Abstract
6β-hydroxytestosterone, a cytochrome P450 1B1-derived metabolite of testosterone, contributes to the development of angiotensin II-induced hypertension and associated cardiovascular pathophysiology. In view of the critical role of angiotensin II in the maintenance of renal homeostasis, development of hypertension and end organ damage, this study was conducted to determine the contribution of 6β-hydroxytestosterone to angiotensin II actions on water consumption and renal function in male Cyp1b1+/+ and Cyp1b1−/− mice. Castration of Cyp1b1+/+ mice or Cyp1b1−/− gene disruption minimized the angiotensin II-induced increase in water consumption, urine output, proteinuria, and sodium excretion and decreases in urine osmolality. 6β-hydroxytestosterone did not alter angiotensin II-induced increases in water intake, urine output, proteinuria, and sodium excretion or decreases in osmolality in Cyp1b1+/+ mice, but restored these effects of angiotensin II in Cyp1b1−/− or castrated mice Cyp1b1+/+ mice. Cyp1b1 gene disruption or castration prevented angiotensin II-induced renal fibrosis, oxidative stress, inflammation, urinary excretion of angiotensinogen, expression of angiotensin II type 1 receptor, and angiotensin converting enzyme. 6β-hydroxytestosterone did not alter angiotensin II-induced renal fibrosis, inflammation, oxidative stress, urinary excretion angiotensinogen, expression of angiotensin II type 1 receptor, or angiotensin converting enzyme in Cyp1b1+/+ mice; however, in Cyp1b1−/− or castrated mice Cyp1b1+/+ mice, it restored these effects of angiotensin II. These data indicate that 6β-hydroxytestosterone contributes to increased thirst, impairment of renal function, and end organ injury associated with angiotensin II-induced hypertension in male mice and that cytochrome P450 1B1 could serve as a novel target for treating renal disease and hypertension in males.
Keywords: 6β-hydroxytestosterone, CYP1B1, renal dysfunction, fibrosis, urinary angiotensinogen
Introduction
Men are more prone to develop hypertension and renal dysfunction compared to premenopausal women of the same age.1–3 Sexual dimorphism in hypertension and renal dysfunction was demonstrated in various animal models of hypertension including angiotensin II (Ang II) and deoxycorticosterone acetate (DOCA)-salt-induced hypertension, Dahl salt-sensitive, and spontaneously hypertensive rats (SHR). In particular, males are more likely to develop hypertension and renal dysfunction compared to females in these models.4–10 Testosterone stimulates the expression of renal angiotensinogen (AGT) or urinary excretion of AGT in SHRs and in Ang II-high salt (HS) diet-induced hypertension.11–13 Moreover, castration decreases expression of renal renin and AGT and hepatic AGT in male SHRs.11,12 Ang II infusion also increases Ang II type 1 receptor (AT1a) expression in male SHRs14 or alters the Ang II type 2 receptor (AT2) to AT1a receptor balance in kidneys of male SHRs.15 Furthermore, Ang II decreases the expression of Mas receptor and angiotensin converting enzyme 2 (ACE 2) mRNA in male SHR and Sprague-Dawley (SD) rats.14,16 Dihydrotestosterone (DHT)-induced hypertension in rats expressing endothelial Cytochrome P-450 4A2 (CYP4A2) depends on the prohypertensive eicosanoid 20-hydroxyeicosatetraenoic acid (20-HETE),17 and is associated with increased vascular expression of angiotensin converting enzyme (ACE).18 Hypertension is also associated with inflammation.19–21 In males, Ang II infusion increases vascular and renal infiltration of CD4+ and CD8+ T-lymphocytes.21, 22 Adaptive transfer of T-cells from male mice into male Rag1−/− mice increases blood pressure (BP), but transfer of T-cells from female mice does not elicit a similar response.23 These observations indicate that in males, testosterone downregulates the antihypertensive components and upregulates the hypertensive components of the renin-angiotensin and immune systems and thereby contributes to increased BP and renal dysfunction.
Cytochrome P-450 (CYP)1B1, which is highly expressed in the cardiovascular and renal systems and is capable of metabolizing fatty acids, retinoids, and sex steroids, contributes to the development of hypertension and renal dysfunction in male mice.24–26 In female mice, where Ang II-induced increases in BP and renal dysfunction are minimized as compared to male mice, inhibition of CYP 1B1 activity with 2,3′,4,5′-tetramethoxystilbene (TMS) or Cyp1b1 gene disruption (Cyp1b1−/−) abrogated this protection against Ang II to increased BP and cause renal dysfunction.27,28 These protective effects of CYP1B1 against Ang II in female mice are diminished by treatment with 2-methoxyestradiol, which is formed from a estradiol metabolite of Cyp1b1, 2-OH estradiol, by catechol-O-methyltransferase.29,30 Testosterone is metabolized into dihydrotestosterone (DHT) by 5-alpha reductase, and 6β-hydroxytestosterone (6β-OHT) and 16α-hydroxytestosterone (16α-OHT) by CYP1B1.31,32 Ang II increases selectively the production of 6β-OHT but not 16α-OH or DHT, which is abolished by Cyp1b1 gene disruption. Recently, we have shown and that 6β-OHT contributes to Ang II-induced increase in BP and associated cardiac pathophysiological changes in male mice.33 These observations led us to hypothesize that 6β-OHT also contributes to Ang II-induced renal dysfunction and end organ damage in male mice. To test this hypothesis, we examined the effects of Ang II and 6β-OHT on Ang II-induced renal dysfunction and end organ damage in intact and castrated Cyp1b1+/+ and Cyp1b1−/− male mice. The results show that Ang II-induced renal dysfunction and end organ damage are associated with increased renal oxidative stress, production of AGT, and inflammation; these effects are minimized in Cyp1b1−/− and castrated Cyp1b1+/+ mice and restored by 6β-OHT.
Methods
All experiments were performed according to in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the protocols approved by our Institutional Animal Care and Use Committee. Experiments were performed in 8 week old, 20–25 g body weight, both intact and castrated Cyp1b1+/+ and Cyp1b1−/− mice. These mice were infused with Ang II (700 ng/kg/min) or saline (vehicle) for 14 days with subcutaneously implanted micro osmotic pumps. In another series of experiments 6β-OHT (15 µg/g i.p. every third day) or its vehicle was given concurrently with Ang II infusion in both intact and castrated mice. Water intake, urine output, renal function, renal oxidative stress, end organ damage and T-cell infiltration were assessed in the same groups of mice that were used in the previous study33 to reduce the number of animals used and to comply with the 3R guidelines set by the IACUC. Detailed experimental methods are available in the online-only Data Supplement.
Statistical Analysis
Data were analyzed by two-way analysis of variance followed by Tukey’s multiple comparison post-hoc test or Student’s t-test. The values of three to eight different experiments are expressed as the mean ± SEM. P<0.05 was considered statistically significant.
Results
Cyp1b1 Gene Disruption Minimized Ang II-Induced Increase in Water Intake and Renal Dysfunction
We have previously shown that 6β-OHT contributes to Ang II-induced increase in (BP) and associated cardiac pathophysiological changes in male mice.33 In the present study, we examined in these animals Ang II-induced changes in renal function and associated pathogenesis. Infusion of Ang II for 2 weeks increased systolic blood pressure (SBP), measured by tail cuff every 3rd day, in Cyp1b1+/+ and Cyp1b1−/− mice, but the increase was significantly less in Cyp1b1−/− than in Cyp1b1+/+ mice (Table S1). Ang II also increased water intake and urine output in Cyp1b1+/+ mice; this increase was minimized in Cyp1b1−/− mice (Table S1). Ang II infusion did not alter plasma levels of creatinine in Cyp1b1+/+ or in Cyp1b1−/− mice (Table S1). Ang II infusion decreased urine osmolality, increased urinary excretion of Na+, and caused proteinuria in Cyp1b1+/+ but not in Cyp1b1−/− mice (Table S1). The difference in the renal functional parameters observed between these two groups was consistent, reproducible, and similar to that reported previously.24
6β-OHT Treatment Restored Ang II-induced Increase in Water Intake and Renal Dysfunction in Cyp1b1−/− and Castrated Cyp1b1+/+ Mice
As previously shown, the effect of Ang II to increase BP was minimized in intact Cyp1b1−/− and castrated Cyp1b1+/+ mice. 6β-OHT, which did not alter basal BP, restored the effect of Ang II to increase SBP in intact Cyp1b1−/− and castrated Cyp1b1+/+ mice (Table S1). Cyp1b1 gene disruption mitigated the effects of Ang II to cause renal dysfunction; treatment with 6β-OHT in Cyp1b1−/− mice restored the ability of Ang II to cause an increase in water intake and renal dysfunction to the levels that were observed in Cyp1b1+/+ mice (Table S1). Castration minimized the effect of Ang II to increase SBP, water intake or cause renal dysfunction in Cyp1b1+/+ mice (Table S1). In castrated Cyp1b1+/+ and Cyp1b1−/− mice, administration of 6β-OHT restored the effects of Ang II to increase SBP, water intake and cause renal dysfunction. 6β-OHT did not alter any changes in water intake or renal function in animals infused with vehicle of Ang II. and treated only with 6β-OHT (Table S1).
Cyp1b1 Gene Disruption Attenuated Ang II Induced Renal Fibrosis but was Restored by 6β-OHT
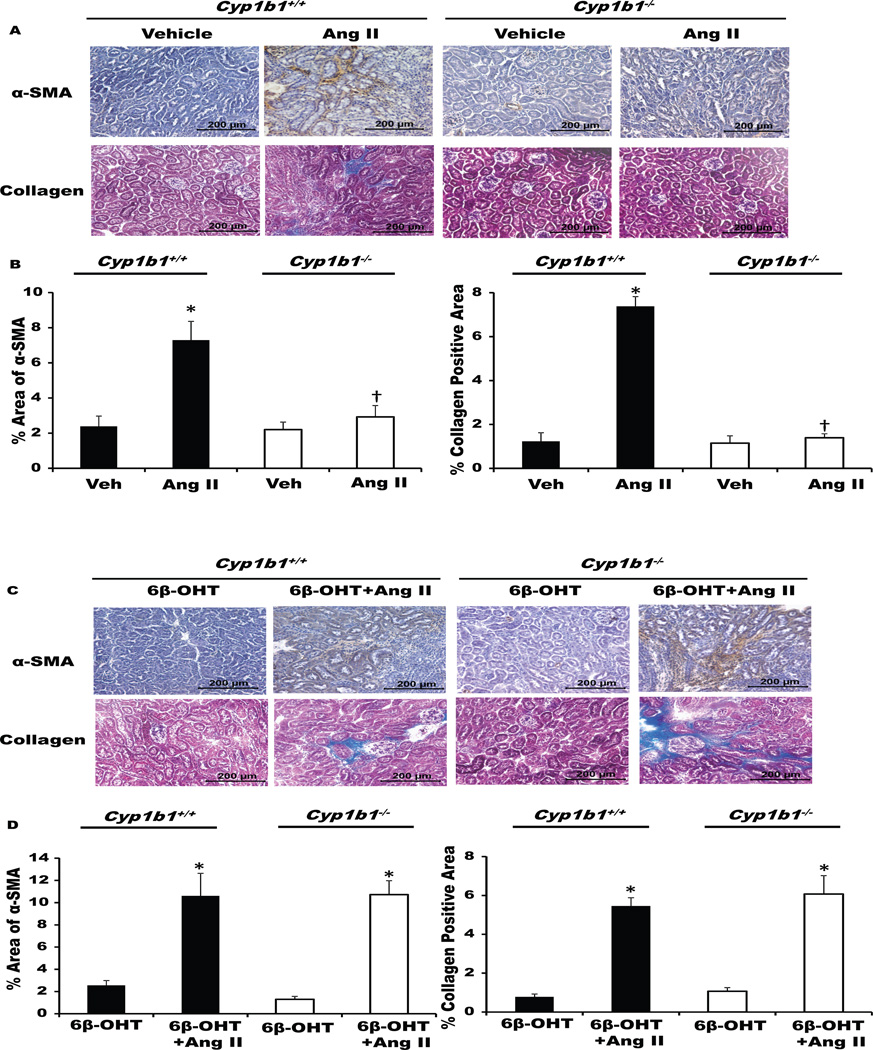
Kidneys from Ang II-infused Cyp1b1+/+ mice, but not from Cyp1b1−/− mice, displayed interstitial fibrosis as indicated by increased interstitial α-smooth muscle actin (α-SMA) staining and collagen deposition in the kidneys (Figure 1A–B, upper panel). Treatment with 6β-OHT restored the effects of Ang II in Cyp1b1−/− mice but did not alter these effects of Ang II in Cyp1b1+/+ mice (Figure 1C–D, lower panel).
Figure 1. Cyp1b1 gene disruption minimizes angiotensin II (Ang II)-induced renal fibrosis that is restored by 6β-hydroxytestosterone (6β-OHT).
Cyp1b1+/+ and Cyp1b1−/− mice were infused with vehicle or Ang II (upper panel) and treated with 6β-OHT (15 µg/g i.p.) or 6β-OHT+Ang II (lower panel) for 2 weeks. After the end of Ang II infusion, kidneys were removed and processed. (A&C) Ang II infusion increased interstitial accumulation of α-smooth muscle actin (α-SMA) and renal collagen deposition in Cyp1b1+/+ mice, which were minimized in Cyp1b1−/− mice, restored by concurrent treatment with 6β-OHT, but not in mice treated with 6β-OHT alone. (B&D) Bar graphs showing quantified data. Data are expressed as means ± SE; n = 3 for each treatment group. *P< 0.05, Ang II vs. vehicle and 6β-OHT vs. 6β-OHT+Ang II; †P< 0.05, Ang II-infused Cyp1b1−/− mice vs. Ang II-infused Cyp1b1+/+ mice.
Castration prevents Ang II-Induced Renal Fibrosis, which is restored by 6β-OHT
Castration reduced Ang II-induced α-SMA and collagen deposition in the kidneys of Ang II infused Cyp1b1+/+ mice, but castration did not have any further effects on Cyp1b1−/− mice infused with Ang II (Figure S1A–B). Concurrent treatment with 6β-OHT restored the ability of Ang II to cause renal fibrosis in castrated Cyp1b1+/+ and Cyp1b1−/− mice (Figure S2A–B). 6β-OHT alone did not cause renal fibrosis in castrated mice of these genotypes (Figure S1A–B and Figure S2A–B).
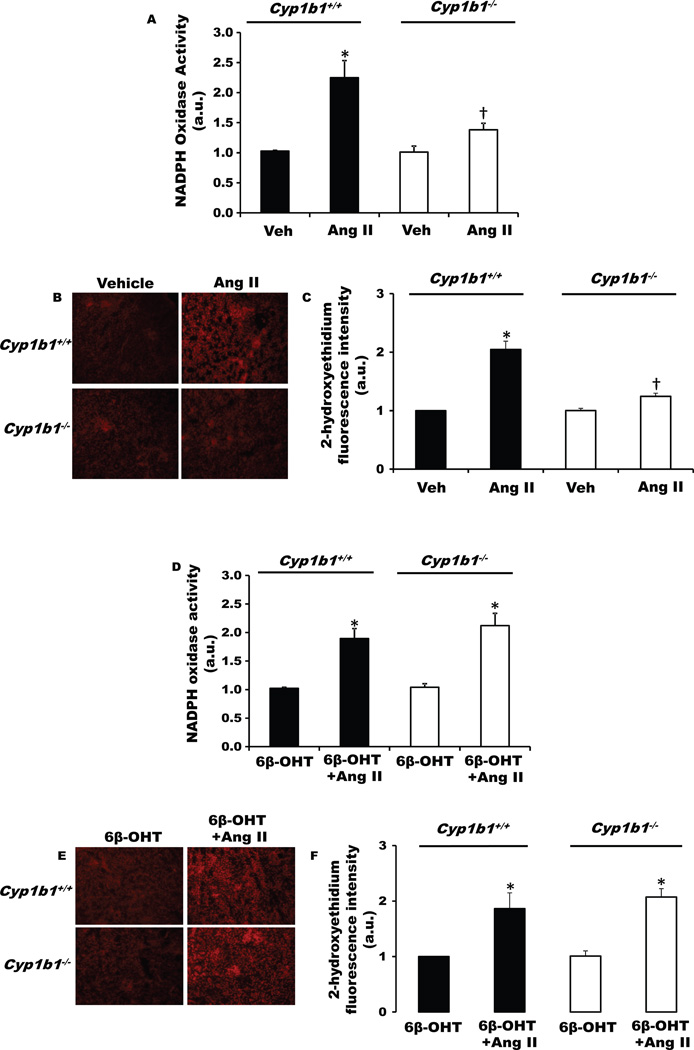
6β-OHT Restored the Ability of Ang II to Increase Renal Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase Activity and Production of Reactive Oxygen Species (ROS), Which was Diminished in Male Cyp1b1−/− Mice
Ang II infusion increased renal NADPH oxidase activity, and ROS production as indicated by increased renal 2-hydroxyethidium fluorescence in Cyp1b1+/+ mice, and this increase was minimized in Cyp1b1−/− mice (Figure 2A–C, upper panel). Treatment with 6β-OHT restored the loss of this action of Ang II in Cyp1b1−/− mice (Figure 2D–F, lower panel) but did not alter Ang II-induced increase in renal NADPH oxidase activity or ROS production in Cyp1b1+/+ mice (Figure 2D–F, lower panel). 6β-OHT did not alter basal levels of NADPH oxidase activity or ROS production in Cyp1b1+/+ or Cyp1b1−/− mice (Figure 2D–F, lower panel).
Figure 2. Cyp1b1 gene disruption minimizes angiotensin II (Ang II)-induced renal nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and superoxide production in Cyp1b1−/− mice, which are restored by concurrent treatment with 6β-hydroxytestosterone (6β-OHT).
Cyp1b1+/+ and Cyp1b1−/− mice were infused with vehicle or Ang II (upper panel) and treated with 6β-OHT or 6β-OHT+Ang II (lower panel) for 2 weeks. (A) NADPH oxidase activity was measured in kidney homogenates. (B) Renal superoxide production was determined by the fluorescence intensity of 2-hydoxyethidium. Photomicrographs are representative of kidneys from mice in each of the different treatment groups following incubation with dihydroethidium. (C) Graph depicts quantified data. *P<0.05 Ang II vs. vehicle; †P<0.05 Cyp1b1−/− Ang II vs. Cyp1b1+/+ Ang II (n=4–5 for each treatment group and data are expressed as mean±SEM).
6β-OHT Restored Loss of Effects of Ang II-Induced Increases in Renal NADPH Oxidase Activity and ROS Production in Castrated Male Cyp1b1+/+ and Cyp1b1−/− Mice
Castration attenuated Ang II mediated increases in renal NADPH oxidase activity or ROS production, as indicated by decreases in 2-hydroxyethidium fluorescence in Cyp1b1+/+ mice (Figure S3 A – C). In intact Cyp1b1−/− mice, Ang II did not increase renal NADPH oxidase activity or ROS production (Figure 2 upper panel A-C), and this was not altered by castration (Figure S3 A – C). Treatment with 6β-OHT restored the ability of Ang II to increase renal NADPH oxidase activity and ROS production in castrated Cyp1b1+/+ and Cyp1b1−/− mice (Figure S4). 6β-OHT did not alter the basal renal NADPH oxidase activity or ROS production in castrated Cyp1b1+/+ or Cyp1b1−/− mice (Figure S4 A – C).
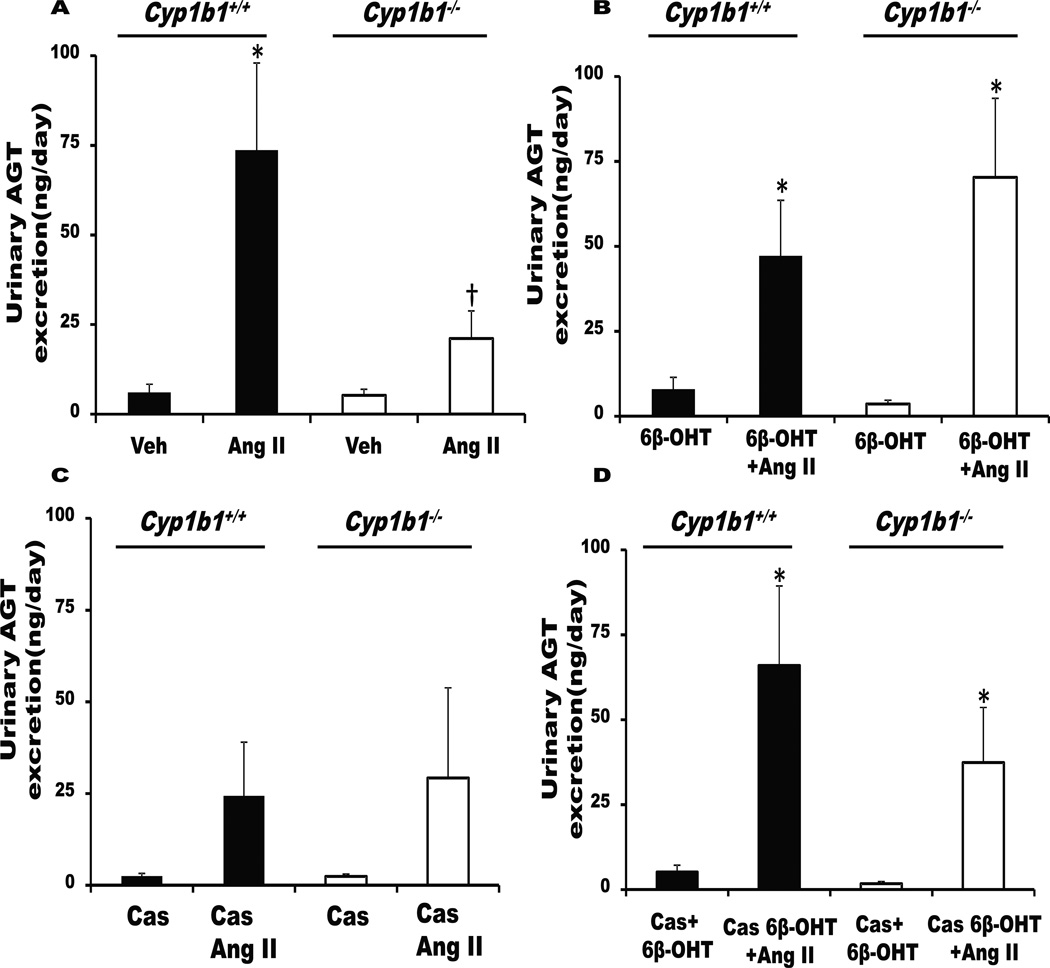
Cyp1b1 Gene Disruption or Castration Minimized Urinary Excretion of AGT, Which was Restored by 6β-OHT Treatment
Ang II infusion increases intrarenal accumulation and urinary excretion of AGT34 and renal production of Ang II, which have been implicated in the development of hypertension.34 Ang II increased urinary excretion of AGT in Cyp1b1+/+ mice (Figure 3A). Cyp1b1 gene disruption or castration minimized urinary AGT increase by Ang II without altering basal levels (Figure 3A,C). (Figure 3B,D).
Figure 3. Cyp1b1 gene disruption or castration in Cyp1b1+/+ mice attenuates urinary angiotensinogen (AGT) excretion, which is restored by 6β-hydroxytestosterone (6β-OHT).
Intact or castrated Cyp1b1+/+ and Cyp1b1−/− mice were infused with vehicle (Veh) or Ang II and given intraperitoneal injections of testosterone metabolite, 6β-OHT, every third day for 2 weeks. At the end of Ang II infusion, urine was collected, and urinary AGT excretion was measured. (A) Intact Cyp1b1+/+ and Cyp1b1−/− mice infused with vehicle or Ang II. (B) Intact Cyp1b1+/+ and Cyp1b1−/− mice infused with vehicle or Ang II and treated with 6β-OHT for 2 weeks. (C) Castrated Cyp1b1+/+ and Cyp1b1−/− mice infused with vehicle or Ang II. (D) Castrated Cyp1b1+/+ and Cyp1b1−/− mice infused with vehicle or Ang II and treated with 6β-OHT. *P<0.05 Ang II vs. vehicle, Ang II+6β-OHT vs. vehicle+6β-OHT, and Cas+6β-OHT+Ang II vs. Cas+6β-OHT; †P<0.05, Cyp1b1+/+ Ang II vs. Cyp1b1−/− Ang II (n=4–8 for each group of experiments; data are expressed as mean±SEM).
Castration Decreased Renal Expression of ACE and AT1a but Not ACE2 or Androgen Receptor (AR), Which Were Restored by 6β-OHT
Castration decreased mRNA expression of AT1a and ACE in both Cyp1b1+/+ and Cyp1b1−/− mice; this effect reversed and increased by treatment with 6β-OHT in both phenotypes. (Figures S5A and S6B). Castration or 6β-OHT did not alter the expression of ACE2 and AR in either Cyp1b1+/+ or Cyp1b1−/− mice (Figures S6A and S6B).
Ang II Infusion Decreased Renal mRNA Expression of CYP4A12A but Not CYP1B1 in Mice
Infusion of Ang II decreased renal mRNA encoding CYP4A12A in both Cyp1b1+/+ and Cyp1b1−/− male mice (Figure S7A). Ang II infusion did not alter Cyp1b1 mRNA expression in Cyp1b1+/+ mice, and, as expected, no Cyp1b1 mRNA was detected in Cyp1b1−/− mice (Figure S7B).
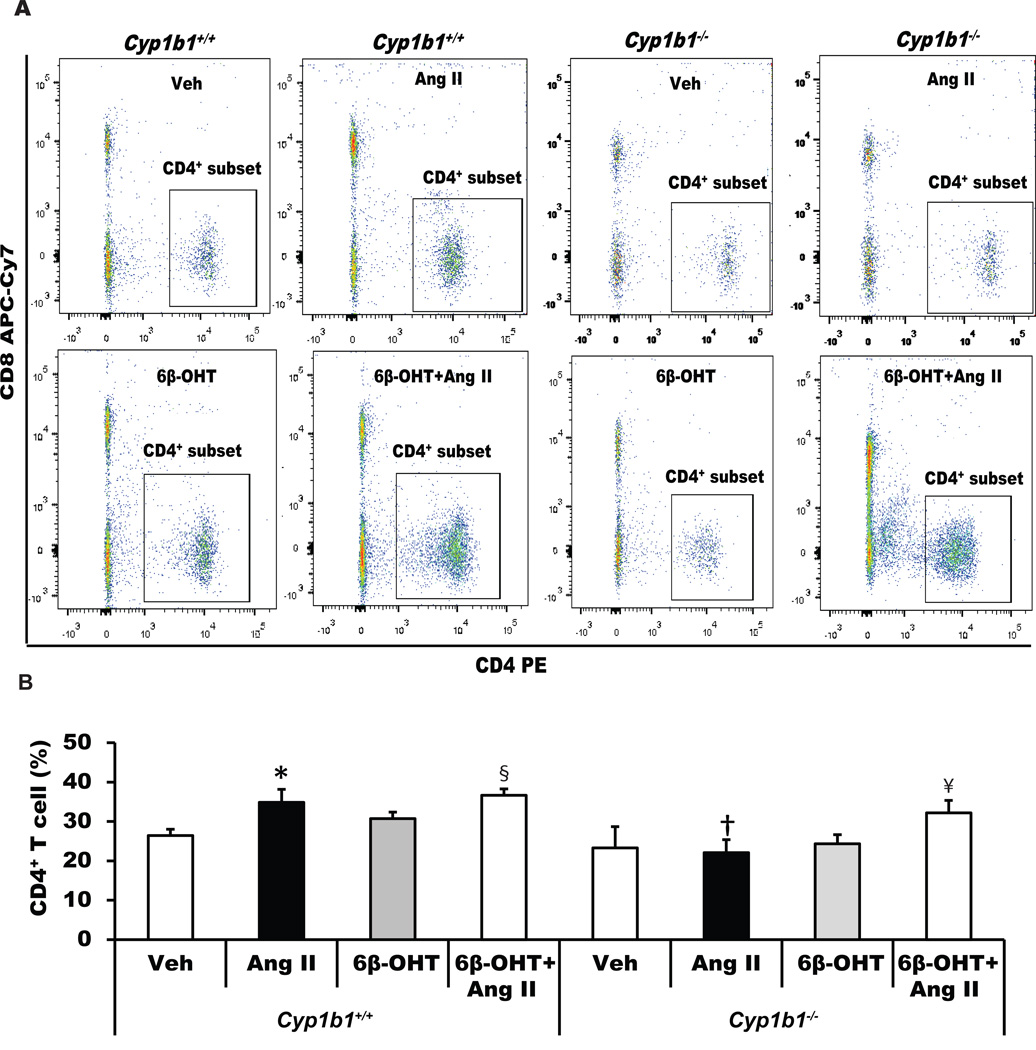
Cyp1b1 Gene Disruption or Castration Minimized Renal Accumulation of CD4+ T-Lymphocytes, Which was Restored by 6β-OHT Treatment in Cyp1b1−/− but Not in Castrated CYP1b1+/+ Mice
Ang II infusion increased renal accumulation of CD4+ T-lymphocytes in Cyp1b1+/+ mice, which was not altered by treatment with 6β-OHT (Figure 4). Cyp1b1 gene disruption or castration of Cyp1b1+/+ mice attenuated the renal accumulation of CD4+ T-cells (Figures 4 and S8). Treatment with 6β-OHT restored the effect of Ang II to increase renal accumulation of CD4+ T-lymphocytes in intact Cyp1b1−/− mice but not in castrated Cyp1b1+/+ mice (Figures 4 and S8).
Figure 4. Cyp1b1 gene disruption minimizes angiotensin II (Ang II)-induced renal accumulation of renal CD4+ T-cells in Cyp1b1−/− mice, which is restored by 6β-hydroxytestosterone (6β-OHT).
Cyp1b1+/+ and Cyp1b1−/− mice were infused with vehicle or Ang II and treated with 6β-OHT or 6β-OHT+Ang II for 2 weeks. At the end of Ang II infusion, kidneys were removed and processed, and cells were isolated, counted, and used for flow cytometry. (A) Dot plot images of intact Cyp1b1+/+ and Cyp1b1−/− mice infused with vehicle or Ang II and treated with 6β-OHT for 2 weeks. (B) the average percentage of CD4+ T cell subset is represented by a histogram. *P<0.05 Ang II vs. vehicle; †P<0.05 Cyp1b1−/− Ang II vs. Cyp1b1+/+ Ang II; § Cyp1b1+/+ 6β-OHT Ang II vs Cyp1b1+/+ 6β-OHT; ¥ P<0.05 Cyp1b1−/− 6β-OHT Ang II vs. Cyp1b1−/− 6β-OHT (n=3 for each treatment group and data are expressed as mean±SEM).
Cyp1b1 Gene Disruption or Castration Decreased IL-6 and IFN-γ mRNA Expression, Which was Restored by 6β-OHT
As shown above, Cyp1b1 gene disruption or castration decreased renal accumulation of CD4+ T-cells, which was restored by 6β-OHT. We examined the effects of Cyp1b1 gene disruption and castration on cytokine IL-6 and IFN-γ mRNA expression and their restoration by 6β-OHT. Cyp1b1 disruption or castration decreased IL-6 and IFN-γ expression (Figure S9A and S9B), and treatment with 6β-OHT restored expression of mRNA encoding IL-6 and IFN-γ in castrated Cyp1b1+/+ and Cyp1b1−/− mice (Figure S9A, B).
Restoration by 6β-OHT of the Effect of Ang II on Renal Fibrosis is Dependent on Systolic BP in Cyp1b1−/− Mice
To determine if the effect of 6β-OHT to restore the effect of Ang II on renal fibrosis in Cyp1b1−/− mice is dependent on systolic BP, we examined the effect of a direct vasodilator hydralazine (250 mg/L in drinking water) on systolic BP and renal fibrosis. Hydralazine reduced SBP (Figure S10A) and renal fibrosis as indicated by decreased absence of interstitial α-smooth muscle actin (α-SMA) staining and collagen deposition in the kidneys of Cyp1b1−/− mice infused with Ang II and concurrently treated with 6β-OHT (Figure S10B).
Renal Fibrosis is independent of Systolic BP Blood Pressure in Cyp1b1+/+ mice
Infusion of Norepinephrine (10 mg/kg/day) increased systolic BP blood pressure in Cyp1b1+/+ mice (Figure S10A) but did not cause and norepinephrine infusion did not increase renal fibrosis as indicated by decreased absence of interstitial α-smooth muscle actin (α-SMA) staining and collagen deposition in the kidneys (Figure S10 B).
Discussion
The main findings of this study are that 6β-OHT, a CYP1B1-generated metabolite of testosterone, contributes to Ang II-induced: a) increase in water intake, b) renal dysfunction, and c) end organ damage by increasing BP and oxidative stress, promoting intrarenal production of AGT, and activating T-cells in male mice. Previously, we reported that Cyp1b1 gene disruption or inhibition of CYP1B1 activity minimized Ang II-induced renal dysfunction and end organ damage in male mice.25 Moreover, we showed that Ang II stimulates the production of 6β-OHT, a testosterone-derived metabolite of CYP1B1, in Cyp1b1+/+, but not in Cyp1b1−/− mice, and contributes to Ang II-induced hypertension and associated cardiac pathophysiology by acting as a permissive factor.33 The current study that Assessment of assessed the renal function and associated pathophysiological changes in these mice33 has revealed that Ang II infusion for 14 days increased water intake and urine output in Cyp1b1+/+ mice, and these effects of Ang II were minimized in Cyp1b1−/− mice thus confirming our previous results.25 Administration of 6β-OHT to Cyp1b1−/− mice that do not generate this testosterone metabolite33 restored the effects of Ang II to increase thirst and urine output, suggesting that 6β-OHT mediates contributes to the this effect of Ang II in male mice , most likely by acting in the brain.
Ang II increased urinary Na+ excretion in Cyp1b1+/+ mice, and Cyp1b1 gene disruption minimized Na+ excretion in male mice.25 In the present study, we found that administering 6β-OHT to Cyp1b1−/− mice infused with Ang II markedly increased Na+ excretion. The increase in urinary Na+ excretion by Ang II in the Cyp1b1+/+ mice, and its restoration by 6β-OHT in Cyp1b1−/− mice is most likely the consequence of increase in BP. Further studies are needed to determine the fractional Na+ excretion and its time-course and total body Na+ to address the role of CYP1B1 and 6β-OHT in Ang II actions to alter Na+ balance in male mice. 6β-OHT also contributed to the effect of Ang II to decrease osmolality, cause proteinuria, and renal fibrosis as indicated by interstitial α-SMA and collagen accumulation, because these effects of Ang II that were abrogated in Cyp1b1 gene disrupted mice, were significantly restored by 6β-OHT. Further support for the role of 6β-OHT in Ang II-induced renal dysfunction and end organ damage was obtained in castrated mice. Castration attenuated Ang II-induced renal dysfunction and fibrosis in Cyp1b1+/+ mice, but, as expected, castration did not have any effect in Cyp1b1−/− mice infused with Ang II. However, concurrent administration of 6β-OHT restored the effects of Ang II to cause renal dysfunction and fibrosis in both castrated Cyp1b1+/+ and Cyp1b1−/− mice. The mechanism by which 6β-OHT mediates the effect of Ang II to cause end organ damage in intact Cyp1b1−/− and castrated Cyp1b1+/+ mice could be due to restoration of the effect of Ang II on BP by 6β-OHT.33 Supporting this view was our observation that hydralazine that abolished the increase in systolic BP prevented renal fibrosis caused by Ang II in Cyp1b1−/− mice treated with 6β-OHT. However, we cannot exclude any possible action of hydralazine on one or more signaling mechanisms involved in the action of Ang II, because Ang II has also been reported to cause fibrosis independent of an increase in BP.35–38
Ang II increases production of ROS, which contributes to the development of hypertension and end organ damage in male SHR rats39–41. Moreover, Cyp1b1 gene disruption and/or inhibition of its activity decreases vascular and renal oxidative stress in rats and mice.23–25 Our finding that administration 6β-OHT to Cyp1b1−/− mice or castrated Cyp1b1+/+ mice infused with Ang II increased renal NADPH oxidase activity and ROS production suggests that 6β-OHT, by restoring the effect of Ang II to increase oxidative stress, contributes to the development of renal dysfunction and end organ damage in male mice.
Treatment with DHT has been shown to increase the renal vascular expression of CYP4A8 and its orthologue CYP4A12 in male mice, thereby stimulating prohypertensive eicosanoid 20-HETE production and increasing the activity of the renin-angiotensin system and BP.18,42,43 DHT-induced hypertension was minimized in AGT-deficient mice.44 Cyp1b1 gene disruption in male mice did not alter renal expression of CYP4A10/12/14 (mouse orthologues of rat CYP4A1/A2/A3).25 In the present study, expression of CYP4A12A mRNA was decreased in the kidneys of both Cyp1b1+/+ and Cyp1b1−/− male mice infused with Ang II, whereas Ang II did not alter renal Cyp1b1 mRNA in Cyp1b1+/+ mice. However, we cannot exclude the possible contribution of CYP4F2 that also metabolizes arachidonic acid (AA) into 20-HETE45 to the actions of Ang II and 6β-OHT. Also, in view of the demonstration that 20-HETE mediates the renal vasoconstrictor effect of Ang II46 but exert natriuretic effect47 further studies are required to assess contribution of 20-HETE to Ang II induced renal dysfunction and end organ damage. Therefore, DHT-generated 20-HETE does not appear to contribute to Ang II-induced renal dysfunction and end organ damage in at least in this genotype of mice. However, in view of the demonstration that 20-HETE mediates the renal vasoconstrictor effect of Ang II45 but exert natriuretic effect46 further studies are required to assess contribution of 20-HETE to Ang II induced renal dysfunction and end organ damage. Moreover, whether alterations in expression and activities of other CYP isoforms including CYP2C and 2J, that metabolize AA into epoxyeicosatrienoic acids, that exert anti-hypertensive, anti-inflammatory and pro-fibrinolytic effects48 are involved in the modulation by 6β-OHT of Ang II actions on BP, renal dysfunction and end organ damage remains to be determined.
Male SHR kidneys have higher AT1a receptor expression in the kidneys as compared to female SHR kidneys, whereas female SHRs have higher AT2 receptor expression in the kidneys.49 Infusion of Ang II increased AT1a receptor expression in male SHRs but not SD rats.14, 16 Renal mRNA expression of ACE2, AT2, and plasma levels of Ang 1–7 are increased in female SHRs compared with male SHRs, and this increase in AT2 mRNA and Ang 1–7 has been proposed to be responsible for the lower BP in female SHRs than in male SHRs.14–16 In the present study, Ang II infusion did not alter renal AT1a or ACE expression in either Cyp1b1+/+ or Cyp1b1−/− mice. However, castration attenuated both AT1a and ACE mRNA expression in both genotypes, and treatment with 6β-OHT increased AT1a and ACE mRNA expression in both mouse genotypes. Castration or 6β-OHT had no effect on expression of ACE2 expression in either group of mice infused with Ang II or its vehicle, suggesting that castration downregulated the prohypertensive arm of the renin-angiotensin system (RAS), and treatment with 6β-OHT upregulated the prohypertensive arm of the RAS.
An increase in urinary excretion of AGT is a marker for increased intrarenal RAS activity.34,50 Ang II infusion increased renal expression and urinary excretion of AGT in male rats, suggesting that there is an increased AGT availability for conversion to Ang II in kidneys.13,34,51–54 In Dahl-salt sensitive male rats, a high salt diet increased intrarenal AGT expression, BP, proteinuria and glomerolosclerosis, which were prevented by castration and restored by administering testosterone, suggesting that Ang II-induced intrarenal AGT production depends on androgens.55 Our previous study showing that Ang II stimulated production of testosterone metabolite 6β-OHT via CYP1B133 together with the demonstration that the Ang II-induced increase in urinary excretion of AGT was inhibited by Cyp1b1 gene disruption or castration in Cyp1b1+/+ mice, and that treatment with 6β-OHT restored the increase in urinary excretion of AGT in these mice suggest that 6β-OHT contributes to Ang II- induced intrarenal AGT production. Increased availability of intrarenal AGT coupled with increased renal ACE expression and AT1a receptor expression may augment the effects of Ang II to cause renal dysfunction and end organ damage. The mechanism by which 6β-OHT mediates increased urinary excretion of AGT that could be due to enhanced transcription of AGT by Ang II remains to be determined.
Ang II also stimulates the immune system and causes infiltration of macrophages and T-cells in blood vessels, kidneys, and central nervous system resulting in increased BP and end organ damage.56 This increase in BP is attenuated in Rag1−/− deficient male mice and restored by adoptive transfer of male wild type (WT) T-cells.57 Recent studies also showed that adoptive transfer of male WT T-cells to Rag1−/− female mice did not increase BP in female mice infused with Ang II58, whereas adoptive transfer of WT female T-cells to male Rag1−/− mice minimized the increase in BP23, indicating that females are protected against inflammation mediated by Ang II. In a recent study, gonadectomy of male and female SHRs increased proinflammatory cells in both sexes.59 However, we found that Cyp1b1 gene disruption or castration of Cyp1b1+/+ mice attenuated Ang II-induced infiltration of CD4+ T-lymphocytes and treatment with 6β-OHT restored renal accumulation of CD4+ T-cells in Cyp1b1−/− mice but failed to do so in castrated mice. These results suggest that 6β-OHT is insufficient to restore Ang II-induced infiltration of CD4+ T-cells, and testosterone and/or its metabolite DHT is required for accumulation of these cells in castrated mice. However, further in-depth studies are required to assess the role of 6β-OHT in the action of Ang II on other subtypes of T-lymphocytes and dendritic cells and production of cytokines in renal dysfunction and end organ damage. Moreover, in view of the recent evidence that the dendritic-cell isoketal-modified proteins activate T-cells and promote hypertension, the possibility that testosterone and/or its metabolite 6β-OHT-dependent activation of T-cells by Ang II might be mediated via formation of dendritic-cell isoketal-modified proteins60 remains to be explored.
The mechanism by which 6β-OHT contributes to the effects of Ang II to increase thirst, renal dysfunction, oxidative stress, inflammation, and end organ damage is unclear. Since 6β-OHT restored these effects of Ang II in both intact Cyp1b1−/− and castrated Cyp1b1+/+ mice without producing these effects alone, it appears that it acts as a permissive factor to increase sensitivity to the action of Ang II, most likely by interacting with AR, which is located on mesangial cells, glomeruli, proximal tubules, and cortical collecting ducts.61,62 Therefore, whether 6β-OHT could restores the renal actions of Ang II through genomic and/or nongenomic AR or independent of AR remains to be determined.63,64 Since expression of AR mRNA in the kidney was not altered either in Cyp1b1 or castrated mice treated with 6β-OHT or its vehicle, the effect of 6β-OHT-mediated Ang II-induced renal pathophysiological changes seems to be independent of AR expression. This observation together with our finding that 6β-OHT did not alter either basal BP or renal function, or Ang II-induced increase in BP or renal dysfunction and end organ damage in Cyp1b1+/+ mice, suggest that its effects are unlikely to be due to competition with testosterone or DHT at the AR.
In conclusion, this study provides evidence that the testosterone metabolite of Cyp1b1, 6β-OHT stimulated by Ang II, contributes to its actions to increase thirst, renal dysfunction, fibrosis, oxidative stress, AGT production, and inflammation in male mice. In contrast, we have shown that, in female mice, CYP1B1 protects against Ang II-induced hypertension and renal dysfunction via metabolism of estradiol to 2-hydroxyestradiol and subsequently its metabolism by catechol-O-methyltransferase to 2-methoxyestradiol29,30 that acts as a permissive factor to suppress Ang II actions.26,27 Therefore, it appears that 17β-estradiol and testosterone metabolites of CYP1B1 contribute to sex differences in Ang II-induced renal dysfunction and end organ damage associated with hypertension. Finally, CYP1B1 could serve as a novel target for developing agents that inhibit CYP1B1 for treating renal dysfunction in males, but inhibitors of CYP1B1 could be detrimental in treating renal dysfunction and hypertension in females. Since CYP1B1 is known to cause congenital glaucoma65 due to a developmental abnormality in the eye 65 and thus acute or chronic inhibition of CYP1B1 in adults may not cause increased ocular pressure. However, this possibility needs to be assessed in the development of CYP1B1 inhibitors in the treatment of renal dysfunction and hypertension in males.
Perspectives
Sex differences in renal dysfunction in various models of experimental hypertension have been attributed to sex chromosome complement and sex hormones. The present study provides evidence that the CYP1B1 metabolite of testosterone, 6β-OHT, in male mice contributes to Ang II-induced increases in thirst, renal dysfunction, and end organ damage most likely by increased oxidative stress and intrarenal RAS activity and activation of T-cells. The levels of testosterone decreases with age in men, and many individuals including females are prescribed testosterone for sexual dysfunction and to increase muscle mass and boost energy. However, the use of testosterone as regards the cardiovascular effects, benefits vs. risk are controversial. Since Ang II increases the production of 6β-OHT that contributes to its hypertensive and pathophysiological effects, it would be important to determine the levels of this testosterone metabolite in individuals of different ages with increased activity of renin-angiotensin system. Moreover, in view of polymorphism in human CYP1B1gene65 and differences in the catalytic properties of polymorphic CYP1B1 variants for steroid hormones including testosterone66 it would be important to determine association of CYP1B1 gene polymorphism to the levels of testosterone and its metabolites, BP and renal function.
Future studies should characterize interactions of 6β-OHT with genomic and nongenomic AR and the mechanism by which it permits Ang II to express its renal pathophysiological effects including possible activation of T-cells via dendritic isoketal-modified proteins.58 Moreover, development of selective inhibitors of the effects of 6β-OHT and CYP1B1 activity could be useful for treating renal dysfunction and end organ damage and hypertension in males.
Supplementary Material
Novelty and Significance.
What is New?
Demonstrates that 6â-hydroxytestosterone (6β-OHT), a testosterone-derived metabolite of CYP1B1, contributes to Ang II-induced increases in thirst and renal dysfunction in male mice.
Provides the evidence that 6β-OHT mediates the effect of Ang II to produce renal fibrosis and oxidative stress in male mice.
Demonstrates that Cyp1b1 gene disruption or castration decreases Ang II-induced urinary excretion of angiotensinogen (AGT), which is restored by 6β-OHT.
Demonstrates that 6β-OHT mediates the effect of Ang II to increase renal infiltration of CD4+ T-cells.
What is Relevant?
This study advances our knowledge of the mechanism by which CYP1B1-testosterone-derived metabolite 6β-OHT contributes to Ang II-induced renal dysfunction, fibrosis, oxidative stress, and inflammation in males.
Our demonstration that 6β-OHT mediates the action of Ang II to stimulate intrarenal AGT production coupled with increased mRNA expression of mRNA encoding AT1a and ACE furthers our understanding of the mechanism of testosterone-dependent activation of intrarenal RAS by Ang II and has important pathophysiological significance in the development of renal disease and hypertension.
Finally, our study has translational significance in developing selective CYP1B1 inhibitors for treating testosterone-dependent Ang II-induced renal dysfunction, end organ damage, and hypertension in men.
Summary.
Ang II increased renal dysfunction, fibrosis, oxidative stress, inflammation, and urinary excretion of AGT in male mice. Cyp1b1 gene disruption or castration in Cyp1b1+/+ mice minimized these effects of Ang II. Administering the testosterone metabolite formed by CYP1B1, 6β-OHT to Cyp1b1−/− or castrated Cyp1b1+/+ mice restored the ability of Ang II to increase thirst and cause renal dysfunction, fibrosis, oxidative stress, inflammation, and urinary excretion of AGT in male mice. Since 6β-OHT increased intrarenal AGT and mRNA expression of AT1a receptor and ACE in Cyp1b1 gene-disrupted or castrated mice infused with Ang II, it appears that 6β-OHT promotes increased formation of Ang II in the kidneys, thereby contributing to renal dysfunction and associated pathogenesis in male mice. Therefore, developing selective inhibitors of CYP1B1 could be useful for treating renin-angiotensin and testosterone-dependent renal disease and hypertension in males.
Acknowledgments
We thank Dr. David Armbruster for editorial assistance.
Sources of Funding
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute grants R01HL-19134–40 and R01HL-079109-09 (K.U.M) and the Department of Veterans Affairs BX001193-02 (D. B.), the National Heart, Lung, and Blood Institute (R01HL-66432) and the Tulane Bridge Fund, (D.S.A.M), the National Institute of General Medical Sciences IDeA Program (COBRE, P30GM103337) and by the National Institutes of Health National Heart, Lung, and Blood Institute grant (R01HL26371) (L.G.N). M.K. was supported by a fellowship from the Department of Pharmacology, Faculty of Medicine, Erciyes University, Kayseri, Turkey 38039. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute.
Footnotes
Conflicts of Interest/Disclosures
None.
References
- 1.Messerli FH, Garavaglia GE, Schmieder RE, Sundgaard-Riise K, Nunez BD, Amodeo C. Disparate cardiovascular findings in men and women with essential hypertension. Ann Intern Med. 1987;107:158–161. doi: 10.7326/0003-4819-107-2-158. [DOI] [PubMed] [Google Scholar]
- 2.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 3.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 4.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of Dahl hypertension. Hypertension. 2000;35:484–489. doi: 10.1161/01.hyp.35.1.484. [DOI] [PubMed] [Google Scholar]
- 5.Ouchi Y, Share L, Crofton JT, Iitake K, Brooks DP. Sex difference in the development of deoxycorticosterone-salt hypertension in the rat. Hypertension. 1987;2:172–177. doi: 10.1161/01.hyp.9.2.172. [DOI] [PubMed] [Google Scholar]
- 6.Remuzzi G, Ruggenenti P, Perico N. Chronic renal disease: renoprotective benefits of renin-angiotensin system inhibition. Ann Intern Med. 2002;136:604–615. doi: 10.7326/0003-4819-136-8-200204160-00010. [DOI] [PubMed] [Google Scholar]
- 7.Sandberg K, Hong Ji. Sex differences in primary hypertension. Biol Sex Diff. 2012;3:3–7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1573–R1579. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 9.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–H2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 10.Fortepiani LA, Yanes L, Zhang H, Racusen LC, Reckelhoff JF. Role of androgens in mediating renal injury in aging SHR. Hypertension. 2003;42:952–955. doi: 10.1161/01.HYP.0000099241.53121.7F. [DOI] [PubMed] [Google Scholar]
- 11.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 12.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Inves. 1989;83:1941–1945. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rands VF, Seth DM, Kobori H, Prieto MC. Sexual dimorphism in urinary angiotensinogen excretion during chronic angiotensin II-salt hypertension. Gend Med. 2012;9:207–218. doi: 10.1016/j.genm.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1–7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension. 2010;56:658–666. doi: 10.1161/HYPERTENSIONAHA.110.153668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilliard LM, Chow CL, Mirabito KM, Steckelings UM, Unger T, Widdop RE, Denton KM. Angiotensin type 2 receptor stimulation increases renal function in female, but not male, spontaneously hypertensive rats. Hypertension. 2014;64:378–383. doi: 10.1161/HYPERTENSIONAHA.113.02809. [DOI] [PubMed] [Google Scholar]
- 16.Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension. 2008;52:666–671. doi: 10.1161/HYPERTENSIONAHA.108.114058. [DOI] [PubMed] [Google Scholar]
- 17.Wu CC, Schwartzman ML. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011;96:45–53. doi: 10.1016/j.prostaglandins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sodhi K, Wu CC, Cheng J, Gotlinger K, Inoue K, Goli M, Falck JR, Abraham NG, Schwartzman ML. CYP4A2-induced hypertension is 20-hydroxyeicosatetraenoic acid- and angiotensin II-dependent. Hypertension. 2010;56:871–878. doi: 10.1161/HYPERTENSIONAHA.110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trott DW, Harrison DG. The immune system in hypertension. Adv Physiol Educ. 2014;38:20–24. doi: 10.1152/advan.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Current Opinion Pharmacol. 2010;10:203–207. doi: 10.1016/j.coph.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci. 2014;126:267–274. doi: 10.1042/CS20130407. [DOI] [PubMed] [Google Scholar]
- 23.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex- specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension. 2014;64:573–582. doi: 10.1161/HYPERTENSIONAHA.114.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jennings BL, Anderson LJ, Estes AM, Fang XR, Song CY, Campbell WB, Malik KU. Involvement of cytochrome P-450 1B1 in renal dysfunction, injury, and inflammation associated with angiotensin II-induced hypertension in rats. Am J Physiol Renal Physiol. 2012;302:F408–F420. doi: 10.1152/ajprenal.00542.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings BL, Anderson LJ, Estes AM, Yaghini FA, Fang XR, Porter J, Gonzalez FJ, Campbell WB, Malik KU. Cytochrome P450 1B1 contributes to renal dysfunction and damage caused by angiotensin II in mice. Hypertension. 2012;59:348–354. doi: 10.1161/HYPERTENSIONAHA.111.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennings BL, George LW, Pingili AK, Khan NS, Estes AM, Fang XR, Gonzalez FJ, Malik KU. Estrogen metabolism by cytochrome P450 1B1 modulates the hypertensive effect of angiotensin II in female mice. Hypertension. 2014;64:134–140. doi: 10.1161/HYPERTENSIONAHA.114.03275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jennings BL, George LW, Pingili AK, Khan NS, Estes AM, Fang XR, Gonzalez FJ, Malik KU. Estrogen metabolism by cytochrome P450 1B1 modulates the hypertensive effect of angiotensin II in female mice. Hypertension. 2014;64:134–140. doi: 10.1161/HYPERTENSIONAHA.114.03275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jennings BL, Moore JA, Pingili AK, Estes AM, Fang XR, Kanu A, Gonzalez FJ, Malik KU. Disruption of the cytochrome P-450 1B1 gene exacerbates renal dysfunction and damage associated with angiotensin II-induced hypertension in female mice. Am J Physiol Renal Physiol. 2015;308:F981–F992. doi: 10.1152/ajprenal.00597.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman M, Sutter CH, Emmert GL, Sutter TR. Regioselective 2-hydroxylation of 17β-estradiol by rat cytochrome P4501B1. Toxicol Appl Pharmacol. 2006;216:469–478. doi: 10.1016/j.taap.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Zacharia LC, Dubey RK, Mi Z, Jackson EK. Methylation of 2-hydroxyestradiol in isolated organs. Hypertension. 2003;42:82–87. doi: 10.1161/01.HYP.0000074702.06466.27. [DOI] [PubMed] [Google Scholar]
- 31.Crespi CL, Penman BW, Steimel DT, Smith T, Yang CS, Sutter TR. Development of a human lymphoblastoid cell line constitutively expressing human CYP1B1 cDNA: substrate specificity with model substrates and promutagens. Mutagenesis. 1997;12:83–89. doi: 10.1093/mutage/12.2.83. [DOI] [PubMed] [Google Scholar]
- 32.Song J, Wadhwa L, Bejjani BA, O’Brien WE. Determination of 3-keto-4-ene steroids and their hydroxylated metabolites catalyzed by recombinant human cytochrome P450 1B1 enzyme using gas chromatography-mass spectrometry with trimethylsilyl derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:127–135. doi: 10.1016/s1570-0232(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 33.Pingili AK, Kara M, Khan NS, Estes AM, Lin Z, Li W, Gonzalez FJ, Malik KU. 6β-hydroxytestosterone, a cytochrome P450 1B1 metabolite of testosterone, contributes to angiotensin II-induced hypertension and its pathogenesis in male mice. Hypertension. 2015;65:1279–1287. doi: 10.1161/HYPERTENSIONAHA.115.05396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intra renal Angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffin SA, Brown WC, MacPherson F, McGrath JC, Wilson VG, Korsgaard N, Mulvany MJ, Lever AF. Angiotensin II causes vascular hypertrophy in part by a non-pressor mechanism. Hypertension. 1991;17:626–635. doi: 10.1161/01.hyp.17.5.626. [DOI] [PubMed] [Google Scholar]
- 36.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102:720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 37.Mervaala E, Müller DN, Schmidt F, Park JK, Gross V, Bader M, Breu V, Ganten D, Haller H, Luft FC. Blood pressure-independent effects in rats with human renin and angiotensinogen genes. Hypertension. 2000;35:587–594. doi: 10.1161/01.hyp.35.2.587. [DOI] [PubMed] [Google Scholar]
- 38.Su EJ, Lombardi DM, Siegal J, Schwartz SM. Angiotensin II induces vascular smooth muscle cell replication independent of blood pressure. Hypertension. 1998;3:1331–1337. doi: 10.1161/01.hyp.31.6.1331. [DOI] [PubMed] [Google Scholar]
- 39.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iliescu R, Cucchiarelli VE, Yanes LL, Iles JW, Reckelhoff JF. Impact of androgen-induced oxidative stress on hypertension in male SHR. Am J Physiol Regul Integr Comp Physiol. 2007;292:731–735. doi: 10.1152/ajpregu.00353.2006. [DOI] [PubMed] [Google Scholar]
- 41.Bhatia K, Elmarakby AA, El-Remessy AB, Sullivan JC. Oxidative stress contributes to sex differences in angiotensin II-mediated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:274–282. doi: 10.1152/ajpregu.00546.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochorme p450 4a expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 2007;50:123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 43.Wu CC, Mei S, Cheng J, Ding Y, Weidenhammer A, Garcia V, Zhang F, Gotlinger K, Manthati VL, Falck JR, Capdevila JH, Schwartzman ML. Androgen-sensitive hypertension associates with upregulated vascular CYP4A12-20-HETE synthase. J Am Soc Nephrol. 2013;24:1288–1296. doi: 10.1681/ASN.2012070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia V, Cheng J, Weidenhammer A, Ding Y, Wu CC, Zhang F, Gotlinger K, Falck JR, Schwartzman ML. Androgen-induced hypertension in angiotensinogen deficient mice: Role of 20-HETE and EETS. Prostaglandins Other Lipid Mediat. 2014;116–117:124–130. doi: 10.1016/j.prostaglandins.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive natriuretic eicosanoid in human kidney. Role of CYP4F2 and CYP4A11. J Biol Chem. 2000;275:4118–4126. doi: 10.1074/jbc.275.6.4118. [DOI] [PubMed] [Google Scholar]
- 46.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:60–68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 47.Williams JM, Sarkis A, Lopez B, Ryan RP, Flasch AK, Roman RJ. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresis. Hypertension. 2007;49:687–94. doi: 10.1161/01.HYP.0000255753.89363.47. [DOI] [PubMed] [Google Scholar]
- 48.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2004;289:496–503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 49.Silva-Antonialli MM, Tostes RCA, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res. 2004;62:587–593. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1558–1565. doi: 10.1681/ASN.2006060554. [DOI] [PubMed] [Google Scholar]
- 51.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Von Thun AM, Vari RC, el-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol Renal Physiol. 1994;266:120–128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 53.Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol. 1997;272:405–409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 54.Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol. 1997;5:412–422. [PubMed] [Google Scholar]
- 55.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009;296:F771–F779. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrison DG, Gongora MC. Oxidative stress and hypertension. Med Clin North Am. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension. 2014;64:384–390. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension. 2014;64:557–564. doi: 10.1161/HYPERTENSIONAHA.114.03512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, 2nd, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elliot SJ, Berho M, Korach K, Doublier S, Lupia E, Striker GE, Karl M. Gender-specific effects of endogenous testosterone: female alphaestrogenreceptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int. 2007;72:464–472. doi: 10.1038/sj.ki.5002328. [DOI] [PubMed] [Google Scholar]
- 62.Quan A, Chakravarty S, Chen JK, Chen JC, Loleh S, Saini N, Harris RC, Capdevila J, Quigley R. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol. 2004;287:F452–F459. doi: 10.1152/ajprenal.00188.2003. [DOI] [PubMed] [Google Scholar]
- 63.Magee J, Chang LW, Stormo GA, Milbrandt J. Direct androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology. 2006;147:590–598. doi: 10.1210/en.2005-1001. [DOI] [PubMed] [Google Scholar]
- 64.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y, Sorenson CM, Sheibani N. Cytochrome P450 1B1 and Primary Congenital Glaucoma. J Ophthalmic Vis Res. 2015;10:60–67. doi: 10.4103/2008-322X.156116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimada T, Watanabe J, Kawajiri K, Sutter TR, Guengrich FP, Gillam EMJ, I K. Catalytic properties of polymorphic human cytochrome P4501B1 variants. Carcinogenesis. 1999;20:1067–1613. doi: 10.1093/carcin/20.8.1607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.