated with laminin. Acinar cells were characterized by measuring viability and amylase release in response to cerulein stimulation. Viability, assessed by trypan blue exclusion, was routinely >98%. Amylase levels were measured using the Phadebas® Amylase Test kit (Lund, Sweden) as described (23).
Mouse PSCs were isolated using the outgrowth method (47,48). Pancreatic tissue was rinsed 3x with DMEM + 1% penicillin/streptomycin (Life Technologies-Invitrogen). The tissue was cut into 1–2 mm3 blocks, washed 3x with DMEM + 1% penicillin/streptomycin and placed in 75-cm2 tissue culture flasks. The tissue was cultured in DMEM containing 10% FBS, 50 μg/ml gentamycin (Life Technologies-Gibco), and 1% penicillin/streptomycin at 37°C. The medium was changed every 3 days starting on day 3. When the colonies reached 80–90% confluence, tissue clumps were removed, and the cells were sub-cultured using 0.025% trypsin/EDTA (Life Technologies). The characterization of mouse PSCs has been described (8,22). The identity of PSCs was confirmed using vimentin as marker.
Cell culture and treatment
For primary acinar cell culture, cells were plated onto 6-well dishes coated with 50 μg/ml laminin (Life Technologies-Invitrogen) and were cultured at 37 °C in a humidified 95% O2/5% CO2 atmosphere in DMEM medium supplemented with L-glutamine and 10% FBS. Treatment was initiated 24 h after plating. Primary PSCs were plated onto 6-well dishes and were cultured under the same conditions as the acinar cells. Treatment was initiated when cells reached 70–80% confluence. Acinar cells were treated with TGF-β (1 and 3 ng/ml), PTHrP (10−7 M), gremlin (1 μg/ml), or BMP-2 (50 and 100 ng/ml) for periods of 0.25 h to 24 h. PSCs were treated with TGF-β (1 and 3 ng/ml), PTHrP (10−7 M), or BMP-2 (50 and 100 ng/ml) for periods of 0.25 h to 48 h. In some experiments, the cells were pre-treated for 1 h with the PTH1R antagonist PTHrP (7-34) (10−5 M; Bachem) or with the Smad3 inhibitor, SIS3 (10 μM). The vehicle control for TGF-β, BMP-2, and gremlin was 4 mM HCl containing 1 mg/ml BSA. PTHrP (1-36) and PTHrP (7-34) were dissolved in water.
AR42J cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA), and were grown at 37 °C in a humidified 95% O2/5% CO2 atmosphere in RPMI-1640 medium supplemented with L-glutamine and 10% FBS. The irPSCc3 rat pancreatic stellate cell line was obtained from Dr. Raul A. Urrutia (Mayo Clinic Cancer Center, MN) (49) and was grown under the same conditions in DMEM high glucose medium supplemented with 10% FBS and L-glutamine. AR42J and irPSCc3 cells were plated in 6-well dishes, and treatment was initiated when cells reached 70–80% confluence. The cells were serum-starved for 16 h prior to treatment, and then treated with PTHrP (1-36), TGF-β, or BMP-2 in the presence or absence of PTHrP (7-34) or SIS3 as described above. AR42J were also treated with gremlin (1 μg/ml). In some experiments, AR42J cells were pre-incubated with anti-gremlin neutralizing antibody (3 μg/ml; R&D Systems) for 2 h before addition of gremlin, TGF-β or PTHrP. For analysis of PTHrP protein levels, acinar cells and PSCs were treated with TGF-β for 18 h in the presence of the proteasome inhibitor lactacystin (5 μM). For analysis of gremlin protein levels, acinar cells were treated with PTHrP (1-36) (10−7 M) for 24 h.
Analysis of mRNA levels
Total RNA was extracted using the RNAqueous® isolation kit (Ambion Inc., Austin, TX), per the manufacturer’s protocol. RNA concentrations were determined by spectrophotometry. RNA (2.0 μg) was reverse transcribed into cDNA using the Applied Biosystems cDNA synthesis kit, following the manufacturer’s protocol. The first-strand cDNA was used as a template for real-time PCR on an Applied Biosystems 7500 Real-Time PCR System using Sybr green Supermix (Applied Biosystems) and the following published primer sequences: mouse PTHrP: forward, CAGTGGAGTGTCCTGGTATT; reverse, GATCTCCGCGATCAGATGGT (50), and rat PTHrP: forward, CAGCCGAAATCAGAGCTACC; reverse, CTCCTGTTCTCTGCGTTTCC (51). The source of the primer sequences for mouse and rat IL-6, ICAM-1, procollagen I, fibronectin, actin and GAPDH is listed in (22). The following gremlin primers were used: mouse: forward, GCAACAGCCGCACTATCA; reverse, CCAAGTCGATGGATATGC (52), and rat: forward: CCAGCAGCTGAAGGGAAAAAGAAA; reverse, TGGCCGTAACAGAAGCGATTGA (53). The threshold cycle (CT) values for each target gene were normalized to levels of β-actin or GAPDH, and the relative expression level of each target gene was calculated using the formula n-fold change = 2−ΔCT, where ΔCT represents CT (target sample) − CT (control).
Western blot analysis
Cells were washed twice with cold PBS on ice and lysed in RIPA buffer containing a Protease Inhibitor cocktail and Phosphatase Inhibitor cocktails A and B (Santa Cruz Biotechnology). Protein concentrations were then estimated using the Bio-Rad protein assay. Protein levels were analyzed by Western blot analysis. GAPDH was used as loading control. The signals were detected using the SuperSignal West Pico Substrate kit (Pierce Biotechnology Inc., Rockford, IL). Densitometric analysis was performed using the Alpha Innotech Image Analysis system (Alpha Innotech Corporation, San Leandro, CA).
Plasmid constructs and cell transfection
Constructs containing regions from the promoter 1, 2 or 3 of the human PTHrP gene, cloned in the luciferase reporter plasmid pGL-2 (Promega, Madison, WI), were obtained from Dr. Z. Bouizar. These constructs have been described (54,55). These constructs, as well as the empty vector control, were electroporated into AR42J and irPSCc3 cells. Cells were co-electroporated with a construct expressing Renilla Luciferase for standardization purposes.
The following conditions were used: 10 μg promoter construct and 0.5 μg Renilla construct, and electroporating ~ 1 × 107 cells at 250 V, 1500 μF, 125 ℧ and 25 ms pulse. After electroporation, an equal number of cells was plated into 24-well plates in complete medium containing 10% FBS. TGF-β (3 ng/ml) was added to irPSCc3 cells 24 h after electroporation. AR42J cells were transferred to serum-free medium 16 h after plating. After 24 h, they were treated with TGF-β (3 ng/ml). HCl (4 mM) containing 1 mg/ml BSA was used as the vehicle control (final volume 0.01% v/v). After 2 h, cell lysates were prepared and promoter activity was assayed using the Dual Luciferase assay kit (Promega). Empty vector control values were subtracted from the respective firefly and Renilla luciferase values. The firefly luciferase activity corresponding to each PTHrP promoter was normalized to Renilla luciferase activity, and the fold differences were plotted as the firefly/Renilla ratio.
Chromatin Immunoprecipitation
AR42J and irPSCc3 cells were plated onto 6-well dishes under conditions described in Cell Culture and Treatment. At 70–80% confluence, the cells (~ 1 × 106) were transferred to serum-free medium for 16 h, then treated with TGF-β for 1–4 h. 4 mM HCl containing 1 mg/ml BSA was used as vehicle control. ChIP was performed following the protocol from Millipore. In brief, the histones were cross-linked by adding 37% formaldehyde to a final concentration of 1%. The cells were then washed twice with PBS containing protease inhibitors, scraped and centrifuged. The cell pellet was resuspended in SDS Lysis Buffer and incubated on ice for 10 min. The resuspended cells were sonicated to shear DNA in order to obtain genomic DNA fragments ranging in size from 200 bp to 1 kb. The sonicated cell extracts were immunoprecipitated using an anti-Smad2/3 antibody (Santa Cruz Biotechnologies) or an anti-acetyl histone H3 antibody (Millipore). Normal mouse IgG was used as negative control. The chromatin immunoprecipitates were washed and cross-links were reverted by heating at 65°C for 4 h. The samples were then treated with proteinase K and DNA was purified by phenol/chloroform extraction and ethanol precipitation in the presence of glycogen as carrier. The DNA was resuspended in 50 μl of TE buffer. Real-time PCR was then performed on an Applied Biosystems 7500 Real-Time PCR System using Sybr green Supermix (Applied Biosystems), 0.5 μl of ChIP DNA and the following primers specific for the PTHrP P3 promoter: forward, GACACACGCACTTGAAACTTG; reverse, ATAGCTGTCTGTCTACCTCCTC. The PCR reaction was performed in triplicate for each independent ChIP preparation. Values were calculated using the fold-enrichment method, where Raw Ct = (Ct IP) − (Ct mock) and Fold Enrichment = (2−DDCt).
Statistics
Numerical data are presented as the mean ± standard error of the mean (S.E.M). The data were analyzed by one-way analysis of variance (ANOVA) followed by a Tukey-Kramer multiple comparisons post-test to determine the statistical significance of differences. All statistical analyses were performed using INSTAT Software (GraphPad Software, Inc., San Diego, CA).
RESULTS
TGF-β upregulates PTHrP expression in acinar cells and PSCs
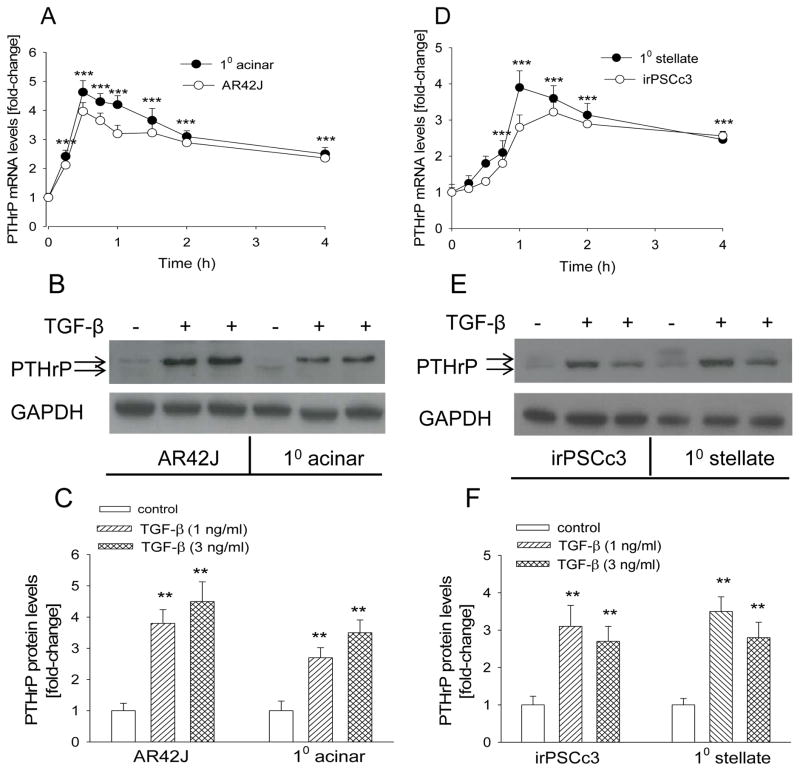
A characteristic of acinar cells is amylase release in response to stimulation with physiological concentrations of the CCK analog cerulein (56). Supplementary Fig. 1A (Supplementary Digital Content 1) shows that treatment of the acinar cells with cerulein increased amylase release, with a peak at 10−10 M cerulein. The response of AR42J cells to cerulein has been described (57). To ask if TGF-β regulates PTHrP expression, acinar cells were treated with TGF-β (3 ng/ml). We show that TGF-β caused a significant 4–5-fold increase in PTHrP mRNA levels in primary acinar cells and AR42J cells (P < 0.001) (Fig. 1A). This effect was evident after 15 min of treatment, peaked at 30 min, and was still evident after 4 h (Fig. 1A). TGF-β also caused a significant increase in PTHrP levels at the protein level (P < 0.01), as monitored by Western blot analysis (Fig. 1, B and C).
Figure 1. Effect of TGF-β on PTHrP mRNA and protein levels in acinar cells and PSCs.
(A,D) Cells were treated with TGF-β (3 ng/ml) for the indicated time intervals. PTHrP mRNA levels were measured by reverse transcription/real-time PCR. Values are expressed relative to the vehicle control value, set arbitrarily at 1.0. (B,E) Western blot analysis for PTHrP in cells treated with TGF-β (1 or 3 ng/ml) for 18 h. − = vehicle control. The figure is representative of data obtained from 3 independent experiments. (C,F) Densitometric analysis of Western blots. 10 = primary cells. Values are expressed relative to the 0 time point (A, D) or the −TGF-β control value (C, F), set arbitrarily at 1.0. Each point or bar is the mean ± SEM of three independent experiments. ***, P < 0.001 vs. 0 time-point (A,D); **, P < 0.01 vs. control (C,F).
We also assessed the effect of TGF-β on PTHrP levels in PSCs. The identity of the primary PSCs was confirmed by staining for vimentin (Supplementary Fig. 1B; see Supplementary Digital Content 2). irPSCc3 cells have been characterized and are responsive to TGF-β (49). TGF-β significantly increased PTHrP mRNA (P < 0.001) and protein (P < 0.01) levels in primary PSCs and irPSCc3 cells (Fig. 1, D–F). A similar time-course profile as in acinar cells was observed.
TGF-β increases transcriptional activity from the PTHrP promoter 3 through a Smad3-dependent pathway
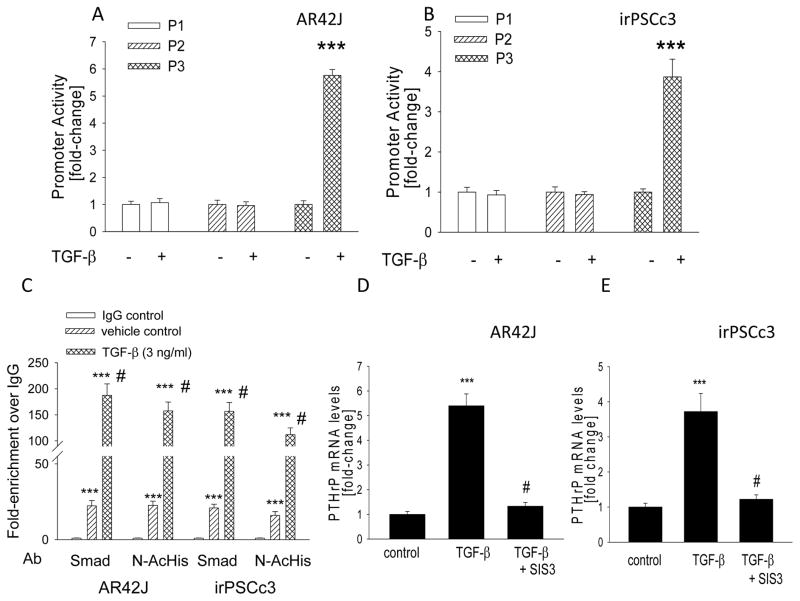
TGF-β regulates the Smad signaling pathway (58), and the PTHrP promoter 3 (P3) contains a Smad3 response element (59). To ask if TGF-β upregulates the activity of this promoter, AR42J and irPSCc3 cells were transfected with a construct encompassing this promoter region. PTHrP promoters 1 and 2, which do not contain putative Smad sites, were used as controls. Treatment with TGF-β resulted in an ~ 6-fold (AR42J cells) and ~4-fold (irPSCc3 cells) increase in Luciferase activity from P3 (P < 0.001) (Fig. 2, A and B). In contrast, TGF-β had no significant effect (P > 0.05) on luciferase activity from promoters 1 and 2 (Fig. 2, A and B).
Figure 2.
(A,B) Effect of TGF-β on PTHrP promoter activity in AR42J and irPSCc3 cells. Cells were transfected with the indicated promoter-driven luciferase reporter constructs plus Renilla luciferase construct, then treated with TGF-β (3 ng/ml). Luciferase activity was measured after 2 h. Empty vector control values were subtracted from the respective firefly and Renilla luciferase values. Values were then normalized to Renilla luciferase activity, and are expressed as the firefly/Renilla ratio. P = promoter. Each bar is the mean ± SEM of three experiments. ***, P < 0.001 vs. −TGF-β. (C) ChIP assay of chromatin from AR42J and irPSCc3 cells treated with TGF-β. Cells were treated with TGF-β for 1 h. Immunoprecipitation was performed using an anti-Smad2/3 antibody (Smad) and an anti-acetyl histone H3 antibody (N-AcHis). Normal IgG was used as antibody control. DNA enriched by ChIP was subjected to real-time PCR using a PTHrP P3 promoter primer. Data are presented as fold-enrichment vs. the IgG value, set arbitrarily as 1.0. Each bar is the mean ± SEM of three experiments. ***, P < 0.001 vs. IgG control, #, P < 0.001 vs. vehicle control. (D,E) Effect of inhibiting Smad3 signaling in AR42J and irPSCc3 cells on TGF-β-mediated upregulation of PTHrP mRNA levels. Cells pre-treated with SIS3 (10 μM) for 1 h were then treated with TGF-β for 2 h. PTHrP mRNA levels were measured by reverse transcription/real-time PCR. Values are expressed relative to the vehicle control value, set arbitrarily at 1.0. Each bar is the mean ± SEM of three independent experiments. ***, P < 0.001 vs. control; #, P < 0.001 vs. TGF-β alone.
Transcriptional activation of the PTHrP P3 promoter by TGF-β was further investigated by ChIP. TGF-β significantly increased recruitment of Smad to the PTHrP P3 promoter (P < 0.001) as early as 1 h after treatment in both AR42J and irPSCc3 cells (Fig. 2C). Levels were still elevated 4 h after treatment (data not shown). TGF-β also significantly increased recruitment of acetyl Histone H3 to the PTHrP P3 promoter (P < 0.001) in both cell lines; again, an effect was observed after 1 h of treatment (Fig. 2C) and was still evident after 4 h (data not shown).
To further assess the role of Smad signaling in the effects of TGF-β on PTHrP expression, AR42J and irPSCc3 were treated with TGF-β in the presence of the specific Smad3 inhibitor SIS3 (60). SIS3 inhibited the effects of TGF-β on PTHrP expression in both cell lines, such that there was no significant difference (P > 0.05) in PTHrP levels in untreated cells vs. cells treated with TGF-β + SIS3 (Fig. 2, D and E).
PTHrP plays a role in the TGF-β-induced upregulation of IL-6 and ICAM-1 levels in acinar cells
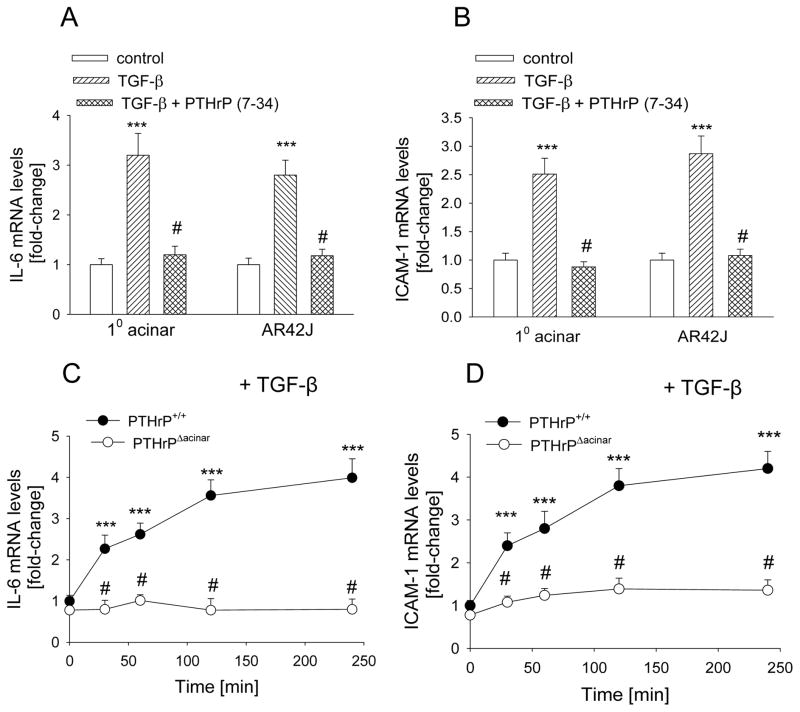
TGF-β levels are upregulated in both AP and CP (14–16). TGF-β plays a role in the inflammatory response associated with pancreatitis (6,7), and upregulates PTHrP levels (Fig. 1). PTHrP regulates levels of the pro-inflammatory cytokines IL-6 and ICAM-1 in acinar cells (22). To ask if PTHrP plays a role in the TGF-β-mediated effects on these cytokines, acinar cells were pre-treated with the PTHrP antagonist PTHrP (7-34), then treated with TGF-β. Treatment with TGF-β (3 ng/ml) for 2 h increased IL-6 and ICAM-1 levels by ~ 3-fold in primary acinar cells and AR42J cells (P < 0.001) (Fig. 3, A and B). When cells were pre-treated with PTHrP (7-34), the effects of TGF-β on IL-6 and ICAM-1 levels were significantly attenuated, such that there was no significant difference (P > 0.05) in IL-6 and ICAM-1 levels in control (vehicle-treated) cells vs. cells co-treated with TGF-β plus PTHrP (7-34) (Fig. 3, A and B). PTHrP (7-34) alone had no significant effect on IL-6 and ICAM-1 mRNA levels (data not shown).
Figure 3.
(A, B) Effect of inhibition of PTHrP signaling on the TGF-β-mediated increase in IL-6 and ICAM-1 mRNA levels in acinar cells. Cells were pre-treated with 10−5 M PTHrP (7-34) for 1 h, then treated in the presence of PTHrP (7-34) with TGF-β (3 ng/ml) for 2 h. 10 = primary cells. (C,D) Effect of TGF-β on IL-6 and ICAM-1 mRNA levels in PTHrPΔacinar cells and PTHrP+/+ acinar cells. IL-6 and ICAM-1 mRNA levels were measured by reverse transcription/real-time PCR. Values are expressed relative to the control value (A, B) or the respective 0 time value (C,D), set arbitrarily at 1.0. Each bar or point is the mean ± SEM of three independent experiments. ***, P < 0.001 vs. control (A,B) or 0 time-point (C,D); #, P < 0.01 vs. TGF-β alone (A,B) or PTHrP+/+ value (C,D).
To further ask if PTHrP plays a role in the TGF-β effects on IL-6 and ICAM-1 levels, the response to TGF-β was compared in PTHrPΔacinar vs. PTHrP+/+ acinar cells. The characterization of these cells has been described (23). Treatment with TGF-β significantly increased IL-6 mRNA levels in PTHrP+/+ acinar cells (P < 0.001). This effect was observed after 30 min of treatment, and was still elevated after 4 h of treatment (Fig. 3C). The effect of TGF-β on IL-6 levels was significantly attenuated in PTHrPΔacinar cells, such that only an ~1.3 fold-increase in IL-6 levels was observed (P > 0.05) (Fig. 3C). A similar profile was observed for ICAM-1, where TGF-β had no significant effect (P > 0.05) on ICAM-1 levels in PTHrPΔacinar cells. In contrast, TGF-β increased ICAM-1 levels by ~ 4-fold in PTHrP+/+ acinar cells (P < 0.001) (Fig. 3D).
PTHrP plays a role in the TGF-β-induced upregulation of procollagen I and fibronectin levels in PSCs
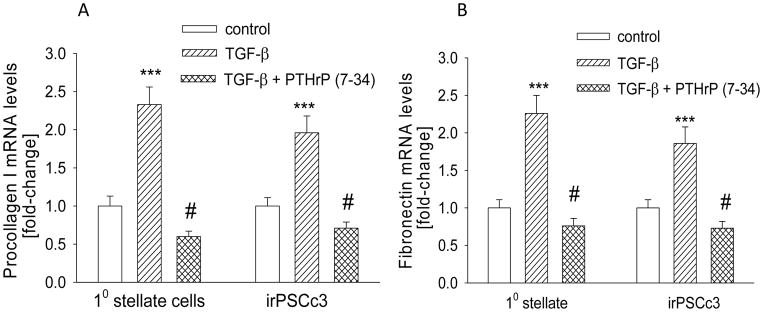
TGF-β plays a significant role in the fibrosis associated with pancreatitis (10–13). TGF-β also induces PTHrP expression in PSCs (Fig. 1, D–F). To determine the role of PTHrP in the TGF-β-mediated regulation of ECM proteins, primary PSCs and irPSCc3 cells were pre-treated with PTHrP (7-34), then treated with TGF-β. TGF-β increased levels of procollagen I and fibronectin by ~2.5-fold in both primary PSCs and irPSCc3 cells (P < 0.001) (Fig. 4, A and B). This effect was significantly attenuated after pretreatment with PTHrP (7-34), such that procollagen I and fibronectin levels were not significantly different (P > 0.05) in vehicle-treated control cells vs. cells treated with TGF-β + PTHrP (7-34) (Fig. 4, A and B). PTHrP (7-34) alone had no significant effect on procollagen I or fibronectin levels (data not shown).
Figure 4.
Effect of inhibition of PTHrP signaling on the TGF-β-mediated increase in procollagen I (A) and fibronectin (B) mRNA levels in PSCs. Cells were pre-treated with 10−5 M PTHrP (7-34) for 1 h, then treated in the presence of PTHrP (7-34) with TGF-β (3 ng/ml) for 2 h. 10 = primary cells. Procollagen I and fibronectin mRNA levels were measured by reverse transcription/real-time PCR. Values are expressed relative to the control value, set arbitrarily at 1.0. Each bar is the mean ± SEM of three independent experiments. ***, P < 0.001 vs. control; #, P < 0.01 vs. TGF-β alone.
PTHrP upregulates gremlin levels in acinar cells
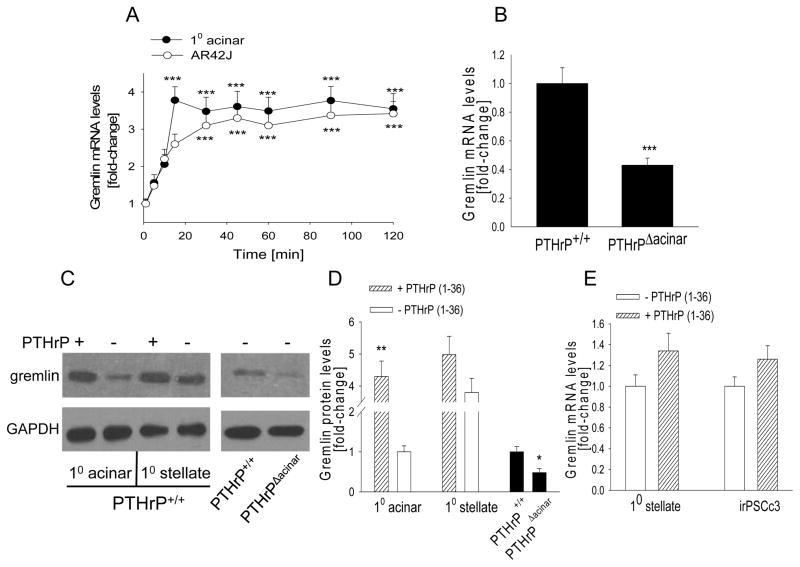
Expression of the BMP antagonist gremlin is regulated by TGF-β (36–38). Since TGF-β upregulates PTHrP expression, we asked if gremlin levels are modulated by PTHrP, indicating the possible involvement of PTHrP in the TGF-β-gremlin signaling axis. Primary acinar and AR42J cells were treated with 10−7 M PTHrP (1-36). PTHrP significantly increased gremlin mRNA (P < 0.001) and protein levels (P < 0.01) in primary acinar cells (Fig. 5, A, C, D) and AR42J cells (Fig. 5A, data not shown). We also compared gremlin mRNA levels in acinar cells from PTHrPΔacinar mice and PTHrP+/+ mice. Deletion of the Pthrp gene in acinar cells resulted in an ~2-fold decrease in gremlin mRNA (P < 0.001) and protein (P < 0.05) levels (Fig. 5, B–D).
Figure 5. Effect of PTHrP on gremlin mRNA levels in acinar cells and PSCs.
(A) Acinar cells were treated with PTHrP (1-36) (10−7 M) for the indicated time intervals. (B) Gremlin mRNA levels in acinar cells isolated from PTHrPΔacinar and PTHrP+/+ mice. (C) Western blot analysis for gremlin in acinar cells and PSCs treated with PTHrP for 24 h, and in cells isolated from PTHrPΔacinar and PTHrP+/+ mice. − = control. The figure is representative of data obtained from 3 independent experiments. (D) Densitometric analysis of Western blots. (E) PSCs were treated with PTHrP (1-36) (10−7 M) for 24 h. 10 = primary cells. (A,B,E) mRNA levels were measured by reverse transcription/real-time PCR. Values are expressed relative to the 0 time point (A), the PTHrP+/+ value (B), the - PTHrP (1-36) value for acinar cells (D), and the - PTHrP value (E), set arbitrarily at 1.0. Each point or bar is the mean ± SEM of three independent experiments. ***, P < 0.001 vs. 0 time-point (A) or PTHrP+/+ value (B); **, P < 0.01 vs. respective - PTHrP (1-36) value (D); *, P < 0.05 vs. PTHrP+/+ (D).
Gremlin mRNA (data not shown) and protein (Fig. 5, C and D) levels were ~ 4-fold higher in PSCs vs. acinar cells. PTHrP treatment increased gremlin mRNA (Fig. 5E) and protein (Fig. 5, C and D) levels by ~1.3-fold in PSCs (P > 0.05) (Fig. 5, C–E); this increase was evident only with longer treatment periods (24 to 48 h). No effect was seen at shorter treatment periods (data not shown). Since PTHrP had no significant effect on gremlin levels in PSCs, investigation of the TGF-β/PTHrP/gremlin axis was only further studied in acinar cells
PTHrP plays a role in the TGF-β-mediated upregulation of gremlin expression in acinar cells
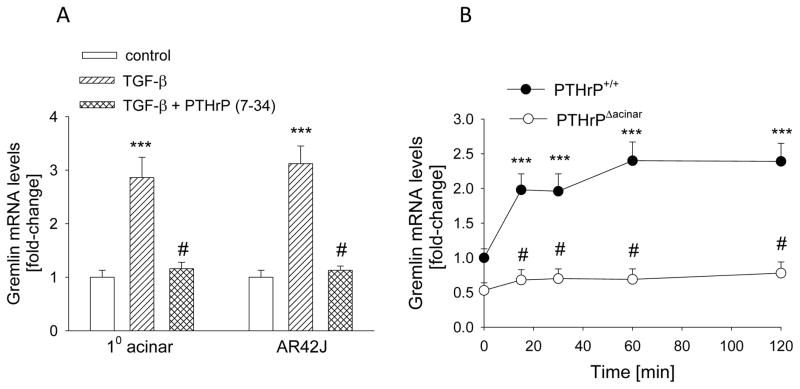
Since TGF-β upregulates PTHrP levels and PTHrP increases gremlin levels, we asked if PTHrP plays a role in the effects of TGF-β on gremlin. Acinar cells were pre-treated with PTHrP (7-34) for 1 h, then treated with TGF-β (3 ng/ml) for 2 h. TGF-β increased gremlin levels by ~ 3-fold in primary acinar and AR42J cells (P < 0.001) (Fig. 6A). When cells were pre-treated with PTHrP (7-34), the effects of TGF-β on gremlin levels were significantly attenuated; there was no significant difference (P > 0.05) in gremlin levels in control cells vs. cells co-treated with TGF-β and PTHrP (7-34) (Fig. 6A). PTHrP (7-34) alone had no significant effect on gremlin mRNA levels (data not shown).
Figure 6. Effect of inhibiting PTHrP signaling on the TGF-β-mediated increase in gremlin levels in acinar cells.
(A) Cells were pre-treated with 10−5 M PTHrP (7-34) for 1 h, then treated in the presence of PTHrP (7-34) with TGF-β (3 ng/ml) for 2 h. 10 = primary cells. (B) Gremlin levels in acinar cells from PTHrPΔacinar mice and PTHrP+/+ mice treated with TGF-β for the indicated time intervals. (A,B) Gremlin mRNA levels were measured by reverse transcription/real-time PCR. Values are expressed relative to the control value (A) or the PTHrP+/+ 0 time-point (B), set arbitrarily at 1.0. Each bar or point is the mean ± SEM of three independent experiments. ***, P < 0.001 vs. control (A) or 0 time-point (B); #, P < 0.01 vs. TGF-β alone (A) or PTHrP+/+ value (B).
To further ask if PTHrP plays a role in the TGF-β-mediated effects on gremlin, the response to TGF-β was compared in PTHrPΔacinar cells and PTHrP+/+ acinar cells. Treatment with TGF-β increased gremlin mRNA levels by ~2.5 fold in PTHrP+/+ acinar cells (P < 0.001) (Fig. 6B). These effects of TGF-β were significantly attenuated in PTHrPΔacinar cells, such that TGF-β had no significant effect (P > 0.05) on gremlin levels in these cells (Fig. 6B).
Gremlin plays a role in TGF-β and PTHrP action in acinar cells
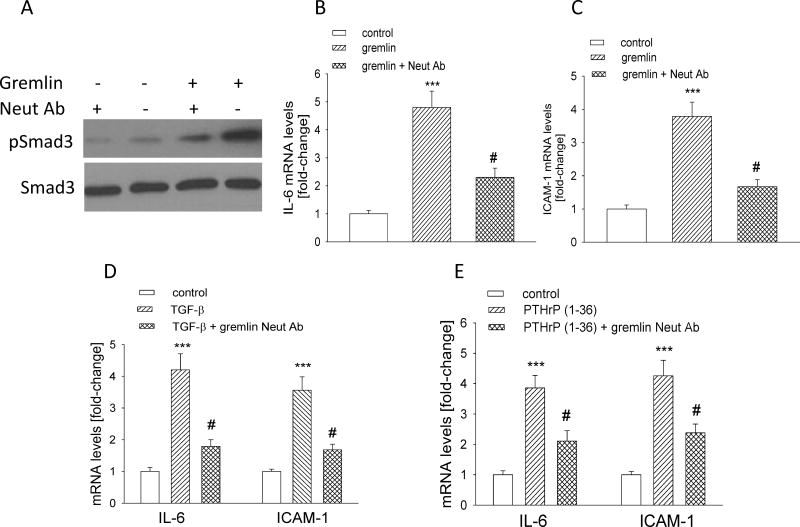
Treating AR42J cells with gremlin (1 μg/ml) increased pSmad levels; this effect was accompanied by an ~ 4–5-fold increase in IL-6 and ICAM-1 mRNA levels (P < 0.001) (Fig. 7, A–C). Pre-incubation with a neutralizing anti-gremlin antibody for 2 h attenuated the gremlin effects on pSmad3, as well as on IL-6 and ICAM-1 mRNA levels (Fig. 7, A–C). We next investigated the role of gremlin in the effects of TGF-β and PTHrP on IL-6 and ICAM-1 levels. AR42J cells were pre-incubated with neutralizing anti-gremlin antibody, then treated with TGF-β (3 ng/ml for 2 h) or PTHrP (10−7 M for 1 h). Inhibiting secreted gremlin action suppressed the effects of both TGF-β and PTHrP on IL-6 and ICAM-1 levels; there was a significant difference (P < 0.05) in IL-6 and ICAM-1 levels in cells treated with TGF-β or PTHrP alone vs. those treated with TGF-β or PTHrP plus neutralizing anti-gremlin antibody (Fig. 7, D and E). Thus, gremlin may function downstream of TGF-β and PTHrP to regulate cytokine levels in acinar cells.
Figure 7. Effect of inhibiting gremlin action on the effects of TGF-β and PTHrP on IL-6 and ICAM-1 levels in AR42J cells.
(A) Western blot analysis for pSmad3 and total Smad3 levels in cells pre-incubated with a neutralizing anti-gremlin antibody (3 μg/ml) for 2 h, then treated with gremlin (1 μg/ml) for 10 min. The figure is representative of data obtained from 3 independent experiments. (B,C) Effect of inhibiting gremlin signaling on IL-6 and ICAM-1 mRNA levels. Cells pre-treated as in (A) were treated with gremlin for 30 min. (D,E) Effect of inhibiting gremlin signaling on the TGF-β- (D) and PTHrP- (E) mediated upregulation of IL-6 and ICAM-1 levels. Cells pre-treated as in (A) were treated with TGF-β (2 h) or PTHrP (1 h). In (B–E), mRNA levels were measured by reverse transcription/real-time PCR. Values are expressed relative to the control value, set arbitrarily at 1.0. Each bar is the mean ± SEM of three independent experiments. *** P, < 0.001 vs. control (B–E); #, P < 0.05 vs. gremlin (B,C), TGF-β (D), or PTHrP (E) alone.
BMP-2 downregulates PTHrP levels in PSCs
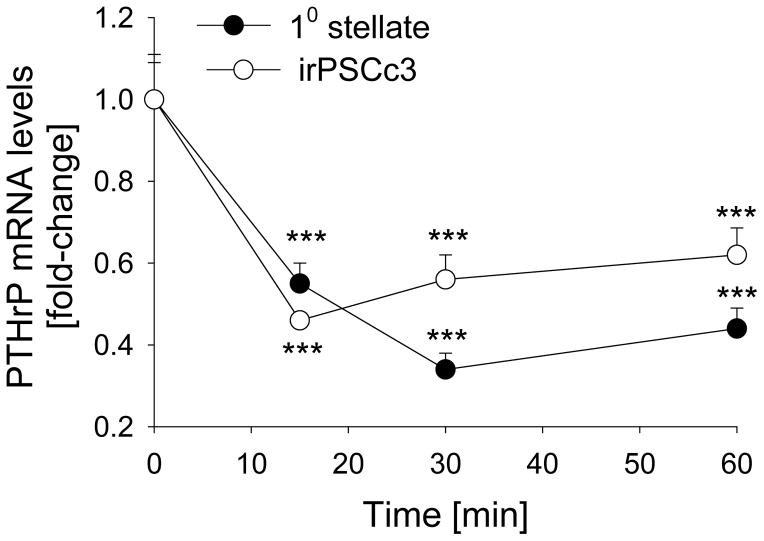
Since TGF-β upregulates PTHrP levels, and BMP-2 attenuates the effects of TGF-β on PSC activation (α-SMA levels) and fibronectin levels (8), we asked if BMP-2 regulates PTHrP mRNA levels in PSCs. We show that treatment with BMP-2 (50 ng/ml) decreased PTHrP mRNA levels by ~ 3-fold (P < 0.001) (Fig. 8). This effect was evident after 15 min of treatment. PTHrP mRNA levels were still suppressed after 1 h, and had returned to baseline levels after 2 h treatment (Fig. 8; data not shown). Similar effects were obtained with 100 ng/ml BMP-2 (data not shown). In contrast, BMP-2 at 50 ng/ml and 100 ng/ml did not decrease PTHrP mRNA levels at any of the time-points tested in primary acinar or AR42J cells (data not shown).
Figure 8. Effect of BMP-2 on PTHrP mRNA levels in PSCs.
Primary (10) PSCs and irPSCc3 cells were treated with BMP-2 (50 ng/ml) for the indicated time intervals. PTHrP mRNA levels were measured by reverse transcription/real-time PCR. Values are expressed relative to the vehicle control value, set arbitrarily at 1.0. Each point is the mean ± SEM of three independent experiments. ***, P < 0.001 vs. 0 time-point.
DISCUSSION
CP is a debilitating disease with no cure, and treatment options are limited to supportive care and symptom palliation. Only a fraction of persons exposed to risk factors develop CP, suggesting differential regulation of endogenous signaling pathways which alter the susceptibility to pancreatic damage after exposure to risk factors. Development of new therapies to target CP can only be achieved through understanding of the interaction between endogenous signaling pathways involved in the pro-inflammatory and pro-fibrotic response.
Levels of TGF-β are elevated in the pancreas of patients with clinical and histological evidence of AP and CP (14–16). PTHrP levels are elevated in mouse models of AP and CP (22,23). Here we show that TGF-β upregulates PTHrP levels in acinar cells and PSCs. These effects of TGF-β on PTHrP expression involve transcriptional activation of the PTHrP-promoter 3 through a Smad3-dependent pathway. In contrast, TGF-β had no significant effect on transcriptional activity from the other two PTHrP promoters. Similar effects of TGF-β on PTHrP expression have been reported in MDA-MB-231 cells (59). Moreover, PTHrP plays a role in the effects of TGF-β on pro-inflammatory cytokine levels in acinar cells and ECM protein levels in PSCs, as evident using cells with suppressed PTHrP signaling or expression.
Damaged acinar cells can activate PSCs both directly, through secretion of pro-inflammatory cytokines which exert paracrine effects in PSCs, and indirectly, via inflammatory cell recruitment (6). TGF-β increases IL-6 release in airway smooth muscle cells (61). We show that TGF-β upregulates IL-6 expression in acinar cells, and that PTHrP plays a role in this effect in that suppressing PTHrP expression or signaling negates these TGF-β effects. We postulate that TGF-β expressed in response to acinar cell injury may upregulate PTHrP levels in these cells, in turn leading to increased IL-6 secretion and PSC activation. In support, previous studies have shown that exogenous addition of PTHrP (1-36) to acinar cells upregulates IL-6 and ICAM-1 levels (22), which in turn activate PSCs (9). Alternatively, PTHrP secreted by acinar cells in response to TGF-β may function directly via a paracrine pathway to activate PSCs and upregulate ECM protein expression. In support, treatment of PSCs with PTHrP (1-36) increases procollagen I and fibronectin levels (22).
TGF-β is secreted by both acinar cells and PSCs in response to injury (7,11). Acinar cell- or PSC-secreted TGF-β may then upregulate PTHrP expression in PSCs. The secreted PTHrP then functions via an autocrine/paracrine pathway to active PSCs. In support, exogenous PTHrP (1-36) increases procollagen I and fibronectin levels in PSCs (22). In this study, we show that TGF-β upregulates PTHrP levels and PTHrP promoter-3 activity in these cells, and that PTHrP (7-34) suppresses the effects of TGF-β on procollagen I and fibronectin mRNA levels. These pathways of TGF-β/PTHrP action support the postulated involvement of two distinct pathways in the pathogenesis of pancreatic fibrosis, with PTHrP potentially playing a role in both pathways. The first is the necro-inflammatory pathway involving cytokine release and PSC activation, and the second involves a non-necro-inflammatory pathway with direct activation of PSCs following pancreatic injury (62,63). TGF-β also exerts anti-inflammatory effects (64); these effects of TGF-β are likely mediated via a PTHrP-independent pathway. The role of PTHrP in the TGF-β effects will be directly addressed in future studies by monitoring pancreatic damage in response to TGF-β in PTHrPΔacinar mice.
TGF-β-induced PSC activation and ECM formation is inhibited by BMP-2 via a Smad1-dependent pathway (8). Using a model of chronic cerulein-induced CP, Gao et al. (8) have shown that pancreatic fibrosis, PSC activation, and leukocyte infiltration was enhanced in mice heterozygous for deletion of the BMP-2 type 2 receptor (BMPR2+/−), indicating a protective effect of BMP signaling in the pancreas. Moreover, BMP-2 inhibited TGF-β-induced fibronectin production in wild-type PSCs; this effect was abolished in BMPR2+/− PSCs (8). BMPs exert anti-fibrogenic functions in multiple organs (32–35). In the kidney, BMP-2 inhibits renal fibrosis in the rat unilateral urethral obstruction model and antagonizes TGF-β-induced renal fibrogenic signals in renal interstitial fibroblast cells (34, 65). BMPs also prevent differentiation and cell migration of lung fibroblasts (66). Here we show that BMP-2 downregulates PTHrP expression in PSCs. Based on these data, we postulate that a feedback mechanism linking TGF-β/BMP-2 signaling with the PTHrP pathway plays a key role in fibrosis in the injured pancreas.
The BMP antagonist gremlin plays a pivotal role in maintaining balanced TGF-β/BMP signaling and modulates BMP activity (33,40). Multiple studies have established a role for gremlin in fibrotic disease. Pancreatic, liver, lung fibrosis is associated with induction of gremlin (33,39,67,68); levels of the BMP antagonists chordin and noggin are unchanged (33). Gremlin levels are elevated in a mouse model of cerulein-induced CP and in pancreata from CP patients (39). Additionally, gremlin induces expression of FN27 and several collagens (36) and Grem1 knockout mice have decreased pancreatic fibrosis after cerulein-induced CP (39). TGF-β increases gremlin levels in PSCs, glaucomatous trabecular meshwork cells, kidney, and vascular smooth muscle cells (36–39). In the kidney, gremlin functions as a downstream mediator of TGF-β’s pro-fibrotic effects (38). We show that TGF-β also upregulates gremlin levels in acinar cells, and that this effect is linked to pro-inflammatory cytokine release. Moreover, the effects of TGF-β on gremlin are suppressed in the absence of PTHrP expression or signaling, and inhibiting gremlin signaling attenuates the effects of TGF-β on IL-6 and ICAM-1. We therefore propose an axis linking PTHrP with the TGF-β-mediated regulation of gremlin.
Given the pro-fibrotic effect of gremlin, its upregulation by PTHrP in acinar cells supports the hypothesis that gremlin may play a role in the pro-fibrotic effects of PTHrP. Since PTHrP primarily regulates gremlin expression in acinar cells, we postulate that gremlin secreted by acinar cells in response to PTHrP may then function via a paracrine pathway to regulate ECM levels in PSCs. Gremlin also exerts a direct effect in acinar cells, and functions downstream of TGF-β and PTHrP to induce IL-6 and ICAM-1 levels. These secreted cytokines may both exert a direct effect in acinar cells and function via a paracrine pathway to activate PSCs. Gremlin specifically binds to and inhibits the actions of BMP-2, -4, and -7 (69,70), and the relationship between BMP signaling and PTHrP is further demonstrated by the observations that BMP-2 downregulates PTHrP expression in PSCs. However, gremlin also functions via BMP-independent pathways (41). Since BMP-2 does not suppress PTHrP expression in acinar cells, the effects of gremlin in these cells may not involve the BMP signaling pathway. Overall, these data also demonstrate the intricate relationship between acinar cells and PSCs in the regulation of fibrosis.
In conclusion, the net increase in PTHrP levels as a result of pancreatic damage sets up a series of cascades that lead to both pro-inflammatory and pro-fibrotic effects. PTHrP may in turn function through different effectors in acinar cells and PSCs. Thus, PTHrP only upregulates gremlin in acinar cells. Secreted gremlin may then function via a paracrine pathway to activate PSCs and increase ECM protein levels, ultimately leading to fibrosis. Gremlin also mediates the effects of TGF-β and PTHrP on pro-inflammatory cytokine levels. BMP-2 suppression of PTHrP levels is only observed in PSCs, this effect may be secondary to inhibition of TGF-β signaling by BMP-2 in these cells (8). Suppressing TGF-β signaling in the pancreas may result in significant deleterious effects. Given the critical role of the TGF-β/BMP/gremlin signaling pathway in pancreatitis, and the intricate relationship of PTHrP signaling in this pathway, targeted inhibition of PTHrP signaling in acinar cells may present a novel therapeutic strategy aimed at preventing pancreatic inflammation and fibrosis, irrespective of the primary risk factor(s) involved. This may ultimately prevent development of CP.
Supplementary Material
Acknowledgments
This work was supported by NIH grant DK035608.
We thank Dr. A.C. Karaplis (McGill University) and Dr. J.J. Wysolmerski (Yale University) for providing the Pthrp+/flox mice. We also acknowledge the expertise of Dr. M. Wakamiya and the Transgenic Mouse Facility at UTMB for generating the PTHrPflox/floxCre+ mice, and the Histology Core at UTMB for immunofluorescence.
Footnotes
The authors declare no conflict of interest.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
Contributor Information
Vandanajay Bhatia, Department of Pharmacology and Toxicology, The University of Texas Medical Branch, Galveston, Texas
Yanna Cao, Department of Surgery, University of Texas Health Science Center at Houston, Houston, Texas
Tien C. Ko, Department of Surgery, University of Texas Health Science Center at Houston, Houston, Texas
Miriam Falzon, Department of Pharmacology and Toxicology, The University of Texas Medical Branch, Galveston, Texas
REFERENCES CITED
- 1.Whitcomb DC. Mechanisms of disease: advances in understanding the mechanisms leading to chronic pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2004;1:46–52. doi: 10.1038/ncpgasthep0025. [DOI] [PubMed] [Google Scholar]
- 2.Leung PS, Ip SP. Pancreatic acinar cells: its role in acute pancreatitis. Int J Biochem Cell Biol. 2006;38:1024–1030. doi: 10.1016/j.biocel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Apte M, Pirola R, Wilson J. The fibrosis of chronic pancreatitis: new insights into the role of pancreatic stellate cells. Antioxidant Redox Signal. 2011;15:2711–2722. doi: 10.1089/ars.2011.4079. [DOI] [PubMed] [Google Scholar]
- 4.Omary MB, Lugea AL, Lowe AW, et al. The pancreatic stellate cell: a star on the rise in pancreatic disease. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 6.Patel M, Fine DR. Fibrogenesis in the pancreas after acinar cell injury. Scand J Surg. 2005;94:108–111. doi: 10.1177/145749690509400205. [DOI] [PubMed] [Google Scholar]
- 7.Yu JH, Kim KH, Kim H. SOCS 3 and PPAR-γ ligands inhibit the expression of IL-6 and TGF-β1 by regulating JAK2/STAT3 signaling in pancreas. Int J Biochem Cell Biol. 2008;40:677–688. doi: 10.1016/j.biocel.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Gao X, Cao Y, Yang W, et al. BMP2 inhibits TGF-β-induced pancreatic stellate cell activation and extracellular matrix formation. Am J Physiol Gastrointest Liver Physiol. 2013;304:G804–G813. doi: 10.1152/ajpgi.00306.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mews P, Phillips P, Fahmy R, et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535–541. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagashio Y, Ueno H, Imamura M, et al. Inhibition of transforming growth factor β decreases pancreatic fibrosis and protects the pancreas against chronic injury in mice. Lab Invest. 2004;84:1610–1618. doi: 10.1038/labinvest.3700191. [DOI] [PubMed] [Google Scholar]
- 11.Shek FW, Benyon RC, Walker FM, et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787–1798. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelmann R, Ruf D, Wagner M, et al. Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-beta1 transgenic mouse model. Am J Physiol Gastrointest Liver Physiol. 2001;280:G164–G172. doi: 10.1152/ajpgi.2001.280.1.G164. [DOI] [PubMed] [Google Scholar]
- 13.Yoo BM, Yeo M, Oh TY, et al. Amelioration of pancreatic fibrosis in mice with defective TGF-beta signaling. Pancreas. 2005;30:e71–e79. doi: 10.1097/01.mpa.0000157388.54016.0a. [DOI] [PubMed] [Google Scholar]
- 14.Slater SD, Williamson RC, Foster CS. Expression of transforming growth factor-beta 1 in chronic pancreatitis. Digestion. 1995;56:237–241. doi: 10.1159/000201249. [DOI] [PubMed] [Google Scholar]
- 15.Van Laethem JL, Deviere J, Resibois A, et al. Localization of transforming growth factor beta 1 and its latent binding protein in human chronic pancreatitis. Gastroenterology. 1995;108:1873–1881. doi: 10.1016/0016-5085(95)90152-3. [DOI] [PubMed] [Google Scholar]
- 16.Friess H, Lu Z, Riesle E, et al. Enhanced expression of TGF-βs and their receptors in human acute pancreatitis. Ann Surg. 1998;227:95–104. doi: 10.1097/00000658-199801000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foley J, Wysolmerski JJ, Broadus AE, et al. Parathyroid hormone-related protein: an update. Clin Endocrinol Metab. 2012;97:2947–2956. doi: 10.1210/jc.2012-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannstadt M, Jüppner H, Gardella TJ. Receptors for PTH and PTHrP. Am J Physiol. 1999;277:F665–675. doi: 10.1152/ajprenal.1999.277.5.F665. [DOI] [PubMed] [Google Scholar]
- 19.Cebrian A, Garcia-Ocano A, Takane KK, et al. Overexpression of parathyroid hormone-related protein inhibits pancreatic β-cell death in vivo and in vitro. Diabetes. 2002;51:3003–3013. doi: 10.2337/diabetes.51.10.3003. [DOI] [PubMed] [Google Scholar]
- 20.Clemens TL, Cormier S, Eichinger A, et al. Parathyroid hormone-related protein and its receptors: nuclear functions and roles in the renal and cardiovascular systems, the placental trophoblasts and the pancreatic islets. Br J Phamacol. 2001;134:1113–1136. doi: 10.1038/sj.bjp.0704378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasavada RC, Cavaliere C, D’Ercole AJ, et al. Overexpression of parathyroid hormone-related protein in the pancreatic islet of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J Biol Chem. 1996;271:1200–1208. doi: 10.1074/jbc.271.2.1200. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia V, Kim SOK, Aronson JF, et al. Role of parathyroid hormone-related protein in the pro-inflammatory and pro-fibrogenic response associated with acute pancreatitis. Reg Pept. 2012;175:49–61. doi: 10.1016/j.regpep.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatia V, Rastellini C, Han S, et al. Acinar cell-specific knockout of the Pthrp gene decreases the pro-inflammatory and pro-fibrotic response in pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G533–G549. doi: 10.1152/ajpgi.00428.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funk JL. A role for parathyroid hormone-related protein in the pathogenesis of inflammatory/autoimmune disease. Immunopharmacology. 2001;1:1101–1121. doi: 10.1016/s1567-5769(01)00040-6. [DOI] [PubMed] [Google Scholar]
- 25.Funk JL, Wei H, Downey KJ, et al. Expression of PTHrP and its cognate receptor in the rheumatoid synovial microcirculation. Biochem Biophys Res Commun. 2002;297:890–897. doi: 10.1016/s0006-291x(02)02263-5. [DOI] [PubMed] [Google Scholar]
- 26.Martin-Ventura JL, Ortego M, Esbrit P, et al. Possible role of parathyroid hormone-related protein as a proinflammatory cytokine in atherosclerosis. Stroke. 2003;34:1783–1789. doi: 10.1161/01.STR.0000078371.00577.76. [DOI] [PubMed] [Google Scholar]
- 27.Rámila D, Ardura JA, Esteban V, et al. Parathyroid hormone-related protein promotes inflammation in the kidney with an obstructed ureter. Kidney Int. 873:835–47. doi: 10.1038/sj.ki.5002775. 200. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida T, Sakamotoa H, Horiuchi T, et al. Involvement of prostaglandin E2 in interleukin-1a-induced parathyroid hormone-related peptide production in synovial fibroblasts of patients with rheumatoid arthritis. J Clin Endocrinol Metab. 2001;86:3272–3278. doi: 10.1210/jcem.86.7.7687. [DOI] [PubMed] [Google Scholar]
- 29.Yin JJ, Selander K, Chirgwin JM, et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serra R, Karaplis A, Sohn P. Parathyroid hormone–related peptide (PTHrP)-dependent and -independent effects of transforming growth factor β (TGF-β) on endochondral bone formation. J Cell Biol. 1999;145:783–794. doi: 10.1083/jcb.145.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Y, Zhang W, Gao X, et al. PTHrP is a novel mediator for TGF-β-induced apoptosis. Regul Pept. 2013;184:40–46. doi: 10.1016/j.regpep.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinoshita K, Iimuro Y, Otogawa K, et al. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut. 2007;56:706–714. doi: 10.1136/gut.2006.092460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myllarniemi M, Lindholm P, Ryynanen MJ, et al. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:321–329. doi: 10.1164/rccm.200706-945OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang YL, Liu YS, Chuang LY, et al. Bone morphogenetic protein-2 antagonizes renal interstitial fibrosis by promoting catabolism of type I transforming growth factor-beta receptors. Endocrinology. 2009;150:727–740. doi: 10.1210/en.2008-0090. [DOI] [PubMed] [Google Scholar]
- 35.Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 36.Sethi A, Jain A, Zode GS, et al. Role of TGFβ/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Invest Ophthalm Vis Sci. 2011;52:5251–5259. doi: 10.1167/iovs.11-7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maciel TT, Melo RS, Schor N, et al. Gremlin promotes vascular smooth muscle cell proliferation and migration. J Mol Cell Cardiol. 2008;44:370–379. doi: 10.1016/j.yjmcc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Carvajal G, Droguett A, Burgos ME, et al. Gremlin: a novel mediator of epithelial mesenchymal transition and fibrosis in chronic allograft nephropathy. Transplant Proc. 2008;40:734–739. doi: 10.1016/j.transproceed.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 39.Staloch D, Gao X, Liu K, et al. Gremlin is a key pro-fibrogenic factor in chronic pancreatitis. J Mol Med (Berl) 2015 Jul 5; doi: 10.1007/s00109-015-1308-9. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 40.Costello CM, Cahill E, Martin F, et al. Role of gremlin in the lung. Development and disease. Am J Respir Cell Mol Biol. 2010;42:517–523. doi: 10.1165/rcmb.2009-0101TR. [DOI] [PubMed] [Google Scholar]
- 41.Lavoz C, Alique M, Rodrigues-Diez R, et al. Gremlin regulates renal inflammation via the vascular endothelial growth factor receptor 2 pathway. J Pathol. 2015;236:407–420. doi: 10.1002/path.4537. [DOI] [PubMed] [Google Scholar]
- 42.Miao D, He B, Jiang Y, et al. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1-34. J Clin Invest. 2005;115:2402–2411. doi: 10.1172/JCI24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Karaplis AC, Huang DC, et al. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. J Clin Invest. 2001;121:4655–4669. doi: 10.1172/JCI46134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He B, Deckelbaum RA, Miao D, et al. Tissue-specific targeting of the PTHrP gene: the generation of mice with floxed alleles. Endocrinology. 2001;142:2070–2077. doi: 10.1210/endo.142.5.8146. [DOI] [PubMed] [Google Scholar]
- 45.Desai BM, Oliver-Krasinski J, De Leon DD, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet β cell, regeneration. J Clin Invest. 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madisen L, Zwingman TA, Sunkin SM. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–432. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- 48.Kruse ML, Hildebrand PB, Timke C, et al. Isolation, long-term culture, and characterization of rat pancreatic fibroblastoid/stellate cells. Pancreas. 2001;23:49–54. doi: 10.1097/00006676-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Mathison A, Liebl A, Bharucha J, et al. Pancreatic stellate cell models for transcriptional studies of desmoplasia-associated genes. Pancreatology. 2010;10:505–516. doi: 10.1159/000320540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bai XH, Wang DW, Kong L, et al. ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol Cell Biol. 2009;29:4201–4219. doi: 10.1128/MCB.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meziani F, Tesse A, Welsch S, et al. Expression and biological activity of parathyroid hormone-related peptide in pregnant rat uterine artery: any role for 8-iso-prostaglandin F2α? Endocrinology. 2008;149:626–633. doi: 10.1210/en.2007-0568. [DOI] [PubMed] [Google Scholar]
- 52.Lau AW, Pringle LM, Quick L, et al. Tre17/ubiquitin-specific protease 6 (USP6) oncogene translocated in aneurysmal bone cyst blocks osteoblastic maturation via an autocrine mechanism involving bone morphogenetic protein dysregulation. J Biol Chem. 2010;285:37111–37120. doi: 10.1074/jbc.M110.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maciel TT, Melo RS, Campos AH. The bone morphogenetic protein antagonist gremlin promotes vascular smooth muscle cell apoptosis. J Vasc Res. 2009;46:325–332. doi: 10.1159/000189793. [DOI] [PubMed] [Google Scholar]
- 54.Cataisson C, Gordon J, Roussiére M, et al. Ets-1 activates parathyroid hormone-related protein gene expression in tumorigenic breast epithelial cells. Mol Cell Endo. 2002;204:155–168. doi: 10.1016/s0303-7207(02)00298-8. [DOI] [PubMed] [Google Scholar]
- 55.Bhatia V, Mula RV, Falzon M. 1,25-Dihydroxyvitamin D3 regulates PTHrP expression via transcriptional, post-transcriptional and post-translational pathways. Mol Cell Endo. 2011;342:32–40. doi: 10.1016/j.mce.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grady T, Liang P, Ernst SA, et al. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology. 1997;113:1966–1975. doi: 10.1016/s0016-5085(97)70017-9. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Yang L, Chen K-L, et al. Knockdown of GRP78 promotes apoptosis in pancreatic acinar cells and attenuates the severity of cerulein and LPS induced pancreatic inflammation. PLoS One. 2014;9:e92389. doi: 10.1371/journal.pone.0092389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signaling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 59.Lindemann RK, Ballschmieter P, Nordheim A, et al. Transforming growth factor β regulates parathyroid hormone-related protein expression in MDA-MB-231 breast cancer cells through a novel Smad/Ets synergism. J Biol Chem. 2001;276:46661–46670. doi: 10.1074/jbc.M105816200. [DOI] [PubMed] [Google Scholar]
- 60.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-β1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 61.Michaeloudes C, Sukkar MB, Khorasani NM, et al. TGF-β regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L295–L304. doi: 10.1152/ajplung.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haber PS, Keogh GW, Apte MV, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087–1095. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Apte MV, Wilson JS. Stellate cell activation in alcoholic pancreatitis. Pancreas. 2003;27:316–320. doi: 10.1097/00006676-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 65.Yang YL, Ju HZ, Liu SF, et al. BMP-2 suppresses renal interstitial fibrosis by regulating epithelial-mesenchymal transition. J Cell Biochem. 2011;112:2558–2565. doi: 10.1002/jcb.23180. [DOI] [PubMed] [Google Scholar]
- 66.Shlyonsky V, Soussia IB, Naeije R, et al. Opposing effects of bone morphogenetic protein-2 and endothelin-1 on lung fibroblast chloride currents. Am J Respir Cell Mol Biol. 2011;45:1154–1160. doi: 10.1165/rcmb.2010-0443OC. [DOI] [PubMed] [Google Scholar]
- 67.Boers W, Aarrass S, Linthorst C, et al. Transcriptional profiling reveals novel markers of liver fibrogenesis. Gremlin and insulin-like growth factor-binding proteins. J Biol Chem. 2006;281:16289–16295. doi: 10.1074/jbc.M600711200. [DOI] [PubMed] [Google Scholar]
- 68.Koli K, Myllarniemi M, Vuorinen K, et al. Bone morphogenetic protein-4 inhibitor gremlin is overexpressed in idiopathic pulmonary fibrosis. Am J Pathol. 2006;169:61–71. doi: 10.2353/ajpath.2006.051263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Q, Shi Y, Wada J, et al. In vivo delivery of Gremlin siRNA plasmid reveals therapeutic potential against diabetic nephropathy by recovering bone morphogenetic protein-7. PLoS One. 2010;5:e11709. doi: 10.1371/journal.pone.0011709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsu DR, Economides AN, Wang X, et al. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998;1:673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.