Abstract
We investigated the role of angiotensin type 1a receptors (AGTR1a) in vascular injury induced by aldosterone activation of mineralocorticoid receptors (MR) in Agtr1a−/− and wild-type mice infused with aldosterone for 14 days while receiving 1% NaCl in drinking water. Aldosterone increased systolic blood pressure by ~30 mmHg in wild-type mice, and ~50 mmHg in Agtr1a−/− mice. Aldosterone induced aortic and small artery remodeling and impaired endothelium-dependent relaxation in wild-type mice, and enhanced fibronectin and collagen deposition, and vascular inflammation. None of these vascular effects were observed in Agtr1a−/− mice. Aldosterone effects were prevented by the AGTR1 antagonist losartan in wild-type mice. In contrast to aldosterone, norepinephrine caused similar BP increase and mesenteric artery remodeling in wild-type and Agtr1a−/− mice. Agtr1a−/− mice infused with aldosterone did not increase sodium excretion in response to a sodium chloride challenge, suggesting sodium retention that could contribute to the exaggerated blood pressure rise induced by aldosterone. Agtr1a−/− mice had decreased mesenteric artery expression of the calcium-activated potassium channel Kcnmb1, which may enhance myogenic tone and together with sodium retention exacerbate BP responses to aldosterone/salt in Agtr1a−/− mice. We conclude that although aldosterone activation of MR raises BP more in Agtr1a−/− mice, AGTR1a is required for MR stimulation to induce vascular remodeling and inflammation, and endothelial dysfunction.
Keywords: mineralocorticoid, vascular remodeling, oxidative stress, inflammation, sodium, calcium-activated potassium channels
Introduction
Beyond its renal effects on sodium reabsorption, aldosterone excess or inappropriately elevated concentrations relative to sodium balance can exert deleterious effects on the cardiovascular system, including cardiovascular remodeling, fibrosis, endothelial dysfunction, inflammation and oxidative stress.1 Aldosterone acts through the mineralocorticoid receptor (MR) via genomic and non-genomic pathways involving transactivation of epidermal growth factor receptor (EGFR) and activation of extracellular signal-regulated kinase (ERK), c-Src, c-jun N-terminal kinases (JNK), and other kinases and pathways.2 MR blockade in angiotensin (Ang) II-infused rats improved cardiovascular remodeling, suggesting that part of Ang II cardiovascular effects are MR-dependent.3 In patients with heart failure, adding MR antagonists on top of renin-angiotensin system (RAS) blockade improves prognosis.4,5
The classical vascular effects of RAS including hypertension, vascular remodeling, oxidative stress, inflammation and fibrosis, are mediated mainly by the Ang type 1 receptor (AGTR1).6 Of the two subtypes present in mice, AGTR1a and AGTR1b, AGTR1a is the major pressor Ang receptor.7-9
In vitro studies suggest that there is crosstalk between MR-stimulated pathways and Ang receptor pathways in vascular smooth muscle cells (VSMC). Aldosterone increases AGTR1 expression10 and potentiates Ang II-induced signaling.11 Ang II has been shown to activate MR in VSMC in vitro in an AGTR1-dependent manner,12 and VSMC MR is necessary in vivo for Ang II-induced hypertension, vasoconstriction and vascular oxidative stress.13 Aldosterone and Ang II exert synergistic effects on cell proliferation through EGFR and ERK activation,14 migration through c-Src-regulated redox-sensitive RhoA pathways,15 and senescence through Ki-ras2A and oxidative stress.16 Knock-down of Agtr1a in VSMC prevents aldosterone activation of ERK1/2, JNK and NF-κB.17 However, the relevance of these in vitro observations has not been investigated in vivo.18,19
In the present study, we hypothesized that the vascular remodeling and functional effects of MR activation would be blunted in Agtr1a null mice.
Methods
The Materials and Methods are described in detail in the online Supplement.
Experimental design
C57BL/6 Agtr null mice (Agtr1a−/−)7 and wild-type (WT) littermate of 14 ± 3 weeks were infused or not with aldosterone (600 μg/Kg/d) for 14 days using ALZET osmotic minipumps while receiving 1% NaCl in drinking water. As previously described,20 a group of mice was anesthetized with isoflurane, surgically instrumented with PA-C10 telemetry transmitters, allowed to recover for 7-10 days, and blood pressure (BP) determined by telemetry for 2 consecutive days before and during the 14-day treatment period. In another group of mice, during the last two days of the study, 24-hour urine was collected using metabolic cages to determine urinary creatinine and proteins. At the end of the protocol, mice were anesthetized with isoflurane. Blood was collected by cardiac puncture for creatinine, urea and potassium determination. Second-order branches of mesenteric arteries were dissected and studied by pressurized myography.21 Portions of aorta were kept for NADPH activity assay using lucigenin chemiluminescence,22 embedded in VWR Clear Frozen Section Compound for determination of reactive oxygen species (ROS) generation with dihydroethidium, expression of fibronectin and monocyte chemotactic protein-1 (MCP-1), and evaluation of tissue monocyte/macrophages by immunofluorescence, or fixed with 4% paraformaldehyde and embedded in paraffin for Sirius red staining for collagen content.23
An additional group of Agtr1a−/− and WT mice were infused with norepinephrine (4.17 μg/Kg/min) using ALZET osmotic minipumps for 14 days as previously described.24 Another group of aldosterone-infused WT mice was treated with the AGTR1 receptor antagonist losartan (10 mg/kg/day) in drinking water from 1 week before aldosterone infusion to the end of the study. Another group of aldosterone-treated Agtr1a−/− mice was treated with the MR antagonist eplerenone (100 mg/kg/day) mixed with food. In other Agtr1a−/− and WT mice, diuretic and natriuretic responses to a saline challenge was determined at baseline and during aldosterone treatment as previously described.25
A separate group of WT and Agtr1a−/− mice was used to examine effects of aldosterone on the expression of Agtr1b, Nr3c2 (MR) and MR target genes regulating VSMC tone and renal Na+ reabsorption by reverse transcription - quantitative PCR in mesenteric arteries and renal cortex and medulla. The mesenteric artery arcade was dissected under RNase-free conditions, rinsed in ice-cold phosphate buffered saline (PBS), and stored in RNAlater. Kidneys were collected in ice-cold PBS, dissected to separate renal cortex and medulla, frozen in liquid nitrogen and stored at −80°C until RNA was extracted.
Data analysis
Results are presented as means ± SEM. Comparisons between multiple groups were done by one- or two-way ANOVA, or two-way ANOVA for repeated measures as appropriate, followed by a Student–Newman–Keuls or Dunnett’s post-hoc tests. P<0.05 was considered statistically significant.
Results
Physiological parameters
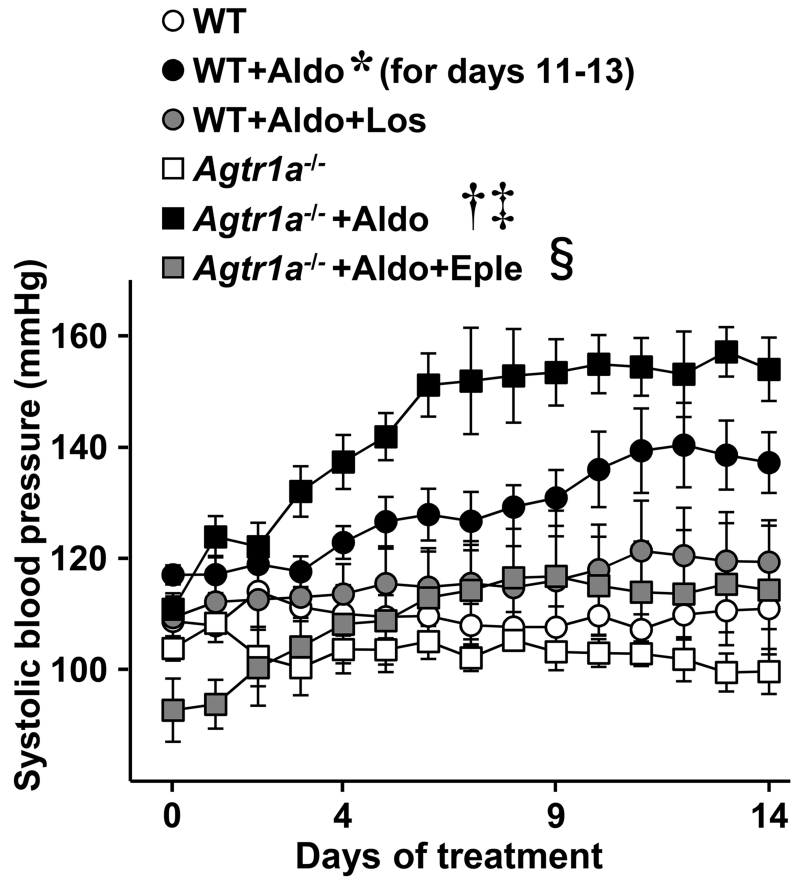
Systolic BP (SBP) tended to be lower in Agtr1a−/− compared to WT mice (Figure 1). Aldosterone infusion with 1% NaCl in the drinking water raised SBP in WT mice from 117 ± 2 to 140 ± 8 mmHg, whereas in Agtr1a−/− mice SBP rose from 110 ± 3 to 154 ± 5 mmHg. In contrast, norepinephrine infusion raised SBP by 25 mmHg at day 14 in WT vs. 23 mmHg in Agtr1a−/− mice (Supplemental Figure S1).
Figure 1.
Aldosterone-induced hypertension in wild-type and Agtr1a−/− mice. Systolic blood pressure (SBP) was assessed by telemetry in wild-type (WT) and Agtr1a−/− mice not treated or infused with aldosterone (+Aldo) for 14 days while receiving 1% NaCl in the drinking water. SBP was also determined in aldosterone-treated WT and Agtr1a−/− mice co-treated respectively with losartan (Los) or eplerenone (Eple). Mean 24-hour SBP data are presented. Values are means ± SEM, n = 4-7, *P<0.05 vs. untreated WT, †P<0.001 vs. untreated Agtr1a−/−, ‡P<0.05 vs. WT+ALDO and §P<0.01 vs. Agtr1a−/−+ALDO.
Aldosterone did not change body weight or tibia length (TL) of WT or Agtr1a−/− mice (Table 1). Aldosterone caused cardiac hypertrophy in Agtr1a−/− but not in WT mice. Renal function in Agtr1a−/− was similar to that of WT mice. Aldosterone caused a similar decrease in plasma potassium in WT and Agtr1a−/− mice. Aldosterone increased urinary protein/creatinine ratio 1.6-fold more in Agtr1a−/− than in WT mice.
Table 1.
Body and tissue weight, aortic and mesenteric artery wall cross-sectional area and kidney function
| Parameters | WT | WT + ALDO | Agtr1a −/− | Agtrla−/− + ALDO |
|---|---|---|---|---|
| Body weight (g) | 25.5 ± 0.9 | 26.7 ± 0.5 | 26.9 ± 0.6 | 27.7 ± 1.0 |
| TL (mm) | 16.9 ± 0.3 | 17.0 ± 0.2 | 16.9 ± 0.5 | 16.8 ± 0.3 |
| Heart weight/TL (mg/mm) | 7.1 ± 0.6 | 7.6 ± 0.6 | 7.9 ± 0.8 | 12.4 ± 1.7†‡ |
| Lung weight/TL (mg/mm) | 12.7 ± 1.0 | 11.9 ± 1.1 | 12.0 ± 1.4 | 19.4 ± 3.4†‡ |
| Kidney weights/TL (mg/mm) | 19.6 ± 1.0 | 25.8 ± 1.1 | 19.5 ± 1.7 | 24.5 ± 3.1 |
| Plasma creatinine (mmol/L) | 24.0 ± 0.4 | 24.2 ± 1.5 | 25.6 ± 0.9 | 25.9 ± 1.7 |
| Plasma urea nitrogen (mmol/L) | 8.6 ± 1.3 | 7.2 ± 1.5 | 10.9 ± 0.9 | 6.5 ± 0.7 |
| Plasma potassium (mmol/L) | 4.4 ± 0.1 | 2.7 ± 0.1* | 4.6 ± 0.3 | 3.6 ± 0.4†‡ |
| Urinary protein/creatinine | 1.3 ± 0.1 | 4.3 ± 0.7* | 2.1 ± 0.4 | 6.7 ± 1.2†‡ |
| Aortic WCSA (μm2 × 1000) | 92.4 ± 7.0 | 127.9 ± 13.2* | 124.3 ± 12.0* | 129.8 ± 6.0 |
| MA WCSA (μm2 × 1000) | 4.5 ± 0.1 | 6.0 ± 0.4 | 3.8 ± 1.0 | 4.3 ± 0.5 |
Body weight, tibia length (TL) and tissue weight, kidney function assessed by measuring plasma creatinine and potassium, urea nitrogen and urinary protein/creatinine ratio, and aortic and mesenteric artery (MA) wall cross-sectional area (WCSA) were determined in wild-type (WT) and Agtr1a−/− mice untreated or infused with aldosterone (+Aldo) for 14 days while receiving 1% NaCl in the drinking water. Values are mean ± SEM, n = 5-7 for body and tissue weight and TL, 4-5 for creatinine, urea nitrogen and potassium, 4-6 for urinary ACR, and 4-6 for aortic WCSA, 6-9 for MA WCSA.
P<0.05 vs. untreated WT
P<0.05 vs. untreated Agtr1a−/−
P<0.05 vs. WT+Aldo.
AGTR1a is required for aldosterone-induced endothelial dysfunction
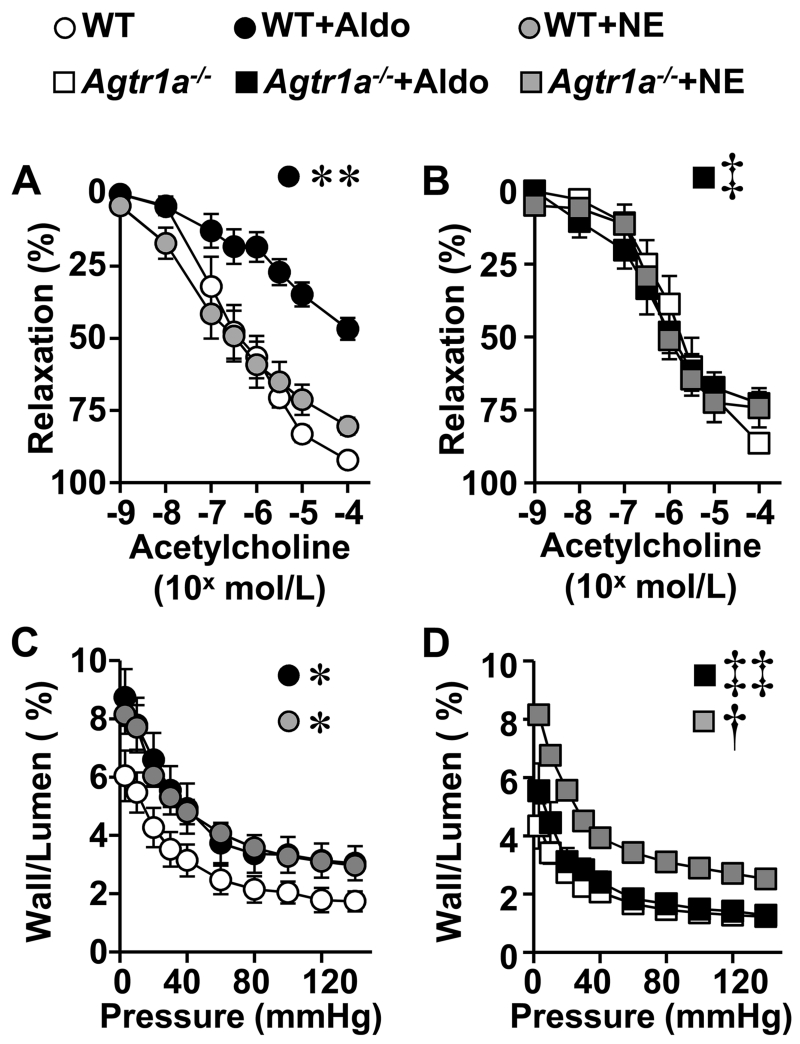
Aldosterone impaired acetylcholine-induced endothelium-dependent relaxation in mesenteric arteries in WT but not in Agtr1a−/− mice (Fig. 2A, B). Norepinephrine did not affect endothelium-dependent relaxation in WT or Agtr1a−/− mice (Figure 2B). Endothelium-independent relaxation to sodium nitroprusside was similar in all groups (Supplemental Figure S2B).
Figure 2.
Agtr1a knockout prevented aldosterone-induced endothelial dysfunction and vascular remodeling in small arteries. Vasodilator responses to acetylcholine (A and B) and wall/lumen ratio in function of intraluminal pressure (C and D) were determined in mesenteric arteries of WT (A and C) and Agtr1a−/− (B and D) mice untreated, infused with aldosterone (+Aldo) while receiving 1% NaCl in the drinking water, or infused with norepinephrine (+NE) for 14 days. Data are means ± SEM, n = 5-8 for A and C, and 6-9 for B and D, *P<0.05 and **P<0.01 vs. untreated WT, †P<0.01 vs. untreated Agtr1a−/− and ‡P<0.05 and ‡‡P<0.01 vs. WT+Aldo.
AGTR1a is required for aldosterone-induced remodeling of conduit and resistance arteries
Agtr1a−/− mice had a 35% greater aortic wall cross-sectional area (WCSA) at baseline than WT mice. However, WCSA was not different in mesenteric arteries (Table 1). Aldosterone increased aortic WCSA by 38% in WT but not in Agtr1a−/− mice. Aldosterone induced a ~2-fold increase in wall/lumen ratio of mesenteric arteries in WT but not in Agtr1a−/− mice (Figure 2C, D and Table 1). Norepinephrine treatment increased wall/lumen ratio ~2-fold in both WT and Agtr1a−/− mice (Figure 2C, D and Supplemental Table S1).
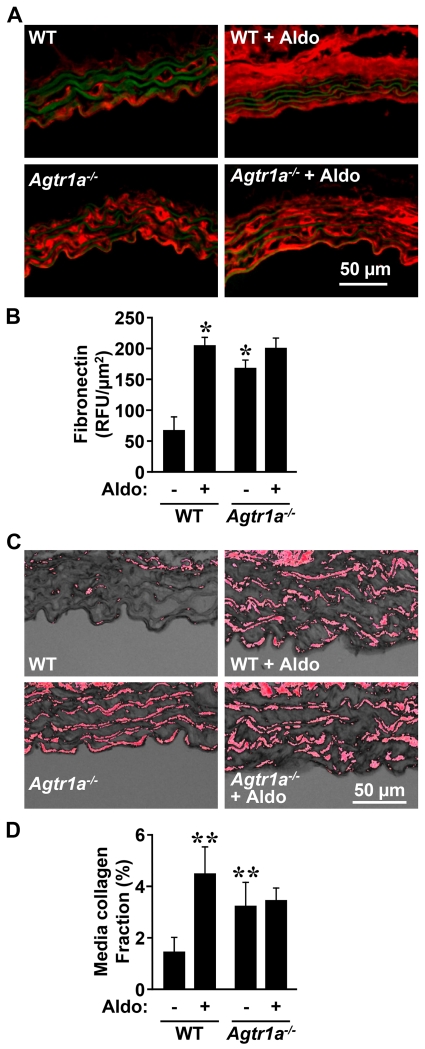
Aorta from Agtr1a−/− mice exhibited ≥2-fold greater fibronectin and collagen content compared to WT mice at baseline (Figure 3). Aldosterone infusion caused a 3-fold increase in fibronectin and collagen content in WT but not in Agtr1a−/− mice.
Figure 3.
AGTR1a is required for aldosterone-induced fibrosis of the media of the descending thoracic aorta. Fibronectin content (A and B, in red) and collagen content (C and D, in red) were determined respectively by immunofluorescence and Sirius red staining in the wall of the descending thoracic aorta of the same groups as in Figure 1. Representative fibronectin immunofluorescence images (A) and RGB thresholded images of Sirius red-stained aortic sections are shown (C). Green fluorescence in A represents autofluorescence of elastin. WT, wild-type; Aldo, aldosterone. Data are means ± SEM, n = 4-6 for A and B and 5-6 for C and D, *P<0.05 and **P<0.05 vs. untreated WT.
AGTR1a is required for aldosterone-induced vascular ROS generation
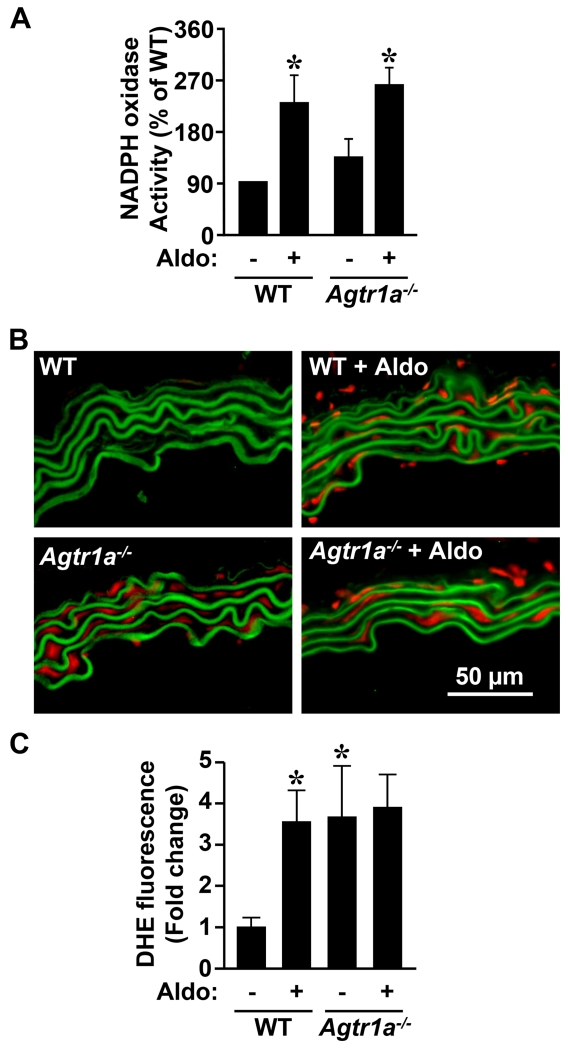
NADPH oxidase activity was enhanced ≥2.5-fold in aorta of WT and Agtr1a−/− mice infused with aldosterone compared to untreated mice (Figure 4A). ROS generation was >3-fold higher in aorta of Agtr1a−/− compared to WT mice (Figure 4B, C). Aldosterone increased ROS generation 3.5-fold in WT but not in Agtr1a−/− mice.
Figure 4.
AGTR1a involvement in aldosterone-induced oxidative stress. NADPH oxidase activity by lucigenin chemiluminescence (A) and reactive oxidative species generation by dihydroethidium (DHE) staining (B and C) were determined in the aorta in the same groups as in Figure 1. Representative images of DHE-stained aortic sections are shown in B. Red and green fluorescence represents respectively DHE fluorescence and elastin autofluorescence. WT, wild-type; Aldo, aldosterone. Data are means ± SEM, n = 4-6 for A and 5-6 for B *P<0.05 vs. untreated WT.
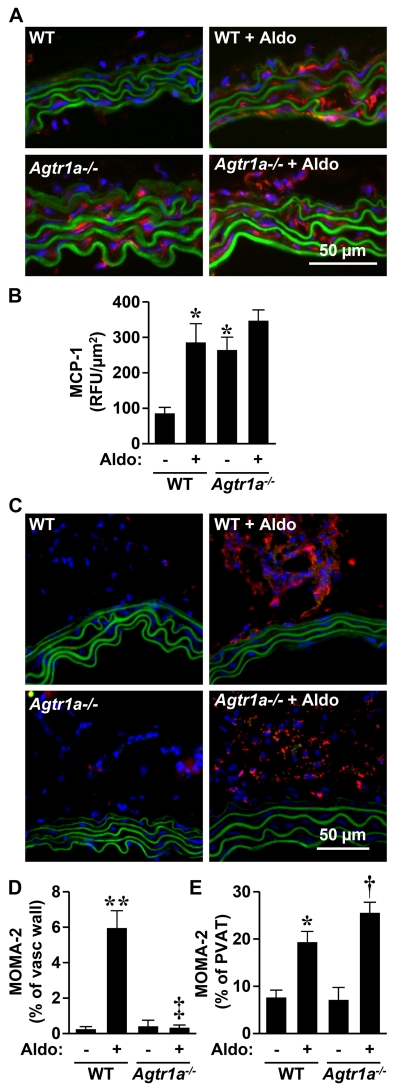
AGTR1a is required for aldosterone-induced inflammation of aorta
Agtr1a−/− mice presented 3-fold greater MCP-1 expression in aorta compared to WT mice (Figure 5A, B). Aldosterone increased aortic MCP-1 expression >3-fold in WT but not in Agtr1a−/− mice. Aldosterone caused monocyte/macrophage infiltration in the adventitia in WT but not in Agtr1a−/− mice. Aldosterone induced monocyte/macrophage infiltration in perivascular fat in both WT and Agtr1a−/− mice, (Figure 5C-E).
Figure 5.
AGTR1a involvement in aldosterone-induced vascular inflammation. MCP-1 staining (A and B) and monocyte/macrophage infiltration (C and D) were measured by immunofluorescence in aorta of the same groups as in Figure 1. Representative MCP-1 (A, in red) and MOMA-2 (C, in red) immunofluorescence images of aortic sections are shown. Green fluorescence represents elastin autofluorescence. Vasc, vascular, PVAT, perivascular fat. WT, wild-type; Aldo, aldosterone. Data are means ± SEM, n = 4-7 for A and 4-5 for B *P<0.05 and **P<0.001 vs. untreated WT, †P<0.001 vs. untreated Agtr1a−/−, ‡P<0.001 vs. WT+ALDO.
Pharmacologic blockade of AGTR1
Aldosterone-infused WT mice were treated with the AGTR1 antagonist losartan, which abrogated the aldosterone-induced BP increase (Figure 1), the remodeling of mesenteric arteries (wall/lumen ratio: 2.8 ± 0.2%, n=7, vs. 2.9 ± 0.5%, n=6, in losartan and aldosterone co-treated and untreated WT mice, respectively) and the impairment of mesenteric artery relaxation responses to acetylcholine (data not shown).
Mechanism of enhanced BP response to aldosterone in Agtr1a−/− mice
The enhanced BP elevation in response to aldosterone was reproducibly observed in additional aldosterone-treated Agtr1a−/− mice (rising to 148±8 mmHg). The exaggerated aldosterone BP response in Agtr1a−/− mice was MR-dependent, since it was prevented by eplerenone (Figure 1). The heart weight/TL of Agtr1a−/− mice treated with eplerenone and aldosterone (8.3±1.8 mg/mm) was similar to that of control Agtr1a−/− mice (Table 1).
To explore the role of the kidney in the enhanced BP response to aldosterone in Agtr1a−/− mice, diuretic and natriuretic responses to a saline challenge were examined. Untreated Agtr1a−/− mice presented a >2-fold increase in excretion of sodium and water compared to untreated WT mice, but body weight was similar in the two groups (Supplemental Figure S3A-C). At day 2 of aldosterone infusion, WT mice presented greater sodium and water excretion compared to baseline due to 1% NaCl contained in the drinking water (Supplemental Figure S3D, E vs S3A, B). This adaptation to an increased sodium load was not observed in Agtr1a−/− mice, suggesting retention of the excess sodium. There was no difference in excretion of sodium and water or body weight during the following days of aldosterone treatment in WT and Agtr1a−/− mice (Supplemental Figure S3D-F).
The mRNA expression of MR (Nr3c2) in the renal medulla was 40% lower in Agtr1a−/− compared to WT mice, and was unaffected by aldosterone in either group (Supplemental Figure S4A). The mRNA expression of key regulators of Na+ reabsorption that are aldosterone/MR targets:26-28 the thiazide-sensitive Na+ Cl− cotransporter (NCC, Slc12a3), the epithelial sodium channel α (ENaCα, Scnn1a), a regulator of ENaC; the glucocorticoid-induced leucine zipper protein 1 (GILZ1, Tsc22d3); and a regulator of ENaC and NCC, the serum- and glucocorticoid-induced kinase-1 (SGK1, Sgk1), were assessed in the renal medulla in WT and Agtr1a−/− mice before and after 2 and 14 days of aldosterone infusion. The mRNA levels of Slc12a3, Scnn1a, Tsc22d3 and Sgk1 were unaffected by Agtr1a knockout compared to WT mice (Supplemental Figure S5). Two days of aldosterone treatment caused a ≥2-fold increase in expression of Slc12a3, Scnn1a, Tsc22d3 and Sgk1. After 14 days of aldosterone treatment, the expression of Slc12a3 and Tsc22d3 returned to untreated levels, whereas expression of Scnn1a and Sgk1 remained increased although at a lower level compared to day 2. In Agtr1a−/− mice, expression levels of Slc12a3 and Tsc22d3 were unchanged at day 2, but decreased by 67% and 92% respectively on day 14 of the aldosterone infusion. Aldosterone-induced expression of Scnn1a was lower at day 2 in Agtr1a−/− mice compared to aldosterone-treated WT mice, and blunted on day 14. Sgk1 changes resembled those in WT.
In mesenteric arteries, Nr3c2 expression was unaffected by Agtr1a knockout and decreased by 19% in the aldosterone-treated Agtr1a−/− mice (Supplemental Figure S4B). mRNA expression of Agtr1b in mesenteric arteries was decreased in Agtr1a−/− compared to WT mice, and was unaffected by aldosterone (Supplemental Figure S6). The voltage-dependent L-type calcium channel alpha 1c subunit (Cav1.2, Cacna1c), the large-conductance calcium-activated potassium channel subfamily M, alpha member 1 (BKCaα1, Kcnma1), and beta member 1 (BKCaβ1, Kcnmb1), contribute to VSMC tone and are regulated by MR.29 Mesenteric artery mRNA expression of Cacna1c, Kcnma1 and Kcnmb1 was unaffected by aldosterone (Supplemental Figure S7). However, expression of Kcnmb1 was 24% lower in Agtr1a−/− compared to WT mice, decrease that could enhance myogenic tone and contribute to exaggerate the BP response to aldosterone/salt in Agtr1a−/−.
Discussion
In the present study, we demonstrate in vivo that crosstalk between aldosterone-stimulated MR and Ang receptor pathways plays a role in vascular effects of aldosterone. AGTR1a is required for MR-induced endothelial dysfunction and vascular remodeling, oxidative stress, and inflammation. In addition, we observed an exaggerated elevation of BP in response to aldosterone infusion in Agtr1a−/− compared to WT mice. Agtr1a−/− infused with aldosterone did not respond with increased sodium excretion to a sodium load. Together with a decreased expression of the large conductance calcium-activated potassium channel Kcnmb1 in VSMC that would be expected to enhance myogenic tone in association with greater sodium retention, these changes may account for the exaggerated BP response to aldosterone/salt in Agtr1a−/−.
Close links between MR and Ang receptor pathways have been shown in VSMC. In vitro, Ang II can activate MR in VSMC in an AGTR1-dependent manner.12 VSMC MR is necessary in vivo for Ang II-induced hypertension, vasoconstriction and vascular oxidative stress.13 Aldosterone and Ang II exert a synergistic effect on VSMC proliferation, senescence, and migration.1 In vitro, Agtr1a knock-down in VSMC impaired aldosterone-induced activation of ERK1/2, JNK and NF-κB.17 We now demonstrate that these in vitro observations occur in vivo, confirming and extending the role of AGTR1a in vascular responses to MR stimulation by aldosterone.
The mechanisms involved in aldosterone-induced vascular remodeling30 may be BP-dependent31,32 and/or independent.33 Since SBP during aldosterone infusion increased more in Agtr1a−/− mice than in WT mice, the absence of vascular remodeling in Agtr1a−/− mice infused with aldosterone must be BP-independent. A greater baseline aortic fibrosis, WCSA and oxidative stress in Agtr1a−/− could have blunted aldosterone-induced remodeling. However, baseline WCSA of small mesenteric arteries was not greater and also remained unaltered by aldosterone. Aldosterone-induced vascular remodeling involves changes in extracellular matrix in the arterial wall.34 In vitro, Min et al. showed that aldosterone and Ang II exerted synergistic effects on VSMC proliferation.14 In VSMC in which Agtr1a was knocked-down, activation of signaling pathways involved in mitogenic effects of aldosterone was blunted.17 Oxidative stress also participates in the remodeling response to aldosterone.35 Although oxidative stress was greater in Agtr1a−/− mice under basal conditions, lack of ROS generation changes in response to aldosterone could contribute to blunt remodeling in Agtr1a−/− mice.
Endothelial dysfunction in response to aldosterone involves MR (Nr3c2) expression, oxidative stress,22,36,37 inflammation,22 as well as participation of glucose-6-phosphate dehydrogenase,38,39 and EGFR.38 The absence of endothelial dysfunction in response to aldosterone in Agtr1a−/− mice could be related to preservation of nitric oxide bioavailability as a consequence of a lower inflammatory response and absence of increased superoxide generation in the vascular wall of Agtr1a−/− mice infused with aldosterone.
Blockade of AGTR1 with losartan in WT mice did not reproduce the exaggerated response to aldosterone observed in Agtr1a null mice. Differences between the effect of losartan and gene deletion of Agtr1a could be due to in utero compensatory developmental changes or adaptation to life-long absence of AGTR1a. Since losartan blocks both AGTR1a and AGTR1b, the difference may relate to the presence of unblocked AGTR1b in Agtr1a−/− mice and blocked AGTR1b in mice treated with losartan. However, expression of AGTR1b was unchanged in Agtr1a−/− mice, and thus AGTR1b does not seem to play a role in our findings. It should be recalled anyway from a translational point of view that humans only have one subtype of Ang type 1 receptor.
The exaggerated BP elevation and cardiac hypertrophy caused by aldosterone in Agtr1a−/− was unexpected, as Agtr1a−/− mice have impaired pressor responses to Ang II.7,18 We reproduced the enhanced BP response in additional Agtr1a−/− mice. Kagiyama et al. showed as well that aldosterone caused greater BP elevation and cardiac hypertrophy in another strain of Agtr1a−/− compared to WT, and cardiac fibrosis only in Agtr1a−/− mice.40 The mechanism of excess BP increase in aldosterone-infused Agtr1a−/− mice could be related to compensatory effects of MR signaling pathways in presence of the Agtr1a gene deletion, or to a kidney defect in sodium and water excretion.
Since the kidney plays a central role in BP regulation,41 Agtr1a−/− mice could present altered renal responses to aldosterone. Aldosterone could have caused renal injury in Agtr1a−/− mice, as proteinuria tended to be higher compared to WT mice. Agtr1a−/− mice have kidney defects since they exhibit mild mesangial expansion and hypertrophy of the juxtaglomerular apparatus19 and polyuria and urinary concentration defects42,43 associated with a reduction in plasma and extracellular volume44 that results in hypotension. Agtr1a−/− mice presented increased sodium and water excretion under normal salt diet, but did not increase sodium excretion in response to the acute increase in sodium intake accompanying aldosterone infusion. This response was not related to MR expression, since mRNA expression Nr3c2 was 40% lower in the renal medulla of Agtr1a null mice.
Aldosterone via MR augments extracellular volume and BP primarily by increasing sodium reabsorption in the distal nephron,26-28 mediated by activation of NCC and ENaC, key Na+ transporters. Aldosterone increases the renal mRNA expression of NCC in rats,45 and activates ENaC in part by stimulating transcription of ENaCα (Scnn1a) and two ENaC activators, SGK1 (Sgk1) and GILZ1 (Tsc22d3).46 SGK1 is also a regulator of NCC. Since MR and AGTR1a are co-expressed in the kidney including the distal tubule,26 the impairment in aldosterone regulation of NCC, ENaCα and GILZ found in Agtr1a−/− mice could be a consequence of the Agtr1a deficiency, and suggest the existence of crosstalk between MR and AGTR1a in the renal medulla. Whether amiloride might block these effects if ENaC activity is increased despite the relative impairment of aldosterone-induced expression of ENaCα in Agtr1a-deficient mice remains to be demonstrated. Shibata et al.47 reported phosphorylation at S843 in the MR ligand-binding domain that prevents ligand binding and activation, which in the kidney is only found in intercalated cells of the distal nephron. In Agtr1a-deficient mice, dephosphorylation of MRS843-P may result in aldosterone-dependent increases of the intercalated cell apical proton pump and Cl−/HCO3− exchangers, increasing Cl− reabsorption and promoting increased plasma volume.47 This mechanism of MR activation could contribute to enhanced sodium retention when challenged with excess sodium during aldosterone infusion in Agtr1a−/− mice, but remains to be demonstrated. An additional possibility to be considered is that cortisol, or in rodents corticosterone, binds to MR and may mimic aldosterone effects, considering that concentrations in blood of the latter are vastly superior to those of aldosterone. The activity of 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2), which converts cortisol and corticosterone to their MR-inactive 11-keto congeners cortisone and 11-dehydrocorticosterone, plays role regulating this effect.48 The activity of 11βHSD2 depends on the concentration of reduced nicotinamide-adenine dinucleotide (NADH) generated from nicotinamide-adenine dinucleotide (NAD), the co-substrate for the cortisol-to-cortisone conversion, which is modulated by the redox state of the cell. In pathophysiological conditions, with enhanced oxidative stress, more NADH could result in agonistic effects of cortisol/corticosterone on MR.48 Whether the pharmacological dose of aldosterone used in these studies to stimulate MR added to the absence of AGTR1a in Agtr1a-deficient mice could affect the actions of these steroids on MR and contribute to our findings remains unclear.
Aldosterone may increase BP by modulating vascular tone through its action on VSMC by altering the expression of the calcium ion channel Cav1.2 or the calcium-activated potassium channels BKCa.29 Smooth muscle cell-specific MR knockout in mice decreased mRNA expression of Cav1.2 in the aorta but not of BKCaα1 and BKCaβ1.13 The expression of BKCaα1 and BKCaβ1 subunits have been shown to be decreased in the heart and coronary arteries of mice with cardiomyocyte-specific aldosterone synthase overexpression and in aldosterone-treated VSMC.49 Since Agtr1a−/− mice exhibited a decrease in expression of BKCaβ1 (Kcnmb1), which could be expected to enhance myogenic tone,50 particularly in salt-loaded aldosterone-infused mice presenting a defect in sodium excretion to a sodium load, the combined alteration could contribute to the exaggerated BP elevation found in aldosterone-infused Agtr1a−/− mice.
We determined whether the AGTR1a-dependent and BP-independent aldosterone-induced vascular damage is selective to aldosterone by testing an alternative hypertensive agent. Norepinephrine did not produce different effects on BP, vascular remodeling or endothelial function in WT and Agtr1a−/− mice, consistent with the earlier findings of Rajagopolan et al,51 which could be due to the inability of NE to stimulate vascular oxidative stress.51-54
A strength of the present study is the evaluation in vivo of the interaction between aldosterone and AGTR1a in the vascular wall, which reinforces the concept of using MR blockers in addition to RAS blockers in hypertension to prevent vascular remodeling of large and small arteries. It underscores potential mechanisms whereby MR blockade, a not very potent antihypertensive, when added to renin-angiotensin blockade may exert powerful BP lowering actions in some clinical conditions such as resistant hypertension.55-57
Limitations
The main limitation of the present study is the use of mice with full Agtr1a knockout, in which all tissues are affected. Even if the renal phenotype is moderate in this model,8 it could influence the vascular phenotype. As well, aortic fibronectin and collagen, and oxidative stress were increased at baseline in Agtr1a null mice, which could limit the remodeling that aldosterone can induce. From a translation viewpoint, comparison of the Agtr1a-deficient mouse with the effects of the Ang receptor blocker losartan to understand human responses to MR activation or blockade is complicated by the fact that humans have only one Ang type 1 receptor in contrast to rodents. Despite these caveats, this study has allowed testing the hypothesis that absence of AGTR1a prevents MR-induced vascular injury even though BP effects are enhanced.
Supplementary Material
Perspectives.
This is the first in vivo study to demonstrate that AGTR1a is critical for aldosterone-induced vascular remodeling and endothelial dysfunction. It extends previous in vitro data suggesting that MR and Ang receptor pathways cross-talk to induce physiological and pathophysiological effects. These results, with the caveats mentioned in the limitations of our study, provide potential mechanisms that could explain findings of clinical trials in which addition of an MR blocker significantly potentiates the therapeutic benefits of RAS blockade, particularly in resistant hypertension and in heart failure.
Novelty and Significance.
1. What Is New?
In vivo, aldosterone infusion in presence of salt intake failed to induce vascular remodeling and endothelial dysfunction in Agtr1a-deficient mice, despite SBP rising more than in WT, indicating that a functional AGTR1a is necessary for aldosterone to exert these pathophysiological actions. This occurred despite greater BP elevation, probably a consequence of renal failure to excrete excess sodium in response to a sodium load under aldosterone treatment combined with a deficiency in the calcium-sensitive potassium channel BKCaβ1 (Kcnmb1), which could be expected to enhance vascular myogenic tone.
2. What Is Relevant?
The results of this in vivo study and of preceding in vitro studies suggest that aldosterone via MR and Ang II via AGTR1a cross-talk in vascular smooth muscle cells, leading to enhanced effects.
These results could explain in part the beneficial result of the use of MR blockers on top of RAS inhibition in cardiovascular conditions.
3. Summary
We investigated in vivo the implication of Ang type 1a receptors on the vascular response to aldosterone. Agtr1a−/− and WT mice were infused with aldosterone for 14 days while receiving 1% NaCl in drinking water. Aldosterone-induced endothelial dysfunction and vascular remodeling and inflammation were reduced in Agtr1a−/− mice, whereas BP rise was enhanced, demonstrating for the first time in vivo a critical role of AGTR1a in the effects of aldosterone.
Acknowledgments
We are grateful to Adriana Cristina Ene and Guillem Colell Dinarès for excellent technical support and animal care.
Funding Sources
This work was supported by Canadian Institutes of Health Research (CIHR) grants 82790 and 123465, a CIHR Foundation Grant, a Canada Research Chair (CRC) on Hypertension and Vascular Research by the CRC Government of Canada/CIHR Program, and by the Canada Fund for Innovation (CFI), all to ELS. Dr. Marie Briet was supported by a Fellowship from the Heart and Stroke Foundation of Canada.
Footnotes
Disclosures
None
References
- 1.Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6:261–273. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- 2.Briet M, Schiffrin EL. Vascular actions of aldosterone. J Vasc Res. 2013;50:89–99. doi: 10.1159/000345243. [DOI] [PubMed] [Google Scholar]
- 3.Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J, Randomized aldactone evaluation study investigators The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 6.Lemarie CA, Schiffrin EL. The angiotensin II type 2 receptor in cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2010;11:19–31. doi: 10.1177/1470320309347785. [DOI] [PubMed] [Google Scholar]
- 7.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliverio MI, Best CF, Kim HS, Arendshorst WJ, Smithies O, Coffman TM. Angiotensin II responses in AT1A receptor-deficient mice: A role for AT1B receptors in blood pressure regulation. Am J Physiol. 1997;272:F515–520. doi: 10.1152/ajprenal.1997.272.4.F515. [DOI] [PubMed] [Google Scholar]
- 9.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci USA. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffrin EL, Franks DJ, Gutkowska J. Effect of aldosterone on vascular angiotensin II receptors in the rat. Can J Physiol Pharmacol. 1985;63:1522–1527. doi: 10.1139/y85-250. [DOI] [PubMed] [Google Scholar]
- 11.Mazak I, Fiebeler A, Muller DN, Park JK, Shagdarsuren E, Lindschau C, Dechend R, Viedt C, Pilz B, Haller H, Luft FC. Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation. 2004;109:2792–2800. doi: 10.1161/01.CIR.0000131860.80444.AB. [DOI] [PubMed] [Google Scholar]
- 12.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96:643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 13.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Min LJ, Mogi M, Li JM, Iwanami J, Iwai M, Horiuchi M. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ Res. 2005;97:434–442. doi: 10.1161/01.RES.0000180753.63183.95. [DOI] [PubMed] [Google Scholar]
- 15.Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive Rhoa pathways. Arterioscler Thromb Vasc Biol. 2008;28:1511–1518. doi: 10.1161/ATVBAHA.108.168021. [DOI] [PubMed] [Google Scholar]
- 16.Min LJ, Mogi M, Iwanami J, Li JM, Sakata A, Fujita T, Tsukuda K, Iwai M, Horiuchi M. Cross-talk between aldosterone and angiotensin II in vascular smooth muscle cell senescence. Cardiovasc Res. 2007;76:506–516. doi: 10.1016/j.cardiores.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Lemarie CA, Simeone SM, Nikonova A, Ebrahimian T, Deschenes ME, Coffman TM, Paradis P, Schiffrin EL. Aldosterone-induced activation of signaling pathways requires activity of angiotensin type 1a receptors. Circ Res. 2009;105:852–859. doi: 10.1161/CIRCRESAHA.109.196576. [DOI] [PubMed] [Google Scholar]
- 18.Gembardt F, Heringer-Walther S, van Esch JH, Sterner-Kock A, van Veghel R, Le TH, Garrelds IM, Coffman TM, Danser AH, Schultheiss HP, Walther T. Cardiovascular phenotype of mice lacking all three subtypes of angiotensin II receptors. FASEB J. 2008;22:3068–3077. doi: 10.1096/fj.08-108316. [DOI] [PubMed] [Google Scholar]
- 19.Oliverio MI, Madsen K, Best CF, Ito M, Maeda N, Smithies O, Coffman TM. Renal growth and development in mice lacking AT1A receptors for angiotensin II. Am J Physiol. 1998;274:F43–50. doi: 10.1152/ajprenal.1998.274.1.F43. [DOI] [PubMed] [Google Scholar]
- 20.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 21.Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: Role of peroxisome proliferator-activated receptor-gamma. Circulation. 2002;105:2296–2302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- 22.Leibovitz E, Ebrahimian T, Paradis P, Schiffrin EL. Aldosterone induces arterial stiffness in absence of oxidative stress and endothelial dysfunction. J Hypertens. 2009;27:2192–2200. doi: 10.1097/HJH.0b013e328330a963. [DOI] [PubMed] [Google Scholar]
- 23.Li MW, Mian MO, Barhoumi T, Rehman A, Mann K, Paradis P, Schiffrin EL. Endothelin-1 overexpression exacerbates atherosclerosis and induces aortic aneurysms in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2013;33:2306–2315. doi: 10.1161/ATVBAHA.113.302028. [DOI] [PubMed] [Google Scholar]
- 24.Hu C, Dandapat A, Sun L, Marwali MR, Inoue N, Sugawara F, Inoue K, Kawase Y, Jishage K, Suzuki H, Hermonat PL, Sawamura T, Mehta JL. Modulation of angiotensin II-mediated hypertension and cardiac remodeling by lectin-like oxidized low-density lipoprotein receptor-1 deletion. Hypertension. 2008;52:556–562. doi: 10.1161/HYPERTENSIONAHA.108.115287. [DOI] [PubMed] [Google Scholar]
- 25.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma−/− and interleukin-17A−/− mice. Hypertension. 2015;65:569–576. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossier BC, Staub O, Hummler E. Genetic dissection of sodium and potassium transport along the aldosterone-sensitive distal nephron: Importance in the control of blood pressure and hypertension. FEBS Lett. 2013;587:1929–1941. doi: 10.1016/j.febslet.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Soundararajan R, Pearce D, Hughey RP, Kleyman TR. Role of epithelial sodium channels and their regulators in hypertension. J Biol Chem. 2010;285:30363–30369. doi: 10.1074/jbc.R110.155341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soundararajan R, Pearce D, Ziera T. The role of the ENaC-regulatory complex in aldosterone-mediated sodium transport. Mol Cell Endocrinol. 2012;350:242–247. doi: 10.1016/j.mce.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DuPont JJ, Hill MA, Bender SB, Jaisser F, Jaffe IZ. Aldosterone and vascular mineralocorticoid receptors: Regulators of ion channels beyond the kidney. Hypertension. 2014;63:632–637. doi: 10.1161/HYPERTENSIONAHA.113.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JB, Schiffrin EL. ET(A) receptor antagonist prevents blood pressure elevation and vascular remodeling in aldosterone-infused rats. Hypertension. 2001;37:1444–1449. doi: 10.1161/01.hyp.37.6.1444. [DOI] [PubMed] [Google Scholar]
- 31.Levy BI, Michel JB, Salzmann JL, Devissaguet M, Safar ME. Remodeling of heart and arteries by chronic converting enzyme inhibition in spontaneously hypertensive rats. Am J Hypertens. 1991;4:240S–245S. doi: 10.1093/ajh/4.3.240s. [DOI] [PubMed] [Google Scholar]
- 32.Park JB, Schiffrin EL. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens. 2001;19:921–930. doi: 10.1097/00004872-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Beggah AT, Escoubet B, Puttini S, Cailmail S, Delage V, Ouvrard-Pascaud A, Bocchi B, Peuchmaur M, Delcayre C, Farman N, Jaisser F. Reversible cardiac fibrosis and heart failure induced by conditional expression of an antisense mRNA of the mineralocorticoid receptor in cardiomyocytes. Proc Natl Acad Sci USA. 2002;99:7160–7165. doi: 10.1073/pnas.102673599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Ramires FJ, Weber KT. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res. 1997;35:138–147. doi: 10.1016/s0008-6363(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 35.Nakano S, Kobayashi N, Yoshida K, Ohno T, Matsuoka H. Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extracellular signal-regulated kinases, NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor-1, and Rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens Res. 2005;28:925–936. doi: 10.1291/hypres.28.925. [DOI] [PubMed] [Google Scholar]
- 36.Favre J, Gao J, Zhang AD, Remy-Jouet I, Ouvrard-Pascaud A, Dautreaux B, Escoubet B, Thuillez C, Jaisser F, Richard V. Coronary endothelial dysfunction after cardiomyocyte-specific mineralocorticoid receptor overexpression. Am J Physiol Heart Circ Physiol. 2011;300:H2035–2043. doi: 10.1152/ajpheart.00552.2010. [DOI] [PubMed] [Google Scholar]
- 37.Garnier A, Bendall JK, Fuchs S, Escoubet B, Rochais F, Hoerter J, Nehme J, Ambroisine ML, De Angelis N, Morineau G, d’Estienne P, Fischmeister R, Heymes C, Pinet F, Delcayre C. Cardiac specific increase in aldosterone production induces coronary dysfunction in aldosterone synthase-transgenic mice. Circulation. 2004;110:1819–1825. doi: 10.1161/01.CIR.0000142858.44680.27. [DOI] [PubMed] [Google Scholar]
- 38.Griol-Charhbili V, Fassot C, Messaoudi S, Perret C, Agrapart V, Jaisser F. Epidermal growth factor receptor mediates the vascular dysfunction but not the remodeling induced by aldosterone/salt. Hypertension. 2011;57:238–244. doi: 10.1161/HYPERTENSIONAHA.110.153619. [DOI] [PubMed] [Google Scholar]
- 39.Leopold JA, Dam A, Maron BA, Scribner AW, Liao R, Handy DE, Stanton RC, Pitt B, Loscalzo J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13:189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagiyama S, Matsumura K, Fukuhara M, Sakagami K, Fujii K, Iida M. Aldosterone-and-salt-induced cardiac fibrosis is independent from angiotensin II type 1a receptor signaling in mice. Hypertens Res. 2007;30:979–989. doi: 10.1291/hypres.30.979. [DOI] [PubMed] [Google Scholar]
- 41.Guyton AC. Renal function curve--a key to understanding the pathogenesis of hypertension. Hypertension. 1987;10:1–6. doi: 10.1161/01.hyp.10.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Li XC, Shao Y, Zhuo JL. AT1a receptor knockout in mice impairs urine concentration by reducing basal vasopressin levels and its receptor signaling proteins in the inner medulla. Kidney Int. 2009;76:169–177. doi: 10.1038/ki.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliverio MI, Delnomdedieu M, Best CF, Li P, Morris M, Callahan MF, Johnson GA, Smithies O, Coffman TM. Abnormal water metabolism in mice lacking the type 1A receptor for ANG II. Am J Physiol Renal Physiol. 2000;278:F75–82. doi: 10.1152/ajprenal.2000.278.1.F75. [DOI] [PubMed] [Google Scholar]
- 44.Cervenka L, Mitchell KD, Oliverio MI, Coffman TM, Navar LG. Renal function in the AT1A receptor knockout mouse during normal and volume-expanded conditions. Kidney Int. 1999;56:1855–1862. doi: 10.1046/j.1523-1755.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- 45.Lai L, Feng X, Liu D, Chen J, Zhang Y, Niu B, Gu Y, Cai H. Dietary salt modulates the sodium chloride cotransporter expression likely through an aldosterone-mediated WNK4-ERK1/2 signaling pathway. Pflugers Arch. 2012;463:477–485. doi: 10.1007/s00424-011-1062-y. [DOI] [PubMed] [Google Scholar]
- 46.Muller OG, Parnova RG, Centeno G, Rossier BC, Firsov D, Horisberger JD. Mineralocorticoid effects in the kidney: Correlation between alphaENaC, GILZ, and Sgk-1 mRNA expression and urinary excretion of Na+ and K+ J Am Soc Nephrol. 2003;14:1107–1115. doi: 10.1097/01.asn.0000061777.67332.77. [DOI] [PubMed] [Google Scholar]
- 47.Shibata S, Rinehart J, Zhang J, Moeckel G, Castaneda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, Lifton RP. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013;18:660–671. doi: 10.1016/j.cmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Funder JW. Reconsidering the roles of the mineralocorticoid receptor. Hypertension. 2009;53:286–290. doi: 10.1161/HYPERTENSIONAHA.108.119966. [DOI] [PubMed] [Google Scholar]
- 49.Ambroisine ML, Favre J, Oliviero P, Rodriguez C, Gao J, Thuillez C, Samuel JL, Richard V, Delcayre C. Aldosterone-induced coronary dysfunction in transgenic mice involves the calcium-activated potassium (BKCa) channels of vascular smooth muscle cells. Circulation. 2007;116:2435–2443. doi: 10.1161/CIRCULATIONAHA.107.722009. [DOI] [PubMed] [Google Scholar]
- 50.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 51.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane nadh/nadph oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 53.Wang D, Chen Y, Chabrashvili T, Aslam S, Borrego Conde LJ, Umans JG, Wilcox CS. Role of oxidative stress in endothelial dysfunction and enhanced responses to angiotensin II of afferent arterioles from rabbits infused with angiotensin II. J Am Soc Nephrol. 2003;14:2783–2789. doi: 10.1097/01.asn.0000090747.59919.d2. [DOI] [PubMed] [Google Scholar]
- 54.Wang D, Jose P, Wilcox CS. Beta(1) receptors protect the renal afferent arteriole of angiotensin-infused rabbits from norepinephrine-induced oxidative stress. J Am Soc Nephrol. 2006;17:3347–3354. doi: 10.1681/ASN.2006030212. [DOI] [PubMed] [Google Scholar]
- 55.Calhoun DA, White WB. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. J Am Soc Hypertens. 2008;2:462–468. doi: 10.1016/j.jash.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Vaclavik J, Sedlak R, Plachy M, Navratil K, Plasek J, Jarkovsky J, Vaclavik T, Husar R, Kocianova E, Taborsky M. Addition of spironolactone in patients with resistant arterial hypertension (aspirant): A randomized, double-blind, placebo-controlled trial. Hypertension. 2011;57:1069–1075. doi: 10.1161/HYPERTENSIONAHA.111.169961. [DOI] [PubMed] [Google Scholar]
- 57.Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ, British Hypertension Society’s PSG Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (pathway-2): A randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068. doi: 10.1016/S0140-6736(15)00257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.