Abstract
Background
Many outcome measures (OM) that assess individuals’ ability or beliefs in their ability to perform tasks exist to evaluate activity and participation after stroke; however, the relationship between various OM and activity/participation is unclear.
Objective
The purpose of this study was to explore the relationships between different OM and activity and participation in people after stroke.
Methods
59 subjects post-stroke participated in an assessment including self-selected walking speed, 6 minute walk test, Timed “Up and Go” Test, Berg Balance Scale, Functional Gait Assessment, Walk 12, and Activity-specific Balance Confidence Scale. Step Activity Monitoring (SAM) was used as a measure of activity and Stroke Impact Scale-Participation (SIS-P) as a measure of participation. Exploratory Factor Analysis was performed including all measures except SAM and SIS-P. Two factors were extracted and termed performance based (PB) and self-efficacy (SE). A Path Analysis assessed the role of SE as a mediator in the relationships of PB and SAM/SIS-P.
Results
In the path analysis, PB significantly predicts SE (p < 0.001, b=0.44), but not SAM or SIS-P (p > 0.05, b=0.25 and b=0.11 respectively). SE significantly predicts both SAM and SIS-P (p < 0.001, b=0.46 and b=0.59 respectively). The Indirect Effects of PB on SAM and SIS-P were significant (p < 0.001; b=0.20 and b=0.26 respectively).
Conclusion
These results suggest that SE mediates the relationship between PB and activity and participation after stroke, reinforcing that improving activity and participation is more complicated than only targeting performance. Clinicians should administer SE and PB measures to determine the most accurate view of patients after stroke and seek to improve SE through interventions.
Keywords: Stroke, Self-efficacy, Mediator, Outcome measures, Activity, Participation
Introduction
Each year approximately 795,000 people in the United States experience a stroke1, making stroke one of the leading causes of disability.2 With the growing number of individuals surviving stroke, limiting post stroke disability is becoming increasingly important. With the advent of the World Health Organization International Classification of Functioning, Disability, and Health model (WHO-ICF), clinicians have been encouraged to evaluate and consider each individual comprehensively, including activity, participation, and personal and environmental factors. Evaluating an individual from this view point will allow clinicians to better comprehend the full impact of the individual’s stroke as well as the factors that affect recovery.
According to the WHO-ICF, activity is defined as the performance of a task or action and participation is defined as involvement in a life situation.3 These domains of the WHO-ICF have drawn close attention from the rehabilitation community treating persons post-stroke, as decreased activity and participation have been found following stroke.4–7 In order to adequately address decreased post-stroke activity and participation, it is important to understand the factors that contribute to this reduction.
An individual’s physical capacity to perform, i.e. how an individual actually functions, is often evaluated clinically and considered a critical factor impacting activity and participation following stroke. Clinically, this is typically measured by objective data including time, distance, or standardized scores. More recent research has begun to evaluate the role that self-efficacy may have on activity and participation. Self-efficacy is an individual’s own belief in their ability to perform and is related to their confidence, motivation, behavior, and environment.8 While some studies have found physical performance measures, such as scores on the Berg Balance Scale, to be strongly related to walking activity after stroke7, other studies have found self-reported measures of self-efficacy to be more highly related to self-reported activity.9 Similarly, some studies have shown performance based measures of gait speed to be highly related to post-stroke participation10; however, other studies have shown a significant relationship between factors such as fatigue and mood with participation after stroke.11 Given the multi-dimensional nature of both activity and participation, it is not surprising that the evaluation of an individual’s performance or self-efficacy may not capture all the factors that impact them. As a result, the use of multiple outcome measures in the clinical setting that evaluate both an individual’s performance as well as the individual’s self-efficacy may be advantageous.
Despite this, limited research evaluating the relationship of outcome measures that assess physical capacity and self-efficacy with activity and participation has been done for those post-stroke. Following the constructs of the WHO-ICF model, it seems likely that different types of outcome measures may better predict activity versus participation. For example, given that activity is defined as the performance of a task or action, it seems possible that a performance-based outcome measure evaluating an individual’s activity capacity may best capture activity after stroke. In contrast, participation is defined as involvement in a life situation, which does not necessarily require a particular level of performance of a task and therefore, may not be as readily captured by measures of performance. Based on the classifications and recommendations made in StrokEDGE, created by the Neurology Section of the America Physical Therapy Association12 as well as the existing evidence, it is reasonable to expect that different outcome measures would relate differently to the activity and participation domains of the WHO- ICF model.
Thus, the purpose of this study was to evaluate the relationship between outcome measures that evaluate physical performance and self-efficacy with those that measure activity and participation in persons following stroke. We hypothesized that performance based measures would be strongly predictive of activity, but less related to participation after stroke; conversely, self-efficacy measures would be strongly related to participation, but less predictive of activity after stroke. Understanding the different relationships between various types of outcome measures and activity and participation after stroke will assist therapists in designing optimal rehabilitation interventions to target recovery and to track a patient’s progress during therapy in each of these domains.
Methods
An analysis was performed on data collected for 2 separate prospective longitudinal studies. The 59 individuals included in this study were selected based on having had data for all of the following outcomes: self-selected walking speed (SSWS), 6 minute walk test (6MWT), Timed “Up and Go” Test (TUG), Berg Balance Scale (BBS), Functional Gait Assessment (FGA), Walk 12, Activity-specific Balance Confidence Scale (ABC), steps per day as measured by the StepWatch Activity Monitor (SAM), and Stroke Impact Scale-Participation (SIS-P). All measures were performed on the same day and scored by one of two raters. These outcome measures are recommended by the StrokEDGE for the use within an outpatient setting for community dwelling individuals following stroke.12 As a result, these measures were selected for this study as evidence based tools to assess an individual’s activity and participation following stroke. Although StrokEDGE does not recommend measuring actual steps per day, there is considerable evidence that the SAM is a reliable method to collect data about an individual’s true daily activity by providing an accurate count of the steps taken per day.6,13
Participants were recruited from local physical therapy clinics, stroke support groups, and newspaper advertisements. Individuals age 21–85 were included in the study if they had sustained a stroke greater than 6 months prior and were able to communicate with the investigators. Subjects were also able to walk without physical assistance, 5 minutes at a self-selected pace on the treadmill, and outside the home prior to stroke. The use of orthotics or assistive devices were allowed. Individuals were not included in the study if they had experienced more than one stroke, had received Botox in lower extremities less than 4 months prior, had pain that limited walking, experienced unexplained dizziness in the past 6 months, or were participating in skilled physical therapy services. Subjects were also excluded if they had evidence of a cerebellar stroke, additional neurologic diseases, and/or a cardiac event less than 3 months prior. All participants received medical clearance prior to beginning the study and provided informed consent. This study was approved by the Human Subjects Review Board at University of Delaware.
All outcome measures included in this study have been validated for use in individuals following stroke12 and were chosen to characterize walking capacity, self-efficacy, activity, and community/social participation. Self-selected walking speed (SSWS), 6 minute walk test (6MWT), Timed “Up and Go” Test (TUG), Berg Balance Scale (BBS), Functional Gait Assessment (FGA), Walk 12, and Activity-specific Balance Confidence Scale (ABC) were the independent variables. Steps per day as measured by StepWatch Activity Monitoring (SAM) and Stroke Impact Scale-Participation (SIS-P) are the dependent variables and represented the activity and participation domains of the WHO-ICF model respectively.12
Independent Outcome Measures
Self-selected walking speed (SSWS) was measured using the 10-meter walk test where the 6 meters in the middle were timed. This was converted to gait velocity.14
The 6 Minute Walk Test (6MWT) assesses total distance walked over 6 minutes and was used as a sub-maximal test of aerobic capacity/ endurance.5,15
The Timed Up and Go (TUG) test was used to measure the subject’s ability to perform transitional movements with ambulation and to assess their risk of falls. Subjects walk 3 meters, turn around and walk back and then sit down. Increased time to perform the test indicates increased fall risk.16,17
The Berg Balance Scale (BBS) assesses balance based on14 functional tasks that are scored on a scale of 0 (unable) to 4 (normal). Lower scores indicate impaired balance and indicate increased risk of falls.18,19
The Functional Gait Assessment (FGA) is a 10-item assessment of postural stability during various walking tasks like ambulating backwards, walking with a narrow base of support, and with eyes closed. Lower scores are indicative of increased fall risks.20,21
The Walking Impact Scale (Walk-12) is a self-reported questionnaire that reports perceived limitations in walking among patients post-stroke. The scale consists of 12-items asking about limitations due to their stroke during the previous 2 weeks specifically in tasks like walking and climbing stairs as well as support needed indoors and outdoors when walking. The total score of the Walk 12 is reported on a 0–100 scale (which yielded a value of self-perceived walking limitation in percentage). A score of 0 indicates no self-perceived limitation in walking and 100 indicates maximum limitation.22,23
The ABC is 16-item questionnaire measuring balance self-efficacy, the confidence an individual has when performing various tasks including changing position, sweeping the flooring, and walking on icy sidewalks. Each task is scored on an 11-point ordinal scale ranging from 0% (“no confidence”) to 100% (“complete confidence”). Item scores are averaged to determine an overall balance confidence score ranging from 0% to 100%. Low scores indicate low levels of confidence to perform functional activities that require balance.24–27
Dependent Outcome Measures
The StepWatch Activity Monitor (SAM) (Orthocare Innovations, Seattle Washington) is a calibrated device used to measure subject’s step activity throughout the day. The SAM measures a variety of data, for this study steps per day serves as the dependent outcome measure representing the activity domain.15 For each subject, the SAM was placed above the ankle on the non-paretic lower extremity and calibrated to the participants' height and walking characteristics per manufacturer's instructions. Participants wore the SAM all day, except when sleeping, bathing, and swimming activities. For each subject, at least 3 days of data were required to calculate the mean steps per day. The SAM data that was collected was representative of the activity domain of the WHO-ICF in our data analysis.13,28–31 Steps per day as measured by the SAM was selected to represent the activity domain because it truly measures steps taken per day. The other outcome measures used in this study have been designated by StrokeEDGE to represent the activity domain of the ICF; however, do not actually measure activity during a typical day.
The Stroke Impact Scale (SIS) is used to measure health status post stroke. The SIS-P subsection of the SIS can be used independently and is representative of the participation domain of the WHO-ICF model.20,32,33
Statistical analysis
An Exploratory Factor Analysis (EFA) was performed including all measures except the dependent variables (SAM and SIS-P). Due to non-normality, the EFA was performed on the Spearman Correlation Matrix using Maximum Likelihood extraction. A simplified orthogonal structure resulted from the extraction and therefore no rotation was needed and factors were not correlated with each other. Two factors were extracted based on examining the Scree plot and running a parallel analysis. The first factor, which we termed performance based (PB), included SSWS, 6MWT, TUG, BBS, and FGA. The second factor included Walk-12, ABC, and FGA. Although FGA loaded onto this factor, it had the weakest loading factor and loaded with two other measures that clearly measure self-efficacy. As a result, this factor was termed self-efficacy (SE). Factor scores were calculated using the Regression Method after standardizing the variables in Table 1.
Table 1.
Exploratory Factor Analysis via Spearman correlation. Values represent the loading r values.
| Performance Based Measures |
Self- Efficacy Measures | ||
|---|---|---|---|
| SSWT | 0.984 | Walk-12 | 0.810 |
| 6MWT | 0.917 | ABC | 0.713 |
| TUG | 0.858 | FGA | 0.511 |
| BBS | 0.740 | ||
| FGA | 0.604 | ||
Factor loadings under 0.4 have been suppressed
Abbreviations: SSWT: Self-selected walking speed; 6MWT: 6 minute walk test; TUG: Timed “Up and Go” Test; BBS: Berg Balance Scale; FGA: Functional Gait Assessment; Walk-12: Walk 12; ABC: Activity-specific Balance Confidence Scale
A Path Analysis was then performed to test the role of SE as a mediator in the relationship between PB and both SAM and SIS-P. A Path Analysis, a special case of Structural Equation Modeling where all variables included in the model are observed (non-latent) variables, is a powerful multivariate statistical technique that permits one to test complicated models. Conceptually, it can be thought of as a set of multiple regressions, with multiple predictors and multiple outcomes. In multiple regression, there would be a separate regression model for each outcome, path analysis allows for the simultaneous estimation of these equations. Additionally, path analysis can involve modeling indirect and direct effects as well as hypothesis tests on the parameters of direct and indirect effects. A direct effect is a regression-like relationship between two variables involving a direct link between them, e.g. A→B. An indirect effect can be defined as a relationship between two variables that operate through other variables, e.g. A→B→C. Significance was determined by a p value of <0.005. Given the relatively small sample size and partially exploratory hypotheses, this two-step approach of first extracting factors and then calculating factor scores to use in a mediation model, was used versus attempting to estimate a full latent variable model.
Results
Demographics
Fifty-nine subjects with the average age of 59±11 years were included. The average time since stroke was 44.3 ± 63.1 months. Of these participants 38 subjects had left sided hemiparesis. Additional clinical data including scores on outcome measures performed are presented in Table 2.
Table 2.
Socio-demographic and clinical characteristics of subjects
| Characteristic | Subjects (mean±SD) |
|---|---|
| Age in years | 59 ±11.2 |
| Side of hemiparesis, # of subject | |
| Right | 21 |
| Left | 38 |
| Time since stroke in months | 44.3 ± 63.1 |
| Assistive device: | |
| Rolling walker | 3 |
| Hemi-walker | 1 |
| Quad-Cane | 5 |
| Single Point Cane | 10 |
| No Assistive Device | 38 |
| Ankle Foot Orthosis (AFO) | 19 |
| Self-Selected Walking Speed (meter/seconds) | 0.71 ± 0.3 |
| Timed Up and Go (seconds) | 20.4±17 |
| 6 Minute Walk Test (meters) | 288.6±186.4 |
| Functional Gait Assessment | 12.5±4.8 |
| Berg Balance Scale | 45.7±9 |
| Activity-specific Balance Confidence Scale (%) | 70.86± 18.7 |
| Walk-12 | 66.3±20.6 |
| Steps per Day | 5607±3275 |
| Stroke Impact Scale-Participation (%) | 63.7±23.2 |
SD=Standard Deviation
Path Analysis
All four criteria for mediation established by Baron and Kenney34 were satisfied. First, PB was found to predict SAM and SIS-P (p=0.002, p<0.001 respectively). Second, SE also predicted activity and participation (p<0.001). Third, PB significantly predicted SE (b=0.44, p < 0.001). In the full model, the indirect effect of PB through SE on activity (b=0.20, p <0.005, R2= 0.38) and participation (b=0.26, p <0.005, R2 =0.43) were significant (Figure 1). The direct effect of the mediator, SE, on both outcomes, for activity (b =0.46, p < 0.001) and participation (b =0.59, p < 0.001), was significant after adjusting for PB. The direct effect of PB on activity, (b = 0.25, p= 0.028), was significant after adjusting for SE. The direct effect of PB on participation was not significant, (b= 0.11, p=0.30), after adjusting for SE. These finding suggest that PB’s impact on activity is partially mediated by SE, but that its impact on participation is fully mediated by SE. A summary of the results of indirect and direct pathways is presented in Table 3.
Figure 1.
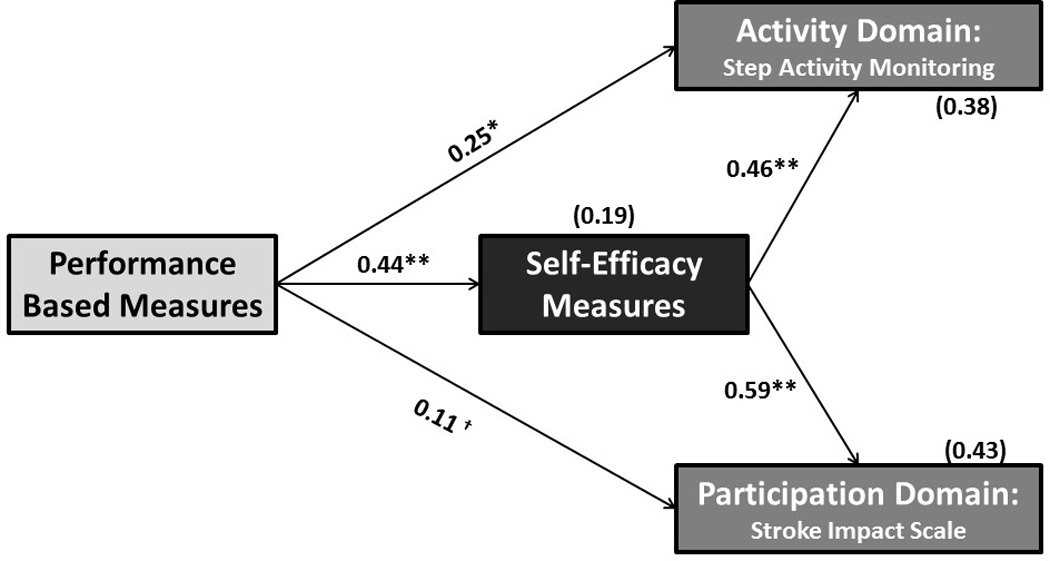
Self- Efficiency mediates the relationship between PB and Activity/Participation. *p = 0.028; **p < 0.001; † p = 0.30
Table 3.
Statistical values for each pathway in Path Analysis
| Pathway | b | p-value | R2 |
|---|---|---|---|
| PB→Activity | 0.45 | 0.002* | 0.14 |
| PB→Participation | 0.37 | <0.001* | 0.21 |
| SE→Activity | 0.46 | <0.001* | 0.38 |
| SE→Participation | 0.59 | <0.001* | 0.43 |
| Indirect Effect of PB on Activity (PB→SE→Activity) | 0.20 | <0.005* | 0.38 |
| Indirect Effect of PB on Participation (PB→SE→Participation) | 0.26 | <0.005* | 0.43 |
| Direct Effect of PB on Activity with SE in model | 0.25 | 0.028* | |
| Direct Effect of PB on Participation with SE in model | 0.11 | 0.30 |
indicates statistical significance
Abbreviations: PB: performance based; SE: self-efficacy
Discussion
These results suggest that although physical capacity as measured by performance based measures is important in predicting both activity and participation after stroke, self-efficacy mediates the relationship of PB with activity and participation. As a result, it seems that activity and participation are best predicted by the assessment of physical capacity as well as self-efficacy. This idea that physical capacity alone does not predict activity and participation post-stroke was shown in a study by Schmid et al35 which found that self-efficacy impacts self-reported activity and participation in persons post stroke.9 The impact of self-efficacy on activity and participation has also been shown in community dwelling manual wheelchair users aged 50 years or older36, individuals with hip osteoarthritis37, adults with arthritis38 and women with fibromyalgia.39 Research in other populations, including spinal cord injury, congestive heart failure, and knee osteoarthritis, also supports the role of self-efficacy as a mediator between performance and activity and participation domains.8,40–43
Previous literature12 has classified outcome measures by whether they evaluate the activity or participation domain for persons post-stroke; however, our results suggest that this may be an over-simplified approach to outcome measures. Rather, our results indicate that utilizing outcome measures that evaluate these domains from various perspectives including physical capacity and self-efficacy, provides valuable information to clinicians about patients’ overall activity or participation post stroke. This is supported by other research, which has found that the use of one outcome measure does not fully explain a person’s activity or participation capacity after stroke.44
Based on the results of this study, particularly the relationship between performance based measures and self-efficacy, it is likely that the impact therapists make on performance may directly improve self-efficacy; thus, have a significant impact on activity and participation. This is supported by a previous study which found that an emphasis on mobility during rehabilitation improves self-efficacy in older adults with history of at least two falls in the past year.45 Other research has also found a relationship between gait and balance performance and self-efficacy post stroke.46–48 These results indicate the complex and interdependent nature of the physical and psychological realms. Despite the impact therapists can have on self-efficacy and the relationship between performance and self-efficacy, it may also be advantageous to utilize other treatment methods to improve self-efficacy and ultimately lead to a larger improvement in activity and participation after stroke.
Despite our findings, our study is not without limitations. One limitation is the complex nature of self-efficacy. While the ABC is recommended by the StrokEDGE and commonly used in clinical practice, the ABC focuses primarily on balance self-efficacy. Similarly, the Walk-12 focuses primarily on walking self-efficacy. There are certainly other forms of self-efficacy and a plethora of factors that impact each individual person’s own self-efficacy. Given our findings on the importance of self-efficacy, future research should be conducted to develop additional measures to assess self-efficacy.
Lastly, based on the fact that FGA loaded onto both the PB and SE factors, FGA is potentially a more complex test than initially thought. Although the FGA is often considered a performance based outcome measure, it has similarities to other performance based measures as well as measures of self-efficacy.21 Although, we do not fully understand the dual loading of the FGA, we hypothesize that patients may have preconceived notions about the difficulty of some of the tasks included in the FGA. For example, a healthy individual as well as an individual post stroke may perceive backwards walking as an inherently difficult task. As a result, they may actually perform worse on the task due to lower confidence in their ability to perform the task. Similarly, an individual may believe that walking with eyes closed is a very challenging task and again not perform as well on it due to a lack of confidence. Based on these thoughts, the FGA may link more directly to self-efficacy than other performance based walking tests, such as TUG, which requires the patient to perform activities commonly encountered in daily life and for which patients may have more confidence in performing. Further research into this link is warranted.
Conclusions
Our results support the role of self-efficacy as a mediator between performance capacity and activity and participation. Although physical capacity as evaluated by performance based outcome measures is important in predicting activity and community participation, self-efficacy further predicts activity and participation in patients post stroke. As a result, it is important for clinicians to utilize multiple outcome measures that assess both physical capacity and self-efficacy when trying to understand the effect of stroke on activity and participation. The use of multiple outcome measures will allow therapists to determine how performance impacts self-efficacy and will ultimately provide a more complete assessment of activity and participation. Additionally, treatment strategies that address both performance and self-efficacy may have the largest impact on activity and participation after stroke.
Acknowledgments
financial support
Financial support: National Institutes of Health: 1R21HD071042-01A1
Footnotes
Conflicts of interest/disclosures
There are no conflicts of interest.
Adherence to ethics and reporting requirements
Study approved by UD Human Subjects Review Board
References
- 1.Writing Group Member. Lloyd-Jones D, Adams RJ, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability? J Stroke Cerebrovasc Dis. 2004;13(4):171–177. doi: 10.1016/j.jstrokecerebrovasdis.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Weltgesundheitsorganisation, editor. Geneva: World Health Organization; 2007. International Classification of Functioning, Disability and Health: Children & Youth Version ; ICF-CY. [Google Scholar]
- 4.Tudor-Locke CE, Myers AM. Methodological Considerations for Researchers and Practitioners Using Pedometers to Measure Physical (Ambulatory) Activity. Res Q Exerc Sport. 2001;72(1):1–12. doi: 10.1080/02701367.2001.10608926. [DOI] [PubMed] [Google Scholar]
- 5.Fulk GD, Reynolds C, Mondal S, Deutsch JE. Predicting Home and Community Walking Activity in People With Stroke. Arch Phys Med Rehabil. 2010;91(10):1582–1586. doi: 10.1016/j.apmr.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Haeuber E, Shaughnessy M, Forrester LW, Coleman KL, Macko RF. Accelerometer monitoring of home- and community-based ambulatory activity after stroke. Arch Phys Med Rehabil. 2004;85(12):1997–2001. doi: 10.1016/j.apmr.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86(8):1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Barlow J. Self-efficacy in the context of rehabilitation. 2010 [Google Scholar]
- 9.Schmid AA, Van Puymbroeck M, Altenburger PA, et al. Balance and Balance Self-Efficacy Are Associated With Activity and Participation After Stroke: A Cross-Sectional Study in People With Chronic Stroke. Arch Phys Med Rehabil. 2012;93(6):1101–1107. doi: 10.1016/j.apmr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Schmid A, Duncan PW, Studenski S, et al. Improvements in Speed-Based Gait Classifications Are Meaningful. Stroke. 2007;38(7):2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 11.Van der Zee CH, Visser-Meily JMA, Lindeman E, Jaap Kappelle L, Post MWM. Participation in the chronic phase of stroke. Top Stroke Rehabil. 2013;20(1):52–61. doi: 10.1310/tsr2001-52. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan JE, Crowner BE, Kluding PM, et al. Outcome Measures for Individuals With Stroke: Process and Recommendations From the American Physical Therapy Association Neurology Section Task Force. Phys Ther. 2013;93(10):1383–1396. doi: 10.2522/ptj.20120492. [DOI] [PubMed] [Google Scholar]
- 13.Macko RF, Haeuber E, Shaughnessy M, et al. Microprocessor-based ambulatory activity monitoring in stroke patients. Med Sci Sports Exerc. 2002;34(3):394–399. doi: 10.1097/00005768-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Collen FM, Wade DT, Bradshaw CM. Mobility after stroke: reliability of measures of impairment and disability. Int Disabil Stud. 1990;12(1):6–9. doi: 10.3109/03790799009166594. [DOI] [PubMed] [Google Scholar]
- 15.Mudge S, Stott NS. Timed Walking Tests Correlate With Daily Step Activity In Persons With Stroke. Arch Phys Med Rehabil. 2009;90(2):296–301. doi: 10.1016/j.apmr.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005;86(8):1641–1647. doi: 10.1016/j.apmr.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- 18.Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88(5):559–566. doi: 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson TJ. Detecting change in patients with stroke using the Berg Balance Scale. Aust J Physiother. 2001;47(1):29–38. doi: 10.1016/s0004-9514(14)60296-8. [DOI] [PubMed] [Google Scholar]
- 20.Lin J-H, Hsu M-J, Hsu H-W, Wu H-C, Hsieh C-L. Psychometric comparisons of 3 functional ambulation measures for patients with stroke. Stroke J Cereb Circ. 2010;41(9):2021–2025. doi: 10.1161/STROKEAHA.110.589739. [DOI] [PubMed] [Google Scholar]
- 21.Wrisley DM, Kumar NA. Functional gait assessment: concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys Ther. 2010;90(5):761–773. doi: 10.2522/ptj.20090069. [DOI] [PubMed] [Google Scholar]
- 22.Brogårdh C, Flansbjer U-B, Lexell J. Self-reported walking ability in persons with chronic stroke and the relationship with gait performance tests. PM R. 2012;4(10):734–738. doi: 10.1016/j.pmrj.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Holland A, O’Connor RJ, Thompson AJ, Playford ED, Hobart JC. Talking the talk on walking the walk: a 12-item generic walking scale suitable for neurological conditions? J Neurol. 2006;253(12):1594–1602. doi: 10.1007/s00415-006-0272-2. [DOI] [PubMed] [Google Scholar]
- 24.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 25.Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci. 1998;53(4):M287–M294. doi: 10.1093/gerona/53a.4.m287. [DOI] [PubMed] [Google Scholar]
- 26.Botner EM, Miller WC, Eng JJ. Measurement properties of the Activities-specific Balance Confidence Scale among individuals with stroke. Disabil Rehabil. 2005;27(4):156–163. doi: 10.1080/09638280400008982. [DOI] [PubMed] [Google Scholar]
- 27.Salbach NM, Mayo NE, Robichaud-Ekstrand S, Hanley JA, Richards CL, Wood-Dauphinee S. Balance Self-Efficacy and Its Relevance to Physical Function and Perceived Health Status After Stroke. Arch Phys Med Rehabil. 2006;87(3):364–370. doi: 10.1016/j.apmr.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Bowden MG, Behrman AL. Step Activity Monitor: accuracy and test-retest reliability in persons with incomplete spinal cord injury. J Rehabil Res Dev. 2007;44(3):355–362. doi: 10.1682/jrrd.2006.03.0033. [DOI] [PubMed] [Google Scholar]
- 29.Danks KA, Roos MA, McCoy D, Reisman DS. A step activity monitoring program improves real world walking activity post stroke. Disabil Rehabil. 2014;36(26):2233–2236. doi: 10.3109/09638288.2014.903303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiauzzi E, Rodarte C, DasMahapatra P. Patient-centered activity monitoring in the self-management of chronic health conditions. BMC Med. 2015;13(1) doi: 10.1186/s12916-015-0319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resnick B, Nahm ES, Orwig D, Zimmerman SS, Magaziner J. Measurement of activity in older adults: reliability and validity of the Step Activity Monitor. J Nurs Meas. 2001;9(3):275–290. [PubMed] [Google Scholar]
- 32.Duncan PW, Lai SM, Bode RK, Perera S, DeRosa J. Stroke Impact Scale-16: A brief assessment of physical function. Neurology. 2003;60(2):291–296. doi: 10.1212/01.wnl.0000041493.65665.d6. [DOI] [PubMed] [Google Scholar]
- 33.Lai S-M, Perera S, Duncan PW, Bode R. Physical and social functioning after stroke: comparison of the Stroke Impact Scale and Short Form-36. Stroke J Cereb Circ. 2003;34(2):488–493. doi: 10.1161/01.str.0000054162.94998.c0. [DOI] [PubMed] [Google Scholar]
- 34.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 35.Schmid AA, Damush T, Tu W, et al. Depression Improvement Is Related to Social Role Functioning After Stroke. Arch Phys Med Rehabil. 2012;93(6):978–982. doi: 10.1016/j.apmr.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Sakakibara BM, Miller WC, Routhier F, Backman CL, Eng JJ. Association between self-efficacy and participation in community-dwelling manual wheelchair users aged 50 years or older. Phys Ther. 2014;94(5):664–674. doi: 10.2522/ptj.20130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold CM, Faulkner RA, Gyurcsik NC. The Relationship between Falls Efficacy and Improvement in Fall Risk Factors Following an Exercise Plus Educational Intervention for Older Adults with Hip Osteoarthritis. Physiother Can. 2011;63(4):410–420. doi: 10.3138/ptc.2010-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mielenz TJ, Kubiak-Rizzone KL, Alvarez KJ, et al. Association of Self-Efficacy and Outcome Expectations with Physical Activity in Adults with Arthritis. Arthritis. 2013;2013:1–8. doi: 10.1155/2013/621396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muto LHA, Sauer JF, Yuan SLK, Sousa A, Mango PC, Marques AP. Postural control and balance self-efficacy in women with fibromyalgia: are there differences? Eur J Phys Rehabil Med. 2015;51(2):149–154. [PubMed] [Google Scholar]
- 40.Arnstein P, Caudill M, Mandle CL, Norris A, Beasley R. Self efficacy as a mediator of the relationship between pain intensity, disability and depression in chronic pain patients. Pain. 1999;80(3):483–491. doi: 10.1016/S0304-3959(98)00220-6. [DOI] [PubMed] [Google Scholar]
- 41.Chen C-W, Chen Y-C, Su W-J, Wang J-K, Lee P-C, Beckstead JW. Social-cognitive determinants of exercise behaviour among adolescents with mild congenital heart disease. Eur J Cardiovasc Nurs. 2013;12(4):368–376. doi: 10.1177/1474515112460797. [DOI] [PubMed] [Google Scholar]
- 42.Craig A, Tran Y, Siddall P, et al. Developing a model of associations between chronic pain, depressive mood, chronic fatigue, and self-efficacy in people with spinal cord injury. J Pain Off J Am Pain Soc. 2013;14(9):911–920. doi: 10.1016/j.jpain.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Shelby RA, Somers TJ, Keefe FJ, Pells JJ, Dixon KE, Blumenthal JA. Domain specific self-efficacy mediates the impact of pain catastrophizing on pain and disability in overweight and obese osteoarthritis patients. J Pain Off J Am Pain Soc. 2008;9(10):912–919. doi: 10.1016/j.jpain.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson CA, Shumway-Cook A, Matsuda PN, Ciol MA. Understanding physical factors associated with participation in community ambulation following stroke. Disabil Rehabil. 2011;33(12):1033–1042. doi: 10.3109/09638288.2010.520803. [DOI] [PubMed] [Google Scholar]
- 45.Bishop MD, Patterson TS, Romero S, Light KE. Improved Fall-Related Efficacy in Older Adults Related to Changes in Dynamic Gait Ability. Phys Ther. 2010;90(11):1598–1606. doi: 10.2522/ptj.20090284. [DOI] [PubMed] [Google Scholar]
- 46.Pang MYC, Eng JJ. Fall-related self-efficacy, not balance and mobility performance, is related to accidental falls in chronic stroke survivors with low bone mineral density. Osteoporos Int. 2008;19(7):919–927. doi: 10.1007/s00198-007-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosén E, Sunnerhagen KS, Kreuter M. Fear of falling, balance, and gait velocity in patients with stroke. Physiother Theory Pract. 2005;21(2):113–120. doi: 10.1080/09593980590922299. [DOI] [PubMed] [Google Scholar]
- 48.Belgen B, Beninato M, Sullivan PE, Narielwalla K. The association of balance capacity and falls self-efficacy with history of falling in community-dwelling people with chronic stroke. Arch Phys Med Rehabil. 2006;87(4):554–561. doi: 10.1016/j.apmr.2005.12.027. [DOI] [PubMed] [Google Scholar]


