Abstract
Motor function in mammalian species depends on the maturation of spinal circuits formed by a large variety of interneurons that regulate motoneuron firing and motor output. Interneuron activity is in turn modulated by the organization of their synaptic inputs, but the principles governing the development of specific synaptic architectures unique to each premotor interneuron are unknown. For example, Renshaw cells receive, at least in the neonate, convergent inputs from sensory afferents (likely Ia) and motor axons raising the question of whether they interact during Renshaw cell development. In other well-studied neurons, like Purkinje cells, heterosynaptic competition between inputs from different sources shapes synaptic organization. To examine the possibility that sensory afferents modulate synaptic maturation on developing Renshaw cells, we used three animal models in which afferent inputs in the ventral horn are dramatically reduced (Er81(−/−) knockout), weakened (Egr3(−/−) knockout) or strengthened (mlcNT3(+/−) transgenic). We demonstrate that increasing the strength of sensory inputs on Renshaw cells prevents their de-selection and reduces motor axon synaptic density and, in contrast, absent or diminished sensory afferent inputs correlate with increased densities of motor axons synapses. No effects were observed on other glutamatergic inputs. We conclude that the early strength of Ia synapses influences their maintenance or weakening during later development and that heterosynaptic influences from sensory synapses during early development regulates the density and organization of motor inputs on mature Renshaw cells.
Keywords: spinal cord, VGLUT1, VAChT, development, calbindin, parvalbumin, Ia afferent, motoneuron
Renshaw cells receive convergent inputs from Ia afferents and motor axons during early development. In this manuscript we show that strengthening Ia afferent inputs decreases the number of synapses from motor axons and that decreasing Ia afferent inputs increases motor axon synapses.
Therefore we conclude that Ia afferents heterosynaptically modulate motor axon synapse numbers on Renshaw cells during postnatal development.
Neurons receive inputs of different densities and strengths from a variety of sources. These can target the cell soma, proximal or distal dendrites, and even the axon initial segment and their synaptic boutons. The patterns of activity that emerge from the integration by individual neurons of these unique combinations of synapses determine their circuit functions. This cell-type specific organization of synaptic inputs on neurons is therefore central to network function and is a core principle of the neuronal doctrine proposed more than 100 years ago (Cajal, 1899, 1904). However, the mechanisms that control the organization, specificity and balance of synaptic inputs of different origins on individual neurons are understudied.
One well-studied example is the Purkinje cell, which integrates information from two excitatory inputs, climbing and parallel fibers, which respectively originate from inferior olive and cerebellar granular cells (Cajal, 1888). Adult Purkinje cells are innervated by a single climbing fiber that targets the shafts of major dendritic branches, and several thousand parallel fibers that synapse on the spines of more distal dendrites (Palay and Chan-Palay, 1974). At birth, several climbing fibers initially target each Purkinje cell and the excess is subsequently pruned postnatally (Crepel et al., 1976; Watanabe and Kano, 2011). In contrast, parallel fibers first establish synapses on newly formed dendritic branches and later translocate to spines in more distal branches. Homosynaptic and heterosynaptic competition between climbing and parallel fibers modulate maturation towards the final synaptic organization (reviewed in Watanabe, 2008; Miyazaki and Watanabe, 2011). In mutant mice in which granule cell development (weaver, staggerer and reeler) or connectivity between parallel fibers and Purkinje cells (Glurδ2 and cerebelin1 knockouts) is perturbed, multiple innervation by climbing fibers is preserved and their dendritic target area extends to regions normally occupied by parallel fibers. In contrast, experimental situations that diminish climbing fiber activity (tetrodotoxin block) or their synaptic strength (α1A subunit of P/Q-type Ca2+ channel knockout) result in ectopic innervation of proximal dendrites by parallel fibers. It is unknown if similar principles govern the development of synaptic organizations in other neurons, in particular the interneurons that form the local networks in brain and spinal cord.
In the spinal cord, the interneuronal premotor network of the ventral horn controls motoneuron output during motor behaviors and locomotion, and it is well known to undergo extensive maturation during postnatal development. Rhythmic and non-rhythmic motor function matures from relatively spastic limb movements in neonates to the well-coordinated contractions around limb joints of juveniles and adults. This network comprises a variety of interneurons that exhibit differences in the type and strength of the excitatory inputs they receive from various sources (Jankowska, 1992, 2001; Brownstone and Bui, 2010). Despite the importance of this network for correct modulation of movement, we know very little about the mechanisms that select and mature specific inputs on different interneurons.
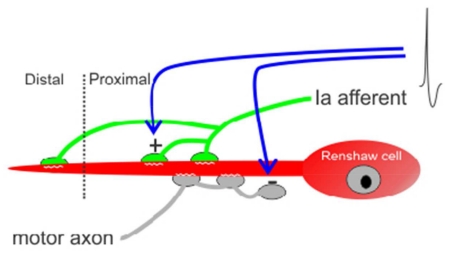
The Renshaw cell (RC) is one example of a specialized spinal interneuron with a relatively well known synaptic architecture and development (Alvarez and Fyffe, 2007; Alvarez et al., 2013). Similar to Purkinje cells, RCs can be identified throughout development by their characteristic location and calbindin-immunoreactivity (Geiman et al., 2000; Siembab et al., 2010). In addition, several different excitatory inputs are distributed along their dendrites while large inhibitory synapses are predominantly found on the soma and most proximal dendritic regions (Alvarez et al., 1997, 1999; Geiman et al., 2000, 2002; Mentis et al., 2005, 2006; Siembab et al., 2010). Renshaw cells are defined functionally by a powerful cholinergic input from motoneurons and by their ability to inhibit those same motoneurons (Renshaw, 1946, Eccles et al., 1954; Van Keulen, 1981). Neonatal RCs receive convergent inputs from motor and sensory axons and both inputs mainly target their dendrites (Mentis et al., 2006). Motor axon synapses on RCs are formed early (E12-E13 in mouse: Alvarez et al., 2013), soon after ventral horn neurogenesis and when little synaptic circuitry has yet been formed. In contrast, proprioceptive sensory inputs on RCs are established in the late embryonic and early postnatal periods (~E18: Mentis et al., 2006), and after a significant amount of connectivity is already established. Both synaptic inputs proliferate on RCs during the first postnatal week but after the second postnatal week, sensory synapses diminish in density on RCs while motor axon synapses continue to proliferate and strengthen (Mentis et al., 2006). The reduction of proprioceptive synapses on RCs correlates with a relatively lower initial density compared to other interneurons like Ia inhibitory interneurons (Siembab et al., 2010). This comparatively lower initial density may relate to their very ventral and slightly medially-shifted location, just at the edge of spinal cord regions containing the majority of Ia afferent axon arbors. A low initial density might influence their later postnatal weakening, but in addition they may play other roles during early postnatal development like shaping the organization of other excitatory synapses on RCs, in particular motor axon cholinergic inputs. To explore these possibilities, we used mouse genetics to systematically vary the density and strength of Ia sensory inputs in the ventral spinal cord and determine the effects of these changes on the density of other excitatory synapses on RCs.
To alter the strength of muscle proprioceptive inputs we took advantage of the fact that the growth of proprioceptive sensory afferents into the ventral horn is regulated by several transcription factors and neurotrophin-3 (NT3). One critical transcription factor expressed by proprioceptive neurons is Er81 (also known as Etv1), a member of the ETS (E twenty-six Transformation Specific) domain family (Lin et al., 1998). Er81(−/−) knockout animals display severe deficits in motor coordination due to the failure of many muscle afferents to project to the ventral horn (Arber et al., 2000; de Nooji et al., 2013). Dorsal root stimulation fails to elicit action potentials in motoneurons in Er81(−/−) mice, demonstrating that the monosynaptic connections of sensory afferents with motoneurons are severely disrupted (Arber et al., 2000). A different transcription factor, Egr3, or early growth response gene 3 is a member of the zinc finger family of transcription factors that is essential for muscle spindle development (Tourtellotte et al., 2001) and maturation of proprioceptive projections in the spinal cord (Chen et al., 2002). Muscle spindles lacking Egr3 fail to develop and differentiate normally causing profound gait ataxia (Tourtellotte and Milbrandt, 1998; Akay et al., 2014; Oliveira Fernandes and Tourtellote, 2015). Peripherally, Ia afferents innervate underdeveloped, abnormal “spindle remnants” (Oliveira Fernandes and Tourtellote, 2015). Centrally, Ia afferent axons initially project to the ventral horn of Egr3(−/−) embryos and neonates and make connections with motoneurons, but thereafter Ia afferent inputs on motoneurons are functionally weaken during postnatal development, possibly because arrested maturation of Ia afferent central arborizations and synapses. It is known that spindle-derived signals maintain and regulate the strength of Ia-motoneuron connections, but not their initial formation (Shneider et al., 2009b). Thus, decreased postnatal expression of NT3 by underdeveloped spindles in Egr3(−/−) mice (Oliveira Fernandes and Tourtellote, 2015) has been suggested to explain the functional deficits observed (Chen et al., 2002). Indeed, injection of NT3 into hindlimbs restores synaptic connectivity between Ia afferents and motoneurons in Egr3(−/−) mice (Chen et al., 2002), emphasizing the importance of NT3 in maintaining or enhancing proprioceptive afferents functional connectivity in the spinal cord (Munson et al., 1997; Taylor et al., 2001; Arvanian et al., 2003). In mlcNT3(+/−) mice, NT3 is constitutively over-expressed by muscle from embryo to adult, which results in supernumerary spindles and an increase in the number of surviving proprioceptors (Wright et al., 2002). In these animals, proprioceptive inputs on motoneurons are strengthened (Wang et al., 2007). In our study we investigated whether proprioceptive input strength on RCs, measured as synaptic densities, is similarly altered in these genetic models and studied whether this modified the developmental downregulation of Ia inputs on RCs (labeled with the VGLUT1 marker: Alvarez et al., 2004; 2011) or the organization of motor axon cholinergic inputs (immunolabeled with VAChT: Alvarez et al., 1999) and other glutamatergic inputs on RCs (labeled with VGLUT2).
Materials and Methods
All animal procedures were performed according to NIH guidelines and reviewed by the local Laboratory Animal Use Committees at Wright State University and NIH.
Animals
Three animal models were used, each with different genetic alterations that either decrease (Er81(−/−)and Egr3(−/−) knockouts) or increase (mlcNT3(+/−) heterozygote) proprioceptive sensory inputs in the ventral horn. Er81 knockout animals were generated by inserting an IRES-taulacZpA-PGK-NEO cassette into exon 11 of the Er81 gene (Arber et al., 2000; RRID: MGI_MGI:2384493). Egr3(−/−)animals were generated by inserting a pMC1NeoA cassette into the Egr3 gene, which leads to deletion of the zinc-finger DNA binding domain and a portion of the C-terminus resulting in a functional null truncated protein (Tourtellotte & Milbrandt, 1998; RRID: MGI_MGI: 3688874). Finally, in mlcNT3(+/−) heterozygotes, NT3 is inserted in the genomic locus of the myosin light chain, a gene highly expressed in muscle fibers (Wright et al., 2002). Therefore, in mlcNT3(+/−) heterozygotes, NT3 expression is elevated during development and retained postnatally throughout adulthood.
Animals were bred at Wright State University (Er81 line was donated by Dr. T.M. Jessell, Columbia University) or NIH (Egr3 and mlcNT3). All mutants displayed significant ataxia, however their survival differed. Er81(−/−) animals usually died in the third postnatal week, Egr3(−/−) survive until adulthood but do not breed, mlcNT3(+/−) heterozygotes, although ataxic, survive and breed normally.
Tissue Preparation
Er81(−/−), Egr3(−/−) and mlcNT3(+/−) mice of different ages were deeply anesthetized with Nembutal (50 mg/kg, i.p.) and perfused transcardially with 4% paraformaldehyde in 0.1M phosphate buffer pH 7.4 (PB). The spinal cords were postfixed in the same fixative, usually overnight, and then placed in a cryoprotective solution of 15% or 30% sucrose and 0.01% sodium azide in 0.1M PB until the tissue was processed. Transverse sections from lumbar 4 and 5 spinal cord segments were acquired using a freezing sliding microtome (40 to 50 μm) or cryostat (20 μm). Tissue sections collected from the freezing sliding microtome were processed free-floating and those collected from the cryostat were processed on slides.
Antibody and immunostaining characterization (see Table 1 for further details and dilutions)
Table 1. Antibodies used in this study.
| Antigen | Immunogen | Host/Type | Manufacturer | Dilution |
|---|---|---|---|---|
| Calbindin | Rat recombinant Calbindin D28k |
Rabbit polyclonal |
Swant (Bellizona, Switzerland), cat. no. CB-38a, lot no. 9.03, |
1:2000-1:5000 |
| NeuN | Purified cell nuclei from mouse brain |
Mouse monoclonal |
Chemicon (Temecula, CA), cat. no. MAB377B, lot no.25040759, |
1:500-1:1,000 |
| Parvalbumin | Purified from frog muscle |
Mouse monoclonal |
Chemicon (Temecula, CA), cat. no. MAB1572, lot no. 070105877, |
1:1000 |
| VAChT | Synthetic peptide from rat VAChT (20 aa: 511-530) |
Goat polyclonal |
Chemicon (Temecula, CA), cat. no. AB1578, lot no.0602021588, |
1:1000-1:2000 |
| VGLUT1 | Synthetic peptide from rat VGLUT1 (18 aa: 542-560) |
Guinea pig polyclonal |
Chemicon (Temecula, CA), cat. no. AB5905, lot no. 0507005922 |
1:1000-1:2000 |
| VGLUT2 | Purified recombinant protein of rat VGLUT2 (72 aa: 510-582) |
Guinea pig polyclonal |
Synaptic Systems (Goettingen, Germany), cat. no. 135 404 lot no. 135404/3 |
1:1000 |
Abbreviations: NeuN, Neuronal Nuclear Protein; VAChT, Vesicular Acetylcholine Transporter; VGLUT1, Vesicular Glutamate Transporter 1; VGLUT2, Vesicular Glutamate Transporter 2;
Parvalbumin antibody
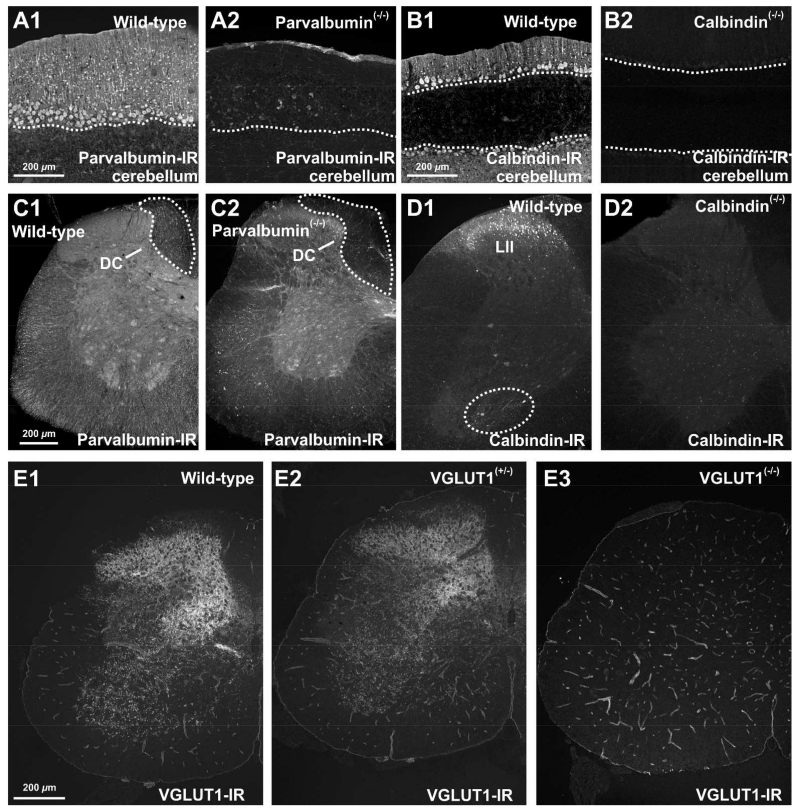
A mouse monoclonal antibody raised against parvalbumin purified from frog muscle was used (Chemicon, Mouse anti Parvalbumin; MAB1572; RRID: AB_2174015). In western blots of mouse brain lysates this antibody detects a single 12 kDa band (manufacture’s information). Immunolabeling with parvalbumin produced labeling patterns identical to those reported previously by us (Alvarez et al., 2005; Siembab et al., 2010) and others (reviewed in Ren and Ruda, 1994) and this immunostaining was abolished in spinal cord sections of parvalbumin knockout mice (Figure 1A,C).
Figure 1. Specificity of calbindin, parvalbumin and VGLUT1 antibodies tested in knockout tissues.
All images are in epifluorescence captured at low magnification with a digital camera. with All images are wide-field epifluorescence captured at low magnification with a digital camera. A-B, Parvalbumin and calbindin-immunoreactivity in the cerebellum of wild-type (A1 and B1), parvalbumin knockout (A2) and calbindin knockout (B2) adult mice. Parvalbumin (A1) and calbindin (B1) immunoreactivities are revealed in Purkinje cells of adult wild-type mice and abolished in the cerebellum of their respective knockout animals (A2, B2)(dashed line indicate the Purkinje cell layer). C-D, Parvalbumin and calbindin-immunoreactivities in the spinal cord of adult wild-type (C1 and D1), parvalbumin knockout (C2) and calbindin knockout (D2) mice. Parvalbumin-IR afferent axons are visible in the dorsal columns (DCs) of the spinal cord of wild-type mice (C1, outlined area), but not in parvalbumin knockout mice (C2). Calbindin antibodies reveal in wild-type spinal cords a large number of immunoreactive cells in lamina II (LII, D1) and in the Renshaw cell area (ventral dashed outline, D1). No immunoreactive neurons are observed in spinal cord sections from knockout animals (D2). E, VGLUT1-immunoreactivity in the spinal cord of wild-type (E1), VGLUT1 heterozygote (E2) and VGLUT1 knockout (E3) mice. A normal pattern of VGLUT1-IR boutons is present in spinal cords from wild type (E1) and heterozygotes (E2). There are no VGLUT1-IR boutons detected in VGLUT1 knockout mice (E3). Some blood vessel immunolabeling remains in knockout mice indicating it is due to a cross-reaction of the primary antibody (it is not revealed by secondary antibodies applied to the section in the absence of primary antibodies). This relatively weaker immunoreaction of blood vessels did not interfere with the identification of VGLUT1-IR synaptic puncta on spinal neurons and was variable from animal to animal. It is also a particular feature of the guinea-pig antibody against VGLUT1 we used since other anti-VGLUT1 antibodies raised in different species (rabbit) did not show blood vessel labeling. We used guinea pig antibodies to better combine with other rabbit-raised primary antibodies for dual and triple immunfluorescence. Scale bars: 200 μm in A1, B1, C1, D1 and E1.
Calbindin antibody
A polyclonal antibody raised in rabbit against recombinant calbindin D-28K was used (Swant, Rabbit anti-Calbindin; CB38; RRID: AB_10000340). This antibody detects a single 28 kDa band in western blots of brain homogenates from various species including rat, rabbit, guinea pig, and mouse (manufacture’s information). The antibody produces no staining in spinal cord sections of calbindin D-28k knockout mice (Figure 1B,D). Spinal cord sections immunostained with this antibody display staining patterns identical to those previously described by us (Alvarez et al., 1999; Geiman et al., 2000; 2002; Siembab et al., 2010) and others (reviewed in Ren and Ruda, 1994; Watson et al., 2009), using a variety of monoclonal and polyclonal antibodies. Calbindin mRNA expression in calbindin-IR Renshaw cells revealed with this antibody has been confirmed with in situ hybridization (Wootz et al., 2013).
VAChT antibody
The antibody used was a polyclonal antibody raised in goat against a recombinant 20 aa peptide in the C-terminus region of the rat VAChT (aminoacids 510-530, Chemicon, Guinea Pig anti-VAChT, AB1588; RRID AB_2187981). The VAChT antibody produced patterns of staining identical to those previously reported by us (Alvarez et al., 1999; Siembab et al., 2010) and others (Arvidsson et al., 1997; Hellstrom et al., 1999). In dual immunostains this VAChT antibody labeled exactly the same terminals as other VAChT antibodies raised in different species against different epitopes (Siembab et al., 2010). This antibody labeled the same neurons shown to express VAChT with in situ hybridization (Wootz et al., 2013).
VGLUT1 antibody
We used a polyclonal antibody raised in guinea pig against an 18 aminoacid synthetic peptide from the rat VGLUT1 sequence (aa: 542-560) (Chemicon, Guinea pig VGLUT1; AB5905; RRID: AB_2301751). This antibody detected a single 62 kDa band in western blots of rat brain lysates (manufacture’s information). The laminar distribution of VGLUT1-IR puncta is similar to that previously reported by us (Alvarez et al., 2004; Mentis et al., 2006; Siembab et al., 2010) and others (Todd et al., 2003; Oliveira et al., 2003) with this antibody or different rabbit polyclonal antibodies. All synaptic puncta immunostaining was abolished in spinal cord sections from VGLUT1 knockout animals (Figure 1E; a weak cross-reaction with blood vessels remained, but did not interfere with our synaptic analyses).
VGLUT2 antibody
In this study we used a polyclonal antibody raised in guinea pig against a recombinant peptide encompassing 72 aminoacids (510-582) of the rat VGLUT2 sequence (Synaptic Systems, Guinea pig anti-VGLUT2; Cat#135 404; RRID:AB_887884). In western blots of rat brain synaptic vesicle fractions, this antibody detects a single 52 kDa band. Spinal cords immunolabeled with the VGLUT2 antibody used in this study produced a pattern of staining similar to those previously reported by us (Alvarez et al., 2004) and others (Todd et al., 2003; Oliveira et al., 2003) with this antibody or different rabbit and guinea pig polyclonal antibodies.
NeuN antibody
A mouse monoclonal antibody (Chemicon, Mouse monoclonal NeuN MAB377B; AB_2314891) raised against isolated mouse brain nuclei (clone mAB A60: Mullen et al., 1992) was used. In western blots mAB A60 recognizes a 40-50 kDa band as well as a ~70 kDa band (Kim et al., 2009; Dredge and Jensen, 2011). The latter corresponds with synapsin I and is abolished by paraformaldehyde fixation; the former corresponds to the RNA splicing regulator fox3 (Rbfox3; feminizing on X) that is expressed in most differentiated neurons, with few exceptions. In the ventral horn NeuN is strongly expressed by interneurons and α-motoneurons, but not γ-motoneurons (Shneider et al., 2009a). Our labeling of spinal cord cell bodies is identical to that reported by us (Alvarez et al., 2004, 2005) as well as others (e.g., Watson et al., 2009). In sections obtained from fixed spinal cords or brains NeuN mAB A60 antibody never recognizes synapsin I puncta.
VGLUT1-immunoreactivity on motoneurons in Er81(−/−), Egr3(−/−) and mlcNT3(+/−) mice
The distribution and density of VGLUT1-immunoreactive boutons contacting motoneuron somata or in the lamina IX neuropil was examined in P20 Er81(−/−), Egr3(−/−) and mlcNT3(+/−) mice (n=2 mutant and 2 control wild-type littermates per transgenic line at P20; n=2 mutants and 2 wild-type littermates at P15 for Er81(−/−) comparisons). Spinal cord sections from lumbar segments 4 and 5 were first blocked with a 10% normal donkey serum diluted in 0.01M PBS with 0.3% Triton X-100 (PBS-T 0.3%; pH 7.4) and then processed for dual immunofluorescence using antibodies against the vesicular glutamate transporter 1 (VGLUT1; guinea pig polyclonal antibody) and neuronal nuclei (NeuN; mouse monoclonal antibody). Following incubation overnight at 4°C in the primary antibody mixture, the immunoreactive sites were revealed using species-specific fluorochrome-conjugated secondary antibodies (FITC for VGLUT1; Cy3 for NeuN) raised in donkey (diluted 1:50 in PBS-T 0.3%; Jackson Laboratories, West Groove, Pennsylvania, US). Sections were then mounted on gelatin-coated slides and cover-slipped with Vectashield (Vector, Burlingame, California, US). Immunofluorescent preparations were imaged with an Olympus FX confocal microscope (Olympus Optical, Tokyo, Japan; excitation lines: argon laser, 488 nm; krypton laser, 568 nm). Sections were first imaged at low magnification (20×1) and from these low magnification confocal images, NeuN-IR motoneurons were randomly selected and imaged using a 60× oil objective (NA 1.35). Series of confocal optical sections (z-step 0.5 μm) were obtained throughout the cell body. VGLUT1-IR synaptic densities were calculated in ~15-20 motoneuron somata per animal. Each motoneuron was sampled at several mid-cell body optical cross sections separated by at least 3-5 μm in the z-axis such that individual boutons were not counted twice. The number of boutons contacting the motoneuron cell body in each optical section was normalized against cell body perimeter and 3-5 estimates per cell were averaged to obtain an estimate of linear density per 100 μm of soma perimeter. In the same confocal image 100 μm2 regions of interest (ROIs) were randomly placed in the lamina IX neuropil and the density of VGLUT1-IR puncta in the neuropil determined. The average densities of contact on motoneurons or immunoreactive boutons in the neuropil ROIs were compared in each mutant animal against a control wild-type average (pooling all motoneurons from both wild type littermates in each line/age) using one-way ANOVA followed by Bonferroni corrected t-tests (SigmaStat V. 2.0, Jandel, Sausalito, California).
Effects of altering primary afferent input on the expression of calcium buffering proteins, calbindin and parvalbumin
Serial sections from the same P20 spinal cords described before were processed for calbindin or parvalbumin immunofluorescence. In addition, a developmental time series was used in the Er81 line where we analyzed two mutant and two wild types at P5, P10 and P15 to test whether there are developmental delays in calcium buffering protein expression regulation when no primary afferents invade the ventral horn. After blocking the sections with 10% normal donkey serum they were incubated overnight in either calbindin (CB; rabbit polyclonal antibody) or parvalbumin (PV; mouse monoclonal antibody) and immunoreactive sites revealed using donkey raised species-specific secondary antibodies coupled to Cy3 (1:50, Jackson Laboratories). Sections were then mounted and cover-slipped as before and analyzed in Neurolucida (MicroBrightField, Colchester, Vermont; RRID: nif_0000_10294) using a BX50 Olympus microscope coupled to a digital color camera (Microfire CCD, Optronics, Goleta, California). The sections were visualized at low magnification and the ventral horn traced following the gray-white matter border. A horizontal line passing through the dorsal tip of the central canal was used as the border between dorsal and ventral spinal cord. All immunofluorescent cells in the ventral horn were plotted at high magnification (40×). We analyzed an average of 14.3 ± 3.8 ventral horns per animal. Only neuronal profiles with their somata fully contained within the 40 μm thick section were counted. In order to determine whether there were any differences in the number of calbindin-IR Renshaw cells, we selected the subset of calbindin-IR cells within 250 μm of the ventral border between gray and white matter for analyses. Since expression of these calcium buffering proteins is developmentally regulated (Zhang et al., 1990; Siembab et al., 2010) and because each mouse line might show small differences in their developmental growth, we compared the two mutant animals against a control wild-type average from each line (obtained from 2 wild-type littermates). One-Way ANOVAs followed by Bonferroni corrected t-tests were used to detect significant differences in cell numbers between both mutant animals and the wild-type average.
Confocal analysis of synaptic marker contact densities on mature calbindin-IR Renshaw cells in wild-type, Er81(−/−), Egr3(−/−) and mlcNT3(+/−) mice
Spinal cord sections of Er81(−/−), Egr3(−/−) and mlcNT3(+/−) animals at P15, P20, and spinal cord of Egr3(−/−) and mlcNT3(+/−) adult animals (>P40) were immunolabeled for calbindin (to identify Renshaw cells) VGLUT1 (to identify primary afferent synapses) VAChT (to identify motor axon synapses) or VGLUT2 (to identify glutamatergic excitatory synapses from mainly local spinal interneurons). Sections were blocked with normal donkey serum and then processed for dual or triple-immunofluorescence by incubating overnight in one of the following primary antisera mixtures: calbindin/VAChT, calbindin/VGLUT1, calbindin/VAChT/VGLUT1, or calbindin/VGLUT2. Immunoreactive sites were revealed using species-specific fluorochrome-conjugated donkey secondary antibodies (1:50, Jackson Laboratories). Calbindin was always revealed with Cy3 and synaptic markers with FITC. In triple-fluorescent preparations VAChT immunoreactivity was visualized with Cy5.
Synaptic densities were obtained by reconstructing calbindin-IR RCs using Neurolucida (RRID: nif_0000_10294). Briefly, low magnification (20×1) confocal images were obtained and RCs randomly sampled for higher magnification scans. Selected RCs were imaged (60× oil N.A., 1.35 digitally zoomed 1.5×) in series of confocal optical sections (z-step 0.5 μm) obtained throughout their cell bodies and dendritic arbors within the field of view and thickness of the tissue sections. The confocal stacks were loaded in Neurolucida and the cells reconstructed in 3D. The accuracy of detecting contacts was confirmed by rendering the surfaces of immunoreactive boutons and dendrites in 3D and rotating them using the Imaris program (Bitplane, Concord, Massachusetts; RRID: nif-0000-00314) (Figs. 4, 6, 8). Synaptic contacts were plotted on reconstructed dendritic arbors and contact densities calculated as number of contacts per 10 μm of linear dendrite. In addition we performed Sholl analyses to detect any possible differential changes at different distances from the cell body. We did not detect significant differences among estimates obtained from RCs sampled from wild types in the different lines. Therefore one single pooled value was used as control value at each age. Sample characteristics are summarized in Tables 3 and 4.
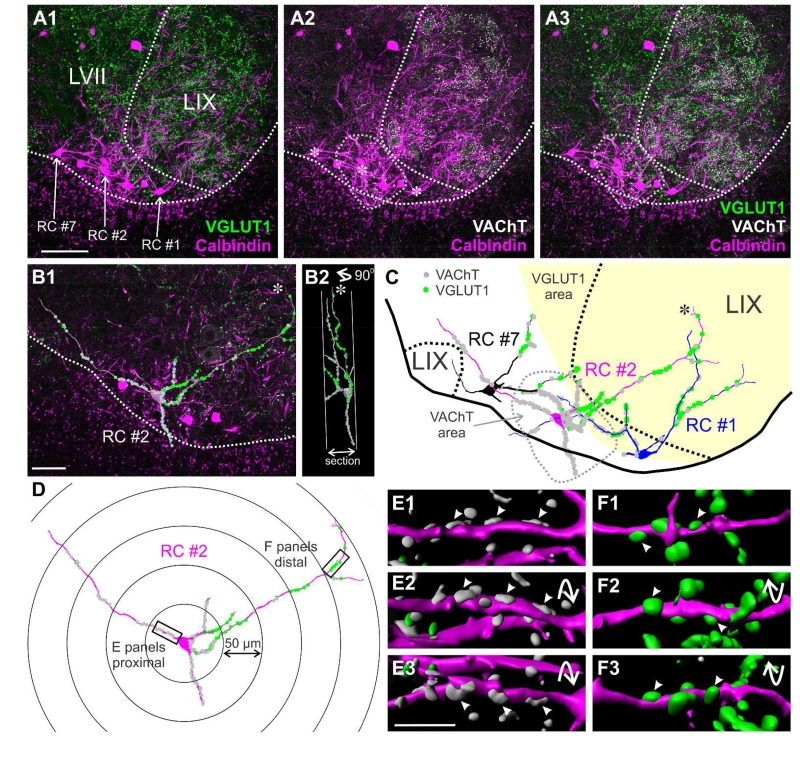
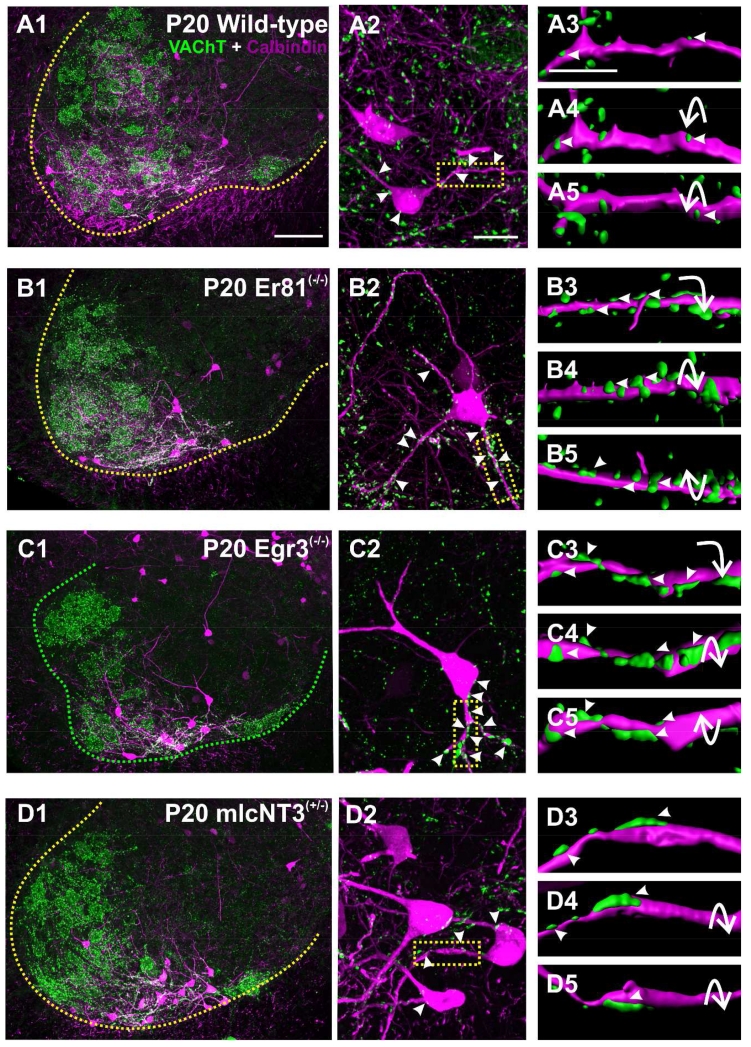
Figure 4. Normal distribution of VGLUT1 and VAChT contacts on calbindin-IR Renshaw cells.
A1, A2, A3, Triple immunofluorescence for calbindin (magenta, Cy3), VGLUT1 (green, FITC) and VAChT (white, Cy5). The images are 2D projections of 4 optical sections (obtained at 20×; 6 μm depth) and show all three immunoreactivities in different combinations. The location of three RCs reconstructed in following panels is indicated in A1 and with asterisks in A2. White dashed lines indicate the edge of the spinal cord gray matter and the boundary between lamina IX and VII. Green dashed line indicates the region with high density of VGLUT1-IR puncta. The dashed grey line indicates the region with the highest concentration of motor axon-derived VAChT-IR synaptic boutons. Each of the three RCs are located in different positions with respect to regions with high density VGLUT1-IR or VAChT-IR boutons. B1, Full reconstruction of RC#2 superimposed on a single optical section (through the RC2 cell body) from a high magnification (60×) tiled image. Contacts from VAChT-IR and VGLUT1-IR boutons are indicated as grey and green circles, respectively. Dashed line is the ventral border of the spinal cord gray matter. B2, Ninety degree rotation of RC#2 showing the section thickness. Dendrites oriented in the longitudinal rostro-caudal axis intersect the section surface faster than dendrites in more or less transverse orientations. Some transversally oriented dendrites reach their natural terminations within the section and usually end with small bi- or trifurcations (see asterisk in B1, B2 and also C). C, Reconstructions of three RCs with VGLUT1-IR (green dots) and VACHT-IR boutons (grey dots) mapped on their dendrites. VAChT-IR contacts are denser on dendrite segments coursing through the VAChT area, while VGLUT1-IR contacts are more frequent in dendrites entering the VGLUT1 area. D, Full reconstruction of RC#2 within Sholl circles. Two dendritic areas are boxed; one is in the first 50 μm Sholl (proximal) from the cell body center and the other at 200-250 Sholl distance (distal). E1, E2, E3 and F1, F2, F3 are Imaris surface renderings of these dendritic segments in three different rotations. Arrowheads indicate different views of the same contacts. The proximal segment (E) has a high density of VAChT-IR contacts, while the distal segment (F) receives predominantly VGLUT1-IR contacts. Scale bars: 100 μm in A1, 50 μm in B1 and 10 μm in E3 (F panels are at the same magnification as E).
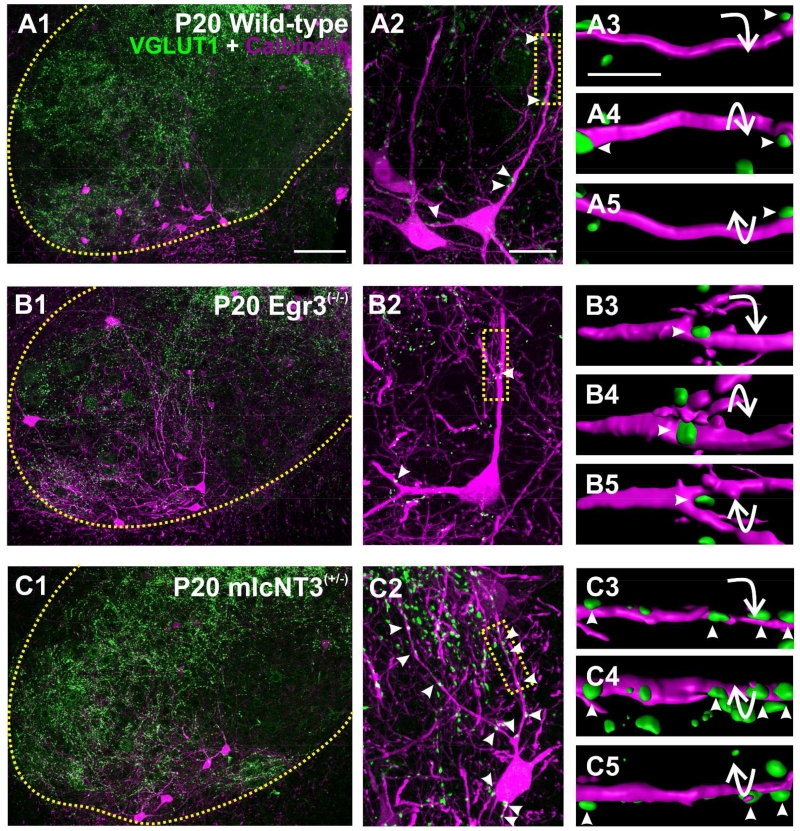
Figure 6. VGLUT1-immunoreactive contacts on Renshaw cells in the ventral horn of wild-type, Egr3(−/−) and mlcNT3(+/−) P20 mice.
A1, B1, C1, Low magnification confocal images of VGLUT1-IR boutons (green, FITC) and CB-IR RCs (magenta, Cy3) in the ventral horn at P20 in wild-type (A1), Egr3(−/−) (B1) and mlcNT3(+/−) (C1) animals. The yellow dotted line indicates the border between the ventral horn and the white matter. A2, B2, C2, Superimposed VGLUT1-IR (green) and CB-IR neurons (magenta, Cy3). Boxes indicate areas shown at high magnification in A3, B3, and C3. A3-5, B3-5, C3-5, VGLUT1-IR boutons on P20 CB-IR RCs surface rendered in Imaris software and shown in three different rotations. Arrowheads indicate the same VGLUT1-IR contacts on RC dendrites at three different rotations. Compared to wild-types there are fewer VGLUT1-IR contacts in Egr3(−/−) RCs and more in mlcNT3(+/−). Scale bars: 100 μm in A1 (A1-A2, B1-B2 and C1-C2 are all at the same magnification); 20 μm in A3 (A3, B3 and C3 are at the same magnification).
Figure 8. VAChT-immunoreactive contacts on Renshaw cells in the ventral horn of wild-type, Er81(−/−) Egr3(−/−), and mlcNT3(+/−) P20 mice.
A1, B1, C1, D1, Low magnification confocal images of VAChT-IR boutons (green, FITC) and CB-IR RCs (magenta, Cy3) in the ventral horn of a P20 wild-type (A1), Er81(−/−) (B1), Egr3(−/−) (C1), and mlcNT3(+/−) (D1) animal. VAChT-IR staining is increased in LIX and in the RC area of Egr3(−/−) and Er81(−/−) mice compared to wild-types. The yellow dotted line indicates the border between the ventral horn and white matter. A2, B2, C2, D2, Superimposed VAChT-IR (green) and CB-IR (magenta, Cy3). Boxes indicate areas of high magnification shown in A3, B3, C3, and D3. A3-5, B3-5, C3-5, D3-5, VAChT-IR contacts on P20 CB-IR RCs surface rendered in Imaris software and shown in three different rotations. Arrowheads indicate VAChT-IR contacts on RC dendrites, shown in different rotations. There appears to be more VAChT-IR contacts on RCs in Er81(−/−) (B3) and Egr3(−/−) (C3) mice compared to wild-type control (A1) animals. In contrast, RCs in mlcNT3(+/−) (D3) mice have fewer VAChT-IR contacts than wild-types. Scale bars: Scale bars: 100 μm in A1 (A1-A2, B1-B2 C1-C2 and D1-D2 are all at the same magnification); 20 μm in A3 (A3, B3 C3 and D3 are at the same magnification).
Table 3. VGLUT1-IR contact densities on Renshaw cells.
| P15 | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Animal Model |
Changes in VGLUT1 |
VGLUT1- IR contacts per 10 μm of dendrite (Average ± SEM) |
Percent change compared to wildtype |
P value (*, significant) t-test |
Total # of VGLUT1-IR contacts per cell (Average ± SEM) |
N (# animals, # Renshaw cells) |
|
| ||||||
| Wild-Type | 1.35 ± 0.15 | 26.72 ± 0.68 | 5, 57 | |||
| Er81 (−/−) | No VGLUT1 |
N/A | N/A | N/A | N/A | 3, 31 |
| Egr3 (−/−) | ↓ | 0.96 ± 0.16 | 30% ↓ | *p<0.001 | 21.08 ± 0.75 | 3, 37 |
| mlcNT3 (+/−) | ↑ | 1.76 ± 0.21 | 30% ↑ | *p<0.001 | 39.83 ± 0.99 | 3, 40 |
|
| ||||||
| P20 | ||||||
|
| ||||||
| Wild-Type | 0.96 ± 0.13 | 18.28 ± 0.57 | 5, 57 | |||
| Er81 (−/−) | No VGLUT1 |
N/A | N/A | N/A | N/A | 3, 37 |
| Egr3 (−/−) | ↓ | 0.83 ± 0.16 | 14% ↓ | *p<0.009 | 16.81 ± 0.72 | 3, 34 |
| mlcNT3 (+/−) | ↑ | 1.99 ± 0.24 | 107% ↑ | *p<0.001 | 35.89 ± 1.01 | 3, 35 |
|
| ||||||
| Adult | ||||||
|
| ||||||
| Wild-Type | 0.98 ± 0.15 | 20.18 ± 0.68 | 4, 44 | |||
| Er81 (−/−) | N/A | N/A | N/A | N/A | N/A | N/A |
| Egr3 (−/−) | ↓ | 0.70 ± 0.15 | 29% ↓ | *p<0.001 | 14.35 ± 0.68 | 3, 31 |
| mlcNT3 (+/−) | ↑ | 1.97 ± 0.24 | 101% ↑ | *p<0.001 | 37.24 ± 1.06 | 3, 33 |
Table 4. VAChT-IR contact densities on Renshaw cells.
| P15 | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Animal Model |
Changes in VGLUT1 |
VGLUT1- IR contacts per 10 μm of dendrite (Average ± SEM) |
Percent change compared to wildtype |
P value (*, significant) t-test |
Total # of VGLUT1-IR contacts per cell (Average ± SEM) |
N (# animals, # Renshaw cells) |
|
| ||||||
| Wild-Type | 1.45 ± 0.15 | 28.50 ± 0.71 | 5, 57 RCs | |||
| Er81 (−/−) | ↑ | 2.44 ± 0.28 | 70% ↑ | *p<0.001 | 47.97 ± 1.24 | 3, 31 RCs |
| Egr3 (−/−) | ↑ | 1.97 ± 0.24 | 18% ↑ | *p<0.001 | 38.09 ± 1.06 | 3, 34 RCs |
| mlcNT3 (+/−) | ↓ | 1.04 ± 0.16 | 30% ↓ | *p<0.001 | 23.75 ± 0.77 | 3, 40 RCs |
|
| ||||||
| P20 | ||||||
|
| ||||||
| Wild-Type | 1.67 ± 0.16 | 29.62 ± 0.67 | 5, 66 RCs | |||
| Er81 (−/−) | ↑ | 2.06 ± 0.27 | 23% ↑ | *p<0.001 | 38.92 ± 1.03 | 3, 37 RCs |
| Egr3 (−/−) | ↑ | 1.97 ± 0.24 | 18% ↑ | *p<0.001 | 38.09 ± 1.06 | 3, 34 RCs |
| mlcNT3 (+/−) | ↓ | 1.24 ± 0.20 | 27% ↓ | *p<0.001 | 22.08 ± 0.87 | 3, 30 RCs |
|
| ||||||
| Adult | ||||||
|
| ||||||
| Wild-Type | 1.54 ± 0.27 | 30.52 ± 1.21 | 2, 21 RCs | |||
| Er81 (−/−) | N/A | N/A | N/A | N/A | N/A | N/A |
| Egr3(−/−) | ↑ | 2.14 ± 0.26 | 39% ↑ | *p<0.001 | 41.61 ± 1.16 | 3, 31 RCs |
| mlcNT3 (+/−) | ↓ | 1.16 ± 0.19 | 23% ↓ | *p<0.001 | 21.48 ± 0.81 | 3, 33 RCs |
The data usually failed normality criteria and therefore a One-Way ANOVA on Ranks was used to compare synaptic densities on RCs of different ages for each genotype. These tests were followed by a Dunn’s post-hoc test in which pair-wise comparisons were done at each age for each of the mutations to the age-matched wild-type control. A similar ANOVA was used to compare changes in density with age within each genotype. In addition, we correlated the density of VGLUT1-IR and VAChT-IR bouton “contact” densities in individual RCs in a subsample of preparations that were triple immunolabeled for VGLUT1, VAChT and calbindin (Fig. 9C). These data was fitted by linear regression using ClampFit (PClamp; Molecular Devices, Sunnyvale, California; RRID: nif_0000_000085).
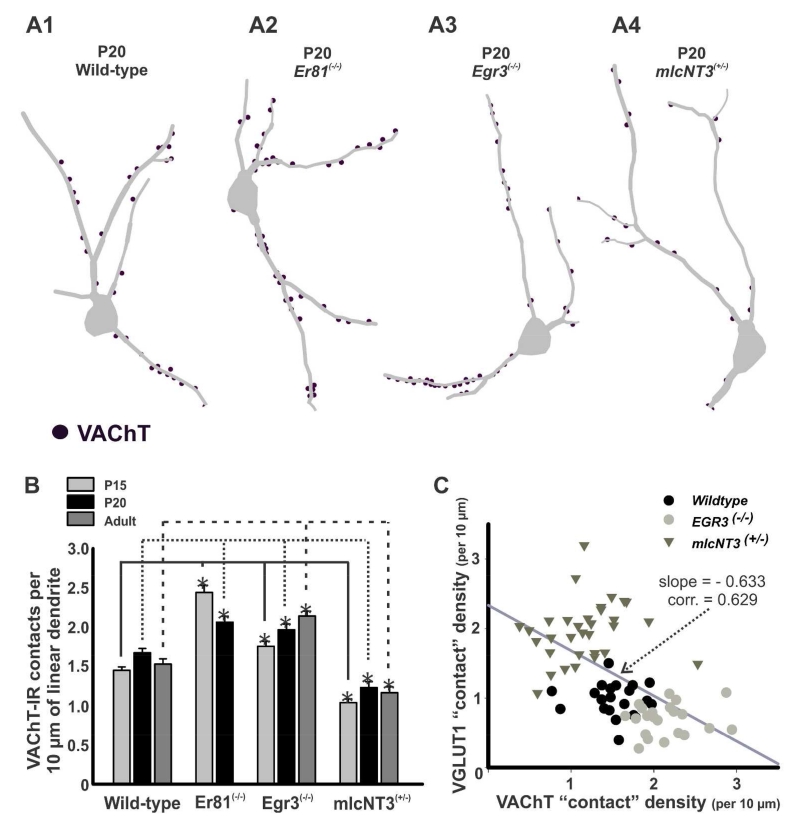
Figure 9. Density of VAChT-immunoreactive contacts on Renshaw cells in wild-type and mutant mice.
A, Three dimensional reconstructions of RCs from P20 wild-type (A1), Er81(−/−) (A2), Egr3(−/−) (A3), and mlcNT3(+/−) (A4) mice. VAChT-IR contacts (dots) are plotted on the traced dendritic arbors. B, Density of VAChT-IR contacts per 10 μm of linear RC dendrite in Er81(−/−), Egr3(−/−), and mlcNT3(+/−) compared to age-matched wild-type controls. During postnatal development RCs from Er81(−/−) animals showed a significant decrease in VAChT-IR contact density from P15 to P20 (p<0.05 in post-hoc Dunn’s comparisons), while it increased in Egr3(−/−) mice from P15 to P20 and adult (p=0.001, one-way ANOVA on Ranks). The density of VAChT-IR contacts was significantly increased at all ages in RCs from Er81(−/−) and Egr3(−/−) animals compared to wild-types (asterisks, p<0.05 in post-hoc Dunn’s comparison). In contrast, VAChT-IR contact densities in mlcNT3(+/−) were significantly lower compared to wild-type animals (asterisks, P15 and P20, p<0.05 in post-hoc Dunn’s comparisons). C, The density of contacts from VGLUT1-IR and VAChT-IR boutons were negatively correlated in a sample population of RCs from preparations in which both synaptic inputs where simultaneously analyzed in single RCs using triple immunofluorescence.
Because dendrite length in these analyses is limited by the field of view at magnifications high enough to discriminate contacts, the analyses we used to compare the effects of the three mutations focused on the first 100 μm of dendritic distance from the RC cell body. To examine the distribution of VGLUT1-IR and VAChT-IR contacts in the full dendritic tree of RCs we sampled eight RCs from three P23 animals processed for triple calbindin, VGLUT1 and VAChT immunofluorescence, but this time we used a motorized stage to tile high magnification 60× confocal optical section stacks throughout the whole ventral horn. Tiles were merged in Neurolucida and RC dendrites and their VGLUT1 and VAChT contacts, traced, reconstructed and mapped as before, joining all segments imaged in different tiles. To analyze the distribution of contacts at different distances from dendrite origins in the cell body, we calculated with Neuroexplorer (MicroBrightField, Colchester, Vermont; RRID: nif-0000-10382) the dendrite path distance location of each contact and estimated the number of contacts, their percentage with respect to each class (VGLUT1 or VAChT) and their density per 100 μm2 of dendrite surface at different distances in the dendrite binned in 50 μm increments. In addition, we correlated changes in number and density of contacts with the locations of ventral horn areas containing high or low densities of VGLUT1-IR or VAChT-IR boutons.
Figure Composition
Figures were composed using CorelDraw (v. 16.0) and graphs were created in SigmaPlot (ver. 12.0, Jandel). Adjustments to images were done only for presentation/publication purposes. All quantification was carried out using unprocessed original images. Images in plates were adjusted for optimal contrast and brightness in Image Pro Plus (ver 5.0 Media Cybernetics, Bethesda, Maryland; RRID: nif_0000_00313). Some images were sharpened using a “high gauss” filter. Manipulations were minimal and the informational content was not altered.
Results
VGLUT1-IR synaptic bouton densities in the ventral horn are altered in mutants with different strengths of proprioceptive inputs
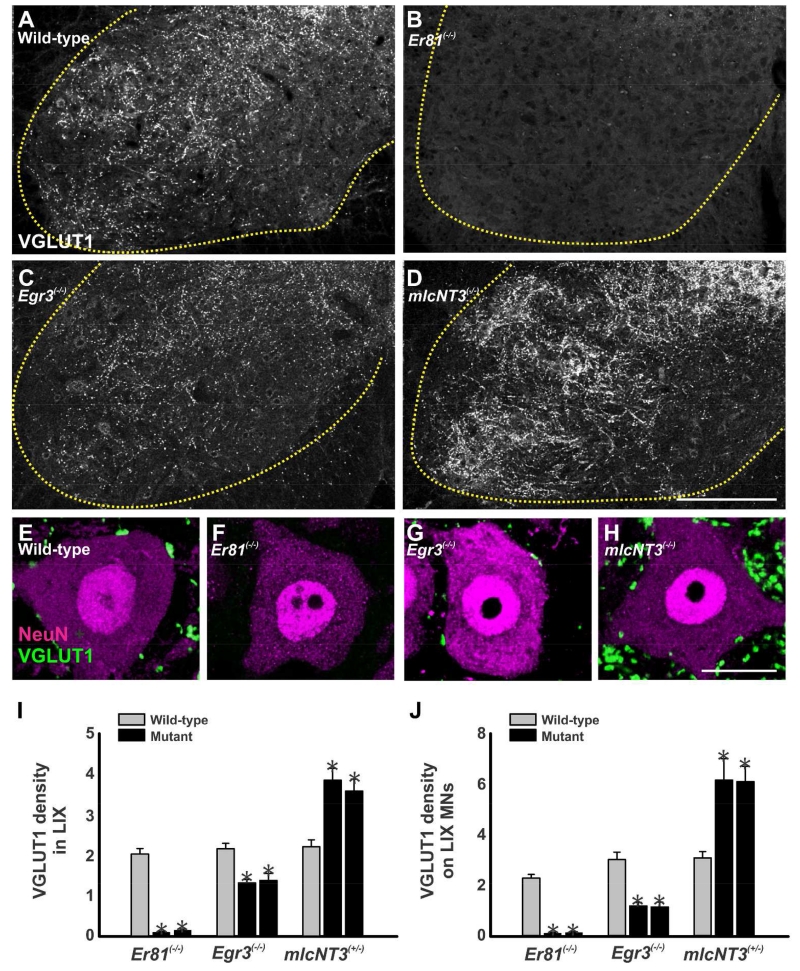
Monosynaptic proprioceptive inputs on motoneurons are absent in Er81 knockout mutants (Arber et al., 2000), weak in Egr3 mutants (Chen et al., 2002) and strengthened in mlcNT3 heterozygous transgenics (Wang et al., 2007). As predicted, these animals showed corresponding variations in the density of VGLUT1-IR varicosities in the ventral horn (Fig. 2A-D), whereas no significant changes were observed in the dorsal horn. VGLUT1 densities were estimated in the Lamina IX (LIX) of 2 mutants of each genotype/age and 2 age-matched wild-type controls (1 wild-type control was analyzed from the Egr3 line). Analyses were done at P20. In addition, we also analyzed P15 Er81(−/−) animals because of the severe ataxia and compromised health we frequently observed at P20 in Er81 mutants (Figure 2 shows only P20 data). The data from two control animals of each transgenic line (or age) were averaged together and VGLUT1-IR densities were estimated for each mutant and compared to the wild-type control average (Fig. 2I). LIX VGLUT1-IR densities were decreased by ~94% of the wild-type average in Er81(−/−) P20 spinal cords and this was similar at P15 (~98% decrease). The density of VGLUT1 varicosities in LIX of Egr3(−/−) mice was also significantly decreased, although to a lesser extent (~38% decrease). In contrast, VGLUT1-IR density increased by ~69% in mlcNT3(+/−) animals compared to their respective controls. In all cases VGLUT1 densities in mutant animals were significantly different from the average wild-type controls, matched for age and mouse line (p<0.001, One-way ANOVA, followed by Bonferroni corrected post-hoc t-test).
Figure 2. Distribution and density of VGLUT1-immunoreactive boutons in LIX and around motoneurons.
A-D, Low magnification confocal images of VGLUT1-immunoreactivity in the ventral horn of wild-type (A), Er81(−/−) (B), Egr3(−/−) (C) and mlcNT3(+/−) (D) animals at P20. The dotted yellow line indicates the border between the ventral grey and the white matter. At P20, VGLUT1-IR boutons are largely absent in the ventral horn of Er81(−/−) mice. Compared to wild-type animals, VGLUT1-immunoreactivity in the ventral horn appears decreased in Egr3(−/−) mice and increased in mlcNT3(+/−) animals. E-H, High magnification images of single confocal planes through the somata of NeuN-immunoreactive motoneurons (magenta, Cy3) and associated VGLUT1-IR boutons (green, FITC) in wild-type (E), Er81(−/−) (F), Egr3(−/−) (G) and mlcNT3(+/−) P20 animals (H). Virtually no VGLUT1-IR boutons were observed around motoneurons in Er81(−/−) mice and these were significantly decreased and of smaller size in Egr3(−/−) mice compared to wild-types. In contrast, the number of VGLUT1-IR varicosities in the neuropil and in contact with motoneurons was significantly increased in mlcNT3(+/−) animals. I, Lamina IX (LIX) neuropil density of VGLUT1-IR puncta (per 100 μm2) in mutant animals compared to wild-type littermates at P20. VGLUT1-IR clusters were nearly absent in the LIX neuropil of Er81(−/−) animals. In Egr3(−/−) animals VGLUT1-IR density was partially reduced, while mlcNT3(+/−) animals showed significant increases in density compared to wild-type littermates (asterisks, p<0.001, One-way ANOVA followed by Bonferroni-corrected t-tests). J, Number of VGLUT1-IR contacts per 100 μm of linear perimeter on NeuN-IR motoneuron somata at P20. Compared to wild-type littermates, VGLUT1-IR contacts on motoneurons were almost absent in Er81(−/−) animals (asterisks, p<0.001, Bonferroni-corrected t-tests) and significantly decreased in Egr3(−/−) mice (asterisk, p=0.015, Bonferroni-corrected t-tests). In contrast, VGLUT1-IR contact density increased in both mlcNT3(+/−) mice compared to wild-type littermates at P20 (asterisks, p<0.001, Bonferroni-corrected t-tests). In all the graphs the error bars represent SEM estimates. Scale bars: 200 μm in D (A to D at the same magnification); 10 μm in E (E to G at the same magnification); 20 μm in H.
The changes in LIX VGLUT1-IR neuropil density were reflected in larger changes in VGLUT1-IR contacts on the cell bodies of NeuN-IR motoneurons (i.e., α-motoneurons; Shneider et al., 2009a). VGLUT1-IR bouton densities were analyzed in 3-5 cell body confocal optical cross-sections (VGLUT1-IR contacts per 100 μm of linear perimeter) in each of 10-19 motoneurons per animal with care taken to allow 3-5 μm separation in the z-axis such that individual boutons were not sampled twice (Fig. 2E-H). Percentages of change in the density of VGLUT1-IR contacts on motoneurons for each mutant animal were calculated against the average density on motoneurons from two age-matched wild-type littermates (only 1 wild-type control was analyzed for the Egr3 line (Fig. 2J). VGLUT1-IR contacts were nearly absent on motoneuron somata of Er81(−/−) mice at P15 (~96% depletion) and P20 (95% decrease). They were also significantly decreased by ~62% in Egr3(−/−) animals. In mlcNT3(+/−) animals they were significantly increased by ~97-100% (Fig. 2J; p<0.001, One way ANOVA followed by Bonferroni corrected t-test comparison vs. control).
In conclusion, the above data show that the densities of VGLUT1-IR synaptic puncta in the ventral horn and the somatic contacts on motoneurons are altered in each of these animals. The decline of VGLUT1-IR contacts is almost complete in Er81(−/−) knockout animals and partial in Egr3(−/−) knockout animals. In contrast, VGLUT1-IR contact density is increased in mlcNT3(+/−) het animals. We also noticed that VGLUT1-IR puncta tend to be smaller in Egr3(−/−) and larger in mlcNT3(+/−) animals. Changes in the size of Ia boutons also affect synaptic strength (Pierce and Mendell, 1993; Shneider et al., 2009b) and influence density estimates because boutons of different sizes have different probabilities of being sampled within the focal depth of any given confocal plane. Thus, the quantitative data presented in figure 1J is best interpreted by a combination of changes in both number and size of boutons, both affecting synaptic strength in the same direction. Changes in input density on the motoneuron cell body are reasonable predictors of variations in the proximal dendritic tree, where most of the Ia afferent input is focused (Rotterman et al., 2014), therefore the changes in VGLUT1-IR contacts on the cell body, not surprisingly, correlate well with reported changes in the strength of monosynaptic sensory inputs on motoneurons in Egr3(−/−) and mlcNT3(+/−) animals (Chen et al., 2002; Wang et al., 2007).
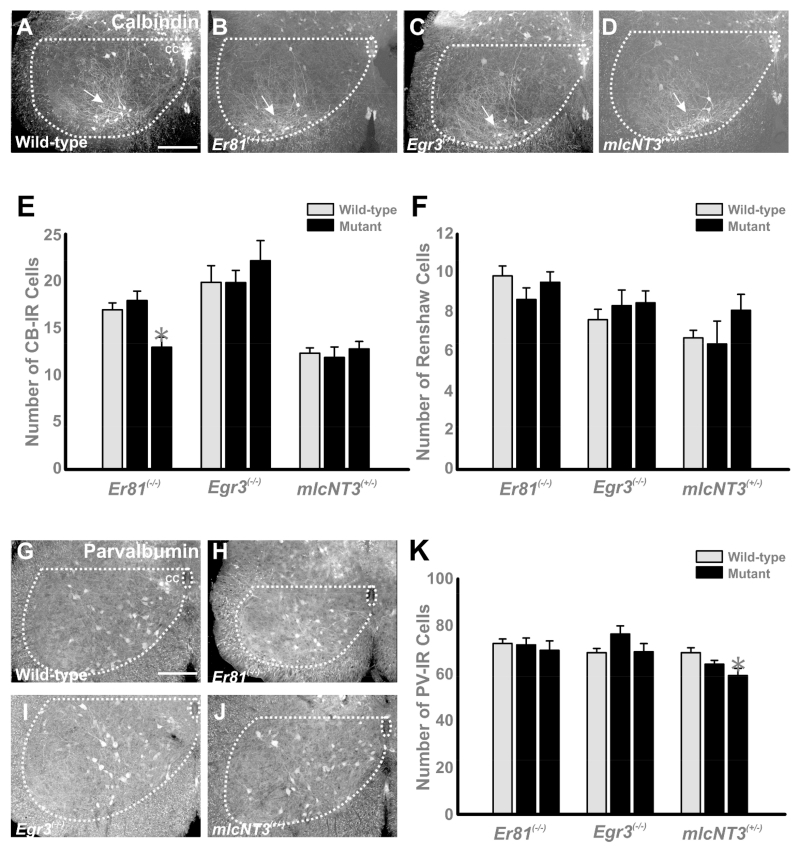
Calbindin and parvalbumin expression in the ventral horn is not altered in Er81(−/−), Egr3(−/−) and mlcNT3(+/−) mice
The calcium buffering proteins calbindin (CB) and parvalbumin (PV) are differentially expressed by distinct subtypes of ventral horn interneurons, including RCs, and they are both developmentally regulated (Zhang et al., 1990; Alvarez et al., 2005; Siembab et al., 2010). The mechanisms that control CB and PV expression in spinal interneurons are unknown, but in other brain regions (visual pathways and barrel cortex), their developmental expression is regulated by patterns of activity induced by the arrival of afferent inputs (Cellerino et al., 1992; Schmidt-Kastener et al., 1992; Alcantara et al., 1996; Philpot et al.,1997; Patz et al., 2004). Therefore, we examined whether decreasing (Er81(−/−) and Egr3(−/−)) or increasing (mlcNT3(+/−)) proprioceptive input could influence CB or PV immunoreactivity in ventral spinal interneurons. Qualitatively we found no major differences in the distribution of CB- or PV-IR cells in the spinal cords of mutants compared to controls (Fig. 3A-D and G-J). In lumbar segments 4 and 5 of P20 animals, ventral horn CB-IR cells were concentrated in the RC cluster, while fewer cells were scattered around the central canal and other ventral horn regions. Parvalbumin-IR cells were distributed at all dorso-ventral and medio-lateral regions of the ventral horn and included some RCs (see also Alvarez et al., 2005). Their numbers and positions were analyzed by plotting in Neurolucida the profiles of CB-IR and PV-IR neurons in 8-22 ventral horns per animal in 20 μm thick cryostat sections of the lumbar 5 spinal cord segments of P20 mice. We counted all immunoreactive interneurons in the ventral horn (see Fig. 3A-D, G-J). To avoid variability due to genetic background or developmental maturation in different mouse lines, comparisons were done against age-matched littermates as controls. Although a significant decrease was found in CB-IR interneurons (but not RCs) in one of two Er81(−/−) animals (Fig. 3E) and a very small change in PV cells was detected in one of two mlcNT3(+/−)animals (Fig. 3K), there was in general little difference between control and mutant animals in the number of CB-IR neurons (Fig. 3E), or the number of CB-IR RCs (defined by location, Fig. 3F) or ventral PV-IR cells (Fig. 3K) at P20. The results however do not rule out possible delays in maturation. Thus, we analyzed a developmental time series of Er81(−/−) mutants lacking all VGLUT1 inputs (data not shown). Two knockout animals were studied at three postnatal ages (P5, P10 and P15) and these were compared to age-matched wild-type littermates. We found no differences in the developmental regulation of PV and CB expression in spinal interneurons. In conclusion, varying the number of primary afferent synapses in the ventral horn does not influence the developmentally regulated expression of CB or PV in ventral interneurons.
Figure 3. Calbindin and parvalbumin expression in the ventral horns of wild-type, Er81(−/−), Egr3(−/−) and mlcNT3(+/−) mice.
A-D, Low magnification confocal images of calbindin-immunoreactive (CB-IR) cells in the ventral horn of wild-type (A), Er81(−/−)(B), Egr3(−/−) (C) and mlcNT3(+/−) (D) animals at P20. The border between white and ventral gray matter is outlined and a white dotted line is drawn horizontally from the dorsal tip of the central canal (cc in A). CB-IR cells in the ventral horn of P20 mice are largely restricted to the Renshaw cell area in the ventral-most regions of LVII and LIX (arrow). A few additional CB-IR neurons (usually with weaker immunofluorescence) are found near the dorsal-ventral border and close to the central canal. Genetic alterations in proprioceptive inputs did not change the number or distribution of CB-IR neurons. E, The number of CB-IR cells per ventral horn in P20 mice remained largely unchanged. A small difference was detected in one Er81(−/−) mutant (asterisks, p<0.01, Bonferroni t-tests compared to average controls). F, Number of CB-IR cells in the ventral most 250 μm of LVII and LIX. Most of these CB-IR cells correspond with RCs and their numbers did not significantly change in any mutant. In E and F the wild-type bar (grey) represents the average estimate from ventral horns pooled from two animals per line (n = 23, 33 and 31 ventral horns in, respectively, the Er81(−/−), Egr3(−/−) and mlcNT3(+/−) samples), while averages in mutant animals (black bars) are represented individually (n = 10-18 ventral horns per animal). Error bars indicate SEM estimates. G-J, Parvalbumin-immunoreactivity (PV-IR) in the ventral horn of P20 wild-type (G), Er81(−/−) (H), Egr3(−/−) (I) and mlcNT3(+/−) (J) mice. PV-IR neurons in P20 mice are distributed throughout the whole ventral horn with a lower density in lateral lamina IX. Similar numbers and distribution of PV-IR cells are found in mutant animals. K, Number of PV-IR cells per ventral horn in P20 mice. Wild-type bar (grey) is the average estimate from control ventral horns from two animals (n = 22, 32 and 33 ventral horns in the Er81(−/−), Egr3(−/−) and mlcNT3(+/−) samples, respectively), while the average mutant animals (black bars) are represented individually (n = 8-22 ventral horns per animal). Error bars in all graphs indicate SEM estimates. Scale bars: 100 μm in A and G (A-D and G-J are at the same magnification).
Normal distribution of VGLUT1-IR and VAChT-IR contacts on Renshaw cells
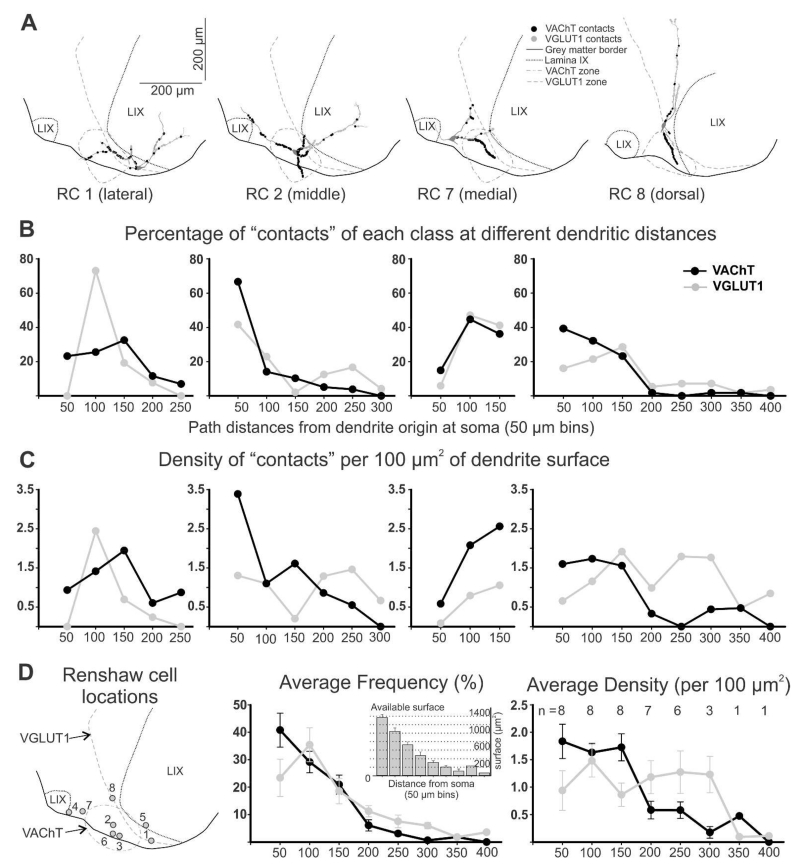
VGLUT1-IR and VAChT-IR inputs preferentially target the dendrites of RCs and few contacts are placed on the cell bodies. To identify the exact distribution of both inputs on the dendrites, and potential regions of overlap, we analyzed VGLUT1-IR and VAChT-IR contacts on dendrites of eight mature (P23) RCs in the lumbar 5 segment sampled from three different wild-type mice. These RCs extended 2 to 4 primary dendrites (3.5 ±0.8, average ±S.D.) with sparse branching (8.8 ±2.3 dendrite endings per RC). Analyses were performed in single 50 μm thick sections and therefore many dendrites were cut by the sectioning plane. To sample dendritic segments at different distances from the cell body we selected RCs with the longest dendrites contained within the section and performed extensive tiling of confocal optical stacks to cover large areas of the ventral horn in order to image the whole dendritic arbor at high magnification (and reveal VGLUT1-R and VAChT-IR contacts; Fig. 4A,B). Total length of the longest dendrite in each RC ranged from 412.3 to 158.9 μm (285.5 ±71.7, average ±S.D.), while the shortest dendrites measured from 134.4 to 18.8 (65.5 ±44.0). Short dendrites were always oriented rostro-caudally and therefore cut at the section surfaces after a short distance (Fig. 4B2). In contrast, long dendrites travelled in the transverse plane and sometimes could be followed to their natural endings within the thickness of the section (asterisk in Figs 4B, C). The analysis was therefore biased towards dendrites in the plane of the transverse histological section. Overall, total dendritic lengths analyzed per RC (combined sum of all dendritic segments traced in each RC) ranged from 521.8 to 1045.8 μm and the average in the RC sample was 783.2 ±160.9 (±S.D.), while the average length of all dendrites analyzed in each RC (average of the mean dendrite lengths of all dendrites from each RC) was 234.2 ±70.6. Natural dendrite endings, when found, were characterized by elongated varicosities and sometimes a branching point from which one or two very short branches emerge (< 5 μm in length). They were located from 66.2 to 412.3 μm from the dendrite origin (average: 219.8 ±99.4). Strong CB immunoreactivity in dendrite endings shows that CB is distributed throughout the whole dendrite, as well as in the cell bodies, nucleus, axons and synaptic varicosities of RCs in neonates and young adults. Although based on RC reconstructions from single histological sections in which a proportion of dendrites are pruned, these data demonstrate that the analyses are representative of all RC dendrite compartments, from origin to termination.
RC dendrites displayed a variety of organizations (see also, Fyffe, 1990). Some RCs had fusiform cell bodies with dendritic arbors extending from each pole of the soma and arborizing predominantly in a transverse orientation, sometimes parallel to the ventral gray matter border, other times they are directed dorso-ventrally. Most RCs were multipolar with the majority of dendrites confined to the ventral most 200 μm of the ventral horn, in ventral LIX and LVII (Fig. 4A-C). Finally, some Renshaw cells extended one or two dorsally-oriented dendrites that traversed most of the ventral horn through either LIX and/or the adjacent regions of LVII (Fig. 5A, RC 8). The distribution and density of VGLUT1-IR or VAChT-IR contacts was influenced by dendrite location and orientation in relation to regions with different densities of VGLUT1 or VAChT synapses (Figs. 4C-F and 5A). The majority of VAChT-IR synapses originating from motor axons are confined to the “Renshaw cell area”. In lumbar 5 this region is located ventromedial to the lateral motor cell column, just above the ventral horn exit points of ventral rootlets containing motor axons (Cullheim and Kellerth, 1978; see also Fig. 4A2). In contrast, VGLUT1-IR synaptic density is high throughout LIX (both lateral and medial motor columns) and the adjacent LVII, gradually decreasing in medial LVII and in LVIII (Alvarez et al., 2004). This parallels the distribution of Ia synaptic varicosities (Ishizuka et al., 1979). Thus, dendritic segments traversing the VAChT-IR rich “Renshaw cell area” contained the highest densities of VAChT-IR contacts, while dendrites extending into lamina IX and, in particular, long and dorsally-directed dendrites received the highest numbers of VGLUT1-IR contacts (Fig. 4B-F and 5A). VGLUT1-IR contacts were found in even the distal tips of dendrites with natural endings contained within the section. That was not the case for VAChT-IR contacts.
Figure 5. Quantification of VGLUT1-IR and VAChT-IR contacts on reconstructed Renshaw cells.
A, Four reconstructed RCs, three with cell bodies located lateral (RC 1), medial (RC 7) or dorsal (RC 8) to the region containing a high density of VAChT-IR boutons and one RC with the cell body in the middle of this region (RC 2). The positions of VAChT-IR contacts are marked in black and VGLUT1 contacts in grey. The highest numbers of contacts occur in dendrite segments entering the VAChT and VGLUT1 areas, independent of distance to cell body, order of dendrite or thickness. B, Distribution of contacts along the dendrites of the four cells shown in A. Y-axis is the percentage of contacts of each class (i.e., percentage of VAChT-IR contacts in each bin with respect to all VAChT contacts), found at different distance bins. X-axis represents path distance; “50” means contacts from 0 (dendrite origins) to 50 μm path distance (in any dendrite), “100” is for contacts located >50 to 100, and so on. Cells located medial, lateral and dorsal to the VAChT regions have variable distributions of VAChT contacts because differences in dendrite locations. Dendrites entering the VAChT region receive a large number of contacts, while dendrites away from this region receive very few. In contrast, RCs with cell bodies in the middle of the VAChT region (i.e., RC 2) display balanced distributions of high numbers of VAChT-IR contacts in all proximal dendrites that then decay with distance. In general, few VAChT-IR contacts are found further than 150 μm from the cell body. VGLUT1-IR contacts are also found in higher frequency in dendrite regions entering the area with high density of VGLUT1-IR boutons. By difference to VAChT-IR contacts they are also frequent in distal dendrites. C, Density of VAChT-IR and VGLUT1-IR contacts (boutons per 100 μm2 of dendrite surface) at different distance bins in the same four cells. D, Average frequency and density of contacts for the eight RCs reconstructed. In the left panel the location of all RCs are indicated on an “average” section profile with the approximate VGLUT1 and VAChT areas indicated. Middle panel represents the average distribution of VAChT- and VGLUT1-IR contacts at different distance bins in the eight RCs (error bars indicate SEMs; not all RCs had dendrite segments at all distances and the number of RCs included in each data point is indicated in the right panel). VAChT-IR contacts decrease in frequency more abruptly than VGLUT1-IR contacts. The distribution of available surface at different distances in indicated in the inset histogram. As a result, on average, the density of VAChT-IR contacts falls abruptly after 150 μm distance from the cell body, while the density of VGLUT1-IR contacts is maintained in the distal dendrite. The largest overlap between VAChT-IR and VGLUT1-IR contacts occurs in the first 100 μm of dendrites.
Another important factor that affected the distribution of putative synaptic contacts, particularly VAChT-IR boutons, is cell body placement (Figs. 4C and 5A). The majority of RCs had cell bodies located within the VAChT-rich “Renshaw cell area” and in this case VAChT-IR contacts were distributed symmetrically on proximal dendrites around the cell body (RC2 in figures 4 and 5). However, when the cell body was located at the edge of the “Renshaw cell area” (RC1), the VAChT-input was biased to the proximal region of dendrites oriented towards this area. Other times the cell body was far from the “Renshaw cell area” (RC7). In this situation the majority of VAChT-IR contacts were placed at a distance from the cell body, on the distal dendritic segments that enter the “Renshaw cell area”.
To obtain a quantitative description of the distribution of VAChT-IR and VGLUT1-IR contacts we estimated the proportion of contacts and their density (per 100 μm2 of dendrite surface) at different distances from the cell body after binning dendritic arbors in 50 μm increments of path distance (i.e., distance along the dendrite from its cell body origin). The results show large cell to cell variability following the topographical rules explained above (Fig. 5A, B, C). However, overall, most VAChT-IR contacts in our sample of RCs were located proximally (Fig. 5D). Their decrease in frequency from origins to 150 μm path distance parallels the available surface membrane and thus their density is maintained in this region. VAChT-IR contact density then significantly decreased after 150 μm, in large part because many dendrites exit the VAChT-IR area at this distance and the frequency of contacts drops faster than the decrease in available surface membrane. The majority of VGLUT1-IR contacts were also detected proximally, peaking in dendrite locations between 50 and 100 μm from the cell body, but with lower numbers than VAChT-IR contacts. Their decay in frequency with distance was, however, not as abrupt as VAChT-IR contacts (Fig. 5D) and in fact, they paralleled reductions in available dendritic surface (due to dendrite tapering combined with relatively low branching); thus VGLUT1-IR contact density was maintained between 1 and 1.5 contacts per 100 μm2 of surface from dendrite origins to 300 μm dendritic distance. VGLUT1-IR density was lower than VAChT-IR in the first 150 μm of dendrite and higher from 150 μm to the end of the dendrite (Fig. 5D, right panel). Within these averages there were individual exceptions explained by cell body positions and dendrite trajectories.
In summary, the analysis of these two inputs reveals that: 1) none of these inputs specifically “seeks” a certain region of the RC dendrite, rather their distribution is best explained by cell body location and dendrite trajectories in relation to the synaptic fields of motor axons and Ia afferents; 2) because of these topographical relations and the fact that many RCs are contained within the VAChT-rich Renshaw cell region, VAChT-IR inputs are most commonly concentrated in the first 150 μm of dendrite, while the VGLUT1 input accesses both proximal and distal dendrites; 3) the highest overlap between VGLUT1-IR and VAChT-IR contacts occurs in the first 100 μm of dendrite.
VGLUT1-IR synaptic density on maturing calbindin-IR Renshaw cells of different genotypes parallels changes observed on motoneuron cell somata and the ventral horn
It is not feasible to perform extensive tiling and full reconstructions of large samples of RCs in the three genotypes studied and at multiple time points during development. Therefore we performed comparisons of input densities in different genotypes and time points in single image confocal stacks. Dendritic length in these analyses was restricted by the field of view at 60×1.5. In these analyses the average more distal dendritic point analyzed varied between 90 and 110 μm from soma, the average length of all dendrites analyzed in the different samples of RCs varied between 58 and 70 μm and the total combined dendritic length per cell averaged in different experimental groups between 164 and 237 μm (Table 2). Therefore, the analyses mainly sampled the first 100 μm of dendrite, the region that displayed the greatest density of VAChT-IR inputs and overlap with VGLUT1 inputs in our previous analysis of full dendritic arbors.
Table 2. Dendritic lengths.
| P15 | P20 | Adult | |||||
|---|---|---|---|---|---|---|---|
| ER81 | WT | KO | WT | KO | WT | KO | |
| n | 31 | 31 | 74 | 37 | 0 | 0 | |
| Maximum | 94.3 ± 25.6 | 91.1 ± 19.9 | 93.3 ± 22.4 | 90.8 ± 27.2 | N/A | N/A | |
| Average | 60.1 ± 19.4 | 57.6 ± 13.5 | 61.4 ± 18.1 | 65.3 ± 21.3 | N/A | N/A | |
| Total | 213.3 ± 62.4 | 200.7 ± 57.2 | 197.3 ± 62.7 | 201.6 ± 81.34 | N/A | N/A | |
| EGR3 | WT | KO | WT | KO | WT | KO | |
| n | 12 | 37 | 27 | 37 | 47 | 62 | |
| Maximum | 99.2 ± 18.8 | 103.6 ± 22.9 | 95.1 ± 28.4 | 95.3 ± 23.6 | 103.3 ± 32.3 | 96.4 ± 26.5 | |
| Average | 65.9 ± 10.5 | 63.4 ± 16.2 | 61.7 ± 20.4 | 63.4 ± 15.2 | 68.0 ± 20.3 | 61.8 ± 14.7 | |
| Total | 212.6 ± 63.2 | 221.0 ± 61.5 | 204.8 ± 66.9 | 201.3 ± 63.1 | 195.0 ± 58.5 | 205.3 ± 68.5 | |
| mlcNT3 | WT | Het | WT | Het | WT | Het | |
| n | 14 | 40 | 31 | 65 | 9 | 33 | |
| Maximum | 92.7 ± 22.6 | 101.6 ± 26.3 | 103.6 ± 24.2 | 106.2 ± 25.1 | 110.1 ± 33.0 | 99.3 ± 27.8 | |
| Average | 67.5 ± 25.0 | 68.0 ± 17.3 | 68.5 ± 19.7 | 70.2 ± 24.3 | 61.8 ± 17.3 | 61.8 ± 19.6 | |
| Total | 164.1 ± 56.6 | 232.0 ± 80.8 | 175.3 ± 49.2 | 189.0 ± 75.5 | 236.9 ± 57.9 | 190.7 ± 73.4 | |
All data is in μm ± SEM. Includes RCs used analysis of both VAChT and VGLUT1.
Frequently, but not always, this was done in single cells triple immunolabeled for both inputs and calbindin-immunoreactivity.
Maximum indicates the average of the farthest dendritic point measured for each RC. Average is the average of mean lengths of all dendrites analyzed in each RC. Rostro-caudally oriented dendrites are cut at the surfaces of the 75 μm thick section and are therefore always shorter than dendrites travelling in the transverse plane. Total is the average of dendritic length analyzed per cell after adding the lengths of all dendritic segments analyzed in each RC.
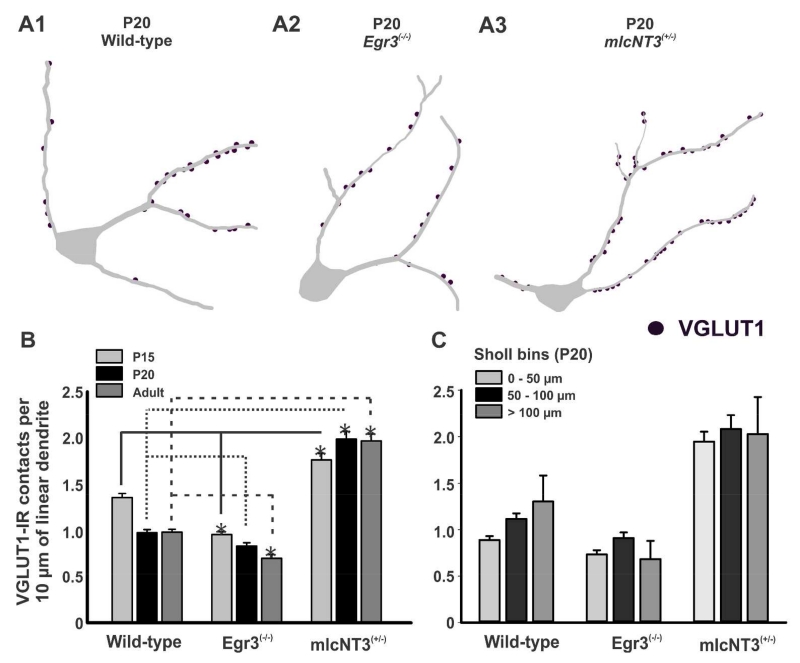
VGLUT1-IR contact density in this region on developing RCs increases from P0 to P15 and then decreases in the adult (Mentis et al., 2006; Siembab et al., 2010). We therefore examined whether decreasing (Er81 and Egr3 knockouts) or increasing (mlcNT3 heterozygotes) primary afferent input strength modified VGLUT1-IR contact number on these dendritic regions in mature RCs. P15, P20 and adult RCs immunolabeled with CB and VGLUT1 were imaged with confocal microscopy (Fig. 6) and their proximal dendrites imaged and traced in 3D using Neurolucida (Fig. 7A). VGLUT1-IR contacts were plotted on these dendrites to estimate VGLUT1-IR contact linear densities (number of terminals per 10 μm of linear dendrite). We analyzed 31-40 RCs in each of three animals for each genotype Er81(−/−), Egr3(−/−), mlcNT3(+/−) and age (P15, P20, adult), and compared them to 44-57 control RCs sampled from 4 or 5 age-matched littermates for each age (data summarized in Table 3). At each time point no differences were observed between wild-types from the three different lines, therefore the data were pooled into one developmental control group. No VGLUT1-IR contacts were observed on RCs from Er81(−/−) animals and therefore these animals are not included in the quantitative analyses.
Figure 7. Density of VGLUT1-immunoreactive contacts on mature Renshaw cells in wild-type mice compared to mutant mice.
A, Three-dimensional reconstructions of RCs from P20 wild-type (A1), Egr3(−/−) (A2), and mlcNT3(+/−) (A3) mice. VGLUT1-IR contacts (dots) are plotted on the dendrites of the traced cells. B, Estimates of VGLUT1-IR contacts per 10 μm of linear dendrite on CB-IR RCs of wild-type, Egr3(−/−) and mlcNT3(+/−) mice of P15 (light grey bars), P20 (black bars) and adult (dark grey bars) postnatal ages. Renshaw cells from wild-type animals show densities and changes with maturation similar to those reported in Mentis et al. (2006) and Siembab et al. (2010). In wild-type and Egr3(−/−) animals, RCs display statistically significant decreases in VGLUT1-IR contact density from P15 to adult (p<0.001, one-way ANOVA). In contrast, VGLUT1-IR contact density increased significantly from P15 to adult in mlcNT3(+/−) mice (p=0.041, one-way ANOVA). Compared to their age-matched wild-types, RCs in Egr3(−/−) animals always had statistically significant lower density of VGLUT1-IR contacts, while in mlcNT3(+/−) mice show significant increases: Lines indicate statistical comparisons at P15 (continuous line), P20 (dotted line), adult (dashed line)(asterisks indicate significance; p<0.05 in post-hoc Dunn’s comparison). In mature mlcNT3(+/−) mice (P20 and adult), VGLUT1-IR contact density on RCs more than doubled compared to wild-types. During postnatal maturation VGLUT1-IR densities significantly decreased in wild-types and Egr3(−/−) mutants and increased in mlcNT3(+/−) mice (p<0.001 One-Way ANOVA on Ranks). C, Densities recorded in three different Sholl bins (aerial distance from the center of the cell body) of 50 μm distances. The densities (within each genotype) were not significantly different among the three different Sholl bins, indicating that the changes in VGLUT1-IR contact density were equally distributed throughout the proximal dendrite.
In Egr3(−/−) mice, VGLUT1-IR contact density on RCs was significantly decreased by 30% at P15 compared to controls (Fig. 7B and Table 3) (p<0.05, post-hoc Dunn’s test). From P15 to adult, VGLUT1 inputs were developmentally reduced in both Egr3(−/−) and wild-type animals (Fig. 5B, P<0.001 One-way ANOVA on Ranks) as shown previously in two other independent samples of different wild-types (Mentis et al., 2006; Siembab et al., 2010). The developmental reduction in VGLUT1 density was however more profound in P20 wild-types compared to P20 Egr3(−/−) mutants and in wild-types we did not observe statistically significant differences between P20 and adult, but in Egr3(−/−) mutants VGLUT1-IR was further reduced from P20 to adult (P<0.05 post-hoc Dunn’s test). As a result, VGLUT1-IR densities in adult Egr3(−/−) animals were significantly lower than in adult wild types (29% reduced; p<0.05, post-hoc Dunn’s test). In summary, VGLUT1-IR contacts in Egr3(−/−) animals are already at low density at P15 and become further reduced during postnatal maturation resulting in significantly fewer VGLUT1-IR contacts than normal in the adult.
As expected, the density of VGLUT1-IR contacts on RCs in mlcNT3(+/−) animals was increased (Fig. 5B), up 30% compared to age-matched wild-types at P15 (Table 3) (p<0.05, post-hoc Dunn’s test). There was also a developmental 12% increase in VGLUT1-IR density from P15 to P20 and adult (p<0.001, one-way ANOVA on Ranks), a period in which this input normally decreases. As a result, the density of VGLUT1-IR contacts on RCs in P20 and adult mlcNT3 animals was doubled (107% and 101% increased) compared to age-matched wild-type controls (p<0.05, post-hoc Dunn’s test).
To assess whether these changes were focused to any particular region, we divided the proximal dendrite in three 50 μm distance Sholl bins (Fig. 3C, only P20 data is shown). Decreases (Egr3(−/−)) and increases (mlcNT3(+/−)) in VGLUT1 density where equally distributed throughout all Sholl bins in the proximal dendrite (Fig. 7C). It is noteworthy that the region of spinal cord containing high densities of VGLUT1-IR puncta contracted and expanded in Egr3(−/−) and mlcNT3(+/−) mutants, respectively, thus including more or less of the ‘Renshaw cell region”. In conclusion, increasing VGLUT1 input strength prevents its developmental downregulation throughout the proximal dendrites of RCs, while decreasing its initial strength causes further losses during postnatal maturation.
The density of VAChT-IR contacts is altered in opposite direction to VGLUT1
Neonatal (P0-P1) RCs receive a larger density of contacts from VAChT-IR boutons derived from motor axon recurrent collaterals than from VGLUT1 boutons. Then, during early postnatal development both inputs increase in synaptic numbers in parallel, but after P15 VGLUT1-IR contacts decrease in density while VAChT-IR contact densities are stably maintained throughout adulthood (Mentis et al., 2006 and Siembab et al., 2010). To examine the fate of VAChT-IR synapses on developing RCs receiving different levels of VGLUT1 input, we analyzed 30 to 40 RCs Neurolucida reconstructions from each genotype and age (P15, P20, adult) and compared them to wild-type littermates (see Table 4 for sample details).
VAChT-IR staining was increased in LIX of Er81(−/−) mice. Compared to controls there was a greater density of VAChT-IR synaptic puncta in the ventral area containing RCs (Fig. 8A1-B1), accordingly, there were parallel changes in VAChT-IR contact densities on the dendrites of RCs (Figs. 8B2-5; 9A1-2). Er81(−/−) RCs at P15 showed a 68% increase in the density of VAChT-IR contacts on their dendrites compared to age-matched wild-type controls (p<0.05, post-hoc Dunn’s test; Fig. 9B). At P20, VAChT-IR contact density was only 23% larger in Er81(−/−) RCs compared to age-matched wild-types because of a significant decrease (16%) in VAChT-IR contact density on RCs in Er81(−/−) animals from P15 to P20 (p<0.05, post-hoc Dunn’s tests). Adults were not analyzed because of their high mortality rate.
VAChT-IR staining was also increased in LIX and the RC area in Egr3(−/−) animals (Fig. 8C1) and more VAChT-IR contacts were observed on RCs in these mutants compared to controls (Figs. 8C2-5, 9A3). VAChT-IR contact density on RCs from Egr3(−/−) animals was significantly higher at all ages and the differences increased with maturation (P15: 21% higher than age-matched wild-types; adult: 39% higher; in all cases p<0.05, post-hoc Dunn’s tests; Fig. 9B). In conclusion, there is a greater density of VAChT-IR contacts on RCs in Er81(−/−) and Egr3(−/−) mice compared to control animals (summarized in Table 3), suggesting that decreased primary afferent input is correlated with an increase in VAChT-IR inputs on RCs. Differences were also noted between both mutant lines. At P15, VAChT-IR input density was much larger in Er81(−/−) than Egr3(−/−) mice, correlating with the larger loss of VGLUT1 inputs in Er81(−/−). However, RCs in Er81(−/−) animals lost VAChT-IR contacts during late postnatal development in contrast to RCs in Egr3(−/−) mice which gained them. This difference is perhaps best explained by the overall decline in viability of the Er81(−/−) mutants. Alternatively, some VGLUT1 input might be necessary for stabilization of VAChT-IR contacts on RCs during late maturation.
These observations suggest that an increase in VGLUT1 inputs might be associated with fewer VAChT-IR contacts. Although at low magnification there was no clear difference in VAChT immunostaining in mlcNT3(+/−) animals compared to wild-types (Fig. 8D1), RCs in P15, P20 and adult mlcNT3(+/−) mice exhibited a 25-28% decrease in VAChT-IR contact density compared to controls (Figs 8D2-5, 9A4-B, Table 3). At all ages the density was significantly lower compared to age matched wild-types (p<0.05, post-hoc Dunn’s tests; Fig. 9B; Table 3). Similar to wild-types, the density of VAChT-IR contacts on RCs remained stable from P15 to adult with no significant differences (p=0.267, One-way ANOVA). Changes in the density of VAChT-IR contacts were similar in all three 50 μm Sholl bins analyzed in the proximal dendrite (not shown).
In summary, the densities of VGLUT1 and VAChT-IR synaptic contacts on RC dendrites appear inversely related in these mutant animals, but the changes in bouton contact densities for VGLUT1 where always larger compared to VAChT. To examine their inverse correlations we selected P20 and adult RCs in mlcNT3(+/−) and Egr3(−/−) animals and their wild-type littermates in which both inputs were simultaneously analyzed using triple immunofluorescence for calbindin, VGLUT1 and VAChT. Er81(−/−) mutants lack VGLUT1, so they were not included in this analysis. Results were very similar at P20 and adult. We only show correlations in adult animals (Fig. 9C). The data could be fitted using linear regression (r = 0.629) to a line with a negative slope in which, on average, for every unitary change in VGLUT1 density, the density of VAChT contacts changed by 0.6 in the opposite direction.
Excitatory glutamatergic inputs from spinal interneurons on Renshaw cells are not influenced by changes in the strength of the VGLUT1 input
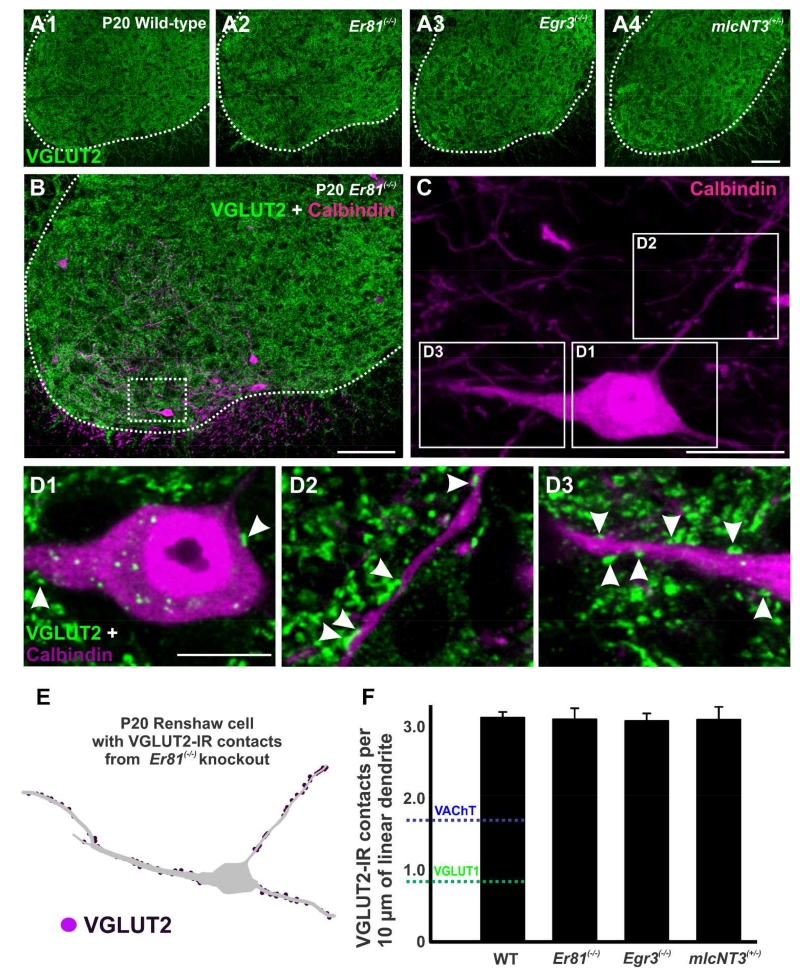
We then investigated whether the changes observed for VAChT-IR contacts were generally applicable to other excitatory synapses. We used vesicular glutamate transporter 2 (VGLUT2) as a marker for excitatory glutamatergic inputs from sources other than primary afferents, including spinal excitatory interneurons (Todd et al., 2003) and possibly, descending systems (Du Beau et al., 2012). In P20 wild-type mice, VGLUT2-IR boutons are distributed throughout the gray matter of the ventral horn and a similar distribution was also detected in all three transgenic mouse lines (Fig. 10A1-A4). The ventral area occupied by RCs contains a high density of VGLUT2-IR synaptic puncta (Fig. 10B). A few VGLUT2-IR contacts were found on the soma of RCs (Fig. 10C and D1) and most targeted the dendrites (Fig. 10C and D2-D3). Neurolucida reconstructions of RCs at P20 (Fig. 10E) revealed that there was no significant difference in the density of VGLUT2-IR contacts on RC dendrites in Er81(−/−), Egr3(−/−) and mlcNT3(+/−) animals compared to wild-type controls (Fig. 10F) (p=0.997, one-way ANOVA). Interestingly, the density of VGLUT2-IR contacts was always greater than that of VGLUT1-IR or VAChT-IR contacts on wild-type averages (Fig. 10F indicated with color dashed lines). In conclusion, VGLUT2 contacts constitute a significant source of excitatory inputs to RCs that likely originate from a variety of spinal interneurons and descending systems and are not affected by changes in proprioceptive input. Therefore, among excitatory synapses on RCs, VAChT inputs seem selectively altered by variations in VGLUT1 input strength.
Figure 10. VGLUT2-immunoreactive contacts on Renshaw cells do not change in any mutation.
A, Low magnification confocal images showing VGLUT2-immunoreactivity (green, FITC) in the ventral horn of wild-type (A1), Er81(−/−) (A2), Egr3(−/−) (A3), and mlcNT3(+/−) (A4) mice at P20. The white dotted line represents the border between the ventral horn and the white matter. VGLUT2-IR bouton density is unchanged. B, Confocal image of the ventral horn of a P20 Er81(−/−) spinal cord immunolabeled for CB (magenta, Cy3) and VGLUT2 (green). C, High magnification two-dimensional projection of the CB-IR RC indicated in a box in B. Boxes indicate the dendritic regions shown at higher magnification in D1-D2. D1-D2, High magnification confocal images of VGLUT2-IR contacts (green, arrowheads) on the dendrites of this CB-IR RC (red). E, Reconstruction of this same CB-IR RC showing VGLUT2-IR contacts on the dendrites. F, VGLUT2-IR contact density (per 10 μm of linear dendrite) in P20 wild-type, Er81(−/−), Egr3(−/−), and mlcNT3(+/−)animals. No significant differences were detected in the density of VGLUT2-IR contacts (p=0.997, one-way ANOVA). The blue line represents the density of VAChT-IR contacts and the green line the density of VGLUT1-IR contacts, both on P20 wild-type RCs. Scale bars: 100 μm in A4 and B (A2-A4 are at the same magnification); 20 μm in C; 10 μm in D2 (D1 at the same magnification.
Discussion
The main findings in this study demonstrate that altering the strength of primary afferent inputs in the ventral horn influences the final density of VGLUT1-IR contacts on RCs and that the density of VAChT-IR inputs is inversely related to the number of VGLUT1 synapses. This reciprocal relationship between sensory afferent (VGLUT1) and motor axon (VAChT) inputs on RCs appears to be specific, since other glutamatergic inputs (labeled by VGLUT2) are unaffected. In addition, we describe for the first time the distribution of VAChT and VGLUT1 inputs throughout the whole dendritic arbor of RCs. The analyses suggest that, in general, VAChT-IR inputs are concentrated proximally while VGLUT1-IR inputs are distributed throughout the dendrite. However, we also found large cell to cell variability in the distribution of both inputs that was best explained by differences in the proximity of different dendrites from different RCs to spinal cord regions rich in VGLUT1 and/or VAChT-IR boutons. The data thus reveal a developmental influence of sensory afferent strength on motor axon input proliferation that shapes the final synaptic organization on RCs, but this is constrained by topographical relations between RC dendrite placements and the termination fields of VGLUT1 Ia afferents and VAChT motor axons.
Development and fate of Ia afferent inputs on Renshaw cells
Based on their ventral location, labeling from dorsal roots and parvalbumin content, VGLUT1-IR synapses on RCs are believed to originate from Ia sensory afferents (Mentis et al., 2006; Siembab et al., 2010). The initial discovery of a robust, likely Ia sensory input on mouse RCs during the first week after birth (Mentis et al., 2006) was somewhat surprising because previous electrophysiology in the adult cat spinal cord found no evidence of monosynaptic Ia afferent inputs (Renshaw, 1946; Ryall and Piercey, 1971). In the study from Mentis and colleagues (2006) it was nevertheless shown that sensory inputs were able to evoke robust firing in neonatal rodent RCs, and that many VGLUT1-IR synapses are physically maintained in adult cats and rodents, although at lower density. A logical conclusion is that these synapses become weakened in the adult, begging the question of whether they have a role in RC development and what factors determine their fate.
Recently we proposed a model in which the migration and positioning of interneurons in the spinal cord during early development determines the inputs they later receive (Benito-Gonzalez and Alvarez, 2012; Alvarez et al., 2013). According to this model, motor axons target RCs preferentially because these interneurons are positioned along the pathway used by motor axons to access the ventral root. In contrast, Ia inhibitory interneurons receive strong projections from proprioceptive sensory afferents because they are located along the trajectory of Ia axons projecting to their respective motor pools. The ventral location of RCs places them at the edge of the projection area of Ia afferent axons, in a region in which the density of VGLUT1 synapses drops quickly. These anatomical relations are reflected in differences in input density over each interneuron subtype in neonates; Ia inhibitory interneurons initially receive more VGLUT1 synapses than RCs (Siembab et al., 2010). Moreover, we show here that the numbers of VGLUT1 synapses on RCs vary depending on the trajectory of their main dendritic branches. Dorsally-directed RC dendrites traverse areas with high density of VGLUT1 synapses and receive more of this input than RC dendrites projecting ventrally or with a horizontal distribution at the base of the ventral horn. RCs are therefore competent to receive Ia afferent synapses as long as their dendrites travel through areas rich on this input. In mlcNT3(+/−) animals the expansion of the ventral horn territory occupied by VGLUT1-IR synapses lead to their increased density on RCs. Thus, Ia input trajectories combined with interneuron placements define an initial Ia afferent to interneuron connectomics map that can later be refined by postnatal mechanisms. These findings parallel differences in the strength of homonymous and heteronymous Ia inputs received by specific motoneurons depending on the exact orientations and extension of their dendritic arbors (Vriesling and Arber, 2006). Similar principles may apply to RCs and to other interneurons as well (Bikoff et al., 2012). That is, given fixed trajectories and locations of Ia axons and Ia synapses, the distribution of interneuron dendrites determine the density of Ia VGLUT1 synapses established during initial synaptogenesis. This initial input strength influences later synapse proliferation and further strengthening (Siembab et al., 2010). Interestingly, Ia input strength on heteronymous motoneurons is also refined in the postnatal period by activity-dependent mechanisms (Mendelsohn et al., 2015).
We now extend this principle to VAChT-IR motor axon inputs, by revealing that the number of contacts is also dependent on dendrite locations. Therefore, we favor a model in which the synaptic organization of different inputs on spinal interneurons is strongly influenced by anatomical relations between the distributions of inputs and dendrite trajectories. This model minimizes a possible role of molecular signals in attracting one or the other input to certain dendrite locations - distal or proximal dendrites receive motor axon or Ia afferent synapses as long as they traverse the terminal field of each input - however, it does not rule out the possibility that molecular signals underlie avoidance of some axons coursing through the same region. Repellent semaphorin-plexin signals have been found to mediate synaptic avoidance between specific Ia afferents and the dendrites of specific motor pools crossing the terminal fields of these Ia afferents (Pecho-Vrieseling et al., 2009; Fukuhara et al., 2013).
In the postnatal period, activity-dependent mechanisms likely operate on a pre-existing complement of synapses by adding (strengthening) or removing (weakening) synapses. The structure of the Ia afferent and motor axon input provides some insights to this process. Both inputs on mature RCs are usually visualized as variable numbers of “en passant” contact boutons interconnected by intervaricose regions. It is therefore likely that resulting densities are the consequence of changes in the number of boutons in these individual synaptic strings. The result is that contacts from VGLUT1-IR and VAChT-IR boutons are not uniformly distributed, but always highly clustered along mature dendrites. Whether or not synapse numbers changed, developmental growth of the target dendrite via increased diameter, more branching or lengthening is an alternative mechanism by which the density of a synaptic input can be reduced. This latter mechanism seems to strongly influence the reduction in Ia afferent synapse density on RCs from P15 to adult (Mentis et al., 2006; Siembab et al., 2010). How synapse numbers are modulated by activity or other mechanisms is at present unclear.
One important feature of Ia afferent projections to the ventral horn is that their fine spatial organization is based on the muscle of origin, which determines Ia afferent trajectories and the preferential dorso-ventral domains occupied by their terminal arbors in the ventral horn (Ishizuka et al., 1979). Similarly, motor pools occupy specific dorso-ventral and medio-lateral positions that are generally maintained across species (Romanes, 1964; McHanwell and Biscoe, 1981; Nicolopoulos-Stournaras and Iles, 1983; Vanderhorst and Hosltege, 1997; Ryan et al., 1998; Bacskai et al., 2014). These topographic relations determine the specificity of Ia afferent connections with homonymous motoneurons as proven by the loss of specificity when motoneuron positions are scrambled by genetic manipulation (Demireva et al., 2011; Surmeli et al., 2011). Similar rules were also recently suggested for interneurons (Bikoff et al., 2012). Thus, the ventral location of RCs constrains the range of muscles from which they can receive Ia afferent inputs. Limitations based on motor pool origin or mechanical action are less likely for the connections between motoneurons and RCs, which are better defined by the “segmental proximity” hypothesis (‘regardless of function’) of Eccles et al. (1961). Thus, motor pools located at the same rostro-caudal level converge on a similar pool of RCs that distributes recurrent inhibition to many different motoneurons located nearby, irrespective of pool identity. The only two restrictions are that distal flexor motor pools receive weak (or no) RC-mediated recurrent inhibition from homonymous or heteronymous motor pools (Hamm, 1990) and that RCs do not innervate strict antagonists of motor pools from which they receive input (Eccles et al., 1961; Hultborn et al., 1971). This organization implies that Ia afferent inputs are unlikely to establish strict reciprocal or homonymous inhibitory circuits with specific motor pools through RCs because the motor pools connected to individual RCs are likely not organized in strict antagonist or synergistic fashion with the muscles of origin of their Ia afferent inputs. The lack of clear relationships between Ia afferent muscle origins and motor pool origins of motor axon inputs on RCs suggest that it is unlikely that specific patterns of motor activity during development could strengthen or weaken Ia afferent inputs on RCs; i.e., motor output and RC firing are likely de-correlated with Ia afferent synaptic activation.
However, the postnatal strengthening of inhibitory synapses on RCs after P10, to balance the maturation of motor axon inputs and activity (Gonzalez-Forero and Alvarez 2005; Gonzalez-Forero et al., 2005), and the decrease in input resistance of mature RCs (415.0 ±49.3 MΩ at P5; n=11 compared to 250.7 ± 27.0 MΩ at P10; n=14; Sonner and Alvarez, 2010) imply that excitatory inputs that initially evoke RC firing in neonates might become subthreshold later in development if they are not strengthened in parallel. In contrast, excess of NT3 in the mlcNT3(+/−) animal causes excessive proliferation of Ia afferent synapses in the ventral horn and strengthens these synapses to the point of disturbing the specificity of connections and resulting in ataxia (Wang et al., 2007). Independent of circuit function, Ia afferent inputs might be preserved in this situation by mechanisms related to the direct actions of target-derived NT3 on Ia afferents, which include preventing programmed cell death, enlarging their central projections during early development (Wright et al., 1997) and enhancing maintenance of Ia afferent synapses during late development (Shneider et al., 2009b). NT3 also strongly potentiates Ia synapses on motoneurons through LTP-like, NMDA-mediated mechanisms (Arvanov et al., 2000) and similar mechanisms might operate on RCs. Thus, excess of NT3 trophic support in mlcNT3(+/−) animals overcomes normal development and preserves strong Ia afferent inputs on mature and adult RCs. These data also shows that chronic excess NT3 during development not only disturbs the normal organization of Ia inputs on motoneurons, but also on interneurons.
Heterosynaptic influence of Ia afferents and motor axon synapse numbers
The formation of motor axon synapses on RCs in early embryos precedes the arrival of Ia afferents inputs, which occurs around birth (Alvarez et al., 2013). Therefore Ia afferents can only modulate the late maturation phase of motor axon synapse formation on RCs. Motor activity increases significantly in rodents from P10 to P20 (Westerga and Gramsbergen, 1990) and during this time motor axon synapses proliferate on RCs to maintain their overall density on the expanding surface of developing dendrites (Mentis et al., 2006; Siembab et al., 2010). Given the tight relationships between motoneuron inputs and RC firing in adults (Eccles et al., 1954; Ross et al., 1979; van Keulen, 1981) and neonates (Mentis et al., 2005; Nishimaru et al., 2005) it is expected that motor axon coupling to RCs will strengthen with increased motoneuron activity during normal development. The data presented here suggest that this latter development can be modified depending on the number of Ia afferent synapses that converge onto the same RCs. Thus, an excess of Ia afferent synapses decreases the number of motor axon synapses and the lack of Ia afferent inputs increases the number of motor axon synapses. It could be argued that motor axon synapses and Ia afferent synapses compete for “synaptic space”, but the nature of this competition is subtle and specific to VGLUT1 and cholinergic synapses, and not to VGLUT2 inputs. One intriguing possibility is that the synaptic formation and maintenance of VGLUT1 and motor synapses involve competition for some critical, limited, RC-derived factor (that could be related to electrical activity or not) on which VGLUT2 synapses do not depend.
There are several potential synaptotropic molecules that could be shared by VGLUT1 and VAChT synapses on RCs and mediate heterosynaptic competition between both inputs. Neuregulin-1 is expressed by both Ia afferents and motoneurons (Fischbach and Rosen, 1997; Hippenmeyer et al. 2002), it is released in an activity-dependent manner (Ozaki et al., 2004) and activates postsynaptic ErbB receptors leading to their internalization (Liu et al., 2007). ErbB activation modulates maturation of glutamatergic synapses on inhibitory interneurons (Ting et al., 2011) and is required for activity-dependent AMPA receptor incorporation and stabilization through interactions with PSD-95 (Garcia et al., 2000; Huang et al., 2000; Li et al., 2007). Similarly, activity-dependent release of neuregulin-1 modulates nicotinic receptor numbers and currents at neuron-to-neuron cholinergic synapses (Kim et al., 2015). Thus, sequestration of ErbB receptors by an excess of Ia afferent VGLUT1 synapses on RCs could reduce availability at cholinergic synapses and weaken nicotinic receptor recruitment and motor axon synapse stabilization, while fewer VGLUT1 synapses could favor motor axon synapse stabilization through a larger pool of available ErbB receptors.
Another mechanism that controls initial synapse numbers is the balanced interaction between presynaptic neurexins and postsynaptic neuroligins (Bang and Owczareck, 2013). In particular, neuroligin-1 availability at individual synapses modulates glutamatergic synapse numbers in a competitive manner (Kwon et al., 2012) and it also regulates neuron to neuron cholinergic nicotinic synapses (Conroy et al., 2007). The exact neuroligins underlying formation and stabilization of the various excitatory synaptic inputs on RCs are unknown, but one possibility is that both motor axon and VGLUT1 synapses compete for neuroligin-1. Moreover, the inhibitory postsynaptic organizer gephyrin can sequester neuroligin-1 and further modulate excitatory synapse numbers (Varley et al., 2011). Thus, the large gephyrin patches that develop postnatally on RCs (Geiman et al., 2000) could also regulate VGLUT1 and/or VAChT inputs. Additional analyses will be necessary to elucidate the molecular mechanisms that regulate VGLUT1 and cholinergic synapse formation and maturation on RCs.
In conclusion, Ia afferents heterosynaptically modify the density of cholinergic motor synapses on the proximal dendrites of RCs, raising the question of what function this mechanism serves during motor circuit maturation. One possibility is that Ia afferents limit the proliferation of motor inputs from different motor pools by regulating the number of motor axon synapses on RCs. It is well known that a single motor axon is capable of eliciting burst firing in RCs (Ross et al., 1979; van Keulen, 1981), so while additional synapses might not provide any functional advantage, reducing the number of synapses could limit motor axon inputs to those originating from nearby motoneurons and prevent the proliferation or maintenance of abnormal motor inputs on RCs, like for example those from more distal motor pools that are presumably weaker. The synaptic fields of individual motor axons are reduced in their rostro-caudal distribution during postnatal development (Cullheim and Ulfhake, 1985), which accounts for focusing recurrent inhibition to motoneurons in narrow rostro-caudal spinal cord regions - a key feature of the mature organization of this motor control circuit. It will be interesting to investigate in the future if excess motor axon synaptogenesis on RCs disturbs this basic organization and development.
Acknowledgements
We thank Mrs. Courtney Smith for her help with histology processing and Mrs. Maria C. Berrocal for maintaining some of the mouse colonies used. Dr. George Z. Mentis read the manuscript and provided invaluable input. The Er81 knockout line was generously provided by Dr. T.M. Jessell (Columbia University, NY).
Support: This work was supported by NIH grant NS047357 to FJA and by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NAS). VCS was in part supported by the Biomedical Sciences PhD program at Wright State University.
Note added in proof: The initial preliminary communication of Bikoff et al., 2012 cited in here has now been accepted for publication in a full length article. “Bikoff JB, Gabitto MI, Rivard AF, Drobac E, Macahdo TA, Miri A, Brenner-Morton S, Famojure E, Diaz C, Alvarez FJ. Mentos GZ, Jessell TM, 2015 Spinal inhibitory interneuron diversity defines variant motor circuits. Cell (in press).
Footnotes
Role of Authors
- Study concept and design: FJA.
- Acquisition of data and statistical analyses: VCS, LG-P, TMR & FJA.
- Drafting and critical revision of manuscript: FJA, VCS & NAS.
- Study supervision: FJA & NAS.
Conflict of Interest
There are no known conflicts of interest that would have inappropriately influenced the work.
Literature Cited
- Akay T, Tourtellote WG, Arber S, Jessell TM. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci USA. 2014;111:16877–16882. doi: 10.1073/pnas.1419045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara S, Soriano E, Ferrer I. Thalamic and basal forebrain afferents modulate the development of parvalbumin and calbindin D28k immunoreactivity in the barrel cortex of the rat. Eur J Neurosci. 1996;8:1522–1534. doi: 10.1111/j.1460-9568.1996.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Benito-Gonzalez A, Siembab VC. Principles of interneuron development learned from Renshaw cells and the motoneuron recurrent inhibitory circuit. Annals NY Acad of Sci. 2013;1279:22–31. doi: 10.1111/nyas.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Dewey DE, Harrington DA, Fyffe RE. Cell-type specific organization of glycine receptor clusters in the mammalian spinal cord. J Comp Neurol. 1997;379:150–170. [PubMed] [Google Scholar]
- Alvarez FJ, Dewey DE, McMillin P, Fyffe RE. Distribution of cholinergic contacts on Renshaw cells in the rat spinal cord: a light microscopic study. J Physiol. 1999;515:787–797. doi: 10.1111/j.1469-7793.1999.787ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Fyffe RE. The continuing case for the Renshaw cell. J Physiol. 2007;584:31–45. doi: 10.1113/jphysiol.2007.136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ, Goulding M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J Comp Neurol. 2005;493:177–192. doi: 10.1002/cne.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;10:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Horner PJ, Gage FH, Mendell LM. Chronic neurotrophin-3 strengthens synaptic connections to motoneurons in the neonatal rat. J Neurosci. 2003;23:8706–8712. doi: 10.1523/JNEUROSCI.23-25-08706.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanov VL, Seebacj BS, Mendell LM. NT-3 evokes an LTP-like facilitation of AMPA/kainate receptor-mediated synaptic transmission in the neonatal rat spinal cord. J Neurophysiol. 2000;84:752–758. doi: 10.1152/jn.2000.84.2.752. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378:454–467. [PubMed] [Google Scholar]
- Bacskai T, Rusznak Z, Paxinos G, Watson C. Musculotopic organization of the motor neurons supplying the mouse hindlimb muscles: a quantitative study using Fluoro-Gold retrograde tracing. Brain Struc. & Function. 2014;219:303–321. doi: 10.1007/s00429-012-0501-7. [DOI] [PubMed] [Google Scholar]
- Benito-Gonzalez A, Alvarez FJ. Renshaw cells and Ia inhibitory interneurons are generated at different times from p1 progenitors and differentiate shortly after exiting the cell cycle. J Neurosci. 2012;32:1156–1170. doi: 10.1523/JNEUROSCI.3630-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang ML, Owczarek S. A matter of balance: role of neurexin and neuroligin at the synapse. Neurochem Res. 2013;38(6):1174–1189. doi: 10.1007/s11064-013-1029-9. [DOI] [PubMed] [Google Scholar]
- Bikoff JB, Machado T, Drobac E, Mentis GM, Jessell TM. Subtype diversity of spinal V1 interneurons defined by transcription factor expression Program No 73412; 2012 Neuroscience Meeting Planner; New Orleans, LA: Society for Neuroscience. 2012; 2012 Online. [Google Scholar]
- Brownstone RM, Bui TV. Spinal interneurons providing input to the final common path during locomotion. Prog Brain Res. 2010;187:81–95. doi: 10.1016/B978-0-444-53613-6.00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal S, Ramón y. Textura del Sistema Nervioso del Hombre y de los Veterbrados. Moya; Madrid: 1899, 1904. [Google Scholar]
- Cajal S, Ramón y. Sobre las fibras nerviosas de la capa molecular del cerebelo. Rev Trim Histol Norm Patol. 1888;1:33–49. [Google Scholar]
- Cellerino A, Siciliano R, Domenici L, Maffei L. Parvalbumin immunoreactivity: a reliable marker for the effects of monocular deprivation in the rat visual cortex. Neuroscience. 1992;51(4):749–753. doi: 10.1016/0306-4522(92)90514-3. [DOI] [PubMed] [Google Scholar]
- Chen HH, Tourtellotte WG, Frank E. Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons. J Neurosci. 2002;22:3512–3519. doi: 10.1523/JNEUROSCI.22-09-03512.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy WG, Nai Q, Ross B, Naughton G, Berg DK. Postsynaptic neuroligin enhances presynaptic inputs at neuronal nicotinic synapses. Developmental Biology. 2007;307:79–91. doi: 10.1016/j.ydbio.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976;7:567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Kellerh J-O. A morphological study of the axons and recurrent axon collaterals of cat α-motoneurons supplying different types of muscle unit. J. Physiol. 1978;281:310–313. doi: 10.1113/jphysiol.1978.sp012423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S, Ulfhake B. Postnatal changes in the termination patterns of recurrent axon collaterals of triceps surae alpha-motoneurons in the cat. Dev. Brain Re. 1985;17:63–73. doi: 10.1016/0165-3806(85)90132-4. [DOI] [PubMed] [Google Scholar]
- Demireva EY, Shapiro LS, Jessell TM, Zampieri N. Motor neuron position and topographic order imposed by beta- and gamma-catenin activities. Cell. 2011;147(3):641–652. doi: 10.1016/j.cell.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nooji JC, Doobar S, Jessell TM. Etv1 inactivation reveals proprioceptor subclasses that reflect the level of NT3 expression in muscle targets. Neuron. 2013;77:1055–1068. doi: 10.1016/j.neuron.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dredge BK, Jensen KB. NeuN/Rbfox3 nuclear and cytoplasmic isoforms differentially regulate alternative splicing and nonsense mediated decay of Rbfox2. PLoS One. 2011;6:e21585. doi: 10.1371/journal.pone.0021585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Beau A, Shakya Shrestha S, Bannatyne BA, Jalicy SM, Linnen S, Maxwell DJ. Neurotransmitter phenotypes of descending systems in the rat lumbar spinal cord. Neuroscience. 2012;227:67–79. doi: 10.1016/j.neuroscience.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Iggo A, Lundberg A. Electrophysiological investigations on Renshaw cells. J Physiol. 1961;159:461–478. doi: 10.1113/jphysiol.1961.sp006821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954;126:524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach GD, Rosen KM. ARIA: a neuromuscular junction neuregulin. Ann Rev Neurosci. 1997;20:429–458. doi: 10.1146/annurev.neuro.20.1.429. [DOI] [PubMed] [Google Scholar]
- Fyffe REW. Evidence for separate morphological classes of Renshaw cell in the cat’s spinal cord. Brain Res. 1990;536:301–304. doi: 10.1016/0006-8993(90)90038-d. [DOI] [PubMed] [Google Scholar]
- Fukuhara K, Imai F, Ladle DR, Katayama K, Leslie JR, Arber S, Jessell TM, Yoshida Y. Specificity of monosynaptic sensory-motor connections imposed by repellent Sema3E-PlexinD1 signaling. Cell Rep. 2013;5:748–758. doi: 10.1016/j.celrep.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiman EJ, Knox MC, Alvarez FJ. Postnatal maturation of gephyrin/glycine receptor clusters on developing Renshaw cells. J Comp Neurol. 2000;426:130–142. doi: 10.1002/1096-9861(20001009)426:1<130::aid-cne9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Geiman EJ, Zheng W, Fritschy JM, Alvarez FJ. Glycine and GABA(A) receptor subunits on Renshaw cells: relationship with presynaptic neurotransmitters and postsynaptic gephyrin clusters. J Comp Neurol. 2002;444:275–289. doi: 10.1002/cne.10148. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Forero D, Alvarez FJ. Differential postnatal maturation of GABAA, glycine receptor, and mixed synaptic currents in Renshaw cells and ventral spinal interneurons. J Neurosci. 2005;25:2010–2023. doi: 10.1523/JNEUROSCI.2383-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Forero D, Pastor AM, Geiman EJ, Benitez-Temino B, Alvarez FJ. Regulation of gephyrin cluster size and inhibitory synaptic currents on Renshaw cells by motor axon excitatory inputs. J Neurosci. 2005;25:417–429. doi: 10.1523/JNEUROSCI.3725-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm TM. Recurrent inhibition to and from motoneurons innervating the flexor digitorum and flexor hallucis longus muscles of the cat. J. Nuerophsyiol. 1990;63:395–403. doi: 10.1152/jn.1990.63.3.395. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. Wiley; New York: 1949. [Google Scholar]
- Hellstrom J, Arvidsson U, Elde R, Cullheim S, Meister B. Differential expression of nerve terminal protein isoforms in VAChT-containing varicosities of the spinal cord ventral horn. J Comp Neurol. 1999;411:578–590. [PubMed] [Google Scholar]
- Hippenmeyer S, Shneider NA, Birchmeier C, Burden SJ, Jessell TM, Arber S. A role for neuregulin1 signaling in muscle spindle differentiation. Neuron. 2002;36:1035–1049. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Jankowska E, Lindstrom S. Relative contributions from different nerves to recurrent depression of Ia IPSPs in motoneurons. J. Physiol. 1971;215:637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Mannen H, Hongo T, Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol. 1979;186:189–211. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog in Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol. 2001;533:31–40. doi: 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, Cho SM, Lee CK, Jeong SW. Neuregulin 1 as an endogenous regulator of nicotinic acetylcholine receptors in adult major pelvic ganglion neurons. Biochem Biophys Res Comm. 2015;463:632–637. doi: 10.1016/j.bbrc.2015.05.113. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Kozorovitskiy Y, Oh WJ, Peixoto RT, Akhtar N, Saulnier JL, Gu C, Sabatini BL. Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nature Neuroscience. 2012;15:1667–1674. doi: 10.1038/nn.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Bio Chem. 2009;284:31052–31061. doi: 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Saito T, Anderson DJ, Lance-Jones C, Jessell TM, Arber S. Functionally related motor neuron pool and muscle sensory afferent subtypes defined by coordinate ETS gene expression. Cell. 1998;95(3):393–407. doi: 10.1016/s0092-8674(00)81770-5. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tao YM, Woo RS, Xiong WC, Mei L. Stimulated ErbB4 internalization is necessary for neuregulin signaling in neurons. Biochem Biophys Res Comm. 2007;354:505–510. doi: 10.1016/j.bbrc.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Phil Trans Royal Soc Lond Series B, Biological Sciences. 1981;293:477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- Mei F, Zhu J, Guo S, Zhou DS, Han J, Yu B, Li SF, Jiang ZY, Xiong CJ. An age-dependent proliferation is involved in the postnatal development of interstitial cells of Cajal in the small intestine of mice. Histochem and Cell Biol. 2009;131:43–53. doi: 10.1007/s00418-008-0515-7. [DOI] [PubMed] [Google Scholar]
- Mendelsohn AI, Simon CM, Abbot LF, Mentis GZ, Jessell TM. Activity regulates the incidence of heteronymous sensory-motor connections. Neuron. 2015;87:111–123. doi: 10.1016/j.neuron.2015.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O’Donovan MJ. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci U S A. 2005;102:7344–7349. doi: 10.1073/pnas.0502788102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Siembab VC, Zerda R, O’Donovan MJ, Alvarez FJ. Primary afferent synapses on developing and adult Renshaw cells. J Neurosci. 2006;26:13297–13310. doi: 10.1523/JNEUROSCI.2945-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Watanabe M. Development of an anatomical technique for visualizing the mode of climbing fiber innervation in Purkinje cells and its application to mutant mice lacking GluRdelta2 and Ca(v)2.1. Anat Sci Int. 2011;86:10–18. doi: 10.1007/s12565-010-0095-1. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nicolopoulos-Stournaras S, Iles JF. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol. 1983;217:75–85. doi: 10.1002/cne.902170107. [DOI] [PubMed] [Google Scholar]
- Munson JB, Johnson RD, Mendell LM. NT-3 increases amplitude of EPSPs produced by axotomized group Ia afferents. J Neurophysiol. 1997;77:2209–2212. doi: 10.1152/jn.1997.77.4.2209. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci U S A. 2005;102:5245–5249. doi: 10.1073/pnas.0501331102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Oliveira Fernandes M, Tourtellotte WG. Egr3-dependent muscle spindle stretch receptor intrafusal muscle fiber differentiation and fusimotor innervation. J. Neusroci. 2015;35:5566–5578. doi: 10.1523/JNEUROSCI.0241-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M, Itoh K, Miyakawa Y, Kishida H, Hashikawa T. Protein processing and releases of neuregulin-1 are regulated in an activity-dependent manner. J Neurochem. 2004;91:176–188. doi: 10.1111/j.1471-4159.2004.02719.x. [DOI] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex. Springer-Verlag; New York: 1974. [Google Scholar]
- Patz S, Grabert J, Gorba T, Wirth MJ, Wahle P. Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an early period of postnatal development. Cerebral Cortex. 2004;14:342–351. doi: 10.1093/cercor/bhg132. [DOI] [PubMed] [Google Scholar]
- Pecho-Vriesling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;450:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Lim JH, Brunjes PC. Activity-dependent regulation of calcium-binding proteins in the developing rat olfactory bulb. J Comp Neurol. 1997;387:12–26. [PubMed] [Google Scholar]
- Pierce JP, Mendell LM. Quantitative ultrastructure of Ia boutons in the ventral horn: scaling and positional relationships. J Neurosci. 1993;13:4748–4763. doi: 10.1523/JNEUROSCI.13-11-04748.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Ruda MA. A comparative study of the calcium-binding proteins calbindin-D28K, calretinin, calmodulin and parvalbumin in the rat spinal cord. Brain Res Rev. 1994;19:163–179. doi: 10.1016/0165-0173(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Renshaw B. Central effects of centripetal impulses in axons of spinal ventral roots. J Neurophysiol. 1946;9:191–204. doi: 10.1152/jn.1946.9.3.191. [DOI] [PubMed] [Google Scholar]
- Romanes GJ. The Motor Pools of the Spinal Cord. Prog Brain Res. 1964;11:93–119. doi: 10.1016/s0079-6123(08)64045-5. [DOI] [PubMed] [Google Scholar]
- Rotterman TM, Nardelli P, Cope TC, Alvarez FJ. Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury. J Neurosci. 2014;34:3475–3492. doi: 10.1523/JNEUROSCI.4768-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross H-G, Cleveland S, Haase J. Contribution of single motoneurons to Renshaw cell activity. Neurosci Lett. 1979;1:105–108. doi: 10.1016/0304-3940(75)90053-1. [DOI] [PubMed] [Google Scholar]
- Ryall RW, Piercey MF. Excitation and inhibition of Renshaw cells by impulses in peripheral afferent nerve fibers. J Neurophysiol. 1971;34:242–251. doi: 10.1152/jn.1971.34.2.242. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Cushman J, Jordan B, Samuels A, Frazer H, Baier C. Topographic position of forelimb motoneuron pools is conserved in vertebrate evolution. Brain, Behavior and Evolution. 1998;51:90–99. doi: 10.1159/000006531. [DOI] [PubMed] [Google Scholar]
- Sapir T, Geiman EJ, Wang Z, Velasquez T, Mitsui S, Yoshihara Y, Frank E, Alvarez FJ, Goulding M. Pax6 and engrailed 1 regulate two distinct aspects of renshaw cell development. J Neurosci. 2004;24:1255–1264. doi: 10.1523/JNEUROSCI.3187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Meller D, Eysel UT. Immunohistochemical changes of neuronal calcium-binding proteins parvalbumin and calbindin-D-28k following unilateral deafferentation in the rat visual system. Exp Neurol. 1992;117:230–246. doi: 10.1016/0014-4886(92)90132-a. [DOI] [PubMed] [Google Scholar]
- Shneider NA, Brown MN, Smith CA, Pickel J, Alvarez FJ. Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived GDNF for postnatal survival. Neural Development. 2009a;4:42. doi: 10.1186/1749-8104-4-42. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider NA, Mentis GZ, Schustak J, O’Donovan MJ. Functionally reduced sensorimotor connections form with normal specificity despite abnormal muscle spindle development: the role of spindle-derived neurotrophin 3. J Neurosci. 2009b;29:4719–4735. doi: 10.1523/JNEUROSCI.5790-08.2009. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siembab VC, Smith CA, Zagoraiou L, Berrocal MC, Mentis GZ, Alvarez FJ. Target selection of proprioceptive and motor axon synapses on neonatal V1-derived Ia inhibitory interneurons and Renshaw cells. J Comp Neurol. 2010;518:4675–4701. doi: 10.1002/cne.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner PM, Alvarez FJ. Developmental divergence of intrinsic firing properties among spinal V1 interneurons; 2010 Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience. 2010; Program No 49013. 2010 Online. [Google Scholar]
- Surmeli G, Akay T, Ippolito GC, Tucker PW, Jessell TM. Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell. 2011;147:653–665. doi: 10.1016/j.cell.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, Liu X, Xiong WC, Mei L. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011;31:15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Vancura R, Patterson CL, Williams JM, Riekhof JT, Wright DE. Postnatal regulation of limb proprioception by muscle-derived neurotrophin-3. J Comp Neurol. 2001;432:244–258. doi: 10.1002/cne.1100. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. The Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Tourtellotte WG, Keller-Peck C, Milbrandt J, Kucera J. The transcription factor Egr3 modulates sensory axon-myotube interactions during muscle spindle morphogenesis. Dev Biol. 2001;232:388–399. doi: 10.1006/dbio.2001.0202. [DOI] [PubMed] [Google Scholar]
- Tourtellotte WG, Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nature Genetics. 1998;20:87–91. doi: 10.1038/1757. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol. 1997;382:46–76. [PubMed] [Google Scholar]
- Van Keulen L. Autogenetic recurrent inhibition of individual spinal motoneurones of the cat. Neurosci Lett. 1981;21:297–300. doi: 10.1016/0304-3940(81)90220-2. [DOI] [PubMed] [Google Scholar]
- Varley ZK, Pizzarelli R, Antonelli R, Stancheva SH, Kneussel M, Cherubini E, Zacchi P. Gephyrin regulates GABAergic and glutamatergic synaptic transmission in hippocampal cell cultures. J Biol Chem. 2011;286:20942–20951. doi: 10.1074/jbc.M111.234641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriesling E, Arber A. Tragte-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452. doi: 10.1016/j.cell.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li LY, Taylor MD, Wright DE, Frank E. Prenatal exposure to elevated NT3 disrupts synaptic selectivity in the spinal cord. J Neurosci. 2007;27:3686–3694. doi: 10.1523/JNEUROSCI.0197-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M. Molecular mechanisms governing competitive synaptic wiring in cerebellar Purkinje cells. Journal of Experimental Medicine. 2008;214:175–190. doi: 10.1620/tjem.214.175. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kano M. Climbing fiber synapse elimination in cerebellar Purkinje cells. The Eur J Neurosci. 2011;34:1697–1710. doi: 10.1111/j.1460-9568.2011.07894.x. [DOI] [PubMed] [Google Scholar]
- Watson C, Paxinos G, Kayalioglu G. The Spinal Cord. A Christopher and Dana Reeve Foundation Text and Atlas. Elsevier; Amsterdam: 2009. [Google Scholar]
- Westerga J, Gramsbergen A. The development of locomotion in the rat. Brain Res Dev Brain Res. 1990;57:163–172. doi: 10.1016/0165-3806(90)90042-w. [DOI] [PubMed] [Google Scholar]
- Wootz H, Fitzsimons-Kantamneni E, Larhammar M, Rotterman TM, Enjin A, Patra K, Andre E, Van Zundert B, Kullander K, Alvarez FJ. Alterations in the motor neuron-renshaw cell circuit in the Sod1(G93A) mouse model. J Comp Neurol. 2013;521:1449–1469. doi: 10.1002/cne.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Williams JM, McDonald JT, Carlsten JA, Taylor MD. Muscle-derived neurotrophin-3 reduces injury-induced proprioceptive degeneration in neonatal mice. J Neurobiol. 2002;50:198–208. doi: 10.1002/neu.10024. [DOI] [PubMed] [Google Scholar]
- Wright DE, Zhou L, Kucera J, Snider WD. Introduction of a Neurotrophin-3 Transgene into Muscle Selectively Rescues Proprioceptive Neurons in Mice Lacking Endogenous Neurotrophin-3. Neuron. 1997;19:503–517. doi: 10.1016/s0896-6273(00)80367-0. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Morita Y, Hironaka T, Emson PC, Tohyama M. Ontological study of calbindin-D28k-like and parvalbumin-like immunoreactivities in rat spinal cord and dorsal root ganglia. J Comp Neurol. 1990;302:715–728. doi: 10.1002/cne.903020404. [DOI] [PubMed] [Google Scholar]