Abstract
Objective
To test the clinimetric properties of the Comprehensive Cervical Dystonia Rating Scale.
Background
This is a modular scale with modifications of the Toronto Western Spasmodic Torticollis Rating Scale (composed of three subscales assessing motor severity, disability and pain) now referred to as the revised Toronto Western Spasmodic Torticollis Scale-2.; a newly developed psychiatric screening instrument; and the Cervical Dystonia Impact Profile-58 as a quality of life measure.
Methods
Ten dystonia experts rated subjects with cervical dystonia using the comprehensive scale. Clinimetric techniques assessed each module of the scale for reliability, item correlation and factor structure.
Results
There were 208 cervical dystonia patients (73% women, age 59±10 years, duration 15±12 years). The internal consistency of the motor severity subscale was acceptable (Cronbach’s alpha = 0.57). Item to total correlations showed that elimination of items with low correlations (<0.20) increased alpha to 0.71. Internal consistency estimates for the subscales for disability and pain were 0.88 and 0.95 respectively. The psychiatric screening scale had a Cronbach’s alpha of 0.84 and satisfactory item to total correlations. When the subscales of the Toronto Western Spasmodic Torticollis scale -2 were combined with the psychiatric screening scale, Cronbach's alpha was 0.88, and construct validity assessment demonstrated four rational factors: motor, disability, pain and psychiatric disorders. The Cervical Dystonia Impact Profile-58 had an alpha of 0.98 and its construction was validated through a confirmatory factor analysis.
Conclusions
The modules of the Comprehensive Cervical Dystonia Rating Scale are internally consistent with a logical factor structure.
Keywords: Cervical dystonia, focal dystonia, rating scale, Toronto Western Spasmodic Torticollis Rating Scale, Cervical Dystonia Impact Profile-58
Introduction
Cervical dystonia (CD) is a complex disorder marked by involuntary movements of neck and shoulders, pain, impaired activities of daily living and reduced quality of life. The abnormal movements often combine head turn, tilt, forward or backward flexion, anterior or sagittal shift and shoulder elevation.1, 2 The involuntary movements are associated with significant disability. In addition, pain occurs in 75% of patients and contributes to a greater degree of disability.3 CD has also been associated with psychiatric disorders, including depression, anxiety, panic disorders and social phobia.4–7 Furthermore, several studies have also demonstrated impaired health-related QOL in CD8–14.
Although there have been many rating scales developed for motor symptoms of CD15, only 3 of these, the Tsui scale16, the Cervical Dystonia Severity Scale17 and the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) have had clinimetric evaluation. None of these scales address the psychiatric symptoms or quality of life. The Tsui rating scale is a 6-item scale that assesses amplitude and duration of involuntary neck movements, shoulder elevation, and head tremor16. This scale is designed to assess head and shoulder postures and head tremor but does not take into account the other manifestations of CD. The Cervical Dystonia Severity Scale uses a protractor and wall chart to rate angles of head deviation from neutral in each of three planes17. This scale does not evaluate shoulder elevation, tremor or sagittal shift. The Tsui rating scale and CDSS do not address pain, activities of daily living, psychiatric symptoms, or quality of life.
The standard TWSTRS consists of three domains that assess motor severity, pain, and disability18. The motor severity subscale consists of 10 items, with variable scaling and weighting. It also includes a disability scale with 6 items, and a pain scale with 3 items. The total score is the sum of each of the subscales. Only the motor domain has undergone evaluation for inter-rater reliability and construct validity, with good to excellent inter-rater reliability19. Despite the limited clinimetric studies of the TWSTRS, it has been used extensively in clinical studies of CD and is the scale currently recommended by the Movement Disorder Society task force on dystonia rating scales.15
There are no psychiatric rating scales validated for use in CD. While the DSM-V criteria are the gold standard for diagnosis of psychiatric disease, their application requires specific training and is impractical for routine use by most CD providers. There are several self-administered scales that are easy to administer, require no examiner training, and have been assessed for clinimetric properties in primary depression and anxiety. The Beck Depression Inventory20 is a self-administered scale with 21 components that takes 10 – 15 min to complete. This scale does not emphasize somatic components and therefore avoids the confounding factors of the movement associated with CD.21 The Hospital Anxiety and Depression Rating Scale is a self-administered scale that consists of 14-item subscales for both depression and anxiety.22, 23 This scale was specifically developed for use in patients with somatic co-morbidity and has no questions related to the physical signs of depression or anxiety. The Beck Anxiety Index is a self-reported scale21 designed as a screening tool for anxiety with good positive predictive value for panic disorders.24 Although these psychiatric rating scales are all well validated in psychiatric practice, they have not been systematically applied to CD.
The effect of CD on quality of life is comparable to that seen in multiple sclerosis, Parkinson's disease, stroke14 and other chronic diseases.25 Standard measures of QOL, including generic health-related QOL,25 EuroQoL, SF-36 and Rosenbergs’ self-esteem scale26 are not consistent in identifying factors predicting reduced quality of life in CD and do not correlate with effective treatment for CD, such as botulinum toxin injections.12, 27 The Craniocervical Dystonia Questionnaire (CDQ-24), although designed specifically for blepharospasm and CD, has not been extensively used or tested against other scales.28 The Cervical Dystonia Impact Profile – 58 item (CDIP-58) was developed using a modified Delphi method with Rasch methodology.29 It is a self-administered scale with 8 subscales measuring the impact of head and neck symptoms on a variety of quality of life items.29 The CDIP-58 has been evaluated for reliability and validity in CD30 and shown to be superior to the SF-36, a widely used but generic QOL measure. The CDIP-58 also demonstrates sensitivity to change following botulinum toxin injections29, 31 and is the recommended scale for quality of life in CD.15
In this study, the original TWSTRS was revised to the TWSTRS-2 to address identified deficiencies, including the variable scaling of items, the lack of an item for head tremor and the weighting of the duration factor by two.32 The TWSTRS-PSYCH was developed to screen for psychiatric disorders associated with CD. The CDIP-58 with previously established reliability and validity was included in its original form. We combined the TWSTRS-2, TWSTRS-PSYCH and the CDIP-58 to produce the modular Comprehensive Cervical Dystonia Rating Scale or CCDRS. The specific aim of the study was to assess the reliability and construct validity of the CCDRS.
Methods
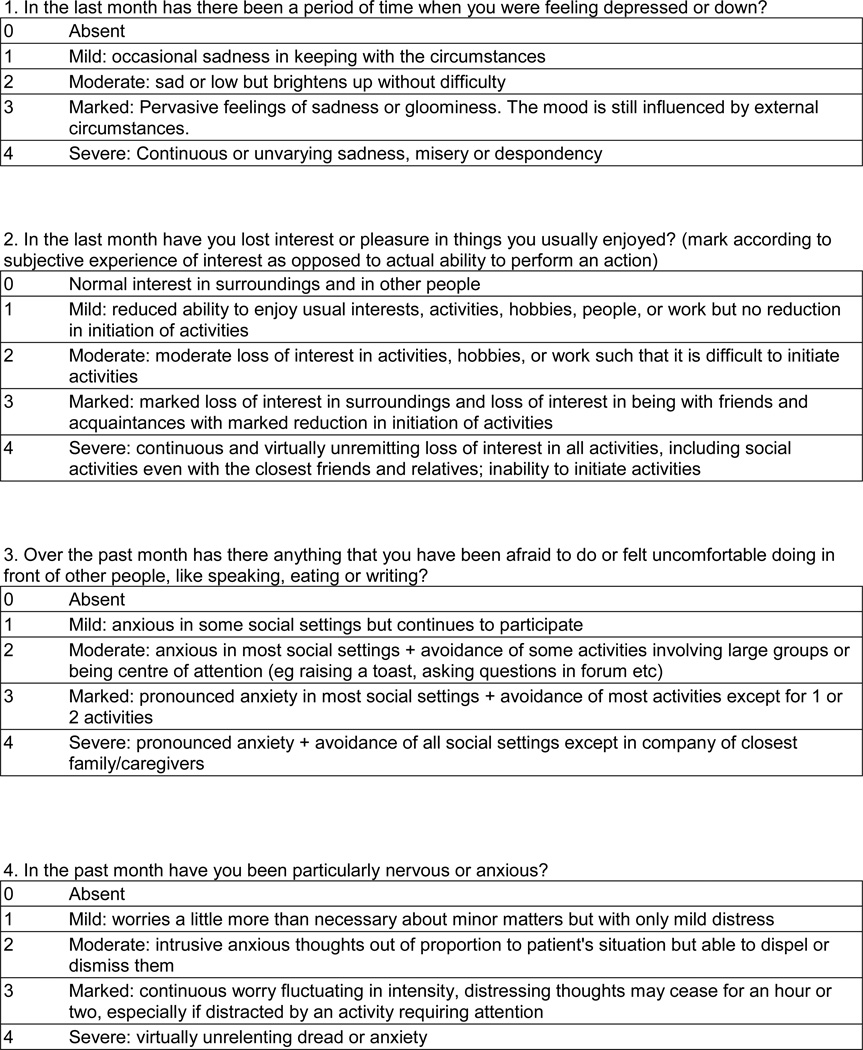
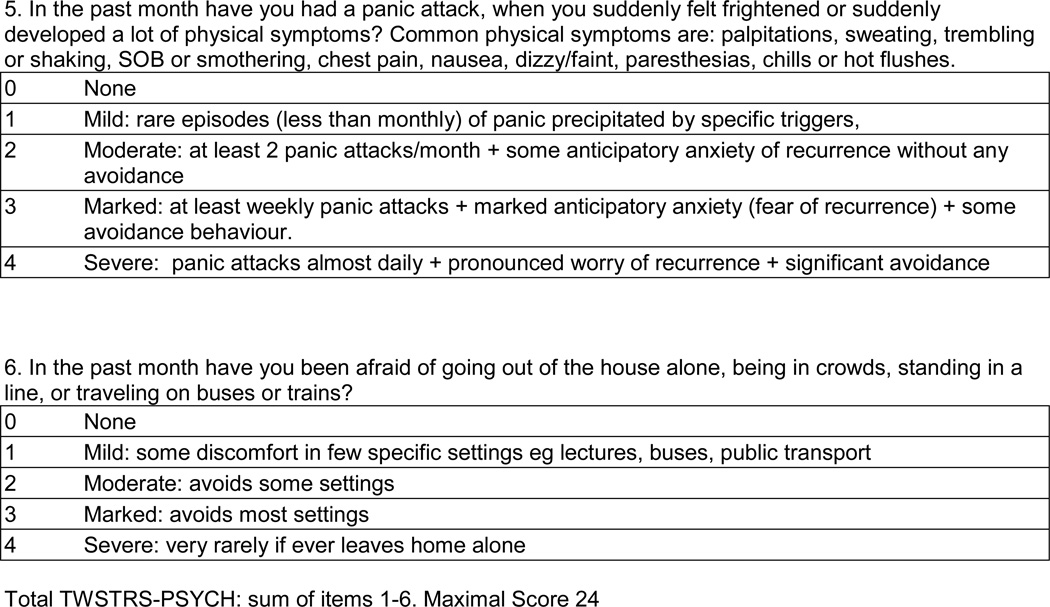
The methods for development of the CCDRS have been described in a prior publication.32 Briefly, the existing TWSTRS motor severity was revised to the TWSTRS-2 motor severity using a modified Delphi method with input from dystonia experts. The TWSTRS–PSYCH was developed using a similar methodology with input from psychiatrists, dystonia experts and patients. The draft TWSTRS-2 included assessments for motor severity (12 items), pain (5 items) and disability (6 items). The TWSTRS-PSYCH included 6 items rated on a 5-point scale from 0 (absent) to 4 (severe) for occurrence over the past month (Figure 2). The maximal score of the TWSTRS-PSYCH was 24. The CDIP-58 includes 58 self-administered questions that define 8 subscales and are transformed into a total score, with a maximal score of 100. The TWSTRS-2, TWSTRS-PYSCH, and CDIP-58 then were combined into the CCDRS and used in the data collection phase of the study along with other demographic and disease-related measures.
Subjects with isolated CD, previously known as primary dystonia, were recruited from 10 sites. Demographic information, including age, gender, and duration of CD were collected. For this study, subjects were videotaped using a standardized protocol during the time that the site investigator rated the subject severity using the TWSTRS-2 motor severity subscale.32 Subjects were interviewed to complete the TWSTRS-2 disability and pain subscales, as well as the TWSTRS-PSYCH. The subjects completed the self-reported CDIP-58.
There are no accepted formulae for calculating required sample sizes for scale validation studies, particularly factor analytic methods, at given levels of power33. Instead, recommended subject-to-item ratios are employed. For the present study, we have a 9.1:1 subject-to-item ratio, which exceeds the recommended 8:1 ratio shown to be adequate for this analysis34, 35.
Rating scores and video were electronically sent to a central database at Washington University, St. Louis, MO36. The video and data were assessed for completeness. Queries regarding missing data were resolved. Accuracy of the data entry was verified through cross referencing electronic data to paper data collection forms in 10% of cases.
Statistical approach
Subject demographics and disease-related variables were examined using frequency counts and measures of central tendency and variability, as appropriate. To assess the reliability and validity of the TWSTRS-2 and TWSTRS - PSYCH components of the CCDRS, we employed both Classical Test Theory (CRT) and Item Response Theory (IRT). Classical Test Theory focuses on the relationships of individual items to the entire scale37 while Item Response Theory focuses on the measurement characteristics of the items in relation to the individual completing the scale37. Using Classical Test Theory we examined Cronbach's alpha, a measure of scale reliability, item-to-total correlations, changes in alpha if selected items were removed and distributional skewness, a measure of potential floor or ceiling effects, for the separate subscales of the TWSTRS-2 (motor severity, disability, pain) and TWSTRS-PSYCH modules of the CCDRS. These analyses were conducted using SPSS (Version 21). Additionally, we examined the construct validity through exploratory factor analyses. Because of the ordered categorical level of measurement of the CCDRS we employed an unweighted least squares approach for the factor estimate and a CF-Varimax orthogonal rotation to improve the interpretability of the factors. MPlus (Version 7) was used for these analyses. For the Item Response Theory approach, we used a graded response model analysis with maximum likelihood parameter estimation38 to examine item discrimination, or the strength of the relationship between the item and the measured domain, and item threshold, or the level of item response to the overall severity of the measured domain. MPlus (version 7) was used for these analyses.
To assess each item's utility in the CCDRS, we identified items with low item-to-total correlations (defined as ≤ 0.3), improvement in Cronbach's alpha if omitted, low factor loading (defined as ≤ 0.4), a skewness outside of the range −1.50 to +1.50 representing possible floor or ceiling effects, non-significant Item Response Theory discrimination scores and thresholds that did not encompass a value of zero. Based on this assessment, each item was considered either as one to keep in the scale or as one to drop or modify. If an item met the criteria for acceptable item-to-total correlation, change in alpha if the item were omitted, appropriate factor loading, skewness and Item Response Theory discrimination and threshold, it was retained. Items not meeting these criteria were deleted.
Because the CDIP-58 module of the CCDRS had already undergone clinimetric examination for reliability and validity, we limited our analysis to assessments of internal consistency (Cronbach's alpha) and confirmatory factor structure (CFA). The CFA was conducted to determine if the 8 factors found in the original publication29 could be confirmed with the data collected for this study. We evaluated the CFA results based on the Comparative Fit Index (CFI)39. To confirm a good fit between the original factor structure and our data, the CFI was required to be 0.90 or greater. Mean and variance adjusted weighted least square (WLSMV) estimator was used to confirm model fit. We also used the root mean square error of approximation (RMSEA) to check the goodness of fit, with values less or equal than 0.10 indicating an acceptable index. MPlus (version 7) was used for these analyses.
Results
A total of 208 CD subjects (73% women, mean age 59 years SD± 9.95), onset of CD 44 years (SD ± 12.11) from 10 sites in the United States were included. The mean severity of CD as measured using the TWSTRS 2 total score was (33.24 (SD ± 13.22)), with subscale scores for motor severity of (16.29 (SD ± 5.54)), disability (9.21 (SD ± 5.72)) and pain (7.88 (SD ± 5.56))
TWSTRS-2 Motor Severity Subscale (Table 1)
Table 1.
Classical test theory and item response analyses results for items on the TWSTRS-2 Motor, TWSTRS-2 Disability, TWSTRS-2 Pain and TWSTRS-2 Psych components of the Comprehensive Cervical Dystonia Rating Scale.
| Item | Item-Total Correlation |
Alpha-if- Item- Removed |
Factor Loading |
Skewness | IRT Discrimination (p value) |
IRT Threshold (Min; Max) |
Action |
|---|---|---|---|---|---|---|---|
| TWSTRS-2 Motor | |||||||
| Rotation | 0.314 | No Increase | 0.420 | 0.314 | 1.00 (<0.0005) | −2.75; 2.50 | Keep |
| Laterocollis | 0.466 | No Increase | 0.519 | 0.608 | 1.40 (<0.0005) | −2.21; 6.22 | Keep |
| Anterocollis | 0.180 | Increased | <0.3 | 1.780 | 0.69 (0.06) | 0.68; 3.53 | Drop/Modify |
| Retrocollis | 0.091 | Increased | <0.3 | 1.536 | 0.17 (0.44) | 0.62; 5.31 | Drop/Modify |
| Lateral Shift | −0.068 | Increased | <0.3 | 1.723 | −0.24 (0.19) | 0.71; 5.35 | Drop/Modify |
| Sagittal Shift | 0.136 | Increased | <0.3 | 1.575 | 0.34 (0.05) | 0.45; 5.37 | Drop/Modify |
| Head Tremor | 0.000 | Increased | <0.3 | 0.472 | 0.04 (0.84) | −0.59; 3.91 | Drop |
| Shoulder Elevation | 0.349 | No Increase | 0.481 | 0.542 | 1.11 (<0.0005) | −1.79; 2.88 | Keep |
| Duration | 0.527 | No Increase | 0.626 | −0.648 | 1.58 (<0.0005) | −6.53; 1.06 | Keep |
| Sensory Trick | 0.011 | Increased | <0.3 | 1.107 | 0.09 (0.63) | −1.42; 2.23 | Drop/Modify |
| ROM | 0.373 | No Increase | 0.428 | 0.877 | 1.16 (<0.0005) | −1.81; 3.88 | Keep |
| Time in Midline | 0.472 | No Increase | 0.631 | −0.563 | 1.60 (<0.0005) | −1.59; 0.49 | Keep |
| TWSTRS- 2 Disability | |||||||
| Work | 0.722 | No Increase | 0.704 | 0.483 | 1.00 (<0.0005) | −1.46; 5.50 | Keep |
| ADL | 0.654 | No Increase | 0.604 | 0.774 | 1.66 (<0.0005) | −0.77; 5.45 | Keep |
| Driving | 0.587 | No Increase | 0.542 | 0.294 | 1.40 (<0.0005) | −1.90; 5.13 | Keep |
| Reading | 0.748 | No Increase | 0.805 | 0.183 | 3.32 (<0.0005) | −3.00; 8.12 | Keep |
| Television | 0.734 | No Increase | 0.759 | −0.052 | 3.35 (<0.0005) | −2.92; 6.80 | Keep |
| Outside of Home | 0.728 | No Increase | 0.690 | 0.685 | 2.22 (<0.0005) | −2.00; 5.91 | Keep |
| TWSTRS- 2 Pain | |||||||
| Pain Best | 0.778 | No Increase | 0.752 | 1.13 | 1.000 (<0.0005) | −0.11; 2.92 | Keep |
| Pain Worst | 0.884 | No Increase | 0.848 | −0.210 | 4.853 (<0.0005) | −1.20; 0.96 | Keep |
| Pain Usual | 0.944 | No Increase | 0.900 | 0.312 | 6.169 (<0.0005) | −0.77; 1.87 | Keep |
| Pain Duration | 0.835 | No Increase | 0.778 | −0.169 | 3.564 (<0.0005) | −1.130; 0.55 | Keep |
| Pain Disability | 0.735 | No Increase | 0.669 | 0.472 | 2.320 (<0.0005) | −0.50; 1.74 | Keep |
| TWSTRS- 2 Psych | |||||||
| Depression | 0.657 | No Increase | 0.724 | 0.557 | 1.00 (< 0.0005) | −0.44; 2.38 | Keep |
| Loss of Interests | 0.673 | No Increase | 0.740 | 1.263 | 2.20 (< 0.0005) | 0.22; 2.08 | Keep |
| Discomfort in Public | 0.570 | No Increase | 0.627 | 0.728 | 1.57 (< 0.0005) | −0.17; 2.34 | Keep |
| Anxiety | 0.672 | No Increase | 0.741 | 0.907 | 2.04 (< 0.0005) | −0.22; 2.24 | Keep |
| Panic | 0.559 | No Increase | 0.626 | 3.646 | 2.37 (< 0.0005) | 1.18; 2.57 | Keep |
| Afraid Going Out | 0.576 | No Increase | 0.645 | 2.673 | 2.16 (< 0.0005) | 0.93; 2.85 | Keep |
Note: Item-To-Total = correct item score to total score correlation; Alpha-If-Item-Removed = increase of decrease in Cronbach's alpha if the item is removed from the analysis; Factor Loading = maximum factor loading value; Skewness = a measure of distributional asymmetry; IRT Discrimination = strength of relationship between item and the measured construct; IRT Threshold = level of item response in relation to severity of measured construct, minimum and maximum thresholds displayed.
Overall Cronbach's alpha for the TWSTRS-2 motor severity subscale was 0.57. Items assessing Rotation, Laterocollis, Shoulder Elevation, Duration, Range of Motion and Time in Midline met criteria for acceptable item-to-total correlation, change in alpha if item omitted, factor loading, skewness, IRT discrimination and IRT threshold. Items assessing anterocollis, retrocollis, lateral shift, sagittal shift, head tremor and effect of a sensory trick failed to meet the criteria for utility in the CCDRS and were deleted from the CCDRS.
TWSTRS-2 Disability Subscale (Table 1)
Overall Cronbach's alpha for the TWSTRS-2 disability subscale was 0.88. Items assessing Work, Activities of Daily Living, Driving, Reading, Television and Outside of Home Disability met criteria for acceptable item-to-total correlation, change in alpha if item omitted, factor loading, skewness, IRT discrimination and IRT threshold. All items met the criteria for utility in the CCDRS and were retained in the CCDRS.
TWSTRS-2 Pain Subscale (Table 1)
Overall Cronbach's alpha for the TWSTRS-2 pain subscale was 0.95. Items assessing Pain at its Best, Pain at its Worst, Usual Pain, Pain Duration and Pain Disability met criteria for acceptable item-to-total correlation, change in alpha if item omitted, factor loading, skewness, IRT discrimination and IRT threshold. All items met the criteria for utility in the CCDRS and were retained in the CCDRS. The revised TWSTRS-2 scale is included in figure 1.
Figure 1.
Toronto Western Spasmodic Torticollis Rating Scale-2 (TWSTRS-2)
TWSTRS- PSYCH (Table 1)
Overall Cronbach's alpha for the TWSTRS-PSYCH was 0.84. Items assessing Depression, Loss of Interest, Discomfort and Anxiety met criteria for acceptable item-to-total correlation, change in alpha if item omitted, factor loading, skewness, IRT discrimination and IRT threshold and were retained in the CCDRS. Items assessing Panic and Afraid of Going Outside met all criteria except for skewness. The skewed distribution appears to be due to the high percentages of zero scores for Panic (88%) and Afraid of Going Outside (82%). The TWSTRS-PSYCH scale is included in figure 2.
Figure 2. TWSTRS-PSYCH.
Circle one number for each question.
Combined TWSTRS-2 and TWSTRS-PSYCH
Overall Cronbach's alpha for the combined TWSTRS2 (after removing items assessing anterocollis, retrocollis, lateral shift, sagittal shift, head tremor and effect of a sensory trick) and TWSTRS-PSYCH was 0.88. All items met criteria for acceptable item-to-total correlation, change in alpha if item omitted, skewness, IRT discrimination and IRT threshold. Factor analysis revealed a satisfactory four-factor solution with items assessing motor severity, disability, pain and psychiatric manifestation loading on separate factors (all factor loadings > 0.40) (Table 2).
Table 2.
Factor solution for combined TWSTRS-2 and TWSTRS-PSYCH after deleting TWSTRS-2 Severity items not meeting criteria for inclusion in the Comprehensive Cervical Dystonia Rating Scale.
| Item | Factor | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Rotation | .41 | |||
| Laterocollis | .51 | |||
| Shoulder elevation | .53 | |||
| Duration | .62 | |||
| Range of movement | .42 | |||
| Time midline | .63 | |||
| Work disability | .59 | |||
| ADL disability | .52 | |||
| Driving disability | .52 | |||
| Reading disability | .80 | |||
| Television disability | .75 | |||
| Outside disability | .63 | |||
| Pain at best | .76 | |||
| Pain at worst | .84 | |||
| Pain at usual | .92 | |||
| Pain duration | .82 | |||
| Pain disability | .67 | |||
| Depression | .68 | |||
| Loss of interest | .70 | |||
| Discomfort in public | .58 | |||
| Anxiety | .71 | |||
| Panic attack | .61 | |||
| Fear of outside | .62 | |||
Note: All factor loadings < 0.40 are not shown in the Table.
CDIP-58
Overall Cronbach's alpha for the CDIP-58 was 0.98. The CFA of the 8-factor solution of the original CDIP-58 resulted in a CFI of 0.97 with a RMSEA of 0.07 and a model fit chi-square of 48.96 (p < 0.0005) using the data from the current study. Thus the pre-specified 8-factor structure was confirmed.
Discussion
This study demonstrates that the CCDRS assesses distinct components of CD and can be applied as a complete scale or used in a modular format. The current study provides a realistic picture of the clinimetric properties of this scale and each of its modules, and allows the deletion of items that do not demonstrate clinical utility.
The revised motor severity subscale of the TWSTRS-2 demonstrated that certain items (anterocollis, retrocollis, lateral shift, sagittal shift, head tremor and effect of sensory trick) had multiple indicators of poor utility on both CCT and IRT analyses. The reasons for the lack of utility of these items are varied. Anterocollis, retrocollis, lateral shift and sagittal shift ratings had highly skewed distributions, suggesting possible floor-effects. Head tremor and effect of sensory trick had more normal-shaped distributions. However, these items had low item-to-total correlations, and increased the alpha if omitted. Further, the low factor loading of these items indicates that they may not directly contribute to overall CD severity in contrast to the other items, although these may be features of the disorder. Hence, these items were deleted from the rating of motor severity, resulting in a simplified scale that can be used efficiently in a clinical study (Figure 1)
The TWSTRS-2 disability subscale, which was unchanged from the standard TWSTRS, was not revised and had good reliability and content validity. The TWSTRS-2 pain subscale was revised, removing the mathematical manipulations (the multiplication of the usual level of pain by 2 and eliminating the division of the pain scores by 4), and was found to be reliable and valid. The first psychiatric screening tool for CD, TWSTRS-PSYCH (Figure 2), demonstrated good clinimetric properties. The CDIP-58, which has previously been assessed for reliability and validity using a different scale development technique, was found to have acceptable internal consistency and a confirmed factor structure of 8 factors. Inclusion of the CDIP-58 provides a patient reported measure of the impact of CD on quality of life that is distinct from information provided by the other scales in the CCDRS.
Although many rating scales have been developed to evaluate CD, none has been comprehensive10, 15, 40, 41. The CCDRS includes measures for motor severity, disability, pain, psychiatric disorders and quality of life measures. Each of these domains may be affected in CD and contribute to overall severity of the condition. The reduction in total items in the TWSTRS-2 motor severity subscale based on these results will allow for easier use. While the deleted items may be useful as descriptors for CD, these items do not contribute to the overall assessment of CD severity
The results of the factor analysis for the modified TWSTRS-2 and TWSTRS-PSYCH suggest that the scores of the four subcomponents (motor severity, disability, pain and psychiatric concerns) can be used either as independent measures or summarized into a single measure of CD impairment. The previously defined factor structure of the CDIP-58 was confirmed in the present analysis.
The CCDRS provides a tool that allows an assessment of all aspects of CD and can be used in modular format. This study provides the framework for development of rating scales that can be used to assess the varied clinical aspects of focal dystonias involving other body regions. As new therapeutic modalities become available for the treatment of the focal dystonias42, it is critical that validated outcome measures capture not only the motor features, but also those related to psychological disorders and impact on quality of life.
Acknowledgments
This study is a part of the Dystonia Coalition (NS065701), which is a part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR), NCATS, funded through collaboration between NCATS and the National Institute of Neurological Disorders and Stroke. The Dystonia Coalition’s program includes clinical features, natural history, a DNA repository and the development of validated rating scales for focal dystonias. The Dystonia Study Group provided additional funding. The Dystonia Medical Research provided coordination of study sites.
We also acknowledge the coordinators at each of the sites: Tracy Waliczek (Rush), Mary Louise Weeks (Emory), Emily Muller, Sara Lewis (Beth Israel Medical Center), Katie Holmes, (University of Maryland), Christine Hunter, Lea Kiefer (Baylor College of Medicine), Laura Wright, Lin Yang (Washington University), Matthew Grana (University of Rochester) Brandon Rothberg (University of Toronto) Kyle Rizer (University of Florida) Amy Duffy (Mayo Clinic of Arizona, Scottsdale)
C. L. Comella serves on the editorial board of Clinical Neuropharmacology, Sleep Medicine and Continuum. She receives research support from the NIH R01NS074343, U54NS065701, Dystonia Medical Research Foundation, Allergan Inc. Ipsen Biopharmaceuticals, Inc and Merz Pharmaceutical. She receives compensation/honoraria for services as a consultant or an advisory committee member: Acorda Therapeutics, Allergan, Inc; Impax Pharmaceuticals; Ipsen Biopharmaceuticals, Inc; Lundbeck Ltd.; Medtronic Inc.; Merz Pharmaceuticals; Acadia Pharmaceuticals; Teva Neurosciences; Neurocrine Biosciences Inc., Revance Therapeutic; and Ultragenyx Pharmaceuticals. She receives royalties from Cambridge, Humana Press; Wolters Kluwer. She receives research support from the Parkinson’s Disease Foundation.
J.S. Perlmutter has received research funding from the NIH.
H. A. Jinnah has active grant support from the US National Institutes of Health, Merz Inc., Ipsen Inc., the Benign Essential Blepharospasm Research Foundation, and Cure Dystonia Now. He also is principle investigator for the Dystonia Coalition, which receives the majority of its support through NIH grant NS065701 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences, and National Institutes of Neurological Disorders and Stroke. The Dystonia Coalition receives additional material or administrative support from industry sponsors (Allergan Inc. and Merz Pharmaceuticals) as well as private foundations (The American Dystonia Society, The Benign Essential Blepharospasm Foundation, Cure Dystonia Now, Dystonia Inc., Dystonia Ireland, The Dystonia Medical Research Foundation, The European Dystonia Federation, The Foundation for Dystonia Research, The National Spasmodic Dysphonia Association, and The National Spasmodic Torticollis Association). Dr. Jinnah serves as a consultant for Psyadon Pharmaceuticals and serves on the Scientific Advisory Boards for the Cure Dystonia Now, the Dystonia Medical Research Foundation, Lesch-Nyhan Action France, the Lesch-Nyhan Syndrome Children’s Research Foundation and Tyler's Hope for a Cure.
C. H. Adler has received consulting fees from Allergan, Abbvie, Acadia, Ipsen, Lilly, Lundbeck, Merz, and Teva, and received research funding from Avid Radiopharmaceuticals, the Michael J. Fox Foundation, and the NIH/NINDS.
R. L. Barbano has served on advisory board for Allergan and has received educational support from Allergan; research support from Dystonia Coalition, Allergan, Merz
S. Factor has received honoraria from Merz, Chelsea Therapuetics, Neurocrine, Lunbeck, Auspex, Avanir and UCB. He has received research grants from Teva, Ipsen Allergan, Medtronics, Auspex, Genzyme Corp, Genzyme A Sanofi, Mucheal J Fox Foundation and the NIH.
C. G. Goetz has served on Consulting or Advisory Boards with AstraZeneca, Avanir, Boston Scientific, Clearview, Health Advances,, Kanter Health, Oxford Biomedica, Pfizer,, Teva, WebMD. He has received funding from NIH, Michael J. Fox Foundation. He directs the Rush Parkinson’s Disease Research Center that receives support from the Parkinson’s Disease Foundation. He directs the translation program for the MDS-UPDRS and UDysRS and receives funds from the International Parkinson and Movement Disorder Society (IPMDS) for this effort. He has received honoraria from the American Academy of Neurology, Captain James A Lovell Federal Health Care Center, University of California, World Parkinson Coalition. He receives royalties from Oxford University Press, Elsevier Publishers, Wolters Kluwer Health- Lippincott, Wilkins and Wilkins
J. Jankovic has received research grants from: Allergan, Inc; Ipsen Limited; Merz Pharmaceuticals. Dr. Jankovic has served as a consultant or as an advisory committee member for: Allergan, Inc.
S. G. Reich has received royalties: Informa (Reich SG. Movement Disorders. 100 instructive Cases) Reviewer (paid): UpToDate
R. L. Rodriguez has received research grants/support from NIH, Dystonia Coalition, Allergan, Merz, Adamas, Abbvie and Ipsen Limited. Dr. Rodriguez has received honoraria for consulting/advisory boards from Auspex, Abbvie, Lundbeck, Pfizer, and National Parkinson Foundation.
W. L. Severt has received honorarium for consulting and/or speakers’ bureaus from Allergan, Teva, Lundbeck, and Impax.
M. Zurowski has received funding from the Dystonia Coalition
G.T. Stebbins has received honoraria for consulting/advisory board activities from Acadia, Pharmaceuticals, Adamas Pharmaceuticals, Inc., Ceregene, Inc., CHDI Management, Inc., Ingenix Pharmaceutical Services (i3 Research), Neurocrine Biosciences, Inc.,Pfizer, Inc. He has received research support from the National Institutes of Health, Michael J. Fox Foundation for Parkinson’s Research, Dystonia Coalition, CHDI, International Parkinson and Movement Disorder Society. He has received honoraria from the International Parkinson and Movement Disorder Society, American Academy of Neurology, Michael J. Fox Foundation for Parkinson’s Research.
Footnotes
Financial disclosures: None
Financial Disclosures (preceding 12 months)
T. A. Waliczek has nothing to disclose
A. R. Rosen: has nothing to disclose
W. Galpern has nothing to disclose
- Research project: a. conception; b. organization; c. execution
- Statistical analysis: a. design; b. execution; c. review and critique
- Manuscript: a. writing first draft; b. review and critique
C. L. Comella: 1a,b,c; 2a,c; 3a
J.S. Perlmutter 1a,b,c;2a;3b
H. A. Jinnah 1a,b,c;2a;3b
T. A. Waliczek 1b,c;2b;3b
A. R. Rosen 1a,b,c;2a;3b
W. Galpern 1a,b,c; 2a,c; 3a
C. H. Adler 1a,b,c; 2a,c; 3a
R. L. Barbano 1a,b,c; 2a,c; 3a
S. Factor 1a,b,c; 2a,c; 3a
C.G. Goetz 1a,b,c; 2a,c; 3a
J. Jankovic 1a,b,c; 2a,c; 3a
S. G. Reich 1a,b,c; 2a,c; 3a
R. L. Rodriguez 1a,b,c; 2a,c; 3a
W. L. Severt 1a,b,c; 2a,c; 3a
M. Zurowski 1a,b,c; 2a,c; 3a
G.T. Stebbins 1a,b,c; 2a,b,c; 3a
References
- 1.Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–1091. doi: 10.1212/wnl.41.7.1088. [DOI] [PubMed] [Google Scholar]
- 2.Bhidayasiri R. Treatment of complex cervical dystonia with botulinum toxin: involvement of deep-cervical muscles may contribute to suboptimal responses. Parkinsonism & related disorders. 2011;17(Suppl 1):S20–S24. doi: 10.1016/j.parkreldis.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: clinical characteristics. Movement disorders : official journal of the Movement Disorder Society. 1991;6:119–126. doi: 10.1002/mds.870060206. [DOI] [PubMed] [Google Scholar]
- 4.Moraru E, Schnider P, Wimmer A, et al. Relation between depression and anxiety in dystonic patients: implications for clinical management. Depress Anxiety. 2002;16:100–103. doi: 10.1002/da.10039. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel T, Schnider P, Wimmer A, Steinhoff N, Moraru E, Auff E. Psychiatric comorbidity in patients with spasmodic torticollis. J Psychosom Res. 1998;44:687–690. doi: 10.1016/s0022-3999(97)00229-8. [DOI] [PubMed] [Google Scholar]
- 6.Queiroz MR, Chien HF, Barbosa ER. Quality of life in individuals with cervical dystonia before botulinum toxin injection in a Brazilian tertiary care hospital. Arquivos de neuro-psiquiatria. 2011;69:900–904. doi: 10.1590/s0004-282x2011000700010. [DOI] [PubMed] [Google Scholar]
- 7.Kuyper DJ, Parra V, Aerts S, Okun MS, Kluger BM. Nonmotor manifestations of dystonia: a systematic review. Movement disorders : official journal of the Movement Disorder Society. 2011;26:1206–1217. doi: 10.1002/mds.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odergren T, Tollback A, Borg J. Efficacy of botulinum toxin for cervical dystonia. A comparison of methods for evaluation. Scand J Rehabil Med. 1994;26:191–195. [PubMed] [Google Scholar]
- 9.Gudex CM, Hawthorne MR, Butler AG, Duffey P. Effect of dystonia and botulinum toxin treatment on health-related quality of life. Mov Disord. 1998;13:941–946. doi: 10.1002/mds.870130613. [DOI] [PubMed] [Google Scholar]
- 10.Lindeboom R, Brans JW, Aramideh M, Speelman HD, De Haan RJ. Treatment of cervical dystonia: a comparison of measures for outcome assessment. Movement disorders : official journal of the Movement Disorder Society. 1998;13:706–712. doi: 10.1002/mds.870130417. [DOI] [PubMed] [Google Scholar]
- 11.Brans JW, Lindeboom R, Aramideh M, Speelman JD. Long-term effect of botulinum toxin on impairment and functional health in cervical dystonia. Neurology. 1998;50:1461–1463. doi: 10.1212/wnl.50.5.1461. [DOI] [PubMed] [Google Scholar]
- 12.Hilker R, Schischniaschvili M, Ghaemi M, Jacobs A, Rudolf J. Health related quality of life is improved by botulinum neurotoxin type A in long term treated patients with focal dystonia. J Neurol Neurosurg Psychiatry. 2001;71:193–199. doi: 10.1136/jnnp.71.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller J, Kemmler G, Wissel J, et al. The impact of blepharospasm and cervical dystonia on health-related quality of life and depression. Journal of neurology. 2002;249:842–846. doi: 10.1007/s00415-002-0733-1. [DOI] [PubMed] [Google Scholar]
- 14.Camfield L, Ben-Shlomo Y, Warner TT Epidemiological Study of Dystonia in Europe Collaborative G. Impact of cervical dystonia on quality of life. Mov Disord. 2002;17:838–841. doi: 10.1002/mds.10127. [DOI] [PubMed] [Google Scholar]
- 15.Albanese A, Sorbo FD, Comella C, et al. Dystonia rating scales: critique and recommendations. Movement disorders : official journal of the Movement Disorder Society. 2013;28:874–883. doi: 10.1002/mds.25579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsui JK, Eisen A, Stoessl AJ, Calne S, Calne DB. Double-blind study of botulinum toxin in spasmodic torticollis. Lancet. 1986;2:245–247. doi: 10.1016/s0140-6736(86)92070-2. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien C, Brashear A, Cullis P, et al. Cervical dystonia severity scale reliability study. Mov Disord. 2001;16:1086–1090. doi: 10.1002/mds.1226. [DOI] [PubMed] [Google Scholar]
- 18.Consky E, Lang AE. Clinical assessments of patients with cervical dystonia. In: Jankovic J, Hallett M, editors. Therapy with botulinum toxin. New York: Marcel Dekker, Inc.; 1994. pp. 211–237. [Google Scholar]
- 19.Comella CL, Stebbins GT, Goetz CG, Chmura TA, Bressman SB, Lang AE. Teaching tape for the motor section of the Toronto Western Spasmodic Torticollis Scale. Movement disorders : official journal of the Movement Disorder Society. 1997;12:570–575. doi: 10.1002/mds.870120414. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 21.Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the Beck Depression Inventory. A review. Psychopathology. 1998;31:160–168. doi: 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale--a review of validation data and clinical results. J Psychosom Res. 1997;42:17–41. doi: 10.1016/s0022-3999(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 24.Leyfer OT, Ruberg JL, Woodruff-Borden J. Examination of the utility of the Beck Anxiety Inventory and its factors as a screener for anxiety disorders. J Anxiety Disord. 2006;20:444–458. doi: 10.1016/j.janxdis.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Shlomo Y, Camfield L, Warner T. What are the determinants of quality of life in people with cervical dystonia? J Neurol Neurosurg Psychiatry. 2002;72:608–614. doi: 10.1136/jnnp.72.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg M. Society and the Adolescent self-image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- 27.Skogseid IM, Malt UF, Roislien J, Kerty E. Determinants and status of quality of life after long-term botulinum toxin therapy for cervical dystonia. Eur J Neurol. 2007;14:1129–1137. doi: 10.1111/j.1468-1331.2007.01922.x. [DOI] [PubMed] [Google Scholar]
- 28.Muller J, Wissel J, Kemmler G, et al. Craniocervical dystonia questionnaire (CDQ-24): development and validation of a disease-specific quality of life instrument. J Neurol Neurosurg Psychiatry. 2004;75:749–753. doi: 10.1136/jnnp.2003.013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cano SJ, Warner TT, Linacre JM, et al. Capturing the true burden of dystonia on patients: the Cervical Dystonia Impact Profile (CDIP-58) Neurology. 2004;63:1629–1633. doi: 10.1212/01.wnl.0000142962.11881.26. [DOI] [PubMed] [Google Scholar]
- 30.Cano SJ, Warner TT, Thompson AJ, Bhatia KP, Fitzpatrick R, Hobart JC. The cervical dystonia impact profile (CDIP-58): can a Rasch developed patient reported outcome measure satisfy traditional psychometric criteria? Health Qual Life Outcomes. 2008;6:58. doi: 10.1186/1477-7525-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cano SJ, Hobart JC, Edwards M, et al. CDIP-58 can measure the impact of botulinum toxin treatment in cervical dystonia. Neurology. 2006;67:2230–2232. doi: 10.1212/01.wnl.0000249310.25427.f2. [DOI] [PubMed] [Google Scholar]
- 32.Comella C, Fox S, Bhatia K, et al. Development of the Comprehensive Cervical Dystonia Rating Scale: Methodology. Movement Disorders Clinical Practice. 2015;2:135–141. doi: 10.1002/mdc3.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osborne J, Costello A. Sample size and subject to item ratio in principal components analysis. Prac Assess Res Eval. 2004;9:1–12. [Google Scholar]
- 34.Hatcher L. A step-by-step approach to using SAS system for factor analysis and structural equation modeling. Cary, NC: SAS Institute, Inc.; 1994. [Google Scholar]
- 35.Grousch R. Factor analysis. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- 36.Yan L, Hicks M, Winslow K, et al. Secured web-based video repository for multicenter studies. Parkinsonism & related disorders. 2015;21:366–371. doi: 10.1016/j.parkreldis.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeVellis R. Classical test theory. Med Care. 2006;44:S50–S59. doi: 10.1097/01.mlr.0000245426.10853.30. [DOI] [PubMed] [Google Scholar]
- 38.Stout W. Psychometrics from practice to theory and back. Psychometrika. 2001;67:485–518. [Google Scholar]
- 39.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 40.Jost WH, Hefter H, Stenner A, Reichel G. Rating scales for cervical dystonia: a critical evaluation of tools for outcome assessment of botulinum toxin therapy. Journal of neural transmission. 120:487–496. doi: 10.1007/s00702-012-0887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarsy D. Comparison of clinical rating scales in treatment of cervical dystonia with botulinum toxin. Movement disorders : official journal of the Movement Disorder Society. 1997;12:100–102. doi: 10.1002/mds.870120117. [DOI] [PubMed] [Google Scholar]
- 42.Jinnah HA, Hess EJ. Experimental therapeutics for dystonia. Neurotherapeutics. 2008;5:198–209. doi: 10.1016/j.nurt.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]