Abstract
Background
Buprenorphine can be abused by the intranasal route. This study sought to examine the relative abuse liability and reinforcing efficacy of intranasal buprenorphine compared to intranasal buprenorphine/naloxone in opioid-dependent individuals.
Methods
Eleven healthy male and female volunteers physically dependent on short-acting opioids resided as inpatients during participation in this double blind, within subject, placebo-controlled study. Participants were maintained on oxycodone (30 mg/q.i.d., p.o.) throughout the 6-week study. Eight pairs of experimental sessions were conducted at ≥48 hour intervals to examine the pharmacodynamic profile (Sample) and reinforcing efficacy (Self-administration the following day) of intranasal placebo, oxycodone (60 mg), buprenorphine (2, 8 & 16 mg) and buprenorphine/naloxone (2/0.5, 8/2 & 16/4 mg). Subjective, observer-rated and physiological measures were collected to assess the magnitude of opioid agonist and antagonist effects. A progressive ratio self-administration procedure assessed choices for drug versus money.
Results
All active doses produced opioid agonist-like effects (e.g., increased ratings of “liking,” and miosis) compared to placebo. The effects of buprenorphine and buprenorphine/naloxone were not reliably dose-dependent. Intranasal buprenorphine/naloxone elicited modest and transient opioid withdrawal-like effects in the first hour post-drug administration, while simultaneously blunting or blocking the early onset of agonist effects seen with buprenorphine alone. All active doses of buprenorphine were self-administered more than placebo, but buprenorphine/naloxone doses were not.
Conclusions
These data confirm that intranasal buprenorphine/naloxone has deterrent properties related to transient withdrawal effects that likely decrease its desirability for misuse compared to buprenorphine in opioid-dependent individuals maintained on short-acting opioids.
Keywords: opioid, mu agonist, opioid withdrawal, abuse, buprenorphine, misuse
1. INTRODUCTION
Buprenorphine (BUP), a partial mu opioid agonist, has been successfully used for the treatment of opioid dependence since the 1990’s (see recent reviews Fullerton et al., 2014; Mattick et al., 2014). Following the passage of the U.S. Drug Addiction Treatment Act of 2000 and FDA approval of Suboxone® and Subutex® in 2002, BUP implementation in the U.S. became widespread. While the majority of BUP sold in the U.S. is buprenorphine/naloxone (BUP/NX), many countries outside the U.S. primarily use BUP alone (e.g., France). In part, this was because BUP was introduced in these countries before BUP/NX was available; however, now that BUP/NX is available, reasons for continued preference for BUP alone may not be empirically justified given growing concerns about diversion, misuse, and adult and pediatric overdose (Hayes et al., 2008; Kintz, 2002; Lai and Teo, 2006; Lavonas et al., 2013; Lovegrove et al., 2014; Reynaud et al., 1998; Simonsen et al., 2011; Tracqui et al., 1998). The preferential use of BUP/NX in the U.S. occurred because the FDA approved both formulations simultaneously and the manufacturer, Reckitt-Benckiser Pharmaceuticals, priced BUP/NX lower than BUP to discourage widespread prescribing of BUP. Also, physician trainings and published treatment guidelines (e.g., CSAT, 2004) preferentially recommended BUP/NX (except for induction and supervised dosing and treatment of pregnant women).
The rationale for the combination BUP/NX development was based upon the assumption that adding naloxone would reduce potential misuse and diversion compared to BUP alone, as has been observed with pentacozine/naloxone and tilidine/naloxone (Johnson and McCagh, 2000). Naloxone has poor sublingual bioavailability (Preston et al., 1990) and is functionally inactive when sublingual BUP/NX is taken as directed. However, if misused parenterally, naloxone becomes 100% bioavailable and can precipitate opioid withdrawal in opioid-dependent individuals (e.g., Fudala et al., 1998; Stoller et al., 2001). However, studies and clinical experience have demonstrated that whether BUP and BUP/NX administration is or is not associated with abuse liability and does or does not precipitate opioid withdrawal in opioid-dependent individuals is complicated and determined by numerous factors, including opioid maintenance drug, maintenance dose, time since last dose, BUP or BUP/NX dose, and route of BUP or BUP/NX administration (Clark et al., 2002; Harris et al., 2000; Larance et al., 2014; Mendelson et al., 1996; Preston et al., 1988; Rosado et al., 2007; Schuh et al., 1996; Stoller et al., 2001; Walsh et al., 1995).
Reports indicate that BUP tablets with and without naloxone are being crushed (and sublingual films dissolved) and subsequently injection or insufflated intranasally (Alho et al., 2007; Bruce et al., 2009; Horyniak et al., 2011; Jenkinson et al., 2005; Lofwall and Walsh, 2014; Nordmann et al., 2012; Young et al., 2010). For instance, two studies report that up to 30% of patients enrolled in BUP therapy were snorting their medication (Barrau et al., 2001; Roux et al., 2008). Case reports describe adverse consequences from intranasal BUP, including acute hepatitis and renal failure (Eiden et al., 2013) and fatal overdose (Ferrant et al., 2011; Megarbane et al., 2011). Few controlled human studies have characterized the response to intranasal BUP, BUP/NX or naloxone. We previously examined the pharmacodynamic and pharmacokinetic profiles of intranasal BUP and BUP/NX in opioid abusers who were not physically dependent on opioids (Middleton et al., 2011). That study demonstrated significant intranasal absorption of buprenorphine (~38–44%) and naloxone (~24–30%) from BUP/NX yielding naloxone plasma concentrations comparable to those produced by doses that precipitate withdrawal in opioid-dependent individuals; however, this did not translate into meaningful pharmacodynamic differences between BUP and BUP/NX in non-dependent abusers. Jones and colleagues recently reported that intranasal BUP and BUP/NX do have reinforcing efficacy in BUP-maintained individuals (mimicking those receiving treatment with BUP), and this declines as a function of maintenance dose (Jones et al., 2015). Further, they reported that BUP/NX produced decreased positive subjective effects compared to BUP and was associated with increased ratings of aversive effects in BUP-maintained individuals. However, as BUP is known to have very high affinity for the mu receptor and a long duration of action, the effects of intranasal BUP/NX in individuals dependent on BUP may differ substantially from its effects in those dependent on short-acting opioids with lower affinity (e.g., see Schuh et al., 1996; Walsh et al., 1995). Thus, the present study sought to examine the effects of intranasal BUP compared to BUP/NX among opioid-dependent individuals maintained on a short-acting full mu opioid agonist, mimicking the typical out-of-treatment scenario for those dependent on heroin or short-acting prescription opioids who may misuse buprenorphine products.
2. MATERIALS AND METHODS
2.1. Participants
Eleven adult volunteers completed this study. All were physically dependent on short-acting opioids, recruited through advertisements, and determined to be in good health by history, physical examination, electrocardiogram and laboratory tests. Inclusion criteria included ages 18–50 years, within 25% of ideal body weight or BMI<30, prior intranasal opioid use, ≥21 of last 30 days short-acting illicit opioid use. Exclusion criteria included: seizure or respiratory disorders, head injury, hypertension, cardiovascular disease, abnormal electrocardiogram, pregnancy, nursing, chronic pain, requiring daily medication, physical dependence on sedatives or alcohol, currently enrolled/seeking treatment for opioid dependence and long-acting opioid as primary opioid of choice. To confirm opioid physical dependence, subjects were required to report regular illicit opioid use confirmed by opioid-positive urine drug screens (UDS) during the screening period. It was expected that opioid withdrawal signs would be present, assessed by the COWS (Wesson and Ling, 2003), if someone presented with a negative opioid screen. A modified timeline-follow back (TLFB) and the Addiction Severity Index (ASI) further characterized their substance abuse (McLellan et al., 1992).
The University of Kentucky IRB approved the study, an NIH Certificate of Confidentiality was obtained, and the Declaration of Helsinki guidelines were followed. Subjects gave written informed consent and were paid for participation.
2.2. Drugs
This study was performed under an Investigational New Drug Application (#69,214). Study medications were prepared by the UK Investigational Pharmacy. Oxycodone hydrochloride tablets (30 mg) (Mallinckrodt, Hazelwood, MO) were overencapsulated and loose-filled with lactose monohydrate powder (Medisca Pharmaceuticals, Plattsburgh, NY) for daily maintenance dosing; identical capsules filled with lactose were placebo. A commercial solution (Hospira; 0.4 mg/mL) was diluted to formulate 0.2 mg/mL for the naloxone challenge; sterile saline was the placebo (Hospira). For the intranasal test doses, placebo was lactose. Oxycodone hydrochloride powder (Spectrum Laboratory, Gardena, CA) was weighed (60 mg). Subutex® (2 and 8 mg tablets) was obtained through NIDA, and Suboxone® (2/0.5 and 8/2 mg tablets) was obtained from Reckitt Benckiser (Hull, England) in order to use all white tablets (as U.S. Suboxone® is orange). For BUP (2, 8 & 16 mg) and BUP/NX (2/0.5, 8/2 & 16/4 mg), tablets were pulverized to powder (2 mg tablets for 2 mg doses; one or two 8 mg tablet(s) for 8 mg or 16 mg doses, respectively). As the 16 mg doses yielded the greatest volume, all other doses were formulated with additional lactose to produce equivalent volume for insufflation to preserve the blind. Doses were placed in a small glass vial and into an envelope with blinded identifying information. For the Self-administration sessions, doses were formulated to produce 1/7th, 2/7th …. to 7/7th of the dose in same manner.
2.3. Experimental Design
A double blind, within-subject, placebo-controlled design was employed. Subjects resided for ~6 weeks on the hospital inpatient research unit to complete the three-phase study: 1) stabilization on oxycodone and naloxone challenge, 2) reinforcing efficacy and abuse liability evaluation [~4 weeks], 3) wash-out, discharge and optional outpatient BUP taper [1 week]. Urine samples were tested daily for potential illicit drug contraband; females were tested weekly for pregnancy (all were negative).
2.3.1. Experimental Sessions: General Methods
Following admission, subjects were trained to perform all experimental tasks. They were allowed a light breakfast 2 hr pre-session and allowed to smoke up to 30 min pre-session (and prohibited from eating or smoking during all sessions). During sessions, subjects’ data were entered directly into a programmed Mac Mini, OSX (Apple Computer, Cupertino, CA); a research assistant seated behind the computer initiated tasks and entered observer-rated measures (Babalonis et al., 2013). Oxygen saturation, heart rate, blood pressure (Dynamap Non-Invasive Patient Monitor) were recorded every minute through computer interface; expired end tidal CO2 and respiratory rate were recorded (Capnograph N-85, Nellcor, Boulder, CO). Pupil diameter was determined using a pupilometer (NeurOptics, San Clemente, CA) in constant lighting conditions.
2.3.2. Phase 1- Stabilization and Physical Dependence Confirmation
Hospital admission was typically around noon, and double blind supervised oxycodone dosing (30 mg, p.o. qid) commenced either after admission or at 6:00 PM depending upon clinical assessment of opioid intoxication/withdrawal and at 8:00 AM, 12:00, 6:00 and 10:00 PM thereafter. After steady-state was achieved (~16 doses), two sessions on the same day commenced at 10:30AM and 2:30PM. Physiological and subjective data were collected for 0.5 hours before and 1.25 hr after intramuscular injection (1 mL) of placebo or naloxone (0.2 mg) in counterbalanced order 3 hr after last oxycodone dose (see description below and Table 1). Naloxone challenge was included to confirm subjects maintained on this oxycodone dosing regimen would exhibit measurable precipitated withdrawal signs, as there is substantial inter-subject variability even with identical maintenance regimens (see reference Rosado et al., 2007).
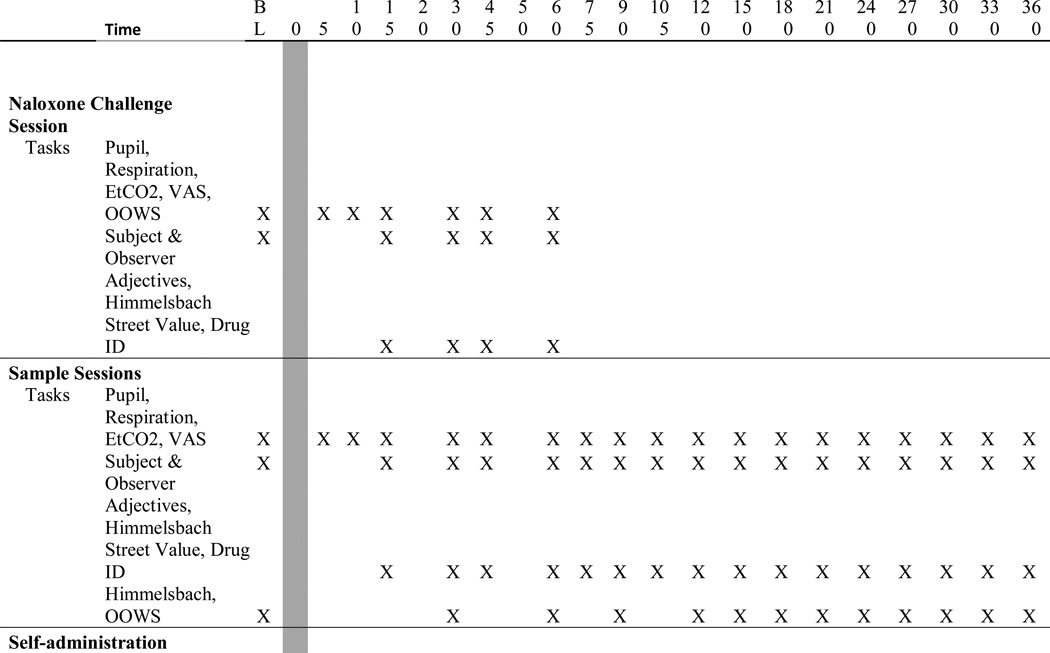
Table 1.
Study timeline for data collection for each of the three types of experimental sessions.
2.3.3. Phase 2- Sample and Self-Administration Sessions
After ≥1 week of stabilization, test sessions commenced to characterize the pharmacodynamic profile of the test drugs (i.e., Sample Session) followed 24-hr later by a Self-Administration Session when participants could work for none, some or all of the preceding day’s sample dose as described previously (Babalonis et al., 2013). Double-blind placebo was substituted for oxycodone the preceding evening and session morning (10PM and 8 AM), with the last active dose 17.5 hr before Sample and Self-Administration sessions. The 12:00 PM dose was also withheld on Sample and Self-administration session days as subjects were likely to receive active drug during the morning of these days. Eight session pairs assessed intranasal placebo, oxycodone (60 mg), BUP (2, 8, 16 mg) and BUP/NX (2/0.5, 8/2, 16/4 mg); session pairs were scheduled ≥48 hr apart. It was hypothesized that BUP/NX would precipitate withdrawal at some dose; thus, lower doses were tested before higher doses. The BUP and BUP/NX conditions were presented in ascending order across weeks with other conditions randomly interspersed; hence, dosing employed a quasi-randomized order. Withdrawal criteria were set to discontinue further BUP or BUP/NX dose escalation for participant protection (Rosado et al., 2007).
Sample sessions (6.5-hr duration) began at 8:00AM with drug administration at 8:30AM. Participants were reminded to pay close attention to their drug experience, as they would have the opportunity to earn that dose the next day. The nurse emptied the sample dose onto a mirror, the participant split the powder into four lines and insufflated those with a straw through alternating nostrils over 5-min. Measures were collected before and after drug administration (described below; Table 1).
Self-administration sessions began at 8:10AM with the first choice trial at 8:40AM. Session duration varied as a function of progressive ratio (PR) responding. Participants could respond for 7 trials on a PR schedule to work and earn portions (1/7th increments) of the sample dose or money ($21 total available, $3 increments) or choose to work for neither. Participants worked via clicking a computer mouse with successively increasing requirements across trials: 50, 250, 500, 1000, 1500, 2000 and 2500 responses, with 7800 responses necessary to earn all drug or money over ≤210 min. Each reinforcer operated under an independent PR schedule. After completion, participants received the earned drug or money. Cash was delivered to the volunteer but kept locked until study completion. Safety was monitored for 2 hr after drug administration; all participants were required to remain in session for 2 hr when no drug was chosen (e.g., money only) or chose not to work.
2.3.4. Phase III: Discharge
Participants remained inpatient for one week after completing these sessions to participate in a secondary study. All were offered a 7-day buprenorphine taper at discharge.
2.4. Subject and Observer-Rated Measures
Subject-rated measures included visual analog scales (VAS), street value, a 38-item adjective Agonist and Antagonist scale (Preston and Bigelow, 1998), and a modified drug identification questionnaire (Babalonis et al., 2013; Jasinski et al., 1977). Observers used a 12-item opioid agonist scale (Preston and Bigelow, 1998), modified Himmelsbach scale (Eissenberg et al., 1996; Himmelsbach, 1941) and Observer Opioid Withdrawal Scale (OOWS)(Handelsman et al., 1987). The VAS were “Do you feel any DRUG EFFECT?” “How HIGH are you?” “Does the drug have any GOOD ….. BAD effects?” “How much do you LIKE the drug…… DESIRE OPIATES right now?” and “How severe is your OPIOID WITHDRAWAL?” Participants placed an arrow along a 100-mm line with “not at all” (0) and “extremely” (100). Adjective checklists used a 5-point Likert scale (0 not at all – 4 extremely).
2.5. Statistical Analysis
All measures were initially analyzed as raw time course with 2-factor within-subject models (dose [8]×time [variable]). Automated min-by-min physiological data were first averaged to yield intervals (5–30 min) corresponding to subjective measures. Peak scores (minimum or maximum) were derived and analyzed with 1-factor models. Significant main and interaction effects (p < 0.05) were further explored with Fisher LSD tests to compare active doses to placebo and matched buprenorphine doses to each other (e.g., 8 BUP vs. 8/2 BUP/NX). All analyses were run with SAS 9.3 Proc Mixed software for Windows.
3. RESULTS
3.1. Participant Characteristics
Sixty-two individuals were screened in-person, twenty were admitted as inpatients, and eleven completed the protocol. Early discharge reasons were: couldn’t tolerate spontaneous withdrawal during first week (n=1), no naloxone response (n=1), personal reasons (n=2), provided poor/inconsistent data (n=3), and contraband activities (n=2). Completers were 7/4 males/females, mean age = 28.4 years; all Caucasian, 11.7 mean years of education, 5/2/4 were single/married/divorced. Based upon TLFB, the average number of days for any opioid use was 27.6 (±0.9) of past 30 days with multiple daily uses. With the ASI, all participants reported prescription opioid abuse with 9 of 11 reporting past month whereas 5 reported past month heroin abuse. All reported abusing intranasally and five reported abusing intravenously.
3.2. Naloxone Challenge Session
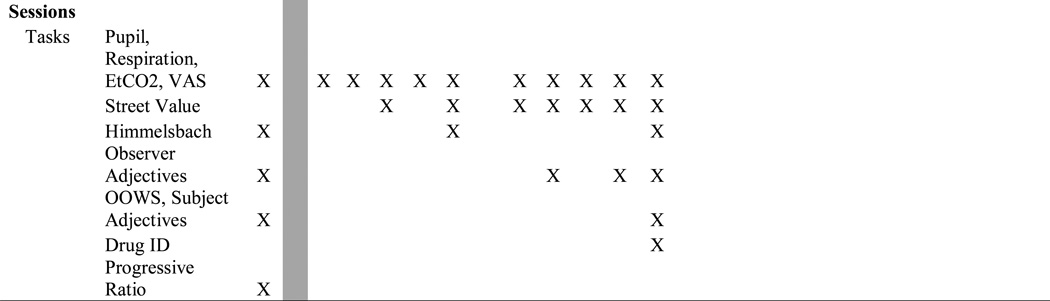
Naloxone (0.2 mg) produced mild but statistically significant precipitated withdrawal compared to placebo. Time course data revealed significant effects of dose and time on opioid withdrawal outcomes (e.g., mydriasis, tachycardia, and increased scores on withdrawal signs and symptoms; p<.05). Figure 1 (statistics in legend) shows data for peak response for VAS “bad drug effects” by naloxone compared to placebo (upper left). Withdrawal ratings (upper right) show mild elevations after placebo (last active dose given 17.5 hr prior) that were increased further by naloxone. Similarly, observer-rated opioid withdrawal signs increased (OOWS; lower left), and naloxone produced significant mydriasis (lower right). Time-to-peak (Tmax) analyses revealed most withdrawal signs/symptoms peaked 20–25 minutes post-naloxone.
Figure 1.
Mean peak data (n=11; ± 1 SEM) are shown for four measures of opioid withdrawal comparing placebo (white bars) versus naloxone (0.2 mg, i.m.; black bars). Significant main effects of drug condition (placebo vs. naloxone; designated by the asterisk *) were found for all four measures (df=10) as follows: “Bad Effects” (t=3.43; p=.006), Opiate Withdrawal (t= −2.74; p =.021), OOWS Score (t= −3.3; p=.008) and pupil diameter (t= −5.47; p<.001).
3.3. Pharmacodynamic Response: Sample Sessions
3.3.1. Opioid Agonist and Withdrawal Outcomes
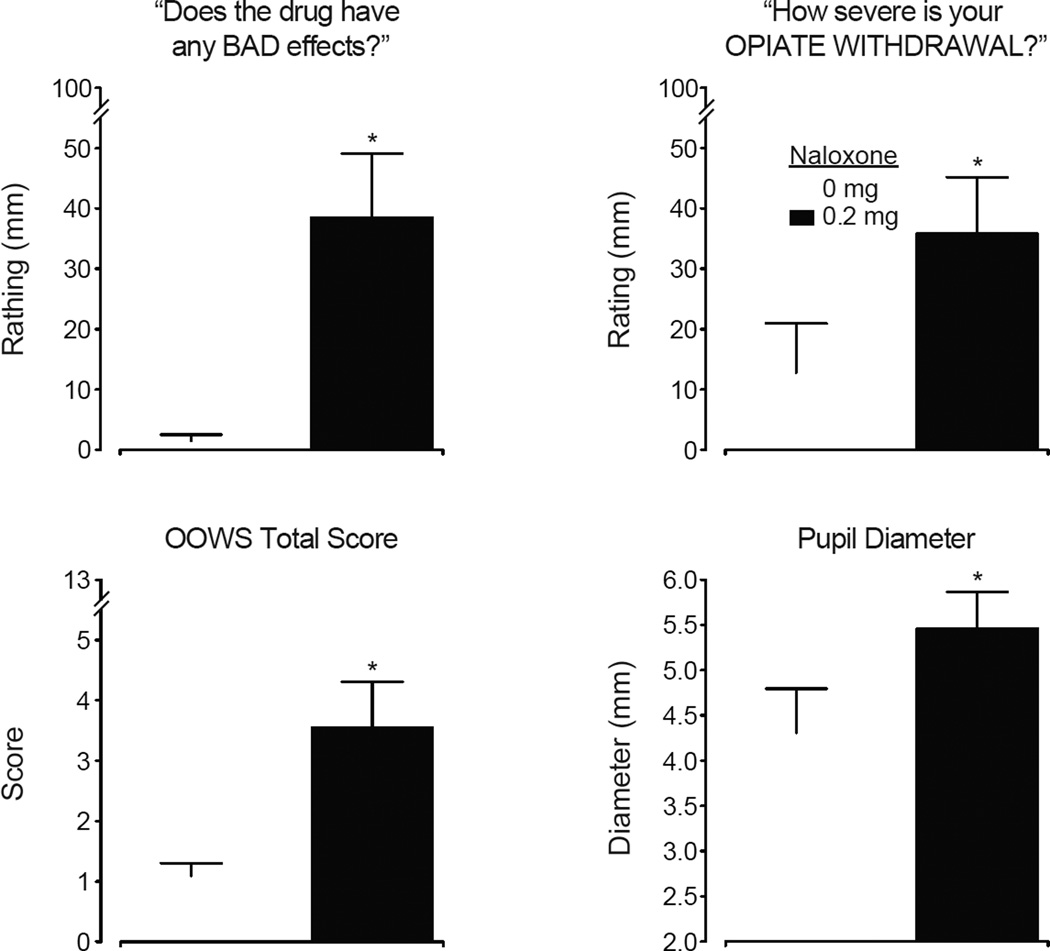
Time course analyses revealed significant main effects of dose for outcomes sensitive to mu opioid agonists. The Opioid Agonist Scales rated by participants and observers revealed significant main effects of dose (Subjects F [7, 70]= 2.15; p=.05; Observers F=3.99; p<.001), time and dose×time interactions (all p<.0001). For participant ratings, the specific symptoms of “skin itchy” and “energetic” also achieved statistical significance (p <.05); while the observers rated significant dose effects for “skin itchy,” “relaxed,” “talkative” and “good mood” (p<.05). The peak score data for these composite measures are shown in Figure 2 (upper panels). All active doses produced modest but significant elevations in ratings compared to placebo by the subjects, whereas all but BUP 2 and BUP/NX 2/0.5 were significantly different from placebo for observer-rated scores (statistical outcomes in legends).
Figure 2.
Mean peak data (n=11; ± 1 SEM) are shown for six measures reflecting opioid agonist effects, abuse potential and opioid withdrawal from the subjects and observers after intranasal administration of placebo (diamond), oxycodone (triangle), BUP alone (square) and BUP/NX (circles). For all measures, there were statistically significant main effects of dose as follows: Subject-rated Agonist Scale (upper left; F [7, 70] = 5.43; p<.0001), Observer-rated Agonist Scale (upper right; F=3.86; p=.001), the VAS “How much do you LIKE the drug?” (center left; F=3.48; p=.003), the VAS “Do you feel any DRUG EFFECT?” (center right; F=3.81; p=.002), Subject-rated Withdrawal Scale (lower left; F=2.45; p=.027) and the observer-rated Himmelsbach Scale (lower right; F=3.41; p=.003). Filled symbols indicate significant post-hoc differences between active doses and placebo; asterisks (*) indicate significant difference between matched doses of BUP vs. BUP/NX (Fisher’s LSD test, p<.05).
The visual analog ratings also revealed significant positive mood effects as a function of dose. Ratings for “good drug effect,” “high” and “liking” all produced significant main effects of dose, time and dose×time interactions. The time course data for “good drug effect” appear in Figure 3 (upper panels). In general, all active doses increased good drug effect ratings with oxycodone reliably producing the highest values. For both BUP and BUP/NX, while the 16 mg & 16/4 mg doses tended to produce the highest ratings, there was little separation among the doses. Peak data for “liking” (prototypic abuse liability measure; Fig. 2 middle left) revealed that all conditions, except for 2 mg BUP and 8 mg BUP/NX) produced ratings significantly greater than placebo (p<.05). Peak ratings on VAS effects were not reliably dose-related in most instances.
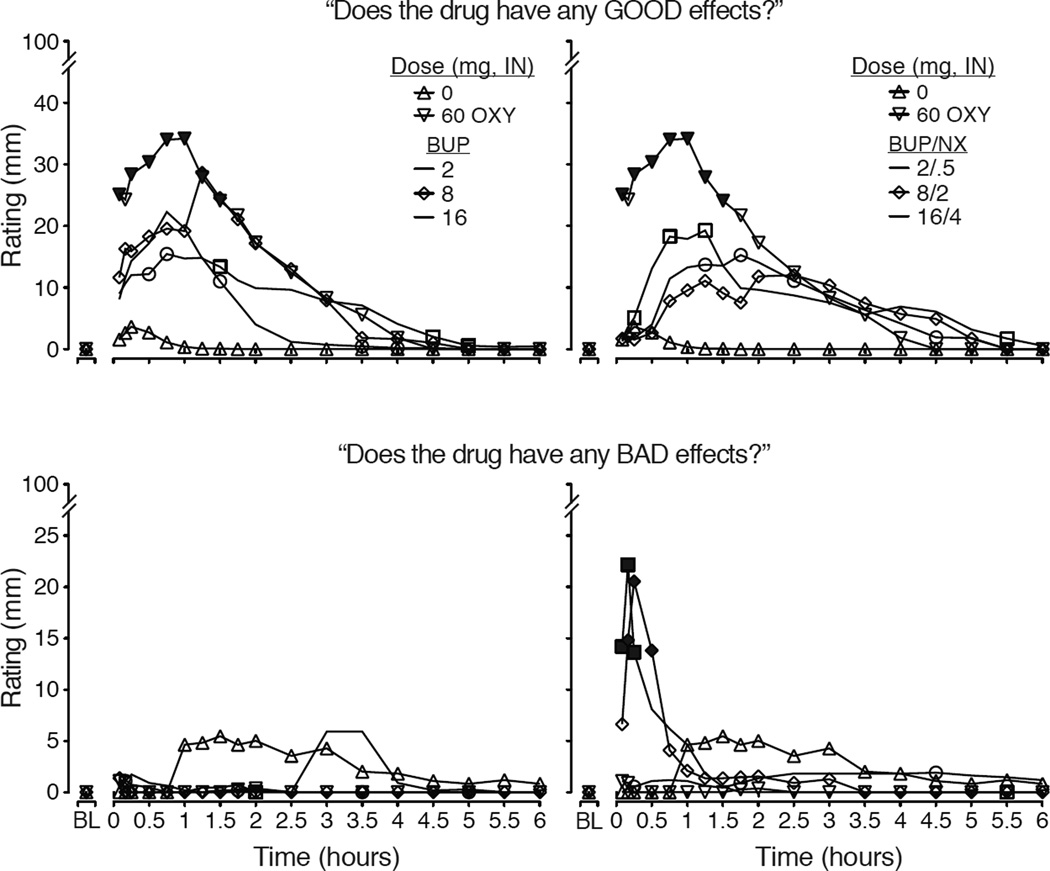
Figure 3.
Data shown depict the time-action curves for mean data (n=11; error bars omitted for clarity) collected during the 6.5 hour Sample sessions for two visual analog scales: Does the drug have any GOOD EFFECTS? (upper panels) and Does the drug have any BAD EFFECTS? (lower panels). On the left side, data are shown for the control conditions (placebo & 60 mg oxycodone) and the BUP doses while, on the right side, data are repeated for the control conditions and shown with the BUP/NX doses. For ratings of “good effects” there was a significant main effect of dose (F[7,70]=4.12; p=.001) and dose×time interaction (F[126, 160]=2.22; p<.0001). For ratings of bad effects, there was a significant main effect of dose (F=2.46; p=.026) and dose×time interaction (F=1.8; p<.0001). For both outcomes, there were significant main effects of time (p<.05). Filled symbols indicate significant differences between matched BUP vs. BUP/NX doses (e.g., 8 versus 8/2 at that time; Fisher’s LSD test; p<.05).
The global measure “any drug effect” shown in Figure 2 (middle right) shows the general magnitude of drug effect. Ratings for all active doses (except for BUP 2 mg and BUP/NX 2 mg) were significantly greater than placebo. There was a significant main effect of dose for ratings of “Desire for opioids” (F[7,70]=2.42; p=.028) with all active doses reducing desire. Peak street value estimates revealed that both oxycodone 60 mg ($23.86) and BUP 8 mg ($20.46) were valued significantly (p<.05) higher than placebo ($4.18); while lower estimates were obtained for all other doses (BUP 2 mg = $9.9; BUP 16 mg = $15.46; BUP 2/0.5 mg = $9.27; BUP 8/2 mg = $8.46; BUP 16/4 mg =$13.09). The drug identification questionnaire (using the most frequently provided answer within each session) revealed that all subjects identified oxycodone and BUP 8 mg as an opioid agonist. BUP 2 mg, BUP/NX 2/0.5 mg and BUP 16 mg were identified as an opioid agonist by about 2/3 and as placebo by about 1/3 of the volunteers. Both BUP/NX 8/2 mg and 16/4 mg were identified primarily as an opioid agonist, but was identified as an opioid antagonist by three and one subject(s), respectively.
The time course analyses for opioid withdrawal measures revealed significant main effects of dose, time and dose×time interactions by both the observers and subjects. For example, significant dose×time interactions were observed for the Himmelsbach (F[84,840]=2.15; p<.001) and OOWS scales (F[84,840]= 2.48; p<.001). Individual items for which significant effects were identified included “watery eyes,” “runny nose,” “yawning,” “sneezing,” “shaky hands,” “chills/gooseflesh” (p<.05). The VAS ratings of “bad effects” (Figure 3; lower panels) exhibit a similar profile that distinguishes BUP and BUP/NX as BUP/NX produces transient but significantly increased ratings of “bad effects” within the first hour. Peak ratings for subject-rated withdrawal (Figure 2, lower left) show reduction of withdrawal by oxycodone and increased withdrawal by BUP/NX 8/2 mg compared to BUP 8 mg. For the Himmelsbach (Figure 2 lower right), all active doses reduced withdrawal compared to placebo, while BUP/NX 8/2 mg produced greater withdrawal compared to BUP 8 mg.
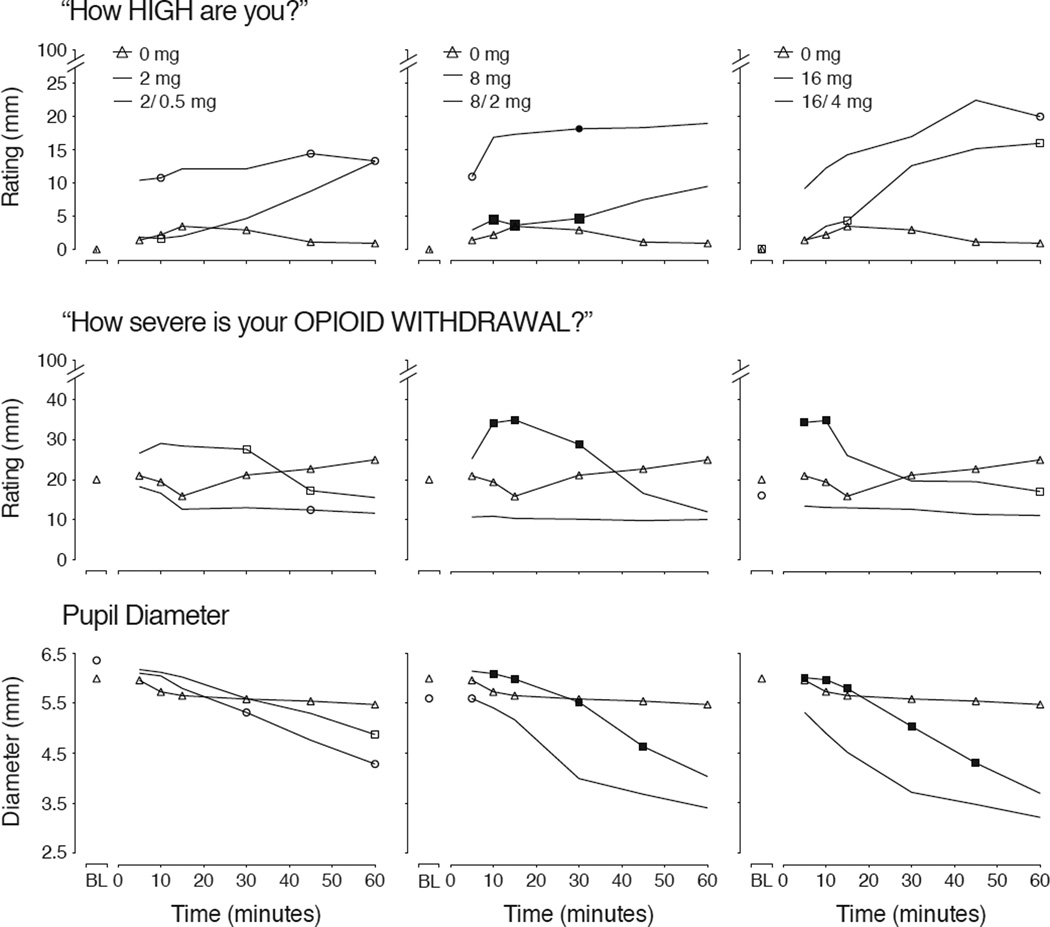
To explore these early observed differences, the first hour after dosing was analyzed for select opioid agonist outcomes. Findings for VAS ratings of “High” are shown in Figure 4 (upper panel) and reveal that the onset of BUP effects can be seen 5 min after insufflation, whereas the onset of BUP/NX is delayed for ~30 minutes. VAS ratings for “Opioid Withdrawal” in Figure 4 (middle panels) show significant increases in withdrawal for 8/2 and 16/4 compared to 8 and 16 emerging soon after insufflation.
Figure 4.
Shown for mean (n=11) data for VAS ratings of “How HIGH are you?” (upper panel), pupil diameter (middle panel) and VAS ratings of “How severe is your OPIOID WITHDRAWAL?” for baseline (BL) and the first hour after intranasal placebo (triangles) or matched doses of BUP (circles) and BUP/NX (squares). For each outcome, there was a significant main effect of dose as follows: (“High?” (F[7,70]=6.61; p<.0001); pupil diameter (F[7,70]=17.43; p<.0001), “Opioid Withdrawal” (F[7,70]=3.01; p=.008). Filled symbols indicate a significant difference at that time point between the matched dose of BUP and BUP/NX (Fisher’s LSD test, p<.05).
3.3.2. Physiological Outcomes
Pupil diameter was significantly reduced by all active doses compared to placebo based upon the time course analysis (F[7,70]=15.02; p<.0001). Data for the first hour after dosing are shown in Figure 4 (lower panel) to show the separation between BUP and BUP/NX whereby the miotic effects of BUP occur earlier and to a greater degree than after BUP/NX for doses higher than 2 mg. Respiratory rate was significantly altered as a function of dose (F[7,70]=3.67; p=.002) and time (p<.0001) based upon the time course analysis. While there was a trend for expired CO2 to increase as a function of dose condition, only the main effect of time reached significance (F[18,180]=4.19; p<.001). For all three measures, Tmax for all active doses was achieved between ~ 1.5 – 2 hours post-dosing.
3.4. Progressive Ratio Self-administration
Of the 88 total progressive ratio sessions, there were 13 sessions when participants completed all seven ratios for the same reinforcer (7 instances for money, 6 instances for drug). In all sessions (with one exception), participants worked for drug, money or a combination of both. Thus, for the majority of sessions (74 of 88), participants distributed their responding between the two available reinforcers.
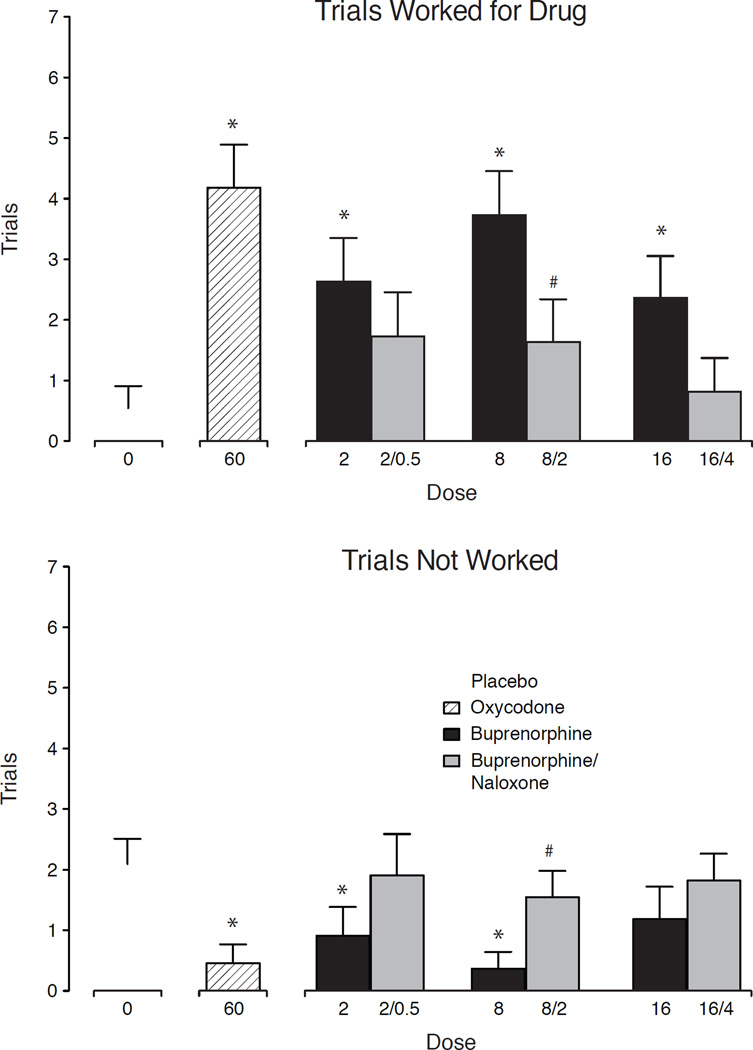
Figure 5 depicts the results from the PR sessions with the number of trials worked for drug (upper panel) and trials not worked (lower panel). For number of trials worked for drug, there was a significant main effect of dose (F [7, 70] =4.7; p < .0001). Placebo engendered modest responding whereby 2 of 11 subjects worked for drug (three trials each). Oxycodone (60 mg) and all active BUP doses produced statistically significant increases in responding compared to placebo (p<.05), while no BUP/NX doses were statistically different from placebo. Moreover, BUP 8 mg produced significantly greater responding compared to BUP/NX 8/2 mg. For trials not worked, oxycodone and BUP 2 and 8 mg were associated with fewer trials not worked compared to placebo, while 8 BUP and 8/2 BUP/NX differed significantly from one another (Fisher’s LSD; p<.05). There were no significant main effects of dose for number of trials worked for money or amount of money earned.
Figure 5.
Data are shown for the mean (n=11) number of trials completed (± 1 SEM) to earn the study drug during the progressive ratio sessions (upper panel) and number of trials not worked (lower panel). Significant main effects of dose were found for both outcomes (F[7,70]=4.71; p<.001 trials completed for drug and (F [7, 70] =2.9; p = .010) trials not worked, respectively. Asterisk (*) indicates significant difference from placebo, while hash (#) indicates significant difference between matched BUP and BUP/NX doses (Fisher’s LSD; p<.05). Number of trials worked for money can be derived by adding trials worked for drug with trials not worked for a given condition and subtracting that total from 7 (the total possible trials within a session).
4. DISCUSSION
This inpatient study examined a range of intranasal BUP and BUP/NX doses in opioid-dependent individuals maintained on the short-acting opioid, oxycodone, to assess opioid agonist and antagonist effects, reinforcing efficacy and safety. It was hypothesized that higher doses (e.g., 16/4) of BUP/NX would potentially precipitate robust and frank withdrawal (Stoller et al., 2001; Walsh et al., 1995) and, thus, a priori safety criteria were set to preclude dose escalation to ensure the safety and comfort of participants. Surprisingly, these criteria were never met, and all subjects were able to safely tolerate all BUP and BUP/NX dose challenges. However, the pharmacodynamic data are very revealing with regard to the effects of naloxone when given in combination with BUP, suggesting that intranasal naloxone exerts meaningful effects by both eliciting modest and transient unpleasant effects related to opioid withdrawal and delaying the early onset of the agonist effects of BUP, both of which may contribute to the observation here that intranasal BUP/NX was self-administered to a lesser extent than matched doses of BUP.
Studies with opioid-dependent participants have used a wide array of opioid maintenance drugs/doses to stabilize enrollees, including methadone, buprenorphine, morphine and hydromorphone. However, we are unaware of other studies that have employed oxycodone for this purpose. Here, oral oxycodone was employed at 30 mg/qid, as it is a commonly abused prescription opioid (Cicero et al., 2013). This was well tolerated with only one participant leaving early because of spontaneous opioid withdrawal. The regimen also produced measurable opioid dependence based upon naloxone challenge results (Figure 1). Omission of two doses before Sample Sessions produced modest opioid withdrawal and was chosen to mimic the scenario whereby an individual may not have access to their usual opioid and misuse BUP as a substitute. It is likely that more profound intranasal BUP and/or BUP/NX withdrawal may have resulted had the omission period been shorter and greater amounts of oxycodone were present (CSAT, 2004).
The dose response curves for both BUP and BUP/NX were not reliably dose-dependent (see Figure 2), and, relying on the statistical outcomes, the dose associated with the greatest abuse liability was the 8 mg BUP dose, with the 16 mg BUP frequently producing ratings of lower magnitude. Moreover, despite the broad range of test doses (8-fold), the dose-functions were often overlapping without great separation among the doses for some outcomes (see Fig. 2, 3). It is unclear whether these findings are attributable to BUP’s partial agonist profile, frequently expressed as reaching a ceiling effect (Cowan et al., 1977; Walsh et al., 1994). This seems plausible as, in many instances, the effects of intranasal oxycodone (60 mg), a full opioid agonist, exceeded those of BUP and BUP/NX both in producing direct agonist effects and suppressing spontaneous withdrawal during the Sample sessions (Figure 2).
Similar to the findings by Jones and colleagues who studied opioid-dependent subjects maintained on buprenorphine itself (Jones et al., 2015), BUP/NX was less reinforcing and produced some dysphoric/unpleasant effects compared to BUP in the present study in individuals maintained on a short-acting opioid. Here, the self-administration data revealed that, while only oxycodone (60 mg) and all BUP doses elicited drug taking that was significantly greater than placebo, BUP/NX was always chosen (and worked for) less than the same dose of BUP alone (significantly so for the 8/2 mg dose) and did not differ from placebo. The time course data suggest that the intranasal absorption of naloxone produced two effects of interest. First, the presence of naloxone was sufficient to delay the early onset of BUP’s opioid agonist effects, which were delayed in comparison to those of BUP alone (Figure 4). The delay in onset of positive mood effects is meaningful because it is widely recognized that the speed of onset of drug action is positively related to abuse potential (Abreu et al., 2001; de Wit et al., 1992, 1993). It is interesting to note in our earlier study examining intranasal BUP and BUP/NX in non-dependent opioid abusers that these same naloxone doses did not appreciably alter the response to BUP/NX compared to BUP (only 2 and 8 mg were tested). Second, naloxone did, indeed, produce modest but significant increased subjective ratings of bad drug effects and opioid withdrawal and increased observer ratings of withdrawal (Figures 2 & 3) that were consistent with naloxone’s short duration of action (Berkowitz, 1976; Watson et al., 1998), suggesting that naloxone-precipitated withdrawal occurred within minutes of insufflation and was readily detected by participants. In summary, this study demonstrated that in individuals dependent on short-acting opioids 1) oral oxycodone can be employed safely as a maintenance regimen, 2) intranasal BUP and BUP/NX both produce opioid-agonist like effects in this population that are not reliably dose-related, and 3) that the absorption of naloxone by the intranasal route is sufficient to produce withdrawal-like symptoms and blunt the onset of agonist effects, both of which are transient. The antagonist properties of intranasal BUP/NX may account for the finding that BUP/NX was less preferable than BUP in the self-administration procedure. Overall, these data confirm that intranasal BUP/NX has some abuse deterrent properties that decrease its positive subjective effects and reinforcing efficacy compared to intranasal BUP.
Highights.
Inpatient study examined persons dependent on short-acting opioids
Intranasal (IN) buprenorphine/naloxone (BUP/NX) precipitated opioid withdrawal
IN naloxone slowed the onset of buprenorphine’s opioid agonist effects
Buprenorphine was self-administered more than BUP/NX
IN BUP/NX has lower abuse potential and reinforcing efficacy than buprenorphine
Acknowledgments
The authors would like to thank the nursing staff at the Center for Clinical and Translational Research, the research staff at the Center on Drug and Alcohol Research, the pharmacy staff at the Investigational Drug Services Pharmacy at the University of Kentucky for support of this project, and Lisa Middleton, Ph.D. for work on an early draft of the protocol. The authors also thank Reckitt Benckiser Pharmaceuticals, especially Dr. Neil Hyde and Dr. Chris Chapleo, for assistance in obtaining the EU buprenorphine/naloxone supply at no cost through the NIDA Research Triangle Institute drug supply program.
Role of Funding Source: This study was supported by a grant from the National Institute on Drug Abuse, R01 DA016718-04 (SLW) and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, UL1TR000117. Reckitt Benckiser and the National Institute on Drug Abuse provided the drug supply at no cost. None of these agencies had any role in the study design; in the collection, analysis and interpretation of the date; in writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: SLW, SB and MRL conceived and designed the study. SLW, SB, VAC and PN provided oversight for the conduct of the study and enrollment of volunteers. MRL provided medical evaluations and medical oversight. VAC and PN managed data collection and conducted data analyses. All authors participated in the preparation and review of the final manuscript.
Conflicts of Interest: Conflicts of interest for SB, VV and PN: none. SLW and MRL have received honoraria and travel reimbursement for developing and delivering educational talks through an arms-length unrestricted educational grant from Reckitt Benckiser Pharmaceuticals to PCM Scientific, UK; SLW has also received honoraria from the same grant for organizing and serving as a conference chairperson. SLW and MRL are also serving as consultants to Braeburn Pharmaceuticals and Camurus in the development of novel buprenorphine formulations. MRL and SLW have received salary support from research grants from Braeburn Pharmaceuticals. SLW has also received consulting fees for advising pharmaceutical companies on product development, study design and abuse deterrence, including Pfizer, Novartis, Sun Pharma, Astra Zeneca, Lightlake Therapeutics and World Meds, Inc.
REFERENCES
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl.) 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Alho H, Sinclair D, Vuori E, Holopainen A. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75–78. doi: 10.1016/j.drugalcdep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Babalonis S, Lofwall MR, Nuzzo PA, Siegel AJ, Walsh SL. Abuse liability and reinforcing efficacy of oral tramadol in humans. Drug Alcohol Depend. 2013;129:116–124. doi: 10.1016/j.drugalcdep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrau K, Thirion X, Micallef J, Chuniaud-Louche C, Bellemin B, San Marco JL. Comparison of methadone and high dosage buprenorphine users in French care centres. Addiction. 2001;96:1433–1441. doi: 10.1046/j.1360-0443.2001.961014337.x. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA. The relationship of pharmacokinetics to pharmacological activity: morphine, methadone and naloxone. Clin. Pharmacokinet. 1976;1:219–230. doi: 10.2165/00003088-197601030-00004. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Govindasamy S, Sylla L, Kamarulzaman A, Altice FL. Lack of reduction in buprenorphine injection after introduction of co-formulated buprenorphine/naloxone to the Malaysian market. Am. J. Drug Alcohol Abuse. 2009;35:68–72. doi: 10.1080/00952990802585406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. Factors influencing the selection of hydrocodone and oxycodone as primary opioids in substance abusers seeking treatment in the United States. Pain. 2013;154:2639–2648. doi: 10.1016/j.pain.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Clark NC, Lintzeris N, Muhleisen PJ. Severe opiate withdrawal in a heroin user precipitated by a massive buprenorphine dose. Med. J. Aust. 2002;176:166–167. [PubMed] [Google Scholar]
- Cowan A, Lewis JW, Macfarlane IR. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br. J. Pharmacol. 1977;60:537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAT. Clinical Guidelines for Buprenorphine in the Treatment of Opioid Addiction. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. [PubMed] [Google Scholar]
- de Wit H, Bodker B, Ambre J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology (Berl.) 1992;107:352–358. doi: 10.1007/BF02245161. [DOI] [PubMed] [Google Scholar]
- de Wit H, Dudish S, Ambre J. Subjective and behavioral effects of diazepam depend on its rate of onset. Psychopharmacology (Berl.) 1993;112:324–330. doi: 10.1007/BF02244928. [DOI] [PubMed] [Google Scholar]
- Eiden C, Ripault MP, Larrey D, Faillie JL, Pinzani V, Pageaux GP, Peyriere H. Acute hepatitis and renal failure related to intranasal buprenorphine misuse: case report and analysis of cases reported to the French network for drug monitoring. Ann. Pharmacother. 2013;47:1721–1726. doi: 10.1177/1060028013507429. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Greenwald MW, Johnson RE, Liebson IA, Bigelow GE, Stitzer ML. Buprenorphine's physical dependence potential: antagonist-precipitated withdrawal in humans. J. Pharmacol. Exp. Ther. 1996;276:449–459. [PubMed] [Google Scholar]
- Ferrant O, Papin F, Clin B, Lacroix C, Saussereau E, Remoue JE, Goulle JP. Fatal poisoning due to snort ing buprenorphine and alcohol consumption. Forensic Sci. Int. 2011;204:e8–e11. doi: 10.1016/j.forsciint.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Yu E, Macfadden W, Boardman C, Chiang CN. Effects of buprenorphine and naloxone in morphine-stablized opioids addicts. Drug Alcohol Depend. 1998;50:1–8. doi: 10.1016/s0376-8716(98)00008-8. [DOI] [PubMed] [Google Scholar]
- Fullerton CA, Kim M, Thomas CP, Lyman DR, Montejano LB, Dougherty RH, Daniels AS, Ghose SS, Delphin-Rittmon ME. Medication-assisted treatment with methadone: assessing the evidence. Psychiatr. Serv. 2014;65:146–157. doi: 10.1176/appi.ps.201300235. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am. J. Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Harris DS, Jones RT, Welm S, Upton RA, Lin E, Mendelson J. Buprenorphine and naloxone co-administration in opiate-dependent patients stabilized on sublingual buprenorphine. Drug Alcohol Depend. 2000;61:85–94. doi: 10.1016/s0376-8716(00)00126-5. [DOI] [PubMed] [Google Scholar]
- Hayes BD, Klein-Schwartz W, Doyon S. Toxicity of buprenorphine overdoses in children. Pediatrics. 2008;121:e782–e786. doi: 10.1542/peds.2007-1774. [DOI] [PubMed] [Google Scholar]
- Himmelsbach CK. The morphine abstinence syndrome, its nature and treatment. Ann. Intern. Med. 1941;15:829–839. [Google Scholar]
- Horyniak D, Dietze P, Larance B, Winstock A, Degenhardt L. The prevalence and correlates of buprenorphine inhalation amongst opioid substitution treatment (OST) clients in Australia. Int. J. Drug Policy. 2011;22:167–171. doi: 10.1016/j.drugpo.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Griffith JD, Pevnick J, Gorodetzky C, Cone E, Kay D. Progress Report From The Clinical Pharmacology Section Of The NIDA Addiction Research Center, 39th Annual Meeting; The Committee on Problems of Drug Dependence. National Research Council, National Academy of Sciences; Washington, DC. 1977. pp. 133–168. [Google Scholar]
- Jenkinson RA, Clark NC, Fry CL, Dobbin M. Buprenorphine diversion and injection in Melbourne, Australia: an emerging issue? Addiction. 2005;100:197–205. doi: 10.1111/j.1360-0443.2004.00958.x. [DOI] [PubMed] [Google Scholar]
- Johnson RE, McCagh JC. Buprenorphine and naloxone for heroin dependence. Curr. Psychiatry Rep. 2000;2:519–526. doi: 10.1007/s11920-000-0012-8. [DOI] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Vosburg SK, Manubay JM, Mogali S, Metz V, Comer SD. Abuse potential of intranasal buprenorphine versus buprenorphine/naloxone in buprenorphine-maintained heroin users. Addict. Biol. 2015;20:784–798. doi: 10.1111/adb.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintz P. A new series of 13 buprenorphine-related deaths. Clin. Biochem. 2002;35:513–516. doi: 10.1016/s0009-9120(02)00304-1. [DOI] [PubMed] [Google Scholar]
- Lai SH, Teo CE. Buprenorphine-associated deaths in Singapore. Ann. Acad. Med. Singapore. 2006;35:508–511. [PubMed] [Google Scholar]
- Larance B, Lintzeris N, Ali R, Dietze P, Mattick R, Jenkinson R, White N, Degenhardt L. The diversion and injection of a buprenorphine-naloxone soluble film formulation. Drug Alcohol Depend. 2014;136:21–27. doi: 10.1016/j.drugalcdep.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Lavonas EJ, Banner W, Bradt P, Bucher-Bartelson B, Brown KR, Rajan P, Murrelle L, Dart RC, Green JL. Root causes, clinical effects, and outcomes of unintentional exposures to buprenorphine by young children. J. Pediatr. 2013;163:1377–1383. doi: 10.1016/j.jpeds.2013.06.058. [DOI] [PubMed] [Google Scholar]
- Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J. Addict. Med. 2014;8:315–326. doi: 10.1097/ADM.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovegrove MC, Mathew J, Hampp C, Governale L, Wysowski DK, Budnitz DS. Emergency hospitalizations for unsupervised prescription medication ingestions by young children. Pediatrics. 2014;134:e1009–e1016. doi: 10.1542/peds.2014-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J. Subst. Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Megarbane B, Vodovar D, Baud FJ. Fatalities in relation to buprenorphine snorting and ethanol co-ingestion: mechanisms of toxicity. Forensic Sci. Int. 2011;207:e59–e60. doi: 10.1016/j.forsciint.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Fernandez I, Welm S, Melby AK, Baggott MJ. Buprenorphine and naloxone interactions in opiate-dependent volunteers. Clin. Pharmacol. Ther. 1996;60:105–114. doi: 10.1016/S0009-9236(96)90173-3. [DOI] [PubMed] [Google Scholar]
- Middleton LS, Nuzzo PA, Lofwall MR, Moody DE, Walsh SL. The pharmacodynamic and pharmacokinetic profile of intranasal crushed buprenorphine and buprenorphine/naloxone tablets in opioid abusers. Addiction. 2011;106:1460–1473. doi: 10.1111/j.1360-0443.2011.03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann S, Frauger E, Pauly V, Orleans V, Pradel V, Mallaret M, Thirion X, Micallef J. Misuse of buprenorphine maintenance treatment since introduction of its generic forms: OPPIDUM survey. Pharmacoepidemiol. Drug Saf. 2012;21:184–190. doi: 10.1002/pds.2263. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Opioid discrimination in humans: discriminative and subjective effects of progressively lower training dose. Behav. Pharmacol. 1998;9:533–543. doi: 10.1097/00008877-199811000-00009. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA. Buprenorphine and naloxone alone and in combination in opioid-dependent humans. Psychopharmacology (Berl.) 1988;94:484–490. doi: 10.1007/BF00212842. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA. Effects of sublingually given naloxone in opioid-dependent volunteers. Drug Alcohol Depend. 1990;25:27–34. doi: 10.1016/0376-8716(90)90136-3. [DOI] [PubMed] [Google Scholar]
- Reynaud M, Petit G, Potard D, Courty P. Six deaths linked to concomitant use of buprenorphine and benzodiazepines. Addiction. 1998;93:1385–1392. doi: 10.1046/j.1360-0443.1998.93913859.x. [DOI] [PubMed] [Google Scholar]
- Rosado J, Walsh SL, Bigelow GE, Strain EC. Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100mg of daily methadone. Drug Alcohol Depend. 2007;90:261–269. doi: 10.1016/j.drugalcdep.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P, Villes V, Bry D, Spire B, Feroni I, Marcellin F, Carrieri MP. Buprenorphine sniffing as a response to inadequate care in substituted patients: results from the Subazur survey in south-eastern France. Addict. Behav. 2008;33:1625–1629. doi: 10.1016/j.addbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Walsh SL, Bigelow GE, Preston KL, Stitzer ML. Buprenorphine, morphine and naloxone effects during ascending morphine maintenance in humans. J. Pharmacol. Exp. Ther. 1996;278:836–846. [PubMed] [Google Scholar]
- Simonsen KW, Normann PT, Ceder G, Vuori E, Thordardottir S, Thelander G, Hansen AC, Teige B, Rollmann D. Fatal poisoning in drug addicts in the Nordic countries in 2007. Forensic Sci. Int. 2011;207:170–176. doi: 10.1016/j.forsciint.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Stoller K, Bigelow GE, Walsh SL, Strain EC. Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology (Berl.) 2001;154:230–242. doi: 10.1007/s002130000637. [DOI] [PubMed] [Google Scholar]
- Tracqui A, Kintz P, Ludes B. Buprenorphine-related deaths among drug addicts in France: a report on 20 fatalities. J. Anal. Toxicol. 1998;22:430–434. doi: 10.1093/jat/22.6.430. [DOI] [PubMed] [Google Scholar]
- Walsh SL, June HL, Schuh KJ, Preston KL, Bigelow GE, Stitzer ML. Effects of buprenorphine and methadone in methadone-maintained subjects. Psychopharmacology (Berl.) 1995;119:268–276. doi: 10.1007/BF02246290. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin. Pharmacol. Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Watson WA, Steele MT, Muelleman RL, Rush MD. Opioid toxicity recurrence after an initial response to naloxone. J. Toxicol. Clin. Toxicol. 1998;36:11–17. doi: 10.3109/15563659809162577. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J. Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Young AM, Havens JR, Leukefeld CG. Route of administration for illicit prescription opioids: a comparison of rural and urban drug users. Harm Reduct. J. 2010;7:24. doi: 10.1186/1477-7517-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]