Abstract
Background
Adolescence and young adulthood are critical vulnerability periods for initiation of tobacco smoking. White matter development is ongoing during this time and may be influenced by exposure to nicotine. Synthesis of findings from diffusion tensor imaging (DTI) studies of adolescent and young adult smokers may be helpful in understanding the relationship between neurodevelopment and initiation and progression of tobacco-use behaviors and in guiding further research.
Methods
A systematic literature review was conducted to identify DTI studies comparing adolescent and young adult (mean age <30 years) smokers versus nonsmokers. A total of 5 studies meeting inclusion criteria were identified. Primary study findings are reviewed and discussed within the context of neurodevelopment and in relation to findings from adult studies. Directions for further research are also discussed.
Results
All identified studies reported increases in fractional anisotropy (FA) among adolescent/young adult smokers in comparison to non-smokers. Increased FA was most frequently reported in regions of the corpus callosum (genu, body and spenium), internal capsule and superior longitudinal fasciculus.
Conclusions
Findings of increased FA among adolescent/young adult smokers are contrary to those from most adult studies and thus raise the possibility of differential effects of nicotine on white matter across the lifespan. Further research including multiple time points is needed to test this hypothesis. Other areas warranting further research include DTI studies of e-cigarette use and studies incorporating measures of pubertal stage.
Keywords: Addiction, Development, White matter, Nicotine dependence, Diffusion magnetic resonance imaging, (dMRI), Adolescence
1. Introduction
1.1. Tobacco smoking and adolescence
Tobacco smoking is addictive and associated with negative health effects including lung cancer, emphysema, and type-II diabetes (Adcock et al., 2011; Caramori et al., 2011; Tuesta et al., 2011; USDHHS, 2014). Despite significant advancements in treatments for smoking cessation (Cahill et al., 2013; Morean et al., 2015; Pbert et al., 2015), tobacco smoking remains a significant public health concern, with the prevalence of past-year tobacco use among US citizens over the age of 12 estimated at 25.5%, equating to 66.9 million individuals (SAMHSA, 2014).
Adolescence is a critical vulnerability period for the initiation of tobacco smoking, with earlier ages of initiation associated with greater severity of nicotine dependence (Riggs et al., 2007; Behrendt et al., 2009; Klein et al., 2013; Lanza and Vasilenko, 2015). In the United States, more than 85% of adult smokers report initiation of cigarette smoking prior to the age of 18 and essentially all adult smokers (i.e., 99.9%) report initiation by the age of 30 (USDHHS, 2014). For most individuals, tobacco-use disorder may therefore be considered an adolescent-onset disorder.
Neural development of both grey- and white-matter tissue structures is ongoing throughout childhood and adolescence and into adulthood (Giedd et al., 1999; Giedd, 2004; Lebel and Beaulieu, 2011; Raznahan et al., 2014). Within the brain, nicotine binds to nicotinic acetylcholine receptors (nAChRs) and influences neurotransmission and neuronal growth (Rüdiger and Bolz, 2008). Stimulation of nicotinic receptors with nAChR agonists (such as nicotine) results in decreased axonal surface areas, whereas nAChR antagonists increase axonal surface areas (Nordman and Kabbani, 2012). Chronic exposure to nicotine is associated with upregulation of nAChRs (Sallette et al., 2005), and preclinical data indicate that the adolescent brain may be more susceptible than the adult brain to nicotine-associated increases in nAChR expression (Goriounova and Mansvelder, 2012). Thus, relationships between neural structural characteristics and nicotine exposure may vary across developmental epochs.
1.2. Diffusion-weighted imaging
Diffusion-weighted magnetic resonance imaging (dMRI) is a widely used method for in vivo quantification of white-matter microstructures at high spatial resolution and is commonly analyzed using an approach referred to as diffusion tensor imaging (DTI; Basser, 1995; Soares et al., 2013). During dMRI, the MR signal is sensitized to the diffusion of water molecules in multiple directions. Due to the presence of physical boundaries such as those imposed by cell membranes or myelin in the axon sheath, diffusion within organized white matter is orientation-dependent, or anisotropic (DaSilva et al., 2003; Hagmann et al., 2006). By contrast, when unrestricted, diffusion of water molecules will be random and non-directional, or isotropic (Hagmann et al., 2006). Using the resultant data, it is possible to quantify diffusion within a given voxel in the brain, and this has been used to infer white-matter microstructural characteristics (Basser, 1995; Basser and Pierpaoli, 1996); for reviews see (Sullivan et al., 2010; Jones et al., 2013; Soares et al., 2013). One of the most widely used methods for quantifying diffusion within a given voxel is DTI.
Using DTI, it is possible to calculate a number of scalar indices. The most widely used index is fractional anisotropy (FA), a scalar measure ranging between 0 (isotropic diffusion) and 1 (anisotropic diffusion), based on the ratio of parallel to perpendicular diffusion within a given voxel (Pierpaoli and Basser, 1996). Another frequently used index is mean diffusivity (MD) which corresponds to the overall magnitude of diffusion, irrespective of direction; reviewed in (Sullivan et al., 2010). Broadly speaking, FA increases and MD decreases during typical development, although these changes are increasingly recognized to be both non-linear and tract-specific and vary across individuals (e.g., Barnea-Goraly et al., 2005; Lebel et al., 2008; Hasan et al., 2009; Lebel and Beaulieu, 2011; Lebel et al., 2012). While individual variability in FA values within the genu during adolescence has been associated with measures of impulsivity and risk-taking, the direction of these associations has not always been consistent across studies (e.g., Berns et al., 2009; Olson et al., 2009). Thus, further research is needed to determine the specific behavioral significance of altered white matter development in relation to substance-use behaviors amongst adolescents.
DTI studies have demonstrated alterations in white-matter tissue structures (including regions of the corpus callosum and cingulum) among adult smokers (Paul et al., 2008; Hudkins et al., 2012; Lin et al., 2012; Savjani et al., 2014; Umene-Nakano et al., 2014). Increases in FA within the genu of the corpus callosum have also been reported following acute nicotine administration in human adults (Kochunov et al., 2013). While several recent studies indicate white-matter alterations among adolescent and young adult tobacco-users, the relationship between tobacco smoking and neural development during adolescence and young adulthood is not yet well understood.
To synthesize existing findings related to tobacco smoking and white-matter development during adolescence and young adulthood, and to identify directions for further research, we here systematically review findings from published DTI studies conducted in adolescents and young adults. We aimed to identify areas requiring further study to guide future research endeavors in this vulnerable population. Implications for development of nicotine dependence in adolescence and for future research are discussed.
2. Methods
To identify DTI studies conducted in adolescent and young adult populations of smokers, Pubmed and Medline databases were searched during the first two weeks of July, 2015 using the following combinations of key words: ‘diffusion tensor’ and (‘smoking’ or ‘cigarette’ or ‘tobacco’ or ‘nicotine’) and (‘adolescent’ or ‘adolescence’ or ‘young’ or ‘youth’ or ‘young adult’). Study Abstracts and Methods sections (when necessary) were then inspected to identify studies meeting the following inclusionary criteria: (1) use of DTI; (2) inclusion of both smokers and non-smokers; (3) mean participant age ≤30 years; (4) direct comparison of smokers versus non-smokers on DTI measures. Studies comparing poly-substance users (e.g., tobacco and marijuana use) were excluded.
3. Results
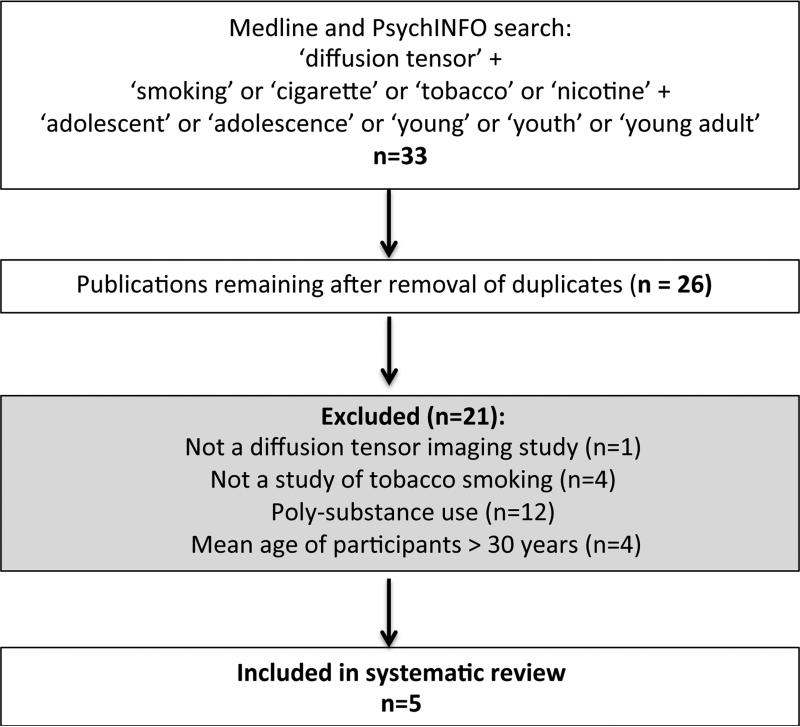
Our initial search yielded a total of 33 results (Fig. 1). After removal of duplicates and application of the inclusion criteria described above, five separate DTI studies were identified for inclusion in this review. The included publications together contained 142 smokers (114 males and 28 females) and 191 non-smokers (129 males and 62 females). The mean age of the smokers in the five studies ranged from 17 to 28 years, with an overall mean age of 21.4 years, whereas the mean age for the non-smokers ranged from 16 to 26 years, with an overall mean age of 20.22 years. The articles’ publication dates ranged from 2007 to 2015.
Fig. 1.
Schematic diagram of systematic literature review.
3.1. Overview of studies
Participant information and primary findings are summarized in Table 1. To facilitate integration across studies, we focus primarily on findings from between-group comparisons of FA values and from correlational analyses assessing associations between FA values and measures of nicotine dependence, as assessed using the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) or other measures.
Table 1.
Participant characteristics and primary findings from identified DTI studies of adolescent/young adult smokers.
| Study | Participants: male/female; age (years; mean ± st. dev) | FTND score (mean ± st. dev.) | Multiple comparison correction | Time since last cigarette | Co-varied for age? | Primary findings |
|---|---|---|---|---|---|---|
| Jacobsen et al. (2007) | Smokers: 7/7 Controls: 8/12 Smokers: 17.0 ± 0.7 Controls: 16.3 ± 1.2 |
3.1 ± 1.8 | FDR p < 0.01 | Not specified | Not specified | FA in smokers f in CC genu, CC splenium, L inferior and R superior longitudinal fasciculus, R forceps minor, L forceps minor, R anterior limb of the internal capsule, R frontal association fibers. Relationship to FTND scores not assessed. |
| Liao et al. (2011) | Smokers: 36/8 Controls: 34/10 Smokers: 28.0 ± 5.6 Controls: 26.3 ± 5.8 |
Not reported | Uncorrected-p < 0.001; k > 100 | 12h | No | FA in smokers ↑ in R & L superior longitudinal fasciculus. Relationship to FTND scores not assessed. FA not associated with other smoking variables (e.g., age-of-onset, cigarettes/day). |
| Huang et al. (2013) | Smokers: 9/2 Controls: 6/4 Smokers: 23.7 ± 1.98 Controls: 22.5 ± 6.78 |
4.0± 1.7 | Not specified (ROI-based) | Not specified | Not specified | FA in smokers ↑ in L anterior cingulum bundle in smokers compared to non-smokers. Whole-brain FA not assessed. No significant associations between FTND and FA. |
| van Ewijk et al. (2015) | Smokers: 39/11 Controls: 59/36 Smokers: 18.8 ± 2.0 Controls: 16.7 ± 2.2 |
Not reported | FWE p < 0.05 | Not specified | Yes | FA ↑ and MD ↓ in smokers in WM in/around basal ganglia, thalamus, internal & external capsule, corpus callosum, anterior corona radiata, superior longitudinal fasciculus and corticospinal tract. Relationship to FTND scores not assessed. |
| Yu et al. (2015) | Smokers: 23/0 Controls: 22/0 Smokers: 19.6 ± 1.9 Controls: 19.3 ± 2.4 |
6.7 ± 1.3 | FWE p < 0.05 | Not specified | Yes | FA in smokers ↑ in CC body, CC splenium, bilateral internal & external capsules, bilateral superior and posterior corona radiate, bilateral posterior thalamic radiations, and L superior longitudinal fasciculus. No differences in MD. Negative association between FTND and FA in R superior corona radiata. |
FA = fractional anisotropy; CC = corpus callosum; L= left; R-right; FDR = false-discovery-rate; FWE = family-wise-error; ROI = region-of-interest; WM = white matter; FTND = Fagerstrom Test for Nicotine Dependence; MD = mean diffusivity.
3.1.1. Jacobsen et al. (2007)
This study assessed the relationship between adolescent tobacco-smoking, prenatal exposure to tobacco and DTI measures. Subgroups of non-prenatally tobacco exposed adolescents included 14 smokers and 20 non-smokers, ranging from 13 to 18 years, with mean(SD) ages of 17.0(0.7) and 16.3(1.2) years, respectively. Diffusion data were analyzed using the BioImage Suite (http://bioimagesuite.yale.edu). Voxel-wise statistics were restricted to white matter using an anatomical mask (MNI Colin brain) and false discovery rate (FDR) corrected for multiple comparisons at pFDR < 0.01 (k > 10). Comparisons of non-prenatally tobacco-exposed adolescents indicated increased FA among smokers in the left inferior and right superior longitudinal fasciculus, bilateral forceps minor, left splenium, right genu, right anterior limb of the internal capsule, right frontal association fibers and right frontal short association fibers. While associations between nicotine dependence (e.g., FTND score) and diffusion indices were not assessed, there was a significant positive association between pack years and FA values within the corpus genu, suggesting that longer durations of smoking are associated with increased FA in this region during adolescence. This study was the first to assess white-matter characteristics among adolescent smokers and included detailed assessment of clinical measures including depressive symptoms and alcohol consumption. Increases in FA among smokers versus non-smokers remained significant after controlling for these and other clinical variables.
3.1.2. Liao et al. (2011)
In the second largest published DTI study of young adult smokers, Liao et al. (2011) compared FA between 44 smokers and 44 non-smokers, ranging in age from 19 to 39 years and with mean(SD) ages of 28.0(5.6) and 26.3(5.8) years, respectively. All participants were asked to abstain from smoking for 12 h prior to scanning and were provided with nicotine patches ‘as needed’ (Liao et al., 2011). Pre-processing and calculation of FA maps were conducted in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). FA maps were then entered into SPM5 (http://www.fil.ion. ucl.ac.uk/spm/) for group-level statistics, which were performed using an inclusive white-matter mask (from WFU PickAtlas) at an uncorrected statistical threshold of p < 0.001 (k > 100). Between-group comparisons indicated significantly increased FA in bilateral fronto-parietal cortices, specifically within bilateral superior longitudinal fasciculus, in smokers compared to non-smokers. The FTND was not used in this study; however, correlational analyses with other smoking-related variables (e.g., age of smoking onset, number of cigarettes/day) did not indicate any significant associations with FA values. Despite the relatively large sample size, this study included only a small number of female participants; thus, further studies using larger samples of female smokers are needed. An additional limitation of this study is the use of an uncorrected threshold of p < 0.001 with a cluster forming threshold of k>100. Caution should be used when interpreting findings from this and other studies not using a formal correction for multiple comparisons.
3.1.3. Huang et al. (2013)
Huang et al. (2013) compared diffusion data between 11 young adult smokers and 10 young adult non-smokers between the ages of 18–39 years and with mean(SD) ages of 23.7(1.98) years and 22.5(6.78) years, respectively. Preprocessing, calculation of FA and group-level statistics (tract-based spatial statistics; TBSS) were conducted in FSL. Statistical analyses were conducted using a region-of interest-based (ROI-based) approach focusing on the anterior cingulum. In comparison to non-smokers, smokers had increased FA within the right anterior cingulum. FA values within the left anterior cingulum were negatively associated with self-report measures of nicotine dependence, although associations with the FTND did not reach statistical significance. Strengths of this study include assessment of associations between FA and multiple smoking-related measures. However, study findings should be interpreted within the context of the relatively small sample size.
3.1.4. van Ewijk et al. (2015)
This study examined the relationship between DTI-derived indices and tobacco smoking among adolescents and young adults aged 16–24 years with and without attention-deficit/hyperactivity disorder (ADHD). The mean(SD) age of regular smokers and non-smokers was 18.8(2.0) and 16.7(2.2) years, respectively. Pre-processing and calculation of FA and MD maps were conducted using a combination of SPM8 and in-house methods. Between-group comparisons constrained to white-matter tracts were conducted using TBSS and FSL's ‘randomise’ and threshold-free-cluster-enhancement for family-wise-error (FWE) correction at pFWE < 0.05. In comparison to non-smokers, regular smokers exhibited increased FA and decreased MD within the thalamus, internal and external capsule, corpus callosum (subregions not specified), anterior corona radiata, right superior longitudinal fasciculus and corticospinal tract. No measures of nicotine dependence (e.g., FTND scores) or assessments of alcohol use were reported. Participants diagnosed with ADHD (smokers and non-smokers) had lower FA than did those without (smokers and non-smokers). While there was some overlap between the tracts associated with ADHD and smoking (including parts of the corpus callosum, bilateral internal capsule, corticospinal tract, right external capsule, superior longitudinal fasciculus, and anterior corona radiata), effects were in opposing directions suggesting independent contributions to FA. Study strengths include a relatively large sample size and assessment of interaction effects involving ADHD diagnosis and smoking. Limitations include the absence of alcohol-use information for participants.
3.1.5. Yu et al. (2015)
Participants included male adolescents and young adults, ranging from 14 to 23 years with mean(SD) ages of 19.6(1.9) and 19.3(2.4) years for 23 smokers and 22 non-smokers, respectively. Pre-processing and calculation of FA were conducted in FSL. Between-group comparisons constrained to white-matter tracts were conducted using TBSS and FSL's ‘randomise’ and threshold-free-cluster-enhancement for correction at pFWE < 0.05.
In comparison to non-smokers, smokers exhibited increased FA in the body and splenium of the corpus callosum, bilateral internal and external capsules, bilateral superior and posterior corona radiata, bilateral posterior thalamic radiata, and left superior longitudinal fasciculus. Increases in FA within these tracts were accompanied by decreases in radial (perpendicular) diffusivity. Increased axial (parallel) diffusion was also reported among smokers versus non-smokers within the right internal and external capsules and superior corona radiata. No between-group differences in MD were observed between smokers and non-smokers. FA values within the right superior corona radiata were negatively associated with FTND scores and positively associated with cigarette consumption (pack years). Study strengths include the young age range of the participants (16–23 years), use of multiple DTI indices and inclusion of age as a covariate in between-group comparisons. Study limitations include the absence of female participants.
4. Discussion
4.1. Fractional anisotropy
All identified studies reported increases in FA among adolescent/young adult smokers in comparison to control participants, and these were most frequently reported in regions of the corpus callosum (genu, body and splenium), internal capsules and superior longitudinal fasciculus (Jacobsen et al., 2007; Liao et al., 2011; van Ewijk et al., 2015; Yu et al., 2015). Taken together, these data suggest that tobacco smoking among adolescents and young adults is associated with increases in white-matter fiber coherence or ‘integrity’, insofar as these may be inferred from FA values.
These findings are somewhat contrary to those from studies conducted in mature adult populations. While increased FA values within the corpus callosum and other tracts have been reported in some studies of adult smokers (Paul et al., 2008; Hudkins et al., 2012), decreased FA values are more commonly reported in the same regions (Lin et al., 2013; Savjani et al., 2014; Umene-Nakano et al., 2014; Viswanath et al., 2015). Taken together, this suggests that the relationship between FA values and tobacco use may differ between early versus middle adulthood, although other possibilities (acute versus chronic effects of smoking, cohort effects) cannot be excluded.
Data suggest significant effects of recency of cigarette smoking on DTI measures in adults, such that recent smoking is associated with greater decreases in FA (Savjani et al., 2014). However, none of the studies reviewed above controlled for acute effects of nicotine, and the majority included no information on time since last cigarette. Liao et al. (2011) required 12 h of smoking abstinence prior to scanning, but also reported providing nicotine replacement therapy to assist with abstinence. Thus, an important direction for further studies will be to disentangle acute versus chronic effects of smoking (and of nicotine administration more generally) among young adults.
In human adult populations, increases in genual corpus callosal FA have been reported following acute nicotine administration (Kochunov et al., 2013). Thus, increased FA among adolescent/young adult smokers may be a consequence of exposure to nicotine. Consistent with this interpretation, positive associations between pack years and FA values within the genu (Jacobsen et al., 2007) and corona radiata (Yu et al., 2015) were reported among adolescent/young adult smokers. In contrast to this, negative associations between FTND scores and FA values within the anterior cingulum (Huang et al., 2013) and corona radiata (Yu et al., 2015) were also reported. Thus, one hypothesis is that lower FA values might be a vulnerability factor for physical dependence during adolescence/young adulthood whereas higher FA values might emerge as a function of exposure to nicotine during this time. However, further research using longitudinal designs is needed to test this hypothesis.
Preclinical data suggest neurogenesis-related effects of nicotine. In mice, nicotine exposure upregulates the expression of nerve growth factor (NGF) and mRNA levels of its receptor tyrosine receptor kinase A (trkA) (Garrido et al., 2003), indicating that nicotine may enhance neuronal development. Other preclinical work suggests that neuroblasts in the cerebellum treated with nicotine increase the precursor cell's DNA synthesis significantly compared to untreated controls (Opanashuk et al., 2001), further indicating an enhancing role for nicotine in neurogenesis and myelination. Given greater rapidity of white-matter development during adolescence versus adulthood (Giedd et al., 1999), such nicotine-induced cell proliferation may differentially influence white-matter tissue structure and function across the lifespan.
Increases in diffusion anisotropy co-occur with increased myelination reviewed in (Le Bihan et al., 2001). Thus, increased FA may reflect increased myelination. However, this interpretation should not be made without direct evidence, as the opposite may also be true; reviewed in (Soares et al., 2013). Instead of an increase in FA indicating increased myelination, some research indicates that increased FA could be the result of vasogenic swelling within white-matter tracts in response to chronic nicotine exposure; reviewed in (Gazdzinski et al., 2005). Further research is therefore needed to characterize the meaning of increased FA at the molecular level among adolescent and young adult tobacco-users.
4.2. Non-FA indices of diffusion
Simultaneous assessment of multiple DTI-derived indices may provide a more comprehensive assessment of white-matter tissues and assist in interpretation of FA values. Two of the five studies identified included assessment of MD; however, findings were not consistent across studies, with one study reporting decreased MD (van Ewijk et al., 2015) and the other no differences (Yu et al., 2015). At the microstructural level, decreased MD has been associated with increases in intracellular fluid content, or cytotoxic edema, due to excess water content within the myelinated axon; reviewed in (Lin et al., 2015). However, the meaning of MD (and other measures) within a developmental context – when changes in diffusion-derived indices may reflect maturational differences – is less clear (Lebel and Beaulieu, 2011).
Only one of the identified studies included assessment of radial and axial diffusion. Typically, the loss of myelin is associated with greater radial diffusion, or water diffusion perpendicular to the axon, and less axial diffusion, or water diffusion parallel to the axon; reviewed in (Mori and Zhang, 2006). Thus, findings from Yu et al. (2015) of decreased radial diffusivity accompanied by increased axial diffusivity within tracts including the internal and external capsules are consistent with the notion of an increase in myelination among adolescent smokers (although more direct evidence is needed to support this hypothesis). However, as this study only included male smokers, additional studies assessing multiple indices of white matter in both male and female adolescent and young adult smokers are needed to confirm and extend these findings. For example, analysis approaches allowing for estimation of diffusion for multiple fiber orientations per voxel may be helpful for assessing diffusion within the context of regions of crossing fibers (Behrens et al., 2007; Jbabdi et al., 2010; Baumgartner et al., 2015; Reveley et al., 2015), as has been done in one recent study of mature adult smokers (Savjani et al., 2014).
4.3. Clinical significance and co-occurring disorders
The functional significance of increased FA values among adolescent and young adult smokers remains unclear. FA values within regions including the corpus genu are negatively associated with delay discounting during adolescence (Olson et al., 2009). Within this context, findings of increased FA values among adolescent smokers – a population that typically exhibits high rates of delay discounting (Imhoff et al., 2014) – are in some ways surprising. By contrast, increased FA values within the corpus genu have also been positively associated with risk-taking behaviors among adolescents (Berns et al., 2009). Such seemingly contrary findings highlight the need for further adolescent research assessing multiple aspects of risk-taking and neural development. For example, longitudinal DTI assessments before and after smoking initiation may provide information on whether individuals with certain white-matter characteristics are predisposed to smoking, whether smoking leads to white-matter changes (and if so, whether the changes relate to specific developmental epochs or contexts), or whether other possibilities occur. Further DTI studies incorporating behavioral assessments of impulsivity and other individual-difference measures will also be important in determining the clinical significance of increased FA values in this population.
Most studies included in this review included healthy adolescents, those without non-smoking-related psychopathology (Liao et al., 2011; Huang et al., 2013; Yu et al., 2015). However, van Ewijk et al. (2015) included adolescent smokers and nonsmokers with and without ADHD. While effects of both ADHD and tobacco smoking on FA values were observed, findings were in opposing directions and did not interact, suggesting independent effects of ADHD and tobacco smoking on FA during adolescence (van Ewijk et al., 2015). As development of white-matter structures is ongoing during early and middle adulthood (Lebel et al., 2008; Lebel and Beaulieu, 2011; Lebel et al., 2012), further research assessing the relationship between white-matter characteristics, tobacco smoking and co-occurring disorders across different developmental epochs is warranted. Given associations between adolescent smoking and depressive symptoms (Audrain-McGovern et al., 2009, 2012), assessment of these factors in relation to mood disorders specifically appears warranted.
4.4. Summary and future directions
Overall, findings from adolescent and young adult DTI studies of tobacco smoking indicate increased FA among smokers versus non-smokers. These findings are contrary to those from most adult studies (Savjani et al., 2014; Lin et al., 2013; Umene-Nakano et al., 2014; Viswanath et al., 2015), highlighting the importance of assessing relationships with white matter across different developmental epochs. Below several other important areas for future research in this area are highlighted.
4.4.1. Acute effects of nicotine
As discussed above, recent data from adult studies suggest effects of acute nicotine (Kochunov et al., 2013) – and of time since last cigarette (Savjani et al., 2014) – on DTI indices. Thus, an important consideration for future studies will be how best to disentangle acute versus chronic effects of nicotine in adolescents and young adults. Such research could employ within-subjects designs to assess intra-individual changes in DTI measures following acute nicotine versus a period of abstinence, but would need to be conducted among individuals 18 years and older.
4.4.2. Pubertal stage
Puberty is an important developmental period, the onset of which is associated with emotional, motivational and behavioral changes, including the initial use of substances such as marijuana, alcohol, and cigarettes (Kong et al., 2013). Puberty is associated with rapid increases in gonadal and adrenal hormones which contribute to sexual maturation and influence brain functioning (Spear, 2000). Emerging data suggest significant effects of these hormones on white-matter microstructures (Garcia-Segura et al., 2001; Perrin et al., 2008; Arevalo et al., 2010; Pesaresi et al., 2015). However, to our knowledge, no studies have assessed interactions between pubertal stage and tobacco smoking in relation to white-matter development.
4.4.3. E-cigarettes
Electronic cigarettes (e-cigarettes) are increasingly popular worldwide and may be particularly appealing to adolescents for multiple reasons including the variety of flavors available and the perceived absence of negative health effects (Kong et al., 2014; Anand et al., 2015; Krishnan-Sarin et al., 2015; Pokhrel et al., 2015). While the long-term health consequences of e-cigarette use during adolescence are largely unknown (Collaco et al., 2015), recent data indicate that e-cigarette use during early adolescence is itself associated with an increased likelihood of initiation of cigarette smoking and use of other combustible tobacco products (e.g., cigars, hookahs; Leventhal et al., 2015). Given possible effects of acute nicotine on white matter (Kochunov et al., 2013), e-cigarette use may confer increased vulnerability for initiation of combustible tobacco products via nicotine-induced changes in neural plasticity. However, none of the identified studies reported information on any form of tobacco-use other than cigarette smoking. Thus, further research into the neurobiology of e-cigarette and other forms of tobacco-use (e.g., hookahs, chewing tobacco) during adolescence is needed.
5. Conclusions
Collectively, findings from DTI studies conducted in adolescent and young adult populations indicate increases in FA among tobacco smokers (Jacobsen et al., 2007; Liao et al., 2011; Huang et al., 2013; van Ewijk et al., 2015; Yu et al., 2015). These findings are opposite to those from most studies of adults (Lin et al., 2013; Savjani et al., 2014; Umene-Nakano et al., 2014; Viswanath et al., 2015), raising the possibility that the relationship between white-matter structures and tobacco smoking may differ across the lifespan. However, no previous studies have controlled for the effects of acute nicotine on DTI indices, nor have they assessed possible effects of pubertal stage and alternative nicotine administration methods (e.g., hookahs, e-cigarettes). Further research is therefore needed to understand the longitudinal relationships between FA and other DTI measures and initiation and progression of smoking. Such work should be extended to treatment-seeking populations, as data suggest that behavioral and pharmacological therapies may influence white-matter tissue characteristics (Harsan et al., 2008; Schlaug et al., 2009).
Acknowledgement
The authors would like to thank Prof. Suchitra Krishnan-Sarin for helpful suggestions regarding this manuscript.
Role of funding source
This work was supported in part by NIH grants T32 DA007238 and P20 DA027844 from the National Institutes of Health; CASAColumbia; the Connecticut State Department of Mental Health and Addiction Services; the Connecticut Mental Health Center; and a Center of Excellence in Gambling Research Award from the National Center for Responsible Gaming.
Footnotes
Contributors
A.G. and S.Y. conducted the literature search. A.G. performed the initial literature review and wrote the first draft of the manuscript. S.Y. and M.P. worked with A.G. on subsequent drafts on the manuscript. All authors have approved the final submission.
Conflict of interest
The authors report no financial conflicts of interest with respect to the content of this manuscript. Dr. Potenza has consulted for Ironwood, Lundbeck, Shire, INSYS and Rivermend pharmaceuticals; has received research support from the National Institutes of Health, Mohegan Sun Casino, the National Center for Responsible Gaming and Pfizer; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse-control disorders or other health topics; has consulted for gambling and legal entities on issues related to impulse-control/addictive disorders; provides clinical care in a problem gambling services program; has performed grant reviews for the National Institutes of Health and other agencies; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
References
- Adcock IM, Caramori G, Barnes PJ. Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration. 2011;81:265–284. doi: 10.1159/000324601. [DOI] [PubMed] [Google Scholar]
- Anand V, McGinty KL, O'Brien K, Guenthner G, Hahn E, Martin CA. E-cigarette use and beliefs among urban public high school students in North Carolina. J. Adolesc. Health. 2015;57:46–51. doi: 10.1016/j.jadohealth.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: implications for neuroprotection. Biochim. Biophys. Acta. 2010;1800:1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Kassel JD. Adolescent smoking and depression: evidence for self-medication and peer smoking mediation. Addiction. 2009;104:1743–1756. doi: 10.1111/j.1360-0443.2009.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Rodgers K, Cuevas J, Sass J, Riley T. Reward expectations lead to smoking uptake among depressed adolescents. Drug Alcohol Depend. 2012;120:181–189. doi: 10.1016/j.drugalcdep.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb. Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Basser P. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Nash K, Hill C, Knoch D. Neuroanatomy of intergroup bias: a white matter microstructure study of individual differences. NeuroImage. 2015;122:345–354. doi: 10.1016/j.neuroimage.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Behrendt S, Wittchen HU, Hofler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug Alcohol Depend. 2009;99:68–78. doi: 10.1016/j.drugalcdep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Moore S, Capra CM. Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. PLoS One. 2009;4:e6773. doi: 10.1371/journal.pone.0006773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst. Rev. 2013:5Cd009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramori G, Casolari P, Cavallesco GN, Giuffre S, Adcock I, Papi A. Mechanisms involved in lung cancer development in COPD. Int. J. Biochem. Cell Biol. 2011;43:1030–1044. doi: 10.1016/j.biocel.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Collaco JM, Drummond MB, McGrath-Morrow SA. Electronic cigarette use and exposure in the pediatric population. JAMA Pediatr. 2015;169:177–182. doi: 10.1001/jamapediatrics.2014.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Tuch DS, Wiegell MR, Hadjikhani N. A primer on diffusion tensor imaging of anatomical substructures. Neurosurg. Focus. 2005;15:E4. doi: 10.3171/foc.2003.15.1.4. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog. Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Garrido R, King-Pospisil K, Son KW, Hennig B, Toborek M. Nicotine upregulates nerve growth factor expression and prevents apoptosis of cultured spinal cord neurons. Neurosci. Res. 2003;47:349–355. doi: 10.1016/s0168-0102(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain mri in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol. Clin. Exp. Res. 2005;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goriounova NA, Mansvelder HD. Nicotine exposure during adolescence alters the rules for prefrontal cortical synaptic plasticity during adulthood. Front. Synaptic Neurosci. 2012;4:3. doi: 10.3389/fnsyn.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen V, Meuli J, R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(Suppl. 1):S205–223. doi: 10.1148/rg.26si065510. [DOI] [PubMed] [Google Scholar]
- Harsan LA, Steibel J, Zaremba A, Agin A, Sapin R, Poulet P, Guignard B, Parizel N, Grucker D, Boehm N, Miller RH, Ghandour MS. Recovery from chronic demyelination by thyroid hormone therapy: myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. J. Neurosci. 2008;28:14189–14201. doi: 10.1523/JNEUROSCI.4453-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski LK, Frecher RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br. J. Addict. 1991;86:119–127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Huang W, DiFranza JR, Kennedy DN, Zhang N, Ziedonis D, Ursprung S, King JA. Progressive levels of physical dependence to tobacco coincide with changes in the anterior cingulum bundle microstructure. PLoS One. 2013;8:e67837. doi: 10.1371/journal.pone.0067837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudkins M, O'Neill J, Tobias MC, Bartzokis G, London ED. Cigarette smoking and white matter microstructure. Psychopharmacology. 2012;221:285–295. doi: 10.1007/s00213-011-2621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff S, Harris M, Weiser J, Reynolds B. Delay discounting by depressed and non-depressed adolescent smokers and non-smokers. Drug Alcohol Depend. 2014;135:152–155. doi: 10.1016/j.drugalcdep.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, Jackowski MP, Constable RT, Mencl WE. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J. Neurosci. 2007;27:13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S, Behrens TE, Smith SM. Crossing fibres in tract-based spatial statistics. Neuroimage. 2010;49:249–256. doi: 10.1016/j.neuroimage.2009.08.039. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Klein H, Sterk CE, Elifson KW. Initial smoking experiences and current smoking behaviors and perceptions among current smokers. J. Addict. 2013 doi: 10.1155/2013/491797. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Du X, Moran LV, Sampath H, Wijtenburg SA, Yang Y, Rowland LM, Stein EA, Hong LE. Acute nicotine administration effects on fractional anisotropy of cerebral white matter and associated attention performance. Front. Pharmacol. 2013;4:117. doi: 10.3389/fphar.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob. Res. 2014;17:847–854. doi: 10.1093/ntr/ntu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Smith AE, McMahon TJ, Cavallo DA, Schepis TS, Desai RA, Potenza MN, Krishnan-Sarin S. Pubertal status, sensation-seeking, impulsivity, and substance use in high school-aged boys and girls. J. Addict. Med. 2013;7:116–121. doi: 10.1097/ADM.0b013e31828230ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Morean ME, Camenga DR, Cavallo DA, Kong G. E-cigarette use among high school and middle school adolescents in Connecticut. Nicotine Tob. Res. 2015;17:810–818. doi: 10.1093/ntr/ntu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Vasilenko SA. New methods shed light on age of onset as a risk factor for nicotine dependence. Addict. Behav. 2015;50:161–164. doi: 10.1016/j.addbeh.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, Stone MD, Khoddam R, Samet JM, Audrain-McGovern J. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA. 2015;314:700–707. doi: 10.1001/jama.2015.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Tang J, Deng Q, Deng Y, Luo T, Wang X, Chen H, Liu T, Chen X, Brody AL, Hao W. Bilateral fronto-parietal integrity in young chronic cigarette smokers: a diffusion tensor imaging study. PLoS One. 2011;6:e26460. doi: 10.1371/journal.pone.0026460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Wu G, Zhu L, Lei H. Heavy smokers show abnormal microstructural integrity in the anterior corpus callosum: a diffusion tensor imaging study with tract-based spatial statistics. Drug Alcohol Depend. 2013;129:82–87. doi: 10.1016/j.drugalcdep.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Lin F, Zhou Y, Du Y, Qin L, Zhao Z, Xu J, Lei H. Abnormal white matter integrity in adolescents with internet addiction disorder: a tract-based spatial statistics study. PLoS One. 2012;7:e30253. doi: 10.1371/journal.pone.0030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, He H, Schifitto G, Zhong J. Simulation of changes in diffusion related to different pathologies at cellular level after traumatic brain injury. Magn. Reson. Med. 2015 doi: 10.1002/mrm.25816. epus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Kong G, Camenga DR, Cavallo DA, Carroll KM, Pittman B, Krishnan-Sarin S. Contingency management improves smoking cessation treatment outcomes among highly impulsive adolescent smokers relative to cognitive behavioral therapy. Addict. Behav. 2015;42:86–90. doi: 10.1016/j.addbeh.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Nordman JC, Kabbani N. An interaction between α7 nicotinic receptors and a G-protein pathway complex regulates neurite growth in neural cells. J. Cell Sci. 2012;125:5502–5513. doi: 10.1242/jcs.110379. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. J. Cogn. Neurosci. 2009;21:1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opanashuk LA, Pauly JR, Hauser KF. Effect of nicotine on cerebellar granule neuron development. Eur. J. Neurosci. 2001;13:48–56. [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Grieve SM, Niaura R, David SP, Laidlaw DH, Cohen R, Sweet L, Taylor G, Clark CR, Pogun S, Gordon E. Chronic cigarette smoking and the microstructural integrity of white matter in healthy adults: a diffusion tensor imaging study. Nicotine Tob. Res. 2008;10:137–147. doi: 10.1080/14622200701767829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pbert L, Farber H, Horn K, Lando HA, Muramoto M, O'Loughlin J, Tanski S, Wellman RJ, Winickoff JP, Klein JD. State-of-the-art office-based interventions to eliminate youth tobacco use: the past decade. Pediatrics. 2015;135:734–747. doi: 10.1542/peds.2014-2037. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J. Neurosci. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaresi M, Soon-Shiong R, French L, Kaplan DR, Miller FD, Paus T. Axon diameter and axonal transport: in vivo and in vitro effects of androgens. Neuroimage. 2015;115:191–201. doi: 10.1016/j.neuroimage.2015.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn. Reson. Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pokhrel P, Fagan P, Kehl L, Herzog TA. Receptivity to e-cigarette marketing harm perceptions, and e-cigarette use. Am. J. Health Behav. 2015;39:121–131. doi: 10.5993/AJHB.39.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, Pipitone J, Chakravarty MM, Giedd JN. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1592–1597. doi: 10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveley C, Seth AK, Pierpaoli C, Silva AC, Yu D, Saunders RC, Leopold DA, Ye FQ. Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E2820–2828. doi: 10.1073/pnas.1418198112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs NR, Chou CP, Li C, Pentz MA. Adolescent to emerging adulthood smoking trajectories: when do smoking trajectories diverge, and do they predict early adulthood nicotine dependence? Nicotine Tob. Res. 2007;9:1147–1154. doi: 10.1080/14622200701648359. [DOI] [PubMed] [Google Scholar]
- Rüdiger T, Bolz J. Acetylcholine influences growth cone motility and morphology of developing thalamic axons. Cell Adhes. Migr. 2008;2:30–37. doi: 10.4161/cam.2.1.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux J-P, Corringer PJ. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD.: 2014. NSDUH Series H-48. HHS Publication No. (SMA) 14-4863. [Google Scholar]
- Savjani RR, Velasquez KM, Thompson-Lake DG, Baldwin PR, Eagleman DM, De La Garza R, Salas R., 2nd Characterizing white matter changes in cigarette smokers via diffusion tensor imaging. Drug Alcohol Depend. 2014;145:134–142. doi: 10.1016/j.drugalcdep.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca's aphasia undergoing intense intonation-based speech therapy. Ann. N. Y. Acad. Sci. 2009;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JM, Marques P, Alves V, Sousa N. A hitchhiker's guide to diffusion tensor imaging. Front. Neurosci. 2013;7:31. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Longitudinal study of callosal microstructure in the normal adult aging brain using quantitative DTI fiber tracking. Dev. Neuropsychol. 2010;35:233–256. doi: 10.1080/87565641003689556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta LM, Fowler CD, Kenny PJ. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem. Pharmacol. 2011;82:984–995. doi: 10.1016/j.bcp.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umene-Nakano W, Yoshimura R, Kakeda S, Watanabe K, Hayashi K, Nishimura J, Takahashi H, Moriya J, Ide S, Ueda I, Hori H, Ikenouchi-Sugita A, Katsuki A, Atake K, Abe O, Korogi Y, Nakamura J. Abnormal white matter integrity in the corpus callosum among smokers: tract-based spatial statistics. PLoS One. 2014;9:e87890. doi: 10.1371/journal.pone.0087890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS . The Health Consequenses of Smoking—50 Years of of Progress: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Preven-tion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. Chapter 13: patterns of tobacco use among U.S. youth, young adults, and adults. pp. 703–770. [Google Scholar]
- van Ewijk H, Groenman AP, Zwiers MP, Heslenfeld DJ, Faraone SV, Hartman CA, Luman M, Greven CU, Hoekstra PJ, Franke B, Buitelaar J, Oosterlaan J. Smoking and the developing brain: altered white matter microstructure in attention-deficit/hyperactivity disorder and healthy controls. Hum. Brain Mapp. 2015;36:1180–1189. doi: 10.1002/hbm.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath H, Velasquez KM, Thompson-Lake DG, Savjani R, Carter AQ, Eagleman D, Baldwin PR, De La Garza R, 2nd, Salas R. Alterations in interhemispheric functional and anatomical connectivity are associated with tobacco smoking in humans. Front. Hum. Neurosci. 2015;9:116. doi: 10.3389/fnhum.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Yuan K, Zhang B, Liu J, Dong M, Jin C, Luo L, Zhai J, Zhao L, Zhao Y, Gu Y, Xue T, Liu X, Lu X, Qin W, Tian J. White matter integrity in young smokers: a tract-based spatial statistics study. Addict. Biol. 2015 doi: 10.1111/adb.12237. epub. [DOI] [PubMed] [Google Scholar]