Introduction
There are an estimated 1.7 million traumatic brain injuries (TBI) per year in the United States, responsible for approximately 55,000 deaths.1,2 Direct costs of treating these patients is projected at over 9 billion US dollars per year.1 Of those patients with a TBI, the frequency of acute subdural hemorrhage (ASDH) in patients is between 10–30%.3–5 Furthermore, patients with ASDH often present with intracranial hypertension or neurologic dysfunction that requires emergent surgical decompression.3 At the time of surgery, provided there is no extracranial herniation, it often remains unclear if the bone flap should be removed (decompressive craniectomy, DC) or replaced (craniotomy). There is wide variation in the clinical practice of neurosurgeons around the world.6,7
There have been several small single-center retrospective cohort studies attempting to address this equipoise.5,8–13 These studies usually represent the practice patterns of a small number of neurosurgeons and contain data from a limited number of cases, limiting their generalizability.
National trends and estimates of the proportion of patients receiving craniotomy or DC for ASDH in the United States are unknown. Given the paucity of data, the goal of this retrospective cohort study is to characterize the surgical management of ASDH in the United States. In addition, we sought to determine the independent association of DC for ASDH, compared to craniotomy, on hospital mortality.
Materials and Methods
We report our study in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.14
Study Population
We obtained data from the Nationwide Inpatient Sample (NIS), a federal all-payer database which captures approximately 20% of all US hospital discharges.15 The NIS is a complex survey designed and powered to produce national estimates and proportions. It is produced by the Agency for Healthcare Research and Quality (AHRQ) via the Healthcare Cost and Utilization Project. As this study employed a retrospective de-identified dataset, a waiver of consent was obtained from the University of British Columbia Clinical Research Ethics Board (H15-01943).
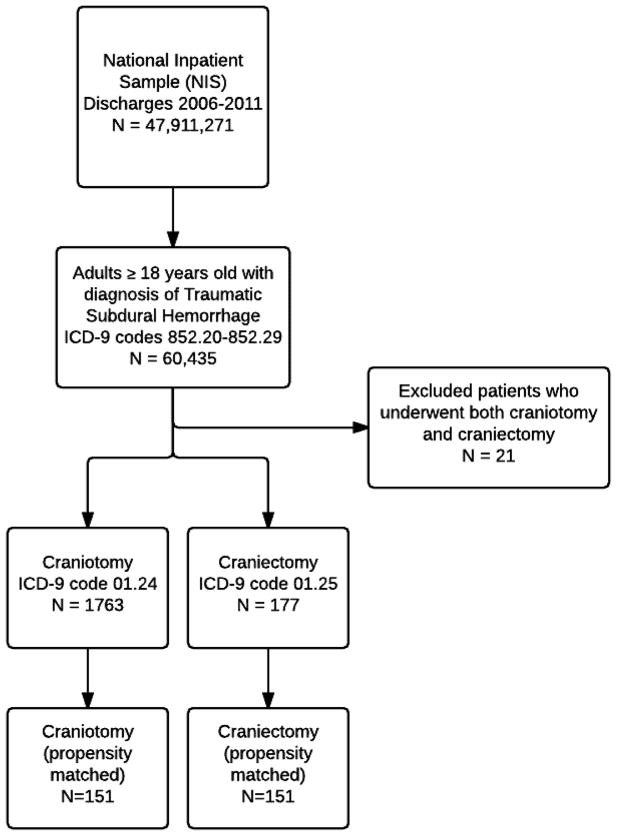
We included patients who were 18 years of age or older with a primary discharge diagnosis of traumatic subdural hemorrhage (ICD9 codes 852.20–852.29) from the 2006–2011 NIS sample. While there are likely more patients who suffered TBI with subdural hematomas that were not captured in these codes, we attempted to be as specific as possible in isolating and defining the ASDH cohort. The sample population was further segregated into patients who underwent craniotomy and craniectomy (ICD9 codes 01.24 and 01.25 respectively). We excluded patients who had codes for both surgeries as well as patients who did not undergo surgery. Figure 1 displays the flow diagram for cohort selection.
Figure 1.
Study design with inclusion and exclusion diagram for patient selection
The primary exposure of interest was craniotomy versus craniectomy for the management of ASDH. We gathered patient level variables including age, sex, race (white, black, hispanic or other), insurance status (coverage or no coverage), length of stay, in-hospital mortality as well as discharge location. Discharge location was defined as normal discharge home versus discharge to a type of facility (skilled nursing facility or rehab facility). We computed the Charlson Co-morbidity index as a method of measuring patient co-morbidities16. In addition, we included the Elixhauser Comorbidity Index17 and the All Patient Refined Diagnosis Related Groups (APRDRG) Severity Index into the final statistical model. The APRDRG indices are created by the AHRQ and are robust scores that adjust for injury severity18. Hospital characteristics were obtained including hospital region (Northeast, Midwest, South, West), size (as defined by the AHRQ15), teaching status (teaching vs non-teaching) as well as rural versus urban location (based on US Census Data).
Statistical Analysis
All analyses were performed using survey procedures in SAS version 9.4 (SAS Institute, Cary, NC). National estimates were obtained with appropriate weights as well as the use of complex survey procedures in SAS. We performed Chi-squared tests to analyze nominal or ordinal outcomes. Independent t-tests were used to analyze normally distributed continuous variables.
Propensity Score
We used a multivariable logistic regression model to estimate the propensity of a craniectomy being performed (compared to craniotomy). The risk of craniectomy was modeled using the following covariates chosen a priori: age, gender, race, insurance status, time to surgery, zip-code income quartile, the 29 Elixhauser co-morbidities, APRDRG Severity Index, hospital size, hospital region, hospital teaching status and urban vs rural hospital status.
Craniectomy patients were then matched in a 1:1 fashion with craniotomy patients by propensity score using a greedy matching algorithm set at a minimum 0.001 level match. The final propensity score model had a c-statistic of 0.872. Following propensity matching, the mortality rate outcomes were compared using a Chi-squared test. The variables included in the propensity score model are available in Appendix 1.
Appendix 1.
Odds ratios from multivariable logistic regression model for odds of undergoing a craniectomy. For zip code income quartile, the first quartile is the reference. For hospital region, “Northeast” is the reference group. For hospital size, “small” is the reference category
| Variable | Odds Ratio | Confidence Interval (95%) | p |
|---|---|---|---|
| Age | 0.96 | 0.94–0.97 | <0.0001 |
| Time to Surgery | 0.99 | 0.94–1.04 | 0.61 |
| Gender | 0.96 | 0.64–1.48 | 0.91 |
| Zip Code Income | |||
| Q1 | 1.0 | 1.0 | |
| Q2 | 1.18 | 0.70–1.99 | 0.55 |
| Q3 | 1.24 | 0.74–2.09 | 0.41 |
| Q4 | 1.22 | 0.71–2.11 | 0.47 |
| Race | |||
| White | 1.0 | 1.0 | |
| Black | 0.87 | 0.43–1.79 | 0.71 |
| Hispanic | 0.93 | 0.47–1.82 | 0.82 |
| Other | 0.67 | 0.29–1.57 | 0.35 |
| Missing | 1.17 | 0.68–1.99 | 0.57 |
| Hospital teaching status | 1.06 | 0.68–1.67 | 0.79 |
| Hospital location (rural vs urban) | 0.66 | 0.19–2.23 | 0.50 |
| Hospital Size | |||
| Small | 1.0 | 1.0 | |
| Medium | 6.57 | 1.36–31.77 | 0.02 |
| Large | 4.46 | 0.96–20.76 | 0.06 |
| Hospital Region | |||
| Northeast | 1.0 | 1.0 | |
| Midwest | 0.41 | 0.23–0.75 | 0.003 |
| South | 0.26 | 0.16–0.44 | <0.0001 |
| West | 0.56 | 0.32–0.96 | 0.04 |
| AIDS Comorbidity | <0.001 | <0.001–>999 | 0.99 |
| Alcohol Comorbidity | 0.68 | 0.42–1.11 | 0.13 |
| Anemia Comorbidity | 0.78 | 0.45–1.34 | 0.36 |
| Arthritis Comorbidity | 1.03 | 0.13–8.31 | 0.98 |
| Blood Loss Comorbidity | 0.96 | 0.25–3.76 | 0.95 |
| Congestive Heart Failure Comorbidity | 0.63 | 0.28–1.44 | 0.27 |
| Chronic Lung Comorbidity | 0.67 | 0.33–1.34 | 0.25 |
| Coagulopathy Comorbidity | 1.12 | 0.65–1.93 | 0.68 |
| Depression Comorbidity | 1.12 | 0.47–2.64 | 0.80 |
| Diabetes Comorbidity | 0.88 | 0.49–1.58 | 0.67 |
| Diabetes Comorbidity (Complicated) | 1.13 | 0.24–5.35 | 0.88 |
| Cardiopulmonary Comorbidity | 4.39 | 1.44–13.49 | 0.01 |
| Drug Abuse Comorbidity | 1.30 | 0.54–3.13 | 0.57 |
| Electrolyte Comorbidity | 1.04 | 0.69–1.56 | 0.85 |
| Hypertension Comorbidity | 0.79 | 0.51–1.23 | 0.30 |
| Hypothyroid Comorbidity | 0.95 | 0.36–2.55 | 0.93 |
| Liver Disease Comorbidity | 1.83 | 0.80–4.18 | 0.15 |
| Lymphoma Comorbidity | 2.03 | 0.37–11.25 | 0.41 |
| Metastatic Cancer Comorbidity | <0.001 | <0.001–>999 | 0.99 |
| Neuro Comorbidity | 0.35 | 0.15–0.78 | 0.01 |
| Obesity Comorbidity | 0.52 | 0.14–1.92 | 0.33 |
| Paralysis Comorbidity | 2.08 | 1.27–3.45 | 0.004 |
| Peripheral Vascular Comorbidity | 1.60 | 0.59–4.34 | 0.36 |
| Psychiatric Comorbidity | 1.55 | 0.60–4.02 | 0.37 |
| Renal Failure Comorbidity | 0.52 | 0.23–1.19 | 0.12 |
| Tumor Comorbidity | <0.001 | <0.001–999 | 0.98 |
| Ulcer Comorbidity | <0.001 | <0.001–999 | 0.99 |
| Valve Comorbidity | 0.63 | 0.18–2.24 | 0.48 |
| Weight Loss Comorbidity | 1.17 | 0.69–1.99 | 0.56 |
| APRDRG Severity Index | 2.36 | 1.92–2.89 | <0.0001 |
Mortality rates and percentage of surgical sample receiving craniectomy were calculated for each year to perform trend analysis. All tests were run with a 5% two-sided significance level. All percentages displayed on tables are obtained from complex survey procedures and estimate national percentages. In accordance with the AHRQ data use agreement, cells containing less than 10 patients are not reported in order to prevent identification of subjects.19 Imputed errors in the counts of patients in cells are displayed to prevent identification of individual patients, however the p values reflect actual data. Linear regression was utilized to determine statistically significant trends in the rates of procedures measured across years.
Results
A total of 47,911,414 (weighted national projection 235,911,271) admissions were examined from the 2006–2011 NIS samples. We isolated a cohort of 60,435 (weighted projection 298,896) patients with a primary diagnosis of traumatic subdural hematoma. From this cohort we identified 1763 patients who underwent craniotomy and 177 who underwent craniectomy. These represent 8,786 craniotomies and 883 craniectomies when weighted for national projections. There were 21 patients who had ICD9 codes for both procedures that were excluded from the analysis.
The baseline patient characteristics for the overall and propensity matched cohorts are presented in table 1. There were 26 craniectomy patients who were unable to be matched to a craniotomy patient at a propensity score match level of 0.001 or better. The mean age of patients who underwent a craniotomy was 68.9 (SD 17.1) years whereas the mean age of patients receiving a craniectomy was 49.5 (SD 20.8) years (p<0.0001). In the overall cohort, craniectomy was associated with increased hospital mortality compared to craniotomy. This increased hospital mortality seen on the univariable analysis persisted after propensity score matching (32% vs. 22%, p=0.044). This represents an adjusted odds ratio of 1.67 (95%CI: 1.02 to 2.76, p=0.044) for death associated with craniectomy compared to craniotomy. The variables included in the logistic regression model to determine the propensity score of receiving a craniectomy (vs. craniotomy) are presented in Appendix 1.
Table 1.
Results of patient characteristics for univariate analysis and propensity score matched analysis.
| Overall Cohort | Propensity Matched Cohort | |||||
|---|---|---|---|---|---|---|
| Craniotomy (n=1763) | Craniectomy (n=177) | p-value | Craniotomy (n=151) | Craniectomy (n=151) | p-value | |
| Age in years, mean (SD) | 68.9 (17.1) | 49.5 (20.8) | <0.0001 | 52.73 (21.5) | 52.37 (20.2) | 0.88 |
| Length of Stay in days, median (IQR) | 10.9 (9) | 14.3 (25) | <0.0001 | 11.0 (16.2) | 15.9 (24.4) | 0.21 |
| Hospital mortality, n(%) | 192 (10.9) | 61 (35.0) | <0.0001 | 33 (22.0) | 48 (32.0) | 0.04 |
| Time to surgery in days, median (IQR) | 0 (0.97) | 0 (0.43) | 0.95 | 0 (0.87) | 0 (0.48) | 0.77 |
| Female sex, n(%) | 613 (34.7) | 50 (28.2) | 0.08 | 45 (30.1) | 43 (28.3) | 0.73 |
| Race, n(%) | ||||||
| White | 1089 (62.0) | 104 (5.9) | 95 (63.2) | 92 (60.7) | ||
| Black | 143 (8.1) | 16 (9.1) | 12 (7.8) | 13 (8.7) | ||
| Hispanic | 121 (6.9) | 16 (8.8) | 11 (7.2) | 14 (9.0) | ||
| Other | 131 (7.5) | <10 | 11 (7.2) | <10 | ||
| Missing | 279 (15.6) | 30 +/− 3 | 0.58 | 22 (14.5) | 23 +/− 3 | 0.83 |
| Zip Code Income Quartile, n(%) | ||||||
| 1 | 504 (29.4) | 49 (29.0) | 40 (26.7) | 41 (28.7) | ||
| 2 | 400 (23.3) | 38 (22.2) | 32 (21.3) | 31 (21.4) | ||
| 3 | 398 (23.0) | 42 (25.0) | 35 (23.6) | 35 (24.4) | ||
| 4 | 416 (24.3) | 41 (23.8) | 0.95 | 41 (28.3) | 37 (25.5) | 0.95 |
| Discharge Location, n(%) | ||||||
| Home | 568 (36.1) | 24 (20.9) | 31 (25.8) | 23 (22.2) | ||
| Facility | 998 (63.9) | 89 (79.1) | 0.0011 | 87 (74.2) | 79 (77.8) | 0.50 |
| Charlson Score, n(%) | ||||||
| 0 | 825 (47.1) | 93 (52.5) | 83 (54.6) | 76 (50.1) | ||
| 1 | 449 (25.3) | 37 (20.8) | 29 (19.4) | 34 (22.4) | ||
| 2 | 259 (14.7) | 28 (15.7) | 18 (12.1) | 23 (15.1) | ||
| ≥3 | 230 (12.9) | 19 (11.0) | 0.39 | 21 (13.9) | 18 (12.1) | 0.75 |
| Insurance coverage, n(%) | 1581 (89.8) | 147 (82.5) | 0.01 | 124 (82.3) | 125 (82.0) | 0.94 |
| Urban Hospital Location, n(%) | ||||||
| Rural | 32 | <10 | <10 | <10 | ||
| Urban | 1712 | 167 +/− 3 | 0.003 | 144 +/− 3 | 145 +/−3 | <0.0001 |
| Teaching hospital, n(%) | 1219 (70.0) | 135 (78.7) | 0.04 | 114 (75.8) | 116 (77.4) | 0.75 |
| Hospital Size, n(%) | ||||||
| Small | 87 | <10 | <10 | <10 | ||
| Medium | 378 | 40 +/− 3 | 35 +/− 3 | 38 +/− 3 | ||
| Large | 1279 | 131 +/− 3 | 0.15 | 110 +/−3 | 110 +/−3 | 0.89 |
| Hospital Region, n(%) | ||||||
| Northeast | 378 (22.6) | 58 (34.1) | 43 (29.6) | 47 (32.5) | ||
| Midwest | 315 (18.2) | 35 (19.7) | 20 (13.4) | 28 (18.6) | ||
| South | 699 (38.8) | 49 (26.8) | 50 (32.4) | 46 (29.5) | ||
| West | 371 (20.5) | 35 (19.4) | 0.02 | 38 (24.6) | 30 (19.4) | 0.54 |
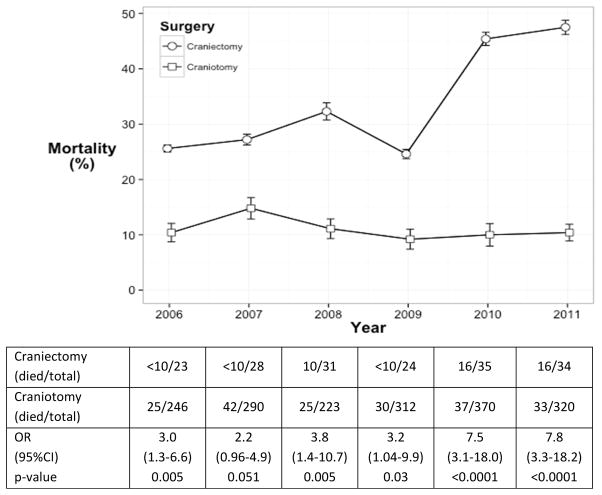
The rates of craniectomy and craniotomy as well as the yearly case mortality are displayed in Figure 2. The case-mortality rate for craniectomy significantly increased from 25.6% in 2006 to 47.5% in 2011 (test for trend p=0.0052). Mortality rates in patients undergoing craniotomy were stable during the study period (p=0.66). The percentage of patients receiving a craniectomy as a percentage of all surgeries was stable between 7.4–11.7% over the time period of the study (p=0.9543).
Figure 2.
Rates of mortality for craniectomy and craniotomy during the study period. Test for trend: craniectomy p=0.0052 and craniotomy p=0.66.
Discussion
In our retrospective cohort study using data from the NIS, we demonstrated that craniotomy is performed approximately 10 times more often than DC in patients with ASDH in the United States. Patients who undergo DC are younger, have more acute comorbid conditions and higher severity indices but are less likely to have a neurologic comorbid condition. When adjusting for all identifiable variables, patients who undergo craniectomy have a 1.7-fold higher risk of dying in the hospital. Management of these patients also varies by geographical location in the United States as well as hospital characteristics. When examining individual years, the mortality rate for craniectomy has steadily risen while the mortality rate for craniotomy has remained steady. This is in light of no significant change in the rates of craniectomy in the sample of patients undergoing surgical intervention.
The significant difference in mortality observed between the two groups confirms findings seen in other smaller studies.5,10,13,20 There may be underlying physiologic basis to account for the increased mortality observed with craniectomy. Removal of the bone segment allows for expansion of swollen brain through the defect leading to axonal stretch.21,22 Axonal stretch has been demonstrated by in-vivo models to cause neuronal injury.23–25 Furthermore, decompressive craniectomy has also been associated with alterations in cerebral auto-regulation and hemodynamics.26 This may lead to pressure passive cerebral blood flow, increasing the risk of cerebral ischemia (from under perfusion) or cerebral edema (from over perfusion), respectively.
The decision to perform a craniectomy is at the discretion of the attending neurosurgeon and reflects a combination of measured and unmeasured patient factors. Craniectomy is likely preformed more often in patients with more severe injuries where post-operative swelling is anticipated. Parenchymal herniation during the operation of patients with more significant injuries may prevent replacement of the bone flap.3 The operating neurosurgeon may have been attempting to perform a craniotomy but due to technical reasons was unable to replace the bone. This is confounding by indication and occurs when variables associated with the outcome in the reference population, are also associated with the exposure.27 We attempted to adjust for confounding using propensity score matching. This results in balancing all of the covariates that are used to generate the score, thereby minimizing confounding by these variables.28 Furthermore, using a propensity score analysis is subject to less bias than conventional logistic regression when the number of events per covariate is low, as in our case.29
It must be stressed however, that the severity of injury is included among the unmeasured patient factors contributing to the signal for increased mortality observed in patients who received a craniectomy. Worse injuries may influence the surgeon to leave the bone flap removed in order to account for anticipated post operative edema and elevated intracranial pressure. Also, craniectomy is often preformed as a lifesaving procedure in order to avoid progression to neurological death. If these patients were not operated on, they would have died and not have been included in this analysis. The increasing mortality rates for patients receiving craniectomy during the study period could also be a reflection of changes over time in traumatic brain injury management where neurosurgeons may increasingly perform craniectomies on more severely injured patients who were formerly considered unsalvageable.
The ratio of ten to one seen in favor of craniotomy versus DC confirms the preferred management technique for ASDH in the United States. The decision to perform a DC over a craniotomy is influenced by surgeon preferences, training, experience as well as patient and hospital characteristics. Some centers have reported only performing DC whereas others only perform craniectomy in patients with ASDH.30–32 While the present analysis does not capture the practices of individual hospitals, it is likely that surgeon preference and patient characteristics influence the decision of surgery selection more than hospital policies. The further characterization of the influences on surgical decision making would be a valuable addition to the literature.
Strengths of this study include the representative sample of ASDH patients treated across the United States. The NIS allows estimations of national trends in the management of patients, unlike previous retrospective studies focusing on single institutions. The large sample size obtained from the dataset allows measurement of patient outcomes on a national scale across many different types of hospitals. This is one of the largest cohorts of patients with exclusively ASDH in the literature.
These data must be interpreted in the context of the study design. The retrospective nature and lack of detailed patient level data to adjust for other factors may confound the results. It is also likely that we were unable to capture all patients with ASDH from the database due to them being coded as non-specific head injury codes. However, the authors attempted to analyze the management of these patients on a homogeneous and specific sample as possible. A lack of a functional outcome assessment is a factor in the interpretation of the findings in this study. An ability to capture 6-month functional status would allow a better characterization of patient outcomes beyond in-hospital mortality and facility discharge.
Further evidence for the best management of these patients will be provided once the RESCUE-ASDH trial results are available.33 This trial based at the University of Cambridge is currently enrolling patients and randomizing to decompressive craniectomy or craniotomy in ASDH. Given the difficulty of recruiting these patients, it may be some time before results become available.
Conclusion
In the United States among patients with ASDH, craniotomy is performed ten times more often than decompressive craniectomy. Patients who undergo craniectomy are younger, have significantly longer hospitalizations, are more likely to be discharged to a facility as well as more likely to die in the hospital. While the optimal management of these patients remains highly debated, the vast majority of surgical management of these patients in the United States involves performing a craniotomy.
Management of acute traumatic subdural hematoma controversial
Craniectomy and Craniotomy are both preformed for ASDH
Craniotomy preformed 10:1 ratio over craniectomy for ASDH in the USA
The case-mortality rate for craniectomy significantly increased from 25.6% in 2006 to 47.5% in 2011
Patients who underwent craniectomy had a 1.7 higher risk of in-hospital mortality than craniotomy patients
Acknowledgments
Funding: Dr. Griesdale is funded by the VGH & UBC Hospital Foundation Best of Health Fund. This study was funded in part by grant #T15LM007092 from the National Library of Medicine.
The authors would like to graciously thank Dr. Ellen McCarthy at the Harvard School of Public Health for her guidance and assistance. This study was funded in part by grant #T15LM007092 from the National Library of Medicine.
Abbreviations
- ASDH
Acute Traumatic Subdural Hemorrhage
- DC
Decompressive Craniectomy
- NIS
Nationwide Inpatient Sample
Footnotes
Conflict of Interest: No authors have any conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Barret RUSH, Email: bar890@mail.harvard.edu.
Justin ROUSSEAU, Email: justin_rousseau@hms.harvard.edu.
Mypinder SEKHON, Email: mypindersekhon@gmail.com.
Donald GRIESDALE, Email: donald.griesdale@vch.ca.
References
- 1.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 21(6):544–8. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Faul M, Xu L, Wald MMCV. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Cent Dis Control Prev Natl Cent Inj Prev Control. 2010:891–904. [Google Scholar]
- 3.Karibe H, Hayashi T, Hirano T, Kameyama M, Nakagawa A, Tominaga T. Surgical management of traumatic acute subdural hematoma in adults: a review. Neurol Med Chir (Tokyo) 2014;54(11):887–94. doi: 10.2176/nmc.ra.2014-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(SUPPL 3) [PubMed] [Google Scholar]
- 5.Chen S-H, Chen Y, Fang W-K, Huang D-W, Huang K-C, Tseng S-H. Comparison of Craniotomy and Decompressive Craniectomy in Severely Head-Injured Patients With Acute Subdural Hematoma. J Trauma Inj Infect Crit Care. 2011;71(6):1632–6. doi: 10.1097/TA.0b013e3182367b3c. [DOI] [PubMed] [Google Scholar]
- 6.Kolias AG, Belli A, Li LM, et al. Primary decompressive craniectomy for acute subdural haematomas: Results of an international survey. Acta Neurochir (Wien) 2012;154(9):1563–5. doi: 10.1007/s00701-012-1349-6. [DOI] [PubMed] [Google Scholar]
- 7.Kolias aG, Scotton WJ, Belli a, et al. Surgical management of acute subdural haematomas: Current practice patterns in the United Kingdom and the Republic of Ireland. Br J Neurosurg. 2013;27(3):330–3. doi: 10.3109/02688697.2013.779365. [DOI] [PubMed] [Google Scholar]
- 8.Ryan CG, Thompson RE, Temkin NR, Crane PK, Ellenbogen RG, Elmore JG. Acute traumatic subdural hematoma. J Trauma Acute Care Surg. 2012;73(5):1348–54. doi: 10.1097/TA.0b013e31826fcb30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massaro F, Lanotte M, Faccani G, Triolo C. One hundred and twenty-seven cases of acute subdural haematoma operated on. Acta Neurochir (Wien) 1996;138(2):185–91. doi: 10.1007/BF01411359. [DOI] [PubMed] [Google Scholar]
- 10.Li LM, Kolias AG, Guilfoyle MR, et al. Outcome following evacuation of acute subdural haematomas: A comparison of craniotomy with decompressive craniectomy. Acta Neurochir (Wien) 2012;154(9):1555–61. doi: 10.1007/s00701-012-1428-8. [DOI] [PubMed] [Google Scholar]
- 11.Walcott BP, Khanna A, Kwon C-S, Phillips HW, Nahed BV, Coumans J-V. Time interval to surgery and outcomes following the surgical treatment of acute traumatic subdural hematoma. J Clin Neurosci. 2014;21(12):2107–11. doi: 10.1016/j.jocn.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitgeb J, Mauritz W, Brazinova A, et al. Outcome after severe brain trauma due to acute subdural hematoma. J Neurosurg. 2012;117(2):324–33. doi: 10.3171/2012.4.JNS111448. [DOI] [PubMed] [Google Scholar]
- 13.Woertgen C, Rothoerl RD, Schebesch KM, Albert R. Comparison of craniotomy and craniectomy in patients with acute subdural haematoma. J Clin Neurosci. 2006;13(7):718–21. doi: 10.1016/j.jocn.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiol Camb Mass. 2007;18(6):805–35. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 15.INTRODUCTION TO THE HCUP NATIONWIDE INPATIENT SAMPLE (NIS) [Internet] Available from: https://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2011.pdf.
- 16.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 17.Elixhauser a, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Averill RF, Goldfield NI, Muldoon J, Steinbeck BA, Grant TM. A closer look at all-patient refined DRGs. J AHIMA Am Health Inf Manag Assoc. 2002;73(1):46–50. [PubMed] [Google Scholar]
- 19.HCUP. HCUP AHRQ Data Use Agreement for the Nationwide Inpatient Sample [Internet] Available from: https://www.hcup-us.ahrq.gov/team/NationwideDUA.pdf.
- 20.Kim KH. Predictors for functional recovery and mortality of surgically treated traumatic acute subdural hematomas in 256 patients. J Korean Neurosurg Soc. 2009;45(3):143–50. doi: 10.3340/jkns.2009.45.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus. 2009;26(6):E7. doi: 10.3171/2009.4.FOCUS0965. [DOI] [PubMed] [Google Scholar]
- 22.Cooper PR, Hagler H, Clark WK, Barnett P. Enhancement of experimental cerebral edema after decompressive craniectomy: implications for the management of severe head injuries. Neurosurgery. 1979;4(4):296–300. doi: 10.1227/00006123-197904000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Chung RS, Staal Ja, McCormack GH, et al. Mild axonal stretch injury in vitro induces a progressive series of neurofilament alterations ultimately leading to delayed axotomy. J Neurotrauma. 2005;22(10):1081–91. doi: 10.1089/neu.2005.22.1081. [DOI] [PubMed] [Google Scholar]
- 24.Tang-Schomer MD, Patel AR, Baas PW, Smith DH. Mechanical breaking of microtubules in axons during dynamic stretch injury underlies delayed elasticity, microtubule disassembly, and axon degeneration. FASEB J Off Publ Fed Am Soc Exp Biol. 2010;24(5):1401–10. doi: 10.1096/fj.09-142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staal Ja, Dickson TC, Gasperini R, Liu Y, Foa L, Vickers JC. Initial calcium release from intracellular stores followed by calcium dysregulation is linked to secondary axotomy following transient axonal stretch injury. J Neurochem. 2010;112(5):1147–55. doi: 10.1111/j.1471-4159.2009.06531.x. [DOI] [PubMed] [Google Scholar]
- 26.Bor-Seng-Shu E, Figueiredo EG, Amorim RLO, et al. Decompressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. J Neurosurg. 2012;117(3):589–96. doi: 10.3171/2012.6.JNS101400. [DOI] [PubMed] [Google Scholar]
- 27.Salas M, Hofman A, Stricker BH. Confounding by indication: an example of variation in the use of epidemiologic terminology. Am J Epidemiol. 1999;149(11):981–3. doi: 10.1093/oxfordjournals.aje.a009758. [DOI] [PubMed] [Google Scholar]
- 28.Seeger JD, Kurth T, Walker AM. Use of propensity score technique to account for exposure-related covariates: an example and lesson. Med Care. 2007;45(10 Supl 2):S143–8. doi: 10.1097/MLR.0b013e318074ce79. [DOI] [PubMed] [Google Scholar]
- 29.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158(3):280–7. doi: 10.1093/aje/kwg115. [DOI] [PubMed] [Google Scholar]
- 30.Kotwica Z, Brzeziński J. Acute subdural haematoma in adults: an analysis of outcome in comatose patients. Acta Neurochir (Wien) 1993;121(3–4):95–9. doi: 10.1007/BF01809257. [DOI] [PubMed] [Google Scholar]
- 31.Paterniti S, Fiore P, Macri E, et al. Extradural haematoma. Report of 37 consecutive cases with survival. Acta Neurochir (Wien) 1994;131(3–4):207–10. doi: 10.1007/BF01808614. [DOI] [PubMed] [Google Scholar]
- 32.Wilberger JE, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg. 1991;74(2):212–8. doi: 10.3171/jns.1991.74.2.0212. [DOI] [PubMed] [Google Scholar]
- 33.Kolias AG, Li LM, Guilfoyle MR, et al. Decompressive craniectomy for acute subdural hematomas: Time for a randomized trial. Acta Neurochir (Wien) 2013;155(1):187–8. doi: 10.1007/s00701-012-1531-x. [DOI] [PubMed] [Google Scholar]