Abstract
Obesity is characterized by increased circulating levels of the adipocyte-derived hormone leptin, which can increase sympathetic nerve activity and raise blood pressure. A previous study revealed that rats fed a high fat diet (HFD) have an enhanced hypertensive response to subsequent angiotensin (Ang) II administration that is mediated at least in part by increased activity of brain renin-angiotensin system (RAS) and proinflammatory cytokines (PICs). The present study tested whether leptin mediates this HFD-induced sensitization of Ang II-elicited hypertension by interacting with brain RAS and PICs mechanisms. Rats fed a HFD for 3 weeks had significant increases in white adipose tissue mass, plasma leptin levels and mRNA expression of leptin and its receptors in the lamina terminalis (LT) and hypothalamic paraventricular nucleus (PVN). Central infusion of a leptin receptor antagonist during HFD feeding abolished HFD sensitization of Ang II-elicited hypertension. Furthermore, central infusion of leptin mimicked the sensitizing action of HFD. Concomitant central infusions of the AT1-R antagonist irbesartan, the TNF-α synthesis inhibitor pentoxifylline, or the inhibitor of microglial activation minocycline prevented the sensitization produced by central infusion of leptin. RT-PCR analysis indicated that either HFD or leptin administration upregulated mRNA expression of several components of the RAS and PICs in the LT and PVN. The leptin antagonist and the inhibitors of AT1-R, TNF-α synthesis and microglial activation all reversed the expression of these genes. The results suggest that HFD-induced sensitization of Ang II-elicited hypertension is mediated by leptin through upregulation of central RAS and PICs.
Keywords: High fat diet, Leptin, Sensitization, blood pressure, Renin-angiotensin system, Proinflammatory cytokine
Introduction
Obesity has become epidemic and is a serious health problem and a major risk factor for the development of hypertension. Accumulating evidence indicates that obesity causes chronic low-grade inflammation associated with systemic metabolic dysfunction that is accompanied by increased renin-angiotensin system (RAS) activity.1,2 Recent studies have demonstrated that the RAS and proinflammatory cytokines (PICs) in the central nervous system (CNS) play a critical role in mediating obesity-induced sensitization and obesity-related hypertension.3,4,5
The adipokine leptin is released in proportion to total fat and primarily acts on the hypothalamus to maintain energy homeostasis and normal body weight.6 Similar to the RAS and PICs, Leptin can act in the CNS to enhance sympathetic nervous system activity (SNA) that contributes to the development of hypertension associated with obesity.7,8 Simonds et al. demonstrated that obesity-induced elevation of circulating leptin increases blood pressure (BP) by acting on leptin receptors (LEPRs) in the dorsomedial hypothalamus (DMH). Furthermore, humans with loss-of-function mutations in leptin and the LEPRs have low BP despite severe obesity.9 Head and colleagues recently reported that 3 weeks of high fat diet (HFD) feeding led to increased mean arterial pressure (MAP), heart rate (HR) and renal SNA in rabbits. This obesity-induced hypertension is accompanied by an increase in plasma leptin levels and can be blocked by central infusion of a leptin antagonist.10,11,12
A recent study showed an interaction between leptin and the RAS in regulating SNA.13 Central infusion of leptin upregulates brain RAS components in specific brain regions. Conversely, central inhibition of angiotensin II type 1 receptor (AT1-R) or global genetic knockout of angiotensin-converting enzyme (ACE) reduces circulating leptin levels.13 These results suggest that the brain RAS plays a facilitatory role in sympathetic nerve responses to leptin. It has been shown that obesity can induce selective leptin resistance whereby the anorexic effect of leptin is attenuated, but its action to increase SNA and BP remains intact.14 One recent study showed that the leptin resistance was induced by obesity-associated activation of the hypothalamic nuclear factor κB/IκB kinase-β (NF-κB/IKK-β) pathway.4 Tumor necrosis factor (TNF)-α also can directly influence leptin signaling activity by modulating LEPR expression.15 These findings suggest that the interactions and synergism between leptin, the RAS and PICs may be responsible for the obesity-induced increase in SNA which occurs early in the process or secondary to long-standing obesity.
Hypothalamic nuclei such as DMH and arcuate nucleus (ARC) are the main sites of leptin action.9,16 However, the presence of the blood–brain barrier (BBB) leads to the obvious question as to how leptin, a 16 kDa peripherally-derived protein, gains access to the central sites to influence BP. Recently, the subfornical organ (SFO), a sensory circumventricular organ lacking the normal BBB, has been demonstrated to be an important target of leptin that mediates leptin-induced increases in renal SNA.17 Our previous work demonstrated that short term HFD feeding can upregulate the RAS and PICs in the lamina terminalis (LT) including the SFO and sensitizes the hypertensive response to a pressor dose of angiotensin (Ang) II.5 However, it is unclear what factors induce upregulation of the RAS and PICs in the SFO under the condition when there is no obvious increase in the plasma levels of Ang II or PICs after short term HFD feeding. Given that the enhanced effect of leptin on SNA and increased plasma levels of leptin in obesity, it is reasonable to hypothesize that HFD feeding may first increase plasma leptin which then acts on the brain to induce sensitization of the Ang II-elicited hypertensive response through upregulation of the brain RAS and PICs signaling pathways.
Methods
Experimental Protocol
Male Sprague-Dawley rats (10–12 weeks old, Harlan, n=96) were used. Rats were prepared with a lateral ventricular cannula, osmotic minipumps for intracerebroventricular (ICV) and subcutaneous drug infusion, and with telemetry probes for continuous BP monitoring, as previously described.18,19 The ICV dose of leptin and doses of agents used to block LEPRs, AT1-R, TNF-α synthesis or microglial activation were chosen on the basis of published in vivo studies.5,18,20–23
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by The University of Iowa Animal Care and Use Committee.
Effects of central blockade of leptin receptor on HFD-induced sensitization and molecular events in the LT and paraventricular nucleus of hypothalamus (PVN)
BP was monitored continuously by telemetry in rats that were fed either normal fat diet (NFD, 10% calories from lard, 3.85 kcal/g, D12450J, Research Diet, NJ) or HFD (60% calories from lard, 5.24 kcal/g, D12492, Research Diet, NJ) for three weeks (Induction period, I), followed by NFD for two weeks which coincided with an Expression period (E). During I, rats received ICV vehicle (V) or rat super-active leptin antagonist (SHLA, 20 µg/d, Cat. SLAN-4, Protein Laboratories, Rehovot, Israel). This antagonist was delivered by osmotic pumps (model 2004, 0.25 µl/h for 4 weeks, Alzet) that were disconnected before Ang II infusion. During E, a slow pressor dose of Ang II (120 ng/kg/min) was delivered subcutaneously by osmotic pump (model 2002, 0.5 µl/h for 2 weeks, Alzet). Thus, the primary study groups (n=6/group) were: 1) I-NFD + ICV V + E-Ang II, 2) I-HFD + ICV V + E-Ang II, 3) I-HFD + ICV SHLA + E-Ang II.
Total adipose tissue mass in three treatment groups (n=6/group) (I-NFD + ICV V, I-HFD + ICV V and I-HFD + ICV SHLA) following I but prior to E was determined by NMR spectroscopy using a Bruker mini-spec LF 90II instrument (Bruker Corporation, Billerica, MA). Plasma, inguinal, retroperitoneal and epididymal white adipose tissue (WAT) and brains were then collected for analysis of plasma levels of leptin, measurement of WAT mass and RT-PCR for mRNA expression, respectively. The microdissected tissue samples for mRNA expression contained the structures lying along the LT [i.e., the SFO, median preoptic nucleus (MnPO), organum vasculosum of the lamina terminalis (OVLT)] and the PVN. Food consumption, water intake and body weight (BW) were measured once a week.
Effects of brain RAS and inflammation on leptin-induced sensitization and molecular events in the LT and PVN
Rats were fed normal rat chow (7013 NIH-31 modified rat diet) ad libitum. BP was monitored continuously during an Induction-Delay-Expression (I-D-E) protocol, as described previously.18,19 During I, ICV vehicle, leptin (20 ng/kg/min), or leptin plus the TNF-α synthesis inhibitor pentoxifylline (PTX, 10 µg/h), the AT1-R blocker irbesartan (Irbe, 125 µg/d) or the inhibitor of microglial activation minocycline (Mino, 5 µg/h) was delivered by osmotic minipump (model 2001, Alzet) for 1 week. To ensure that any exogenous leptin and antagonists were metabolized, the rats then rested for 1 week (D). After this time, a second pump (model 2002, 0.5µl/h Alzet) was implanted to deliver a slow pressor dose of Ang II (120 ng/kg/min) for 2 weeks (E). Thus, the primary study groups (n=6/group) were: 1) I-ICV V + E-Ang II, 2) I-ICV leptin + E-Ang II, 3) I-ICV leptin/Irbe + E-Ang II, 4) I-ICV leptin/PTX + E-Ang II, and 5) I-ICV leptin/Mino + E-Ang II. Food consumption, water intake and BW were measured once a week.
Five additional groups ( n=6/group) underwent identical treatment during I (I-ICV V, I-ICV leptin, I-ICV leptin/Irbe, I-ICV leptin/PTX and I-ICV leptin/Mino) but were euthanized at the end of the D period to collect LT and PVN for analysis of mRNA expression.
Data Analysis
The significance of differences in MAP and HR among groups was analyzed by 2-way ANOVAs followed by Tukey multiple comparison tests. One-way ANOVAs were used to analyze the differences in plasma leptin level, adipose tissue mass and in mRNA expression of brain leptin and its receptors, RAS components and PICs in all groups. All data are expressed as means ± SE. Statistical significance was set at P < 0.05.
Additional Methods
Please see the online-only Data Supplement.
Results
Effects of HFD on Adipose Tissue Mass, Plasma Leptin Level and mRNA Expression of Leptin and LEPRs
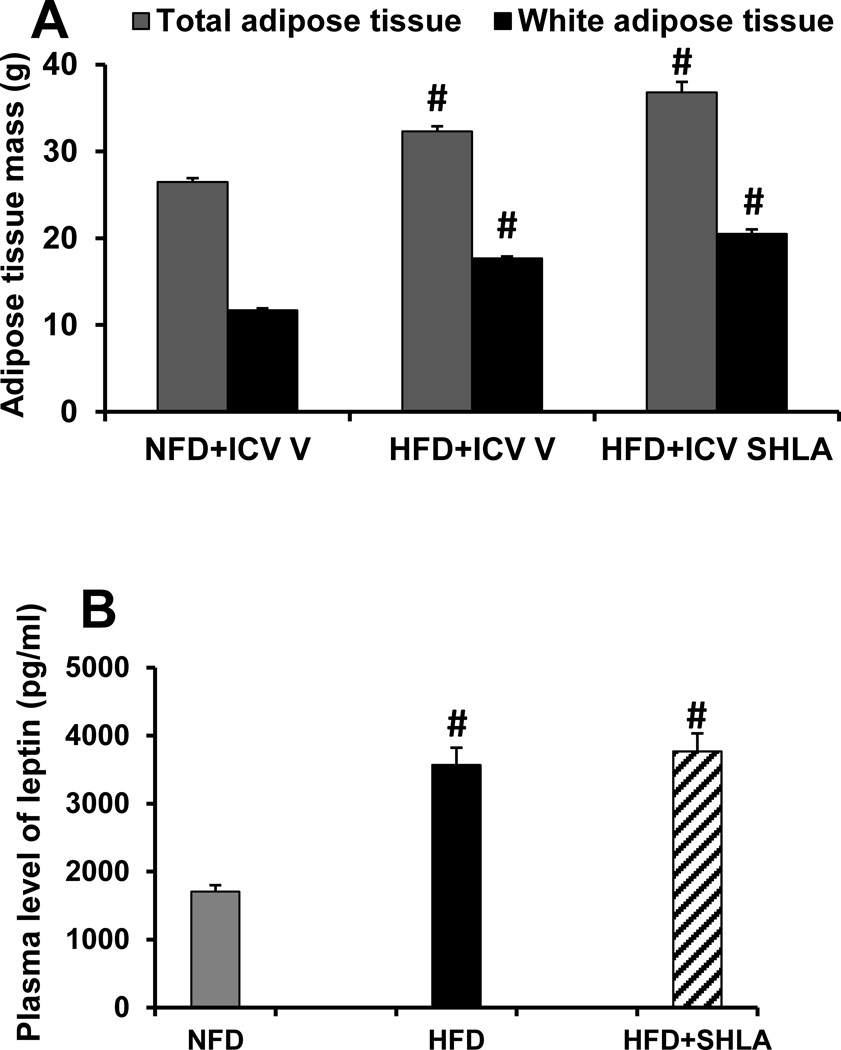
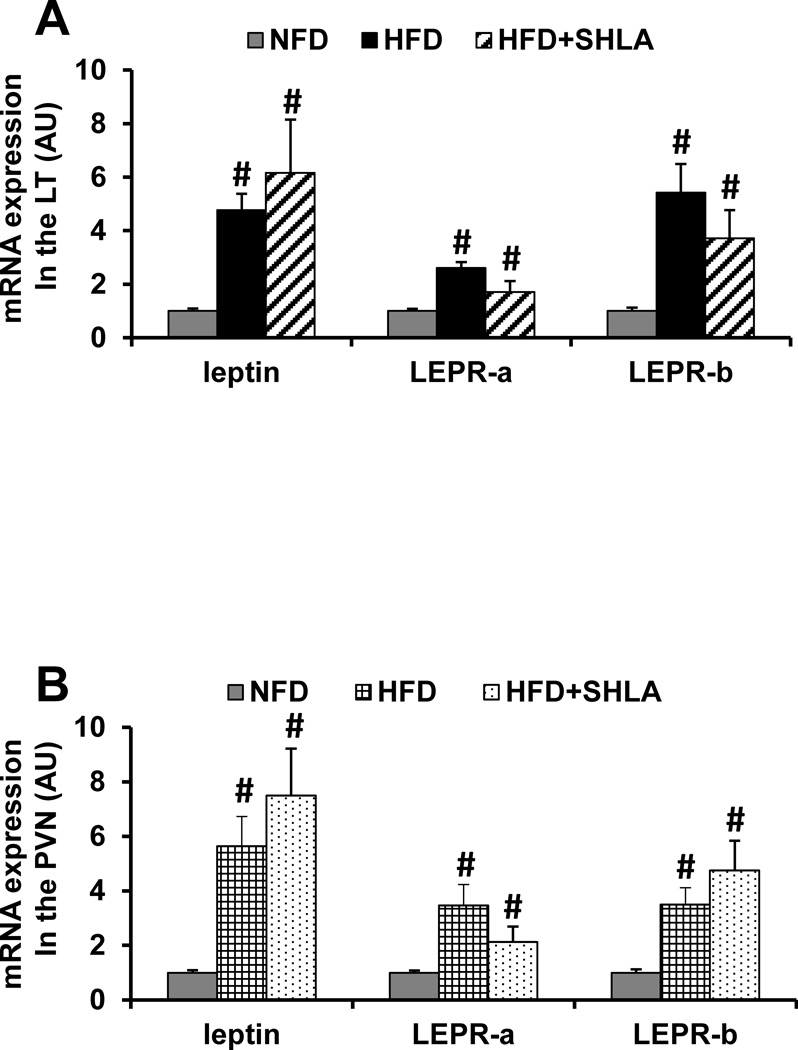
Total adipose tissue mass, WAT mass (Fig. 1A) and plasma leptin concentration (Fig. 1B) were significantly increased after 3 weeks of HFD when compared to those of NFD (p<0.05). Central infusion of the leptin antagonist SHLA slightly enhanced HFD-induced increase in total adipose mass and WAT mass, but had no influence on increased plasma leptin concentration. Likewise, significant increases in mRNA expression of leptin, LEPR-a and LEPR-b were evident in the SFO (Fig. 2A) and PVN (Fig. 2B) of HFD fed rats compared with rats fed NFD (p<0.05). Central infusion of the leptin antagonist SHLA during HFD had no influence on these increases in mRNA expression.
Figure 1.
Changes in total adipose tissue mass, sum of inguinal, retroperitoneal and epididymal white adipose tissue mass (Fig. 1A) and plasma level of leptin (Fig. 1B) in normal fat diet (NFD) rats and high fat diet (HFD) rats treated with central (ICV) vehicle (V) or leptin receptor antagonist (SHLA). (# p<0.05 vs. NFD rats)
Figure 2.
Quantitative comparison of the mRNA expression of leptin and leptin receptors (LEPR-a and LEPR-b) in the lamina terminalis (LT, Fig 2A) and paraventricular nucleus (PVN, Fig 2B) of high fat diet (HFD) feeding rats receiving central (ICV) infusion of vehicle or leptin receptor antagonist (SHLA) as compared with those receiving normal fat diet (NFD) feeding. (# p<0.05 vs HFD with ICV vehicle).
Effect of SHLA on HFD Sensitization of Ang II-induced Hypertension
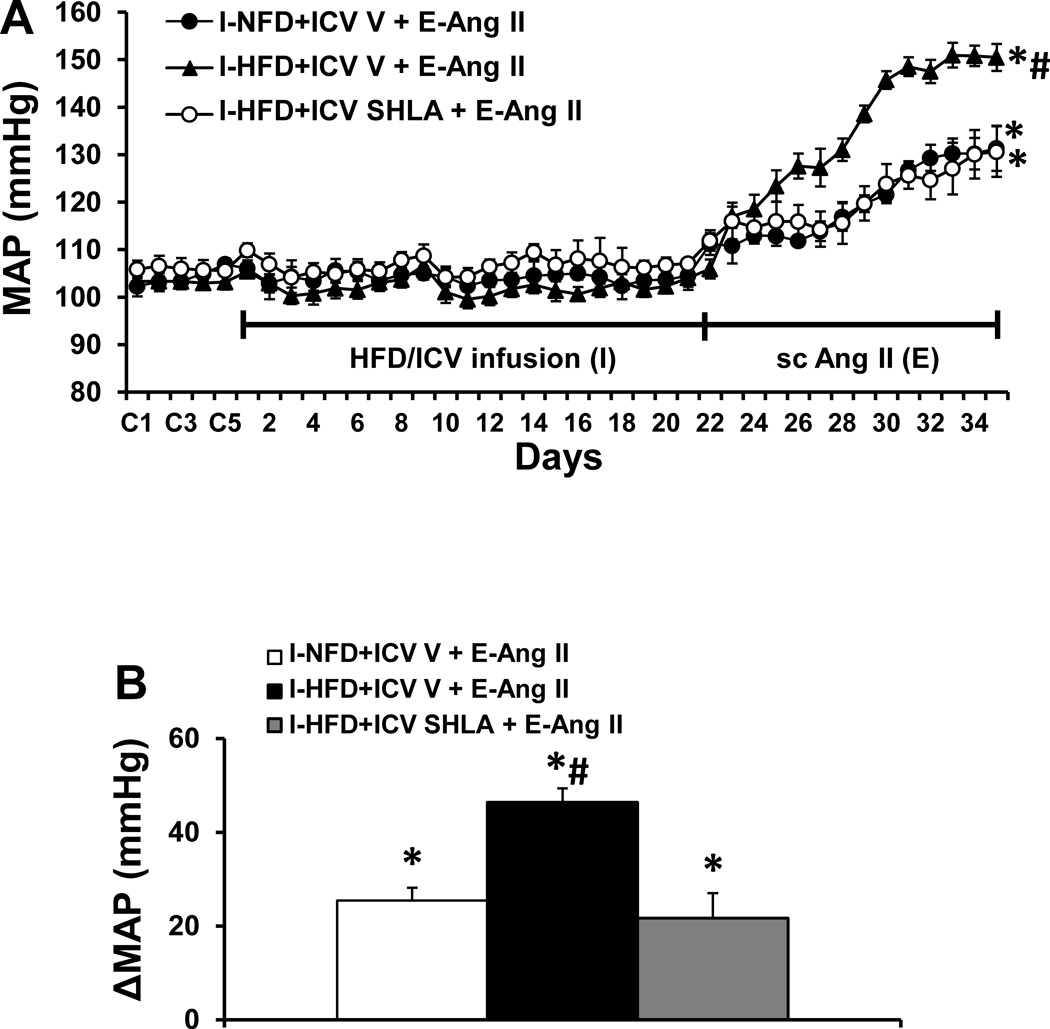
Baseline values for MAP (103.8±1.9 mmHg) and HR (348.4±6.6 beats/min) were comparable prior to and following application of HFD or HFD plus ICV infusion of leptin antagonist in all groups of rats. However, over the course of E, Ang II induced a greater increase in MAP in the rats that received the HFD pretreatment (Δ46.4 ± 3.9 mmHg, p<0.05, Fig. 3A, 3B) as compared to the rats pretreated with NFD (Δ24.9 ± 3.8 mmHg). This augmentation of the pressor effect induced by Ang II was blocked by concurrent ICV infusion of leptin antagonist (Δ21.7 ± 5.3 mmHg) along with the HFD pretreatment (p<0.05). Systemic Ang II infusions produced slight, comparable decreases in HR in all groups (Fig. S2).
Figure 3.
Augmented pressor effects induced by Ang II during the expression (E) period in rats after pretreatment with HFD during the induction (I) period (Fig. 3A). This effect was attenuated by central (ICV) blockade of leptin receptors. Figure 3B shows the changes in MAP after infusion of Ang II during E in all groups. I-NFD = pretreatment with normal fat diet during I; I-HFD = pretreatment with high fat diet during I; ICV V = central treatment with vehicle during I; I-HFD+ICV SHLA = pretreatment with HFD plus central treatment with leptin receptor antagonist during I; E-Ang II = peripheral treatment with a pressor dose of Ang II during E. (* p<0.05 vs baseline or after diet treatment; # p<0.05 vs I-NFD+ICV V + E-Ang II and I-HFD+ICV SHLA+ E-Ang II)
Effect of ICV Leptin Antagonist on HFD-induced mRNA Expression of RAS Components, PICs and Microglial Marker in the LT and PVN
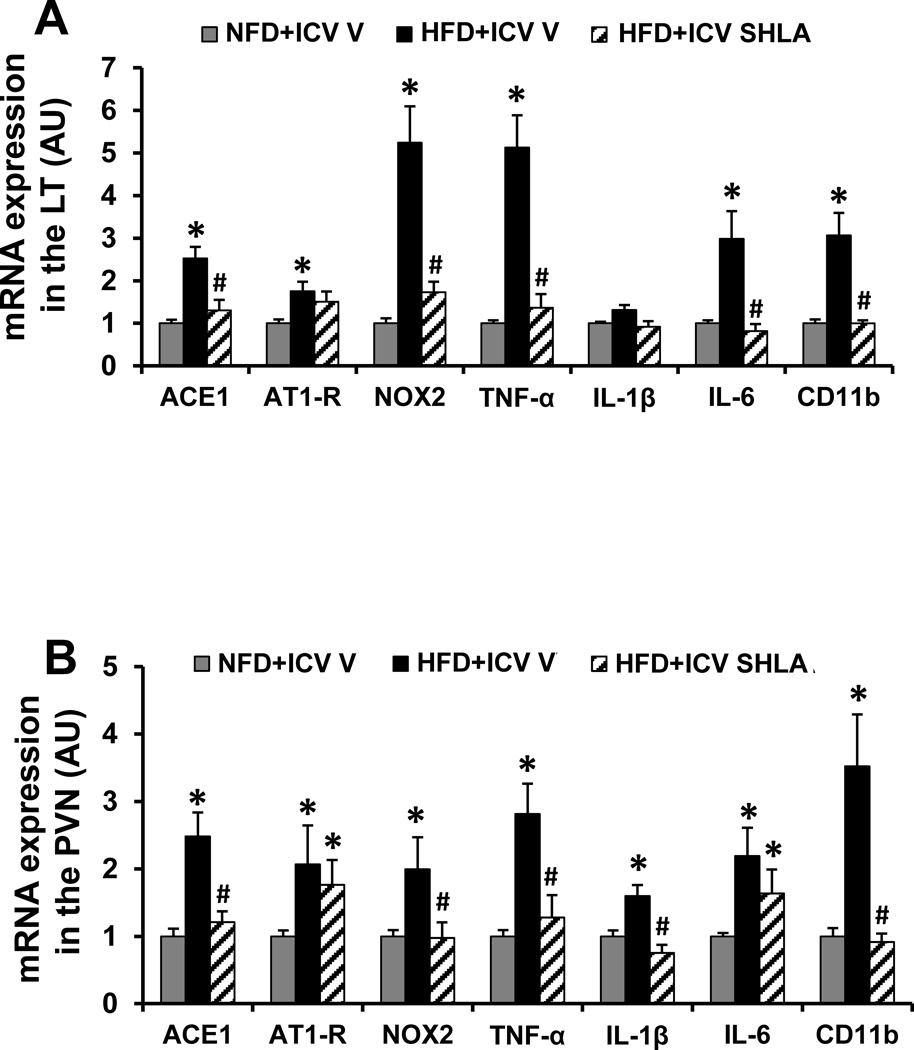
In LT tissue collected at the end of HFD feeding, HFD induced a significant increase in the mRNA expression of the RAS components (AT1-R and ACE1), the NADPH oxidase subunit (i.e. NOX2) and the inflammatory elements (i.e. TNF-α, IL-6, the microglial marker CD11b) in the LT when compared with controls (p<0.05, Fig. 4A). The expression of IL-1β in the LT was not higher after HFD (p>0.05, Fig 4A). Central infusion of SHLA significantly attenuated the increased mRNA expression of ACE1, NOX2, TNF-α, IL-6 and CD11b (p<0.05, Fig. 4A).
Figure 4.
Quantitative comparison of the mRNA expression of renin-angiotensin system components, proinflammatory cytokines and microglial marker in the lamina terminalis (LT, Fig 4A) and paraventricular nucleus (PVN, Fig 4B) of high fat diet (HFD) feeding rats receiving central (ICV) infusion of leptin receptor antagonist SHLA as compared with those receiving normal fat diet (NFD) feeding. (* p<0.05 vs NFD; # p<0.05 vs HFD with ICV vehicle).
In PVN tissue collected at the end of HFD feeding, HFD also induced a significant increase in the mRNA expression of the RAS components (AT1-R and ACE1), the NADPH oxidase subunit (i.e. NOX2) and the inflammatory elements (i.e. TNF-α, IL-1β, IL-6, the microglial marker CD11b) when compared with controls (p<0.05, Fig. 4B). Central infusion of SHLA normalized the increased mRNA expression of ACE1, NOX2, TNF-α, IL-1β and CD11b, but had no effect on the increased expression of AT1-R and IL-6 (p<0.05, Fig. 4B).
Effect of ICV PTX, Irbe or Mino on ICV Leptin Sensitization of Ang II hypertension
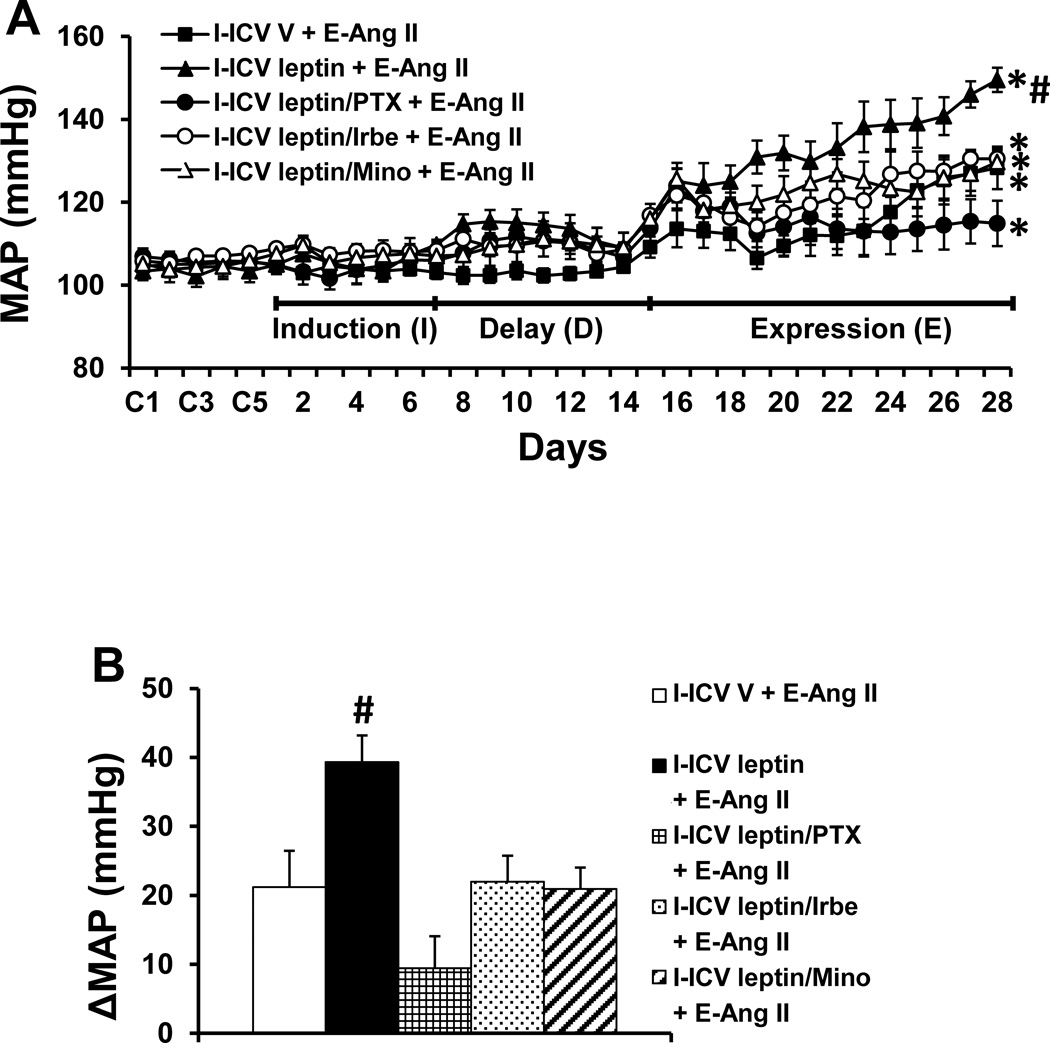
To confirm the direct sensitizing actions of central leptin and the RAS and PICs mediation of central leptin-induced sensitization, leptin alone or leptin combined with TNF-α synthesis inhibitor, AT1-R blocker or inhibitor of microglial activation was infused ICV for 1 week during I. These treatments resulted in a mild but significant increase in MAP during D which gradually returned to the basal level by the end of D (Fig. 5A). Subsequent Ang II-induced hypertension during E was greater in the groups receiving leptin during I in comparison to those groups receiving vehicle (leptin Δ39.3±3.8 mmHg vs. vehicle Δ21.2±5.2 mmHg, p<0.05, Fig. 5A, 5B), which mimicked the HFD sensitization of Ang II-induced hypertension (Δ46.4 ± 3.9 mmHg). This augmentation of the pressor effect induced by leptin was prevented by concurrent ICV infusion of PTX, Irbe or Mino along with the ICV leptin administrated during I (PTX Δ9.5±4.6 mmHg, Irbe Δ21.9±3.8 mmHg, Mino Δ20.9±3.1 mmHg, p<0.05, Fig. 5A, 5B).
Figure 5.
Augmented pressor effects induced by Ang II during the expression (E) period in rats after central treatment with leptin during the induction (I) period (Fig. 5A). The sensitizing effect of leptin was attenuated by central (ICV) inhibition of TNF-α synthesis, AT1-R or microglial activation. Figure 5B shows the changes in MAP after infusion of Ang II during E in all groups. ICV V = central treatment with vehicle during I; ICV leptin = central treatment with leptin during I; I-ICV leptin/PTX = central concurrent treatment with leptin and TNF-α synthesis inhibitor pentoxifylline during I; ICV leptin/Irbe = central concurrent treatment with leptin and AT1-R blocker irbesartan during I; I-ICV leptin/Mino = central concurrent treatment with leptin and inhibitor of microglial activation minocycline during I; E-Ang II = peripheral treatment with a pressor dose of Ang II during E. (* p<0.05 vs baseline; # p<0.05 vs I-ICV V + E-Ang II and other groups treated with central blockers during I).
ICV leptin infusion during I produced a significant increase in HR, which was sustained throughout I and D and most of E, but then gradually returned to the basal level by the end of E. Concomitant ICV infusion of either PTX or Mino had no effect on this leptin-induced increase in HR, whereas Irbe blocked it (Fig. S3).
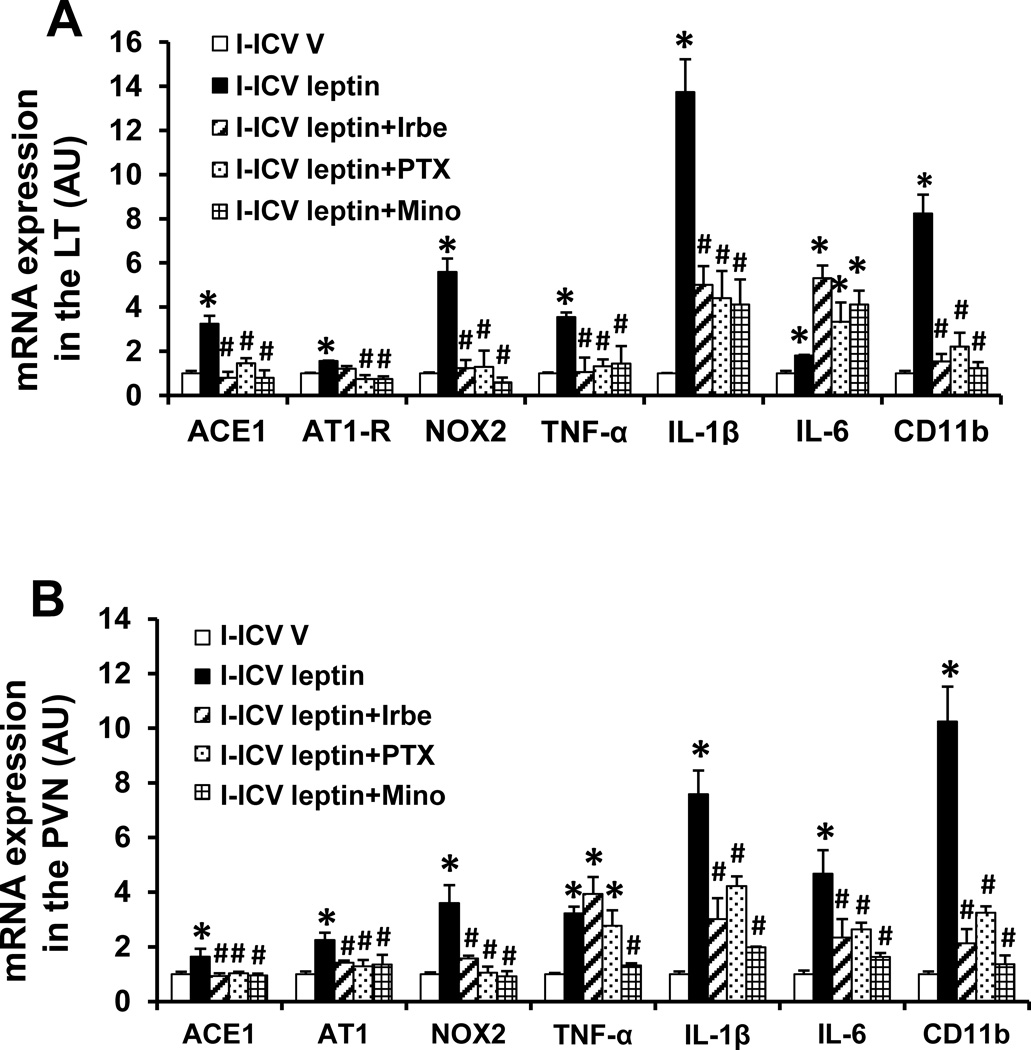
Effect of ICV PTX, Irbe or Mino on Leptin-induced mRNA Expression of RAS Components, PICs and microglial marker in the LT and PVN
In LT and PVN tissue collected at the end of D, the ICV leptin given during I produced a significant increase in the mRNA expression of AT1-R, ACE1, NOX2, TNF-α, IL-1β, IL-6 and CD11b when compared with controls (p<0.05, Fig. 6A, 6B). Central concurrent infusion of PTX, Irbe or Mino along with a sensitizing dose of leptin blocked the enhanced increase in mRNA expression in most cases with the exception of IL-6 in the LT and of TNF-α in the PVN (p<0.05, Fig 6A, 6B).
Figure 6.
Quantitative comparison of the mRNA expression of renin-angiotensin system components, proinflammatory cytokines and microglial marker in the lamina terminalis (LT, Fig 6A) and paraventricular nucleus (PVN, Fig 6B) of rats receiving central (ICV) infusion of leptin, or leptin plus TNF-α synthesis inhibitor pentoxifylline (PTX), AT1-R blocker irbesartan (Irbe) or inhibitor of microglial activation minocycline (Mino) during induction (I). (* p<0.05 vs ICV vehicle, # p<0.05 vs ICV leptin).
Discussion
The major findings of this study are: (1) WAT mass, plasma leptin levels and mRNA expression of leptin and its receptors in both the LT and PVN were elevated in rats by 3 weeks of HFD feeding; (2) ICV administration of leptin mimicked HFD sensitization of Ang II-induced hypertension, and ICV leptin antagonist abolished this HFD-induced sensitizing action; (3) central inhibition of AT1-R, TNF-α synthesis or microglial activation significantly attenuated leptin-induced upregulation of RAS activity, inflammation in the LT and PVN, and leptin sensitization of Ang II-induced hypertension. These observations indicate that HFD can predispose rats to display an enhanced Ang II-elicited hypertensive response through leptin-mediated upregulation of RAS components and PICs in the LT and PVN.
Leptin is primarily produced in WAT and secreted into the circulation. Leptin exerts its biological action through binding to and activating the long form of leptin receptor (LEPR-b), but not through the short form of LEPRs (a,c–f).24 Recently, the SFO, a sensory circumventricular organ devoid of the BBB, has been shown to possess functional LEPRs and its neurons can directly be activated by leptin.25,26,27 Young and colleagues demonstrated that leptin acts within the SFO to selectively increase sympathetic outflow to the kidney and that deletion of SFO LEPRs abolishes ARC LEPR-mediated increase in renal SNA response to leptin, highlighting an emerging role for the SFO in the regulation of circulating energy signals such as leptin.17 Therefore, it is likely that large circulating peptides such as leptin can reach brain metabolic and cardiovascular nuclei inside the BBB through sensory circumventricular organs including the SFO. Furthermore, the PVN, a nucleus downstream from the SFO, has recently been demonstrated to be a site responsible for leptin-induced increases in lumbar SNA, MAP and HR; bilateral inhibition of the PVN with muscimol completely reversed the effects of central leptin.28 Beside LEPRs, the SFO and PVN also possesses AT1-R and PIC receptors that respond to the corresponding agents.29,30 The functional and anatomical coincidence of leptin, the RAS and PICs in the SFO and PVN suggest that these structures are sites for the convergence of leptin signaling with other cardiovascular active agents.
In the present study, we found that 3 weeks of HFD feeding increased WAT mass, plasma leptin levels and mRNA expression of leptin and LEPR-a and b in both the LT and PVN. Central administration of leptin mimicked HFD sensitization of Ang II-induced hypertension while ICV leptin antagonist prevented the sensitization. These results suggest that the LT functions to integrate converging information related to leptin signaling. Leptin mediates the HFD inducing effect to sensitize the response to Ang II-induced hypertension by upregulating leptin signaling within the LT and PVN. Our results are consistent with the recent studies from Head and colleagues. These investigations excluded the effects of insulin and dyslipidemia on the SNA and BP during HFD feeding and further showed that circulating leptin concentrations were unaltered by a HFD at day 3 but were markedly increased by week 3.31 This timing of changes in leptin levels coincided with the increase in visceral fat deposits. The effects of leptin on the SNA and BP were confirmed by finding that central leptin antagonist administration to HFD-fed rabbits failed to elicit a reduction in either hemodynamic or sympathetic parameters at week 1 of the diet but produced large sympathoinhibitory and depressor responses at week 3.12 Taken together, these findings are consistent showing that the enhanced cardiovascular responses during HFD are primarily due to increased circulating leptin.
Although there are strong correlations between plasma leptin concentration and RSNA, hypertension, and tachycardia in rabbits within 3 weeks of fat feeding, the enhanced cardiovascular responses still remained high after the withdrawal of HFD, and after plasma leptin concentration was rapidly normalized.10,11,12 This finding raises a possibility that HFD-induced leptin may activate other sensitizing pressor factors that act centrally to maintain the elevated RSNA and BP. Hilzendeger and colleagues recently demonstrated that ICV injection of leptin can upregulate the brain RAS in the SFO and that the brain RAS plays a facilitatory role in the sympathetic nerve response to leptin.13 Similarly, Thieme et al. reported that chronic systemic infusion of leptin resulted in elevated renin and Ang II plasma levels and increased systolic BP. Concurrent infusion of AT1-R antagonist blocked the leptin-induced increase in BP.32 Moreover, blockade of AT1-R inhibited leptin resistance and restored leptin sensitivity in diet-induced obese rats33 These results indicate that obesity-associated hyperleptinemia contributes to the development of hypertension through crosstalk between leptin and the RAS.
Either HFD feeding or systemic infusion of leptin significantly increased hypothalamic and renal PIC expression including IL-1β, IL-6 and TNF-α, and several components of the RAS such as AT1-R. The increased gene expression for RAS components and PICs were accompanied by increased microglial activation in the hypothalamus including the ARC, SFO and PVN.32,34,35 Conversely, TNF-α upregulates LEPR-b protein level and cell surface expression, which can lead to an increased cellular response to leptin and soluble LEPR production in cultured cells.15 These results suggest that in both the periphery and CNS, PICs also mediate the cardiovascular and metabolic effects of leptin. In the present study, we found that 7 days of central leptin infusion during I upregulated the expression of the RAS components and several PICs in the LT and downstream in the PVN, which was accompanied by the elevated expression of a microglial marker and NADPH oxidase. Central inhibition of AT1-R, TNF-α synthesis and microglial activation during I reversed the leptin-elicited increase in gene expression and the sensitization of Ang II-elicited hypertension. This study extends our previous studies and provides novel evidence that obesity-associated leptin primarily augments brain RAS activity and inflammation which further sensitize Ang II-elicited hypertension. This leptin-dependent increase in RAS activity and PICs within the CNS may offer a causal link between obesity and hypertension.
Although our results suggest a causal role for leptin in HFD sensitization of Ang II-induced hypertension through upregulation of brain RAS and PICs, there still are several issues that need to be addressed. 1) The alterations of the mRNA expression of RAS and PICs occurred not only in the LT but also in the downstream PVN during HFD feeding. Given the roles of hypothalamic nuclei such as the ARC, DMH and VMH and hindbrain nucleus such as the nucleus tractus solitarii in mediating leptin actions,9,16,25,36 the involvement of hypothalamic and hindbrain nuclei in the sensitizing effects of leptin cannot be excluded. 2) The HFD feeding increased plasma leptin levels. Whether this increased circulating leptin induces the central production of leptin that contributes to the sensitizing effects of circulating leptin needs to be studied in the future. 3) In animal experiments, the ranges of effective doses of leptin to produce changes in hemodynamic and sympathetic activity are approximately 2–10 µg for ICV injection and roughly 100–150 µg for intravenous bolus injection.10–13,16,17,32 Also it is important to note that the sensitivity to leptin is altered by diet.10–12 In light of these results, it is assumed that a pharmacological dose of leptin would have been needed for ICV or systemic infusion of leptin to induce a sensitization effect rather than the physiological concentration produced in rats fed HFD. In the present study, we used a dose of 20 ng/kg/min for ICV infusion of leptin, which is also higher than that in the plasma after HFD feeding. ICV infusion of this pharmacological dose of leptin produced a slight increase in BP, but gradually returned to the basal level by the end of D while the increased HR gradually returned to the basal level by the end of E although a pressor dose of Ang II was infused during E. Therefore, it is not likely that the changes of BP and HR produced by leptin pretreatment have an influence on leptin’s sensitizing actions. Moreover, an increasing number of studies indicate that the CNS is mainly responsible for the leptin-mediated overactivity of the hemodynamic parameters produced by HFD feeding. The present study employed the central infusion of the pharmacological doses of leptin peptide or leptin antagonist to give further insight into the findings from our HFD study.
Perspectives
The present study investigated the mechanisms responsible for HFD upregulation of brain RAS and PICs. The results indicate that leptin mediates HFD-induced upregulation of the brain RAS and PICs both of which can exert sensitizing actions. The LT including the SFO is a primary site for convergence of cardiovascular and metabolic signals. These observations suggest that there is a vicious cycle involving leptin, RAS, inflammation and sympathetic activation triggered by feeding a HFD to produce a progressive rise in BP. A diet-induced increase of leptin elicits a state of RAS activation and inflammation, which in turn leads to leptin resistance. This type of mutually enhanced signaling eventually results in both obesity and hypertension. Furthermore, the present findings imply that the sensitizing effects of increased leptin and upregulated RAS and PICs on the development of hypertension will predispose obese individuals to a greater risk of hypertension when they are exposed to many other types of hypertension-eliciting challenges (i.e. stressors). However, due to involvement of multiple brain regions and pressor factors in the sensitization process, the precise central mechanism underlying these interactions that ultimately leads to obesity-related hypertension still need to be determined.
Supplementary Material
Novelty and Significance.
What is New?
These studies demonstrate that high fat diet (HFD)-induced sensitization of Ang II-elicited hypertension is mediated by leptin through upregulation of central renin angiotensin system (RAS) and proinflammatory cytokines (PICs). Central infusion of leptin mimics HFD-elicited sensitization of Ang II hypertension while central blockade of leptin receptors abolished the sensitization produced by HFD feeding, which involves regulation of brain PICs and RAS activity.
What is Relevant?
HFD-induced increase in leptin elicits a state of RAS activation and inflammation, which in turn leads to leptin resistance. These mutually enhanced signaling mechanisms within the CNS offer a causal link between obesity and hypertension and play a pivotal role in the pathogenesis and progression of obesity-related hypertension.
Summary
The study indicates that HFD-induced increase of leptin acts on the brain to sensitize the hypertensive response to Ang II and that sensitization is associated with altered expression of RAS and PICs within forebrain neural network controlling cardiovascular function.
Acknowledgments
Sources of Funding
This work was supported by the NIH grants HL-14388 (AKJ), HL-98207 (AKJ), and MH-80241(AKJ), HL-096671 (RBF) HL-073986 (RBF), with partial support from the Department of Veterans Affairs (RBF).
Footnotes
Disclosures: None
References
- 1.Thaler JP, Guyenet SJ, Dorfman MD, Wisse BE, Schwartz MW. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes. 2013;62:2629–2634. doi: 10.2337/db12-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claflin KE, Grobe JL. Control of energy balance by the brain Renin-Angiotensin system. Curr Hypertens Rep. 2015;17:38. doi: 10.1007/s11906-015-0549-x. [DOI] [PubMed] [Google Scholar]
- 3.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat Med. 2011;17:883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, Johnson AK. Central renin-angiotensin system activation and inflammation induced by high fat diet sensitizes angiotensin II-elicited hypertension. Hypertension. 2016;67:163–170. doi: 10.1161/HYPERTENSIONAHA.115.06263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farooqi IS, O'Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;89:980–984. doi: 10.3945/ajcn.2008.26788C. [DOI] [PubMed] [Google Scholar]
- 7.Rahmouni K. Obesity-associated hypertension: recent progress in deciphering the pathogenesis. Hypertension. 2014;64:215–221. doi: 10.1161/HYPERTENSIONAHA.114.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG, Jr, Licinio J, Brown RD, Enriori PJ, O'Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS, Cowley MA. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404–1416. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55:862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 11.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60:163–171. doi: 10.1161/HYPERTENSIONAHA.111.190413. [DOI] [PubMed] [Google Scholar]
- 12.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension. 2013;61:628–634. doi: 10.1161/HYPERTENSIONAHA.111.00705. [DOI] [PubMed] [Google Scholar]
- 13.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303:H197–H206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305:R566–R581. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gan L, Guo K, Cremona ML, McGraw TE, Leibel RL, Zhang Y. TNF-α up-regulates protein level and cell surface expression of the leptin receptor by stimulating its export via a PKC-dependent mechanism. Endocrinology. 2012;153:5821–5833. doi: 10.1210/en.2012-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young CN, Morgan DA, Butler SD, Mark AL, Davisson RL. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension. 2013;61:737–744. doi: 10.1161/HYPERTENSIONAHA.111.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (Ang II)-initiated hypertension: induction of sensitization by prior AngII treatment. Hypertension. 2012;59:459–466. doi: 10.1161/HYPERTENSIONAHA.111.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Aldosterone acting through the central nervous system sensitizes angiotensin II-induced hypertension. Hypertension. 2012;60:1023–1030. doi: 10.1161/HYPERTENSIONAHA.112.196576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubinion JH, da Silva AA, Hall JE. Chronic blood pressure and appetite responses to central leptin infusion in rats fed a high fat diet. J Hypertens. 2011;29:758–762. doi: 10.1097/HJH.0b013e328344280b. [DOI] [PubMed] [Google Scholar]
- 21.Tümer N, Erdös B, Matheny M, Cudykier I, Scarpace PJ. Leptin antagonist reverses hypertension caused by leptin overexpression, but fails to normalize obesity-related hypertension. J Hypertens. 2007;25:2471–2478. doi: 10.1097/HJH.0b013e3282e9a9fd. [DOI] [PubMed] [Google Scholar]
- 22.Leenen FH, Yuan B. Prevention of hypertension by irbesartan in Dahl S rats relates to central angiotensin II type 1 receptor blockade. Hypertension. 2001;37:981–984. doi: 10.1161/01.hyp.37.3.981. [DOI] [PubMed] [Google Scholar]
- 23.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H227–H236. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 25.Mimee A, Smith PM, Ferguson AV. Circumventricular organs: targets for integration of circulating fluid and energy balance signals? Physiol Behav. 2013;121:96–102. doi: 10.1016/j.physbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Smith PM, Ferguson AV. Cardiovascular actions of leptin in the subfornical organ are abolished by diet-induced obesity. J Neuroendocrinol. 2012;24:504–510. doi: 10.1111/j.1365-2826.2011.02257.x. [DOI] [PubMed] [Google Scholar]
- 27.Smith PM, Chambers AP, Price CJ, Ho W, Hopf C, Sharkey KA, Ferguson AV. The subfornical organ: a central nervous system site for actions of circulating leptin. Am J Physiol Regul Integr Comp Physiol. 2009;296:R512–R520. doi: 10.1152/ajpregu.90858.2008. [DOI] [PubMed] [Google Scholar]
- 28.Shi Z, Li B, Brooks VL. Role of the Paraventricular Nucleus of the Hypothalamus in the Sympathoexcitatory Effects of Leptin. Hypertension. 2015;66:1034–1041. doi: 10.1161/HYPERTENSIONAHA.115.06017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol. 1997;24:96–101. doi: 10.1111/j.1440-1681.1997.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 30.Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65:1126–1133. doi: 10.1161/HYPERTENSIONAHA.114.05112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barzel B, Weir JM, Meikle PJ, Burke SL, Armitage JA, Head GA. Short term fat feeding rapidly increases plasma insulin but does not result in dyslipidaemia. Front Physiol. 2014;5:469. doi: 10.3389/fphys.2014.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thieme K, Oliveira-Souza M. Renal Hemodynamic and Morphological Changes after 7 and 28 Days of Leptin Treatment: The Participation of Angiotensin II via the AT1 Receptor. PLoS One. 2015;10:e0122265. doi: 10.1371/journal.pone.0122265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller-Fielitz H, Hübel N, Mildner M, Vogt FM, Barkhausen J, Raasch W. Chronic blockade of angiotensin AT1 receptors improves cardinal symptoms of metabolic syndrome in diet-induced obesity in rats. Br J Pharmacol. 2014;171:746–760. doi: 10.1111/bph.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Kloet AD, Pioquinto DJ, Nguyen D, Wang L, Smith JA, Hiller H, Sumners C. Obesity induces neuroinflammation mediated by altered expression of the renin-angiotensin system in mouse forebrain nuclei. Physiol Behav. 2014;136:31–38. doi: 10.1016/j.physbeh.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 36.Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53:375–380. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.