Abstract
Prior research illustrates that memory can guide value-based decision-making. For example, previous work has implicated both working memory and procedural memory (i.e., reinforcement learning) in guiding choice. However, other types of memories, such as episodic memory, may also influence decision-making. Here we test the role for episodic memory—specifically item versus associative memory—in supporting value-based choice. Participants completed a task where they first learned the value associated with trial unique lotteries. After a short delay, they completed a decision-making task where they could choose to re-engage with previously encountered lotteries, or new never before seen lotteries. Finally, participants completed a surprise memory test for the lotteries and their associated values. Results indicate that participants chose to re-engage more often with lotteries that resulted in high versus low rewards. Critically, participants not only formed detailed, associative memories for the reward values coupled with individual lotteries, but also exhibited adaptive decision-making only when they had intact associative memory. We further found that the relationship between adaptive choice and associative memory generalized to more complex, ecologically valid choice behavior, such as social decision-making. However, individuals more strongly encode experiences of social violations—such as being treated unfairly, suggesting a bias for how individuals form associative memories within social contexts. Together, these findings provide an important integration of episodic memory and decision-making literatures to better understand key mechanisms supporting adaptive behavior.
Keywords: episodic memory, value, decision-making, dictator game, associative memory
INTRODUCTION
Successfully navigating through the world requires individuals to integrate details from previous experiences with current environmental demands. The seemingly ubiquitous need for memory in decision-making would suggest that these two fields intimately collaborate to decompose the complex relationship between the representation of past experiences and the subsequent effects on choice. While previous research has demonstrated that memory can influence decisions (Daw & Doya, 2006; Fischhoff, 1975; Hawkins & Hastie, 1990; E. J. Johnson, Häubl, & Keinan, 2007; Madan, Ludvig, & Spetch, 2014; Montague & Berns, 2002; Tversky & Kahneman, 1973; E. U. Weber et al., 2007), empirical research directly integrating detailed assays of episodic memory and value-based decision-making has remained largely elusive, leaving critical questions unanswered. How does episodic memory influence value-based choice? How do individuals encode value into episodic memory and how are these memories then accessed during choice? And finally, is value encoding and retrieval domain general such that the same mechanism is deployed across various situational contexts? To address these questions, here we directly explore the interface between episodic memory processes and value-based decision-making.
Research on episodic memory and decision-making has predominantly been studied independently (Elke U. Weber, Goldstein, & Barlas, 1995). Behavioral and neural investigations of episodic memory have revealed that representations of the past can be supported by at least two distinct processes, which has successfully fractionated episodic memory into discrete components (Brown & Aggleton, 2001; Davachi, 2006; Yonelinas & Jacoby, 2012). For instance, individuals can have memories for individual features of an experience without any retrieving any details of the context, which is known as item memory. Alternatively, individuals can recall the associative relationships between discrete features of an experience, resulting in memory for an item and the context in which it occurred. Unlike item memory, these associative memories can involve the recovery of dynamic multidimensional information reflecting specific, detailed experiences. Decades of rigorous trans-species research has elucidated the process by which these memory components are stored, represented, and retrieved (Davachi, 2006; Eichenbaum, Sauvage, Fortin, Komorowski, & Lipton, 2012). Yet, how item and associative memory guide decision-making has not been formally tested or described.
Historically, classic judgment and decision-making research has only loosely linked memory (broadly construed) with value-based choice. For example, some theorists have loosely drawn on working memory research to explain strategic decision-making. Early work demonstrating that working memory is limited (Miller, 1956) has been used as evidence that individuals do not act as pure ‘rationalists’, as decisions are often made under conditions where there is finite information (Simon, 1956) (for a more comprehensive review see Weber et al 1995). However, the transient nature of working memory representations may not be an ideal memory system to support decision-making in isolation, both because of its capacity limitations and fast decay rate.
More recently, the burgeoning field of value based decision-making has examined the intersection of choice and memory within the context of many, repeated experiences (Doll, Shohamy, & Daw, 2015; Palombo, Keane, & Verfaellie, 2015). Results reveal that across hundreds of trials, individuals can form simple stimulus-response associations that can guide subsequent choice (Daw & Doya, 2006). The processes underlying stimulus response learning are more characteristic of procedural memory (e.g., skill and condition memory, see Schacter & Tulving, 1994). Although this work has robustly identified a role for types of procedural memory in supporting stimulus-response learning, it does not preclude the possibility that associative memory may also be influencing choice. Problematically, however, exposing individuals to many, repeated experiences limits the ability to directly disambiguate incremental procedural memory from episodic memory, making it difficult to extract exactly how—and at what stage—episodic memory might influence choice. Despite these limitations, empirical work has demonstrated that the retrieval of prior experiences during choice shape decision-making (Gonzalez & Dutt, 2011; E. J. Johnson et al., 2007; Ludvig, Madan, & Spetch, 2015; Madan et al., 2014; Elke U. Weber et al., 1995). However, many of these prior studies do not characterize the nature of these retrieved memories, making it difficult to extrapolate the exact role of episodic memory in decision-making.
While these research fields indicate that memory and choice are intimately linked (Johnson et al., 2007; Elke U. Weber et al., 1995; Ludvig, Madan, & Spetch, 2015; Madan et al., 2014), little is known about the quality of these memories, or the detailed nature of how they shape value based choice. Given the challenges of testing episodic memory in the context of repeated exposures, it is more efficacious to investigate episodic memory processes during decision-making by exploring choices based on a single prior experience. This approach harks back to more classic models of decision-making where single shot games were considered the archetypal mode of understanding behavior (Camerer, 2003). Indeed, in many everyday situations, individuals do not have the luxury of using many past experiences to guide choice. Instead, individuals routinely make decisions with limited prior information, where they have only experienced one previous relevant episode.
Based on previous work from the fields of episodic memory, decision-making, and learning, there are two candidate mechanisms that may underpin value-based choice. One possibility is that value-based choice does not rely on associative memory. In this case, only a simple representation of the previous experience is retrieved without any specific details (Schacter & Tulving, 1994). In line with this, a large literature on impression formation suggests that individuals infer the value of an individual or object without retrieving any specific details (Lee & Harris, 2013, Uleman et al, 2005). Indeed, patients with amnesia who have deficits in associative memory, still have intact impression formation (M. K. Johnson, Kim, & Risse, 1985). An alternative possibility is that at the time of a decision, individuals may retrieve enriched associative memories that consist of flexible relationships between specific features of the past experience (Davachi, 2006; Schacter & Tulving, 1994). In this case, retrieved memories are contextualized, containing complex details that are explicitly remembered (i.e., value, place, person, etc.). Theoretically, these types of associative memories would provide a highly informative representation of the previous experience that could optimally guide future choice. For example, when deciding whether or not to trust someone, it may be most efficient and beneficial to retrieve the specific actions and consequences of the past experience with that individual.
Decomposing whether value-based choice relies on associative-rich memories—as opposed to less detailed forms of item memory or even no episodic memory—will help characterize the dynamic processes and interplay between memory and choice. Our aim here was three-fold: first, to identify and characterize whether individuals encode value representations into episodic memory. Second, to explore whether these episodic memories subserve value based decisions. And third, to test if this mechanism is deployed across contexts, including during simple, non-social choices and during more complex, social choices. In the current study, participants performed a decision-making task where they first encountered trial-unique lotteries that varied in their reward outcomes (‘Reward Task’, Figure 1). After a short delay, participants completed a decision-making task in which they decided between selecting a previously encountered lottery or a new, never before seen lottery. Adaptive decisions are indicated by selecting lotteries that were previously associated with high rewards more often than those associated with low rewards. To probe episodic memory representations for these lotteries, participants completed a surprise memory test for the lottery and their associated value. We predicted that individuals would make adaptive choices during the decision-making phase (i.e., choose high value and avoid low value), and that this would be related to associative memory for the lotteries and their specific outcomes. We further test whether these predictions generalize to a more complex, ecologically valid social context where participants dyadically interact with other participants.
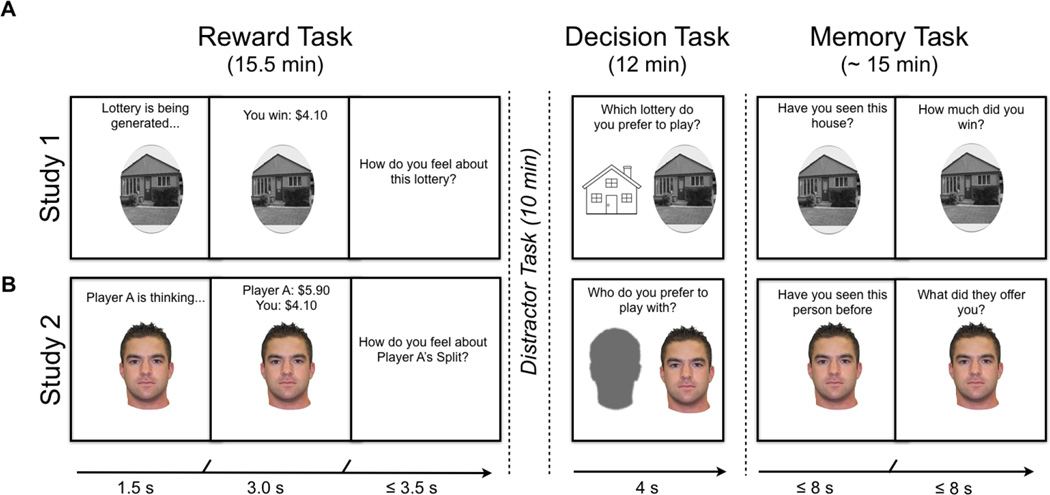
Figure 1. Task structure for Experiment 1 & 2.
A) In the non-social Reward task (lottery game), participants won high and low value amounts. B) In the social Reward task (dictator game), participants received high and low offers. Across experiments, after the Reward tasks, participants completed a 10-minute Distracter task (a simple math test) before completing a Decision task where they were asked which lottery or partner they would prefer to play with. Finally, at the end of the Experiment, participants completed a surprise Memory test in which we explicitly tested item memory and associative memory. To test for item memory, participants had to first indicate whether they had seen the face/house during the Reward task (i.e., old or new), and then state their confidence (1 = high confidence old, 2 = low confidence old, 3 = not sure, 4 = low confidence new, 5 = high confidence new). To test for associative memory, if participants responded with a 1, 2, or 3, participants had to indicate how much money was associated with the house’s outcome during the Reward task on a 5-point likert scale ($0-$5 with $1 increments).
Experiment 1
METHODS
Participants
Participants were recruited from New York University and the surrounding New York City community. Informed consent was obtained from each participant in a manner approved by the University Committee on Activities Involving Human Subjects. Participants were paid an initial $10 and an additional monetary bonus accrued during the task. Experiment 1 included 30 participants (18 females; mean age 23.6±4.0 SD). Sample size was determined by pilot data (not presented here) demonstrating that N=30 was sufficient to procure significant results.
Stimuli
Stimuli for Experiment 1 consisted of 120 gray-scale images of houses (Krebs, Boehler, De Belder, & Egner, 2015). We randomized the presentation of stimuli appearing in the Reward, Decision, and Memory tasks across participants.
Task Design
In Experiment 1, participants completed 4 tasks in the following order: Reward, Distracter, Decision, and Memory. During the Reward task, participants were instructed that they would be playing 60 trials of a game involving 60 independent lotteries which could pay up to $5 (see supplemental methods for details). They were told that each lottery would be denoted by a particular trial-unique, house stimulus. Participants were instructed to pay attention to the houses they encountered because they might encounter them later in the experiment. Prior to starting the experiment, participants were required to correctly answer questions about the nature of the task to ensure comprehension.
Reward Task
On each trial of the Reward task, participants observed for 1.5 seconds a screen that read, “Lottery is being generated” alongside a picture of a trial-unique house (Fig 1a). The text on the top of the screen was then replaced with the reward outcome associated with that lottery for 3 seconds. Finally, the participants had up to 3.5 seconds to indicate how they felt about the outcome of the lottery (1 = Good, 2 = Neutral, 3 = Bad). Following their response, a fixation cross was shown with a jittered ITI varying between 1–3 seconds. Lottery outcomes on each trial were randomly selected without replacement from a range of values between $0.10-$1.50 (low value) and $3.60-$5.00 (high value), each in 10 cent increments. Participants were told that one trial would be randomly selected to be paid out at the end of the experiment.
Distractor Task
Following the Reward task, participants completed a Distracter task—a 10-minute task consisting of various math problems—to introduce a short delay before the Decision and Memory tasks (Duncan, Tompary, & Davachi, 2014). On each trial of the Distracter task, participants were asked to solve a math problem, the solutions to which were all single digit responses. Subjects were given 9 seconds to solve each problem and were instructed to use the number keys on the keyboard to indicate their response. Participants did not receive feedback on their performance for this task. All participants had above chance performance on the Distracter task.
Decision Task
After the Distracter task, participants completed the Decision task. In the Decision task participants were instructed that they would see two house stimuli representing two different lotteries (Fig 1a) and would have to decide which lottery they would prefer to play. On each trial, participants were presented with a pair of images and asked to choose between a trial-unique house and a schematic line drawing of a house (this house schematic was the same on every trial). The trial unique house was either a previously encountered house (from the Reward task) or novel, never before seen house. The schematic line drawing of a house was indicative of a new lottery that would be selected at random. In other words, on each trial, participants could select between a house stimulus (i.e. a previously encountered stimulus or completely novel stimulus) and a schematic line drawing of a house (see supplement methods for more details). Participants had 4 seconds to respond and completed 90 trials. The trial-unique houses were selected randomly without replacement from the 60 houses presented during the reward task and 30 never before seen houses. During this task, participants only selected which lottery they preferred to play, and feedback about the lottery value was never given. This allowed us to gather information on individual preferences for lotteries.
Memory Task
Finally, participants completed a surprise Memory task, where we probed item memory (i.e., recognition memory for houses) and associative memory (i.e., memory for the value associated with individual houses). On each trial, participants were shown a picture of a house, which was either previously shown during the Reward task (i.e., old) or was never shown (i.e., new). To probe item memory, participants had to indicate whether they had seen the house during the Reward task including their confidence (1 = high confidence old, 2 = low confidence old, 3 = not sure, 4 = low confidence new, 5 = high confidence new). To probe associative memory, if participants responded with a 1, 2, or 3, participants had to indicate how much money was associated with the house’s outcome during the Reward task on a 5-point likert scale ($0-$5 with $1 increments). Participants had up to 8 seconds to make each response. We additionally tested whether individuals remembered which house they selected during the decision task, but these responses are not included in the current manuscript. After completing these four tasks, a single trial was selected from the Reward task to be paid out (this was in addition the to $10 show up fee). Finally, participants were debriefed about the task.
Data Analysis
Self report
We first tested whether individuals reported feelings that were congruent with the lottery outcomes during the Reward task, with the prediction that low value wins would engender neutral to bad feelings. For each participant we ran a simple regression between reward outcome and individuals’ self-report scores (1 = Good, 2 = Neutral, 3 = Bad). To test for significance, we submitted r-to-z transformed scores to one-sample t-tests.
Choice
We next tested whether individuals were more likely to choose high versus low value houses during the Decision task, which would indicate that individuals use prior experience to guide decision-making. We ran a Generalized Linear Model (GLM), as implemented by MATLAB’s ‘glmfit’ function, with participants’ choice behavior during the Decision task (selecting a previously seen house = 1, selecting the schematic house = 0) as the dependent variable, and z-scored outcomes (i.e. the reward of the house) during the Reward task as the independent variable. Trials in which participants had the opportunity of selecting the novel house stimuli was not entered into the GLM or included in reported analyses.
Memory
Item memory was delineated by correct recognition memory for the house stimuli alone. Associative memory was correct recognition of both the house stimuli and the associated value of the lottery (within a $1 range). For the item memory analysis, we did not include responses in which participants indicating a ‘not sure’ response (i.e., a 3). Further, we collapsed across high and low confidence responses because preliminary analysis showed that confidence did not influence across group comparisons (social versus non-social) or the influence of item memory on adaptive decision-making. We calculated successful item memory as d’, in which the higher the d’, the better discrimination between old and new houses. d’ was calculated as z-score(Hit Rate) – z-score(False Alarm Rate). We implemented standard correction procedures to account for hit rates of 1 and false alarm rates of 0 by adjusting extreme values by 1/2N, where N is the number of old images for hit rates and novel foils for false alarms. To test for significance, d’ were submitted to 1-sample t-tests. Additionally, we estimated C, which measures biases in response criterion, as defined by −.5*(z-score(Hit Rate) + z-score(False Alarm Rate). We calculated successful associative memory as the proportion of items in which the correct value was selected out of the items correctly identified as old (% correct associative memory / % correct item memory). Because (1) participants did not evenly distribute their responses across the 5-point scale, and (2) source memory was conditionalized by item memory, we tested for significance of associative memory using the following permutation-based boot strapping procedure: First, for each participant we shuffled associative memory responses (i.e., responses to the question ‘how much money was associated with the house?’) and recalculated accuracy. This was repeated 10,000 times to generate a distribution of ‘chance’ performance. Then, to determine significance, we selected a random value from each participant’s distribution of ‘chance’ performance to calculate the average ‘Group Chance’ performance. This too was repeated 10,000 times to generate a ‘Group Chance’ distribution. P-values were calculated by determining the probability of the mean accuracy according to the ‘Group Chance’ distribution.
Interaction of choice and memory
To explicitly investigate the role of different types of memory on choice behavior during the Decision task, we ran an ANOVA where the dependent variable was choice, and value outcome and memory were within-subjects predictors. Outcome was split into binary categories of high ($3.6-$5.00) and low ($0.10-$1.50) values. Memory was split into three categories: no memory, item memory, and associative memory. For this analysis, participants were only included if they had at least two responses in each category of the ANOVA.
RESULT
Self-Report
During the Reward task, participants viewed lotteries that ranged in outcome values from $0.10-$5.00. Participants reported feeling more positively about high versus low value lotteries (t(29)=16.5, p<0.001, Cohen’s d=1.56), indicating that they were sensitive to the outcome value of the lotteries.
Choice
During the Decision task participants were asked to choose to play one of two lotteries, a specific, trial-unique lottery or a lottery chosen at random (Figure 1a). Since participants had already seen a portion of the houses during the Reward task, our first question was whether the outcomes of these past lotteries, i.e. how much a participant previously won on a particular lottery, would guide future decisions to re-engage with those lotteries. Using a logistic regression where value (i.e., outcomes from the Reward task) predicts choice, we found that participants selected lotteries more often when they had been previously associated with larger outcomes (mean 0.13±0.05se, one sample t-test, t(29)=2.79, p=0.009, d=0.26). That the value outcomes from the Reward task predicts choice during the Decision task suggests that individuals use previous experiences to guide their current choice.
Memory
Before testing how episodic memory influences choice, we first characterized the content of individuals’ memory for the lotteries. Participants successfully encoded lotteries from the Reward task, such that item memory (t(29)=3.71, p=0.001, d=0.42, Table 1) and associative memory (permutation-based testing: p=0.01) were both significantly greater than chance. Next, we tested whether the value of the reward itself influenced memory. We did not find any significant differences in item memory (t(29)=0.64, p=0.53, d=0.11) or associative memory (t(29)=1.6, p=0.12, d=0.29) for high versus low reward lotteries.
Table 1.
In the lottery task, the outcome of each lottery (i.e., high versus low) did not influence item or associative memory. However, in the social domain participants showed a significant bias towards better associative memory for individuals’ offer amounts when they were low versus high, without any difference in item memory. This pattern of results was demonstrated across-subjects including the decision phase (Experiment 1,2), across subjects excluding the decision phase (Experiment 3,4), and within subjects including the decision phase (Experiment 5). [HR = hit rate, FAR = false alarm rate)
| Condition | Item Memory | Response Bias | Item HR | Item FAR | Associative Memory |
|---|---|---|---|---|---|
| (d’ ±SE) | (C ±SE) | (mean ±SE) | (mean ±SE) | (mean ±SE) | |
| Experiment 1 | |||||
| high | 0.29 ±0.07 | −0.31 ±0.11 | 0.67 ±.04 | 0.48 ±0.04 | 0.23 ±0.02 |
| low | 0.24 ±0.09 | −0.33 ±0.12 | 0.67 ±0.04 | 0.22 ±0.03 | |
| Experiment 2 | |||||
| high | 0.58 ±0.07 | −0.23 ±0.12 | 0.65 ±0.03 | 0.55 ±0.04 | 0.13 ±0.03 |
| low | 0.61 ±0.08 | −0.24 ±0.12 | 0.64 ±0.04 | 0.30 ±0.03 | |
| Experiment 3 | |||||
| high | 0.63 ±0.12 | 0.15 ±0.12 | 0.49 ±0.03 | 0.21 ±0.04 | 0.18 ±0.02 |
| low | 0.56 ±0.10 | 0.18 ±0.11 | 0.49 ±0.03 | 0.23 ±0.02 | |
| Experiment 4 | |||||
| high | 0.99 ±0.11 | 0.52 ±0.12 | 0.55 ±0.04 | 0.34 ±0.04 | 0.19 ±0.03 |
| low | 0.99 ±0.14 | 0.52 ±0.11 | 0.54 ±0.05 | 0.40 ±0.04 | |
| Experiment 5 | |||||
| Non-social | |||||
| high | 0.45±0.09 | 0.09±0.07 | 0.54 ±0.03 | 0.39 ±0.03 | 0.25 ±0.02 |
| low | 0.48±0.07 | 0.08±0.08 | 0.55 ±0.03 | 0.26 ±0.03 | |
| Social | |||||
| high | 1.23±0.07 | 0.13 ±0.07 | 0.66 ±0.03 | 0.25 ±0.02 | 0.16 ±0.02 |
| Low | 1.29±0.08 | 0.11 ±0.07 | 0.68 ±0.03 | 0.27 ±0.02 |
Interaction of choice and memory
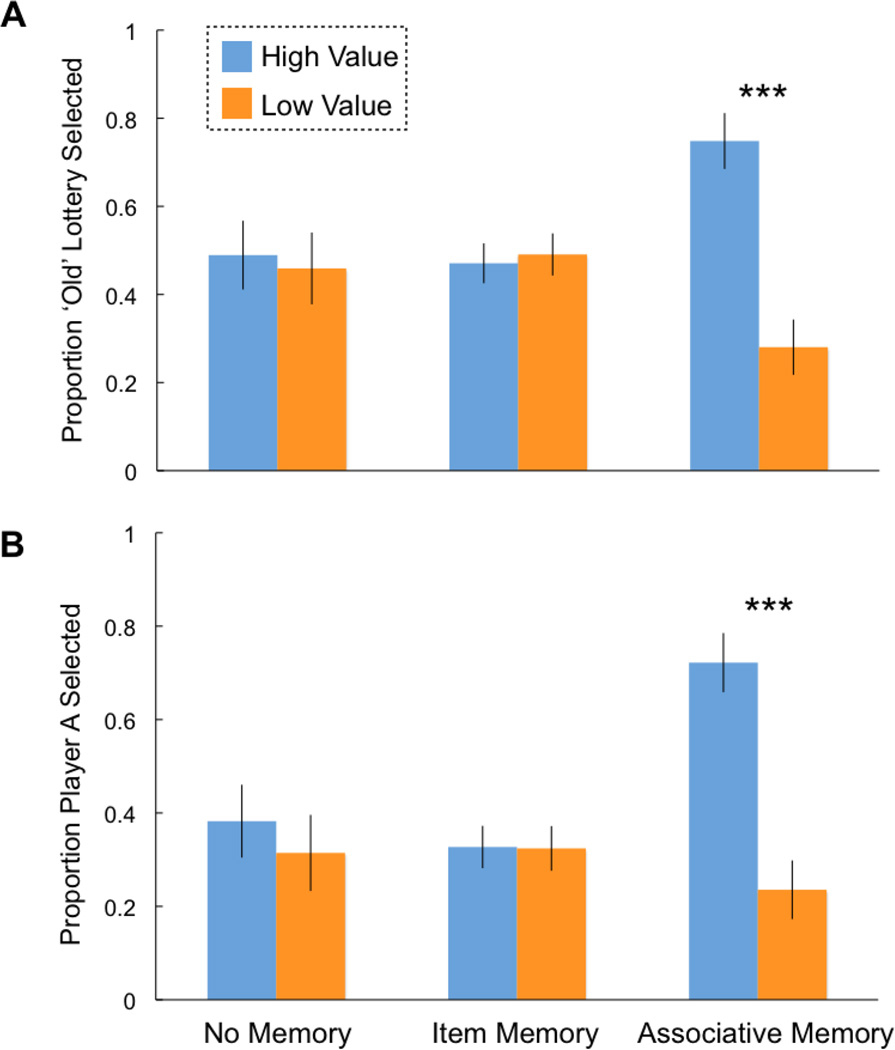
The prior analyses illustrate that not only do participants use prior experience to guide choice, but that they also have significant memories for lottery stimuli and their associated outcomes. However, they do not address what types of memories are deployed to support adaptive decision-making. In other words, do individuals rely on episodic memory to successfully select high value lotteries and avoid low value lotteries? To answer this, we ran an ANOVA on participants’ choice behavior during the Decision task as the dependent variable, with outcome from the Reward task (high, low), and memory from the Memory task (no memory, item memory, associative memory) as within-subjects, repeated-measures predictors (Fig 2A). This analysis revealed no main effect of memory (F(2)=0.43, p=0.23, η2=0.12), a significant main effect of reward (F(1)=40.58, p<0.001, η2=0.66) and a significant interaction of reward and memory (F(2)=18.20, p<0.001, η2=0.59). Post-hoc t-tests revealed that individuals only discriminated between choosing high and low value lotteries when they had intact associative memory. That is, when individuals remembered both the lottery stimulus and the associated value, they chose high lotteries more often than low lotteries (paired samples t-test, t(21)=6.65, p<0.001, d=0.89). When participants only had item memory or no memory, we observed no differences between selecting high versus low lotteries (item: paired samples t-test, t(21)=-1.17, p=0.26, d=-0.10; no memory: paired samples t-test, t(21)=0.99, p=0.28, d=0.11). This benefit of associative memory for adaptive choice was driven by individuals both selecting high reward lotteries (associative > item memory: all paired samples t-tests, t(28)=3.85, p<0.001, d=0.93; associative > no memory: t(28)=3.52, p=0.002, d=0.81) and avoiding low reward lotteries (associative > item memory: t(28)=3.39, p=0.002, d=0.84; associative > no memory: t(28)=3.22, p=0.003, d=0.48). In short, adaptive choice necessitates intact associative memory.
Figure 2. Adaptive choice is predicted by associative—but not item—memory for the prior encounter.
A) In the lottery task (Experiment 1), we found that participants equally endorsed high and low value lotteries when they did not have any memory or only had item memory for the lottery alone. In contrast, when participants had associative memories of the lotteries and their outcomes, they chose high value lotteries more often than low value lotteries. B) In the dictator game (Experiment 2), the same pattern of results was observed. Error bars reflect one standard error of the mean. *** indicates p < 0.001.
Experiment 2
In Experiment 1 we show that within the non-social domain, individuals have contextually enriched associative memories for past lotteries, and these memories are used to adaptively guide future choices to gamble. However, does the same mechanism guiding episodic memory and decision-making within the non-social domain contribute to more complex, ecologically valid decisions within the social domain? Based on the impression formation literature which illustrates that individuals can evaluate others in the absence of episodic memory (M. K. Johnson et al., 1985), one possibility is that value based decisions made in the social domain do not require episodic memories. In Experiment 2, we test this question by asking participants to interact with trial-unique players in a dictator game (Forsythe, Horowitz, Savin, & Sefton, 1994; Kahneman, Knetsch, & Thaler, 1986; Fig 1B).
METHODS
Participants
Recruitment, consent, and payment were identical to Experiment 1. In an attempt to match data collection for Experiment 1, we recruited 40 participants, as participants who failed to believe the social manipulation would be excluded from analysis. Experiment 2 included 32 participants (18 females; mean age 23.6±4.0 SD) after removing 8 participants for not believing the social manipulation (see supplemental methods for details).
Stimuli
Stimuli consisted of 120 color images of white male faces. These 120 images were selected from a larger sample of 179 faces to be the most neutral in terms of ‘Attractiveness’, ‘Approachability’ and ‘Overall Positive or Negative Feeling’, as determined by a separate behavioral cohort (N=24; see supplemental methods).
Task Design
We modified the task from Experiment 1 to investigate dyadic social interactions (Fig 1b). As in the previous study, participants completed the same tasks as Experiment 1 only this time the first and third tasks each contained a social component. The Reward task was the same as the lottery task in every respect except for a social component of ‘intent’, which was manipulated by stimulus type (faces versus houses) and instructions. Effectively, the Reward task was modeled after the dictator game, in which an initial player, Player A, is endowed with $10. Player A can then divide that money however he sees fit with a second player, Player B. Player B must always accept the division. In our task, participants were instructed that they would be playing multiple rounds of the game as Player B, and would encounter 60 different Player As, indicated by a trial unique face on each trial. In reality, each division was randomly generated and paired with a unique face. On each trial, participants first saw the text “Player A is thinking” and a picture of a trial-unique face for 1.5 seconds. The text on the top of the screen was then replaced with the amount of money Player A decided to keep (e.g., Player A: $9.10) and how much money was being offered to Player B (e.g., You: $0.90) for 3 seconds. Finally, the participants viewed a screen asking them to indicate how they felt about the outcome of Player A’s offer (1 = Good, 2 = Neutral, 3 = Bad), and had up to 3.5 seconds to respond. Following their response, a fixation cross was shown with a jittered ITI varying between 1–3 seconds. As in the lottery game explained above, Player A’s monetary division on each trial was randomly selected without replacement from a range of high (ranging from $3.60-$5.00 in 10 cent increments) to low outcomes (ranging from $0.10-$1.50, in 10 cent increments).
Following the Reward task (i.e., dictator game), participants completed the Distracter, Decision, and Memory tasks, which were identical to Experiment 1, except house stimuli were replaced with face stimuli (Fig 1b). Given this, in the Decision task participants were making choices between trial-unique faces and a schematic gray face indicating the selection of a new Player A at random.
Data Analysis
Data analyses were identical to Experiment 1 with one additional analysis. To explore the differences between memory in non-social and social conditions, we conducted a repeated-measures GLM predicting memory performance (both item and associative memory) with value (high, low) as a repeated measure, and group (Experiment 1, Experiment 2) as a between-subjects factor.
RESULT
Self-Report
During the reward task, participants viewed outcomes from Player As that ranged in values from $0.10-$5.00. Replicating Experiment 1, participants reported more positive feelings towards the high versus low value outcomes (t(31)=17.3, p<0.001, d=1.55), indicating that they were sensitive to Player As’ offers. There was no difference in self-report ratings of lottery outcomes in Experiment 1 and dictator offers in Experiment 2 (t(60)=0.11, p=0.91, d=0.03).
Choice
During the Decision task, we observed that participants selected to play with previously encountered players more often when they had made higher value offers during the Reward task (mean 0.24±.10 se, paired samples t-test; t(32)=2.39, p=0.02, d=0.33).
Memory
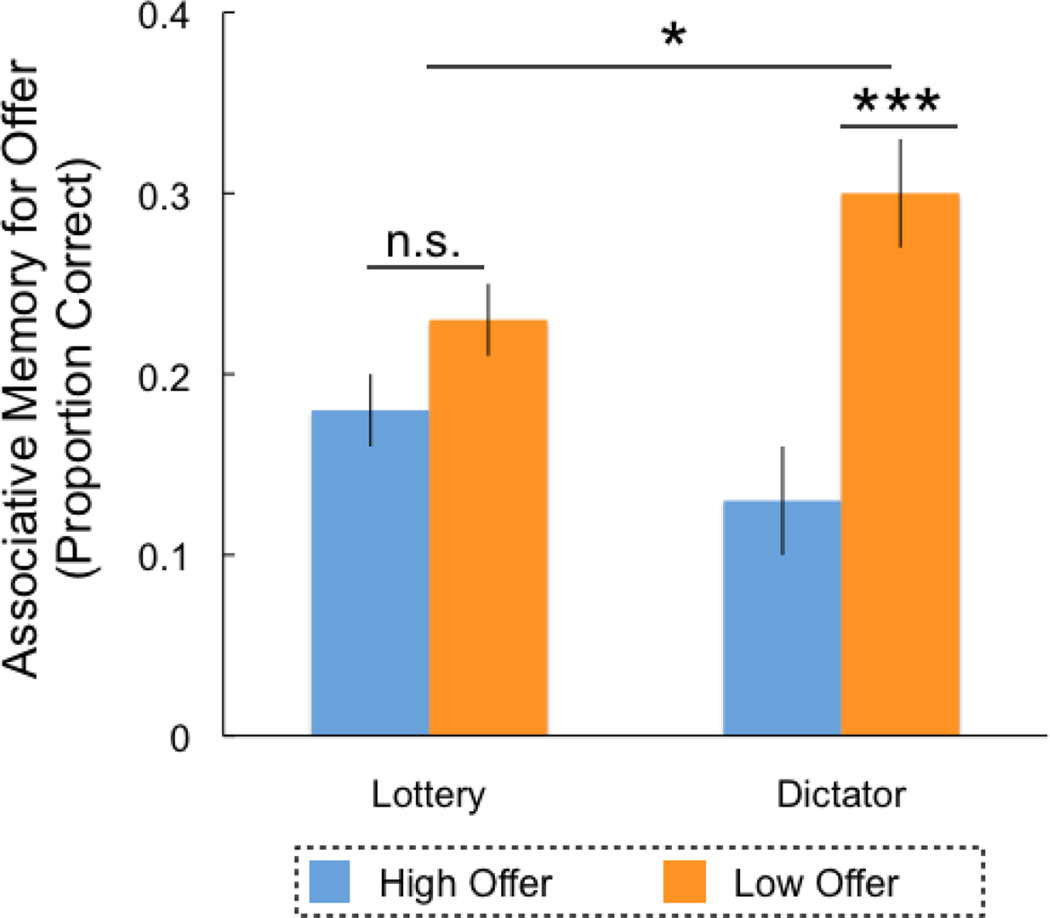
During the memory task, we found that participants successfully encoded the identity and offers of players from the Reward task (Table 1), such that item memory (t(31)=9.09, p=0.001, d=0.98) and associative memory (permutation-based testing: p=0.03) were both significantly greater than chance. Next, we tested whether the offer value influenced memory. While there was no significant difference in item memory (t(31)=0.40, p=0.69, d=0.07), we found that participants had better associative memory for players that offered low versus high values (t(31)=3.69, p=0.001, d=0.65; Fig 3). Simply put, unlike in the non-social domain, in a social context where intention is salient, participants had better associative memory for low versus high outcomes.
Figure 3. Memory is influenced by social intent.
In the non-social condition (Experiment 1), we found no influence of outcome value on associative memory. However, in the matched social condition (i.e., the dictator game), associative memory was stronger for low versus high offers. Value’s influence on associative memory was significantly larger in the social versus non-social domain. Error bars reflect standard error of the mean. *** indicates p < 0.001, * indicates p = 0.05
In fact, comparing memory across Experiments 1 and 2, we found that associative memory was stronger for low value outcomes when the task was social compared to non-social (Value*Group, F(1)=5.08, p=0.03, η2=0.08; Fig 3). However, the influence of value on item memory did not differ across groups (Value*Group, F(1)=0.55, p=0.83, η2=0.001), as both groups showed no effect of value on item memory. Critically, there were no differences in response criteria (C) across groups (paired samples t-test; t(60)=0.45, p=0.65, d=0.22), thus differences in response biases could not explain the item memory results.
Interaction of choice and memory
Next we quantified how different types of memory influenced decisions in the social domain. An ANOVA probing participants’ choice behavior during the Decision task as the dependent variable with reward and memory as within-subjects, repeated-measures predictors (Fig 2B) revealed a main effect of memory (F(2)=5.07, p=0.01, η2=0.33), a main effect of reward (high and low; F(1)=11.92, p=0.004, η2=0.44), and a significant interaction of reward and memory (F(2)=16.50, p<0.001, η2=0.60). Post-hoc t-tests again revealed that individuals only discriminated between choosing high and low value partners when they had intact associative memory. That is, when individuals remembered both the face and the associated offer, they preferred to play with partners who offered high values compared to low values (paired samples t-test; t(15)=4.39, p<0.001, d=0.95). In contrast, when participants exhibited item memory or no memory, we observed no differences between selecting high versus low value offers (item memory: paired samples t-test, t(15)=0.57, p=0.57, d=0.04; no memory: paired samples t-test, t(15)=0.71, p=0.48, d=0.08). In Experiment 2, the benefit of associative memory for adaptive choice was primarily driven by individuals selecting high value offers (associative > item memory: all paired samples t-tests, t(15)=3.79, p=0.002, d=0.95; associative > no memory: t(15)=4.02, p=0.001, d=1.0). There was a trend between avoiding low value offers when comparing associative versus item memory (t(15)=1.98, p=0.07, d=0.5), and a non-significant difference when comparing associative versus no memory (t(15)=0.49, p=0.63, d=0.12).
Experiment 3 & 4
In Experiments 1 and 2 we provide a novel characterization of how individuals bind value to stimuli of past experiences (i.e. associative memory) to guide adaptive choice. However, in these experiments, prior to testing memory, participants completed a decision task in which items were re-presented in the absence of their value. Because of this, prior item and associative memory results could have been influenced by re-exposure to items during the Decision task. To control for this, in Experiment 3 we eliminate the Decision task to test item and associative memory for lotteries without re-exposing houses. In Experiment 4, we eliminate the Decision task to test item and associative memory for players in the dictator game without re-exposing the faces. Analyses focused on determining (1) whether participants still had significant item and associative memory, and (2) whether participants continued to have biases in associative memory towards low value partners in the social dictator task, but not in the non-social lottery task.
METHODS
Participants
Recruitment, consent, and payment were identical to Experiment 1 and 2. Experiment 3 included 31 participants (20 females; mean age 22.9±4.3 SD). Experiment 4 included 30 participants (18 females; mean age 23.9±5.0 SD) after removing 8 participants for not believing the social manipulation (see supplemental materials for details).
Stimuli & Task Design
The stimuli and design in Experiments 3 and 4 were identical to Experiments 1 and 2, respectively, except the Decision task was removed. Thus, participants only completed the Reward, Distracter, and Memory task.
Data Analysis
We probed item and associative memory as described in Experiments 1 & 2. To directly compare the main effect of memory across experiments, we conducted two-sample t-tests. To compare differences in high versus low value memory across groups, we conducted a repeated-measures GLM predicting memory performance (both item and associative memory) with value (high, low) as a repeated measure and group as a between-subjects factor.
RESULT
Memory
As in Experiment 1, in Experiment 3, we found that participants successfully encoded house stimuli and their associated values (Table 1), such that item memory (t(30)=5.54, p<0.001, d=1.0) and associative memory (permutation-based testing: p=0.01, one-tailed) were both significantly greater than chance. There was no significant difference in item memory (paired samples t-test: t(30)=0.9, p=0.37, d=0.16) or associative memory (paired samples t-test: t(30)=0.28, p=0.78, d=0.05) for high versus low lotteries. We also did not find that high versus low value wins differentially influenced item or associative memory across Experiments 1 and 3 (item memory: Value*Group, F(1)=0.02, p=0.89, η2<0.001; associative memory: Value*Group, F(1)=1.67, p=0.2, η2=0.03).
In Experiment 4, we found that participants successfully encoded players and their associated offer values (Table 1), such that item memory (one-sample t-test: t(29)=8.3, p<0.001, d=1.51) was significantly greater than chance, and a trend towards significant associative memory (permutation-based testing: p=0.11). There was no significant difference in item memory for high versus low offers (paired samples t-test: t(29)=0.04, p=0.97, d=0.004). However, as in Experiment 2, participants in Experiment 4 had better associative memory for individuals that offered low versus high offers (paired samples t-test: t(29)=4.01, p<0.001, d=0.73). Comparing the influence of an offer’s value on item and associative memory across Experiments 2 and 4 did not reveal any value*group interactions (item memory: Value*Group, F(1)=0.09, p=0.77, η2=0.001; associative memory: Value*Group, F(1)=0.35, p=0.56, η2=0.006).
Comparing memory across the non-social and social domains (Experiments 3 and 4, respectively), we again observed that associative memory for low value outcomes is greater in the social versus non-social task (Value*Group, F(1)=5.08, p=0.03, η2=0.08). This was not the case for item memory, as we did not observe differences across groups (Value*Group, F(1)=0.55, p=0.83, η2=0.001). However, there was a difference in response criteria (C) for item memory across groups (paired-samples t-test: t(60)=2.17, p=0.03, d=0.56), which could in part be due to differences in response biases. Together, these findings suggest that the bias in associative memory towards low value outcomes in the social versus non-social domain persists even when items were only presented once during the decision phase.
Experiment 5
Given that all previous Experiments were run between subjects, and that the instructions for the social manipulations in Experiments 2 and 4 included additional text on the presentation screen describing Player A’s payout relative to Player B’s payout, we ran an additional follow-up experiment. Experiment 5 was a within subjects design (both the Social and Non-social conditions were presented) and the instructions and task presentation for each condition were matched.
METHODS
Participants
Participants were recruited from Amazon Mechanical Turk (AMT) (Crump, McDonnell, & Gureckis, 2013; Mason & Suri, 2010). Informed consent was obtained from each participant in a manner approved by the University Committee on Activities Involving Human Subjects at New York University. Participants were paid an initial $7 and an additional monetary bonus (up to $2) accrued during the task. 55 participants completed the task, however due to incomplete data or failure to demonstrate source memory, four participants were removed. The remaining sample included 45 participants (27 females; mean age 39.1±12.9 SD).
Stimuli & Task Design
Experiment 5 was a within subjects design in which participants completed a Reward task (both a Lottery and Dictator game) which were exactly the same as those described in Experiments 1 and 2, respectively; a Distractor Task which was a 10 minute anagram puzzle, a Decision task (both a Non-social and Social Decision task which again was structurally identical to Experiments 1 and 2); and finally a Retrieval task (the same surprise memory test used in Experiments 1 and 2). For the reward task, participants completed 2 blocks (social, non-social), and condition order was counterbalanced across participants. For the decision and memory tests social and non-social stimuli were intermixed. This design allowed us to make within-subject comparisons across social and non-social conditions.
Given that Experiment 2 used a social manipulation in which there was additional text on the presentation screen describing Player A’s payout (e.g., “Player A receives $5.90”) relative to Player B’s payout (e.g., “You receive $4.10”), Experiment 5 provided participants with identical numerical information during the Reward task without emphasizing the participant’s payout relative to Player A’s payout (a form of social anchoring). In Experiment 5, the additional wording that referred to Player A’s payout was removed, and instead participants only observed “You receive $.41” during the Reward task. In addition, to ensure that the instructions between the two conditions were as similar as possible, we removed all references to words that could be construed as social anchoring (i.e. split). The instructions for the Social and Non-social tasks followed an identical structure and can be found in full in the supplement.
Data Analysis
We probed ratings, memory, choice, and memory*choice interactions as described in prior experiments with the exception that across condition comparisons (social, non-social) were tested as a within-subject factor.
RESULT
Self-Report
During the Reward task, participants viewed outcomes that ranged in values from $0.01-$.05. Replicating prior experiments, participants reported more positive feelings towards the high versus low value outcomes (non-social: t(44)=32.2, p<0.001, d=9.7; social: t(44)=13.3, p<0.001, d=4.0). However, unlike the prior experiments relationships between self-report ratings and outcomes were stronger for lottery outcomes than dictator offers (t(44)=3.99, p<0.001, d=0.77).
Choice
During the Decision task, we found a main effect of reward (F(1)=9.26, p=0.004, η2=0.17), such that participants selected to play with previously encountered lotteries and players more often when they were associated with higher rewards. Further, we found a main effect of condition (F(1)=8.8, p=0.005, η2=0.17), such that individuals selected old houses more often than old faces. Critically, the interaction between reward and condition was non-significant (F(1)=0.08, p=0.78, η2=0.002), indicating that reward influenced choice equivocally across social and non-social conditions.
Memory
As in prior experiments, we found that participants successfully encoded the identity and of both lotteries and players (Table 1), such that item memory was above chance (non-social: t(44)=6.8, p<0.001, d=2.1; social: t(44)=18.12, p<0.001, d=5.5). Across group comparisons revealed a main effect of condition (F(1)=25.5, p<0.001, η2=0.37), such that players were recognized better than lotteries. In line with Experiments 1–4, there was no main effect of reward (F(1)=0.67, p=0.42, η2=0.02) or a reward*condition interaction (F(1)=0.03, p=0.86, η2=0.001), indicating their was no influence of reward outcome on item memory in either condition.
For associative memory we found that individuals had significant memory for lotteries and their associated value (permutation-based testing p<0.001), and were trending towards significance for players and their offers (permutation-based testing p=0.11). Across-condition comparisons revealed a main effect of condition (F(1)=5.03, p=0.03, η2=0.10) such that associative memory was better for houses than faces, and a main effect of reward (F(1)=5.25, p=0.03, η2=0.11) such that associative memory was better for low value compared to high value items. Critically, we replicated prior experiments by demonstrating a reward*condition interaction (F(1)=5.3, p=0.03, η2=0.11), such that associative memory was biased towards low versus high value players (t(44)=4.02, p<0.001, d=0.87), without any significant differences between low and high value lotteries (t(44)=0.10, p=0.92, d=0.01).
Interactions of choice and memory
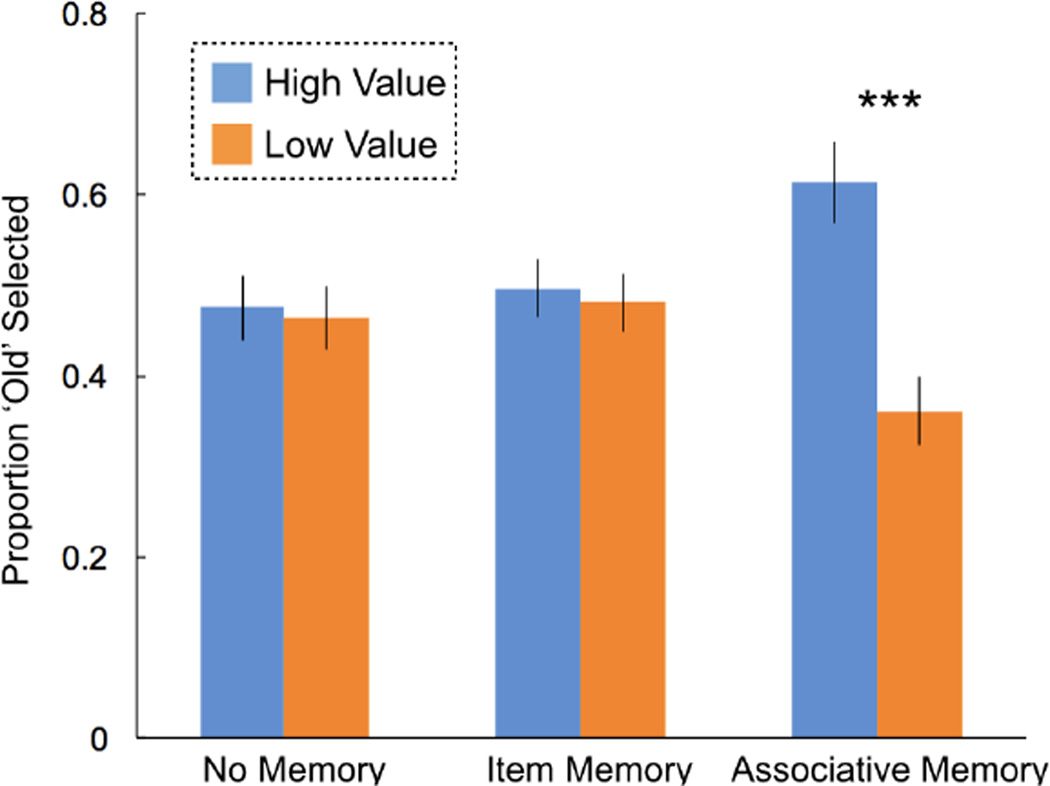
Finally, we probed choice behavior during the decision task as the dependent variable with reward, memory, and group as within-subjects, repeated measures predictors. This analysis revealed a main effect of reward (F(1)=23.4, p<0.001, η2=0.54), such that high value items were selected more often than low value items. Further, replicating the prior experiments, results indicated a reward*memory interaction (F(1)=8.3, p=0.003, η2=0.47, Figure 4). All other terms were non-significant, including memory (p=0.23), condition (p=0.22), reward*memory (p=0.53), reward*condition (p=0.76), memory*condition (p=0.18), and reward*memory*condition (p=0.66). Critically, this last term indicates that memory similarly influenced adaptive choice behavior across social and non-social contexts. Post-hoc t-tests again revealed that individuals reliably discriminated between choosing high and low value items when they had intact associative memory (t(40)=5.27, p<0.001, d=0.94). We observed no differences between selecting high versus low items when participants exhibited only item memory (t(40)=0.71, p=0.48, d=0.1) or no memory (t(40)=0.55, p=0.59, d=0.09).
Figure 4. Replication that adaptive choice is predicted by associative—but not item—memory for the prior encounter.
In a separate cohort of participants, we replicate findings from Experiment 1 & 2. Specifically, we found that participants endorsed high and low reward items equally when they did not have any memory or only had item memory. In contrast, when participants had associative memories of the item and their value, they chose high reward items more often than low reward items. Critically, within this within-subjects design we did not see any differences in the pattern of results (p=0.66) as a function of whether participants were selecting between lotteries (houses) or players (faces). Error bars reflect one standard error of the mean. *** indicates p < 0.001.
DISCUSSION
To make adaptive decisions, an individual should rely on the past as a predictor of the future. This requires that memories be retrieved and deployed when making a decision. Until now, prior work has mainly explored the effects of procedural and working memory on value based choice, without explicitly testing the role of episodic memory. We demonstrate that episodic memory—specifically, associative memory—supports domain general value-based decisions. Our results are three fold. First, individuals formed discrete associations between trial-unique stimuli and their value in both social and non-social domains. Second, individuals were able to use these associative memories to choose adaptively in a subsequent decision-making task, selecting stimuli previously associated with higher, compared to lower, rewards. Finally, individuals were not able to discriminate between high and low value rewards unless they had intact associative memory. In other words, item memory did not appear to contribute to adaptive choice. These results support a new model of adaptive decision-making where enriched episodic memories—as opposed to simple, decontextualized signals—guide behavior.
Why would adaptive value based choice rely on associative memory as opposed to more impoverished forms of memory? Unlike item memory, associative memories provide contextually enriched representations of prior experiences (Eichenbaum et al., 2012; Tulving & Markowitsch, 1998). Critically, these detailed associative memories allow flexible access to all of the relevant information that could prove consequential for future choice (Davachi, 2006; Ranganath, 2010). Indeed, research that has probed the link between retrieval and value based preferences (E. J. Johnson et al., 2007; Elke U. Weber et al., 1995; E. U. Weber et al., 2007) and associative priming and risky choice (Ludvig, Madan, & Spetch, 2015; Madan et al., 2014) implies that rich, associative memories likely support adaptive choice. Our findings confirm and extend these theoretical proposals: when deciding which lottery to play, remembering that you have previously seen a lottery (i.e., item memory) is insufficient in and of itself to make an adaptive decision. Rather, being able to retrieve specific, accurate details of the event (the lottery stimuli) and it’s associated outcomes (i.e. the payout the last time you gambled), allows an individual to use this information to select which lottery will optimize the highest payouts. We found this mechanism to be true for both approach and avoidance behavior; individuals systematically selected highly rewarding outcomes and avoided low reward outcomes. That this mechanism underpinned both approach and avoidance behavior suggests that associative memories are broadly utilized in supporting value based choice.
Previous work, however, has also demonstrated that an individual can exhibit adaptive behavior in the absence of episodic memory (Shohamy & Wagner, 2008; Wimmer & Shohamy, 2012), suggesting that other forms of memory, such as procedural memory, can support value-based choice. Critically, these prior studies utilized paradigms where information was repeated over multiple trials, allowing individuals to incrementally learn information. Although there are times when many past experiences can collectively guide a choice, individuals must often make a decision after limited relevant experiences. Given this, we tried to capture a fairly simple decision space that would provide a robust model for how humans make decisions outside the laboratory. This perspective provided the framework for this work, where we asked individuals to rely on one past experience to guide future choice. An open question for future research is to characterize which domains of value-based decision-making rely on episodic memory.
Our results also shed light onto how individuals form memories about valuable events. While prior research has demonstrated that episodic memory is enhanced in situations in which encoding is explicitly incentivized (Adcock, Thangavel, Whitfield-Gabrieli, Knutson, & Gabrieli, 2006; Callan & Schweighofer, 2008; Shigemune, Tsukiura, Kambara, & Kawashima, 2013; Wolosin, Zeithamova, & Preston, 2012), relatively little research has investigated how individuals associate reward outcomes with items in episodic memory. In essence, do individuals have episodic memories for the value of discrete items? Here, we provide a novel characterization of associative memory, illustrating that individuals have the capacity to bind reward values to items. Critically, we find that individuals encode the discrete values of items with a high degree of accuracy, as opposed to a simplified binary value (i.e., high and low). Importantly, we assayed source memory in a paradigm where participants were anticipating rewards, and future work is needed to delineate the contributions of reward anticipation and outcome to episodic memory.
Further, we illustrate that associative memory for stimuli and their value is highly sensitive to the context in which they are encoded. Specifically, we found that individuals interacting with others in the social condition exhibited a strong associative memory bias for the type of players they encountered. Unlike in the non-social domain where individuals had comparable memory for high and low rewards acquired from lotteries, in the social domain, individuals had much stronger associative memories for partners who treated them unfairly, supporting previous work that memory is asymmetrically biased for negative intent in the social domain (Bell & Buchner, 2011; Bell, Buchner, & Musch, 2010) . That these tasks were matched in all aspects except for the social component provides compelling evidence that individuals more robustly encode associative memories of situations where social connections and relationships have been compromised by harmful intentions. In other words, low value offers—in the context of potentially high value offers (i.e. social intent)—have a greater lasting effect on memory. The fact that episodic memory is stronger for low values in the social domain challenges the long-held assumption that higher reward values always dictate better memory performance. Instead, interpreting the actions of another individual can dictate when individuals incorporate reward into their representations of the environment.
Although prior work has emphasized the importance of integrating prior experiences with current task demands to guide choice (Doll et al., 2015; King-Casas et al., 2005; Shohamy & Adcock, 2010; E. U. Weber et al., 2007; Wimmer & Shohamy, 2012), rarely has research probed the specificity of episodic memory, particularly item versus associative memory, in guiding choice behavior. Across diverse contexts, our results demonstrate that individuals use rich, detailed episodic memories of prior experiences to guide value-based decision-making. Whereas classic models of value-based choice mainly focused on procedural memory, the data here provide a conceptual broadening of more nascent models of decision-making in which contextualized representations of a past experience underlie choice (Dolan & Dayan, 2013; Doll, Simon, & Daw, 2012). Further, these findings mark an important integration of episodic memory and decision-making literatures. Our findings dovetail with models of adaptive memory encoding where associative memories are specifically enhanced for valuable information to support optimal decisions in the future (Shohamy & Adcock, 2010). More mechanistic approaches, such as functional imaging and behavioral modeling, can help further unpack how episodic memory systems interact with known decision-making systems to support future choice.
Supplementary Material
REFERENCES
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-Motivated Learning: Mesolimbic Activation Precedes Memory Formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. http://doi.org/10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Bell R, Buchner A. Source memory for faces is determined by their emotional evaluation. Emotion (Washington, D.C.) 2011;11(2):249–261. doi: 10.1037/a0022597. http://doi.org/10.1037/a0022597. [DOI] [PubMed] [Google Scholar]
- Bell R, Buchner A, Musch J. Enhanced old-new recognition and source memory for faces of cooperators and defectors in a social-dilemma game. Cognition. 2010;117(3):261–275. doi: 10.1016/j.cognition.2010.08.020. http://doi.org/10.1016/j.cognition.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2(1):51–61. doi: 10.1038/35049064. http://doi.org/10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Callan DE, Schweighofer N. Positive and negative modulation of word learning by reward anticipation. Human Brain Mapping. 2008;29(2):237–249. doi: 10.1002/hbm.20383. http://doi.org/10.1002/hbm.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer C. Behavioral Game Theory: Experiments in Strategic Interaction. Princeton University Press; 2003. [Google Scholar]
- Crump MJC, McDonnell JV, Gureckis TM. Evaluating Amazon’s Mechanical Turk as a Tool for Experimental Behavioral Research. PLoS ONE. 2013;8(3):e57410. doi: 10.1371/journal.pone.0057410. http://doi.org/10.1371/journal.pone.0057410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. http://doi.org/10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Daw ND, Doya K. The computational neurobiology of learning and reward. Current Opinion in Neurobiology. 2006;16(2):199–204. doi: 10.1016/j.conb.2006.03.006. http://doi.org/10.1016/j.conb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Dayan P. Goals and habits in the brain. Neuron. 2013;80(2):312–325. doi: 10.1016/j.neuron.2013.09.007. http://doi.org/10.1016/j.neuron.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Shohamy D, Daw ND. Multiple memory systems as substrates for multiple decision systems. Neurobiology of Learning and Memory. 2015;117:4–13. doi: 10.1016/j.nlm.2014.04.014. http://doi.org/10.1016/j.nlm.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Simon DA, Daw ND. The ubiquity of model-based reinforcement learning. Current Opinion in Neurobiology. 2012;22(6):1075–1081. doi: 10.1016/j.conb.2012.08.003. http://doi.org/10.1016/j.conb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Tompary A, Davachi L. Associative encoding and retrieval are predicted by functional connectivity in distinct hippocampal area CA1 pathways. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014;34(34):11188–11198. doi: 10.1523/JNEUROSCI.0521-14.2014. http://doi.org/10.1523/JNEUROSCI.0521-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neuroscience and Biobehavioral Reviews. 2012;36(7):1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. http://doi.org/10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff B. Hindsight is not equal to foresight: The effect of outcome knowledge on judgment under uncertainty. Journal of Experimental Psychology: Human Perception and Performance. 1975;1(3):288–299. http://doi.org/10.1037/0096-1523.1.3.288. [Google Scholar]
- Forsythe R, Horowitz JL, Savin NE, Sefton M. Fairness in Simple Bargaining Experiments. Games and Economic Behavior. 1994;6(3):347–369. http://doi.org/10.1006/game.1994.1021. [Google Scholar]
- Hawkins SA, Hastie R. Hindsight: Biased judgments of past events after the outcomes are known. Psychological Bulletin. 1990;107(3):311–327. http://doi.org/10.1037/0033-2909.107.3.311. [Google Scholar]
- Johnson EJ, Häubl G, Keinan A. Aspects of endowment: a query theory of value construction. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2007;33(3):461–474. doi: 10.1037/0278-7393.33.3.461. http://doi.org/10.1037/0278-7393.33.3.461. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Kim JK, Risse G. Do alcoholic Korsakoff’s syndrome patients acquire affective reactions? Journal of Experimental Psychology. Learning, Memory, and Cognition. 1985;11(1):22–36. doi: 10.1037//0278-7393.11.1.22. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Knetsch J, Thaler R. Fairness and the Assumptions of Economics. The Journal of Business. 1986;59(4):S285–S300. [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science (New York, N.Y.) 2005;308(5718):78–83. doi: 10.1126/science.1108062. http://doi.org/10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, De Belder M, Egner T. Neural conflict-control mechanisms improve memory for target stimuli. Cerebral Cortex (New York, N.Y.: 1991) 2015;25(3):833–843. doi: 10.1093/cercor/bht283. http://doi.org/10.1093/cercor/bht283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VK, Harris LT. How social cognition can inform social decision making. Frontiers in Neuroscience. 2013;7:259. doi: 10.3389/fnins.2013.00259. http://doi.org/10.3389/fnins.2013.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvig EA, Madan CR, Spetch ML. Priming memories of past wins induces risk seeking. Journal of Experimental Psychology. General. 2015;144(1):24–29. doi: 10.1037/xge0000046. http://doi.org/10.1037/xge0000046. [DOI] [PubMed] [Google Scholar]
- Madan CR, Ludvig EA, Spetch ML. Remembering the best and worst of times: memories for extreme outcomes bias risky decisions. Psychonomic Bulletin & Review. 2014;21(3):629–636. doi: 10.3758/s13423-013-0542-9. http://doi.org/10.3758/s13423-013-0542-9. [DOI] [PubMed] [Google Scholar]
- Mason W, Suri S. Conducting Behavioral Research on Amazon’s Mechanical Turk. Rochester, NY: Social Science Research Network; 2010. (SSRN Scholarly Paper No. ID 1691163) Retrieved from http://papers.ssrn.com/abstract=1691163. [DOI] [PubMed] [Google Scholar]
- Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychological Review. 1956;63(2):81–97. http://doi.org/10.1037/h0043158. [PubMed] [Google Scholar]
- Montague PR, Berns GS. Neural Economics and the Biological Substrates of Valuation. Neuron. 2002;36(2):265–284. doi: 10.1016/s0896-6273(02)00974-1. http://doi.org/10.1016/S0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Palombo DJ, Keane MM, Verfaellie M. How does the hippocampus shape decisions? Neurobiology of Learning and Memory. 2015;125:93–97. doi: 10.1016/j.nlm.2015.08.005. http://doi.org/10.1016/j.nlm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20(11):1263–1290. doi: 10.1002/hipo.20852. http://doi.org/10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Tulving E. Memory Systems 1994. MIT Press; 1994. [Google Scholar]
- Shigemune Y, Tsukiura T, Kambara T, Kawashima R. Remembering with Gains and Losses: Effects of Monetary Reward and Punishment on Successful Encoding Activation of Source Memories. Cerebral Cortex. 2013 doi: 10.1093/cercor/bhs415. bhs415. http://doi.org/10.1093/cercor/bhs415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends in Cognitive Sciences. 2010;14(10):464–472. doi: 10.1016/j.tics.2010.08.002. http://doi.org/10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60(2):378–389. doi: 10.1016/j.neuron.2008.09.023. http://doi.org/10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon HA. Rational choice and the structure of the environment. Psychological Review. 1956;63(2):129–138. doi: 10.1037/h0042769. http://doi.org/10.1037/h0042769. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8(3):198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. http://doi.org/10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Availability: A heuristic for judging frequency and probability. Cognitive Psychology. 1973;5(2):207–232. http://doi.org/10.1016/0010-0285(73)90033-9. [Google Scholar]
- Weber EU, Goldstein WM, Barlas S. And let us not Forget Memory: The Role of Memory Processes and Techniques in the Study of Judgment and Choice. In: Jerome Busemeyer RHDLM, editor. Psychology of Learning and Motivation. Vol. 32. Academic Press; 1995. pp. 33–81. Retrieved from http://www.sciencedirect.com/science/article/pii/S0079742108603072. [Google Scholar]
- Weber EU, Johnson EJ, Milch KF, Chang H, Brodscholl JC, Goldstein DG. Asymmetric discounting in intertemporal choice: a query-theory account. Psychological Science. 2007;18(6):516–523. doi: 10.1111/j.1467-9280.2007.01932.x. http://doi.org/10.1111/j.1467-9280.2007.01932.x. [DOI] [PubMed] [Google Scholar]
- Wimmer GE, Shohamy D. Preference by association: how memory mechanisms in the hippocampus bias decisions. Science (New York, N.Y.) 2012;338(6104):270–273. doi: 10.1126/science.1223252. http://doi.org/10.1126/science.1223252. [DOI] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR. Reward Modulation of Hippocampal Subfield Activation during Successful Associative Encoding and Retrieval. Journal of Cognitive Neuroscience. 2012;24(7):1532–1547. doi: 10.1162/jocn_a_00237. http://doi.org/10.1162/jocn_a_00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. The process-dissociation approach two decades later: Convergence, boundary conditions, and new directions. Memory & Cognition. 2012;40(5):663–680. doi: 10.3758/s13421-012-0205-5. http://doi.org/10.3758/s13421-012-0205-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.