Abstract
Objective
To evaluate prevalence, severity, and co-occurrence of, and risk factors for depression, anxiety, and post-traumatic stress disorder (PTSD) symptoms over the first year after ARDS.
Design
Prospective longitudinal cohort study.
Settings
41 ARDS Network hospitals across the U.S.
Patients
698 ARDS survivors.
Interventions
None.
Measurements and Main Results
Psychiatric symptoms were evaluated using the Hospital Anxiety and Depression Scale (HADS) and Impact of Event Scale–Revised (IES-R) at 6 and 12 months. Adjusted prevalence ratios for substantial symptoms (binary outcome) and severity scores were calculated using Poisson and linear regression, respectively. During 12 months, a total of 416 of 629 patients (66%) with at least one psychiatric outcome measure had substantial symptoms in at least one domain. There was a high and almost identical prevalence of substantial symptoms (36%, 42%, and 24% for depression, anxiety and PTSD) at 6 and 12 months. The most common pattern of co-occurrence was having symptoms of all 3 psychiatric domains simultaneously. Younger age, female sex, unemployment, alcohol misuse, and greater opioids use in the ICU were significantly associated with psychiatric symptoms, while greater severity of illness and ICU length of stay were not associated.
Conclusions
Psychiatric symptoms occurred in two-thirds of ARDS survivors with frequent co-occurrence. Sociodemographic characteristics and in-ICU opioids administration, rather than traditional measures of critical illness severity, should be considered in identifying patients at highest risk for psychiatric symptoms during recovery. Given high co-occurrence, ARDS survivors should be simultaneously evaluated for a full spectrum of psychiatric sequelae to maximize recovery.
Keywords: Depression, Anxiety, Stress Disorder, Post-Traumatic, Prospective Studies, Critical Illness, Respiratory Distress Syndrome, Adult
INTRODUCTION
Survivors of acute respiratory distress syndrome (ARDS) frequently experience substantial long-term psychiatric symptoms after hospital discharge.(1–10) Pro-inflammatory cytokines, frightening memories experienced in the intensive care unit (ICU), and stressful life changes after hospital discharge may be potential causes for psychiatric symptoms.(6) In ARDS survivors, systematic reviews have reported point prevalences of 17% to 43% for depression, 23% to 48% for anxiety, and 8% to 35% for post-traumatic stress disorder (PTSD) symptoms,(6;7;9) with common risk factors including younger age, female sex, obesity, pre-existing psychiatric illness, lower educational attainment, baseline unemployment, and lower blood glucose in the ICU.(1;3;4;7;9)
However, most existing publications are single-center studies with modest sample sizes that evaluate a single psychiatric domain in isolation. Few studies have evaluated multiple psychiatric outcomes in multiple institutions with large sample sizes.(6;11) These limitations contribute, in part, to conflicting results in evaluating risk factors for psychiatric symptoms after ARDS. To better inform clinicians’ and researchers’ efforts to effectively identify and treat these important morbidities, additional research is required regarding the prevalence, severity, co-occurrence and risk factors for psychiatric symptoms, using larger-sized, multi-centered, longitudinal studies to simultaneously evaluate commonly reported symptoms.
Hence, our study longitudinally evaluates, over the first year after ARDS, the prevalence, severity and co-occurrence of depression, anxiety, and PTSD symptoms, as well as patient- and critical illness-related risk factors for these symptoms.
METHODS
Study Population
Participants were part of the ARDS Network (ARDSNet) Long-term Outcome Study (ALTOS), a national, multi-center, prospective cohort study evaluating outcomes at 6 and 12 months after enrollment into three recent ARDSNet clinical trials evaluating ICU-based therapies for ARDS patients. The eligibility criteria for these three ARDSNet trials have been published previously and are summarized herein.(12;13) Patients were eligible for recruitment within 48 hours of ARDS onset and within 72 hours of initiation of mechanical ventilation. Major exclusion criteria included severe comorbid malnutrition; lung, liver or neuromuscular diseases; or limitations in life support at time of eligibility.(12;13) For follow-up evaluations in ALTOS, we further excluded survivors from the ARDSNet trials if they had potential cognitive impairment prior to admission (ascertained via medical records and/or patient/proxy report), or were non-English speaking, homeless, or younger than 18 years old. Across all participating sites, patients were managed with simplified versions of lung protective mechanical ventilation and fluid conservative hemodynamic management protocols, with blood glucose control aimed at 80–150 mg/dL (tighter glucose control was permitted). Informed consent was obtained from the patient or a proxy. This study was approved by the Institutional Review Board at Johns Hopkins University and all participating hospitals.
Measurement of Patient- and Critical Illness-Related Exposures
Both patient- and critical illness-related exposures were obtained from ALTOS and the ARDSNet trials. Patient-related baseline exposures included demographics, employment status (unemployed vs. employed), body mass index (BMI), medical comorbidities (diabetes mellitus, prior stroke, and use of hemodialysis), and alcohol misuse. Alcohol misuse was defined by zones 3 and 4 from the Alcohol Use Disorders Identification Test (AUDIT), which indicate alcohol consumption in excess of recommended limits. (14) Critical illness-related baseline exposures included admission to a medical (versus surgical) ICU, severity of illness (i.e., Acute Physiology and Chronic Health Evaluation (APACHE) III score, and partial pressure of oxygen in arterial blood to fraction of inspired oxygen (PaO2/FiO2) ratio), and ARDS risk factor (sepsis versus all others). While in the ICU, the following were collected daily for up to 12 days after enrollment: (1) the Brussels organ failure status for the cardiovascular, pulmonary, coagulation, renal and hepatic systems(15) (modeled as the mean number of organ failures during data collection), (2) use of hemodialysis and vasopressors (modeled as binary variables), (3) use of opioids, corticosteroids, and neuromuscular blockers (modeled as the percentage of ICU days with use), and (4) morning and daily minimum blood glucose (modeled as mean values). Mechanical ventilation duration and ICU length of stay data were also collected.
Measurement of Psychiatric Symptoms
The outcome variables of interest were symptoms of depression, anxiety, and PTSD at 6 and 12 months after ARDS. Depression and anxiety symptoms were measured using the respective subscales of the Hospital Anxiety and Depression Scale (HADS) instrument.(16) Each HADS subscale ranges from 0 to 21, with a higher score indicating worse symptoms and a score ≥8 indicating substantial symptoms.(16) PTSD symptoms were measured using the Impact of Event Scale-Revised (IES-R). The IES-R score ranges from 0 to 4, with a higher score indicating worse PTSD symptoms(17) and a score ≥1.6 indicating substantial PTSD symptoms in ARDS survivors.(18) Both scales were administered to patients only (i.e. no proxy respondents) by trained research staff via phone for 98% of assessments, otherwise via mail or in-person administration. These scales have evidence of good reliability and validity, and have been frequently used in prior studies evaluating survivors of critical illness.(1;9;18;19)
Statistical Analysis
We compared all exposure variables (see Table 1) by binary categorization of the three psychiatric domains at 6 months using Fisher’s exact tests and t-tests. For both the 6- and 12-month time points, correlations of continuous psychiatric symptom scores were calculated using the Spearman correlation coefficient, and co-occurrence of substantial psychiatric symptoms was evaluated by calculating the proportion of patients who had substantial symptoms in more than one domain. The prevalence and co-occurrence were illustrated via a proportioned Venn diagram utilizing the “pvenn” command (Stata version 13.0, StataCorp, College Station, TX).
Table 1.
Baseline characteristics for all patients and by psychiatric symptoms at 6 months after acute respiratory distress syndrome
| Depression Symptoms, n (%) | Anxiety Symptoms, n (%) | PTSD Symptomsb, n (%) | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Variablea | Total (n=613) | Positive (HADS ≥8) n=222 (36%) | Negative (HADS <8) n=391 (64%) | Positive (HADS ≥8) n=260 (42%) | Negative (HADS <8) n=353 (58%) | Positive (IES-R ≥1.6) n=148 (24%) | Negative (IES-R <1.6) n=457 (76%) |
| Baseline patient data | |||||||
| Age, mean (SD) year | 49 (15) | 48 (12) | 49 (16) | 48 (12) | 50 (16) | 47 (12) | 50 (15) |
| Female, N (%) | 316 (52) | 127 (57) | 189 (48) | 155 (60) | 161 (46) | 100 (68) | 213 (47) |
| White, N (%) | 485 (82) | 174 (81) | 311 (82) | 211 (84) | 274 (80) | 108 (78) | 371 (83) |
| Unemployed, N (%) | 296 (49) | 130 (59) | 166 (43) | 143 (56) | 153 (44) | 88 (60) | 205 (45) |
| BMI, mean (SD) kg/m2 | 30 (8) | 31 (8) | 30 (8) | 31 (8) | 30 (8) | 31 (8) | 30 (8) |
| Diabetes, N (%) | 146 (24) | 52 (23) | 94 (24) | 62 (24) | 84 (24) | 32 (22) | 110 (24) |
| Stroke, N (%) | 9 (2) | 5 (2) | 4 (1) | 4 (2) | 5 (1) | 4 (3) | 4 (1) |
| Hemodialysis, N (%) | 14 (2) | 9 (4) | 5 (1) | 7 (3) | 7 (2) | 6 (4) | 8 (2) |
| Alcohol misuse, N (%) | 126 (22) | 52 (25) | 74 (20) | 60 (24) | 66 (20) | 36 (26) | 88 (21) |
| Baseline intensive care data | |||||||
| Admission to medical ICU, N (%) | 350 (57) | 124 (56) | 226 (58) | 151 (58) | 199 (56) | 86 (58) | 259 (57) |
| APACHE III, mean (SD) | 86 (26) | 82 (25) | 88 (26) | 83 (26) | 88 (25) | 83 (26) | 87 (25) |
| PaO2/FiO2, mean (SD) | 204 (73) | 209 (76) | 200 (72) | 202 (65) | 205 (79) | 207 (71) | 202 (74) |
| Sepsis as ARDS risk factor, N (%) | 467 (76) | 157 (71) | 310 (79) | 195 (75) | 272 (77) | 108 (73) | 352 (77) |
| Daily intensive care data | |||||||
| No. of organ failuresc, mean (SD) | 2 (1) | 2 (1) | 2 (1) | 2 (1) | 2 (1) | 2 (1) | 2 (1) |
| Any hemodialysis, N (%) | 76 (14) | 22 (11) | 54 (15) | 24 (10) | 52 (17) | 19 (14) | 56 (14) |
| Any vasopressor use, N (%) | 325 (53) | 110 (50) | 215 (55) | 137 (53) | 188 (53) | 81 (55) | 240 (53) |
| % of days with opioids, mean (SD) | 73 (30) | 78 (28) | 70 (31) | 76 (28) | 70 (32) | 79 (27) | 71 (31) |
| % of days with corticosteroids, mean (SD) | 21 (35) | 23 (37) | 20 (34) | 21 (36) | 20 (34) | 24 (37) | 20 (34) |
| % of days with neuromuscular blocker, mean (SD) | 5 (13) | 5 (14) | 5 (13) | 6 (14) | 4 (13) | 6 (15) | 5 (13) |
| Morning glucose, mean (SD) mg/dl, | 127 (26) | 127 (26) | 127 (26) | 126 (26) | 128 (26) | 126 (26) | 128 (26) |
| Minimum glucose, mean (SD) mg/dl | 110 (23) | 110 (25) | 111 (21) | 108 (23) | 112 (22) | 108 (20) | 111 (23) |
| Other intensive care data | |||||||
| Ventilation duration, mean (SD) day | 11 (10) | 11 (10) | 11 (10) | 10 (8) | 11 (11) | 11 (9) | 11 (10) |
| ICU length of stay, mean (SD) day d | 14 (11) | 14 (11) | 14 (11) | 13 (10) | 15 (12) | 14 (10) | 14 (11) |
Abbreviations: ARDS (acute respiratory distress syndrome), PTSD (post-traumatic stress disorder), HADS (Hospital Anxiety and Depression Scale), IES-R (Impact of Event Scale – Revised), SD (standard deviation), BMI (body mass index), ICU (intensive care unit), APACHE III (Acute Physiology and Chronic Health Evaluation III), PaO2/FiO2 (ratio between partial pressure of oxygen in arterial blood and fraction of inspired oxygen).
Percentages may not add up to 100% due to rounding. Missing data for each variable (N): white (19), unemployed (10), BMI (2), alcohol misuse (42), APACHE III score (17), PaO2/FiO2 ratio (19), any hemodialysis (58), % of days with opioids (130), % of days with corticosteroids (120), % of days with neuromuscular blocker (130), morning glucose (1), and minimum glucose (101).
Missing PTSD assessments for 8 (1%) of 613 patients.
Data represent the average number of organ failures during ICU stay, using the Brussels scoring system(20) for the following five organ systems (with definition of organ failure): cardiac (systolic blood pressure ≤90 mmHg or use of vasopressor), pulmonary (PaO2/FiO2 ratio ≤300), coagulation (platelets ≤80 × 109/L), renal (creatinine ≥2.0 mg/dL) and hepatic (bilirubin ≥2.0 mg/dL).
Median and inter-quartile range (IQR) of ICU length of stay was 10 (7–16) days.
Associations between each exposure variable and substantial symptoms for each of the three psychiatric domains (binary outcome variables, as previously defined) were evaluated using Poisson regression. We evaluated these associations separately at 6- and 12-month follow-up; however, due to statistically similar associations at both time points, we adopted a simplified approach combining 6- and 12-month follow-up visits. For this combined approach, the models included an indicator for time (12- vs. 6-month) and were fitted using generalized estimating equations (GEE), with an exchangeable correlation structure and robust variance estimate to account for within-patient clustering over 6- and 12-month follow-ups. For continuous measures of symptom severity for each of the three psychiatric domains, a similar statistical approach was employed using linear regression models. Multivariable regression models were constructed in the same manner as the bivariable models and included all exposure variables that had a potentially significant bivariable association of p<0.20 with each psychiatric domain. Regardless of bivariable association, ICU length of stay was included in the multivariable models to standardize evaluation of daily ICU exposure variables, and baseline use of hemodialysis was included to adjust for pre-existing renal disease when including hemodialysis in the ICU in the multivariable models. To evaluate the effect of potential confounding by pre-existing psychiatric comorbidity on the results, we conducted sensitivity analysis using a subgroup of patients (n=203) from 5 of the 12 study sites on whom we prospectively collected baseline psychiatric comorbidity based on medical records. In this analysis, we evaluated the exposure-outcome assessments with vs. without this comorbidity in the original multivariable model.
Standard regression diagnostics were conducted for all models. The linearity assumption was verified by assessing locally weighted scatterplot smoothing of each exposure variable against residuals from the regression model. Only age demonstrated a non-linear relationship with outcomes, so we categorized age into four quartiles (18–39, 40–49, 50–59, and 60–89 years), with the last quartile (60–89) as the reference. We confirmed that there was no multi-collinearity by evaluating variance inflation factors.(20) Since missing data were rare (<5% of all survivors at each of 6 and 12 months), regression analysis was done using the available data without imputation. P-values were two-sided, and statistical significance was defined as p<0.05. All statistical analyses were performed using Stata 13.0 (StataCorp, College Station, TX).
RESULTS
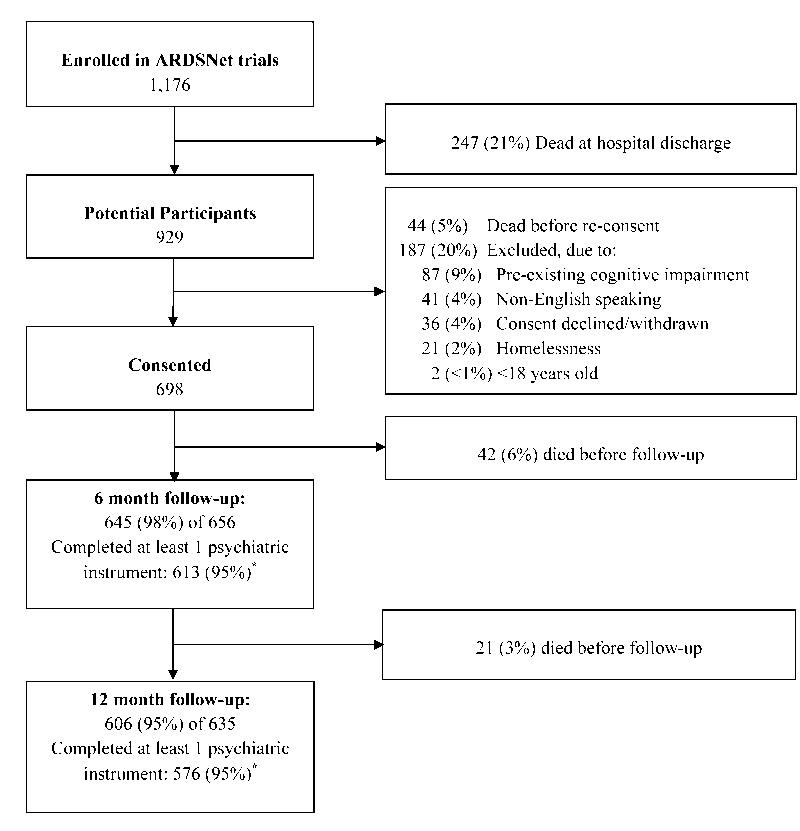
Among 1,176 patients enrolled in the three ARDSNet trials, 247 (21%) died by hospital discharge, an additional 44 (5% of hospital survivors) died before re-consent, and 187 (20% of hospital survivors) met ALTOS exclusion criteria, leaving a total of 698 (59%) who survived hospital stay and were eligible and consented for follow-up (Figure 1). A total of 645 (98%) of 656 and 606 (95%) of 635 survivors had follow-up visits at 6 and 12 months, respectively. Among them, 613 (95%) and 576 (95%) completed at least one psychiatric instrument at 6- and 12-month follow-up, respectively, with 629 patients completing at least one psychiatric instrument over one-year longitudinal follow-up.
Figure 1.
Patient flow diagram.
*Reasons for not completing psychiatric assessments at 6 and 12 months, respectively: declined 8 and 7, physically incapable 7 and 5, cognitively incapable 6 and 4, receiving mechanical ventilation 3 and 3, psychiatric issues 2 and 3, incarcerated 2 and 2, unable to contact 3 and 1, lack of time 0 and 1, and other reason 1 and 4.
Participating patients had a mean (standard deviation) age of 49 (15) years, with 52% female, 82% white and 49% unemployed prior to hospital admission (Table 1). Patients with substantial psychiatric symptoms during follow-up were more likely (p<0.05 based on Fisher’s exact and t-tests) to be younger, female, unemployed prior to ARDS, have alcohol misuse history, be less acutely ill (i.e. lower APACHE III score at ICU admission), and receive opioids for a greater proportion of ICU stay (Table 1).
Individual Psychiatric Domains and their Co-occurrence
Among 629 patients with at least one psychiatric measure, 416 (66%) had substantial symptoms in ≥1 domain during one-year follow-up. At 6 months (Table 1), the prevalence of substantial symptoms of depression, anxiety, and PTSD was 36% (222/613), 42% (260/613), and 24% (148/605), respectively, with almost identical prevalence at 12 months (36% (204/574), 42% (241/575), and 23% (132/573)). Across all three domains, the multivariable models demonstrated no significant change in prevalence or severity of psychiatric morbidity over time (Tables 2 and 3). Of patients who had substantial symptoms of depression, anxiety, and PTSD at 6 months, 57%–66% still had the same symptoms at 12 months, and <15% of patients without substantial symptoms at 6 months developed symptoms later.
Table 2.
Variables associated with presence versus absence of substantial psychiatric symptoms
| Prevalence ratio (95% CI) of substantial psychiatric symptomsa,b,c | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Depression | Anxiety | PTSD | ||||
|
|
||||||
| Variables | Bivariable | Multivariable | Bivariable | Multivariable | Bivariable | Multivariable |
| Baseline patient data | ||||||
| Age quartile (younger vs. older)d | 1.03 (0.95, 1.12) | 1.13 (1.05, 1.21) | 1.16 (1.07, 1.26) | 1.22 (1.10, 1.36) | 1.23 (1.08, 1.41) | |
| Female | 1.24 (1.02, 1.50) | 1.26 (1.01, 1.58) | 1.41 (1.19, 1.66) | 1.43 (1.18, 1.74) | 1.78 (1.37, 2.32) | 1.80 (1.31, 2.48) |
| White | 0.99 (0.78, 1.26) | 1.12 (0.89, 1.40) | 0.83 (0.62, 1.13) | |||
| Unemployed | 1.50 (1.24, 1.83) | 1.35 (1.09, 1.69) | 1.31 (1.11, 1.55) | 1.26 (1.05, 1.52) | 1.51 (1.17, 1.95) | 1.40 (1.03, 1.90) |
| BMI, per 10 kg/m2 | 1.07 (0.96, 1.20) | 1.11 (1.01, 1.22) | 1.14 (1.03, 1.26) | 1.07 (0.93, 1.24) | ||
| Diabetes | 1.06 (0.86, 1.30) | 0.99 (0.82, 1.20) | 0.85 (0.63, 1.14) | |||
| Stroke | 1.39 (0.74, 2.58) | 1.04 (0.49, 2.18) | 1.61 (0.76, 3.43) | |||
| Hemodialysis | 1.29 (0.82, 2.03) | 1.54 (0.86, 2.75) | 1.05 (0.63, 1.77) | 1.26 (0.75, 2.12) | 1.39 (0.72, 2.67) | |
| Alcohol misuse | 1.22 (0.99, 1.50) | 1.39 (1.09, 1.77) | 1.19 (0.99, 1.44) | 1.45 (1.18, 1.79) | 1.35 (1.02, 1.79) | 1.79 (1.31, 2.46) |
| Baseline intensive care data | ||||||
| Admission to medical ICU | 0.99 (0.82, 1.20) | 1.02 (0.87, 1.21) | 1.00 (0.77, 1.28) | |||
| APACHE III, per 20 unit | 0.90 (0.83, 0.97) | 0.90 (0.82, 0.99) | 0.89 (0.84, 0.96) | 0.95 (0.88, 1.02) | 0.89 (0.80, 0.98) | 0.88 (0.79, 0.98) |
| PaO2/FiO2, per 20 unit | 1.01 (0.98, 1.04) | 0.99 (0.97, 1.01) | 1.01 (0.98, 1.04) | |||
| Sepsis as ARDS risk factor | 0.88 (0.71, 1.08) | 0.99 (0.82, 1.20) | 0.91 (0.68, 1.21) | |||
| Daily intensive care data | ||||||
| No. of organ failures | 0.90 (0.78, 1.05) | 0.94 (0.76, 1.18) | 0.84 (0.73, 0.96) | 0.88 (0.73, 1.07) | 0.99 (0.81, 1.21) | |
| Any hemodialysis | 0.66 (0.47, 0.92) | 0.69 (0.45, 1.04) | 0.67 (0.48, 0.91) | 0.77 (0.53, 1.12) | 0.95 (0.64, 1.43) | |
| Any vasopressor use | 0.94 (0.78, 1.14) | 0.98 (0.83, 1.16) | 1.12 (0.87, 1.44) | |||
| % of days with opioids, per 20% | 1.10 (1.02, 1.19) | 1.11 (1.03, 1.20) | 1.07 (1.01, 1.14) | 1.08 (1.01, 1.15) | 1.13 (1.02, 1.25) | 1.09 (0.98, 1.22) |
| % of days with corticosteroids, per 20% | 1.02 (0.96, 1.08) | 1.02 (0.97, 1.07) | 1.05 (0.98, 1.13) | 1.04 (0.96, 1.12) | ||
| % of days with neuromuscular blocker, per 20% | 1.00 (0.87, 1.17) | 1.12 (1.02, 1.24) | 1.12 (0.99, 1.27) | 1.14 (0.96, 1.35) | 1.23 (1.02, 1.49) | |
| Morning glucose, per 20 mg/dL | 1.01 (0.94, 1.09) | 0.98 (0.92, 1.05) | 0.96 (0.87, 1.06) | |||
| Minimum glucose, per 20 mg/dL | 1.00 (0.91, 1.10) | 0.96 (0.87, 1.05) | 0.94 (0.84, 1.06) | |||
| Other intensive care data | ||||||
| Mechanical ventilation duration, per week | 1.02 (0.95, 1.08) | 0.99 (0.93, 1.05) | 1.02 (0.94, 1.11) | |||
| ICU length of stay, per week | 1.01 (0.95, 1.07) | 1.00 (0.93, 1.08) | 0.98 (0.93, 1.03) | 0.95 (0.88, 1.02) | 1.00 (0.92, 1.08) | 0.93 (0.83, 1.04) |
| Change at 12 vs. 6 month follow-upe | 1.00 (0.89, 1.13) | 0.91 (0.83, 1.01) | 0.90 (0.77, 1.06) | |||
Abbreviations: CI (confidence interval), PTSD (post-traumatic stress disorder), BMI (body mass index), ICU (Intensive Care Unit), APACHE III (Acute Physiology and Chronic Health Evaluation III), PaO2/FiO2 (ratio between partial pressure of oxygen in arterial blood and fraction of inspired oxygen), ARDS (acute respiratory distress syndrome).
Presence of substantial symptoms of depression, anxiety and PTSD was defined by Hospital Anxiety and Depression Scale (HADS) depression and anxiety subscale scores ≥8 and an Impact of Event Scale – Revised (IES-R) score ≥1.6. Results are presented as prevalence ratios, calculated by Poisson regression models using generalized estimating equations (GEE) with robust variance estimate, an exchangeable correlation structure, and an indicator for time (12- vs. 6-month follow-up).
Variables included in multivariable analyses are those from bivariable analyses that were associated (at p <0.20) with each outcome measure of depression, anxiety, or PTSD symptoms.
All the significant associations (p<0.05) in multivariable models are highlighted in bold.
Age quartiles in years: 18–39 (quartile 1), 40–49 (quartile 2), 50–59 (quartile 3), 60–89 (quartile 4). Quartile 4 (60–89) was used as the reference group.
Represents the change in outcome proportions, between 12- vs. 6-month follow-up, after adjusting for the other variables.
Table 3.
Variables associated with severity of psychiatric symptoms
| Increase (95% CI) in psychiatric symptom scorea,b,c | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| HADS-Depression | HADS-Anxiety | IES-R | ||||
|
|
||||||
| Variables | Bivariable | Multivariable | Bivariable | Multivariable | Bivariable | Multivariable |
| Baseline patient data | ||||||
| Age quartile (younger vs. older) d | 0.03 (−0.30, 0.36) | 0.67 (0.34, 1.01) | 0.70 (0.30, 1.10) | 0.13 (0.07, 0.19) | 0.14 (0.07, 0.21) | |
| Female | 1.28 (0.57, 1.99) | 0.91 (0.11, 1.71) | 1.63 (0.90, 2.36) | 1.81 (0.94, 2.67) | 0.33 (0.19, 0.46) | 0.38 (0.22, 0.54) |
| White | −0.13 (−1.06, 0.81) | 0.24 (−0.72, 1.20) | −0.08 (−0.25, 0.09) | |||
| Unemployed | 2.04 (1.34, 2.75) | 1.74 (0.95, 2.54) | 1.30 (0.56, 2.04) | 1.22 (0.36, 2.09) | 0.21 (0.08, 0.35) | 0.20 (0.05, 0.36) |
| BMI, per 10 kg/m2 | 0.47 (0.03, 0.91) | 0.15 (−0.35, 0.65) | 0.33 (−0.12, 0.79) | 0.39 (−0.16, 0.95) | 0.06 (−0.02, 0.14) | 0.10 (−0.01, 0.20) |
| Diabetes | 0.16 (−0.68, 1.00) | −0.39 (−1.26, 0.48) | −0.13 (−0.29, 0.02) | −0.02 (−0.21, 0.17) | ||
| Stroke | 0.95 (−2.08, 3.99) | 0.47 (−2.66, 3.61) | 0.14 (−0.45, 0.73) | |||
| Hemodialysis | 0.42 (−2.01, 2.85) | 0.82 (−1.91, 3.55) | −0.92 (−3.42, 1.59) | 0.03 (−3.06, 3.12) | 0.19 (−0.26, 0.64) | |
| Alcohol misuse | 0.55 (−0.34, 1.43) | 0.88 (−0.04, 1.80) | 1.88 (0.81, 2.95) | 0.20 (0.03, 0.36) | 0.40 (0.21, 0.60) | |
| Baseline intensive care data | ||||||
| Admission to medical ICU | 0.13 (−0.60, 0.85) | −0.01 (−0.75, 0.74) | −0.04 (−0.17, 0.10) | |||
| APACHE III, per 20 unit | −0.39 (−0.67, −0.12) | −0.36 (−0.68, −0.03) | −0.52 (−0.81, −0.23) | −0.44 (−0.79, −0.08) | −0.06 (−0.11, −0.01) | −0.07 (−0.13, −0.01) |
| PaO2/FiO2, per 20 unit | 0.05 (−0.05, 0.15) | −0.01 (−0.12, 0.09) | 0.00 (−0.02, 0.02) | |||
| Sepsis as ARDS risk factor | −0.41 (−1.25, 0.43) | −0.22 (−1.09, 0.65) | −0.12 (−0.27, 0.04) | −0.03 (−0.23, 0.17) | ||
| Daily intensive care data | ||||||
| No. of organ failures | −0.61 (−1.18, −0.04) | −0.51 (−1.30, 0.28) | −0.69 (−1.28, −0.11) | −0.28 (−1.13, 0.57) | −0.04 (−0.15, 0.07) | |
| Any hemodialysis | −1.45 (−2.55, −0.35) | −0.64 (−2.01, 0.74) | −1.31 (−2.43, −0.18) | −0.69 (−2.16, 0.78) | −0.03 (−0.24, 0.17) | |
| Any vasopressor use | −0.06 (−0.78, 0.65) | 0.30 (−0.44, 1.04) | 0.07 (−0.06, 0.21) | |||
| % of days with opioids, per 20% | 0.41 (0.14, 0.67) | 0.42 (0.16, 0.69) | 0.39 (0.12, 0.67) | 0.39 (0.10, 0.68) | 0.09 (0.04, 0.13) | 0.07 (0.02, 0.12) |
| % of days with corticosteroids, per 20% | 0.09 (−0.14, 0.32) | 0.08 (−0.16, 0.32) | 0.03 (−0.01, 0.08) | 0.02 (−0.02, 0.06) | ||
| % of days with neuromuscular blocker, per 20% | 0.10 (−0.50, 0.70) | 0.68 (0.07, 1.30) | 0.67 (0.03, 1.31) | 0.12 (0.01, 0.23) | 0.14 (0.02, 0.25) | |
| Morning glucose, per 20 mg/dL | 0.01 (−0.27, 0.28) | −0.17 (−0.46, 0.11) | −0.01 (−0.07, 0.04) | |||
| Minimum glucose, per 20 mg/dL | −0.13 (−0.48, 0.22) | −0.31 (−0.67, 0.06) | −0.32 (−0.73, 0.10) | −0.03 (−0.09, 0.04) | ||
| Other intensive care data | ||||||
| Mechanical ventilation duration, per week | 0.07 (−0.19, 0.33) | −0.07 (−0.34, 0.19) | 0.02 (−0.03, 0.07) | |||
| ICU length of stay, per week | 0.03 (−0.20, 0.26) | 0.01 (−0.26, 0.27) | −0.09 (−0.33, 0.14) | −0.25 (−0.54, 0.05) | 0.01 (−0.03, 0.06) | −0.04 (−0.09, 0.01) |
| Change at 12 vs. 6 month follow-up e | −0.01 (−0.35, 0.33) | −0.30 (−0.70, 0.09) | −0.06 (−0.14, 0.01) | |||
Abbreviations: CI (confidence interval), HADS (Hospital Anxiety and Depression Scale), IES-R (Impact of Event Scale – Revised), BMI (body mass index), ICU (Intensive Care Unit), APACHE III (Acute Physiology and Chronic Health Evaluation III), PaO2/FiO2 (ratio between partial pressure of oxygen in arterial blood and fraction of inspired oxygen), ARDS (acute respiratory distress syndrome).
Results are presented as the mean difference in HADS-depression, HADS-anxiety, and IES-R scores, calculated by linear regression models using generalized estimating equations (GEE), an exchangeable correlation structure, and an indicator for time (12- vs 6-month follow-up).
Variables included in multivariable analyses are those from bivariable analyses that were associated (at p < 0.20) with each outcome measure of psychiatric symptom score.
All the significant associations (p<0.05) in multivariable models are highlighted in bold.
Age quartiles in years: 18–39 (quartile 1), 40–49 (quartile 2), 50–59 (quartile 3), 60–89 (quartile 4). Quartile 4 (60–89) was used as the reference group.
Represents the difference in mean outcome scores, between 12- vs. 6-month follow-up, after adjusting for the other variables.
Continuous scores for psychiatric symptoms during follow-up were correlated as follows: depression and anxiety (Spearman’s rho: 0.70–0.72, p<0.001), anxiety and PTSD (0.69 at both time points, p<0.001), and depression and PTSD (0.58–0.59, p<0.001). The majority of survivors (63%) with any psychiatric morbidity (i.e. depression, anxiety, and/or PTSD symptoms) had substantial symptoms in two or more domains. At 6 months, 325 (53%) of 613 patients had substantial symptoms in at least 1 of the 3 domains assessed in this study. Among these symptomatic patients, the most common pattern of co-occurrence of symptoms involved simultaneously having substantial symptoms of depression, anxiety, and PTSD (n=106, 33%), followed by substantial depression and anxiety symptoms (18%), substantial anxiety and PTSD symptoms (7%), and substantial depression and PTSD symptoms (3%) (Figure 2). Similar results were observed at 12 months.
Figure 2.
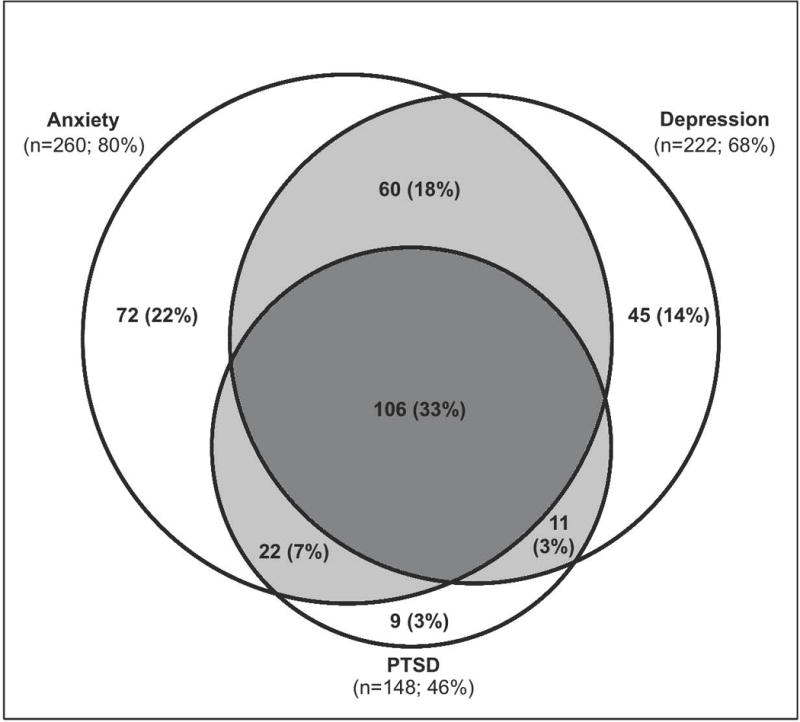
Venn diagram of co-occurrence of anxiety, depression, and PTSD symptoms among 325 patients with any psychiatric morbidity at 6-month follow-up. (Light grey area indicates co-occurrence of two psychiatric symptoms; dark grey area indicates co-occurrence of all three psychiatric symptoms).
Risk Factors for Substantial Psychiatric Symptoms and Severity
The bivariable and multivariable prevalence ratios for each psychiatric domain by risk factors are reported in Table 2. Female sex, unemployment prior to hospital admission, and alcohol misuse were associated with substantial symptoms in all three psychiatric domains. Younger age was significantly associated with substantial anxiety and PTSD symptoms, and a greater proportion of ICU days with opioids administration was significantly associated with substantial anxiety and depression symptoms (Table 2). Risk factor associations were similar when psychiatric symptoms were modeled as continuous variables (Table 3). Specifically, female sex, unemployment, and a greater in-ICU opioid use were associated with symptoms in all three domains. Younger age, alcohol misuse, and greater in-ICU neuromuscular blocker use were associated with more severe anxiety and PTSD symptoms.
Sensitivity analysis demonstrated that pre-existing psychiatric comorbidity was associated with each psychiatric domain in continuous analyses and only with anxiety in binary analyses. Adding psychiatric comorbidity in both continuous and binary multivariable models demonstrated relatively little change in the overall results with the risk factors for depression remaining significant, unemployment no longer being significant for anxiety, and female sex no longer being significant for PTSD.
In both binary and continuous analyses, greater severity of illness (i.e., APACHE III, PaO2/FiO2 ratio, number of organ failures, hemodialysis, and vasopressors), consistently was not associated with, or was negatively associated with, psychiatric symptoms. Moreover, there was no association between type of ICU, ARDS risk factor (sepsis vs. others), mechanical ventilation duration, and ICU length of stay with psychiatric symptoms in any analysis (Tables 2 and 3).
DISCUSSION
In this national, multicenter, longitudinal follow-up study evaluating psychiatric symptoms in over 600 ARDS survivors, two-thirds of participants had substantial symptoms in at least one psychiatric domain during 12-month follow-up. There was high prevalence, persistence, and co-occurrence of depression, anxiety, and PTSD symptoms. Severity of illness, mechanical ventilation duration and ICU length of stay were not associated with worse psychiatric symptoms. Younger age, female sex, baseline unemployment, alcohol misuse, and greater in-ICU use of opioids were consistently significant markers for post-ICU psychiatric symptoms.
At 6 months, the prevalence of substantial symptoms of depression, anxiety, and PTSD were high at 36%, 42%, and 24%, without improvement at 12 months. These prevalence rates are much higher than those in the general population,(21;22) but comparable with prior smaller, single-centered studies of ICU survivors.(4–7) Moreover, our findings of the persistence, frequent co-occurrence, and moderate to high correlation of psychiatric symptoms are also built upon prior studies of ICU survivors.(2;5;8;23–26)
Notably, anxiety and depression symptoms observed in this study may reflect pre-ARDS psychiatric morbidity; however, when accounting for pre-ICU psychiatric status, critical illness may remain an independent risk factor for new post-ICU psychiatric morbidity.(27;28) Moreover, PTSD symptoms, reported in approximately one-quarter of survivors in this study, likely represent incident psychiatric symptoms post-ARDS, since the IES-R instrument used in this study specifically addresses patients’ symptoms in relation to their critical illness and ICU experience.
Our findings add to the growing body of evidence that psychiatric symptoms are a significant and prolonged burden for ICU survivors, and that patient and critical illness factors may be markers for these symptoms. The positive associations of younger age, female sex and unemployment with psychiatric symptoms are recognized in ICU survivors and other patient populations.(1;4;29–35) Notably, however, our sensitivity analysis suggests that the association of unemployment with anxiety and female sex with PTSD may be a marker for patients who have pre-existing psychiatric comorbidity rather than independent risk factors. While existing findings have been inconsistent about alcohol use disorder as a predictor for developing psychiatric conditions, our study suggests that it is strongly associated with psychiatric symptoms and severe outcomes.(36–40)
A longer duration of opioid exposure was the only ICU-related risk factor consistently and positively associated with symptoms in all three psychiatric domains. A prior study of ARDS survivors demonstrated that a high mean daily dose of opioids (≥100mg of morphine equivalents per day) was positively associated with PTSD symptoms measured by IES-R, but a greater proportion of ICU days with opioid was negatively associated with PTSD symptoms.(9) It has been speculated that adequate pain control using opioids may have a preventive effect on PTSD.(9;41–43) Unfortunately, data on opioid dosing was not available to further evaluate this issue in our study. Future research in survivors of critical illness should investigate, in greater detail, the potentially complex role of in-ICU opioid administration and dosage.
Of note, our study demonstrated that five different measures of severity of illness, along with mechanical ventilation duration and ICU length of stay, had no positive association with psychiatric symptoms. Notably, a higher APACHE III score was associated with lower symptom scores, which might reflect that patients with higher severity of illness who survived their ICU stay had less pre-existing psychiatric illness or had another unmeasured factor that was protective against post-ICU psychiatric symptoms. Despite these findings contradicting positive associations of illness severity with post-ARDS physical impairments and mortality,(11;44–48) our findings agree with prior studies evaluating psychiatric symptoms in both ARDS and other ICU survivors.(30–32;49;50) Hence, it is critical for clinicians to recognize that patients with anticipated better physical outcomes, due to a lower severity of illness and shorter length of stay, should not be overlooked when considering risk for post-ICU psychiatric symptoms.(9;49;50)
The strengths of this study include being a national, longitudinal, prospective study with large sample size, high retention rate (≥95%), and simultaneous evaluation of prevalence, severity, co-occurrence and risk factors for three common psychiatric morbidities. However, the study has potential limitations. First, our study focused on relatively young ARDSNet trials survivors with exclusions for severe comorbid diseases. Hence, the findings may not be generalizable to other populations. However, comparisons of our findings to the existing literature generally revealed consistency of results, which may support generalizability. Second, this evaluation was restricted to the variables collected as part of the ARDSNet trials, omitting potentially relevant variables, such as baseline neuropsychological and physical functioning status, ICU exposure to sedatives, dose-related data for the opioid and corticosteroid, and daily pain, sedation and delirium assessments. Inclusion of such variables in future large-scale studies is highly recommended. Third, given its observational design, our study cannot demonstrate cause-effect relationships; hence, the results should be recognized as markers of risk for post-ARDS psychiatric symptoms, rather than as direct causal associations. We could be underpowered to identify a true association between severity of illness and psychiatric symptoms, but prior literature supports our findings. Finally, we used self-reported measures of psychiatric symptoms without ascertaining clinical psychiatric diagnoses and could only determine the prevalence, rather than incidence of depression and anxiety symptoms. However, the HADS and IES-R are commonly used and well-validated instruments.(1;9;18;19) Moreover, they have the advantages of providing both a binary result and a continuous measure of symptom severity, and being feasible to administer to a large geographically dispersed national patient cohort.(9) In addition, the IES-R instrument used in this study evaluates PTSD symptoms with respect to patients’ critical illness, thus likely evaluating incident symptoms. The IES-R instrument has high discrimination (area under the receiver operating characteristics = 95%) in screening for a clinical diagnosis of PTSD in ARDS survivors.(18)
CONCLUSION
Two-thirds of ARDS survivors had substantial symptoms of depression, anxiety or PTSD during 12-month follow-up. We observed high co-occurrence among these psychiatric domains, particularly co-occurrence of all three morbidities. Younger age, female sex, unemployment prior to hospital admission, alcohol misuse, and greater in-ICU use of opioids were significant markers for these symptoms. However, traditional risk factors for post-ICU physical impairment were not associated with worse symptoms. These findings have value in identifying patients at greatest risk of psychiatric symptoms during recovery from critical illness, and emphasize the need to simultaneously evaluate for a full spectrum of potential sequelae to maximize patient recovery.
Acknowledgments
We thank all of the patients and their proxies who participated in this study. We thank Mardee Merrill, Melissa McCullough, Jonathan Gellar, Elizabeth Vayda, Gita Byraiah, Laura Methvin, Vanessa Stan, Shirani Rajan, Cassie Wicken, Meg Shanahan, Elizabeth Baer, and Anita Chandra who assisted with data collection; and William Flickinger and Christopher Mayhew who assisted with data management.
Funding/Support: National Heart, Lung and Blood Institute funded this follow-up study (N01HR56170, R01HL091760 and 3R01HL091760-02S1) and the ALTA and EDEN trials (contracts HHSN268200536165C to HHSN268200536175C and HHSN268200536179C) as well as provided support via 5T32HL00753432.
Copyright form disclosures: Dr. Parker received support for article research from the National Institutes of Health (NIH) and 5 T32 HL007534 32. Her institution received grant support from 5 T32 HL007534 32 (Salary support). Dr. Bienvenu received support for article research from the NIH. His institution received grant support from the NIH. Dr. Colantuoni received support for article research from the NIH. Her institution received grant support from the NIH. Dr. Hopkins lectured for the Michigan Hospital Association ICU Meeting(Presentation on ICU Outcomes) and received grant support from the NIH. Her institution received grant support from the NIH (Peer reviewed grant) and Intermountain Medical and Research Foundation (peer review grants). Dr. Needham received support for article research from the NIH. His institution received grant support from the NHLBI, NIH, AHQR, and Moore Foundation (peer-reviewed grants).
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Author Contributions: MH had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the final manuscript. DMN and ROH developed the study concept and design. MH conducted statistical analysis and all authors have interpreted the data. MH, AMP, OJB, and DMN drafted the manuscript and all authors have provided critical revisions for important intellectual content. This study was supervised by DMN.
Reference List
- 1.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med. 2012 Mar 1;185(5):517–24. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012 Jun 15;185(12):1307–15. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevenson JE, Colantuoni E, Bienvenu OJ, et al. General anxiety symptoms after acute lung injury: predictors and correlates. J Psychosom Res. 2013 Sep;75(3):287–93. doi: 10.1016/j.jpsychores.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davydow DS, Gifford JM, Desai SV, et al. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008 Sep;30(5):421–34. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davydow DS, Gifford JM, Desai SV, et al. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009 May;35(5):796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davydow DS, Desai SV, Needham DM, et al. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008 May;70(4):512–9. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wade D, Hardy R, Howell D, et al. Identifying clinical and acute psychological risk factors for PTSD after critical care: a systematic review. Minerva Anestesiol. 2013 Aug;79(8):944–63. [PubMed] [Google Scholar]
- 8.Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014 May;2(5):369–79. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bienvenu OJ, Gellar J, Althouse BM, et al. Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med. 2013 Feb 26;:1–15. doi: 10.1017/S0033291713000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker AM, Sricharoenchai T, Raparla S, et al. Posttraumatic stress disorder in critical illness survivors: a meta-analysis. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 11.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003 Feb 20;348(8):683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 12.Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011 Oct 12;306(14):1574–81. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012 Feb 22;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babor T, Higgins-Biddle J, Saunders J, Monteiro M. AUDIT: The Alcohol Use Disorder Identification Test: Guidelines for Use in Primary Care. 2001 [Google Scholar]
- 15.Bernard GR. The Brussels Score. Sepsis. 1997;1:43–4. [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983 Jun;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Weiss DS. The Impact of Event Scale – Revised. In: Wilson JP, Keane TM, editors. Assessing Psychological Trauma and PTSD: A Practitioner’s Handbook. Second. New York: Guilford Press; 2004. pp. 168–89. [Google Scholar]
- 18.Bienvenu OJ, Williams JB, Yang A, et al. Posttraumatic stress disorder in survivors of acute lung injury: evaluating the Impact of Event Scale-Revised. Chest. 2013 Jul;144(1):24–31. doi: 10.1378/chest.12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck JG, Grant DM, Read JP, et al. The impact of event scale-revised: psychometric properties in a sample of motor vehicle accident survivors. J Anxiety Disord. 2008;22(2):187–98. doi: 10.1016/j.janxdis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton LC. Statistics with STATA – Update for Version 10. Belmont, CA: Brooks/Cole; 2009. [Google Scholar]
- 21.Crawford JR, Henry JD, Crombie C, et al. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol. 2001 Nov;40(Pt 4):429–34. doi: 10.1348/014466501163904. [DOI] [PubMed] [Google Scholar]
- 22.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005 Jun;62(6):617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davydow DS, Katon WJ, Zatzick DF. Psychiatric morbidity and functional impairments in survivors of burns, traumatic injuries, and ICU stays for other critical illnesses: a review of the literature. Int Rev Psychiatry. 2009 Dec;21(6):531–8. doi: 10.3109/09540260903343877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson JC, Mitchell N, Hopkins RO. Cognitive functioning, mental health, and quality of life in ICU survivors: an overview. Crit Care Clin. 2009 Jul;25(3):615–28. x. doi: 10.1016/j.ccc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Paparrigopoulos T, Melissaki A, Tzavellas E, et al. Increased co-morbidity of depression and post-traumatic stress disorder symptoms and common risk factors in intensive care unit survivors: a two-year follow-up study. Int J Psychiatry Clin Pract. 2014 Jan;18(1):25–31. doi: 10.3109/13651501.2013.855793. [DOI] [PubMed] [Google Scholar]
- 26.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Co-occurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davydow DS, Hough CL, Russo JE, et al. The association between intensive care unit admission and subsequent depression in patients with diabetes. Int J Geriatr Psychiatry. 2012 Jan;27(1):22–30. doi: 10.1002/gps.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wunsch H, Christiansen CF, Johansen MB, et al. Psychiatric diagnoses and psychoactive medication use among nonsurgical critically ill patients receiving mechanical ventilation. JAMA. 2014 Mar 19;311(11):1133–42. doi: 10.1001/jama.2014.2137. [DOI] [PubMed] [Google Scholar]
- 29.Samuelson KA, Lundberg D, Fridlund B. Stressful memories and psychological distress in adult mechanically ventilated intensive care patients - a 2-month follow-up study. Acta Anaesthesiol Scand. 2007 Jul;51(6):671–8. doi: 10.1111/j.1399-6576.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 30.Cuthbertson BH, Hull A, Strachan M, et al. Post-traumatic stress disorder after critical illness requiring general intensive care. Intensive Care Med. 2004 Mar;30(3):450–5. doi: 10.1007/s00134-003-2004-8. [DOI] [PubMed] [Google Scholar]
- 31.Girard TD, Shintani AK, Jackson JC, et al. Risk factors for post-traumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit Care. 2007;11(1):R28. doi: 10.1186/cc5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rattray JE, Johnston M, Wildsmith JA. Predictors of emotional outcomes of intensive care. Anaesthesia. 2005 Nov;60(11):1085–92. doi: 10.1111/j.1365-2044.2005.04336.x. [DOI] [PubMed] [Google Scholar]
- 33.North CS, Oliver J, Pandya A. Examining a comprehensive model of disaster-related posttraumatic stress disorder in systematically studied survivors of 10 disasters. Am J Public Health. 2012 Oct;102(10):e40–e48. doi: 10.2105/AJPH.2012.300689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.North CS, Nixon SJ, Shariat S, et al. Psychiatric disorders among survivors of the Oklahoma City bombing. JAMA. 1999 Aug 25;282(8):755–62. doi: 10.1001/jama.282.8.755. [DOI] [PubMed] [Google Scholar]
- 35.Myhren H, Ekeberg O, Toien K, et al. Posttraumatic stress, anxiety and depression symptoms in patients during the first year post intensive care unit discharge. Crit Care. 2010;14(1):R14. doi: 10.1186/cc8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller T, Lavori P, Keller M, et al. Prognostic effect of the variable course of alcoholism on the 10-year course of depression. The American journal of Psychiatry. 1994;151(5):701–6. doi: 10.1176/ajp.151.5.701. [DOI] [PubMed] [Google Scholar]
- 37.Boschloo L, Vogelzangs N, van den Brink W, et al. Alcohol use disorders and the course of depressive and anxiety disorders. The British Journal of Psychiatry. 2012;200(6):476–84. doi: 10.1192/bjp.bp.111.097550. [DOI] [PubMed] [Google Scholar]
- 38.Rhebergen D, Beekman A, De Graaf R, et al. The three-year naturalistic course of major depressive disorder, dysthymic disorder and double depression. Journal of affective disorders. 2009;115(3):450–9. doi: 10.1016/j.jad.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Kushner M, Sher K, Erickson D. Prospective analysis of the relation between DSM-III anxiety disorders and alcohol use disorders. The American journal of Psychiatry. 1999;156(5):723–32. doi: 10.1176/ajp.156.5.723. [DOI] [PubMed] [Google Scholar]
- 40.Hopkins RO, Key CW, Suchyta MR, et al. Risk factors for depression and anxiety in survivors of acute respiratory distress syndrome. Gen Hosp Psychiatry. 2010 Mar;32(2):147–55. doi: 10.1016/j.genhosppsych.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Saxe G, Stoddard F, Courtney D, et al. Relationship between acute morphine and the course of PTSD in children with burns. J Am Acad Child Adolesc Psychiatry. 2001 Aug;40(8):915–21. doi: 10.1097/00004583-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Bryant RA, Creamer M, O’Donnell M, et al. A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biol Psychiatry. 2009 Mar 1;65(5):438–40. doi: 10.1016/j.biopsych.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 43.Holbrook TL, Galarneau MR, Dye JL, et al. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med. 2010 Jan 14;362(2):110–7. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 44.Miller EA, Weissert WG. Predicting elderly people’s risk for nursing home placement, hospitalization, functional impairment, and mortality: a synthesis. Med Care Res Rev. 2000 Sep;57(3):259–97. doi: 10.1177/107755870005700301. [DOI] [PubMed] [Google Scholar]
- 45.Needham DM, Wozniak AW, Hough CL, et al. Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am J Respir Crit Care Med. 2014 May 15;189(10):1214–24. doi: 10.1164/rccm.201401-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schandl A, Bottai M, Holdar U, et al. Early prediction of new-onset physical disability after intensive care unit stay: a preliminary instrument. Crit Care. 2014;18(4):455. doi: 10.1186/s13054-014-0455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holbrook TL, Anderson JP, Sieber WJ, et al. Outcome after major trauma: 12-month and 18-month follow-up results from the Trauma Recovery Project. J Trauma. 1999 May;46(5):765–71. doi: 10.1097/00005373-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011 Oct;10(10):931–41. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- 49.Nelson BJ, Weinert CR, Bury CL, et al. Intensive care unit drug use and subsequent quality of life in acute lung injury patients. Crit Care Med. 2000 Nov;28(11):3626–30. doi: 10.1097/00003246-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Kapfhammer HP, Rothenhausler HB, Krauseneck T, et al. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychiatry. 2004 Jan;161(1):45–52. doi: 10.1176/appi.ajp.161.1.45. [DOI] [PubMed] [Google Scholar]