Abstract
This study tested the hypothesis that supportive family environments during adolescence buffer exposure to racial discrimination, reducing its impact on biological weathering and its manifestation in cellular aging. Perceived racial discrimination, support in the family environment, and confounder variables were assessed for 3 consecutive years across adolescence in two independent cohorts of African American youth from rural Georgia. DNA was extracted from peripheral blood mononuclear cells collected during young adulthood. Patterns of methylation were used to index the epigenetic ages of these cells and the extent to which they differed from participants’ chronological ages. Among youth in supportive family environments, exposure to higher levels of racial discrimination did not forecast greater epigenetic aging. Among youth in less supportive family environments, exposure to higher levels of racial discrimination did forecast greater epigenetic aging. The associations emerged independently of confounder variables, and the results were replicated across the two cohorts.
Keywords: adolescent development, environmental effects, minority groups, racial and ethnic attitudes and relations, relationship quality
Compared with members of other racial groups, African Americans experience more aging-related diseases earlier in life, at greater severity, and with more serious consequences (Schuster et al., 2012). According to life-course and developmental perspectives, this disproportionate disease risk can be traced to systematic disadvantage and social inequities starting at conception and continuing throughout childhood and adolescence (Priest et al., 2013). According to a “weathering” hypothesis that has been advanced to explain how disadvantage and inequities presage health disparities (Geronimus, Hicken, Keene, & Bound, 2006), cumulative stress experienced early in life weathers physiological systems, resulting in the premature aging of cells and leading eventually to a shortened life expectancy.
The health-risk inequities that African Americans experience likely arise from more than class disadvantage alone. Psychological stressors that disproportionately affect African Americans have been suggested as a mechanism that weathers physiological systems and increases vulnerability to poor health. Racial discrimination presents daily challenges in the lives of many African American youth and their families. Landrine and Klonoff (1996) found that 98% of the African American adults in their sample reported experiencing a discriminatory event during the past year. All participants reported that, at some point in their lives, they had been treated badly or their intentions had been misinterpreted because of their race. Although this study was conducted 20 years ago, recent reports indicate that racial discrimination remains a persistent, if not escalating, experience for African Americans (Williams & Mohammed, 2013). Discriminatory incidents are demeaning and induce stress, frustration, depression, and anxiety in adults (Pascoe & Smart Richman, 2009) and in adolescents (Priest et al., 2013).
An association between racial discrimination and health-relevant physiological outcomes is also plausible. Research conducted primarily with adults has demonstrated that discrimination is associated with a range of physiological health markers, including neuroendocrine risk markers for poor birth outcomes, elevated glucocorticoids, pro-inflammatory cytokines, C-reactive protein (a measure of systemic inflammation), and other markers of inflammation (Lewis, Cogburn, & Williams, 2015; Pascoe & Smart Richman, 2009). Even worrying about the prospect of encountering discrimination is associated with heightened cardiovascular responses, poor sleep quality, and hypertension (Lewis et al., 2015). Indices of social rejection and discrimination have also been found to be associated with health indicators in Caucasians (Pascoe & Smart Richman, 2009). Together, these studies support the importance of understanding the biological weathering effects of exposure to racial discrimination during adolescence, a developmental stage in which African American youth become keenly aware of their treatment by other individuals and are particularly cognizant of targeted rejection (Stevenson, 2004).
A review of recent cross-sectional and longitudinal studies involving adolescents reveals considerable heterogeneity in their amount of exposure to interpersonal discrimination (Brody et al., 2014; Smith-Bynum, Lambert, English, & Ialongo, 2014). These studies show that African American adolescents’ histories of interpersonal discrimination are best characterized in terms of qualitatively different categories; many youth experience initially low levels of racial discrimination that increase over time, and others experience more frequent racial discrimination that remains stable over time. Moreover, studies (Brody et al., 2014; Greene, Way, & Pahl, 2006) have found associations between high exposure to interpersonal discrimination and indicators of psychological adjustment and cardiometabolic risk. These results suggest that an understanding of the contribution of racial discrimination to health-relevant biological-weathering outcomes requires attention to different levels of exposure to discrimination.
Given the heterogeneity reported in the literature, we did not expect a single continuous distribution to represent adequately adolescents’ level of exposure to discrimination. Instead, we expected some youth to experience discrimination at initially low levels that increased across adolescence, and others to encounter initially high levels that either remained stable or increased across time. None of the literature we reviewed led us to believe that a subgroup of adolescents would experience decreases in interpersonal discrimination. We hypothesized that youth who encountered more frequent exposures to interpersonal discrimination across adolescence would be at greater risk of biological weathering. This conjecture was based on the premise that frequent exposure to discrimination leads to frequent activation of stress-response systems, whose hormonal end products (cortisol, epinephrine, norepinephrine) contribute to the weathering process, in which the body’s cells, tissues, and organs age prematurely (Geronimus et al., 2006).
Not all adolescents exposed to high levels of interpersonal discrimination, however, will evince biological weathering. The stress-coping model that has framed much of the research on the effects of discrimination (Clark, Anderson, Clark, & Williams, 1999) posits that discriminatory experiences, like other recurrent stressors, deplete both adults’ and adolescents’ coping resources, and that the impact of discrimination on health depends on the availability of countervailing contextual supports and the protection they confer. For African American youth living in either nonmetropolitan or urban areas, supportive family environments, characterized by emotional support, low levels of conflict, and an organized, predictable home environment, promote psychological resilience to cumulative contextual stressors (Brody, Kogan, & Grange, 2012). Evidence from several recent studies also suggests that family support can offset some of the hormonal, metabolic, inflammatory, and cardiovascular changes that follow childhood adversity. In particular, family support buffers the effects of childhood maltreatment on allostatic load (Carroll et al., 2013) and the effects of low childhood socioeconomic status (SES) on genomic pro-inflammatory signaling profiles (Chen, Miller, Kobor, & Cole, 2011) and on metabolic profiles (Miller et al., 2011) in adulthood. It is thus plausible that supportive family environments during adolescence have a buffering effect that reduces the impact of elevated exposure to racial discrimination on biological weathering and its manifestation in cellular aging.
Using a multiwave prospective research design with two samples of African American youth, we tested the hypothesis that supportive family environments reduce the likelihood of cellular aging among African American youth who report frequent encounters with racial discrimination. A secondary purpose of this study was to test the replicability of our results across two independent samples of African American youth. To measure cellular aging, we used a recently developed epigenetic biomarker derived from the DNA methylation pattern of immune-system cells. Epigenetics refers to modifications in DNA, such as methylation, that do not involve changes in the DNA nucleotide sequence. Methylation is a process whereby methyl groups bind to the cytosine residues found in DNA, and in so doing alter a cell’s ability to activate particular genes. On the basis of the notion that methylation reflects, in part, the amount of turmoil to which a cell has been exposed, researchers have used methylation to assess cellular aging. This marker has been validated in cells from diverse tissues and reflects the disparity between an individual’s biological and chronological ages (Hannum et al., 2013). The markers that make up the epigenetic-aging index lie within or near genes with known functions in aging-related conditions, including Alzheimer’s disease, cancer, DNA damage, tissue degradation, and oxidative stress. Consistent with the weathering hypothesis, the results of a study of five longitudinal cohorts comprising more than 4,500 Caucasian participants showed that those whose immune cells were more epigenetically aged experienced higher rates of all-cause mortality across time (Marioni et al., 2015).
Method
Overview
Data reported in this study were drawn from two longitudinal research projects: the Strong African American Healthy Adult Project (SHAPE; n = 322; Brody et al., 2013) and the Adults in the Making project (AIM; n = 294; Brody, Yu, Chen, Kogan, & Smith, 2012). Racial discrimination and support in the family environment, along with confounder variables, were assessed in SHAPE when the youth were 16 to 18 years of age and in AIM when the youth were 17 to 19 years of age. Participants in each cohort provided antecubital blood samples (at age 20 in SHAPE and age 22 in AIM) that were used to assess epigenetic aging. Information on demographic and biobehavioral confounder variables was gathered to rule out some alternative explanations for the study’s results.
SHAPE and AIM participants
SHAPE
The SHAPE participants and their families resided in nine rural counties in Georgia, in small towns and communities in which poverty rates are among the highest in the nation and unemployment rates are above the national average (Proctor & Dalaker, 2003). Participants were selected randomly from lists of students that schools provided (see Brody et al., 2013, for a full description of recruitment procedures). Recruitment procedures were designed to obtain a sample that was representative of the communities from which it was drawn; no selection criteria were used. Although primary caregivers worked an average of 39.4 hr per week, 46.3% of the participants lived below federal poverty standards, with a median family income of $1,655 per month. From a sample of 561 at the age-18 data collection, 500 participants were selected randomly for subsequent blood draws. The selection of a random subsample was necessary because of financial constraints associated with the costs of DNA methylation assays. Of these 500 participants, 399 (79.8%) provided blood samples. The sample for the present study was composed of 322 participants (138 men and 184 women) from whom blood was drawn at age 20 and for whom data on the other study measures were available from ages 16 to 18. Comparisons between these 322 participants and the 77 participants who were excluded because of incomplete data revealed no significant differences on any of the key or confounder variables.
AIM
For AIM, 496 youth were randomly recruited from public-school lists in six rural Georgia counties; none of these counties were included in SHAPE. Again, the recruitment strategy was designed to obtain a representative sample of youth and families. The primary caregivers worked an average of 38.5 hr per week; 42.0% of the youth lived below federal poverty standards, with a median family income of $2,016 per month. Of the 496 youth who were recruited, 294 (59.3%) provided blood samples at age 22 and had complete data on the other study measures from ages 17 to 19. These 294 participants (107 men and 187 women) constituted our AIM sample. Comparisons of their families with the AIM families not included in the present study revealed no differences on any variables.
Data-collection procedures for SHAPE and AIM
Racial discrimination and support in the family environment, along with confounder variables, were assessed for 3 consecutive years for each cohort—in SHAPE when the youth were 16, 17, and 18 years of age, and in AIM when the youth were 17, 18, and 19 years of age. All data were collected in participants’ homes using standardized protocols. Interviews were conducted privately, with no other family members present or able to overhear the conversation. Participants were compensated $100 at each wave of data collection. Informed-consent forms were completed at all data-collection points. All procedures in both SHAPE and AIM were approved by the University of Georgia Institutional Review Board.
Measures
Perceived racial discrimination
Participants completed nine items from a version of the Schedule of Racist Events (Landrine & Klonoff, 1996) that was designed to be developmentally appropriate for adolescents. This measure has been used previously in longitudinal studies (Brody, Chen, & Kogan, 2010; Brody, Kogan, & Chen, 2012). Items assessed the frequency with which respondents had perceived specific discriminatory events during the previous year, on a scale from 0 (never happened) to 2 (happened a lot). These events included racially based slurs and insults, disrespectful treatment from community members, physical threats, and false accusations from business employees or law-enforcement officials. For example, the items included “Have you been ignored, overlooked, or not given service because of your race?” and “Have you been treated rudely or disrespectfully because of your race?” This questionnaire was completed annually for 3 years. Responses were summed across items to form the perceived-discrimination scale. Alpha coefficients for this scale for SHAPE were .87 to .89 at ages 16 to 18; the coefficients for AIM were .86 to .88 at ages 17 to 19.
Support in the family environment
The family-environment measure was derived from assessments of perceived parental emotional support, parent-child conflict, and disorganization in the home. Parental support was measured using parents’ reports on two scales. The first was the four-item Emotional Support subscale from the Carver Support Scale (Carver, Scheier, & Weintraub, 1989). On a scale ranging from 1 (not at all true) to 5 (very true), parents responded to items such as, “My child discusses his/her feelings with me” and “My child gets sympathy and understanding from me.” Alpha coefficients for this scale for SHAPE were .79 to .81 at ages 16 to 18; for AIM, they were .79 to .82 at ages 17 to 19. Responses were summed across items and were averaged across three assessments. The second scale was the five-item emotional-support subscale from the Family Support Inventory (Wills, Vaccaro, & McNamara, 1992). Using a scale ranging from 1 (not at all true) to 5 (very true), parents rated statements about the support they provided to their children, such as “My child can trust me as someone to talk to” and “When my child feels bad about something, I will listen.” Alpha coefficients for this scale for SHAPE were .82 to .84 at ages 16 to 18; for AIM, they were .79 to .80 at ages 17 to 19. Responses were summed across items and were averaged across three assessments. Ratings on the two emotional-support measures were highly correlated (p < .001); they were standardized and summed to form an indicator of parental emotional support.
Parent-child conflict was measured using an adaptation of the Ineffective Arguing Inventory (Kurdek, 1994). On a scale ranging from 1 (disagree strongly) to 5 (agree strongly), parents rated statements about the conflicts they had with their children, such as “You and your child’s arguments are left hanging and unsettled” and “You and your child go for days being mad at each other.” Alpha coefficients for this scale for SHAPE were .77 to .81 at ages 16 to 18; for AIM, they were .77 to .79 at ages 17 to 19. Responses were summed across items and were averaged across three assessments to form the parent-child conflict scores.
Disorganization in the home was assessed using the 15-item Confusion, Hubbub, and Order Scale (Matheny, Wachs, Ludwig, & Phillips, 1995). Parents were asked to indicate whether each of the 15 statements was true (1) or false (0) about life in their home. The items included “There is often a fuss going on at our home” and “No matter what our family plans, it usually doesn’t seem to work out.” Alpha coefficient for this scale for SHAPE were .71 to .77 at ages 16 to 18; for AIM, they were .72 to .76 at ages 17 to 19. Responses were summed across items and were averaged across three assessments to form the scores for disorganization in the home.
Scores for parental emotional support, parent-child conflict, and disorganization in the home were highly correlated (rs = −.34 to .79 for SHAPE; rs = −.31 to .82 for AIM; all ps < .001). They were standardized, and the conflict and disorganization scores were then subtracted from the parental-support scores to form the scores for support in the family environment. Thus, high scores indicated family environments characterized by high levels of emotional support, low levels of conflict, and high levels of organization and predictability.
Epigenetic aging
When SHAPE participants were 20 years of age and AIM participants were 22 years of age, antecubital blood was drawn by phlebotomists to assess epigenetic aging of immune cells. Peripheral blood mononuclear cells (PBMC) were isolated via density-gradient centrifugation. DNA was extracted (DNA Miniprep Kit; Qiagen, Valencia, CA) and subjected to bisulfite conversion and global amplification (Infinium DNA Methylation Kit; Illumina, Inc., San Diego, CA), followed by hybridization to HumanMethylation450 Beadchips (Illumina, Inc., San Diego, CA). The plates were read on an IScan Station (Illumina, Inc., San Diego, CA) at the University of Minnesota’s Genome Center. Using Genome Studio Version 2009.2 (Illumina, Inc., San Diego, CA) and customized scripts, we normalized the data and excluded unreliable probes. We then derived beta values for the remaining targets (for additional details, see Dogan et al., 2014).
PBMC epigenetic aging was calculated following the methodology of Hannum et al. (2013). Using several large cohorts of adults, they developed and validated a predictive model of aging in cells of the immune system. The model uses a set of 71 methylation values, identified in penalized regression models and subsequent bootstrap analyses as providing the best-fitting diagnostic model of cellular aging. The values derived from this methodology are residuals that form a continuous measure reflecting the discrepancy between an individual’s estimated cellular age and chronological age. For each cohort, these values had a mean of zero; positive and negative deviations reflected faster and slower aging of cells, respectively. As mentioned in the introduction, faster aging as assessed by this metric was shown to presage heightened all-cause mortality risk in five large cohorts (Marioni et al., 2015).
Confounder variables
All analyses controlled for participants’ gender, cumulative SES-related risk, perceived life stress, depressive symptoms, and BMI. Cumulative SES-related risk was defined as the sum of six indicators (0 if absent, 1 if present; see Evans, 2003): current family poverty according to U.S. government criteria, noncompletion of high school or an equivalent by the primary caregiver, current unemployment of the primary caregiver, single-parent family structure, current receipt of Temporary Assistance for Needy Families, and income rated by the primary caregiver as currently inadequate to meet all needs. The average of the indicators across ages (16 to 18 for SHAPE, 17 to 19 for AIM) constituted the measure of SES-related risk.
Participants assessed their life stress using a checklist of 12 events (e.g., acute economic stress, death of a friend, parental divorce, serious injury or illness; Brody et al., 2010), indicating whether each event had occurred during the previous 6 months. The number of endorsed items was tallied at each wave, and these sums were averaged across ages 17 and 18 for SHAPE and ages 17 to 19 for AIM. Exposure to discrimination was not included on the life-stress checklist.
Depressive symptoms were assessed with two instruments. The Child Depression Inventory (CDI; Kovacs, 1979) was administered to SHAPE participants at ages 16 and 17, αs = .86 and .84, respectively, and the 20-item Center for Epidemiologic Studies Depression scale (CES-D; Radloff, 1977) was administered to SHAPE participants at age 18, α = .86, and to AIM participants at ages 17 to 19, α = .85 at each assessment. Depression scores for SHAPE were calculated by averaging the standardized CDI scores at ages 16 and 17 and the standardized CES-D score at age 18. Depression scores for AIM were calculated by averaging the CES-D scores across ages 17 through 19.
BMI was assessed at age 20 for SHAPE participants and at age 22 for AIM participants. The weight and height of each participant were recorded and used to calculate BMI (weight in kilograms divided by the square of height in meters).
Plan of analysis
The data analyses were conducted in three steps. First, latent growth curve models (LGCMs) were executed to characterize the overall experience of racial discrimination across the three waves for each sample. Linear growth models were fit with two individual growth parameters: an intercept parameter representing the level of racial discrimination at the starting point (age 16 for SHAPE and age 17 for AIM) and a linear slope parameter representing the rate at which racial discrimination changed across the three waves of data collection.
Second, latent growth mixture modeling (LGMM) was executed within each sample to identify distinct groups characterized by different levels of exposure to racial discrimination. LGMM determines whether the population under study is composed of distinct classes of individuals based on variation in intercepts, slopes, or both. We compared model fits of one-, two-, and three-class LGMMs, assessing relative fit with conventional indices, including the Bayesian information criterion (BIC) and entropy values. Lower BIC scores indicate better fit, whereas higher entropy scores reflect greater accuracy in classification. Optimal models were chosen on the basis of goodness of fit and parsimony.
Third, we tested our hypotheses with linear regression analyses, including membership in the classes of racial discrimination and the measure of support in the family environment as predictors. Two linear regressions were executed for each sample. Cumulative SES-related risk, gender, perceived life stress, depressive symptoms, and BMI were controlled in all analyses to rule out plausible rival explanations for the findings. The first model was designed to examine the main effects of racial discrimination and support in the family environment on PBMC epigenetic aging. The second model tested the hypothesized interaction of racial discrimination with support in the family environment in forecasting this outcome. All interaction analyses followed the conventions prescribed by Aiken and West (1991); the family-environment variable was first mean centered, and then interaction terms were created as the product of the centered variable and racial-discrimination class membership.
Results
Modeling of perceived racial discrimination
The LGCM analysis for SHAPE revealed that for this sample, racial discrimination increased over time (mean slope = 0.784, p < .001). The analysis also revealed significant variation in the intercept (variance = 10.390, p < .001) and the slope (variance = 2.171, p < .001). For AIM, the LGCM analysis revealed significant variation in the intercept (variance = 12.412, p < .001). Given these findings, the identification of different classes of exposure to discrimination was based on the intercept and slope for SHAPE and the intercept for AIM.
The results for the LGMM analyses are summarized in Table 1. Although the three-class models had the lowest BIC statistics for both studies, the entropy values indicated that the two-class models fit the data better. The two-class models were therefore chosen for both studies.
Table 1.
Results for the Latent Growth Mixture Modeling
| Sample and model | Model-fit indices |
Percentage of sample in
class |
||||
|---|---|---|---|---|---|---|
| Log likelihood | BIC | Entropy | Class 1 | Class 2 | Class 3 | |
| SHAPE | ||||||
| One class | −3,467.68 | 6,983.99 | — | 100 | ||
| Two classes | −3,427.23 | 6,921.34 | .81 | 21.5 | 78.5 | |
| Three classes | −3,409.87 | 6,904.85 | .77 | 16.0 | 18.1 | 65.9 |
| AIM | ||||||
| One class | −3,079.65 | 6,208.74 | — | 100 | ||
| Two classes | −3,057.18 | 6,169.97 | .72 | 34.4 | 65.6 | |
| Three classes | −3,040.71 | 6,155.57 | .61 | 15.7 | 28.6 | 55.7 |
Note: SHAPE = Strong African American Healthy Adult Project; AIM = Adults in the Making project; BIC = Bayesian information criterion.
For SHAPE, the majority of participants (78.5%) were assigned to the low-and-increasing class, which had a low intercept at age 16 (mean intercept = 2.169, SE = 0.186, t = 11.640, p < .001) and increasing exposure to discrimination from ages 16 to 18 (mean slope = 0.961, SE = 0.096, t = 9.986, p < .001). The second group, which comprised 21.5% of the sample, was the high-and-stable class. Youth in this group reported a high and stable level of perceived discrimination over time (mean intercept = 8.610, SE = 0.469, t = 18.343, p < .001; mean slope = 0.160, SE = 0.228, t = 0.700, p = .484). In the regression analyses, membership in the low-and-increasing class was assigned a value of 0; membership in the high-and-stable class was assigned a value of 1.
For AIM, the majority of participants (65.6%) were assigned to the low-and-stable class, which had a relatively low intercept at age 17 (mean intercept = 2.717, SE = 0.316, t = 8.598, p < .001) and little change in discrimination over time (mean slope = 0.013, SE = 0.177, t = 0.073, p = .942). As in SHAPE, the second group (34.4% of the sample), the high-and-stable class, reported a high and stable level of perceived discrimination over time (mean intercept = 9.011, SE = 0.906, t = 9.946, p < .001; mean slope = −0.174, SE = 0.237, t = −0.735, p = .463). Membership in the low-and-stable class was assigned a value of 0; membership in the high-and-stable class was assigned a value of 1. Figure 1 illustrates the discrimination trajectories for the classes identified in the SHAPE and AIM samples.
Fig. 1.
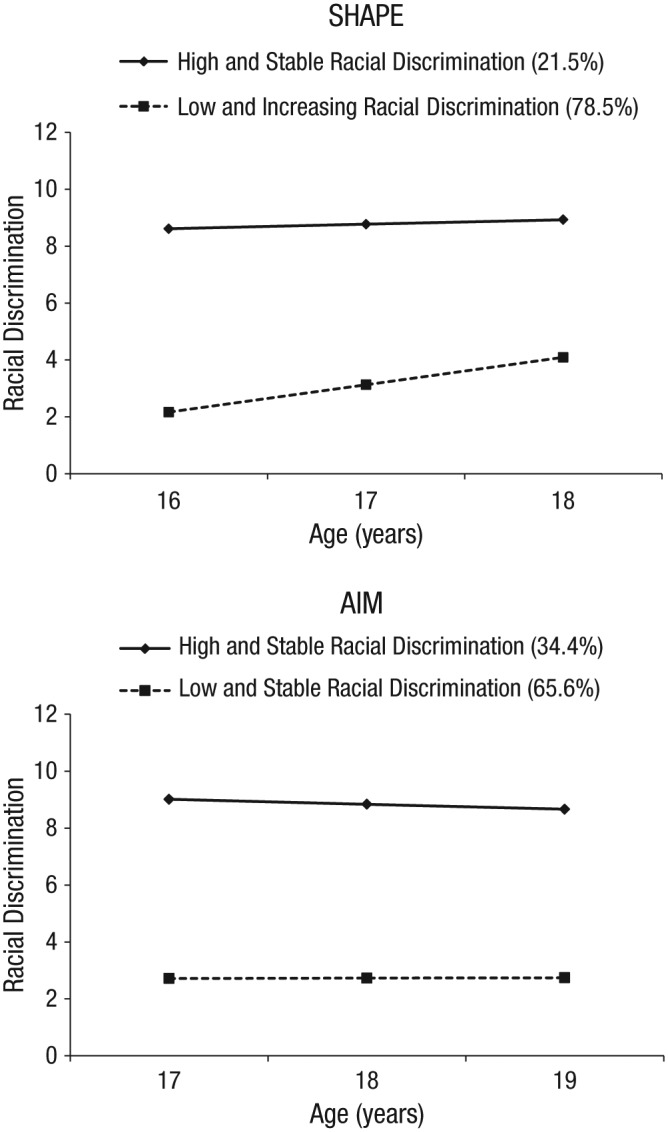
The estimated trajectories of racial discrimination for each class in the Strong African American Healthy Adult Project (SHAPE; top panel) and the Adults in the Making project (AIM; bottom panel).
Test of the hypothesis
Tables 2 and 3 present descriptive statistics for and correlations among the variables for the SHAPE and AIM samples, respectively. In the regression models, PBMC epigenetic aging was predicted from the confounder variables, racial-discrimination class, support in the family environment, and the interaction of the latter two variables. For each sample, Model 1 included racial-discrimination class and support in the family environment only, Model 2 added the confounder variables, and Model 3 added the interaction term. The results of the SHAPE models are presented in Table 4, and the results of the AIM models are presented in Table 5.
Table 2.
Descriptive Statistics and Correlations Among the Variables: Strong African American Healthy Adult Project
| Variable | M | SD | Correlations |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| 1. Epigenetic aging (age 20) | 0 | 5.44 | — | ||||||
| 2. Discrimination classa (ages 16–18) | .21 | .41 | .074 | — | |||||
| 3. Support in the family environment (ages 16–18) | 0.02 | 3.24 | −.064 | −.020 | — | ||||
| 4. Gender (male = 1) | .43 | .50 | .156** | .007 | −.119* | — | |||
| 5. Depressive symptoms (ages 16–18) | 0 | 1.73 | .123* | .222*** | −.232*** | −.039 | — | ||
| 6. Perceived life stress (ages 17–18) | 2.66 | 1.46 | .020 | .224*** | −.035 | .123* | .304*** | — | |
| 7. SES-related risk (ages 16–18) | 2.34 | 1.23 | .073 | −.052 | −.173** | −.056 | .122* | .081 | — |
| 8. Body mass index (age 20) | 28.58 | 8.78 | .139* | .169** | .070 | −.159** | .012 | .050 | .070 |
Note: N = 322. SES = socioeconomic status.
Membership in the high-and-stable class was coded as 1, and membership in the low-and-increasing class was coded as 0.
p ≤ .05, two-tailed. **p ≤ .01, two-tailed. ***p ≤ .001, two-tailed.
Table 3.
Descriptive Statistics and Correlations Among the Variables: Adults in the Making Project
| Variable | M | SD | Correlations |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| 1. Epigenetic aging (age 22) | 0 | 5.26 | — | ||||||
| 2. Discrimination classa (ages 17–19) | .36 | .48 | .127* | — | |||||
| 3. Support in the family environment (ages 17–19) | 0 | 3.16 | −.045 | −.046 | — | ||||
| 4. Gender (male = 1) | .36 | .48 | .119* | .036 | .010 | — | |||
| 5. Depressive symptoms (ages 17–19) | 11.92 | 7.12 | .119* | .209*** | −.219*** | −.085 | — | ||
| 6. Perceived life stress (ages 17–19) | 2.04 | 1.18 | .010 | .218*** | −.110 | .033 | .435*** | — | |
| 7. SES-related risk (ages 17–19) | 2.00 | 1.20 | .129* | −.090 | −.105 | .036 | .195** | .118* | — |
| 8. Body mass index (age 22) | 30.18 | 9.55 | .155** | −.004 | .049 | −.086 | .057 | .030 | −.057 |
Note: N = 294. SES = socioeconomic status.
Membership in the high-and-stable class was coded as 1, and membership in the low-and-stable class was coded as 0.
p ≤ .05, two-tailed. **p ≤ .01, two-tailed. ***p ≤ .001, two-tailed.
Table 4.
Results of the Linear Regression Models Predicting Epigenetic Aging: Strong African American Healthy Adult Project
| Predictor | Epigenetic aging (age
20) |
||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Main effect | |||
| Racial discrimination classa | 0.988 [−0.423, 2.399] | 0.610 [−0.857, 2.076] | 0.623 [−0.834, 2.079] |
| Moderator | |||
| Support in the family environment | −0.072 [−0.251, 0.107] | −0.013 [−0.198, 0.172] | 0.082 [−0.118, 0.282] |
| Two-way interaction | |||
| Racial Discrimination × Support in the Family Environment |
−0.535* [−0.990, –0.080] | ||
| Confounder variables | |||
| Gender (male = 1) | 2.000** [0.806, 3.195] | 1.951** [0.764, 3.138] | |
| Depressive symptoms | 0.320 [−0.046, 0.686] | 0.307 [−0.056, 0.671] | |
| Perceived life stress | −0.297 [−0.716, 0.122] | −0.325 [−0.742, 0.092] | |
| SES-related risk | 0.137 [−0.343, 0.617] | 0.127 [−0.350, 0.603] | |
| Body mass index | 0.089* [0.022, 0.156] | 0.084* [0.017, 0.151] | |
Note: The table presents unstandardized regression coefficients, with 95% confidence intervals in brackets. All models controlled for methylation batch assignment. N = 322. SES = socioeconomic status.
Membership in the high-and-stable class was coded as 1, and membership in the low-and-increasing class was coded as 0.
p ≤ .05, two-tailed. **p ≤ .01, two-tailed.
Table 5.
Results of the Linear Regression Models Predicting Epigenetic Aging: Adults in the Making Project
| Predictor | Epigenetic aging (age
22) |
||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Main effect | |||
| Racial discrimination classa | 1.376* [0.160, 2.593] | 1.470* [0.236, 2.704] | 1.449* [0.224, 2.674] |
| Moderator | |||
| Support in the family environment | −0.053 [−0.238, 0.132] | −0.036 [−0.220, 0.149] | 0.158 [−0.088, 0.403] |
| Two-way interaction | |||
| Racial Discrimination × Support in the Family Environment |
−0.430* [−0.792, –0.067] | ||
| Confounder variables | |||
| Gender (male = 1) | 1.556* [0.364, 2.749] | 1.463* [0.277, 2.649] | |
| Depressive symptoms | 0.054 [−0.039, 0.148] | 0.065 [−0.029, 0.158] | |
| Perceived life stress | −0.338 [−0.881, 0.205] | −0.408 [−0.950, 0.135] | |
| SES-related risk | 0.537* [0.048, 1.026] | 0.514* [0.028, 0.999] | |
| Body mass index | 0.100** [0.040, 0.160] | 0.103** [0.043, 0.162] | |
Note: The table presents unstandardized regression coefficients, with 95% confidence intervals in brackets. All models controlled for methylation batch assignment. N = 294. SES = socioeconomic status.
Membership in the high-and-stable class was coded as 1, and membership in the low-and-stable class was coded as 0.
p ≤ .05, two-tailed. **p ≤ .01, two-tailed.
For the SHAPE sample, no main effects of the key variables were detected (Table 4, Model 2); for the AIM sample, high and stable levels of racial discrimination were associated with a higher epigenetic-aging score at age 22, b = 1.470, 95% confidence interval (CI) = [0.236, 2.704], p = .020 (Table 5, Model 2). In other words, AIM participants who experienced high levels of discrimination had cellular ages greater than would be expected given their chronological ages. When the multiplicative interaction terms were added (Models 3 in Tables 4 and 5), the analyses revealed significant interactions for both samples, F(1, 311) = 5.361, ΔR2 = .015, p = .021, for SHAPE and F(1, 284) = 5.432, ΔR2 = .016, p = .020, for AIM. To decipher these interactions, we performed simple-slopes analyses, estimating the association between discrimination class and PBMC epigenetic aging at low (1 SD below the mean) and high (1 SD above the mean) levels of support in the family environment. Figure 2 presents these results. In both SHAPE and AIM, for participants with low support in the family environment, a higher level of discrimination forecast greater epigenetic aging during young adulthood—SHAPE: b = 2.356, 95% CI = [0.276, 4.435], p = .027; AIM: b = 2.808, 95% CI = [1.142, 4.475], p = .001. Again, this indicates that participants who had experienced high levels of discrimination were epigenetically older than would be expected given their chronological ages. Results for participants from highly supportive family environments were consistent with the buffering hypothesis: No association between discrimination and epigenetic aging was detected in either sample—SHAPE: b = −1.111, 95% CI = [−3.174, 0.953], p = .290; AIM: b = 0.089, 95% CI = [−1.601, 1.780], p = .917. Note that these observed associations were independent of SES-related risk, life stress, depressive symptoms, and BMI.
Fig. 2.
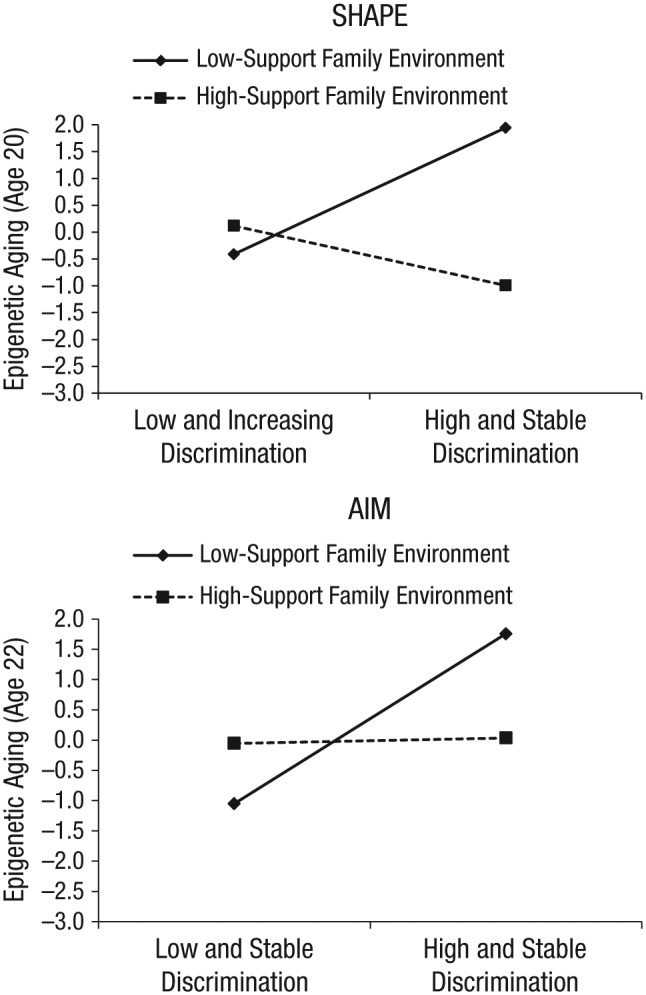
Results of the simple-slopes analyses of the interactive effect of racial discrimination and family-environment support on epigenetic aging among participants in the Strong African American Healthy Adult Project (SHAPE; top panel) and the Adults in the Making project (AIM; bottom panel). Low and high levels of support from the family environment refer to values 1 SD below and above the mean, respectively. Higher values for epigenetic aging indicate a greater discrepancy between participants’ estimated cellular age and their chronological age.
Additional analyses were executed to determine whether similar results would be obtained when continuous measures of discrimination, rather than discrimination classes, were used in the models described previously. These analyses are presented in Table S1 and Figure S1 in the Supplemental Material available online. Individual-level intercepts (individual values of discrimination at the first wave) and slopes of racial discrimination were saved from latent growth models executed using Mplus (Muthén & Muthén, 1998–2015). For both cohorts, correlations showed that individual-level exposure to interpersonal discrimination at each wave was strongly interrelated with individual-level exposure to interpersonal discrimination at the other two waves (SHAPE: range = .57 to .67; AIM: range = .59 to .71). These correlations indicate that individual-level intercept was a robust predictor of exposure to discrimination across time. When we used the individual-level intercepts in our models, the results mirrored those of the regression analyses presented earlier. Specifically, the effect of racial discrimination depended on the level of family-environment support for youth in both SHAPE and AIM. Among youth with low support in the family environment, exposure to high levels of racial discrimination was associated with an acceleration of epigenetic aging. In contrast, among youth living in supportive family environments, racial discrimination was not associated with epigenetic aging. Analyses of the individual-level slopes did not reveal statistically significant results. The high levels of consistency in exposure to racial discrimination across time appeared to attenuate the sensitivity of these data.
Discussion
During adolescence, African American youth become keenly aware of their treatment by other individuals and are particularly cognizant of targeted rejection (Stevenson, 2004). Against this developmental backdrop, we tested a stress-buffering hypothesis with two samples of African American youth. The results indicated that exposure to interpersonal racial discrimination interacted with support in the family environment to forecast PMBC epigenetic age. Moreover, this interaction retained its significance when confounder variables were included in the data analyses, and the results were replicated across two independent samples of African American youth. To our knowledge, this is the first study to detect and replicate associations between racial discrimination and PBMC epigenetic aging.
The results are also pertinent to research indicating that some youth who experience chronic stressors do not experience negative consequences because they live in a supportive family environment (Luthar, 2006). Indeed, within the high-discrimination groups in our study, a 1-SD increase in family-environment support was associated with a PBMC epigenetic age 1.47 years lower for SHAPE participants and a PBMC epigenetic age 0.84 years lower for AIM participants. Although the clinical significance of these differences awaits further study, the pattern of findings extends existing research indicating that parental nurturance and responsiveness are beneficial physiologically for individuals facing adversity. For example, high levels of parental warmth buffer the effects of poverty on allostatic load, a composite of physiological risk markers, in adolescents (Evans, Kim, Ting, Tesher, & Shannis, 2007). Parental warmth has also been found to buffer the effects of low SES in early life on adult metabolic symptoms (Miller, Chen, & Cole, 2009). Among adults who experienced low SES in childhood, the activity of pro-inflammatory gene networks and interleukin-6 inflammatory responses are lower in those who also experienced high maternal warmth than in those who did not (Chen et al., 2011). In this study as well, parents’ provision of emotional warmth and support interacted with discrimination to forecast epigenetic aging, independently of the other indicators included in the family-environment variable (see Table S2 and Figure S2 in the Supplemental Material available online).
The next step in this research process is to understand the mechanisms responsible for the protective capacities of a supportive family environment. We suggest that this research begin with a focus on the regulation of negative emotions. Supportive family environments have been found to forecast adolescents’ enhanced emotion regulation, including emotion-related physiological responses, motivational states, and associated behaviors (Morris, Silk, Steinberg, Myers, & Robinson, 2007). One consistent consequence of perceived discrimination is the development and expression of anger and hostility (Priest et al., 2013), which have been shown to influence physiological functioning across several subsystems. For example, hostility has been associated with amplified blood pressure reactions to stress (Fredrickson et al., 2000), elevated fasting glucose (Shen, Countryman, Spiro, & Niaura, 2008), and heightened plasma lipid levels (Weidner, Sexton, McLellarn, Connor, & Matarazzo, 1987).
We conjecture that racial discrimination may have an effect on neuroendocrine processes. Frequent exposure to interpersonal discrimination can be expected to occasion persistent activation of stress-response systems, in particular, the sympathetic nervous system and hypothalamic-pituitary-adrenal system. The hormonal end products of these systems, catecholamines and glucocorticoids, can be expected to be elevated in youth who frequently encounter interpersonal discrimination. These hormones can affect DNA methylation and, potentially, epigenetic aging by altering the enzymatic activity of DNA methyltransferases, as well as histone deacetylases and acetyltransferases (Barnes & Adcock, 2009). Adiposity is another important mediator to consider. Though our findings were independent of BMI, future work should include more refined measures of adiposity (e.g., abdominal fat accumulation), as well as profiles of dietary intake and physical activity. All of these lifestyle variables have been found to be correlated with racial discrimination and successful aging (Lewis et al., 2015). Finally, gender effects should be explored systematically because past research has suggested that associations between racial discrimination and some outcomes are stronger for male than for female youth (Brody, Kogan, & Chen, 2012).
Despite the strengths of this study, which include a multiwave prospective research design and the replication of the results across samples, several limitations should be addressed in future research. First, because epigenetic aging was not assessed at the first wave of data collection, the study design precluded conclusions regarding its change over time. Second, the discrimination measure assessed interpersonal discrimination only and did not assess structural discrimination; thus, it provided a limited assessment of the range of discriminatory experiences that adolescents encounter. Finally, although the findings are compelling, little research on the long-term impact of epigenetic aging has been conducted. Future research should consider the health consequences of the associations described here. Research exploring the links among discrimination, epigenetic aging, and subsequent health problems would be valuable. For example, are the disparities we observed associated with subsequent development of diabetes, hypertension, or other cardiovascular risks that emerge during early and middle adulthood? These cautions notwithstanding, this study highlights the benefits of supportive family environments in preventing discrimination from weathering young bodies.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Funding: The research reported in this article was supported by Award No. R01 HD030588 from the National Institute of Child Health and Human Development to G. H. Brody and by Award No. P30 DA027827 from the National Institute on Drug Abuse to G. H. Brody.
Supplemental Material: Additional supporting information can be found at http://pss.sagepub.com/content/by/supplemental-data
References
- Aiken L. S., West S. G. (1991). Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage. [Google Scholar]
- Barnes P. J., Adcock I. M. (2009). Glucocorticoid resistance in inflammatory diseases. The Lancet, 373, 1905–1917. doi: 10.1016/S0140-6736(09)60326-3 [DOI] [PubMed] [Google Scholar]
- Brody G. H., Chen Y.-F., Kogan S. M. (2010). A cascade model connecting life stress to risk behavior among rural African American emerging adults. Development and Psychopathology, 22, 667–678. doi: 10.1017/S0954579410000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G. H., Kogan S. M., Chen Y.-f. (2012). Perceived discrimination and longitudinal increases in adolescent substance use: Gender differences and mediational pathways. American Journal of Public Health, 102, 1006–1011. doi: 10.2105/AJPH.2011.300588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G. H., Kogan S. M., Grange C. M. (2012). Translating longitudinal, developmental research with rural African American families into prevention programs for rural African American youth. In King R. B., Maholmes V. (Eds.), The Oxford handbook of poverty and child development (pp. 553–570). New York, NY: Oxford University Press. [Google Scholar]
- Brody G. H., Lei M.-K., Chae D. H., Yu T., Kogan S. M., Beach S. R. H. (2014). Perceived discrimination among African American adolescents and allostatic load: A longitudinal analysis with buffering effects. Child Development, 85, 989–1002. doi: 10.1111/cdev.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G. H., Yu T., Chen Y.-f., Kogan S. M., Evans G. W., Beach S. R. H., . . . Philibert R. A. (2013). Cumulative socioeconomic status risk, allostatic load, and adjustment: A prospective latent profile analysis with contextual and genetic protective factors. Developmental Psychology, 49, 913–927. doi: 10.1037/a0028847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G. H., Yu T., Chen Y.-f., Kogan S. M., Smith K. (2012). The Adults in the Making program: Long-term protective stabilizing effects on alcohol use and substance use problems for rural African American emerging adults. Journal of Consulting and Clinical Psychology, 80, 17–28. doi: 10.1037/a0026592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J. E., Gruenewald T. L., Taylor S. E., Janicki-Deverts D., Matthews K. A., Seeman T. E. (2013). Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proceedings of the National Academy of Sciences, USA, 110, 17149–17153. doi: 10.1073/pnas.1315458110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver C. S., Scheier M. F., Weintraub J. K. (1989). Assessing coping strategies: A theoretically based approach. Journal of Personality and Social Psychology, 56, 267–283. doi: 10.1037/0022-3514.56.2.267 [DOI] [PubMed] [Google Scholar]
- Chen E., Miller G. E., Kobor M. S., Cole S. W. (2011). Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry, 16, 729–737. doi: 10.1038/mp.2010.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Anderson N. B., Clark V. R., Williams D. R. (1999). Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist, 54, 805–816. doi: 10.1037/0003-066X.54.10.805 [DOI] [PubMed] [Google Scholar]
- Dogan M. V., Shields B., Cutrona C. E., Gao L., Gibbons F. X., Simons R. L., . . . Philibert R. A. (2014). The effect of smoking on DNA methylation of peripheral blood mononuclear cells from African American women. BMC Genomics, 15, Article 151. doi: 10.1186/1471-2164-15-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. W. (2003). A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology, 39, 924–933. doi: 10.1037/0012-1649.39.5.924 [DOI] [PubMed] [Google Scholar]
- Evans G. W., Kim P., Ting A. H., Tesher H. B., Shannis D. (2007). Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology, 43, 341–351. doi: 10.1037/0012-1649.43.2.341 [DOI] [PubMed] [Google Scholar]
- Fredrickson B. L., Maynard K. E., Helms M. J., Haney T. L., Siegler I. C., Barefoot J. C. (2000). Hostility predicts magnitude and duration of blood pressure response to anger. Journal of Behavioral Medicine, 23, 229–243. doi: 10.1023/A:1005596208324 [DOI] [PubMed] [Google Scholar]
- Geronimus A. T., Hicken M., Keene D., Bound J. (2006). “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health, 96, 826–833. doi: 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. L., Way N., Pahl K. (2006). Trajectories of perceived adult and peer discrimination among Black, Latino, and Asian American adolescents: Patterns and psychological correlates. Developmental Psychology, 42, 218–236. doi: 10.1037/0012-1649.42.2.218 [DOI] [PubMed] [Google Scholar]
- Hannum G., Guinney J., Zhao L., Zhang L., Hughes G., Sadda S., . . . Zhang K. (2013). Genome-wide methylation profiles reveal quantitative views of human aging rates. Molecular Cell, 49, 359–367. doi: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. (1979). Treating depressive disorders: The efficacy of behavior and cognitive therapies. Behavior Modification, 3, 496–517. doi: 10.1177/014544557934004 [DOI] [Google Scholar]
- Kurdek L. A. (1994). Conflict resolution styles in gay, lesbian, heterosexual nonparent, and heterosexual parent couples. Journal of Marriage and the Family, 56, 705–722. doi: 10.2307/352880 [DOI] [Google Scholar]
- Landrine H., Klonoff E. A. (1996). The Schedule of Racist Events: A measure of racial discrimination and a study of its negative physical and mental health consequences. Journal of Black Psychology, 22, 144–168. doi: 10.1177/00957984960222002 [DOI] [Google Scholar]
- Lewis T. T., Cogburn C. D., Williams D. R. (2015). Self-reported experiences of discrimination and health: Scientific advances, ongoing controversies, and emerging issues. Annual Review of Clinical Psychology, 11, 407–440. doi: 10.1146/annurev-clinpsy-032814-112728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar S. S. (2006). Resilience in development: A synthesis of research across five decades. In Cicchetti D., Cohen D. J. (Eds.), Developmental psychopathology: Vol. 3. Risk, disorder, and adaptation (2nd ed., pp. 739–795). Hoboken, NJ: Wiley. [Google Scholar]
- Marioni R. E., Shah S., McRae A. F., Chen B. H., Colicino E., Harris S. E., . . . Deary I. J. (2015). DNA methylation age of blood predicts all-cause mortality in later life. Genome Biology, 16(1), Article 25. doi: 10.1186/s13059-015-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny A. P., Jr., Wachs T. D., Ludwig J. L., Phillips K. (1995). Bringing order out of chaos: Psychometric characteristics of the Confusion, Hubbub, and Order Scale. Journal of Applied Developmental Psychology, 16, 429–444. doi: 10.1016/0193-3973(95)90028-4 [DOI] [Google Scholar]
- Miller G. E., Chen E., Cole S. W. (2009). Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology, 60, 501–524. doi: 10.1146/annurev.psych.60.110707.163551 [DOI] [PubMed] [Google Scholar]
- Miller G. E., Lachman M. E., Chen E., Gruenewald T. L., Karlamangla A. S., Seeman T. E. (2011). Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychological Science, 22, 1591–1599. doi: 10.1177/0956797611419170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. S., Silk J. S., Steinberg L., Myers S. S., Robinson L. R. (2007). The role of the family context in the development of emotion regulation. Social Development, 16, 361–388. doi: 10.1111/j.1467-9507.2007.00389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L. K., Muthén B. O. (1998. –2015). Mplus user’s guide (7th ed.). Los Angeles, CA: Author. [Google Scholar]
- Pascoe E. A., Smart Richman L. (2009). Perceived discrimination and health: A meta-analytic review. Psychological Bulletin, 135, 531–554. doi: 10.1037/a0016059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest N., Paradies Y., Trenerry B., Truong M., Karlsen S., Kelly Y. (2013). A systematic review of studies examining the relationship between reported racism and health and wellbeing for children and young people. Social Science & Medicine, 95, 115–127. doi: 10.1016/j.socscimed.2012.11.031 [DOI] [PubMed] [Google Scholar]
- Proctor B. D., Dalaker J. (2003). Poverty in the United States: 2002 (Current Population Reports P60–222). Washington, DC: U.S. Census Bureau. [Google Scholar]
- Radloff L. S. (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Schuster M. A., Elliott M. N., Kanouse D. E., Wallander J. L., Tortolero S. R., Ratner J. A., . . . Banspach S. W. (2012). Racial and ethnic health disparities among fifth-graders in three cities. New England Journal of Medicine, 367, 735–745. doi: 10.1056/NEJMsa1114353 [DOI] [PubMed] [Google Scholar]
- Shen B.-J., Countryman A. J., Spiro A., III, Niaura R. (2008). The prospective contribution of hostility characteristics to high fasting glucose levels: The moderating role of marital status. Diabetes Care, 31, 1293–1298. doi: 10.2337/dc07-1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Bynum M., Lambert S. F., English D., Ialongo N. S. (2014). Associations between trajectories of perceived racial discrimination and psychological symptoms among African American adolescents. Development and Psychopathology, 26, 1049–1065. doi: 10.1017/S0954579414000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson H. C. (2004). Racial socialization. In Jones R. L. (Ed.), Black psychology (4th ed., pp. 176–189). Hampton, VA: Cobb & Henry. [Google Scholar]
- Weidner G., Sexton G., McLellarn R., Connor S. L., Matarazzo J. D. (1987). The role of Type A behavior and hostility in an evaluation of plasma lipids in adult women and men. Psychosomatic Medicine, 49, 136–145. [DOI] [PubMed] [Google Scholar]
- Williams D. R., Mohammed S. A. (2013). Racism and health I: Pathways and scientific evidence. American Behavioral Scientist, 57, 1152–1173. doi: 10.1177/0002764213487340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills T. A., Vaccaro D., McNamara G. (1992). The role of life events, family support, and competence in adolescent substance use: A test of vulnerability and protective factors. American Journal of Community Psychology, 20, 349–374. doi: 10.1007/BF00937914 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


