Abstract
Rationale
Accumulating evidence supports a role of adaptive immunity and particularly T cells in the pathogenesis of hypertension. Formation of memory T cells, which requires the co-stimulatory molecule CD70 on antigen presenting cells, is a cardinal feature of adaptive immunity.
Objective
To test the hypothesis that CD70 and immunological memory contribute to the blood pressure elevation and renal dysfunction mediated by repeated hypertensive challenges.
Methods and Results
We imposed repeated hypertensive challenges using either L-NAME/high salt or repeated ang II stimulation in mice. During these challenges effector memory T (TEM) cells accumulated in the kidney and bone marrow. In the L-NAME/high salt model, memory T cells of the kidney were predominant sources of IFN-γ and IL-17A, known to contribute to hypertension. L-NAME/high salt increased macrophage and dendritic cell surface expression of CD70 by 3 to 5-fold. Mice lacking CD70 did not accumulate TEM cells and did not develop hypertension to either high salt or the second ang II challenge and were protected against renal damage. Bone marrow residing TEM cells proliferated and redistributed to the kidney in response to repeated salt feeding. Adoptively transferred TEM cells from hypertensive mice homed to the bone marrow and spleen and expanded upon salt feeding of the recipient mice.
Conclusions
Our findings illustrate a previously undefined role of CD70 and long-lived TEM cells in the development of blood pressure elevation and end-organ damage that occur upon delayed exposure to mild hypertensive stimuli. Interventions to prevent repeated hypertensive surges could attenuate formation of hypertension-specific TEM cells.
Keywords: Adaptive immunity, immunological memory, Interleukin 17A, Interferon gamma, CD70, immune system, inflammation, bone marrow, kidney
INTRODUCTION
During the past several years, emerging evidence has demonstrated that the adaptive immune system plays an important role in the pathogenesis of hypertension.1 In several experimental models of hypertension there is accumulation of T cells in the kidney that release inflammatory cytokines, such as IL-17A and IFN-γ, which promote renal and vascular dysfunction.2 We and others have shown that these cytokines contribute to salt and volume retention by the kidneys and stimulate vascular production of reactive oxygen species and promote vascular stiffening.3-6 In keeping with this, mice deficient in IL-17A and IFN-γ are protected against the anti-diuretic and anti-natriuretic effects of ang II.2, 3
A cardinal feature of adaptive immunity is immunological memory, which provides protection against repeated antigenic exposure. Upon initial antigen presentation the classical T cell immune response is characterized by stimulation of naïve T cells to proliferate and form effector T cells.7 The majority of effector T cells ultimately die, but a few remaining cells become long-lived memory T cells. Some of these return to secondary lymphoid organs such as lymph nodes and the spleen and are referred to as central memory (TCM) cells which are characterized by the surface markers CD44hi/CD62Lhi/CCR7+. Others remain in the periphery as effector memory (TEM) cells bearing the surface markers CD44hi/CD62Llo/CCR7−. A third recently identified subset are resident memory (TRM) cells, which are CD44hi/CD62Llo/CD103+/CD69+. The presence of CD69 inhbits the sphingosine-1 phosphate receptor and keeps activated TRM cells within specific tissues. Formation of memory cells requires the interaction of CD27 on T cells with CD70 on activated antigen presenting cells (APCs). This co-stimulatory interaction is analogous to that of T cell CD28 with the B7 ligands which is requried for naïve T cell activation. Mice lacking either CD27 or CD70 fail to develop memory T cells upon antigen rechallenge.8, 9
We hypothesized that the development of memory T cells in response to an initial hypertensive stimulus could sensitize the host to the development of hypertension in response to subsequent modest stimuli that would otherwise not raise blood pressure. In the current study, we show that repeated hypertensive stimuli promote accumulation of TEM cells in the kidney and bone marrow and that these cells are primarily responsible for production of IFN-γ and IL-17A. This study illustrates a previously undefined role of CD70 in the genesis of hypertension in response to rather mild, repeated hypertensive challenges.
METHODS
Animals and blood pressure measurement
Wild type, and interferon-gamma deficient mice (IFN-γ −/−) on a C57Bl/6J background were purchased from Jackson Laboratories (Bar Harbor, Maine). CD70-deficient mice (CD70−/−) on a C57Bl/6J background were obtained from Dr. Ross Kedl (University of Colorado). Mice were provided regular chow and water ad libitum. At 12 weeks of age, male mice were randomly selected for treatment.
Mice were sacrificed at the end of all experiments by CO2 inhalation. All animal procedures were approved by Vanderbilt University Institutional Animal Care and Use Committee (IACUC), and mice were housed and cared for in accordance with the Guide for the Care and Use of Laboratory Animals.
Statistics
Data are expressed as mean ± standard error of the mean. Blood pressures were analyzed by ANOVA for repeated measures. For comparisons of experiments involving a 2×2 design two-way ANOVA was used. When individual comparisons were made within this 2×2 design, Newman-Keuls or Holm-Sidak post-hoc tests were employed. When variances between groups were unequal, a Mann Whitney comparison followed by a Bonferroni correction was used.
For complete description of methods, please see the on-line supplemental data.
RESULTS
Repeated hypertensive stimuli induce formation of effector memory T cells that reside in the kidney and bone marrow
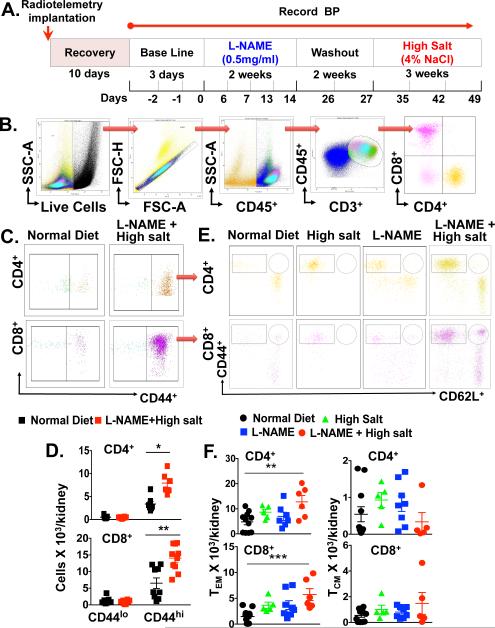
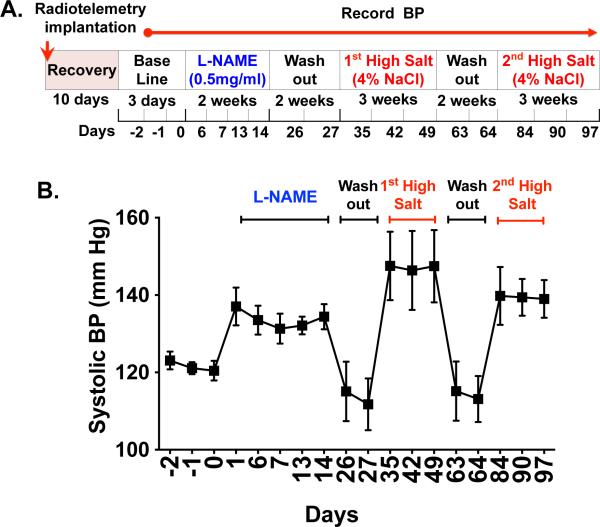
To test the hypothesis that repeated hypertensive stimuli lead to formation of memory T cells and their accumulation in critical tissues such as the kidney, we employed an experimental model that involves an initial exposure of the NOS inhibitor L-NAME (0.5mg/mL) for 2 weeks, followed by a two-week washout and subsequent high salt diet (4% NaCl) feeding for three weeks (Figure 1A). This model permits repeated hypertensive challenges without surgical interventions and recapitulates salt-sensitive hypertension that is common in humans. Flow cytometry analysis of single cell homogenates from kidneys of these mice revealed a dramatic increase in both CD4+ and CD8+ CD44hi memory cells within the kidney (Figure 1B-D). In contrast, CD44lo primary effector T cells were not increased by exposure to L-NAME/high salt. Exposure to either L-NAME or high salt diet alone caused only a modest increase in the number of memory T cells. Surface staining with CD62L indicated that L-NAME followed by high salt diet caused a progressive increase in both CD4+ and CD8+ CD62Llo TEM cells (Figure 1E and 1F), but did not change the small number of CD62Lhi TCM nor TRM cells (Online Figure IA) present in the kidney. It has been reported that a subpopulation of memory T cells express the surface receptor Ly-6C.10 In keeping with this, we found that 40% of CD4+ and 80% of CD8+ TEM cells in the kidney are Ly-6C+. The renal CD8+/Ly-6C+ TEM population was markedly increased by the L-NAME/HS protocol (Online Figure IB). There was no increase in memory T cells in the aorta or spleen of L-NAME/high salt mice (Online Figure IC - F).
Figure 1. Effect of repetitive hypertensive challenges on renal memory T cell formation.
(A) Experimental paradigm employed. (B) Representative flow cytometry dot plots show sequential gating for live and single cells, followed by total leukocytes (CD45+), total T lymphocytes (CD3+) and CD4+ and CD8+ T cells. (C) CD4+ and CD8+ memory T cells (CD44lo and CD44hi) reperesentative dot plots and (D) quantitation are shown. (E) CD4+ and CD8+ representative flow cytometry dot plots and graphs showing infiltrating CD4+ and CD8+ TEM (F) in the kidney are shown respectively at baseline or in response to LNAME/highsalt challenge. Data are expressed as mean ± SEM (n=5-9 per group), P values for the effect of L-NAME/high salt as calculated by one-way ANOVA is shown *P<0.05, **P<0.01, ***P<0.001 vs. Normal Diet.
Effector memory cells are the major source of IFN-γ and IL-17A in the kidney of hypertensive mice
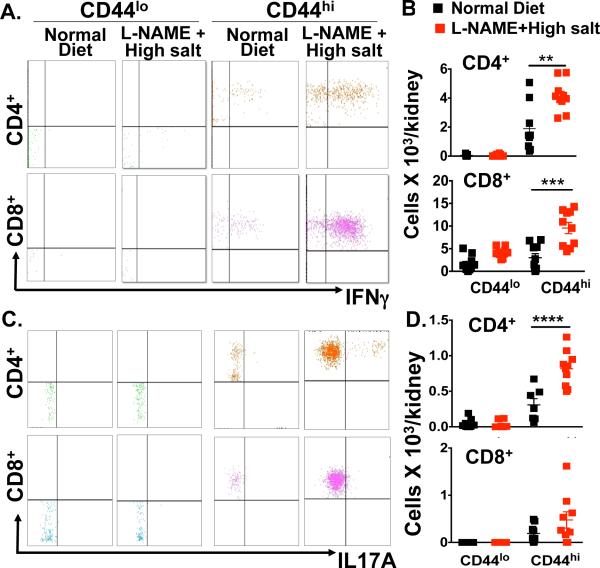
Data from our group and others indicate that cytokines such as IL-6, IFN-γ, and IL-17A affect renal tubular function, modulating expression and activity of the sodium/chloride co-transporter, the sodium potassium chloride co-transporter and the sodium hydrogen exchanger 3.3, 11 Mice lacking IFN-γ and IL-17A are likewise partly protected from ang II-induced hypertension and have preserved natriuresis in response to a sodium challenge during ang II infusion, further emphasizing a role of these cytokines in altering renal function in hypertension.3 We therefore sought to determine if TEM cells within the kidney play a role in production of these cytokines in hypertension. Intracellular staining indicated that IFN-γ production increased significantly in both CD4+ and CD8+ CD44hi memory T cells in hypertensive mice compared to mice fed a normal diet. In contrast, IFN-γ production was minimal in CD44lo primary effector T cells and was not changed by the L-NAME/hypertensive challenge (Figure 2A and 2B). In a similar fashion, we found that renal CD44hi memory T cells are predominantly responsible for IL-17A production in this hypertensive model (Figure 2C and 2D). Thus, among CD4+ and CD8+ T cells in the kidney, memory T cells are predominantly responsible for production of these injurious cytokines.
Figure 2. Subsets of T cells within the kidney producing IFN-γ and IL-17A.
Intracellular staining was performed on renal-infiltrating memory T cells, at baseline or in response to L-NAME/high salt. Data were obtained on cells studied in figure 1D. (A, B) Representative flow cytometry dot plot showing CD4+ and CD8+ memory T cells producing IFN-γ and (C, D) IL-17 by intracellular staining. Data are expressed as mean ± SEM (n=9-15 per group). P values for the effect of L-NAME/high salt as calculated by one-way ANOVA is s*hown; **P<0.001, ***P<0.001, ****P<0.001 vs. Normal Diet.
CD70 is increased on antigen-presenting cells and participates in memory T cell formation and hypertension
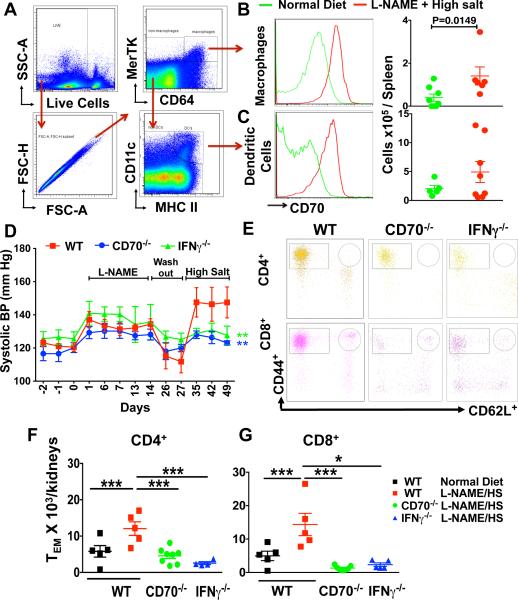
The interaction of CD70 on APCs with CD27 on T cells plays a critical role in the formation of memory T cells, and in particular CD8+ memory T cells.8, 12 Using a gating strategy described by Jakubziak et al,13 which discriminates between DCs and macrophages (figure 3A), we found that L-NAME/high salt increases CD70 expression by 5-fold and 3-fold on macrophages and DCs respectivley (Figure 3B and 3C). To further define a role of the CD70 and CD27 in hypertension, we employed radiotelemetry to monitor blood pressure in WT and CD70−/− mice during the L-NAME/high salt protocol. In WT mice systolic pressure increased to 134 ± 3 mmHg during L-NAME administration and returned to baseline levels during the subsequent washout period (Figure 3D). This exposure to L-NAME caused WT mice to become salt-sensitive, such that the subsequent addition of 4% salt to their diet increased systolic pressure to 147.5 ± 9 mmHg. In contrast, while the increase in systolic pressure caused by L-NAME in CD70−/− mice was similar to that observed in WT mice, the hypertension induced by subsequent salt administration was markedly attenuated (Figure 3D). Because memory T cells are a major source of IFN-γ, we examined the hypertensive response to the L-NAME/high salt protocol in IFN-γ−/− mice. Similar to CD70−/− mice, mice lacking IFN-γ failed to develop hypertension during the high salt-feeding phase of this protocol. Of note, the accumulation of TEM cells within the kidney was completely prevented in mice lacking CD70 and likewise attenuated in IFN-γ−/− mice (Figure 3E-G).
Figure 3. Role of CD70 and IFN-γ in hypertension.
(A) Representative dot plots show gating strategy to identify CD70 on splenic macrophages (B) and DCs (C) from WT mice in response to normal diet or L-NAME/high salt. (D) Systolic BP was measured by telemetry in WT, CD70−/−, and IFN-γ −/− mice exposed to the L-NAME/high salt protocol (n=7-11 per group). Representative flow cytometry dot plots (E) and summary data (F and G) showing WT, CD70−/−, and IFN-γ −/− renal–infiltrating CD4+ and CD8+ TEM cells. Data are expressed as mean ± SEM (n=5-8 per group). P values for the effect of L-NAME/HS (High Salt) as calculated by one-way ANOVA are shown *P<0.05, ****P<0.0001 **P<0.01.
We have recently demonstrated a role for isoketal-adducted peptides in DCs immunogenicity.14 In the present study we found that L-NAME increased the presence of isoketal protein adducts in DCs and this persisted during subsequent high salt feeding (Online Figure IIA). We have also shown that norepinephrine stimulates formation of these adducts in DCs.15 In keeping with this, we found that both the L-NAME and high salt increased the low frequency to high frequency ratio of heart rate variability, a parameter of sympathetic tone (Online Figure IIB). Thus, antigen-presenting cells promote hypertension not only by providing the initial stimulus of isoketal formation, but also by increasing expression of CD70, which facilitates memory T cell formation.
The role of memory T cells in glomerular and tubular injury
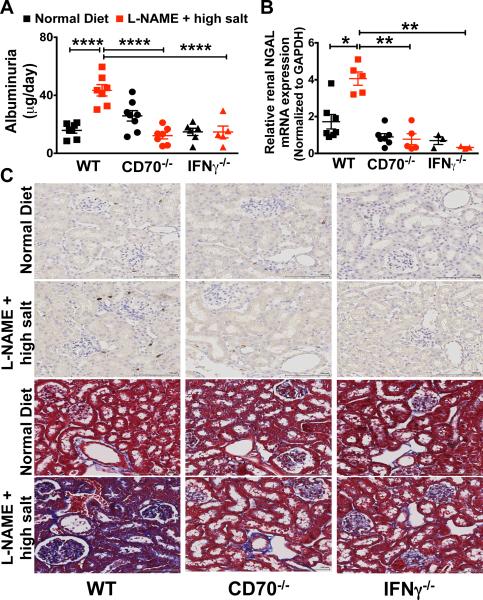
In additional experiments, we sought to define how memory T cells and IFN-γ modulate renal damage. Exposure of wild type mice to the L-NAME/high salt protocol caused a marked increase in albuminuria and renal mRNA levels of neutrophil gelatinase associated lipocalin (N-GAL). In contrast, CD70−/− and IFN-γ−/− mice were completely protected against the development of albuminuria (Figure 4A) and did not exhibit increases in renal NGAL mRNA expression (Figure 4B), indicating that IFN-γ producing memory cells promote glomerular and tubular injury.16 Likewise, CD70−/− and IFN-γ−/− mice had less renal fibrosis than WT mice following the L-NAME/high salt protcol as detected by Masson's Trichrome staining (Figure 4C). It has been proposed that IFN-γ can stimulate angiotensinogen production and promote sodium reabsorption within the renal tubule.11 We therefore examined kidney and urinary angiotensinogen (UAGT) and creatinine (UCre) concentration and found no difference between WT, CD70−/− and IFN-γ−/− mice (Online Figure IIIA and IIIB).
Figure 4. Parameters of renal injury in response to L-NAME/high salt.
(A) Glomerular injury was assessed by quantifying 24-hour urinary excretion of albumin in WT, CD70−/−, and IFNγ −/− mice. (B) NGAL mRNA was detected by quantitative real-time PCR. (C) Masson's Trichrome staining was employed to detect renal fibrosis. Data are expressed as mean ± SEM, (A) n = 5-6, (B and C) n = 6–11 per group. P values analyzed by one-way ANOVA; *P < 0.1, **P < 0.01; ***P < 0.0001, ****P < 0.0001.
TEM cells reside in the bone marrow and are responsive to high salt feeding
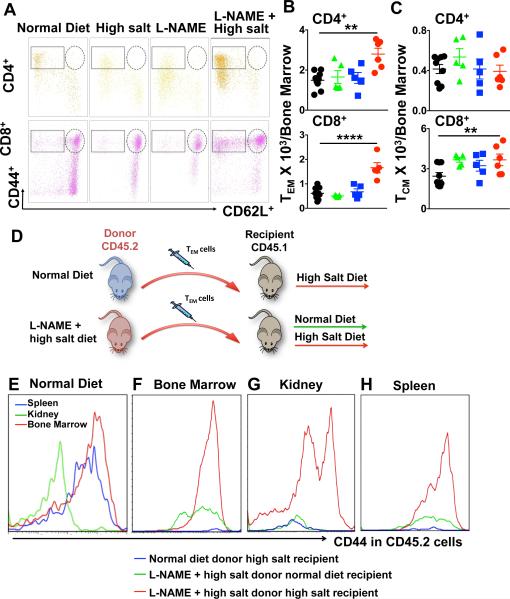
It has recently been appreciated that following an initial antigenic exposure, TEM cells do not simply circulate, but persist in a quiescent state in the bone marrow for months in the mouse and decades in humans. In keeping with this, we observed that there was a 1.8-fold increase in CD4+ TEM cells and a 3-fold increase in CD8+ TEM cells in the bone marrow following the L-NAME/high salt exposure compared to control mice (Figure 5A and 5B). Bone marrow memory T cell maintenance and survival is dependent on stromal cell production of IL-7 and IL-15.17-19 We did not observe a change in expression of either of these within the bone marrow of hypertensive mice (Online Figure IV). In contrast to TEM cells, there was no increase in the number of TCM or TRM cells present in the bone marrow (Figure 5A, 5C and Online Figure VA). In keeping with prior reports,10 bone marrow residing TEM cells expressed Ly-6C and in the case of CD8+ T cells, were markedly increased by the L-NAME/HS challenge (Online Figure VB). Bone marrow-derived TEM cells exist largely in a quiescent state.18 In keeping with this, we found that relatively few bone marrow TEM cells were positive for the proliferation marker Ki-67, and this was not changed by the hypertensive challenge (Online Figure VC-D).
Figure 5. Bone marrow effector memory T cells exhibit salt-sensitivity. Mice received the L-NAME/High salt protocol as in Figure 1.
Representative flow cytometry dot plot showing CD4+ and CD8+ TEM cells in the bone marrow (A). Summary data of the absolute numbers of indicated T cell types at baseline or in response to L-NAME, high salt, and LNAME/highsalt challenges in CD4+ and CD8+ are shown in bone marrow (B-C). Experimental paradigm (D). Bone marrow TEM cells from donor CD45.2 mice fed either a normal diet or the L-NAME/high salt protocol were adoptively transferred to recipient CD45.1 mice. Recipient mice were then fed either normal diet (E) or high salt diet for three weeks. Representative plots of CD45.2 TEM cells from mice exposed to the L-NAME/high salt protocol to recipient CD45.1 mice fed either normal or high salt are shown in panel E-H. Representative plots for adoptively transferred CD45.2 TEM cells in the bone marrow (F), kidney (G) and spleen (H) are shown. Examples are representative of 3 separate experiments. Data are expressed as mean ± SEM (n=5-9 per group). P values for the effect of L-NAME/high salt as calculated by one-way ANOVA are shown **P<0.01, ****P<0.0001.
Because repeated antigenic exposure has been shown to reactivate bone marrow-residing TEM cells, we sought to determine if these cells might exhibit salt-sensitivity. To perform these experiments, we adoptively transferred 0.5 × 106 TEM cells of CD45.2 mice that had undergone the L-NAME/high salt protocol to recipient CD45.1 mice (Figure 5D). In recipient mice fed a normal diet for the ensuing 3 weeks, these adoptively transferred TEM cells were detected in the bone marrow and to a lesser extent in the spleen but not in the kidney (Figure 5E). In contrast, in recipient mice fed a high salt diet there was expansion of TEM cells in the bone marrow (Figure 5F). Importantly, there was also marked accumulation of these adoptively transferred TEM cells in the kidney in response to high salt feeding (figure 5G). We observed a modest increase in the number of TEM cells in the spleen, but these were relatively few compared to the bone marrow and kidney (Figure 5H). Of note, bone marrow TEM cells from control mice fed a normal diet were not detectable in the spleen, bone marrow or kidney of recipient mice (Figures 5F-H).
In additional experiments, we exposed WT mice to the L-NAME/high salt protocol followed by 3 weeks of normal diet. The mice were then re-exposed to a second high salt challenge (Figure 6A). Radiotelemetry recording of blood pressure revealed that these animals maintain salt-sensitivity for as long as 3 months after the initial L-NAME exposure, as reflected by an increase in blood pressure to 140 mmHg (Figure 6B). A striking finding in these animals was that there was a further increase in CD4+ and CD8+ TEM cells in the kidney in response to this second salt challenge (Table 1), and a concomitant decrease in the bone marrow residing TEM cells (Table 2). Taken together with our adoptive transfer experiments, these data suggest that bone marrow-residing TEM cells are salt-sensitive, and redistribute to the kidney upon salt feeding.
Figure 6. Hypertensive effect of a second high salt challenge following L-NAME.
(A) Experimental design. WT mice that had undergone the L-NAME/high salt protocol followed by 2 weeks washout period were re-exposed to second high salt challenge. Systolic BP was measured by telemetry (B). Data are expressed as mean ± SEM (n= 6).
Table 1.
Memory T cells in the kidney following normal diet or L-NAME with either 1 or 2 high salt challenges.
| Normal Diet | L-NAME + HS1 | L-NAME + HS1 +HS2 | |
|---|---|---|---|
| CD8 TEM | 2.4 ± 0.6 (29±1.6) | 8.4 ± 1.5** (36.6±2.5)* | 23.1 ± 4****, ††† (68.8±1.9)****, ††† |
| CD8 TCM | 0.5±0.14 (12.8±2.1) | 1.5±0.8 (17.1±2.8) | 5.3 ± 0.8****, ††† (15.9±0.8) |
| CD4 TEM | 4.7 ± 0.8 (68±3.8) | 12 ± 1.3**** (67.6±3.8) | 18 ± 2.3*** (74.4±2.1) |
| CD4 TCM | 0.5 ±0.2 (8.5±1.7) | 0.3±0.3 (8.1±1.4) | 1.6 ± 0.2†† (6.8±0.5) |
Data are mean ± SEM × 103 (n=5 - 20 per group). Parentheses denote the percent of total CD8+ or CD4+ T cells for the various populations. P values for the effect of L-NAME/high salt as calculated by one-way ANOVA are shown. HS1 and HS2 respectively refer to the first and second high salt challenges.
P<0.05 vs ND
P<0.01 vs ND
P<0.001vs ND
P<0.0001 vs ND.
†p< 0.01 vs HS1
p< 0.001 vs HS1
p < 0.0001
Table 2.
Memory T cells in the bone marrow following normal diet or L-NAME with either 1 or 2 high salt challenges.
| Normal Diet | L-NAME + HS1 | L-NAME + HS1 +HS2 | |
|---|---|---|---|
| CD8 TEM | 0.6 ± 0.1 (10±0.6) | 1.4 ± 0.2**** (19.8±0.9)** | 1.0 ± 0.01† (19.8 ± 1.0)** |
| CD8 TCM | 2.4±0.27 (50±1.4) | 3.7 ± 0.4* (56±1.5) | 2.9 ± 0.1 (58.7±1.3)** |
| CD4 TEM | 1.5 ± 0.4 (48±3.9) | 2.6 ± 0.7*** (64.3±1.2)** | 1.7 ± 0.2 (62.9±1.06)* |
| CD4 TCM | 0.4±0.04 (34.4±1.4) | 0.4 ± 0.2 (26.5±1.0)*** | 0.14 ± 0.01 (5.3±0.5)****, ††† |
Data are mean ± SEM × 103 (n=5 - 20 per group). Parentheses denote the percent of total CD8+ or CD4+ T cells for the various populations. P values for the effect of L-NAME/high salt as calculated by one-way ANOVA are shown. HS1 and HS2 respectively refer to the first and second high salt challenges.
P<0.05 vs ND
P<0.01 vs ND
P<0.001vs ND
P<0.0001 vs ND.
p< 0.01 vs HS1
††p< 0.001 vs HS1
p < 0.0001
Induction of memory T cells in angiotensin II-induced hypertension
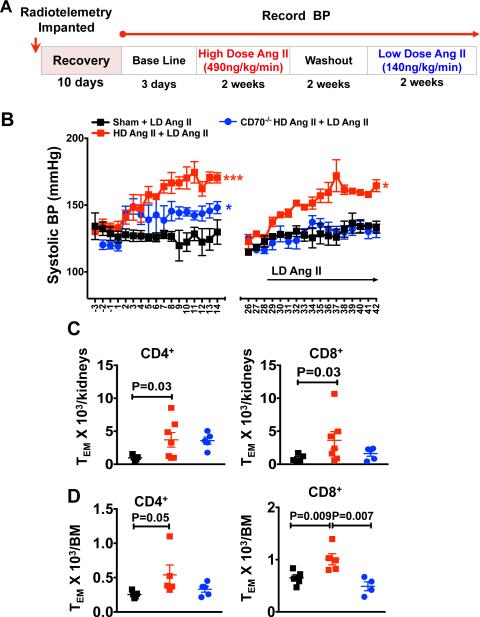
In additional experiments, we also investigated a potential role of T cell memory in ang II-induced hypertension. WT mice were implanted with an osmotic pump for infusion of either ang II (490 ng/kg/min) or vehicle for two weeks, followed by a two week washout period (Figure 7A). The mice were then implanted with a second osmotic minipump for infusion of low dose of ang II (140 ng/kg/min) and blood pressure was monitored by radiotelemetry. As shown in figure 7B, WT mice that initially received a sham infusion had minimal increase in blood pressure in response to this low dose of ang II. In contrast mice that had previously received high dose ang II exhibited an increase in blood pressure to 160 mmHg. CD70−/− mice developed a modest hypertensive response during the first two weeks of high dose ang II infusion, however had essentially no increase in blood pressure during the subsequent infusion of low-dose ang II (Figure 7B). During this two week exposure to high dose ang II, there was a markedly blunted increase in CD8+ TEM cells and no change in CD4+ TEM cells in the kidneys of CD70−/− mice (Online Figure VIA). Of note there was a modest increase in renal CD44lo cells, which represent a primary effector population and in double negative T cells (Online Figure VIB and VIC). In keeping with our findings in the L-NAME/high salt model, in WT mice that had previously received high dose ang II, there was almost a 3-fold increase in CD4+ and CD8+ TEM cells in the kidney (Figure 7C) and bone marrow (Figure 7D) compared to mice that had previously received a sham infusion. In contrast there was no increase in renal or bone marrow TEM cells of CD70−/− mice at the end of this high dose/low dose ang II protocol. Thus, prior exposure to ang II primes severe hypertension in response to a subsequent normally subpressor dose of this octapeptide and leads to accumulation of TEM cells in the kidney and bone marrow. This memory response to ang II is dependent on CD70.
Figure 7. Effect of ang II on memory T cell infiltration in kidney and bone marrow.
(A) WT and CD70−/− mice were infused with either a pressor dose of ang II (490 ng/kg/min) or vehicle for two weeks. Two weeks later, the mice were treated with a low dose of ang II (140 ng/kg/min) and BP was monitored by telemetry (B). Summary data of the absolute numbers renal (C) and bone marrow (D) of CD4+ and CD8+ TEM cells. Data are expressed as mean ± SEM, n = 5-7 per group. P values analyzed by t-test are shown. P values are calculated by one-way ANOVA is shown * <0.05, *** <0.001.
DISCUSSION
In the current study, we show that TEM cells formed during an initial hypertensive challenge enhance sensitivity to a second mild hypertensive challenge, leading to elevations in blood pressure and renal damage that does not occur in the absence of these cells. Our data are compatible with the paradigm illustrated in online figure VII, in which TEM cells accumulate in the kidney and mediate renal dysfunction and sensitize the host to both salt-sensitive and ang II-induced hypertension. These TEM cells can be long lived and can potentially sensitize the host to repeated hypertensive stimuli encountered clinically, such as recurrent episodes of emotional stress, catecholamine surges in sleep apnea or repeated bouts of excess sodium intake.
To mimic salt-sensitive hypertension, which is common in humans, we employed a model of L-NAME followed by high salt feeding. We found that this form of hypertension is associated with the accumulation of memory T cells in the kidney and bone marrow and that these cells are major sources of IL-17A and IFN-γ, which we and others have shown to be important in mediating hypertension and its end-organ damage.3, 20, 21 The formation of memory T cells in hypertension was dependent on CD70, and we found that mice lacking this co-stimulatory ligand, or lacking IFN-γ did not demonstrate salt-induced hypertension following L-NAME exposure. To confirm these findings we employed a second model in which we initially exposed mice to a two week infusion of a pressor dose of ang II (490 ng/kg/min). This also sensitized the mice to develop severe hypertension upon re-exposure to a second low dose of ang II that normally does not raise blood pressure. As in the case of the L-NAME/high salt model, TEM cells accumulated in the kidney and bone marrow, and mice lacking CD70 were protected against the second exposure to ang II. Thus CD70, and by inference its role in promoting formation of memory T cells, plays a critical role in two forms of hypertension.
The development of salt-sensitivity after exposure to L-NAME is of interest because the loss of NO might promote formation of isoketal-protein adducts, which we have previously shown to be immunogenic when formed in DCs.14 In this prior work, we demonstrated that isoketal protein adducts have many features of neoantigens, in that they drive memory T cell proliferation and cytokine production. NO rapidly reacts with lipid peroxy-radicals,22 and in doing so can limit formation of lipid oxidation products such as isoprostanes and isoketals. Indeed in the present study, we found isoketal adducts in DCs were increased after L-NAME and were further increased after exposure to salt. This sequence of events could therefore provide repeated neo-antigen exposure leading to expansion of memory T cells. Of note, many conditions linked to hypertension, such as diabetes, obesity and hypercholesterolemia are associated with loss of endothelial NO production, and in some cases an increase in the endothelial production of the strong oxidant peroxynitrite.23 The administration of L-NAME thus mimics aspects of endothelial dysfunction observed in these common diseases. Myeloid derived DCs, such as those studied in the present paper, are formed when monocytes traverse the vascular endothelium,24 and the interaction with either NO or peroxynitrite producing endothelial cells might modulate formation of intracellular isoketal adducts within the DCs and thus lead to formation of salt-sensitive hypertension. In keeping with this concept, Kopkan et al have shown that eNOS deficient mice develop hypertension upon salt feeding in a fashion analogous to our mice that had previously received L-NAME.25
Related to the above, we recently showed that sympathetic innervation is important for formation of isoketal-adducts in dendritic cells of the kidney, and that this is prevented by renal denervation.15 In this prior study, we showed that DCs possess adrenergic receptors and that norepinephrine can directly stimulate formation of isoketal-adducts in these cells. In our current study, both L-NAME and L-NAME/high salt increased the low frequency to high frequency ratio of heart rate variability, reflecting increased sympathetic tone. It is therefore conceivable that in addition to a loss of endothelial NO, increased sympathetic outflow could stimulate formation of these immunogenic adducts in DCs.
An interesting finding in the present study is that the L-NAME/high salt protocol increases surface expression of CD70 on macrophages and dendritic cells within the kidney. T cell activation requires not only engagement of the T cell receptor but also costimulation. In the case of naïve T cells, a common costimulatory event involves interaction of CD28 on T cells with the B7 ligands CD80 and CD86 on APCs.26 We have previously shown that blockade of this interaction with Abatacept prevents ang II and DOCA-salt hypertension.27 In addition to this interaction, the interplay between CD70 on APCs and CD27 on T cells plays a critical role in formation of memory T cells.8 An important result of the present study was that mice lacking CD70 do not develop salt sensitivity following L-NAME exposure. These animals also do not accumulate memory T cells in the kidney and seem to be protected against the development of albuminuria reflecting preserved glomerular function. These effects are likely due to the absence of injurious cytokines in mice lacking CD70, and indeed were mimicked in IFN-γ deficient mice.
We also employed a model of high dose/low dose ang II as a means to study a potential role of immunological memory in WT and CD70−/− mice. These studies confirmed that there is an immunological memory response to a second dose of ang II that is normally subpressor, and that this response is absent in mice that are unable to generate TEM cells. It is of interest that CD70−/− mice develop modest hypertension during the initial two-week infusion of high dose ang II, and during this time exhibited an increase in primary effector T cells and double negative T cells in their kidneys. It is possible that these cells contribute to the initial high dose response to ang II. In keeping with this, we previously showed that either Abatacept or genetic deletion of B7 ligands, which block co-stimulation of naïve T cells, prevents the acute effect of ang II.27 Thus, taken with our current results, it is possible that the acute two-week response to high dose ang II depends on a different population of immune cells as compared to the recall response a much lower dose of this hormone.
It is likely that the renal accumulation of memory T cells that produce IFN-γ and IL-17A plays an important role in the development of salt sensitivity. We recently found that these cytokines modulate renal tubular sodium transporters including the sodium chloride co-transporter (NCC), the sodium potassium chloride co-transporter (NKCC) and the sodium hydrogen exchanger-3 (NHE3).3 Likewise, Garcia et al demonstrated a blunted hypertensive response to chronic aldosterone infusion in IFNγ−/− mice compared to wild-type animals.21 Satou et al have shown that IFN-γ modulates production of angiotensinogen by proximal tubular cells.11 This leads to intrarenal produciton of ang II, which in turn activates Na+ transport in the distal nephron. In addition to these changes in renal sodium transport, it is likely that these cytokines promote frank renal injury. We observed less renal damage as reflected by albuminuria, NGAL expression and fibrosis in mice lacking either CD70 or IFN-γ. In keeping with this, Marko et al showed that mice lacking the IFN-γ receptor are protected against tubulointerstitial damage, have lower NGAL mRNA and exhibited preserved glomerular filtration rates when given ang II.20 In Dahl-salt sensitive rats, T cells accumulate in perivascular regions and near glomeruli, and are associated with renal fibrosis.28 Deletion of either the RAG-1 gene or the CD3+ zeta subunit prevents renal injury in these animals.29 We previously demonstrated that IL-17A stimulates collagen production by murine fibroblasts in a p38 MAP kinase-dependent fashion,6 perhaps explaining how TEM cells that produce this cytokine might promote renal collagen as observed in Figure 4. Thus, the production of IFN-γ and IL-17A by memory CD4+ and CD8+ T cells likely mediates renal dysfunction, renal damage and ultimately hypertension.
The current study further supports the concept that the kidney is an important site of immune activation in hypertension. Recently, we showed that renal denervation prevents formation of isoketal adducts in dendritic cells not only in the kidney but also in the spleen during ang II-induced hypertension.15 We also showed that renal denervation prevents accumulation of memory cells in the kidney and reduces inflammatory cells in vessels outside of the kidney. Taken together with our current results, these findings suggest that cells activated in the kidney can migrate to other sites, such as the spleen, vasculature and the bone marrow to mediate systemic inflammation. Findings such as these might help explain the frequency of vascular disease among patients with chronic kidney disease.30
An interesting observation in the current study is that TEM cells accumulate in the bone marrow of mice with hypertension. Likewise, adoptively transferred TEM cells from the bone marrow of L-NAME/High salt treated mice accumulated in the bone marrow of recipient mice, and expanded upon salt feeding of the recipients. This result is compatible with the recent recognition that TEM cells can reside in the bone marrow in a quiescent state for prolonged periods and can be reactivated by a repeated antigenic challenge.18 It is interesting to note that several recent lines of evidence support the concept that bone marrow cells contribute to hypertension. As examples, we demonstrated that transplant of bone marrow from hypertension-prone LNK−/− mice markedly enhances hypertension in recipient wild-type mice. 31 Likewise, Santisteban et al recently showed that bone marrow transplantation from normal rats to spontaneously hypertensive rats reduces hypertension in the latter and attenuates both peripheral and central nervous system inflammation.32
A caveat to our observations is that the number of T cells detected by flow cytometry represent the recovered cells, and are unlikely a precise estimate of the cells present in the kidney, vessels or bone marrow in vivo. These experiments involve tissue homogenization, gradient centrifugation, and exclusion of dead cells and selection of single cells by flow cytometry. The latter two steps are necessary to avoid artifactual identification of cells. Thus, there is almost certainly loss of a substantial number of immune cells before a final estimate is made. We therefore caution that while our reported ratios of naïve and memory cells and the relative differences in various hypertensive states are correct, the precise number of cells in various tissues are likely much higher than reported.
Our findings likely have clinical relevance. The recent SPRINT trial showed that strict blood pressure control is superior to modest blood pressure lowering.33 Long-lived hypertension-specific TEM cells in the bone marrow and secondary lymphoid organs could place humans at risk for enhanced blood pressure elevation and renal damage in response to stimuli like salt, stress or angiotensin II for many years after an initial challenge. Thus, rigorous treatment of hypertension and efforts to prevent these repeated hypertensive exposures might be necessary to prevent reactivation of these cells and limit their injurious effects. Likewise it is known that transient hypertension during pregnancy places women at risk for cardiovascular events later in life via mechanisms that are poorly understood.34, 35 It is interesting to speculate that such an insult could lead to formation of long-lived TEM cells that can persist for decades and promote vascular and renal disease upon reactivation.
Supplementary Material
Novelty and Significance.
What Is Known?
Increasing evidence has implicated adaptive immunity in the genesis of hypertension.
A cardinal feature of immunity is the development of memory, which allows rapid, robust response to repeated antigen exposure.
What New Information Does This Article Contribute?
Repeated hypertensive stimuli lead to accumulation of effector memory T cells in the kidney, and these cells are major sources of IL-17A and IFN-γ.
Effector memory T cells also accumulate in the bone marrow and can be reactivated by salt feeding.
The formation and accumulation of effector memory T cells in the kidney and bone marrow is dependent on the presence of CD70 on antigen-presenting cells. Mice lacking CD70 develop blunted hypertension and renal dysfunction in response to a second hypertensive stimulus.
Increasing evidence supports a role of T cells in the genesis of hypertension. Memory T cells can live for decades in humans and might predispose to blood pressure elevations and end-organ damage upon exposure to even mild hypertensive stimuli. We used two models of repeated hypertensive stimulation, L-NAME followed by high salt exposure and high dose followed by low dose ang II infusion, and showed that the hypertensive response to the second stimulus in both of these models is dependent on reactivation of memory T cells. We found that effector memory T cells accumulate in the kidney and bone marrow, and upon repeated salt feeding expand in the kidney, promoting renal dysfunction and severe hypertension. Mice lacking CD70, which is critical for formation of memory T cells were resistant to the second hypertensive stimulus. These studies provide a previously unidentified role of memory T cells and CD70 in sensitizing the host to even mild repeated stimuli, like salt feeding or low concentrations of angiotensin II. Clinically, these finding might provide rationale for strict blood pressure control to prevent formation of these long-lived pro-hypertensive memory T cells.
Acknowledgments
SOURCES OF FUNDING
This work is supported by National Institutes of Health grants R01HL039006, P01HL058000, P01HL095070, P01GM015431, R01HL108701, R01HL105294, VITA award HHSN268201400010C and the Strategically Focused Research Network Award from the American Heart Association. Dr. Itani was funded by the T32 GM007569 and is the recipient of a National Institute of Health Ruth L. Kirschstein Individual National Research Service Award (1F32HL124972-01).
Nonstandard Abbreviations and Acronyms
- TCM
central memory
- TEM
effector memory
- TRM
resident memory
- D
dendritic cell
- IL
interleukin
- IFN-γ
interferon-γ
- UAGT
urinary angiotensinogen
- UCre
creatinine
- N-GAL
neutrophil gelatinase associated lipocalin
Footnotes
DISCLOSURES
The authors have no conflicts of Interest to disclose.
REFERENCES
- 1.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal CD8+ T Cells Play a Critical Role in the Development of Hypertension. Hypertension. 2014;64:1108–1115. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma−/− and interleukin-17A−/− mice. Hypertension. 2015;65:569–576. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res. 2013;97:696–704. doi: 10.1093/cvr/cvs422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG. Inflammation and Mechanical Stretch Promote Aortic Stiffening in Hypertension Through Activation of p38 Mitogen-Activated Protein Kinase. Circ Res. 2014;114:616–625. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 8.Denoeud J, Moser M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J Leukoc Biol. 2011;89:195–203. doi: 10.1189/jlb.0610351. [DOI] [PubMed] [Google Scholar]
- 9.Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. The CD4(+) T-cell help signal is transmitted from APC to CD8(+) T-cells via CD27-CD70 interactions. Nat Commun. 2012;3:1–9. doi: 10.1038/ncomms1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M, Radbruch A. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Satou R, Miyata K, Gonzalez-Villalobos RA, Ingelfinger JR, Navar LG, Kobori H. Interferon-gamma biphasically regulates angiotensinogen expression via a JAK-STAT pathway and suppressor of cytokine signaling 1 (SOCS1) in renal proximal tubular cells. FASEB J. 2012;26:1821–1830. doi: 10.1096/fj.11-195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934–946. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma'ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, 2nd, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J, 2nd, Osborn JW, Itani HA, Harrison DG. Renal Denervation Prevents Immune Cell Activation and Renal Inflammation in Angiotensin II-Induced Hypertension. Circ Res. 2015;117:547–557. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coers W, Vos JT, Van der Meide PH, Van der Horst ML, Huitema S, Weening JJ. Interferon-gamma (IFN-gamma) and IL-4 expressed during mercury-induced membranous nephropathy are toxic for cultured podocytes. Clinical and experimental immunology. 1995;102:297–307. doi: 10.1111/j.1365-2249.1995.tb03781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 18.Sercan Alp O, Durlanik S, Schulz D, McGrath M, Grun JR, Bardua M, Ikuta K, Sgouroudis E, Riedel R, Zehentmeier S, Hauser AE, Tsuneto M, Melchers F, Tokoyoda K, Chang HD, Thiel A, Radbruch A. Memory CD8(+) T cells colocalize with IL-7(+) stromal cells in bone marrow and rest in terms of proliferation and transcription. Eur J Immunol. 2015;45:975–987. doi: 10.1002/eji.201445295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marko L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Muller DN. Interferon-gamma signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension. 2012;60:1430–1436. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- 21.Garcia AG, Wilson RM, Heo J, Murthy NR, Baid S, Ouchi N, Sam F. Interferon-gamma ablation exacerbates myocardial hypertrophy in diastolic heart failure. Am J Physiol Heart Circ Physiol. 2012;303:H587–596. doi: 10.1152/ajpheart.00298.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Donnell VB, Chumley PH, Hogg N, Bloodsworth A, Darley-Usmar VM, Freeman BA. Nitric oxide inhibition of lipid peroxidation: kinetics of reaction with lipid peroxyl radicals and comparison with alpha-tocopherol. Biochemistry. 1997;36:15216–15223. doi: 10.1021/bi971891z. [DOI] [PubMed] [Google Scholar]
- 23.Le Brocq M, Leslie SJ, Milliken P, Megson IL. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1631–1674. doi: 10.1089/ars.2007.2013. [DOI] [PubMed] [Google Scholar]
- 24.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 25.Kopkan L, Hess A, Huskova Z, Cervenka L, Navar LG, Majid DS. High-salt intake enhances superoxide activity in eNOS knockout mice leading to the development of salt sensitivity. Am J Physiol Renal Physiol. 2010;299:F656–663. doi: 10.1152/ajprenal.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, Romani L, Puccetti P. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5:1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 27.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1136–1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 2013;304:R407–414. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106:100–105. doi: 10.1161/01.cir.0000020222.63035.c0. [DOI] [PubMed] [Google Scholar]
- 31.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest. 2015;125:1189–1202. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santisteban MM, Ahmari N, Marulanda Carvajal J, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J. Involvement of Bone Marrow Cells and Neuroinflammation in Hypertension. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.117.305853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright JT, Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, Lawlor DA. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125:1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skjaerven R, Wilcox AJ, Klungsoyr K, Irgens LM, Vikse BE, Vatten LJ, Lie RT. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ. 2012;345:e7677. doi: 10.1136/bmj.e7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.